Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Flash phase engineering of MoS2 nanofilms for enhanced photoelectrochemical performance†
Rong Tanab,
Yuxin Liu ac,
Yifeng Tu
ac,
Yifeng Tu *b and
Felix F. Loeffler
*b and
Felix F. Loeffler *a
*a
aDepartment of Biomolecular Systems, Max Planck Institute of Colloids and Interface, 14476 Potsdam, Germany. E-mail: Felix.Loeffler@mpikg.mpg.de
bCollege of Chemistry, Chemical Engineering and Material Science, Soochow University, 215123 Suzhou, China. E-mail: tuyf@suda.edu.cn
cDepartment of Chemistry and Biochemistry, Freie Universität Berlin, 14195 Berlin, Germany
First published on 5th February 2024
Abstract
A heterophase structure combining semiconducting 2H- and metallic 1T-MoS2 exhibits significantly enhanced photoelectrochemical performance due to the electrical coupling and synergistic effect between the phases. Therefore, site-selective effective phase engineering is crucial for the fabrication of MoS2-based photoelectrochemical devices. Here, we employed a flash phase engineering (FPE) strategy to precisely fabricate a 2H-1T heterophase structure. This technique allows simple, efficient, and precise control over the micropatterning of MoS2 nanofilms while enabling site-selective phase transition from the 1T to the 2H phase. The detection of reduced glutathione (GSH) showed an approximately 5-fold increase in sensitivity when using the electrode fabricated by FPE.
Introduction
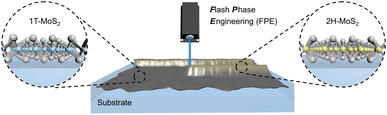
Two-dimensional transition metal dichalcogenides have attracted considerable attention due to their broad potential in electronics, catalysis, biosensors, and energy storage.1–5 Among them, molybdenum disulfide (MoS2) emerges as the most prominent, featuring a metallic 1T phase and semiconducting 2H phase.6,7 While 1T-MoS2 is a good electrical conductor, it lacks sufficient optical response capabilities. Conversely, 2H-MoS2 exhibits an exceptional optical response but suffers from poor electrical conductivity.8–11 These inherent limitations in each phase pose a challenge to their practicality in photoelectrochemical (PEC) devices.6,10 To fully exploit the potential of MoS2 in PEC applications, it is necessary to couple semiconducting and metallic regions within a single MoS2 film by fabricating a 2H-1T heterophase structure.A direct and effective idea is to drive the partial phase transition in a homophase 1T- or 2H-MoS2 film. The in situ transition could be realized by electron injection into a thermodynamically stable 2H-MoS2 film or annealing treatment of an active 1T-MoS2 film.12–15 However, both phase transition pathways are challenging in terms of precise control of the extent and location of the phase transition, as they always occur simultaneously throughout the entire film. Therefore, a method to selectively phase transition MoS2 by a controllable extent and location would enable tunable electrode properties.
In this work, we propose a practical flash phase engineering (FPE) strategy to fabricate 2H-1T heterophase nanofilms, achieving a controllable 1T to 2H phase transition. 1T-MoS2 is a good electrical conductor, but it lacks sufficient optical response capabilities. In contrast, 2H-MoS2 exhibits an exceptional optical response for PEC with photogenerated holes. Glutathione (GSH) can act as an electron donor through oxidization in a PEC process, reacting with the holes located on the 2H-MoS2. These electrons can be quickly injected into the conduction band by 1T-MoS2. The resulting increase in photocurrent enables quantitative detection of GSH.
Results
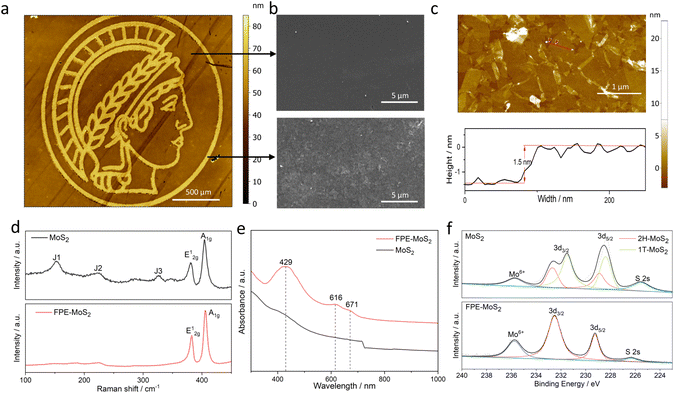
A 1T-MoS2 nanofilm was used as the basal substrate, which was obtained via wet-chemistry exfoliation and vacuum filtration with a 25 nm pore size membrane.14 Different nanofilm thicknesses were controlled by adjusting the volume of 1T-MoS2 during the fabrication process. Then, the desired micro-/nanopattern coupling of two phases was achieved by FPE (Fig. 1). A 488 nm laser beam with a focus diameter of 15 μm (1/e2) was employed, and its power and scan speed were flexibly tuned for precise control.15,16 For example, a logo pattern was achieved by a predefined laser scan path (Fig. 2a). A distinct contrast could be observed in the scanning electron microscope (SEM) image before and after the laser treatment (Fig. 2b). The atomic force microscopy (AFM) image and thickness profile show the ultrathin (nanometer) thickness of the fabricated heterophase MoS2 nanofilm (Fig. 2c).Raman and UV-vis spectroscopy were used to verify the laser-induced phase transition in MoS2. The Raman spectra show characteristic peaks originating from the in-plane (E12g) and out-of-plane (A1g) Mo-S phonon modes both before and after laser treatment (Fig. 2d). However, in the flash phase engineered MoS2 (FPE-MoS2), the specific peaks corresponding to 1T-MoS2 are absent, designated as J1 (153.2 cm−1), J2 (225.1 cm−1), and J3 (327.9 cm−1) modes, indicating a phase transition from 1T- to 2H-MoS2.14,17,18 The UV-vis absorption spectrum of FPE-MoS2 clearly shows 2H-MoS2 excitonic features (Fig. 2e). A and B excitonic peaks were observed at approximately 616 nm and 671 nm, respectively, while C and D excitonic peaks appeared at around 429 nm.14
To further validate the phase transition, X-ray photoelectron spectroscopy (XPS) was used (Fig. 2f). Two characteristic peaks were observed in both MoS2 and FPE-MoS2 at approximately 232.5 eV and 228.9 eV, corresponding to Mo 3d3/2 and Mo 3d5/2, respectively. Additional peaks at 231.4 eV and 228.2 eV could be assigned to 1T-MoS2, while peaks at 232.5 eV and 228.9 eV suggest the presence of the 2H phase.6,19 In addition, all Mo 3d and S 2p peaks of the FPE-MoS2 sample shifted to a slightly higher binding energy (Fig. S1†). The appearance of all of the above features indicates the successful laser-induced 1T to 2H phase transition.
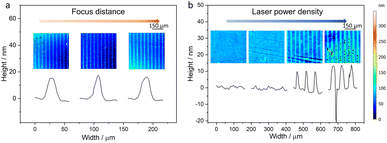
In the FPE process, the incident laser beam irradiates the 1T-MoS2 nanofilm, which serves as an absorbing layer, resulting in a rapid conversion of laser energy into localized thermal energy. Consistent with our previous investigations, the estimated temperature at the center of the laser irradiation spot on each absorber exceeds 343 °C (Fig. S2†),20 which triggers the phase transition process.21 The precise location of the laser focus was optimized to hit the nanofilm interface (Fig. 3a).
It should be noted that the thickness of the 1T-MoS2 substrate nanofilm also influences the effect of phase transition, as evidenced by the patterns observed in a simple microline design (Fig. 3b and S3†). The sharpness/clarity of the lines increases with thicker layers and higher laser power densities, indicating that more MoS2 undergoes phase transition, until some defects start to emerge at ≥0.631 mW μm−2. This phenomenon is more pronounced in the PEC performance, which is strongly influenced by the 2H-1T heterophase structure. FPE shows minimal PEC enhancement on the 20 nm 1T-MoS2 nanofilm, while it becomes more pronounced on the thicker 1T-MoS2 nanofilms. In this context, the 1T-MoS2 nanofilm serves as an effective charge carrier acceptor and transporter, suppressing the recombination of photogenerated charge carriers and facilitating their rapid transport. Consequently, this leads to enhanced PEC performance due to the larger number of charge carriers effectively contributing to the photoelectrochemical process.
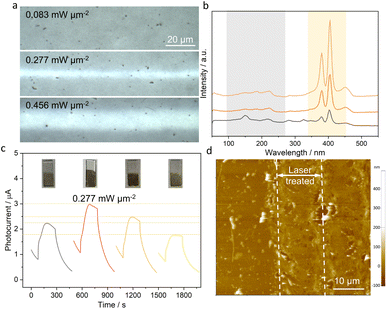
In addition, due to the more obvious difference in PEC response between different laser densities, the 35 nm thin 1T-MoS2 nanofilm was used as a model platform to study the effect of the laser-induced flash phase transition and the underlying mechanisms driving the enhancement of PEC performance. As the laser power density increases, a greater amount of 1T-MoS2 undergoes phase transition to the 2H-MoS2 (Fig. 4a), as evidenced by the Raman spectra showing weaker peaks in the 1T phase and stronger peaks in the 2H phase (Fig. 4b). The photocurrent response shows an initial increase and then decreases with increasing laser power density, reaching a maximum at 0.277 mW μm−2 (Fig. 4c). This behavior can be mainly attributed to the degree of phase transition to 2H-MoS2, which has a high optical absorption but a limited charge transfer capability. Less 2H-MoS2 (potentially a mono- or few layer transition) was achieved under very low irradiation (0.083 mW μm−2), which suffers from low light absorption since it is too thin (as shown in Fig. 4a). In contrast, too much 2H-MoS2 in the film hinders electron transport, leading to diminished PEC performance. Thus, the introduction of moderate amounts of 2H-MoS2 within the 2H-1T heterophase structure provides a favorable balance between optical absorption and efficient electronic transport. In addition, other laser parameters were also studied, such as laser scan speed and line spacing (Fig. S4†). Specifically, the optimized PEC performance was achieved under the laser power density of 0.277 mW μm−2, 10 mm s−1 laser scan speed, and 0.05 mm line spacing. Additionally, the FPE process results in thermal and morphological folding (Fig. 4d), increasing the specific surface area of the electrode for enhanced optical absorption.
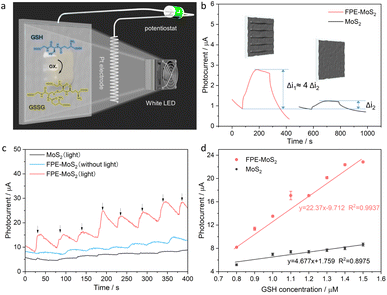
Then, this optimized FPE-MoS2 structure was used as a photoanode in a PEC device, as shown in Fig. 5a. The results show that the photocurrent of the optimized FPE-MoS2 electrode exhibits a substantially higher response (Δi1) compared to the non-engineered counterpart (Δi2) during the on–off cycles of white light irradiation (Fig. 5b). Finally, the optimized PEC device was used for biosensing applications. In this study, reduced GSH was used as a model analyte due to its relevance in various diseases, including aging and cancer.22,23 To evaluate the biosensing activity of the 2H-1T heterophase structure, chronoamperometry (i–t) measurements (Fig. 5c) were conducted. The results show a substantial increase in the photocurrent response with increasing GSH concentration, specifically at 100 nM intervals. Upon the addition of GSH to the system, the reducing agent serves as the electron donor, which quickly scavenges the holes, suppressing the recombination of photogenerated charge carriers. These electrons were injected into the conduction band of the MoS2 and subsequently transferred to the FTO electrode, leading to a relatively large photocurrent response. During this process, GSH is oxidized to glutathione disulfide. The 1T-MoS2 electrode with light was compared with the FPE-MoS2 electrode with and without light, which highlights the significant enhancement in biosensing of the 2H-1T hetero-phase electrode under light irradiation. Further examination revealed that the photocurrent exhibited a linear relationship with physiological GSH concentrations (Fig. 5d). This linear relationship demonstrates high sensitivity (S = Δi/Δc), which is approximately five times higher than that observed in MoS2 biosensing systems without phase engineering. In addition, the stability was studied with an electrode which was kept at 4 °C, periodically measuring its performance (Fig. S5a†). After 7 days, the photocurrent response of the FPE-MoS2 electrode decreased by less than 13%, indicating good storage stability. Moreover, the reproducibility of the optimized PEC device was verified by measuring the photocurrent response of three electrodes in parallel under successive addition of GSH, showing an RSD of 5.36% (Fig. S5b†). This, indicates a high reproducibility of the FPE-MoS2 electrode.
Conclusions
This study demonstrates a facile and efficient method to achieve a controllable 1T to 2H phase transition in a MoS2 nanofilm through FPE. The fabrication of a 2H-1T MoS2 heterophase structure has led to a significant improvement in PEC performance, which is contributed to the synergistic coupling of metallic 1T-MoS2 and semiconducting 2H-MoS2 phases. The inclusion of 2H-MoS2 as a light absorber and the utilization of 1T-MoS2 as an excellent electron acceptor and transporter collectively enhance the photoelectrochemical activity of the material. This work introduces a practical strategy to achieve the phase transition and offers promising potential for large-area fabrication of site-selective heterophase structures. Moreover, the findings open up opportunities for a wide application range, including biosensing. The ability to control the phase transition in MoS2 through this approach may pave the way for advanced biosensing platforms with improved sensitivity and performance, facilitating early disease diagnostics and other (bio)analytical applications.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the China Scholarship Council, the German Federal Ministry of Education and Research (BMBF, 13XP5050A), the Max-Planck-Fraunhofer cooperation (Glyco3Display), the Max Planck Society, and the Natural Science Foundation of China (21675115, 21375091).References
- Q. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- Y. Lee, Y. Q. Hu, X. Lang, D. Kim, K. Li, Y. Ping, K. M. C. Fu and K. Cho, Nat. Commun., 2022, 13, 7501 CrossRef CAS PubMed.
- J. E. Q. Bautista, C. L. A. V. Campos, M. L. da Silva-Neto, C. B. de Araujo, A. M. Jawaid, R. Busch, R. A. Vaia and A. S. L. Gomes, ACS Photonics, 2023, 484–492 CrossRef CAS.
- X. Huang, Z. Zeng and H. Zhang, Chem. Soc. Rev., 2013, 42, 1934–1946 RSC.
- S. Aftab and H. H. Hegazy, Small, 2023, 19, 2205778 CrossRef CAS PubMed.
- W. Wang, X. Zeng, J. Warner, Z. Guo, Y. Hu, Y. Zeng, J. Lu, W. Jin, S. Wang, J. Lu, Y. Zeng and Y. Xiao, ACS Appl. Mater. Interfaces, 2020, 12, 33325–33335 CrossRef CAS.
- T. Sun, H. Zhang, X. Wang, J. Liu, C. Xiao, S. Nanayakkara, J. Blackburn, M. Mirkin and E. M. Miller, Nanoscale Horiz., 2019, 4, 619–624 RSC.
- M. Kertesz and R. Hoffmann, J. Am. Chem. Soc., 1984, 106, 3453–3460 CrossRef CAS.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- Y. Qi, Q. Xu, Y. Wang, B. Yan, Y. Ren and Z. Chen, ACS Nano, 2016, 10, 2903–2909 CrossRef CAS PubMed.
- X. J. Cheng, W. Xu, H. Wen, J. Zhang, H. Zhang, H. W. Li, F. M. Peeters and Q. Chen, Front. Phys., 2023, 18, 53303 CrossRef.
- D. Voiry, A. Mohite and M. Chhowalla, Chem. Soc. Rev., 2015, 44, 2702–2712 RSC.
- S. Cho, S. Kim, J. H. Kim, J. Zhao, J. Seok, D. H. Keum, J. Baik, D. H. Choe, K. J. Chang, K. Suenaga, S. W. Kim, Y. H. Lee and H. Yang, Science, 2015, 349, 625–628 CrossRef CAS PubMed.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. W. Chen and M. Chhowalla, Nano Lett., 2012, 12, 526 CrossRef CAS.
- S. Ronneberger, J. Zhang, Y. Liu and F. F. Loeffler, Adv. Funct. Mater., 2023, 33, 2210116 CrossRef CAS.
- J. Zhang, Y. Zou, S. Eickelmann, C. Njel, T. Heil, S. Ronneberger, V. Strauss, P. H. Seeberger, A. Savateev and F. F. Loeffler, Nat. Commun., 2021, 12, 3224 CrossRef CAS PubMed.
- J. Lu, J. Lu, H. Liu, B. Liu, K. Chan, J. Lin, W. Chen, K. P. Loh and C. H. Sow, ACS Nano, 2014, 8, 6334–6343 CrossRef CAS PubMed.
- Y. Rho, J. Pei, L. Wang, Z. Su, M. Eliceiri and C. P. Grigoropoulos, ACS Appl. Mater. Interfaces, 2019, 11, 39385–39393 CrossRef CAS PubMed.
- K. Liu, W. Zhang, Y. Lee, Y. Lin, M. Chang, C. Su, C. Chang, H. Li, Y. Shi, H. Zhang, C. Lai and L. Li, Nano Lett., 2012, 12, 1538–1544 CrossRef CAS PubMed.
- S. Eickelmann, S. Ronneberger, J. Zhang, G. Paris and F. F. Loeffler, Adv. Mater. Interfaces, 2021, 8, 2001626 CrossRef CAS.
- N. Papadopoulos, J. O. Island, H. S. J. van der Zant and G. A. Steele, IEEE Trans. Electron Devices, 2018, 65, 4053–4058 CAS.
- J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. H. Ryu and J. Yoon, Nat. Protoc., 2015, 10, 1742–1754 CrossRef CAS PubMed.
- J. J. Ge, Y. Zhao, X. Gao, H. Li and G. Jie, Anal. Chem., 2019, 91, 14117–14124 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, investigation of laser-induced MoS2 surface temperature, PEC responses of different electrodes with different parameters (PDF). See DOI: https://doi.org/10.1039/d3ra07759d |
| This journal is © The Royal Society of Chemistry 2024 |