Open Access Article
Open Access ArticleAdsorption of cationic/anionic dyes and endocrine disruptors by yeast/cyclodextrin polymer composites†
Zhikun Lva,
Zhaoyang Wanga,
Huaiguang Wanga,
Jianbin Li *abc and
Kai Li*abc
*abc and
Kai Li*abc
aCollege of Light Industry and Food Engineering, Guangxi University, Nanning 530004, Guangxi, China. E-mail: lijb0771@sina.com; gxlikai@gxu.edu.cn; Tel: +86 13978609908 Tel: +86 13877115103
bProvincial and Ministerial Collaborative Innovation Center for Sugar Industry, Nanning 530004, China
cEngineering Research Center for Sugar Industry and Comprehensive Utilization, Ministry of Education, Nanning 530004, China
First published on 22nd February 2024
Abstract
Factory and natural wastewaters contain a wide range of organic pollutants. Therefore, multifunctional adsorbents must be developed that can purify wastewater. Phytic acid-cross-linked Baker's yeast cyclodextrin polymer composites (IBY-PA-CDP) were prepared using a one-pot method. IBY-PA-CDP was used to adsorb methylene blue (MB), bisphenol A (BPA), and methyl orange (MO). Studies on the ionic strength and strongly acidic ion salts confirmed that IBY-PA-CDP adsorbs MO through hydrophobic interactions. This also shows that Na+ was the direct cause of the increased MO removal. Adsorption studies on binary systems showed that MB/MO inhibited the adsorption of BPA by IBY-PA-CDP. The presence of MB increased the removal rate of MO by IBY-PA-CDP due to the bridging effect. The Langmuir isotherm model calculated the maximum adsorption capacities for MB and BPA to be 630.96 and 83.31 mg g−1, respectively. However, the Freundlich model is more suitable for fitting the experimental data for MO adsorption. To understand the rate-limiting stage of adsorption, a mass-transfer mechanism model was employed. The fitting results show that adsorption onto the active sites is the rate-determining step. After five regeneration cycles, IBY-PA-CDP could be reused with good stability and recyclability.
1. Introduction
The current increase in human demand for industrial chemicals has led to rapid social development and a substantial increase in living standards; however, it has led to some adverse impacts. For example, the discharge of large quantities of pollutants into aquatic environments depletes the quality of water resources.1 Dyes are important sources of water pollution and are produced by textile, paper, rubber, leather, plastic, and printing industries.2 Textile mills are among the largest industrial sectors in terms of dye use and water consumption.3 Over 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of artificial dyes are manufactured annually, of which 10–15% is discharged into wastewater.4 Dyes are mainly categorized as cationic (e.g., methylene blue [MB]) and anionic (e.g., methyl orange [MO]) based on the electrical properties of the molecules.5,6 Both dyes have several applications in printing and research laboratories. Meanwhile, additives with endocrine-disrupting effects (e.g., bisphenol A [BPA]) are employed during dyeing to prevent oxidation and degradation of textiles.7 Dyes in industrial wastewater degrade water quality and cause toxicity, causing the death of aquatic life and human diseases.8 Endocrine disruptors may also be carcinogenic at very low concentrations, and can cause hormonal imbalances.9 Therefore, these dyes and endocrine disruptors must be removed from wastewater.
000 tons of artificial dyes are manufactured annually, of which 10–15% is discharged into wastewater.4 Dyes are mainly categorized as cationic (e.g., methylene blue [MB]) and anionic (e.g., methyl orange [MO]) based on the electrical properties of the molecules.5,6 Both dyes have several applications in printing and research laboratories. Meanwhile, additives with endocrine-disrupting effects (e.g., bisphenol A [BPA]) are employed during dyeing to prevent oxidation and degradation of textiles.7 Dyes in industrial wastewater degrade water quality and cause toxicity, causing the death of aquatic life and human diseases.8 Endocrine disruptors may also be carcinogenic at very low concentrations, and can cause hormonal imbalances.9 Therefore, these dyes and endocrine disruptors must be removed from wastewater.
Adsorption has been used to purify wastewater owing to advantages such as simple operation, low energy consumption, low susceptibility to secondary pollution, and use of regenerable adsorbents.10 Wastewater dyes or phenolic chemicals can be removed using natural biomass as an adsorbent.11,12 Yeast is an inexpensive, readily available raw material and environmentally friendly biosorbent.13 Yeast cell walls contain many functional groups, such as carboxyl, amino, and hydroxyl groups, and yeast has been used to treat pollutants in water, such as phenol,14 Congo red,13 acid orange 7,15 and reactive red 11.16 However, yeast has drawbacks such as low adsorption efficiency, tendency to disperse in water, and difficulty in recollection from aqueous solutions after adsorption, that limits its applications in wastewater treatment.17 Therefore, the yeast must be modified to address these issues. Fortunately, the numerous functional groups (carboxyl, amino, and hydroxyl groups) present on its surface facilitate its ability to be chemically modified. Duan et al.18 prepared an insoluble polymer by grafting β-cyclodextrin (β-CD) onto the surface of Baker's yeast, using thiomalic acid as a crosslinker. Liu et al.19 prepared phosphate-modified Baker's yeast (PMBY). The maximum adsorption capacity of Pb2+ on PMBY was found to be 92 mg g−1, which was about three times that of the pristine Baker's yeast.
Phytic acid (PA) is a naturally occurring cyclic organic acid and a non-toxic polymeric organic compound containing six phosphate groups and 12 hydroxyl groups. Owing to the six phosphate groups in its molecular structure, PA can be used as a cross-linking agent. In addition, this highly negatively charged chemical can enhance adsorption through electrostatic interactions with dyes or metal ions.20 You et al.21 modified wheat straw (WS) with PA to improve adsorption capacity for MB. The maximum adsorption quantity of PA-WS for MB was up to 205.4 mg g−1 at 25 °C. Bouaouina et al.22 modified carob waste using phytic acid. Adsorption capacity of Cr(VI) increased from 243.9 to 434.8 mg g−1 after modification. These results showed that the adsorption capacity could be greatly improved using a PA-cross-linked adsorbent.
β-CD is a macrocyclic oligosaccharide comprising seven glucose units with a truncated cone cavity shape.23 β-CD is of interest because of its natural nontoxicity, hydrophobic cavity, hydrophilic outer wall, and the numerous hydroxyl groups outside the macrocycle. The size of the dye is larger than the cavity of the β-CD, therefore only a part of the dye molecule can be accommodated within the cavity.24 Furthermore, the cavities of multiple CDs create a macromolecular network, which enables the diffusion of dye molecules into the porous structure.25 These properties promote the formation of host–guest complexes or inclusion complexes, allowing them to trap specific organic molecules.26 This makes β-CD a potentially viable option for removing various organic micropollutants. Because natural β-CD is water-soluble, it must be converted into a water-insoluble material using a linker. Zhou et al.27 prepared β-CD/activated carbon hydrogels by mixing β-CD with epichlorohydrin and activated carbon powder. The maximum adsorption capacity of MB can reach 166.67 mg g−1. Alsbaiee et al.28 reported a porous β-CD polymer by using rigid tetrafluoroterephthalonitrile as the crosslinker, and obtained maximum adsorption capacity toward BPA can achieve at 88 mg g−1. Qiu et al.29 grafted acrylamide and 2-acrylamido-2-methylpropane sulfonic acid onto β-CD to prepare a novel hydrogel with high adsorption capacity for MB. β-CD-based adsorbents are often prepared using toxic and carcinogenic compounds as cross-linking or grafting agents. Additionally, some adsorbents can be complicated to prepare and require a large number of organic reagents.30 Thus, this study presents the synthesis of a new adsorbent through a simple one-step process using an eco-friendly cross-linking agent.
Currently, researchers have developed many adsorbents for removing single pollutants such as anionic, cationic dyes or nonionic endocrine disruptors. However, aqueous solutions in natural environments are mixed solutions containing various contaminants. This results in narrow application range for most adsorbents. Therefore, it is necessary to develop new multifunctional adsorbents for the simultaneous removal of anionic, cationic dyes, and nonionic endocrine disruptors.
Herein, a novel Baker's yeast-cyclodextrin polymer (IBY-PA-CDP) composite was developed. MB, MO, and BPA were used to represent cationic, anionic dyes, and nonionic endocrine disruptors, respectively. Then the adsorption properties and mechanisms of MB, MO, and BPA were investigated using batch adsorption experiments, binary pollutant systems, mathematical modeling, and Fourier infrared spectroscopy (FTIR)/X-ray photoelectron spectroscopy (XPS) characterization. This study provides ideas for the simultaneous removal of cationic, anionic, and nonionic organic contaminants.
2. Materials and methods
2.1 Materials and reagents
Baker's yeast was obtained from Hubei Angel Yeast Co., Ltd. β-Cyclodextrin (β-CD, purity >97%) was purchased from Beijing Soleberg Technology Co., Ltd. Phytic acid (PA, 70% in H2O) was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. BPA (λmax = 276 nm) and humic acid (HA, purity ≥90%) were purchased from Shanghai Maclin Biochemical Technology Co., Ltd. Methylene blue (MB, λmax = 665 nm), cobalt chloride hexahydrate (CoCl2·6H2O), methyl orange (MO, λmax = 464 nm), and copper chloride dihydrate (CuCl2·2H2O) were obtained from Tianjin Aupsheng Chemical Co., Ltd. Manganese chloride tetrahydrate (MnCl2·4H2O) and sodium acetate (CH3COONa) were purchased from Tianjin Kemao Chemical Reagent Co. Ltd. Methanol (CH3OH) and anhydrous ethanol (C2H5OH) were purchased from Tianjin Komeo Chemical Reagent Co., Ltd. All reagents were of analytical grade.2.2 Preparation of IBY
Ten grams of dry Baker's yeast was weighed and added to 100 mL of deionized water. The mixture was incubated at room temperature with shaking at 140 rpm for 15 min. The yeast solution was then inactivated in an autoclave (121 °C for 20 min). The inactivated yeast solution was centrifuged at 4000 rpm for 10 min and washed thrice. Cyclic washing was performed to separate impurities from the yeast surface to avoid any influence on the experiment. The inactivated Baker's yeast (IBY) was lyophilized and stored.2.3 Synthesis of IBY-PA-CDP
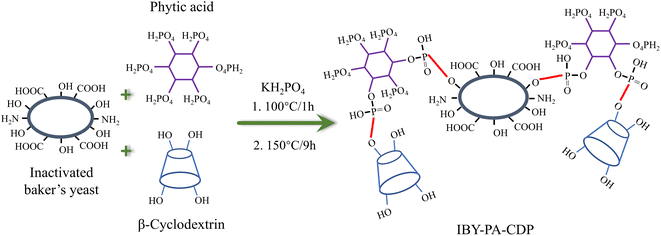
According to researcher previously reported work with minor modification,21 the modified IBY using PA and β-CD (IBY-PA-CDP) was synthesized via esterification as shown in Scheme 1. First, 1 g, 3.97 g, 7.09 g, and 0.56 g of IBY, PA, β-CD, and KH2PO4 respectively were added to deionized water and stirred at 100 °C for 1 h. Then the solutions were placed in an electrically heated desiccator at 150 °C for 9 h. The resulting product was placed in 200 mL of 0.1 mol per L NaOH solution and stirred at room temperature for 2 h. The product was washed with deionized water until the pH of the filtrate was neutral. The product was then placed in a vacuum oven at 55 °C overnight to obtain IBY-PA-CDP biomass adsorbent.3. Results and discussion
3.1 Sample characterization
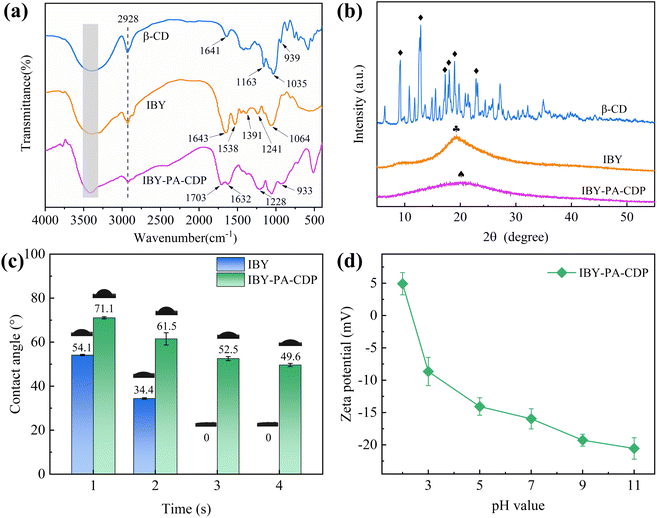
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in –OPO32− (phosphoryl) on the IBY surface and C
O in –OPO32− (phosphoryl) on the IBY surface and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the protein carboxyl group, respectively.33 The adsorption band of IBY-PA-CDP at –OH (3400 cm−1) was reduced compared to those of β-CD and IBY, suggesting that the –OH group of IBY or β-CD reacts with PA.31 The adsorption band of IBY-PA-CDP at 1632 cm−1 was the overlapping peak of the hydroxyl group bending vibration of β-CD and the amide I band of IBY. The new peak at 1703 cm−1 corresponds to the –OH bending vibration of the phosphate group in PA.21 The absorption peaks at 1228 cm−1 was the overlapping peak of the stretch vibrations of amide III band in IBY and P
O stretching of the protein carboxyl group, respectively.33 The adsorption band of IBY-PA-CDP at –OH (3400 cm−1) was reduced compared to those of β-CD and IBY, suggesting that the –OH group of IBY or β-CD reacts with PA.31 The adsorption band of IBY-PA-CDP at 1632 cm−1 was the overlapping peak of the hydroxyl group bending vibration of β-CD and the amide I band of IBY. The new peak at 1703 cm−1 corresponds to the –OH bending vibration of the phosphate group in PA.21 The absorption peaks at 1228 cm−1 was the overlapping peak of the stretch vibrations of amide III band in IBY and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in PA. A broader new peak at 933 cm−1 (P–O–C) was also observed, which was attributed to esterification of the phosphate group on PA with the hydroxyl group on IBY and β-CD.34,35 Then the chemical structures of IBY-PA-CDP before and after loading MB/BPA/MO were analyzed by FTIR spectroscopy in the ESI† file of the manuscript.
O bonds in PA. A broader new peak at 933 cm−1 (P–O–C) was also observed, which was attributed to esterification of the phosphate group on PA with the hydroxyl group on IBY and β-CD.34,35 Then the chemical structures of IBY-PA-CDP before and after loading MB/BPA/MO were analyzed by FTIR spectroscopy in the ESI† file of the manuscript.
XRD was used to analyze the crystallographic features of the surface of the target material. The surface XRD patterns (diffraction angle 2θ) of β-CD, IBY, and IBY-PA-CDP are shown in Fig. 1b. Sharp and intense peaks are observed in the XRD patterns of β-CD, with the strongest diffraction angles (2θ): 9.21°, 12.85°, 17.27°, 17.99°, 18.97°, 23.13° (icon (♦)), which are characteristic peaks of β-CD. This confirmed the nature of the crystal structure. In contrast, IBY shows a broad peak at 2θ of 19.33° (icon ( )). This is a characteristic of noncrystalline structures.33 The observation of a broad peak at 20.01° (icon (♠)) in IBY-PA-CDP indicates that it is amorphous. The broad peaks in the amorphous phase were deflected owing to the cross-linking reactions of β-CD, IBY, and PA.36 However, this is an additional advantage of this method. This is because the amorphous region of the adsorbent favors the adsorption of target compounds.37
)). This is a characteristic of noncrystalline structures.33 The observation of a broad peak at 20.01° (icon (♠)) in IBY-PA-CDP indicates that it is amorphous. The broad peaks in the amorphous phase were deflected owing to the cross-linking reactions of β-CD, IBY, and PA.36 However, this is an additional advantage of this method. This is because the amorphous region of the adsorbent favors the adsorption of target compounds.37
As shown in Fig. 1d, the pH increases from 2 to 11, while the zeta potential of the IBY-PA-CDP surface decreases from 4.92 mV to −20.55 mV. The zeta potential of IBY-PA-CDP was positive at pH 2. Baker yeast cell walls contain various functional groups, including carboxyl, amide, amino, and phosphate groups.42 Therefore, IBY-PA-CDP contained amide and amino groups that were positively charged when protonated under strongly acidic conditions. However, the zeta potential of IBY-PA-CDP was negative at pH > 3. This is because IBY-PA-CDP introduced a large number of phytate molecules with phosphate groups that underwent deprotonation.43 The higher the pH, the more negative the charge on the surface of IBY-PA-CDP.
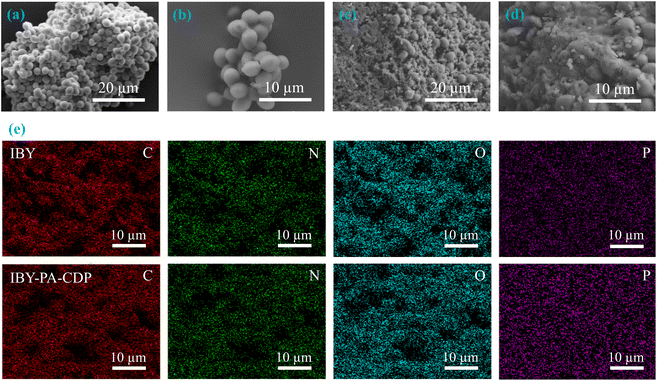 | ||
| Fig. 2 SEM micrographs of (a and b) IBY, (c and d) IBY-PA-CDP; (e) elemental mapping of IBY, and IBY-PA-CDP. | ||
The elemental mapping images of IBY and IBY-PA-CDP in Fig. 2e confirm that the four elements were uniformly distributed on the surfaces of IBY and IBY-PA-CDP. The weight percentage of each element in the samples before and after adsorption was recorded, and the data are presented in Table S1.† The elemental weight percentages showed an increase in the O and P contents on the surface of IBY-PA-CDP, indicating the successful cross-linking of β-CD and PA to the IBY surface. Based on the results for the IBY-PA-CDP model pollutants, the S weight percentages of IBY-PA-CDP/MB and IBY-PA-CDP/MO reached 1.97% and 0.13%, respectively. The weight percentage of O in IBY-PA-CDP/BPA increased by 5.51%. These results further demonstrate the effectiveness of IBY-PA-CDP in adsorbing the modeled pollutants.
3.2 Batch adsorption studies
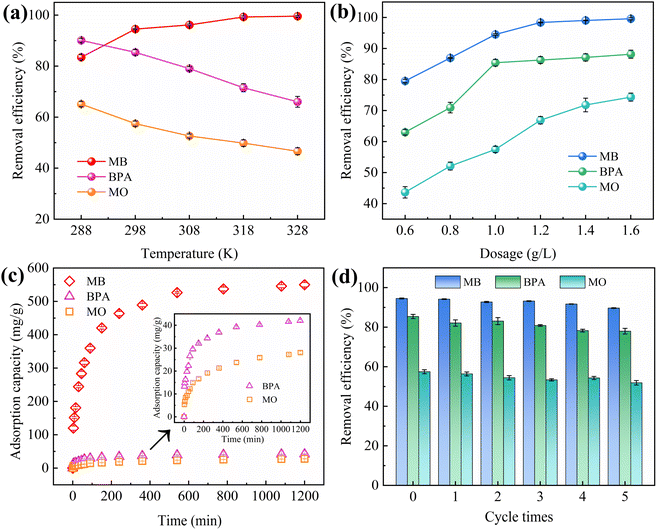
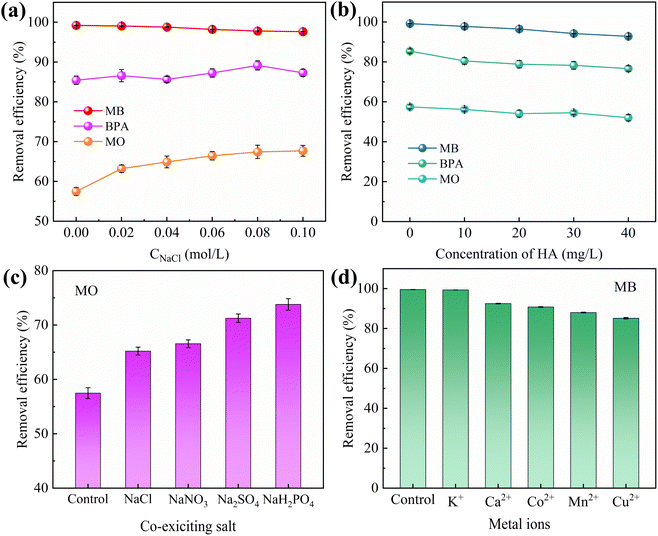
The effects of adsorbent dosage on MB/BPA/MO are shown in Fig. 4b. When the adsorbent dose was less than 1.0 g L−1, MB/BPA/MO exhibited a higher adsorption capacity but a lower removal rate. Owing to the small amount of adsorbent, a portion of the target pollutant in the solution occupied almost all the active sites of the adsorbent, which caused the active sites to become saturated. However, a higher amount of the target contaminant was still present in the solution. When the dose of IBY-PA-CDP exceeded 1.0 g L−1, the adsorbents provided more active adsorption sites to fully adsorb the target pollutants in the solution, thereby enhancing the removal rate. Furthermore, the dose of adsorbent added was greater than that required to remove the total amount of the target pollutant, which resulted in saturation of the active sites becoming difficult and decreasing the adsorption capacity.49 Based on the cost of the adsorbent and removal rate of the modeled contaminants, the IBY-PA-CDP dose was set to 1.0 g L−1 in subsequent experiments.
HA is a complex organic matter present in natural aquatic environments that usually coexists with dyes and endocrine disruptors, which may affect the adsorption performance of IBY-PA-CDP. Fig. 5b shows that as the initial concentration of HA increased, the removal of MB and MO decreased by 6.46% and 5.32%, respectively, and BPA showed a maximum removal decrease of 8.27%. HA is a macromolecule with many functional groups, including hydroxyl, aryl ring, carboxyl, and carbonyl groups.53 Therefore, HA may be bound to the surface of IBY-PA-CDP through hydrogen bonding and hydrophobic and electrostatic interactions, thus competing for active sites. MO was less strongly inhibited by HA than by MB. According to Wang et al.,54 HA forms a spherical structure under acidic conditions, and this form of HA will occupy fewer active sites and reduce the spatial site resistance to favor its adsorption. The results showed that IBY-PA-CDP maintained good adsorption performance for removing MB, BPA, and MO, even in the presence of a large amount of HA.
3.3 Removal of pollutants from binary mixture by the IBY-PA-CDP
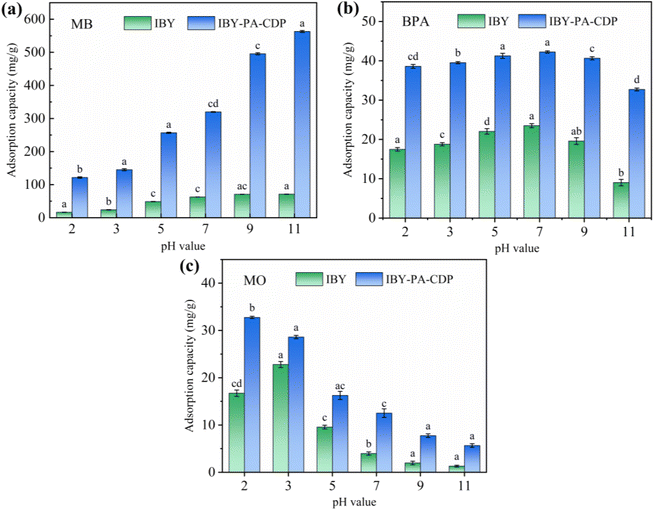
Although IBY-PA-CDP can effectively adsorb a single target pollutant, its adsorption performance for each pollutant in a binary organic pollutant system remains unclear. Fig. 6a and c–e show that the removal rates of BPA and MO in the binary system were still characterized by the pH response, and the trends were the same as those in the single system. The BPA removal efficiency decreased in the presence of MB/MO (Fig. 6a and c). This is because BPA, MB, and MO can be stably contained in the cavity of β-CD through host-guest interactions.59–61 Hydrophobic interactions also promote the adsorption of both BPA and MO by IBY-PA-CDP (Section 3.2.5). Moreover, IBY-PA-CDP adsorbed MB faster than BPA (Section 3.2.3). Therefore, IBY-PA-CDP preferentially adsorbed MB in a mixed solution of BPA + MB. The large amount of MB adsorbed on the surface of IBY-PA-CDP may increase the spatial site resistance to intra-particle diffusion, thereby hindering the adsorption of BPA to the active sites.54 Similarly, the spatial site resistance and competition for the same active site led to a decrease in the removal of MO in the BPA + MO binary system (Fig. 6d). However, very high MB removal efficiencies (Fig. 6b and f) were achieved despite the change in solution pH and the addition of BPA/MO. This indicates that MB was not affected by the presence of BPA/MO. This was due to the high adsorption of MB by the adsorbent, which completely removed low concentrations of MB from the mixed solution.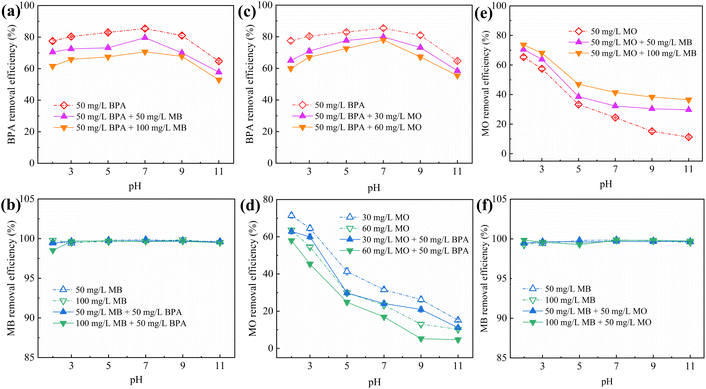 | ||
| Fig. 6 Effect of pH on the adsorption of BPA (a and c), MB (b and f) and MO (d and e) by IBY-PA-CDP in binary systems. | ||
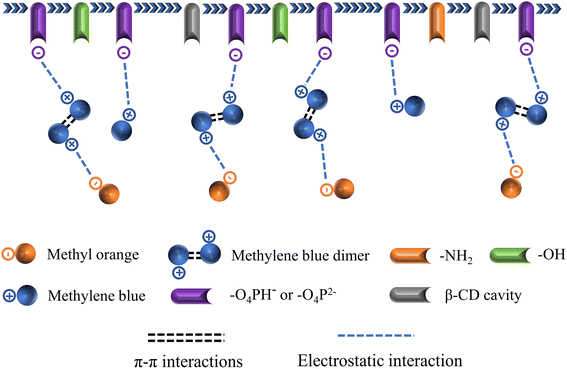
In the BPA/MO and BPA/MB binary systems, the removal rate of BPA/MO decreased when another modeled pollutant was added. However, substantial amounts of MO were adsorbed when MO and MB co-existed (Fig. 6e). That is, MB promoted IBY-PA-CDP adsorption by MO. A plausible explanation for this is that the MB molecules adsorbed on the surface of IBY-PA-CDP act as bridges (Fig. 7). The specific mechanisms are as follows: due to the aromatic ring structure of MB, two MB molecules can form stacked, head-to-tail adjacent dimers in solution by π–π stacking.62 The protonated tertiary amino group of one MB molecule in the MB dimer was attracted by the deprotonated carboxyl and phosphate groups of IBY-PA-CDP, whereas the protonated tertiary amino group of the other MB molecule attracted MO with a sulfonic acid group.63 Therefore, the MO removal rate of IBY-PA-CDP was higher in the MO + MB dimer system. Compared to the acidic system (pH 2–7), the alkaline system (pH 7–11) better promoted the adsorption of MO. The more alkaline the solution, the more negatively charged the IBY-PA-CDP and the greater the drive to adsorb MB. This strong driving force quickly pulled all the MB molecules into the solution, and the fast-moving MB molecules carried more MO molecules to the adsorbent surface.
In summary, MB, BPA, and MO could be adsorbed onto IBY-PA-CDP in a mixed solution. Moreover, the addition of BPA hindered the adsorption of MO. The presence of BPA/MO did not considerably affect MB adsorption. However, the presence of MB can improve the removal efficiency of MO by the adsorbent owing to the bridging effect.
3.4 Adsorption kinetics
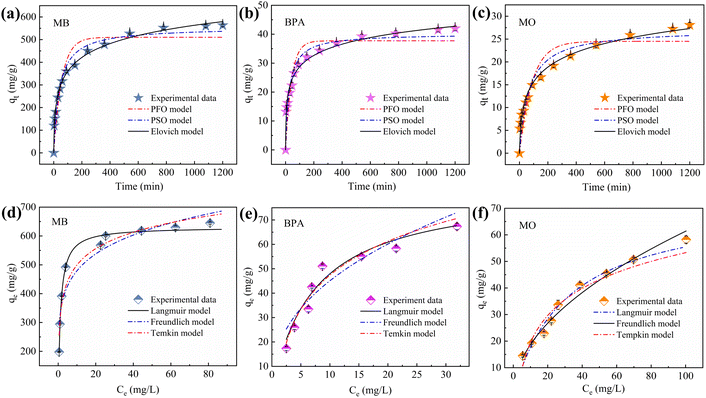
Plots of the curves and associated parameters fitted using the kinetic data and model are in Fig. 8a–c and Table S3.† The correlation coefficients R2 (0.986–0.944) of the Elovich model are higher than those of the PFO (0.862–0.906) and PSO (0.930–0.961). This suggests that the removal of MB, BPA, and MO by IBY-PA-CDP is a chemical process occurring on nonhomogeneous surfaces.64 The fitting of the Elovich model to the experimental data yielded the following values for the three target pollutants: MB (51.054), BPA (9.303), and MO (1.233). Compared with BPA and MO, IBY-PA-CDP was superior for MB adsorption. The presence of more effective MB-binding sites on the adsorbent surface resulted in a stronger driving force for MB with IBY-PA-CDP.3.5 Adsorption isotherms
The fitted curves and parameters of the three isothermal models are shown in Fig. 8d–f and Table S4.† For MB and BPA, the Langmuir model had the best fit among the three models (R2 = 0.992, 0.941), indicating that the adsorption process for MB and BPA was mainly homogeneous monolayer adsorption. The calculated RL values for MB and BPA ranged between 0.0015–0.0056 and 0.0714–0.2778, respectively, indicating that the Langmuir isotherm was highly favorable under the experimental conditions studied. For MO, the Freundlich isotherm model revealed a higher correlation coefficient value (R2 = 0.990) closer to 1, suggesting that MO adsorption on IBY-PA-CDP is a non-homogeneous multilayered adsorption process and that the energy of biosorption decreases exponentially as the adsorption sites on the adsorbent become saturated.65 Meanwhile, the parameter 1/n = 0.52 confirms the advantage of this model in fitting the adsorption of MO.663.6 Mass transfer mechanisms
A mass transfer mechanism model was used to explore the mechanisms and rate-limiting steps in the adsorption of target pollutants by the adsorbents. The uptake of the adsorbate in solution by the adsorbent is essentially a mass-transfer process, that is, the adsorbent is transferred from the solution to the surface of the adsorbent and bound to the adsorption site by physical or chemical forces. This process consists of three main stages. External diffusion: transfer of MB/BPA/MO from the solution to the interior of the liquid film on the surface of IBY-PA-CDP; internal diffusion: transfer of MB/BPA/MO in the internal pores of IBY-PA-CDP upon reaching the interior of the liquid film and adsorption of MB/BPA/MO in the pores to the active sites of IBY-PA-CDP.67
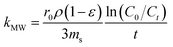 | (1) |
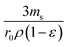 can be replaced by the external surface coefficient S (cm−1). Rearranging eqn (1) yields
can be replaced by the external surface coefficient S (cm−1). Rearranging eqn (1) yields
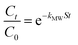 | (2) |
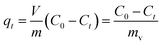 | (3) |
This is obtained by combining eqn (2) and (3):
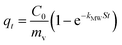 | (4) |
| qt = kit0.5 + C | (5) |
In the actual experiment, the absorption reaction did not begin at t = 0. Therefore, qt=0 = 0, C = 0. However, the C value obtained by most researchers using eqn (5) was not zero. This is not consistent with observed results. Thus, a point (t = 0, qt=0 = 0) is required as an initial condition for the IND model.
In the present study, a new model of intra-particle diffusion is proposed. We assumed that the adsorbate in the solution diffuses into the pores at the beginning of the experiment, and then diffusion within the pores reaches equilibrium at t1, which is the first stage of the internal diffusion model (IND model-1). This is represented by a curve through (0, 0) at time t ∈ [0, t1]:
| qt = k1t0.5 | (6) |
After time t1, the adsorbate in the pores binds to the active site, the equilibrium of internal diffusion in the pores is broken, and the adsorbate in the solution diffuses into the pores until it binds to the active sites. This was the second stage of internal diffusion (IND model-2). For time t ∈ [t1, t2], IND model-2 is described as follows:
| qt − qt1 = k2(t − t1)0.5 | (7) |
In summary, the modified form of the intra-particle diffusion model is:
| qt = k1t0.5, 0 ≤ t ≤ t1 | (8) |
| qt − qt1 = k2(t − t1)0.5, t1 < t ≤ t2 | (9) |
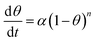 | (10) |
For n = 1,
| θ = 1 − e−αt | (11) |
For n ≠ 1,
| θ = 1 − [1 + (n − 1)αt]1/1−n | (12) |
For n = 1,
| qt = qm(1 − e−αt) | (13) |
For n ≠ 1,
| qt = qm − qm[1 + (n − 1)αt]1/1−n | (14) |
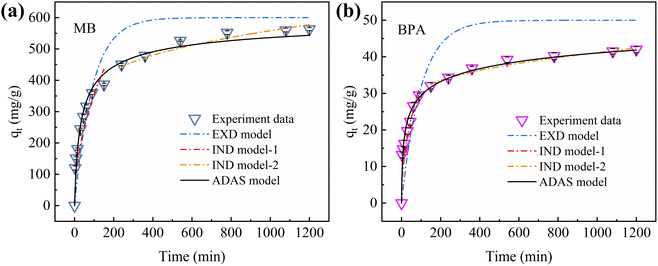
The Freundlich isotherm model did not yield the maximum adsorption capacity qm. Therefore, the ADAS model cannot be fitted using an experimental model. The mass transfer kinetics were fitted to the experimental data for MB and BPA using the EXD, IND, and ADAS models. Fig. 9 shows the plots of the fitted mass-transfer kinetic models, and Table S5† summarizes the calculated parameters for each model. Among the three mass-transfer kinetic models, the ADAS model exhibited the highest correlation coefficients for the kinetic data fitted to MB and BPA. These results indicate that adsorption on the active sites was the main rate-limiting step in the mass transfer of MB and BPA. Since IBY-PA-CDP contains numerous phosphate groups and β-cyclodextrin cavities. Therefore, a longer time was required to saturate the active adsorption sites. These results also illustrate that the external and internal diffusion of MB and BPA into IBY-PA-CDP is rapid. The hydrophilicity and hydrophobicity of IBY-PA-CDP led to the rapid passage of MB and BPA molecules through the liquid membrane between IBY-PA-CDP and the aqueous solution, respectively (i.e., rapid external diffusion). The small specific surface area allows the MB and BPA molecules to quickly reach the active adsorption sites through an intra-particle path (i.e., rapid internal diffusion). Moreover, the electrostatic, hydrophobic, and host–guest interactions between IBY-PA-CDP, MB, and BPA effectively increased the diffusion rates of MB and BPA in the liquid membrane and pores. Therefore, the rate-limiting step must be investigated to better understand the mechanism of adsorption onto the adsorbent.
3.7 Proposed sorption mechanism
To further explore the possible interactions between MB/BPA/MO and IBY-PA-CDP, the high-resolution XPS and FTIR spectra before and after adsorption were analyzed (Fig. 10 and S1†).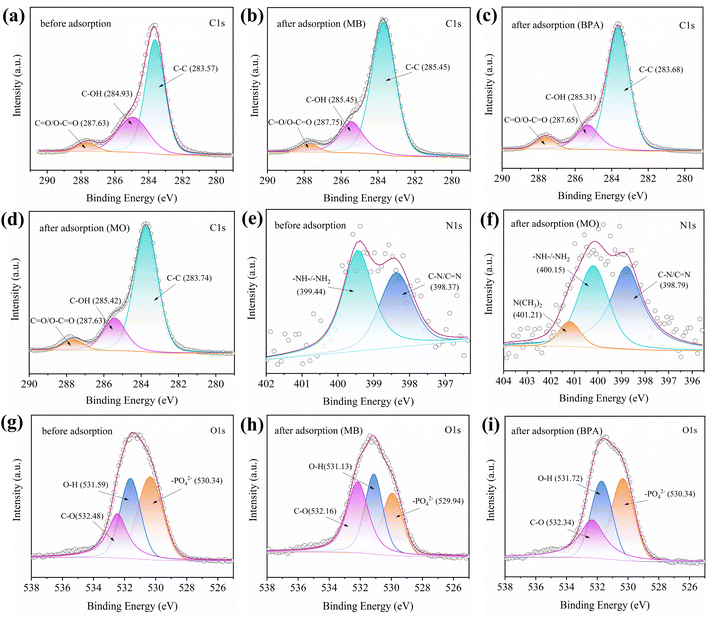 | ||
| Fig. 10 Spectra of (a–d) C 1s, (e and f) N 1s and (g–i) O 1s for IBY-PA-CDP before and after of adsorption MB, BPA, and MO. | ||
Hydrogen bonding is one of the most common mechanisms involved in the adsorption of organic pollutants. Fig. S1† shows that after MB and MO were adsorbed by IBY-PA-CDP, the peaks shifted to 3413 and 3418 cm−1 at 3424 cm−1, respectively, suggesting that –OH is involved in the formation of hydrogen bonds.71 The disappearance of the –OH bending vibrational peak of PA at 1704 cm−1 in IBY-PA-CDP/BPA indicated that the hydroxyl group on BPA was hydrogen-bonded to IBY-PA-CDP. As shown in Fig. 10a–d, when MB, BPA, and MO were adsorbed, the peak at C–OH shifted from 284.93 eV to 285.45 eV, 285.31 eV, and 285.42 eV, respectively, which suggests that hydroxyl groups on the adsorbent were involved in adsorption of the modeled pollutants. C–OH can act as a hydrogen donor to form hydrogen bonds.72 A deconvoluted peak corresponding to O–H was observed in the O 1s spectrum at 531.59 eV (Fig. 10g). When MB and BPA are adsorbed, the peaks at O–H are shifted to 531.13 eV and 537.72 eV, respectively, which is attributed to the oxygen atoms in the O–H that act as hydrogen acceptors to provide lone electron pairs for hydrogen-bonding interactions with MB and BPA.
Electrostatic interactions are considered important mechanisms for dye adsorption. As presented in Fig. S1,† for IBY-PA-CDP/MO, the peak at 1632 cm−1 shifted to 1627 cm−1, suggesting that the N–H groups in the adsorbent can be used to adsorb MO.50 The protonated amino group of IBY-PA-CDP can be assumed to be involved in electrostatic interactions with the sulfonic acid group of MO. The disappearance of the peak near 1704 cm−1 for IBY-PA-CDP/MB indicated that the phosphate group was used to adsorb MB. We observed a deviation of 0.12 eV in the binding energy of IBY-PA-CDP/MB at C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/O–C
O/O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O compared to the original binding energy (Fig. 10a and b), which might have been caused by the electrostatic interaction between MB and the carboxyl group. As shown in Fig. 5 and 6e, after the adsorption of MO, a new characteristic peak at 401.21 eV in the N 1s spectrum correlates with the N(CH3)2 of MO.73 Meanwhile, the peak of IBY-PA-CDP/MO at 399.44 eV of the N 1s spectrum is shifted to 400.15 eV, which implies that –NH–/–NH2 is involved in the electrostatic interaction. For O 1s, the peak at 530.34 eV is attributed to the –PO42− group of phytate (Fig. 10g). After MB adsorption, the peak at –PO42− becomes 529.94 eV, which may be attributed to the electrostatic attraction between the negatively charged oxygen group (–PO42−) of IBY-PA-CDP and the positively charged nitrogen atoms of dimethylimine (
O compared to the original binding energy (Fig. 10a and b), which might have been caused by the electrostatic interaction between MB and the carboxyl group. As shown in Fig. 5 and 6e, after the adsorption of MO, a new characteristic peak at 401.21 eV in the N 1s spectrum correlates with the N(CH3)2 of MO.73 Meanwhile, the peak of IBY-PA-CDP/MO at 399.44 eV of the N 1s spectrum is shifted to 400.15 eV, which implies that –NH–/–NH2 is involved in the electrostatic interaction. For O 1s, the peak at 530.34 eV is attributed to the –PO42− group of phytate (Fig. 10g). After MB adsorption, the peak at –PO42− becomes 529.94 eV, which may be attributed to the electrostatic attraction between the negatively charged oxygen group (–PO42−) of IBY-PA-CDP and the positively charged nitrogen atoms of dimethylimine (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+Me2) on MB.74
N+Me2) on MB.74
However, considering the presence of the hydrophobic cavity of β-CD, IBY-PA-CDP may effectively adsorb hydrophobic pollutants through strong hydrophobic interactions. Ionic strength studies suggested that hydrophobic interactions may be the main driving force for the adsorption of BPA and MO (Section 3.2.5). A study of strongly acidic ionic salts further verified that the main factor for MO adsorption by IBY-PA-CDP was the hydrophobic interactions (Section 3.2.6).
Finally, MB, BPA, and MO have linear structures that match the cavity of β-CD.60 The β-CD cavity was found to contain part of the structure of MB/MO molecules, or multiple β-CD molecules can form a network structure to capture dye molecules.25 However, the β-CD cavity forms a stable inclusion complex with BPA.26 As shown in Fig. S1,† the change in the displacement of the –OH bending vibration from 1632 cm−1 to 1609, 1623, and 1627 cm−1 may be a result of the inclusion of MB/BPA/MO into the-CD cavity. Therefore, MB, BPA, and MO can be inferred to be adsorbed by IBY-PA-CDP as “guests” through host–guest interactions.
4. Conclusion
In this study, Baker's yeast, β-CD, and phytic acid were used to synthesize insoluble and multifunctional biomass polymer (IBY-PA-CDP) composites via a simple cross-linking reaction and this composite was used to treat MB, BPA, and MO in aqueous solutions. This study showed that MB, BPA, and MO could be adsorbed onto IBY-PA-CDP in single and binary systems solutions. The addition of humic acid and metal ions decreased the adsorption capacity of IBY-PA-CDP for MB owing to competitive adsorption. Adsorption studies on model pollutant binary systems showed that MB/MO inhibited the adsorption of BPA by IBY-PA-CDP. In contrast, the presence of MB enhanced the removal rate of MO by IBY-PA-CDP owing to the bridging effect. The adsorption models demonstrated that the adsorption of MB/BPA by IBY-PA-CDP was dominated by monolayer chemisorption, and that the adsorption of MO was multilayer chemisorption. Electrostatic interactions were the main driving force of MB adsorption by IBY-PA-CDP. Hydrophobic interactions were the main factors leading to the adsorption of BPA and MO. In addition, the β-CD cavity contained in IBY-PA-CDP can trap MB/BPA/MO based on the host-guest interactions. The present study analyzed and investigated the adsorption behavior and mechanism of three organic pollutants with different electrical properties. The synthesized Baker's yeast–cyclodextrin polymer composites can potentially be employed to treat wastewater containing MB, BPA, and MO.Author contributions
Zhikun Lv: investigation, conceptualization, writing of the original draft, formal analysis and methodology. Zhaoyang Wang & Huaiguang Wang: data curation, investigation and formal analysis, conceptualization, methodology. Jianbin Li & Kai Li: supervision, funding acquisition, and project administration.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number: 31760469), the Scientific Research and Technology Development Program of Guangxi (AA22117014-1 and AA22117002-5), the Central Guided Local Science and Technology Local Development Funding Program (2023ZYZX3016), and the Agriculture Research System of China (CARS-17-0502). We also thank Elsevier Premium Language Editing Services (https://webshop.elsevier.com/language-editing-services/language-editing/) for its linguistic assistance during the preparation of this manuscript.References
- F. T. Geldasa, M. A. Kebede, M. W. Shura and F. G. Hone, RSC Adv., 2023, 13, 18404–18442 RSC.
- M. I. Khan, T. K. Min, K. Azizli, S. Sufian, H. Ullah and Z. Man, RSC Adv., 2015, 5, 61410–61420 RSC.
- D. Bhatia, N. R. Sharma, J. Singh and R. S. Kanwar, Crit. Rev. Environ. Sci. Technol., 2017, 47, 1836–1876 CrossRef CAS.
- M. D. Khan, D. Li, S. Tabraiz, B. Shamurad, K. Scott, M. Z. Khan and E. H. Yu, Sci. Total Environ., 2021, 756, 143752 CrossRef CAS PubMed.
- J. Xiao, W. Lv, Z. Xie, Y. Tan, Y. Song and Q. Zheng, J. Mater. Chem. A, 2016, 4, 12126–12135 RSC.
- C. Lei, F. Wen, J. Chen, W. Chen, Y. Huang and B. Wang, Polymer, 2021, 213, 123316 CrossRef CAS.
- Z. Zhou, Q. Guo, Z. Xu, L. Wang and K. Cui, Environ. Eng. Sci., 2015, 32, 203–211 CrossRef CAS.
- A. Kanwal, R. Rehman, M. Imran, G. Samin, M. M. Jahangir and S. Ali, RSC Adv., 2023, 13, 26455–26474 RSC.
- N. A. H. Ismail, S. Y. Wee, N. H. Kamarulzaman and A. Z. Aris, Environ. Pollut., 2019, 249, 1019–1028 CrossRef CAS PubMed.
- D. Aksu Demirezen, D. Demirezen Yılmaz and Y. Ş. Yıldız, Int. J. Biol. Macromol., 2023, 239, 124311 CrossRef CAS PubMed.
- Z. M. Lazim, T. Hadibarata, M. H. Puteh and Z. Yusop, Water, Air, Soil Pollut., 2015, 226, 34 CrossRef.
- M. El Khomri, N. El Messaoudi, A. Dbik, S. Bentahar, Y. Fernine, A. Lacherai and A. Jada, Chem. Afr., 2022, 5, 1083–1095 CrossRef CAS.
- E. Y. S. Soh, S. S. Lim, K. W. Chew, X. W. Phuang, V. M. V. Ho, K. Y. H. Chu, R. R. Wong, L. Y. Lee and T. J. Tiong, Environ. Res., 2022, 204, 112385 CrossRef CAS PubMed.
- K. Grace Pavithra, P. Sundarrajan, J. Arun, K. Brindhadevi, Q. Hoang Le and A. Pugazhendhi, Environ. Res., 2023, 117005, DOI:10.1016/j.envres.2023.117005.
- Y. Wu, Y. Hu, Z. Xie, S. Feng, B. Li and X. Mi, Appl. Biochem. Biotechnol., 2011, 163, 882–894 CrossRef CAS PubMed.
- P. Saravanan, S. Kumaran, S. Bharathi, P. Sivakumar, P. Sivakumar, S. R. Pugazhvendan, W. Aruni and S. Renganathan, Environ. Technol. Innovation, 2021, 22, 101442 CrossRef CAS.
- I. Shahzadi, Y. Wu, H. Lin, J. Huang, Z. Zhao, C. Chen, X. Shi and H. Deng, J. Hazard. Mater., 2023, 453, 131312 CrossRef CAS PubMed.
- Z. Duan, M. Song, T. Li, S. Liu, X. Xu, R. Qin, C. He, Y. Wang, L. Xu and M. Zhang, RSC Adv., 2018, 8, 31542–31554 RSC.
- S. Liu, Z. Duan, C. He, X. Xu, T. Li, Y. Li, X. Li, Y. Wang and L. Xu, RSC Adv., 2018, 8, 8026–8038 RSC.
- M. A. E. Bakhite, N. J. Sithole, L. S. Magwaza, A. O. Odindo, S. T. Magwaza and K. Ncama, Heliyon, 2021, 7, e07912 CrossRef CAS PubMed.
- H. You, J. Chen, C. Yang and L. Xu, Colloids Surf., A, 2016, 509, 91–98 CrossRef CAS.
- K. Bouaouina, A. Barras, N. Bezzi, M. A. Amin, S. Szunerits and R. Boukherroub, Chemosphere, 2022, 297, 134188 CrossRef CAS PubMed.
- G. Crini, Chem. Rev., 2014, 114, 10940–10975 CrossRef CAS PubMed.
- Y.-L. Hong, J. Sun, X.-Q. Fang, Q.-W. Liu, C. Wang and C.-M. Liu, Carbohydr. Polym., 2023, 316, 121059 CrossRef CAS PubMed.
- J. Lagiewka, A. Nowik-Zajac, A. Pajdak and I. Zawierucha, Carbohydr. Polym., 2023, 307, 120615 CrossRef CAS PubMed.
- Z. Li, C. Hu, Z. Hu, Y. Fu and Z. Chen, Carbohydr. Polym., 2022, 276, 118786 CrossRef CAS PubMed.
- K. Zhou, Y. Li, Q. Li, Q. Du, D. Wang, K. Sui, C. Wang, H. Li and Y. Xia, J. Polym. Environ., 2018, 26, 3362–3370 CrossRef CAS.
- A. Alsbaiee, B. J. Smith, L. Xiao, Y. Ling, D. E. Helbling and W. R. Dichtel, Nature, 2016, 529, 190–194 CrossRef CAS PubMed.
- J.-w. Wang, D. Lan, L. Yong-qiang, R.-f. Li, X.-t. Yang, G.-h. Lan, H.-y. Qiu and B. Xu, Carbohydr. Res., 2021, 501, 108276 CrossRef CAS PubMed.
- Q. Liu, Y. Zhou, J. Lu and Y. Zhou, Chemosphere, 2020, 241, 125043 CrossRef CAS PubMed.
- F. M. Mpatani, A. A. Aryee, A. N. Kani, Q. Guo, E. Dovi, L. Qu, Z. Li and R. Han, Chemosphere, 2020, 259, 127439 CrossRef CAS PubMed.
- S. Tonk and E. Rápó, Int. J. Mol. Sci., 2022, 23, 126334 Search PubMed.
- Y. Xia, L. Meng, Y. Jiang, Y. Zhang, X. Dai and M. Zhao, Chem. Eng. J., 2015, 259, 927–935 CrossRef CAS.
- A. Mora-Boza, M. L. López-Donaire, L. Saldaña, N. Vilaboa, B. Vázquez-Lasa and J. San Román, Sci. Rep., 2019, 9, 11491 CrossRef PubMed.
- K.-H. Sun, Z. Liu, C. Liu, T. Yu, T. Shang, C. Huang, M. Zhou, C. Liu, F. Ran, Y. Li, Y. Shi and L. Pan, Sci. Rep., 2016, 6, 23931 CrossRef CAS PubMed.
- L. Wang, X. Zhu, X. Chen, Y. Zhang, H. Yang, Q. Li and J. Jiang, Ind. Crops Prod., 2022, 182, 114921 CrossRef CAS.
- K. Hemine, N. Łukasik, M. Gazda and I. Nowak, J. Hazard. Mater., 2021, 418, 126286 CrossRef CAS PubMed.
- P. Awasthi, R. S. Bangari and N. Sinha, J. Mol. Liq., 2023, 370, 120970 CrossRef CAS.
- J. C. Serrano-Niño, A. Cavazos-Garduño, F. Cantú-Cornelio, A. F. González-Córdova, B. Vallejo-Córdoba, A. Hernández-Mendoza and H. S. García, LWT–Food Sci. Technol., 2015, 64, 1334–1341 CrossRef.
- A. Sánchez-Iglesias, M. Grzelczak, T. Altantzis, B. Goris, J. Pérez-Juste, S. Bals, G. Van Tendeloo, S. H. Donaldson Jr, B. F. Chmelka, J. N. Israelachvili and L. M. Liz-Marzán, ACS Nano, 2012, 6, 11059–11065 CrossRef PubMed.
- N. S. Tezerji, M. M. Foroughi, R. R. Bezenjani, N. Jandaghi, E. Rezaeipour and F. Rezvani, Food Chem., 2020, 311, 125747 CrossRef CAS PubMed.
- M. J. Puchana-Rosero, E. C. Lima, S. Ortiz-Monsalve, B. Mella, D. da Costa, E. Poll and M. Gutterres, Environ. Sci. Pollut. Res., 2017, 24, 4200–4209 CrossRef CAS PubMed.
- H. A. Said, I. Ait Bourhim, A. Ouarga, I. Iraola-Arregui, M. Lahcini, A. Barroug, H. Noukrati and H. Ben youcef, Int. J. Biol. Macromol., 2023, 225, 1107–1118 CrossRef CAS PubMed.
- M. Xiao, F. Yang, S. Im, D. S. Dlamini, D. Jassby, S. Mahendra, R. Honda and E. M. V. Hoek, J. Membr. Sci. Lett., 2022, 2, 100022 CrossRef.
- L. Xu, T. Bai, X. Yi, K. Zhao, W. Shi, F. Dai, J. Wei, J. Wang and C. Shi, Int. J. Biol. Macromol., 2023, 238, 124131 CrossRef CAS PubMed.
- N. Du, L.-Y. Huang, Y.-S. Xiong, R. Tian, J.-Y. Yin, D.-Y. Cao, D.-B. Hu, H.-Q. Lu, W. Li and K. Li, Carbohydr. Polym., 2023, 313, 120855 CrossRef CAS PubMed.
- S.-z. Hu, T. Huang, N. Zhang, Y.-z. Lei and Y. Wang, Sep. Purif. Technol., 2022, 297, 121470 CrossRef CAS.
- N. Tahari, P. L. de Hoyos-Martinez, N. Izaguirre, N. Houwaida, M. Abderrabba, S. Ayadi and J. Labidi, Int. J. Biol. Macromol., 2022, 210, 94–106 CrossRef CAS PubMed.
- M. K. Uddin and U. Baig, J. Cleaner Prod., 2019, 211, 1141–1153 CrossRef CAS.
- K. Li, X. Li and B. Li, Sep. Purif. Technol., 2022, 289, 120737 CrossRef CAS.
- G. Xu, X. Xie, L. Qin, X. Hu, D. Zhang, J. Xu, D. Li, X. Ji, Y. Huang, Y. Tu, L. Jiang and D. Wei, Green Chem., 2019, 21, 6062–6072 RSC.
- H. Ding, Z. Zhang, Y. Li, L. Ding, D. Sun and Z. Dong, Bioresour. Technol., 2022, 364, 128018 CrossRef CAS PubMed.
- X. Hu, G. Xu, H. Zhang, M. Li, Y. Tu, X. Xie, Y. Zhu, L. Jiang, X. Zhu, X. Ji, Y. Li and A. Li, ACS Appl. Mater. Interfaces, 2020, 12, 12165–12175 CrossRef CAS PubMed.
- J. Wang, C. Wang, A. Shi, Y. Shi, D. Yue, L. Zhang, J. Wang, H. Wang, C. Wang and D. Cui, Chem. Eng. J., 2023, 461, 142090 CrossRef CAS.
- M. V. Dinu, D. Humelnicu and M. M. Lazar, Gels, 2021, 7, 116847 CrossRef PubMed.
- K. Li, J. Yan, Y. Zhou, B. Li and X. Li, J. Mol. Liq., 2021, 335, 116291 CrossRef CAS.
- Z. Yin, C. Cui, H. Chen, K. Duoni, X. Yu and W. Qian, Small, 2020, 16, 1902301 CrossRef CAS PubMed.
- H. Zhang, S. Zhu, J. Yang, A. Ma and W. Chen, J. Membr. Sci., 2021, 636, 119591 CrossRef CAS.
- J. Liu, J. Zhou, Z. Wu, X. Tian, X. An, Y. Zhang, G. Zhang, F. Deng, X. Meng and J. Qu, J. Hazard. Mater., 2022, 432, 128758 CrossRef CAS PubMed.
- Y. Jiang, B. Liu, J. Xu, K. Pan, H. Hou, J. Hu and J. Yang, Carbohydr. Polym., 2018, 182, 106–114 CrossRef CAS PubMed.
- Y. Liu, D. Wu, X. Wang, J. Yu and F. Li, RSC Adv., 2018, 8, 37715–37723 RSC.
- H. Li, X. Cao, C. Zhang, Q. Yu, Z. Zhao, X. Niu, X. Sun, Y. Liu, L. Ma and Z. Li, RSC Adv., 2017, 7, 16273–16281 RSC.
- B. Chen, F. Long, S. Chen, Y. Cao and X. Pan, Chem. Eng. J., 2020, 385, 123926 CrossRef CAS.
- B. Wang, B. Gao and Y. Wan, Environ. Sci. Pollut. Res., 2019, 26, 11535–11541 CrossRef CAS PubMed.
- M. E. Mahmoud, R. M. El-Sharkawy and G. A. A. Ibrahim, J. Mol. Liq., 2022, 368, 120676 CrossRef CAS.
- L.-Y. Guo, H.-Q. Lu, D. Rackemann, C. Shi, W. Li, K. Li and W. O. S. Doherty, Chem. Eng. J., 2021, 416, 129084 CrossRef CAS.
- X. Guo, Y. Liu and J. Wang, J. Hazard. Mater., 2020, 400, 123324 CrossRef CAS PubMed.
- N. E. Dávila-Guzmán, F. de Jesús Cerino-Córdova, E. Soto-Regalado, J. R. Rangel-Mendez, P. E. Díaz-Flores, M. T. Garza-Gonzalez and J. A. Loredo-Medrano, Clean: Soil, Air, Water, 2013, 41, 557–564 Search PubMed.
- J. Wang and X. Guo, Chemosphere, 2022, 309, 136732 CrossRef CAS PubMed.
- J. Wang and X. Guo, J. Hazard. Mater., 2020, 390, 122156 CrossRef CAS PubMed.
- E. Haque, J. W. Jun and S. H. Jhung, J. Hazard. Mater., 2011, 185, 507–511 CrossRef CAS PubMed.
- Y. Lv, J. Ma, K. Liu, Y. Jiang, G. Yang, Y. Liu, C. Lin, X. Ye, Y. Shi, M. Liu and L. Chen, J. Hazard. Mater., 2021, 403, 123666 CrossRef CAS PubMed.
- X. Wan, Z. Rong, K. Zhu and Y. Wu, Int. J. Biol. Macromol., 2022, 222, 725–735 CrossRef CAS PubMed.
- H. Li, V. L. Budarin, J. H. Clark, M. North and X. Wu, J. Hazard. Mater., 2022, 436, 129174 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07682b |
| This journal is © The Royal Society of Chemistry 2024 |