Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlled Sr(II) ion release from in situ crosslinking electroactive hydrogels with potential for the treatment of infections
Melike Fırlak Demirkan *a,
Dilek Öztürka,
Zeynep Sude Çifçibaşıa,
Fatma Ertana,
John George Hardy
*a,
Dilek Öztürka,
Zeynep Sude Çifçibaşıa,
Fatma Ertana,
John George Hardy *b,
Aslı Nurşeval Oyunluc and
Hakan Darıcıcdef
*b,
Aslı Nurşeval Oyunluc and
Hakan Darıcıcdef
aDepartment of Chemistry, Gebze Technical University, Gebze, Kocaeli 41400, Turkey. E-mail: mfirlak@gtu.edu.tr
bDepartment of Chemistry, Lancaster University, Lancaster LA1 4YB, UK
cHD Bioink Biotechnology Corp., İstanbul, Turkey
d3D Bioprinting Design & Prototyping R&D Center, Istinye University, Istanbul, Turkey
eFaculty of Medicine, Dept. of Histology & Embryology, Istinye University, Istanbul, Turkey
fStem Cell, and Tissue Engineering R&D Center, Istinye University, Istanbul, Turkey
First published on 31st January 2024
Abstract
The development of electrochemical stimuli-responsive drug delivery systems is of both academic and industrial interest due to the ease with which it is possible to trigger payload release, providing drug delivery in a controllable manner. Herein, the preparation of in situ forming hydrogels including electroactive polypyrrole nanoparticles (PPy-NPs) where Sr2+ ions are electrochemically loaded for electrically triggered release of Sr2+ ions is reported. The hydrogels were characterized by a variety of techniques including Fourier-transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), thermogravimetric analysis (TGA), X-ray diffraction (XRD), cyclic voltammetry (CV), etc. The cytocompatibility towards human mesenchymal stem cells (MSCs) and fibroblasts were also studied. The Sr2+ ion loaded PEC-ALD/CS/PPy-NPs hydrogel showed no significant cytotoxicity towards human mesenchymal stem cells (MSCs) and fibroblasts. Sr2+ ions were electrochemically loaded and released from the electroactive hydrogels, and the application of an electrical stimulus enhanced the release of Sr2+ ions from gels by ca. 2–4 fold relative to the passive release control experiment. The antibacterial activity of Sr2+ ions against E. coli and S. aureus was demonstrated in vitro. Although these prototypical examples of Sr2+ loaded electroactive gels don't release sufficient Sr2+ ions to show antibacterial activity against E. coli and S. aureus, we believe future iterations with optimised physical properties of the gels will be capable of doing so.
Introduction
Controlled release technology generates economic, health and societal impacts.1,2 Controlled release technology can ensure high efficacy of treatment,3 at low cost,4 with reduced risks of toxicity and other side effects of the drug,5 providing stable levels of drugs in blood and plasma and greater patient comfort,6 potentially with delivery of precise quantities of drugs at specific locations and times, hence they can be tailored to each person's needs.7 There are various external stimuli being explored including light, pH, temperature, electricity, enzymes and magnetic fields,8 that can potentially be triggered externally from the body. Each type of stimuli has its own advantages; in the case of electrical triggers including switchable ON–OFF release,9 the magnitude of current (and concomitantly amount of drug) can be controlled precisely.10Conducting polymers (CPs) are a family of polymers with extended π-bonding that can confer electrical properties close to semiconductors/metals, and potentially interesting electrochemical and optical properties,11 useful for a variety of technologies/industries. CPs (e.g., polyaniline (PANI), polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), and their derivatives) can be synthesised by a variety of methods.12–14
There is a market need for biocompatible materials capable of well controlled drug delivery,15 hydrogels are one class of materials used for drug delivery,16 tissue engineering17 and biosensors.18 In situ forming hydrogels can be used in the emerging field of patient-specific treatments (e.g. drug delivery, cell therapy, etc.).19 Biopolymers (e.g., chitosan, dextran, gelatin, hyaluronic acid, pectin, etc.) can be used to produce in situ forming hydrogels for drug delivery studies,20 often employing Schiff base reactions.19
Metal ions (e.g., Ca2+, Cu2+, Fe2+/Fe3+, K+, Mg2+, Na+, Zn2+) play a variety of important roles in biology,21–23 motivating the investigation of therapeutic systems incorporating metal ions.24–32 Elegant examples of ion delivery systems include organic electronic ion pumps which have been used to deliver a variety of metal ions including K+,33 Ca2+ (ref. 34) and are the subject of a review.35
Sr2+ ions play a significant role during new bone formation by stimulating osteoblasts36 and simultaneous inhibition of bone resorption by suspending the function of osteoclasts.37 For this reason, several formulations have been used to treat bone diseases and other bone related conditions.38 Some Sr containing minerals display antibacterial activity, however, the effects of Sr2+ are highly related to its concentration, with excess amounts of Sr2+ causing osteonecrosis,39 therefore, the amount of Sr2+ ions released should be well controlled.40
Furthermore, we note that these nanostructured electroactive biomaterials have potential dual use for delivery of bioactive species,41 and moreover, regenerative bone formation triggered by electrical stimulation of cells.42–45 Consequently, we believe that controlled release of Sr2+ ions from biomaterials (e.g., in situ crosslinking electroactive hydrogels) have great promise for the treatment of bone diseases/trauma. Additionally, such biomaterials may be easily repurposed for the delivery of other divalent cations such as Zn2+ and Ca2+ ions.46 By using electroactive hydrogels, a portion of the market demand for dosage-controlled release of divalent ions may be met.
Here we report the preparation of in situ forming electroactive composite hydrogels formed by Schiff base formation between aldehydes displayed on an oxidized derivative of pectin (PEC-ALD) and amines displayed on chitosan (CS) including PPy-NPs, and the characterisation of their physicochemical properties. Sr2+ ions were loaded electrochemically into the hydrogels, and the electrically triggered release of Sr2+ ions was investigated. The cytocompatibility of the hydrogels towards Human Mesenchymal Stem Cells (MSCs) and fibroblasts were studied, as was the antibacterial activity of the Sr2+ ions released from the gels towards E. coli and S. aureus.
Experimental
Materials
Unless otherwise stated, all chemicals and consumables were purchased from either Sigma Aldrich or Acros Organics, of analytical grade, and used as supplied.Synthesis of pectin displaying aldehydes
In order to introduce aldehyde groups, pectin from citrus peel (poly-D-galacturonic acid methyl ester, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–70
000–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) was oxidized by adaptation of the literature.19,47 1.0 g of pectin from citrus peel was dissolved in 100 mL ultrapure (Millipore) water. Then, the oxidant agent sodium periodate (0.535 g, 2.5 mM) was added, and the reaction was stirred for 24 h at room temperature in the dark. Finally, ethylene glycol (140 μL, 2.5 mM) was added to the reaction mixture to eliminate the unreacted periodate and stirred the reaction for 1 h at room temperature in the dark. Low molecular weight contaminants were removed by dialysis using ultrapure water in dialysis tubing cellulose membrane (MWCO 3500). Dialysis was performed for four days with water exchange every 2 hours during the daytime. Then PEC-ALD was freeze-dried. After freeze-drying PEC-ALD was obtained with an 80.54% yield.
000 Da) was oxidized by adaptation of the literature.19,47 1.0 g of pectin from citrus peel was dissolved in 100 mL ultrapure (Millipore) water. Then, the oxidant agent sodium periodate (0.535 g, 2.5 mM) was added, and the reaction was stirred for 24 h at room temperature in the dark. Finally, ethylene glycol (140 μL, 2.5 mM) was added to the reaction mixture to eliminate the unreacted periodate and stirred the reaction for 1 h at room temperature in the dark. Low molecular weight contaminants were removed by dialysis using ultrapure water in dialysis tubing cellulose membrane (MWCO 3500). Dialysis was performed for four days with water exchange every 2 hours during the daytime. Then PEC-ALD was freeze-dried. After freeze-drying PEC-ALD was obtained with an 80.54% yield.
Preparation of chitosan stock solution
Commercial chitosan with medium molecular weight (190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–310
000–310![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, 75–85% deacetylated) was used as –NH2 source for the in situ crosslinking hydrogel. A 2 w% chitosan (CS) stock solution was prepared adding the CS powder into a 1 vol% acetic acid aqueous solution at ambient temperature and stirred until CS was completely dissolved. After complete dissolution, the pH of the solution was adjusted to 5.5 by drop-wise addition of 1 M sodium hydroxide. The formulation was then stirred for 24 h.48
000 Da, 75–85% deacetylated) was used as –NH2 source for the in situ crosslinking hydrogel. A 2 w% chitosan (CS) stock solution was prepared adding the CS powder into a 1 vol% acetic acid aqueous solution at ambient temperature and stirred until CS was completely dissolved. After complete dissolution, the pH of the solution was adjusted to 5.5 by drop-wise addition of 1 M sodium hydroxide. The formulation was then stirred for 24 h.48
Preparation of polypyrrole nanoparticles (PPy-NPs)
PPy-NPs were prepared by adaptation of the literature.49 Briefly, sodium dodecyl sulphate (SDS, 0.86 g) was dissolved in 30 mL distilled water by stirring for 30 minutes. Then, 1 g of pyrrole was added to the solution drop by drop and 5.55 g of FeCl3 was dissolved in 5 mL distilled water which was added dropwise to the mixture. The polymerization process was carried out for 3 hours at room temperature. The black PPy precipitate was filtered off and washed with water and ethanol until clear wash liquid obtained. The black PPy-NPs powder was dried in a vacuum oven at 40 °C overnight. After drying PPy-NPs were obtained with 88.44% yield.Preparation of in situ crosslinking hydrogel (PEC-ALD/CS)
For preparation of in situ crosslinking hydrogels, 1 w% solutions of PEC-ALD in phosphate buffered saline (PBS, pH 7.4) and 2 w% solution of CS in acidified water (pH 5.5) were used. By mixing the stock solutions at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for PEC-ALD/CS and stirring for 2 minutes crosslinking translucent gels were obtained.
2 for PEC-ALD/CS and stirring for 2 minutes crosslinking translucent gels were obtained.
Preparation of electroactive in situ crosslinking hydrogel (PEC-ALD/CS/PPy-NPs)
The stock solutions of CS and PEC-ALD were prepared as described above. Then, four different percentages of PPy-NPs were added to the composition of PEC-ALD/CS hydrogel. The percentage of the PPy-NPs was calculated according to the total mass of the polysaccharides and presented in Table 1. The pre-weighed amount of PPy-NPs was transferred into a container and the stock solutions of the polysaccharides at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for PEC-ALD
2 for PEC-ALD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS were added into the container and the mixture was stirred for 2 minutes yielding homogenous black electroactive gels.
CS were added into the container and the mixture was stirred for 2 minutes yielding homogenous black electroactive gels.
| Formulation | PPy-NPs: polysaccharide percentage (w/w) |
|---|---|
| PEC-ALD/CS | 0 |
| PEC-ALD/CS/PPy-NPs-1 | 2.5 |
| PEC-ALD/CS/PPy-NPs-2 | 5.0 |
| PEC-ALD/CS/PPy-NPs-3 | 7.5 |
| PEC-ALD/CS/PPy-NPs-4 | 10.0 |
Fourier transform infrared spectroscopy (FTIR)
Infrared spectroscopy was carried out on a PerkinElmer Spectrum 100 spectrometer. Spectra were recorded as an average of 16 scans in ATR mode at room temperature, with a 1 cm−1 resolution.In vitro swelling studies
The hydrogels were dried in a vacuum oven at 40 °C overnight and dry hydrogels weighed and immersed in PBS solutions (pH 7.4) at 37 °C for 24 hours. Then, the swollen hydrogels were removed and immediately weighed after the excess water on their surfaces was blotted away with filter paper, until the weight of hydrogels reached an equilibrium value. The swelling percentage (SP) was calculated as follows: SP (%) = ((W24 − W0)/W0) × 100, where W24 and W0 are the weights of the hydrogels in the swollen state and dry state, respectively.X-ray diffraction (XRD)
X-ray diffraction patterns of the samples were obtained using a Bruker powder diffractometer (D8 Advance, Bruker, Germany). Analyses were carried out with Cu Kα radiation (0.15406 nm) at accelerating current of 40 mA and voltage of 45 kV and data were collected with a scan range of 2θ = 10°–80° for determination of crystallographic properties for the samples.Particle size distribution analysis for PPy-NPs
Particle size distribution of PPy-NPs was analysed using a Mastersizer 2000 analyser (Malvern Panalytical Ltd, United Kingdom).Scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS)
The surface morphologies of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogels were investigated by SEM-EDS (Philips XL 30 S-FEG, the Netherlands) equipped with an EDAX detector (Ametek, USA, Apollo X) for energy dispersive X-ray analysis.Thermal analysis
Thermal gravimetric analysis (TGA) and differential scanning calorimetry analysis (DSC) of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogels were performed using a TGA/SDTA851e model TGA instrument (Mettler Toledo, Switzerland) and DSC822e model DSC instrument (Mettler Toledo, Switzerland), respectively. Temperatures observed were from 25 to 700 °C with a heating rate of 10 °C min−1 under an argon flow (50 mL min−1) and −40 to 300 °C with a heating rate of 10 °C min−1 for TGA and DSC, respectively.Cyclic voltammetry (CV)
Cyclic voltammetry measurements were performed using DropSens μSTAT 400 potentiostat connected to a personal computer using the Dropview 8400 software. For CV measurements, a three-electrode system was used with an Ag/AgCl reference electrode, a platinum-wire counter electrode, and a glassy carbon electrode with the PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogel working electrode. The electrodes were in a biomedically relevant buffer (4 mL of phosphate-buffered saline [PBS] at pH 7.4). For CV measurements, the potential was swept between −1.0 V and +1.0 V vs. the Ag/AgCl electrode at a scan rate of 0.05 V s−1.Electrochemical Sr2+ loading studies
Sr2+ loading into the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs electroactive hydrogels was carried out electrochemically using a DropSens μSTAT 400 potentiostat connected to a personal computer using the Dropview 8400 software (amperometric technique). A three-electrode system was used with an Ag/AgCl reference electrode, a platinum-wire counter electrode, and a glassy carbon electrode with the electroactive in situ crosslinking hydrogel (PEC-ALD/CS/PPy-NPs) working electrode in an aqueous solution of Sr2+ ion (100 mM SrCl2·6H20). Prior to electrical stimulation, there was a quiet time of 20 s at a potential of −0.1 V, after which the PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogel working electrodes were stimulated for 30 minutes with a potential of −0.6 V. The concentrations of Sr2+ solutions before and after loading were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) (PerkinElmer, USA, Optima DV).Electrochemical Sr2+ release studies
The electrochemically triggered release of Sr2+ ions from the hydrogels was achieved using a DropSens μSTAT 400 potentiostat connected to a personal computer using the Dropview 8400 software (amperometric technique), and a three-electrode system (described above) in a biomedically relevant buffer (4 mL of PBS at pH 7.4). The data are reported as cumulative release as a percentage of the total mass of the Sr2+ ions in the hydrogels (hydrogels were individually weighed and the loaded concentration of Sr2+ ions were individually determined prior to Sr2+ ion release experiments).Antibacterial activity studies
The antibacterial activity of the Sr2+ ions was tested against Gram-negative (E. coli ATCC 8739) and Gram-positive (S. aureus ATCC 29213) bacterial strains using the agar-well diffusion method.50,51 The bacterium strains were provided by the Department of Microbiology, Gebze Technical University. E. coli and S. aureus were cultured in Luria–Bertani (LB) and Nutrient agar (NA) at 37 °C (180 rpm), respectively.52 The bacterial concentrations were adjusted to approximately 0.1 optical density at 600 nm. Subsequently, nutrient and LB agar plates were inoculated uniformly by spreading 100 μL cell suspension of bacteria with a sterile dragalski spatula all over the plate in one direction. 100 mg Sr2+ ions were dissolved in 1 mL of physiological solution (0.9% NaCl). Four holes with a diameter of 5 mm were punched with a sterile tip and 10–100 μL of strontium solution was introduced into the wells. For this test, chloramphenicol discs (30 μg) were used as a positive control. Agar plates were incubated for 24 h, at 37 °C. The antibacterial effect of Sr2+ ions solution diffused into the agar medium was determined by measuring the zone of inhibition in mm. In addition, the electrically triggered released Sr2+ ions solutions were also separately introduced into the wells and antibacterial activity of the released Sr2+ ions concentration was investigated. The concentration of the released Sr2+ ions was determined by using ICP-OES. The test was performed with three replicates for each Sr2+ ions concentration.CCK8 cell viability assay
ATCC PSC-500-010 Human Mesenchymal Stem Cells (MSCs) and ATCC PCS-201-012 fibroblasts were cultured with DMEM/F12 media with 10% fetal bovine serum and 1% penicillin–streptomycin addition in a 37 °C incubator with 5% CO2 until cells reach enough number. The cells to be treated were seeded at 0.01 × 106 cells per well in a 96-well plate and cultured overnight for adhesion.Hydrogels were weighed and autoclaved at 121 °C for 15 minutes for sterilization.53 Sterile gel beads were divided into equal pieces then placed on top of the cells in a laminar flow hood. At least five samples were used for each weight while no gels were added to control group cells. Cells were cultured with or without hydrogels for 48 hours to observe any harmful effect. Since the hydrogels are black in color which may cause interference in spectroscopy, they were removed along with media. Culture media was renewed with 190 μL of DMEM/F12 with addition of 10 μL CCK8 solution for each well. Cells were kept in the incubator for another 2 hours. Absorption measurements were taken using the spectrophotometry (SPECTROstar) device and SPECTROstar nano application. Graphics and standard deviations (SDs) were processed in excel.
Results and discussion
Synthesis of pectin displaying aldehydes
The chemically crosslinked hydrogels were synthesised via Schiff base reaction between aldehyde displaying PEC-ALD and amine displaying chitosan. The Schiff base reaction between these functional groups rapidly occurs and enables formation of the hydrogels in seconds to minutes.19,54–57 In order to obtain aldehyde displaying pectin (PEC-ALD), pectin was oxidized using sodium periodate.19Preparation of in situ crosslinking hydrogel (PEC-ALD/CS) and electroactive in situ crosslinking hydrogel (PEC-ALD/CS/PPy-NPs)
A 1 w% solution of PEC-ALD in phosphate buffered saline (PBS, pH 7.4) was prepared. A 2 w% CS solution was prepared in acidified water and its pH was adjusted to pH 5.5. The stock solutions described above were mixed at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for PEC-ALD
2 for PEC-ALD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS, crosslinking occurred within seconds and complete gelation was obtained after mixing the components for two minutes, yielding either translucent PEC-ALD/CS electroactive gels or black and PEC-ALD/CS/PPy-NPs electroactive gels by the addition of PPy-NPs. The electroactive behaviour of the PEC-ALD/CS/PPy-NPs electroactive gels including different amount of PPy-NPs was investigated by CV technique. The conductivity of the hydrogels was observed to increase as the amount of PPy-NPs increases (Fig. 1). 5% PPy-NPs (PEC-ALD/CS/PPy-NPs-2) was decided to use for further studies, since the increased amount of PPy-NPs causes deterioration in the physical structure of the hydrogel.
CS, crosslinking occurred within seconds and complete gelation was obtained after mixing the components for two minutes, yielding either translucent PEC-ALD/CS electroactive gels or black and PEC-ALD/CS/PPy-NPs electroactive gels by the addition of PPy-NPs. The electroactive behaviour of the PEC-ALD/CS/PPy-NPs electroactive gels including different amount of PPy-NPs was investigated by CV technique. The conductivity of the hydrogels was observed to increase as the amount of PPy-NPs increases (Fig. 1). 5% PPy-NPs (PEC-ALD/CS/PPy-NPs-2) was decided to use for further studies, since the increased amount of PPy-NPs causes deterioration in the physical structure of the hydrogel.
Fourier transform infrared spectroscopy
The FTIR spectra of CS, PEC, PEC-ALD, PPy-NPs and corresponding hydrogels are displayed in Fig. 2. The characteristic O–H and C–H stretching vibration peaks were observed at 3370 and 2932 cm−1. The peaks at 1733 cm−1 and 1610 cm−1 were attributed to the carboxymethyl (−COOCH3) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks of pectin. The peak at 1017 cm−1 was attributed the C–O stretch of the secondary alcohol groups.58 The FTIR spectrum of PEC-ALD showed that the intensity of peaks increased after oxidizing pectin yielding PEC-ALD.
O peaks of pectin. The peak at 1017 cm−1 was attributed the C–O stretch of the secondary alcohol groups.58 The FTIR spectrum of PEC-ALD showed that the intensity of peaks increased after oxidizing pectin yielding PEC-ALD.
The FTIR spectrum of CS showed a broad band at around 3353–3291 cm−1 which was attributed to O–H and N–H stretching vibrations. A small peak at 2870 cm−1 corresponded C–H symmetric and asymmetric stretching vibrations. These bands are characteristic bands for polysaccharides and found in other polysaccharides such as glucans, xylan, etc., too.59 The peak at 1648 cm−1 related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of amide I and bending vibrations of the N–H (N-acetylated residues, amide II band) absorption band was observed at 1562 cm−1. The absorption band at 1150 cm−1 can be attributed to asymmetric stretching of the C–O–C bridge. The skeletal C–O stretching peaks were observed at 1065–1027 cm−1.60 The FTIR spectrum of the PEC-ALD/CS hydrogel which was obtained by mixing PEC-ALD and CS exhibited a weak shoulder and sharp peaks at 3330 cm−1 is attributed to amine groups on CS, 1730 cm−1 and 1630 cm−1 are attributed to C
O group of amide I and bending vibrations of the N–H (N-acetylated residues, amide II band) absorption band was observed at 1562 cm−1. The absorption band at 1150 cm−1 can be attributed to asymmetric stretching of the C–O–C bridge. The skeletal C–O stretching peaks were observed at 1065–1027 cm−1.60 The FTIR spectrum of the PEC-ALD/CS hydrogel which was obtained by mixing PEC-ALD and CS exhibited a weak shoulder and sharp peaks at 3330 cm−1 is attributed to amine groups on CS, 1730 cm−1 and 1630 cm−1 are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of carboxylic acid on both PEC-ALD and CS. The PEC-ALD/CS/PPy-NPs hydrogel was prepared by mixing PEC-ALD, CS and PPy-NPs and the FTIR spectrum of the hydrogel showed characteristic vibration peaks at 1529–1431 cm−1 that can be attributed to the pyrrole ring stretching of the PPy-NPs. The peak at 1280 cm−1 due to the C–C in-ring stretching and C–N deformation mode.61 The FTIR spectrum of the PEC-ALD/CS/PPy-NPs hydrogel confirmed that PPy-NPs were well mixed with the hydrogel.
O stretching of carboxylic acid on both PEC-ALD and CS. The PEC-ALD/CS/PPy-NPs hydrogel was prepared by mixing PEC-ALD, CS and PPy-NPs and the FTIR spectrum of the hydrogel showed characteristic vibration peaks at 1529–1431 cm−1 that can be attributed to the pyrrole ring stretching of the PPy-NPs. The peak at 1280 cm−1 due to the C–C in-ring stretching and C–N deformation mode.61 The FTIR spectrum of the PEC-ALD/CS/PPy-NPs hydrogel confirmed that PPy-NPs were well mixed with the hydrogel.
In vitro swelling studies
The swelling behaviour of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels in PBS was investigated. The swelling percentage for PEC-ALD/CS hydrogel was found to be 208 ± 10, whereas that of PEC-ALD/CS/PPy-NPs hydrogel was found to be 525 ± 7. The addition of PPy-NPs to the gel formulation resulted in higher swelling percentages than the PEC-ALD/CS hydrogel formulation, likely due to the presence of PPy-NPs that diminishes the number of Schiff base crosslinks that form, thereby increasing the pore sizes within the hydrogel.19,54–56X-ray diffraction
XRD was employed to provide more information on the individual components of the gels, and PEC-ALD/CS and the PEC-ALD/CS/PPy-NPs gels' structures. XRD patterns of CS, PEC-ALD, PPy-NPs, PEC-ALD/CS gel, and PEC-ALD/CS/PPy-NPs gel are shown in Fig. 3. The XRD pattern of CS is in agreement with literature showing a broad peak with a sharp top at 2θ = 20° which is a clear evidence of a semi-crystalline structure.19 A sharp peak at 2θ = 15.57° and a broad peak centred around 2θ = 25° of PEC-ALD were observed indicating PEC-ALD has mostly amorphous structure with partial crystallinity.19,62,63 The XRD pattern of the PPy-NPs showed a broad peak centred at 2θ value ca. 26° confirming amorphous nature of PPy-NPs.63,64 The XRD pattern of PEC-ALD/CS gel showed a very broad peak at around 2θ = 30° with a shoulder centred around at 2θ = 40° that indicates PEC-ALD/CS gel has an amorphous structure,19 whereas the pattern of the PEC-ALD/CS/PPy-NPs gel showed a broad peak at around 2θ = 22° and four sharp peaks at 2θ = 31.78, 45.57, 58.59 and 75.49° correspond to NaCl (2θ = 31.78, 45.57 and 58.59)65 and KCl (2θ = 31.78, 58.59 and 75.49°)66 in the gel.Particle size distribution analysis for PPy-NPs
The particle size of the nanoparticles is an important parameter underpinning the injectability of the hydrogels including the nanoparticles. In order to understand suitability of the PPy-NPs for in situ crosslinking hydrogels and needle gauge necessary, particle size distribution analysis was performed using a Mastersizer. The particle size of the PPy-NPs was found to be 112 ± 29 nm, however a few particles were observed to have agglomerated. The size of the nanoparticles was also confirmed using scanning electron microscopy and it can be seen that particle size is mostly around 110 nm and the diameters of aggregates are around 250 nm which is still injectable via needles of a variety of gauge sizes used in surgical procedures.67–74Scanning electron microscopy (SEM)
The surface morphology of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels were investigated using SEM are shown in Fig. 5. As it can be seen from Fig. 4a and b, PEC-ALD/CS gels have smooth surfaces with heterogeneously distributed pores, while PEC-ALD/CS/PPy-NPs gels have larger pores. SEM images of PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels are in good agreement with the swelling percentage data. On the other hand, as it can be seen in Fig. 4, comparing the PEC-ALD/CS gel with the PEC-ALD/CS/PPy-NPs gel, it is evident the presence of agglomerated PPy-NPs in the electroactive gel. However, the SEM images of PEC-ALD/CS/PPy-NPs gel, show homogeneously distributed aggregates inside the gel matrix. It is also possible to observe the difference of the network structure when polymerized in the absence or presence of the nanoparticles. In Fig. 4c and d, evidence of agglomerated PPy-NPs can be observed. By comparison in the case of PEC-ALD/CS gels, it is possible to observe in Fig. 4a and b, the presence of fibrillar structures in the hydrogel network. Additionally, the particle size of PPy-NPs and agglomerates in the SEM images are in good agreement with particle size distribution data determined using a Mastersizer. | ||
| Fig. 4 SEM images of PEC-ALD/CS (a and b) and PEC-ALD/CS/PPy-NPs gels (c and d). Scale bars represent 2, 2, 0.5, 0.5 μm in (a)–(d), respectively. | ||
Energy dispersive spectroscopy (EDS)
The EDS analysis of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels was performed, and results are given in Fig. 5 and Table 2. The elemental analysis results confirm the presence of PPy-NPs in PEC-ALD/CS/PPy-NPs gel as there is a clear increase in the percentages of C and N atoms while the percentage of O atoms decreased (Table 2). Analysis of the EDS data for PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels (Fig. 5a and b, respectively) showed elemental signals characteristic of carbon (Kα at 0.277 keV), nitrogen (Kα at 0.392 keV) and oxygen (Kα at 0.525 keV) found on CS, PEC-ALD and PPy, respectively.| Element | Composition (%) – PEC-ALD/CS gel | Composition (%) – PEC-ALD/CS/PPy-NPs gel |
|---|---|---|
| C | 44.43 | 55.16 |
| N | 10.56 | 23.46 |
| O | 45.01 | 21.38 |
Thermogravimetric analysis
Fig. 6 shows TGA thermograms of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs hydrogels. Main mass loss due to the evaporation of the water was observed from 20 °C to approximately 100 °C for PEC-ALD/CS gels whereas that of for PEC-ALD/CS/PPy-NPs gels was from 20 °C to approximately 135 °C. All gels showed major weight loss due to evaporation of water. Then the peak at ca. 200 °C and ca. 220 °C correspond to the mass loss upon degradation of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs hydrogels, respectively. Pure chitosan shows a distinct weight loss at between 200–300 °C, attributable to the degradation of chitosan chains. Addition of other components into the structure showed that thermal stability of the gel was decreased.75,76 PEC-ALD shows two steps degradation, first step occurs at between 150–250 °C and second step occurs at between 250–300 °C.77 The thermal stability of the in situ crosslinking hydrogels can be attributed to the presence of strong contact between –CHO groups of PEC-ALD and –NH2 group of CS that have reacted to form Schiff bases.77 The TG analysis of the hydrogels is in a good agreement with the literature and show that addition of the electroactive PPy-NPs to the hydrogel matrix increased the thermal stability of the hydrogels (potentially due to polysaccharide-PPY interactions).In order to provide information about the physical properties (i.e. crystalline or amorphous nature, and the possible interactions between biopolymers) of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels, DSC measurements were performed.78 DSC thermograms of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels are shown in Fig. 7. Two endothermic peaks were observed for both gels. The endothermic peak between −30 and 50 °C corresponds to the melting phenomenon ascribable to water.79
The second detected endotherm peak at temperatures higher than 100 °C is attributed to the evaporation of water tightly linked through polar interactions to ionic groups and can be assigned to the excision of an ion pair between the carboxylic group (–COOH) of the PEC and the ammonium group (–NH3+) of the CS.80,81 Additionally this peak can be attributed to the decomposition of PEC-ALD (105 °C for PEC-ALD/CS/PPy-NPs gel and 115 °C for PEC-ALD/CS gel) and chitosan (145 °C for both gels).82
Cyclic voltammetry
The electrochemical properties of the PEC-ALD/CS and PEC-ALD/CS/PPy-NPs gels were studied via cyclic voltammetry. Fig. 8 shows cyclic voltammograms of bare GCE (a), PEC-ALD/CS in situ crosslinking hydrogel (b) and PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogel (c) (1st to 10th cycles). As evident from the CV curves, the charge-storage capacities of the hydrogels decreased steadily on repeated cycling, and the areas under the curves of the 1st and 10th cycles were almost the same. The currents evolved in the PEC-ALD/CS/PPy-NPs gel were higher than those in the PEC-ALD/CS gel, and the oxidation and reduction peaks were more prominent. Occurrences of reduction and oxidation peaks correspond to the de-doping of the hydrogel (i.e., release of drug molecules), and the hydrogels were subsequently re-doped by other anions (either the anionic drug, or anions from the PBS buffer: H2PO4− and HPO42−, and Cl−).Sr2+ release studies
The electrochemically triggered release of Sr2+ ions from PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogels was studied using DropSens μSTAT 400 potentiostat connected to a personal computer using the Dropview 8400 software and released amount of Sr2+ ions were measured with ICP-OES, where the release was either passive (i.e., in the absence of an electrochemical stimulus) or electrochemically triggered (i.e., in the presence of one or more rounds of electrochemical stimulation: 30 s of stimulation at a reducing potential of 0.6 V, followed by 10.5 min of rest (Fig. 9)). The quantity of the released Sr2+ ions in solution was quantified at various time points, and the data are reported as cumulative release as a percentage of the total mass of the Sr2+ ions in the hydrogel (hydrogels were individually weighed; and Sr2+ ions concentrations of the solutions before and after Sr2+ ion loading were determine using ICP-OES for each hydrogel), and compared to passive drug release from unstimulated films every 11 minutes (Fig. 10). | ||
| Fig. 9 Electrochemically loading Sr2+ ions (a) and electrochemically enhanced delivery (b) of Sr2+ ions from PEC-ALD/CS/PPy-NPs in situ crosslinked hydrogels in PBS (pH = 7.4). | ||
Antibacterial activity
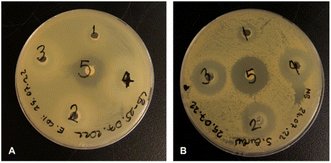
Antibacterial activity tests showed that none of the tested concentrations (1.0 mg, 5.0 mg, 7.5 mg and 10 mg) of the strontium showed antibacterial activity against E. coli ATCC 8739 while positive control chloramphenicol showed antibacterial activity with 20 mm inhibition zone. We found that the 7.5 and 10.0 mg strontium have antibacterial effect against S. aureus ATCC 29213, giving inhibition zones with values 27.8 and 28.3 mm, respectively. The inhibition zone of chloramphenicol against S. aureus was found to be 26 mm (Fig. 11).According to antibacterial test results, the strontium has antibacterial activity against S. aureus better than control antibiotic chloramphenicol while it shows no antibacterial activity against E. coli. The antibacterial properties of hydroxyapatite (HA) nanoparticles on E. coli and S. aureus were improved substituted by strontium.83 In another study, it was reported that strontium ranelate (SR)-loaded poly(lactic-co-glycolic acid) (PLGA) microspheres (PM) with assembled silver nanoparticles (AgNPs) and hydroxyapatite nanoparticles (HANPs) (SR-PM-Ag-HA) showed antibacterial activity against Staphylococcus epidermidis (ATCC 12228) and methicillin resistant S. aureus (MRSA; ATCC 43300), and have potential application in the treatment of bone-related infections.84 Different studies have shown that strontium has an antibacterial effect against S. aureus.85–87 The antibacterial activities of electrically triggered released Sr2+ ions from PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogels against E. coli and S. aureus were also investigated and results are shown in Fig. 12, as can be observed the released concentrations of Sr2+ ions were not sufficient enough for any antibacterial activity; this observation is due to the physicochemical properties of the gels.
Cell viability assay
No significant toxic effects were observed in an MSC viability test in vitro. All fibroblast plus gel groups were found to be slightly higher in viability although there was no significant difference (Fig. 13). Therefore, the hydrogels are safe with human cells within the tested weight range (10–90 μg) in vitro.Conclusions
Herein, we report Sr2+ ions-loaded PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogels which electrochemically release antibacterial Sr2+ ions. The gels were characterized by FTIR, SEM-EDX, TGA, DSC, XRD and CV, which confirm the successful preparation of the Sr2+ ions-loaded PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogels. Thermal analysis results showed that hydrogels show good thermal stability. The swelling percentage for PEC-ALD/CS hydrogel was found to be 208 ± 10, whereas that of PEC-ALD/CS/PPy-NPs hydrogel was found to be 525 ± 7. These results are in good agreement with SEM images. PEC-ALD/CS/PPy-NPs hydrogel has larger pores while PEC-ALD/CS gels have smooth surfaces. According to CV results that demonstrating the electrochemical behaviour of the PEC-ALD/CS/PPy-NPs hydrogel, the conductivity of the hydrogels was observed to increase as the amount of PPy-NPs increases. On the other hand, addition of higher amount of PPy-NPs causes deterioration in the physical structure of the hydrogel. Due to this limitation, 5% PPy-NPs (PEC-ALD/CS/PPy-NPs-2) was decided to use for studies. The Sr2+ ions loaded PEC-ALD/CS/PPy-NPs in situ crosslinking hydrogel showed no significant cytotoxicity towards human mesenchymal stem cells (MSCs) and fibroblasts. This is the first example of Sr2+ loaded in situ crosslinking PEC-ALD/CS/PPy-NPs electroactive hydrogels that deliver the Sr2+ ions in a controllable manner in response to the application of electricity. The antibacterial activity of Sr2+ ions against E. coli and S. aureus was demonstrated and their minimum inhibitory concentrations (MIC) were determined. While these prototypical examples of Sr2+ loaded electroactive gels don't release sufficient Sr2+ ions to show antibacterial activity against E. coli and S. aureus, we believe future iterations with optimised physical properties of the gels will be capable of doing so, likewise the gels will be capable of the delivery of other divalent cations such as Ca2+ and Zn2+ ions, and therefore such in situ crosslinking conducting hydrogels have great promise for treatment of bone related diseases.Author contributions
M. F. D. conceptualized the paper and prepared the original draft. Methodology, formal analysis, and investigation were carried out by all authors. M. F. D., D. Ö. and Z. S. Ç. carried out materials and characterization. F. E. conducted antibacterial activity studies. H. D. and A. N. O. performed cell culture. M. F. D., D. Ö. and Z. S. Ç. were involved in drug delivery. M. F. D., J. G. H. and D. Ö. carried out data curation. All authors were involved in writing, reviewing, and editing. M. F. D. and J. G. H. supervised the experiments. M. F. D. led project administration and funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Royal Society of Chemistry (Research Fund, R20-4000) awarded to MFD. JGH thanks the UKRI Biotechnology and Biological Sciences Research Council (BBSRC) Networks in Industrial Biotechnology and Bioenergy (NIBB) for support, specifically: FoodWasteNet NIBB (BB/L0137971/1), IBCarb NIBB (BB/L013762/1), P2P NIBB (BB/L013819/1), and the Royal Society for a Research Grant (RG160449).References
- D. Malik, R. Joshi, H. Kaur, A. Prakash and B. Medhi, in Advanced Drug Delivery Systems in the Management of Cancer, ed. K. Dua, M. Mehta, T. de Jesus Andreoli Pinto, L. G. Pont, K. A. Williams and M. J. Rathbone, Academic Press, 2021, pp. 521–531, DOI:10.1016/B978-0-323-85503-7.00035-3.
- F. Zamani, F. Jahanmard, F. Ghasemkhah, S. Amjad-Iranagh, R. Bagherzadeh, M. Amani-Tehran and M. Latifi, in Nanostructures for Drug Delivery, ed. E. Andronescu and A. M. Grumezescu, Elsevier, 2017, pp. 239–270, DOI:10.1016/B978-0-323-46143-6.00007-5.
- H. Wen, H. Jung and X. Li, AAPS J., 2015, 17, 1327–1340 CrossRef CAS PubMed.
- A. M. Vargason, A. C. Anselmo and S. Mitragotri, Nat. Biomed. Eng., 2021, 5, 951–967 CrossRef PubMed.
- P. Kumari and A. Meena, Environ. Sci. Pollut. Res., 2021, 28, 46343–46355 CrossRef CAS PubMed.
- J. V. Natarajan, C. Nugraha, X. W. Ng and S. Venkatraman, J. Controlled Release, 2014, 193, 122–138 CrossRef CAS PubMed.
- X. Lin, J. Wu, Y. Liu, N. Lin, J. Hu and B. Zhang, Molecules, 2022, 27(3), 9482022 Search PubMed.
- Y. Wang, M. S. Shim, N. S. Levinson, H. W. Sung and Y. Xia, Adv. Funct. Mater., 2014, 24, 4206–4220 CrossRef CAS PubMed.
- W. Xu, Y. Zhang, Y. Gao and M. J. Serpe, ACS Appl. Mater. Interfaces, 2018, 10, 13124–13129 CrossRef CAS PubMed.
- Y. T. Yi, J. Y. Sun, Y. W. Lu and Y. C. Liao, Biomicrofluidics, 2015, 9, 022401 CrossRef CAS PubMed.
- A. A. Baleg, M. Masikini, S. V. John, A. R. Williams, N. Jahed, P. Baker and E. Iwuoha, Functional Polymers, ed. M. A. Jafar Mazumder, H. Sheardown and A. Al-Ahmed, Springer International Publishing, Cham, 2019, pp. 551–604, DOI:10.1007/978-3-319-95987-0_17.
- S. A. A. Shah, M. Firlak, S. R. Berrow, N. R. Halcovitch, S. J. Baldock, B. M. Yousafzai, R. M. Hathout and J. G. Hardy, Materials, 2018, 11, 1123 CrossRef PubMed.
- M. D. Ashton, I. C. Appen, M. Firlak, N. E. Stanhope, C. E. Schmidt, W. R. Eisenstadt, B. Hur and J. G. Hardy, Polym. Int., 2021, 70, 467–474 CrossRef CAS.
- G. JosephJagur, Polym. Adv. Technol., 2002, 13, 615–625 CrossRef.
- O. S. Fenton, K. N. Olafson, P. S. Pillai, M. J. Mitchell and R. Langer, Adv. Mater., 2018, e1705328, DOI:10.1002/adma.201705328.
- L. L. Palmese, R. K. Thapa, M. O. Sullivan and K. L. Kiick, Curr. Opin. Chem. Eng., 2019, 24, 143–157 CrossRef PubMed.
- S. Mantha, S. Pillai, P. Khayambashi, A. Upadhyay, Y. Zhang, O. Tao, H. M. Pham and S. D. Tran, Materials, 2019, 12(20), 3323 CrossRef CAS PubMed.
- J. Tavakoli and Y. Tang, Polymers, 2017, 9(8), 364 CrossRef PubMed.
- F. Leone, M. Firlak, K. Challen, W. Bonnefin, B. Onida, K. L. Wright and J. G. Hardy, J. Funct. Biomater., 2019, 10(4), 50 CrossRef CAS PubMed.
- N. Raina, R. Pahwa, J. Bhattacharya, A. K. Paul, V. Nissapatorn, M. de Lourdes Pereira, S. M. R. Oliveira, K. G. Dolma, M. Rahmatullah, P. Wilairatana and M. Gupta, Pharmaceutics, 2022, 14(3), 574 CrossRef CAS PubMed.
- D. G. J. Smethurst and N. Shcherbik, J. Biol. Chem., 2021, 297, 101374 CrossRef CAS PubMed.
- D. J. Hare, E. J. New, M. D. de Jonge and G. McColl, Chem. Soc. Rev., 2015, 44, 5941–5958 RSC.
- M. Moustakas, Materials, 2021, 14(3), 549 CrossRef CAS PubMed.
- Y. Liu, Y. Wang, S. Song and H. Zhang, Chem. Sci., 2021, 12, 12234–12247 RSC.
- V. Mouriño, J. P. Cattalini and A. R. Boccaccini, J. R. Soc., Interface, 2012, 9(68), 401–419 CrossRef PubMed.
- I. Yousuf, M. Bashir, F. Arjmand and S. Tabassum, Coord. Chem. Rev., 2021, 445, 214104 CrossRef CAS.
- H. Safiaghdam, H. Nokhbatolfoghahaei and A. Khojasteh, Iran. J. Pharm. Res., 2019, 18, 101–118 CAS.
- M. Awais, A. Aizaz, A. Nazneen, Q. U. Bhatti, M. Akhtar, A. Wadood and M. Atiq Ur Rehman, Prosthesis, 2022, 4, 263–316 CrossRef.
- A. R. Farmani, M. A. Salmeh, Z. Golkar, A. Moeinzadeh, F. F. Ghiasi, S. Z. Amirabad, M. H. Shoormeij, F. Mahdavinezhad, S. Momeni, F. Moradbeygi, J. Ai, J. G. Hardy and A. Mostafaei, J. Funct. Biomater., 2022, 13(4), 1622022 Search PubMed.
- L. Freage, A. Trifonov, R. Tel-Vered, E. Golub, F. Wang, J. S. McCaskill and I. Willner, Chem. Sci., 2015, 6, 3544–3549 RSC.
- Y. Wang, J. Chen, J. Lu, J. Xi, Z. Xu, L. Fan, H. Dai and L. Gao, J. Nanobiotechnol., 2022, 20, 199 CrossRef CAS PubMed.
- J. Koo, S. B. Kim, Y. S. Choi, Z. Xie, A. J. Bandodkar, J. Khalifeh, Y. Yan, H. Kim, M. K. Pezhouh, K. Doty, G. Lee, Y.-Y. Chen, S. M. Lee, D. D'Andrea, K. Jung, K. Lee, K. Li, S. Jo, H. Wang, J.-H. Kim, J. Kim, S.-G. Choi, W. J. Jang, Y. S. Oh, I. Park, S. S. Kwak, J.-H. Park, D. Hong, X. Feng, C.-H. Lee, A. Banks, C. Leal, H. M. Lee, Y. Huang, C. K. Franz, W. Z. Ray, M. MacEwan, S.-K. Kang and J. A. Rogers, Sci. Adv., 2020, 6(35), eabb1093 CrossRef CAS PubMed.
- A. Williamson, J. Rivnay, L. Kergoat, A. Jonsson, S. Inal, I. Uguz, M. Ferro, A. Ivanov, T. A. Sjöström, D. T. Simon, M. Berggren, G. G. Malliaras and C. Bernard, Adv. Mater., 2015, 27, 3138–3144 CrossRef CAS PubMed.
- J. Isaksson, P. Kjäll, D. Nilsson, N. Robinson, M. Berggren and A. Richter-Dahlfors, Nat. Mater., 2007, 6, 673–679 CrossRef CAS PubMed.
- T. Arbring Sjöström, M. Berggren, E. O. Gabrielsson, P. Janson, D. J. Poxson, M. Seitanidou and D. T. Simon, Adv. Mater., 2018, 3, 1700360 Search PubMed.
- B. Kołodziejska, N. Stępień and J. Kolmas, Int. J. Mol. Sci., 2021, 22, 6564 CrossRef PubMed.
- E. Bonnelye, A. Chabadel, F. Saltel and P. Jurdic, Bone, 2008, 42, 129–138 CrossRef CAS PubMed.
- M. Schumacher, A. Lode, A. Helth and M. Gelinsky, Acta Biomater., 2013, 9, 9547–9557 CrossRef CAS PubMed.
- D. Gunson, K. E. Gropp and A. Varela, in Haschek and Rousseaux's Handbook of Toxicologic Pathology, ed. W. M. Haschek, C. G. Rousseaux and M. A. Wallig, Academic Press, Boston, 3rd edn, 2013, pp. 2761–2858, DOI:10.1016/B978-0-12-415759-0.00063-7.
- A. D. Anastasiou, M. Nerantzaki, E. Gounari, M. S. Duggal, P. V. Giannoudis, A. Jha and D. Bikiaris, Sci. Rep., 2019, 9, 14469 CrossRef CAS PubMed.
- V. Pillay, T.-S. Tsai, Y. E. Choonara, L. C. du Toit, P. Kumar, G. Modi, D. Naidoo, L. K. Tomar, C. Tyagi and V. M. K. Ndesendo, J. Biomed. Mater. Res., Part A, 2014, 102, 2039–2054 CrossRef PubMed.
- C. Ning, Z. Zhou, G. Tan, Y. Zhu and C. Mao, Prog. Polym. Sci., 2018, 81, 144–162 CrossRef CAS PubMed.
- T. Zheng, Y. Huang, X. Zhang, Q. Cai, X. Deng and X. Yang, J. Mater. Chem. B, 2020, 8, 10221–10256 RSC.
- X. Zhang, T. Wang, Z. Zhang, H. Liu, L. Li, A. Wang, J. Ouyang, T. Xie, L. Zhang, J. Xue and W. Tao, Mater. Today, 2023, 68, 177–203 CrossRef CAS.
- J. G. Hardy, M. K. Villancio-Wolter, R. C. Sukhavasi, D. J. Mouser, D. Aguilar Jr, S. A. Geissler, D. L. Kaplan and C. E. Schmidt, Macromol. Rapid Commun., 2015, 36, 1884–1890 CrossRef CAS PubMed.
- A. Ozols, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1993, 105, 785–791 CrossRef CAS PubMed.
- E. Lallana, F. Fernandez-Trillo, A. Sousa-Herves, R. Riguera and E. Fernandez-Megia, Pharm. Res., 2012, 29, 902–921 CrossRef CAS PubMed.
- J. Nie, Z. Wang and Q. Hu, Sci. Rep., 2016, 6, 36053 CrossRef CAS PubMed.
- B. Sevil and K. Zuhal, Macromol. Symp., 2010, 295, 59–64 CrossRef CAS.
- L. L. Zaika, J. Food Saf., 1988, 9, 97–118 CrossRef.
- N. A. Dafale, U. P. Semwal, R. K. Rajput and G. N. Singh, J. Pharm. Anal., 2016, 6, 207–213 CrossRef PubMed.
- M. Moradi, S. A. Kousheh, R. Razavi, Y. Rasouli, M. Ghorbani, E. Divsalar, H. Tajik, J. T. Guimarães and S. A. Ibrahim, Trends Food Sci. Technol., 2021, 111, 595–609 CrossRef CAS.
- F. Munarin, S. Bozzini, L. Visai, M. C. Tanzi and P. Petrini, Food Hydrocolloids, 2013, 31, 74–84 CrossRef CAS.
- S. P. Hudson, R. Langer, G. R. Fink and D. S. Kohane, Biomaterials, 2010, 31, 1444–1452 CrossRef CAS PubMed.
- X. Jia, J. A. Burdick, J. Kobler, R. J. Clifton, J. J. Rosowski, S. M. Zeitels and R. Langer, Macromolecules, 2004, 37, 3239–3248 CrossRef CAS.
- D. D. McKinnon, D. W. Domaille, J. N. Cha and K. S. Anseth, Adv. Mater., 2014, 26, 865–872 CrossRef CAS PubMed.
- F. Rizzo and N. S. Kehr, Adv. Healthcare Mater., 2021, 10, 2001341 CrossRef CAS PubMed.
- R. K. Mishra, A. B. A. Majeed and A. K. Banthia, Int. J. Plast. Technol., 2011, 15, 82–95 CrossRef CAS.
- M. Fernandes Queiroz, K. R. Melo, D. A. Sabry, G. L. Sassaki and H. A. Rocha, Mar. Drugs, 2014, 13, 141–158 CrossRef PubMed.
- S. Yasmeen, M. K. Kabiraz, B. Saha, M.-D. Qadir, M. Gafur and S. Masum, Int. Res. J. Pure Appl. Chem., 2016, 10, 1–14 CrossRef CAS.
- E. Effati, B. Pourabbas and M. S. Zakerhamidi, RSC Adv., 2019, 9, 16977–16988 RSC.
- T. Nisar, Z.-C. Wang, A. Alim, M. Iqbal, X. Yang, L. Sun and Y. Guo, CyTA--J. Food, 2019, 17, 695–705 CrossRef CAS.
- B. Hastuti and S. Hadi, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 858, 012014 CrossRef CAS.
- R. Devi, K. Patel, V. Sahu, K. Kashyap, K. Tapadia and T. Maharana, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 798, 012034 CrossRef CAS.
- K. Ahmad, M. B. Kakakhel, S. Hayat, M. Wazir-Ud-Din, M. M. Mahmood, S. Ur Rehman, M. T. Siddique and S. M. Mirza, Radiat. Environ. Biophys., 2021, 60, 365–375 CrossRef CAS PubMed.
- D. N. Krishnakumar and R. N. Perumal, J. Mater. Sci.: Mater. Electron., 2020, 31, 4294–4300 CrossRef CAS.
- M. Vishnu Priya, A. Sivshanmugam, A. R. Boccaccini, O. M. Goudouri, W. Sun, N. Hwang, S. Deepthi, S. V. Nair and R. Jayakumar, Biomed. Mater., 2016, 11, 035017 CrossRef CAS PubMed.
- H. Yu, N. Ingram, J. V. Rowley, D. C. Green and P. D. Thornton, Chem.–Eur. J., 2020, 26, 13352–13358 CrossRef CAS PubMed.
- D. G. Cacione, F. do Carmo Novaes and D. H. Moreno, Cochrane Database Syst. Rev., 2018, 10(10), CD012794 Search PubMed.
- M. H. Amer, F. R. A. J. Rose, K. M. Shakesheff, M. Modo and L. J. White, npj Regener. Med., 2017, 2, 23 CrossRef PubMed.
- M. H. Amer, L. J. White and K. M. Shakesheff, J. Pharm. Pharmacol., 2015, 67, 640–650 CrossRef CAS PubMed.
- M. H. Amer, F. R. A. J. Rose, L. J. White and K. M. Shakesheff, Stem Cells Transl. Med., 2016, 5, 366–378 CrossRef CAS PubMed.
- F. Moreno Sancho, Y. Leira, M. Orlandi, J. Buti, W. V. Giannobile and F. D'Aiuto, Stem Cells Transl. Med., 2019, 8, 1286–1295 CrossRef PubMed.
- B. A. Aguado, W. Mulyasasmita, J. Su, K. J. Lampe and S. C. Heilshorn, Tissue Eng., Part A, 2011, 18, 806–815 CrossRef PubMed.
- A. G. Yavuz, A. Uygun and V. R. Bhethanabotla, Carbohydr. Polym., 2009, 75, 448–453 CrossRef CAS.
- Y. Serap and C. Nevin, in Nanoclay, ed. O. Walid, IntechOpen, Rijeka, 2022, ch. 5, DOI:10.5772/intechopen.106379.
- A. Chetouani, M. Elkolli, M. Bounekhel and D. Benachour, Polym. Bull., 2014, 71, 2303–2316 CrossRef CAS.
- P. B. Sutar, R. K. Mishra, K. Pal and A. K. Banthia, J. Mater. Sci.: Mater. Med., 2008, 19, 2247–2253 CrossRef CAS PubMed.
- U. Einhorn-Stoll, Food Hydrocolloids, 2007, 21, 1101–1112 CrossRef CAS.
- A. J. Ribeiro, C. Silva, D. Ferreira and F. Veiga, Eur. J. Pharm. Sci., 2005, 25, 31–40 CrossRef CAS PubMed.
- S. D. Pasini Cabello, N. A. Ochoa, E. A. Takara, S. Mollá and V. Compañ, Carbohydr. Polym., 2017, 157, 1759–1768 CrossRef CAS PubMed.
- A. Chetouani, N. Follain, S. Marais, C. Rihouey, M. Elkolli, M. Bounekhel, D. Benachour and D. Le Cerf, Int. J. Biol. Macromol., 2017, 97, 348–356 CrossRef CAS PubMed.
- R. M. Mathew, S. Hegde, S. Mathew, N. Shruthi and S. J. M. T. P. Geevarghese, Mater. Today: Proc., 2021, 46, 5975–5979 CAS.
- Z. Mao, Y. Li, Y. Yang, Z. Fang, X. Chen, Y. Wang, J. Kang, X. Qu, W. Yuan, K. Dai and B. Yue, Front. Pharmacol., 2018, 9, 368 CrossRef PubMed.
- T. S. Sampath Kumar, K. Madhumathi, Y. Rubaiya and M. Doble, Front. Bioeng. Biotechnol., 2015, 3, 59 CAS.
- D. Q. Pham, S. Gangadoo, Z. Lu, C. C. Berndt, E. T. Newsom, H. Zreiqat, V. K. Truong and A. S. M. Ang, Mater. Today Chem., 2022, 24, 100822 CrossRef CAS.
- I. Mutreja, D. Kumar, K. Hogan, E. Campbell, K. Mansky and C. Aparicio, Biomater. Adv., 2022, 135, 212735 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |