Open Access Article
Open Access ArticleQuinoline–sulfonamides as a multi-targeting neurotherapeutic for cognitive decline: in vitro, in silico studies and ADME evaluation of monoamine oxidases and cholinesterases inhibitors†
Saquib Jalilab,
Zahid Hussaina,
Syed Mobashir Ali Abidab,
Abdul Hameed d and
Jamshed Iqbal
d and
Jamshed Iqbal *abc
*abc
aDepartment of Pharmacy COMSATS University Islamabad, Centre for Advanced Drug Research, Abbottabad Campus, Abbottabad-22060, Pakistan. E-mail: drjamshed@ciit.net.pk; jamshediqb@googlemail.com
bDepartment of Pharmacy, COMSATS University Islamabad, Abbottabad Campus, Abbottabad 22060, Pakistan
cDepartment of Chemistry, COMSATS University Islamabad, Abbottabad Campus, Abbottabad 22060, Pakistan
dDepartment of Chemistry, University of Sahiwal, Sahiwal, 57000, Pakistan
First published on 15th March 2024
Abstract
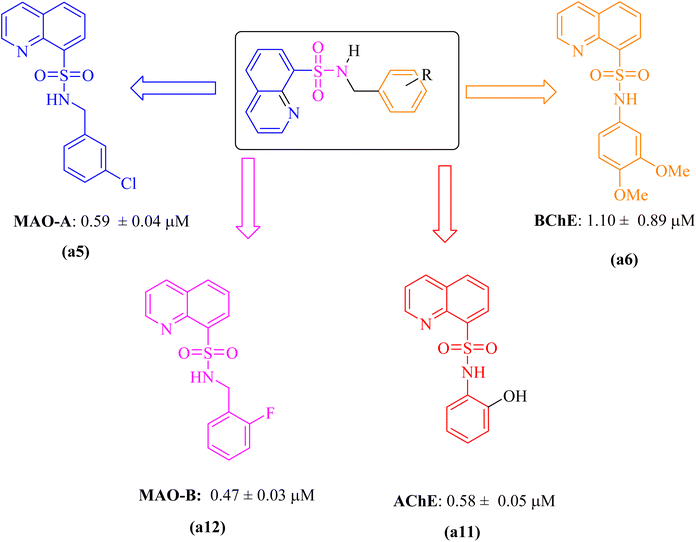
Alzheimer's disease (AD) is a multifactorial irreversible neurological disorder with multiple enzymes involved. In the treatment of AD, multifunctional agents targeting cholinesterase (ChE) and monoamine oxidase (MAO) inhibitors have shown promising results. Herein, a series of novel quinoline–sulfonamides (a1–18) were designed and synthesized as a dual inhibitor of MAOs and ChEs. The in vitro results showed that compounds a5, a12, a11, and a6 exhibited the most potent compounds against specific enzymes. They had IC50 value 0.59 ± 0.04 for MAO-A, 0.47 ± 0.03 for MAO-B, 0.58 ± 0.05 for BChE and 1.10 ± 0.77 for AChE μM respectively. Furthermore, kinetic studies revealed that these compounds are competitive. Molecular docking studies enhanced the understanding of the in silico component, unveiling critical interactions, specifically the hydrogen bonding interaction, π–π, π–alkyl, π–amid and π–sulfur interactions between the ligand and enzymes. These findings suggest that compounds a5, a6, a11, a12, a15, and a18 may be potent multifunctional candidates for AD treatment.
1 Introduction
Alzheimer's disease (AD) is a multifactorial neurodegenerative disease that causes abnormal behavior, impaired cognitive abilities such as learning, memory, perception, and problem-solving.1,2 The pathophysiology of the disease is quite complicated and two hypotheses have been proposed, such as “cholinergic” and “amyloid”. According to the Amyloid hypothesis, the hallmarks of AD include amyloid beta aggregates which lead to neural cells deaths.3 According to the second hypothesis, the cholinergic hypothesis, acetylcholine (ACh) is not produced in sufficient quantities in AD due to less production of a neurotransmitter that plays a significant role in sleep, learning, attention, and sensitivity.4 AD is caused by aberrant expression of cholinesterase's (acetylcholinesterase: AChE and butyrylcholinesterase: BChE) and monoamine oxidases (MAO-A and MAO-B).5,6 Inhibiting the enzyme can raise the level of MAO and AChE in the presynaptic cleft and improve signaling.7Monoamine oxidase (MAO) is an enzyme that regulates mood, emotions, and behavior. As a result of amines degradation, reactive oxygen species (ROS) and hydrogen peroxide (H2O2) were produced via Fenton reaction, which were responsible for neural cell death.8 Neurotransmitter deficiencies, can causes the neurodegenerative diseases, Alzheimer's, disease, Parkinson's disease and depression. Monoamine oxidase inhibitors (MAO-Is) play a crucial role in neurodegenerative diseases. By reducing the breakdown of neurotransmitters such as dopamine, serotonin, and noradrenaline in nerve endings, increases the level of monoamine oxidase. MAO-A inhibitors are primarily used to treat depression, while MAO-B inhibitors are commonly used to treat Alzheimer's, Parkinson's and other neurological diseases.9
Choline esterase is found in the central nervous system. It exists in two isoforms acetylcholinesterase (AChE EC 3.1.1.7) and butyrylcholinesterase (BChE EC 3.1.1.8) which are responsible for metabolizing AChE into acetic acid and choline, leads to neural cell death.10 Alzheimer's disease is characterized by decreased neurotransmitter levels, especially acetylcholine (ACh) in the brain. One of the most prominent biochemical changes in AD is the reduction in brain acetylcholine levels. Cholinergic inhibitors are used to inhibit and can increase AChE levels in the brain improve signaling.5 Different class of compounds have been identified as a MAO and ChE inhibitors among them quinoline gain much attention.11
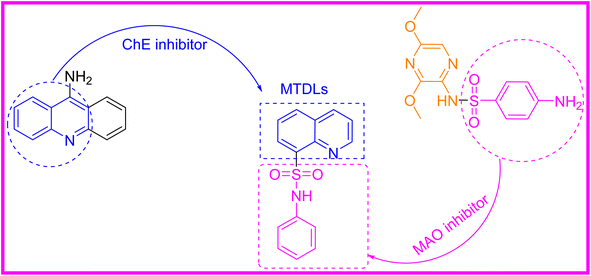
The quinoline is a bicyclic chemical compound containing a pyridine ring fused to a benzene ring. This compound is found in several natural compounds (Cinchona alkaloids) and in drugs, with a wide range of biological effects, anti-malarial,12 anti-bacterial,13 anti-fungal,14 anti-cancer,15 anti-convulsant,16 anti-inflammatory,17 neurodegenerative18,19 and analgesic activities.20 Whereas, sulfonamide moiety has emerged as a prominent focus of research in recent years. Numerous review articles highlighting the significance of this nucleus is displaying a broad spectrum of biological activities. Various studies have shown that sulfonamide, when combined with heterocycles such as coumarin (a)21 isatin22,23 thiazole (c)24 and quinoline (d, e and f),25,26 exhibit excellent anti-Alzheimer properties as shown in Fig. 1.
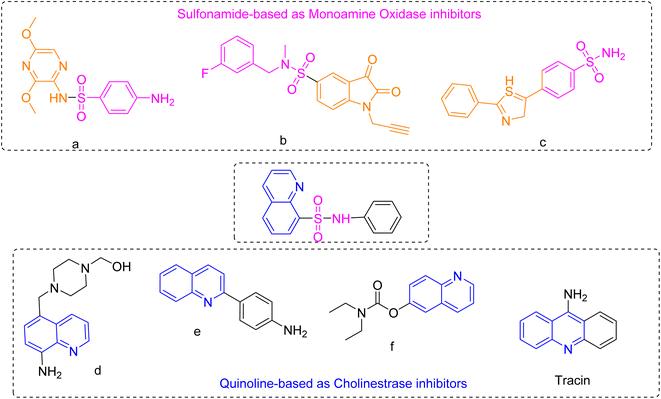 | ||
| Fig. 1 Some similar derivatives containing quinoline and sulfonamide hybrids possessing anti-Alzheimer's activities. | ||
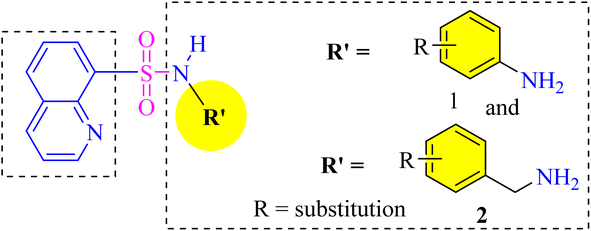
Herein we plan to introduce sulfonamide moiety into the quinoline skeleton to obtain novel quinoline–sulfonamide hybrids for MTDLs.
Presently, there is no cure for AD, but several treatments have been found that slow down the progression and ease the symptoms.27 It is a well-known fact that monoamine oxidase and cholinesterase play critical roles in cognitive function and decline. Therefore, a more comprehensive approach that targets a variety of enzymes simultaneously is needed for complex diseases. Rather than traditionally treating one disease with only one drug, the concept of one drug, multiple targets has become commonplace. In recent years, multiple target drug ligands (MTDLs) have been developed, which are molecules that bind simultaneously to multiple enzymes (Fig. 2).28–30 Multitarget approaches can also reduce side effects and improve treatment outcomes. Few types of chemicals have been identified as MAO and ChEs inhibitors.31
In the present work, we have design and synthesized quinoline–sulfonamide derivatives and studied their biological non-selective potential against targeted enzymes. Kinetic studies have been used to determine the mode of inhibition of potent compounds. The compound was also explored in silico for its interactions with different amino acids residues at the catalytic site.
2 Results and discussion
2.1. Chemistry
2.2. Biological activity
The compounds were investigated for inhibition of MAOs and ChEs. Enzyme inhibition assays of reference and test compounds on MAO (MAO-A and MAO-B) and ChE (AChE and BChE) were performed. Positive controls included clorgyline for MAO-A and deprenyl for MAO-B, as well as Donepezil for AChE and BChE. IC50 values for newly synthesized compounds and reference inhibitors are summarized in Table 1. General structure of quinoline-8-sulfonamide were present in Fig. 3.| Codes | R′ | IC50a (μM) | ||||
|---|---|---|---|---|---|---|
| MAO-A | MAO-B | AChE | BChE | |||
| a IC50 (μM).b Not active.c Standard inhibitors. | ||||||
| a1 |  |
1.17 ± 0.77 | 2.73 ± 0.16 | 1.11 ± 0.57 | N/Ab | |
| a2 |  |
2.74 ± 1.09 | 1.81 ± 0.98 | 2.58 ± 0.97 | N/A | |
| a3 |  |
0.61 ± 0.04 | 1.69 ± 0.16 | 1.01 ± 0.59 | N/A | |
| a4 |  |
0.78 ± 0.03 | 2.29 ± 0.50 | 2.18 ± 1.17 | N/A | |
| a5 |  |
0.59 ± 0.04 | 0.73 ± 0.08 | 1.95 ± 1.07 | 2.84 ± 1.07 | |
| a6 |  |
2.34 ± 0.91 | 1.07 ± 0.65 | 1.11 ± 0.47 | 1.10 ± 0.89 | |
| a7 |  |
2.45 ± 1.01 | 2.23 ± 1.08 | 1.73 ± 0.87 | N/A | |
| a8 |  |
2.75 ± 0.79 | 1.26 ± 0.98 | 1.15 ± 0.72 | N/A | |
| a9 |  |
2.25 ± 0.94 | 1.20 ± 0.45 | 3.78 ± 0.92 | N/A | |
| a10 |  |
2.44 ± 0.98 | 2.26 ± 1.12 | 1.36 ± 0.77 | N/A | |
| a11 | 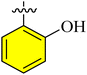 |
1.25 ± 0.45 | 1.09 ± 0.65 | 0.58 ± 0.05 | 1.72 ± 0.68 | |
| a12 | 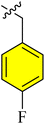 |
1.34 ± 0.67 | 0.47 ± 0.03 | 2.65 ± 0.97 | 1.16 ± 0.77 | |
| a13 | 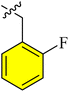 |
1.04 ± 0.67 | 1.22 ± 0.99 | 1.65 ± 0.97 | N/A | |
| a14 | 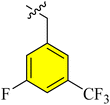 |
2.58 ± 1.23 | 0.91 ± 0.08 | 3.06 ± 0.77 | 1.176 ± 0.57 | |
| a15 |  |
1.16 ± 0.49 | 1.20 ± 0.50 | 1.40 ± 0.54 | N/A | |
| a16 |  |
1.92 ± 0.76 | 1.48 ± 0.76 | 1.92 ± 0.87 | N/A | |
| a17 |  |
2.59 ± 1.10 | 1.79 ± 0.88 | 2.34 ± 0.67 | N/A | |
| a18 |  |
1.03 ± 0.56 | 2.22 ± 0.99 | 1.01 ± 0.61 | 2.784 ± 0.92 | |
| Clorgylinec | 0.045 ± 0.03 | 61.35 ± 1.13 | — | — | ||
| Deprenylc | 67.25 ± 1.02 | 0.0196 ± 0.001 | — | — | ||
| Donepezilc | — | — | 0.032 ± 0.003 | 6.41 ± 0.34 | ||
2.3. Enzyme inhibition structure–activity relationship analysis
The structure of a compound can be systematically modulated to investigate the effect of different functional groups or modifications on its activity in vitro. A remarkable inhibitory effect was observed on the newly synthesized hybrids. The benzyl group substituents are responsible for the differential inhibition activities observed in these compounds.26 All synthetic analogues showed variable results in IC50 = 0.59 ± 0.04 to 2.85 ± 1.09 μM for MAO-A and 0.47 ± 0.03 to 2.73 ± 0.16 μM for MAO-B. The introduction of the electro-withdrawn group (EWG) and electron donating group (EDG) to the phenyl ring resulted in a substantial inhibitory effect.Chloro substitution at meta (m-), ortho (o-) and para (p-) position affects the compound's inhibition potential against selected targets. Meta substitution is the most active substitution. As a result of di-chloro substitution, o- and p- substituted compounds have a lower inhibition potential (a15). Furthermore, when di-substitution at o- and m- with chlorine and p-hydroxyl, the potency also decreased 4-fold on the same enzyme. When chlorine is replaced by fluorine, the di-fluoro substitution at the o-position makes it more potent toward the enzyme, but its activity is less than m-chloro. When there is di-substitution at o- and p- (a12 and a13), its activity was nearly the same and decreased 2-fold compared to (a3), also the activity of (a14) and (a2) dropped 4-fold. Replacing chlorine with m-iodine (a1) results in low potency, whereas changes in position from meta to para (a8) result in a 2-fold decrease in potency with respect to the m-position. The potency of the compounds is also affected when the halogen is replaced by other functional groups, such as methoxy. The m-substitution of methoxy (a18) was 2-fold more potent than di-substitution (a6), indicating that di-substitution decreases the potency of the targeted enzyme. The introduction of m-cyano (a9), benzimidazole ring (a7), and hexane ring (a10) resulted in almost the same potency. Results are summarized in Table 1.
The compound featuring o-fluoro substitution (a12) demonstrated the highest potency against MAO-B, while the di-substituted compound (a14) exhibited nearly equivalent efficacy. Notably, altering the position from ortho to para (a13) led to a 3-fold reduction in potency. Di-substitutions of phenyl rings (a2 and a3) resulted in a substantial 4-fold decrease in potency. Intriguingly, replacing fluorine with chlorine revealed interesting behavior, with m-chloro substitution yielding almost the same potency as (a12). Similarly, transitioning from m-chloro (a5) to o-chloro (a16) resulted in a 2-fold potency decrease, while the shift to p-chloro (a15) led to a 3-fold decrease. Moreover, di-substitutions (a15 and a17) resulted in a nearly 2-fold decrement in potency.
In comparison, substituting p-iodine (a8) resulted in a compound that is 3-times less potent than (a12), while a shift from p-iodine to m-iodine (a1) caused a two-folds reduction in potency. Additionally, p-methoxy substitution (a18) exhibited nearly 2.5-fold lower potency compared to di-substitution (a6). Minimal distinctions were observed among the outcomes of compounds (a7), (a9), (a11), and (a10), all demonstrating a potency 2-fold less than the most potent compound (a12). These findings are summarized in Table 1.
Similarly, introducing o-hydroxy (a11) enhanced potency against AChE. However, substituting o-hydroxy with o-chloro (a16), m-chloro (a5), and p-chloro (a4) resulted in an approximate 4-fold decrease in activity compared to (a11). Furthermore, compound (a15) exhibited almost 2.5-fold less potency than (a11). The introduction of hydroxyl groups in di-chloro (a17) led to a substantial 5-fold decrease in substitution activity, underscoring that mono-substitution (a11) is the most favorable configuration. In summary, these observations emphasize the crucial role of specific substitutions in determining the efficacy of compounds against AChE, with mono-substitution at o-hydroxy position proving to be the most favorable. Replacing chloro with fluoro had a notable impact on compound potency. Compound (a13) showed a 3-fold decrease compared to the most potent, while positional changes further diminished activity. Compounds (a3 and a2) were nearly 3-fold less active than (a11). Substituting fluoro with iodio (a1 and a8) yielded compounds with similar, albeit almost 2-fold less, potency than the most potent compound. Halogen substitution with methoxy (a18 and a6) resulted in compounds 2-fold less potent than (a11). Compound (a9) exhibited a significant 7-fold potency decrease. BChE inhibition revealed di-methoxy substitution (a5) as more potent, though activity decreased 2-fold with mono-substitution (a18). Compound (a14) matched (a5) in potency, but (a5) showed almost 2-fold less activity. These findings, detailed in Table 1.
2.4. Potent inhibitor against targeted enzymes
Potency of the compounds depends upon the structural confirmation and position of the substituent attached with them in the given series the most potent inhibitor was illustrated in the Fig. 4. Compound (a5) showed more potency towards MAO-A having chloro group at m-position and compound (a12) showed more potency towards MOA-B due to presence of fluoro group at o-position same pattern was also observed in case of AChE and BChE but the substituent were different the potent inhibitor (a6) against BChE contain o-, m-methoxy (–OCH3) groups and compound (a11) against AChE contain hydroxyl (OH) group at o-position the results shows that both ortho and meta position play important role in the inhibition of the targeted enzymes.2.5. Enzyme kinetic studies
Kinetic studies of the most potent inhibitors were conducted to find the modes of inhibition against MAO-A, MAO-B, AChE, and BChE. The most potent inhibitors were tested at different concentrations. Several concentrations of compound vs. substrate were used to determine the mode of inhibition. Different concentrations of compounds a5 (0.500, 0.600, 0.700, 0.800 μM), a12 (0.500, 0.600, 0.700, 0.800 μM), a11 (0.10, 0.20, 0.30, 0.40 μM) and a6 (0.60, 0.80, 1, 1.20, 1.40 μM) and substrates (p-tyramine) (0.75, 1.5, 3, 4.5 and 6 mM), ATCII (1.25, 2.5, 5, 7.5, and 10 mM), and BTCII (1.25, 2.5, 5, 7.5, and 10 mM) were used to perform the assay as previously reported method.26 Line Weaver–Burk graphs were used to analyze the inhibitory effect and determine the type of inhibition. All the tested compounds (a5, a12, a11, a6) showed competitive inhibition toward MAO-A, MAO-B, AChE and BChE respectively as displayed in Fig. 5.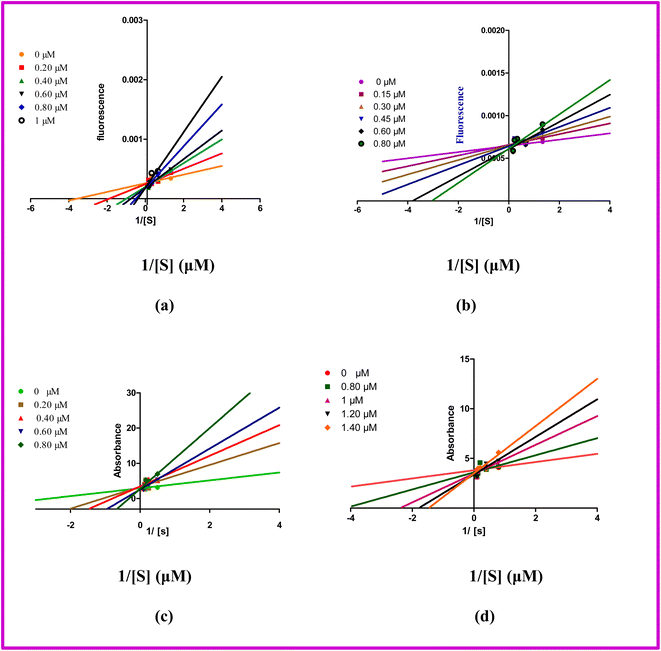 | ||
| Fig. 5 Double reciprocal plot of MAO-A (a), MAO-B (b), AChE (c), and BChE (d) by compound a5, a12, a11, and a6 respectively. | ||
2.6. Molecular docking studies
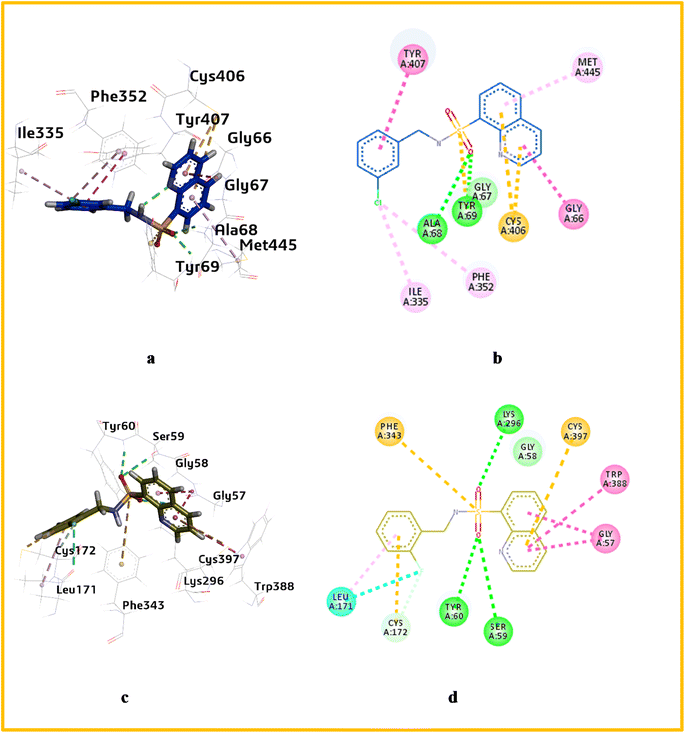
Similarly, prior to docking the potent compounds, a cognate ligand (safinamide) was docked within MAO-B's active pocket, and an RMSD 1.5 Å was obtained. Several interactions were observed between catalytic site amino acids and the most potent compound a12. Hydrogen bonding interaction was also observed between the sulfonamide oxygen and the amino acids TYR60, SER59 and ILY296 with a bond distance of 2.98 Å, 2.90 Å and 3.10 Å respectively, with binding energy −10.57 kcal mol−1. Additionally, carbon–hydrogen bonds were found between fluorine and CYS172. This bond is also strong due to the electronegativity of the fluorine atom, which is higher than that of the carbon atom. Finally, π–alkyl and π–π interactions were present between the phenyl ring and quinoline ring with the amino acids LEU 171, and TRP 388. This interaction is possible due to the aromaticity of the rings, allowing them to form strong π-bonds with the amino acids as shown in Fig. 6.
The quinoline derivatives used in this study form strong interactions with enzyme active sites, resulting in adducts with increased stability and activity. The majority of derivative molecules form H-bonds with MAO-A's TYR444 and TYR69. In the catalytic mechanism of MAO-A, TYR444 and TYR69 are involved. The quinoline compounds a3, a5, a6 and, and a8 form H-bonds with amino acids. The binding energy of compound a6 was the highest, −10.40 kcal mol−1.
All quinoline derivatives have an active interaction with enzyme active sites. The interactions were predominantly hydrophobic and van der Waals in nature. Hydrogen bonds have been formed in most derivative molecules with TYR434 and TYR60, involved in the catalytic mechanism of the MAO-B. It has been observed that compounds a2, a18, a3, a4, a17, a5, a12, a6,a9, anda10 form H-bond with LEU171, GLY434, 435, and TYR60, amino acid residues play a key role in catalytic site. The binding energy of compound a4 was the highest, −10.57 kcal mol−1.
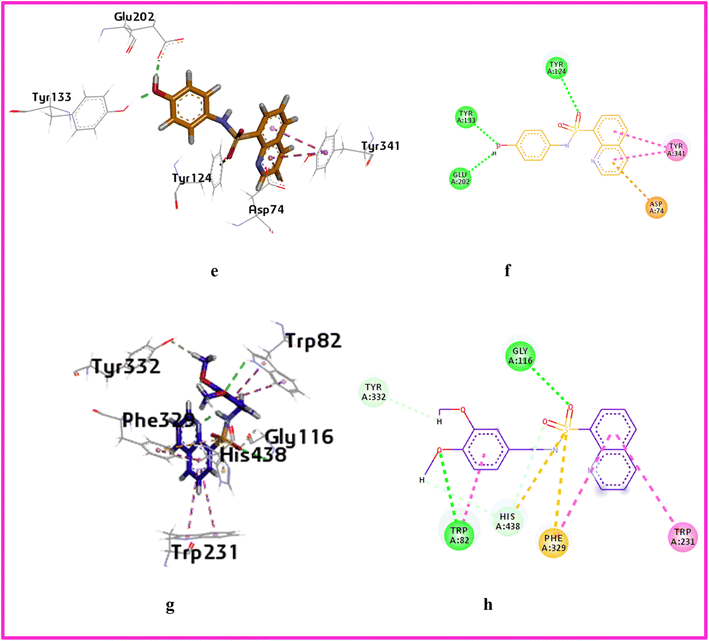 | ||
| Fig. 7 3D and 2D interaction of compound a11 left side AChE (e and f) and a6 right side BChE (g and h). | ||
On the other hand, compound a11 showed hydrogen bonding interactions between the oxygen atom and the sulfonamide with the amino acids TRP82 and GLY116 with distances 2.86 Å, 2.99 Å respectively, with binding energy −7.68 kcal mol−1. Furthermore, a carbon hydrogen bond was observed between the hydrogen of methoxy and the oxygen of the sulfonamide with the amino acids TYR332 and HIS 438. Lastly, a π–sulfur interaction was seen between the sulfur of the sulfonamide and the amino acid PHE329 as shown in Fig. 7.
Several compounds formed hydrogen bonds with amino acid residues involved in the catalytic mechanism of the AChE enzyme. Among them a1, a5, a10, a12, a15, and a16 formed hydrogen bonds with PHE 338 residue. The binding score of compound a11 is highest, −8.68 kcal mol−1.
2.7. Pharmacokinetic studies
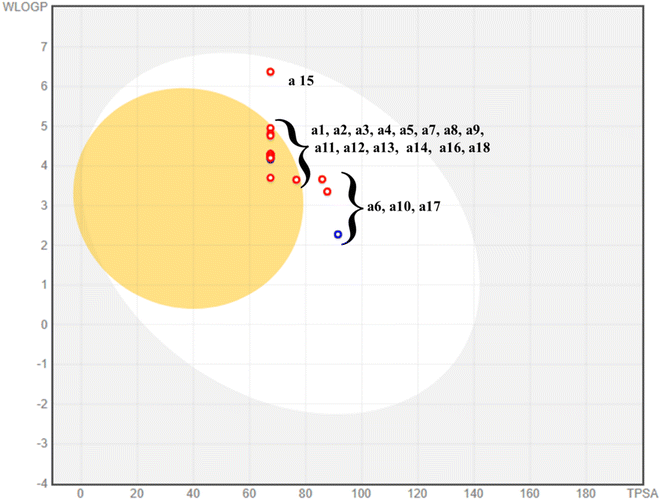
Many predicted enzyme inhibitors are unable to proceed to the clinical trial stage due to their undesirable pharmacokinetic characteristics. Therefore, in silico approaches provide a reliable substitute for trials in earlier phases of studying a compound's fundamental pharmacokinetic features, and it's a sensible strategy to cut both the labor and associated costs. Using a web-based SwissADME, the absorption, distribution, metabolism, and excretion (ADME) profile of the produced compounds (a1–18) was developed. The results are described in Table 2. The boiled egg plot included in SwissADME is a quick, simple, and incredibly reliable way to predict a compound's passive gastrointestinal absorption.33 The egg-shaped plot offers a visual summary of gastrointestinal absorption; it is represented by the white portion of the egg and the BBB penetration of the compound; it is shown as the inner yolk-like component of the egg and presents the boiled-egg plot of the recently synthesized derivatives as shown in Fig. 8.34 The majority of the synthetic analogues exhibits brain penetration, as is shown from the plot. All compounds have the ability to penetrate the gastrointestinal tract and the BBB. The SWISSADME website generated the plot.33 Every scaffold complied with Lipinski's law of drug resemblance.35 Furthermore, the PAINS (Panassay Interference Compounds) filter was used to evaluate the promiscuity of the prepared hits. According to the data, each substance is distinct and does not share any chemical properties with the PAINS.36 The topological polar surface area of all the compounds ranged from 87 Å to 67 Å, and they all showed good gastrointestinal absorption. All of these factors indicate that synthetic hits could be promising treatments for the treatment of severe neurological diseases including Parkinson's and Alzheimer's.| Molecule | TPSAa | Lipinski violations | PAINSb | WLOGPc | HBAd | HBDe | GI absorption | BBBf permeant | NRBg |
|---|---|---|---|---|---|---|---|---|---|
| a Topological polar surface area.b Pan-assay interference.c Logarithm of partition coefficient between n-octanol and water.d Hydrogen bond acceptor.e Hydrogen bond donor.f Blood–brain barrier.g Number of rotatable bonds. | |||||||||
| a1 | 67.44 | 0 | 0 | 4.25 | 4 | 1 | High | Yes | 4 |
| a2 | 67.44 | 0 | 0 | 4.81 | 5 | 1 | High | Yes | 4 |
| a3 | 67.44 | 0 | 0 | 5.04 | 5 | 1 | High | No | 3 |
| a4 | 67.44 | 0 | 0 | 4.3 | 4 | 1 | High | Yes | 4 |
| a5 | 67.44 | 0 | 0 | 4.3 | 4 | 1 | High | Yes | 4 |
| a6 | 85.9 | 0 | 0 | 3.66 | 6 | 1 | High | No | 6 |
| a7 | 91.5 | 0 | 0 | 2.27 | 4 | 3 | High | No | 3 |
| a8 | 67.44 | 0 | 0 | 3.7 | 4 | 1 | High | Yes | 4 |
| a9 | 67.44 | 0 | 0 | 4.25 | 4 | 1 | High | Yes | 4 |
| a10 | 67.44 | 0 | 0 | 4.17 | 4 | 1 | High | Yes | 4 |
| a11 | 87.67 | 0 | 0 | 3.35 | 5 | 2 | High | No | 4 |
| a12 | 67.44 | 0 | 0 | 4.2 | 5 | 1 | High | Yes | 4 |
| a13 | 67.44 | 0 | 0 | 4.2 | 5 | 1 | High | Yes | 4 |
| a14 | 67.44 | 0 | 0 | 6.37 | 8 | 1 | High | No | 5 |
| a15 | 67.44 | 0 | 0 | 4.95 | 4 | 1 | High | Yes | 4 |
| a16 | 67.44 | 0 | 0 | 4.3 | 4 | 1 | High | Yes | 4 |
| a17 | 76.67 | 0 | 0 | 3.65 | 5 | 1 | High | Yes | 5 |
| a18 | 76.67 | 0 | 0 | 3.65 | 5 | 1 | High | Yes | 5 |
3 Material and methods
All the chemicals were purchased from Merck and Sigma-Aldrich (Steinheim, Germany). The thin layer chromatography (TLC) was used with pre-coated silica gel plates Keisel 60. The Bruker Advance NMR instrument (400 MHz) was used to measure 1H and 13C NMR in DMSO-d6. Chemical shift in with respect to tetramethylsilane as a reference compound was used and data reported in parts-per-million. Moreover, FTIR spectra was obtained on an Agilent Technology 630. Open capillary method (Gallenkamp melting point apparatus) was used to measure the melting point.3.1. Experimental data
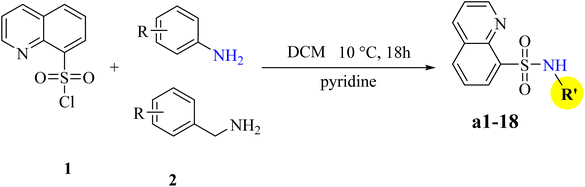
The synthesis of quinoline-8-sulfonamide derivative (a1–18) were discussed in Scheme 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 250–252 °C, FTIR (ν cm−1), 3202 (stretching, NH), 1604 (stretching C
3), M.P: 250–252 °C, FTIR (ν cm−1), 3202 (stretching, NH), 1604 (stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1348 (S:O, stretching), 681 (C–I, stretching) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.11 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 9.02 (NH, 1H, brs), 8.57 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.37–8.30 (Ar-H, 2H, m), 7.79–7.69 (Ar-H, 3H, m), 7.18 (Ar-H, app td, J = 8.0, 4.0 Hz, 1H), 7.11 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 6.81 (Ar-H, td, J = 8.0, 1.8 Hz, 1H). 13C NMR (100 MHz, DMSO) δC 152.2 (C), 142.8, 139.9, 138.9, 137.7, 135.8, 134.9, 131.7, 129.5, 129.0, 127.5, 126.2, 123.3, 122.7, 94.8 (Ar-C). Anal. calculated for C15H11IN2O2S, %: C, 43.92; H, 2.70; N, 6.83; found, %: C, 43.81; H, 2.66; N, 6.85.
C), 1348 (S:O, stretching), 681 (C–I, stretching) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.11 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 9.02 (NH, 1H, brs), 8.57 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.37–8.30 (Ar-H, 2H, m), 7.79–7.69 (Ar-H, 3H, m), 7.18 (Ar-H, app td, J = 8.0, 4.0 Hz, 1H), 7.11 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 6.81 (Ar-H, td, J = 8.0, 1.8 Hz, 1H). 13C NMR (100 MHz, DMSO) δC 152.2 (C), 142.8, 139.9, 138.9, 137.7, 135.8, 134.9, 131.7, 129.5, 129.0, 127.5, 126.2, 123.3, 122.7, 94.8 (Ar-C). Anal. calculated for C15H11IN2O2S, %: C, 43.92; H, 2.70; N, 6.83; found, %: C, 43.81; H, 2.66; N, 6.85.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 260–262 °C, FTIR (ν cm−1), 3288 (stretching, NH), 1636 (C
3), M.P: 260–262 °C, FTIR (ν cm−1), 3288 (stretching, NH), 1636 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, stretching), 1348 (stretching S:O), 642 (stretching, C–I) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.42 (1H, NH, brs), 9.12 (Ar-H, dd, J = 4.0, 1.6 Hz, 1H), 8.57 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.30 (Ar-H, ddd, J = 12.0, 8.0, 1.4 Hz, 2H), 7.79–7.65 (Ar-H, m, 2H), 7.04 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 6.81 (Ar-H, m, 2H), 1.94 (CH2, 2H, s). 13C NMR (101 MHz, DMSO-d6) δC 161.6 (C), 151.98, 143.1, 137.7, 137.1, 137.0, 136.2, 134.8, 132.2, 132.2, 131.9, 129.0, 128.4, 128.3, 126.2, 123.3, 112.5, 112.4, 110.9, 110.7 (Ar C), 17.28 (CH2). Anal. calculated for C17H15FN2O2S, %: C, 61.80; H, 4.58; N, 8.48; found, %: C, 61.84; H, 4.60; N, 8.52.
C, stretching), 1348 (stretching S:O), 642 (stretching, C–I) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.42 (1H, NH, brs), 9.12 (Ar-H, dd, J = 4.0, 1.6 Hz, 1H), 8.57 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.30 (Ar-H, ddd, J = 12.0, 8.0, 1.4 Hz, 2H), 7.79–7.65 (Ar-H, m, 2H), 7.04 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 6.81 (Ar-H, m, 2H), 1.94 (CH2, 2H, s). 13C NMR (101 MHz, DMSO-d6) δC 161.6 (C), 151.98, 143.1, 137.7, 137.1, 137.0, 136.2, 134.8, 132.2, 132.2, 131.9, 129.0, 128.4, 128.3, 126.2, 123.3, 112.5, 112.4, 110.9, 110.7 (Ar C), 17.28 (CH2). Anal. calculated for C17H15FN2O2S, %: C, 61.80; H, 4.58; N, 8.48; found, %: C, 61.84; H, 4.60; N, 8.52.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 254–257 °C, FTIR (ν cm−1), 3211 (stretching, NH), 1682 (stretching, C
3), M.P: 254–257 °C, FTIR (ν cm−1), 3211 (stretching, NH), 1682 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1356 (stretching, S:O), 712 (stretching, CF), cm−1. 1H NMR (400 MHz, DMSO-d6) 9.67 (NH, 1H, brs), 9.09 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.65 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.30 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.20 (Ar-H, dd, J = 8.0, 1.6 Hz, 1H), 7.74 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 7.68 (Ar-H, app t, J = 8.0, 1H), 7.20–7.05 (Ar-H, 2H, m), 6.98–6.88 (Ar-H, 1H, m). 13C NMR (100 MHz, DMSO) δC 161.5/161.4/158.9, 158.0/157.9/155.5/115.4, 151.8, 143.2, 137.5, 136.3, 134.7, 131.5, 129.10/129.01/128.98, 128.94, 128.9, 126.0, 123.1, 121.80/121.76/121.67/121.63, 112.02/111.98/111.80/111.76 (ArC), 105.07/104.83/104.90/104.59. Anal. calculated for C15H10F2N2O2S, %: C, 56.25; H, 3.15; N, 8.75; found, %: C, 56.29; H, 3.18; N, 8.67.
C), 1356 (stretching, S:O), 712 (stretching, CF), cm−1. 1H NMR (400 MHz, DMSO-d6) 9.67 (NH, 1H, brs), 9.09 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.65 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.30 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.20 (Ar-H, dd, J = 8.0, 1.6 Hz, 1H), 7.74 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 7.68 (Ar-H, app t, J = 8.0, 1H), 7.20–7.05 (Ar-H, 2H, m), 6.98–6.88 (Ar-H, 1H, m). 13C NMR (100 MHz, DMSO) δC 161.5/161.4/158.9, 158.0/157.9/155.5/115.4, 151.8, 143.2, 137.5, 136.3, 134.7, 131.5, 129.10/129.01/128.98, 128.94, 128.9, 126.0, 123.1, 121.80/121.76/121.67/121.63, 112.02/111.98/111.80/111.76 (ArC), 105.07/104.83/104.90/104.59. Anal. calculated for C15H10F2N2O2S, %: C, 56.25; H, 3.15; N, 8.75; found, %: C, 56.29; H, 3.18; N, 8.67.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 258–260 °C, FTIR (ν cm−1), 3209 (stretching, NH), 1674 (stretching, C
3), M.P: 258–260 °C, FTIR (ν cm−1), 3209 (stretching, NH), 1674 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1325 (stretching, S:O), 781 (stretching C–Cl) 1H NMR (400 MHz, DMSO-d6) δ 9.03 (Ar-H, dd, J = 4.2, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.3, 1.8 Hz, 1H), 8.21 (Ar-H, m, 2H), 7.81 (NH, t, J = 6.5 Hz, 1H), 7.67 (Ar-H, m, 2H), 7.05 (Ar-H,d, J = 8.6 Hz, 2H), 7.00 (Ar-H, d, J = 8.6, 2H), 4.08 (CH2, d, J = 6.5 Hz, 2H). 13C NMR (100 MHz, DMSO) δ 151.6, 143.0, 137.4, 137.3, 136.9, 133.8, 131.8, 131.0, 129.8, 128.8, 127.8, 126.0, 122.9 (Ar-C), 46.4 (CH2). Calculated for C16H13ClN2O2S, %: C, 57.74; H, 3.94; N, 8.42; found, %: C, 57.85; H, 3.61; N, 8.70.
C), 1325 (stretching, S:O), 781 (stretching C–Cl) 1H NMR (400 MHz, DMSO-d6) δ 9.03 (Ar-H, dd, J = 4.2, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.3, 1.8 Hz, 1H), 8.21 (Ar-H, m, 2H), 7.81 (NH, t, J = 6.5 Hz, 1H), 7.67 (Ar-H, m, 2H), 7.05 (Ar-H,d, J = 8.6 Hz, 2H), 7.00 (Ar-H, d, J = 8.6, 2H), 4.08 (CH2, d, J = 6.5 Hz, 2H). 13C NMR (100 MHz, DMSO) δ 151.6, 143.0, 137.4, 137.3, 136.9, 133.8, 131.8, 131.0, 129.8, 128.8, 127.8, 126.0, 122.9 (Ar-C), 46.4 (CH2). Calculated for C16H13ClN2O2S, %: C, 57.74; H, 3.94; N, 8.42; found, %: C, 57.85; H, 3.61; N, 8.70.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 256–258 °C, FTIR (ν cm−1), 3209 (stretching, NH), 1674 (stretching, C
3), M.P: 256–258 °C, FTIR (ν cm−1), 3209 (stretching, NH), 1674 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1325 (stretching, S:O), 781 (stretching C–Cl) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.05 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.24–8.14 (Ar-H, 2H, m), 7.89 (HN, app t, J = 6.6, 1.4 Hz, 1H), 7.70–7.63 (Ar-H, 2H, m), 7.07 (Ar-H, app q, J = 4.0 Hz, 1H), 7.03–6.98 (Ar-H, 3H, m), 4.13 (CH2, d, J = 4.0 Hz, 2H). 13C NMR (100 MHz, DMSO) δC 151.6, 142.9, 140.4, 137.4, 137.3, 133.9, 132.9, 130.8, 129.8, 128.8, 127.8, 127.1, 126.6, 125.9, 122.8 (Ar-C), 46.42 (CH2). Anal. calculated for C16H13ClN2O2S, %: C, 57.74; H, 3.94; N, 8.42; found, %: C, 57.85; H, 3.61; N, 8.70.
C), 1325 (stretching, S:O), 781 (stretching C–Cl) cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.05 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.24–8.14 (Ar-H, 2H, m), 7.89 (HN, app t, J = 6.6, 1.4 Hz, 1H), 7.70–7.63 (Ar-H, 2H, m), 7.07 (Ar-H, app q, J = 4.0 Hz, 1H), 7.03–6.98 (Ar-H, 3H, m), 4.13 (CH2, d, J = 4.0 Hz, 2H). 13C NMR (100 MHz, DMSO) δC 151.6, 142.9, 140.4, 137.4, 137.3, 133.9, 132.9, 130.8, 129.8, 128.8, 127.8, 127.1, 126.6, 125.9, 122.8 (Ar-C), 46.42 (CH2). Anal. calculated for C16H13ClN2O2S, %: C, 57.74; H, 3.94; N, 8.42; found, %: C, 57.85; H, 3.61; N, 8.70.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 260–263 °C, FTIR (ν cm−1), 3402 (stretching, NH), 2932 (stretching, CH), 1693 (stretching, C
3), M.P: 260–263 °C, FTIR (ν cm−1), 3402 (stretching, NH), 2932 (stretching, CH), 1693 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1373 (stretching, S:O), 1176 (stretching, C–O), 738 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 9.00 (Ar-H, dd, J = 4.0, 1.7 Hz, 1H), 8.44 (Ar-H, dd, J = 8.0, 1.7 Hz, 1H), 8.21–8.10 (Ar-H, 2H, m), 7.71–7.59 (Ar-H, 2H, m), 7.05 (NH, app t, J = 8.0 Hz, 1H), 6.75 (Ar-H, d, J = 8.0, 1.5 Hz, 1H), 6.04–5.91 (Ar-H, 2H, m), 3.97 (2H, d, J = 8.0 Hz), 3.57 (CH3, 3H, s), 3.41 (CH3, 3H, s). 13C NMR (100 MHz, DMSO) δC 160.2, 157.7, 151.4, 142.8, 137.3, 136.8, 133.7, 130.5, 130.3, 128.7, 125.9, 122.7, 116.4, 103.9, 97.5 (Ar-C), 55.5 (OCH3), 55.3 (OCH3), 43.13 (CH2). Anal. calculated for C18H18N2O4S, %: C, 60.32; H, 5.06; N, 7.82; found, %: C, 60.58; H, 5.01; N, 7.62.
C), 1373 (stretching, S:O), 1176 (stretching, C–O), 738 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 9.00 (Ar-H, dd, J = 4.0, 1.7 Hz, 1H), 8.44 (Ar-H, dd, J = 8.0, 1.7 Hz, 1H), 8.21–8.10 (Ar-H, 2H, m), 7.71–7.59 (Ar-H, 2H, m), 7.05 (NH, app t, J = 8.0 Hz, 1H), 6.75 (Ar-H, d, J = 8.0, 1.5 Hz, 1H), 6.04–5.91 (Ar-H, 2H, m), 3.97 (2H, d, J = 8.0 Hz), 3.57 (CH3, 3H, s), 3.41 (CH3, 3H, s). 13C NMR (100 MHz, DMSO) δC 160.2, 157.7, 151.4, 142.8, 137.3, 136.8, 133.7, 130.5, 130.3, 128.7, 125.9, 122.7, 116.4, 103.9, 97.5 (Ar-C), 55.5 (OCH3), 55.3 (OCH3), 43.13 (CH2). Anal. calculated for C18H18N2O4S, %: C, 60.32; H, 5.06; N, 7.82; found, %: C, 60.58; H, 5.01; N, 7.62.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), M.P: 257–259 °C, 1H NMR (400 MHz, DMSO-d6) δ 1H NMR (400 MHz, DMSO-d6) δH 9.21 (NH, d, J = 4.0 Hz, 1H), 9.02 (NH, d, J = 8.0 Hz, 1H), 8.94–8.88 (Ar-H, m, 1H), 8.80–8.73 (Ar-H, m, 1H), 8.52 (Ar-H, dd, J = 8.0, 1.5 Hz, 1H), 8.45–8.36 (Ar-H, m, 2H), 8.29 (Ar-H, d, J = 8.0 Hz, 1H), 7.97 (Ar-H, dd, J = 8.0, 5.2 Hz, 1H), 7.91–7.83 (Ar-H, m, 2H), 7.68 (dd, J = 8.4, 4.2 Hz, 1H), 7.34 (d, J = 7.4 Hz, 2H), 7.09 (d, J = 7.6 Hz, 1H), 6.86 (t, J = 7.4 Hz, 1H, CH). 13C NMR (101 MHz, DMSO) δC 154.1, 152.3, 143.1, 137.8, 136.8, 133.9, 133.4, 131.2, 128.9, 126.3, 124.5, 123.7, 122.5, 120.6, 116.0, 112.1.
2), M.P: 257–259 °C, 1H NMR (400 MHz, DMSO-d6) δ 1H NMR (400 MHz, DMSO-d6) δH 9.21 (NH, d, J = 4.0 Hz, 1H), 9.02 (NH, d, J = 8.0 Hz, 1H), 8.94–8.88 (Ar-H, m, 1H), 8.80–8.73 (Ar-H, m, 1H), 8.52 (Ar-H, dd, J = 8.0, 1.5 Hz, 1H), 8.45–8.36 (Ar-H, m, 2H), 8.29 (Ar-H, d, J = 8.0 Hz, 1H), 7.97 (Ar-H, dd, J = 8.0, 5.2 Hz, 1H), 7.91–7.83 (Ar-H, m, 2H), 7.68 (dd, J = 8.4, 4.2 Hz, 1H), 7.34 (d, J = 7.4 Hz, 2H), 7.09 (d, J = 7.6 Hz, 1H), 6.86 (t, J = 7.4 Hz, 1H, CH). 13C NMR (101 MHz, DMSO) δC 154.1, 152.3, 143.1, 137.8, 136.8, 133.9, 133.4, 131.2, 128.9, 126.3, 124.5, 123.7, 122.5, 120.6, 116.0, 112.1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 260–262 °C, FTIR (ν cm−1), 2964 (stretching, NH), 1634 (stretching C
3), M.P: 260–262 °C, FTIR (ν cm−1), 2964 (stretching, NH), 1634 (stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1342 (stretching, S:O), 681 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 10.29 (NH, 1H, brs), 9.11 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.51 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 8.36 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.27 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 7.73–7.69 (Ar-H, 2H, m), 7.46–7.38 (Ar-H, 2H, m), 7.41 (Ar-H, d, J = 8.0 Hz, 1H). 13C NMR (101 MHz, DMSO) δC 151.9 (C), 143.1, 138.2, 137.9, 137.5, 135.3, 134.9, 132.8, 128.9, 126.1, 123.2, 122.0, 88.1 (Ar-C). Anal. calculated for C15H11IN2O2S, %: C, 43.92; H, 2.70; N, 6.83; found, %: C, 43.72; H, 2.51; N, 6.55.
C), 1342 (stretching, S:O), 681 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 10.29 (NH, 1H, brs), 9.11 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.51 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 8.36 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.27 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 7.73–7.69 (Ar-H, 2H, m), 7.46–7.38 (Ar-H, 2H, m), 7.41 (Ar-H, d, J = 8.0 Hz, 1H). 13C NMR (101 MHz, DMSO) δC 151.9 (C), 143.1, 138.2, 137.9, 137.5, 135.3, 134.9, 132.8, 128.9, 126.1, 123.2, 122.0, 88.1 (Ar-C). Anal. calculated for C15H11IN2O2S, %: C, 43.92; H, 2.70; N, 6.83; found, %: C, 43.72; H, 2.51; N, 6.55.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 257–259 °C, FTIR (ν cm1), 3296 (stretching, NH), 2266 (stretching, CN), 1649 (stretching, C
3), M.P: 257–259 °C, FTIR (ν cm1), 3296 (stretching, NH), 2266 (stretching, CN), 1649 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1344 (stretching, S:O), 681 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δC, 10.98 (NH, 1H, brs), 9.09 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.50 (Ar-H, td, J = 8.0, 2.4 Hz, 2H), 8.30 (Ar-H, d, J = 8.0 Hz, 1H), 7.76 (Ar-H, t, J = 8.0 Hz, 1H), 7.69 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 7.57 (Ar-H, d, J = 8.0 Hz, 2H), 7.23 (Ar-H, d, J = 8.0 Hz, 2H). 13C NMR (101 MHZ, DMSO) δH 152.1 (C), 143.1, 142.9, 137.5, 135.3, 135.2, 133.8, 133.1, 128.9, 126.2, 123.2, 119.2, 118.6, 105.3 (Ar–C). Anal. calculated for C16H11N3O2S, %: C, 62.12; H, 3.58; N, 13.58; found, %: C, 62.36; H, 3.31; N, 13.38.
C), 1344 (stretching, S:O), 681 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δC, 10.98 (NH, 1H, brs), 9.09 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.50 (Ar-H, td, J = 8.0, 2.4 Hz, 2H), 8.30 (Ar-H, d, J = 8.0 Hz, 1H), 7.76 (Ar-H, t, J = 8.0 Hz, 1H), 7.69 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 7.57 (Ar-H, d, J = 8.0 Hz, 2H), 7.23 (Ar-H, d, J = 8.0 Hz, 2H). 13C NMR (101 MHZ, DMSO) δH 152.1 (C), 143.1, 142.9, 137.5, 135.3, 135.2, 133.8, 133.1, 128.9, 126.2, 123.2, 119.2, 118.6, 105.3 (Ar–C). Anal. calculated for C16H11N3O2S, %: C, 62.12; H, 3.58; N, 13.58; found, %: C, 62.36; H, 3.31; N, 13.38.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 264–266 °C, FTIR (ν cm−1), 3373 (NH, stretching), 1625 (stretching, C
3), M.P: 264–266 °C, FTIR (ν cm−1), 3373 (NH, stretching), 1625 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1348 (stretching, S:O), cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.07 (Ar-H, dd, J = 4.0, 1.4 Hz, 1H), 8.55 (Ar-H, dd, J = 8.4, 4.0 Hz, 1H), 8.30 (Ar-H, d, J = 8.0 Hz, 1H), 7.79–7.68 (Ar-H, 2H, m), 7.14 (NH, 1H, app t, J = 8.0 Hz), 2.58 (CH2, t, J = 8.0 Hz, 2H), 1.55–1.49 (cyclohexyl-H, 5H, m), 1.27 (cyclohexyl-H, 1H, m), 1.02–0.93 (cyclohexyl-H, 3H, m), 0.74–0.64 (cyclohexyl-H, 2H, m). 13C NMR (101 MHz, DMSO) δC 151.8 (C), 143.1, 137.6, 136.8, 134.0, 131.1, 128.9, 126.2, 123.0, 49.5, 40.6, 40.4, 40.2, 39.9, 39.8, 39.6, 39.3, 37.6, 30.6, 26.3, 25.7. Anal. calculated for C16H20N2O2S, %: C, 63.13; H, 6.62; N, 9.20; found, %: C, 63.43; H, 6.40; N, 9.30.
C), 1348 (stretching, S:O), cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.07 (Ar-H, dd, J = 4.0, 1.4 Hz, 1H), 8.55 (Ar-H, dd, J = 8.4, 4.0 Hz, 1H), 8.30 (Ar-H, d, J = 8.0 Hz, 1H), 7.79–7.68 (Ar-H, 2H, m), 7.14 (NH, 1H, app t, J = 8.0 Hz), 2.58 (CH2, t, J = 8.0 Hz, 2H), 1.55–1.49 (cyclohexyl-H, 5H, m), 1.27 (cyclohexyl-H, 1H, m), 1.02–0.93 (cyclohexyl-H, 3H, m), 0.74–0.64 (cyclohexyl-H, 2H, m). 13C NMR (101 MHz, DMSO) δC 151.8 (C), 143.1, 137.6, 136.8, 134.0, 131.1, 128.9, 126.2, 123.0, 49.5, 40.6, 40.4, 40.2, 39.9, 39.8, 39.6, 39.3, 37.6, 30.6, 26.3, 25.7. Anal. calculated for C16H20N2O2S, %: C, 63.13; H, 6.62; N, 9.20; found, %: C, 63.43; H, 6.40; N, 9.30.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 260–263 °C, FTIR (ν cm−1), 3458 (stretching, OH), 3174 (stretching, NH), 1570 (stretching, C
3), M.P: 260–263 °C, FTIR (ν cm−1), 3458 (stretching, OH), 3174 (stretching, NH), 1570 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1267 (stretching, S:O) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.94 (NH, 1H, brs), 9.31 (OH, 1H, brs), 9.13 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.51 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.34 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.26 (Ar-H, dd, J = 8.0, 1.5 Hz, 1H), 7.73–7.69 (Ar-H, m, 2H), 6.83 (Ar-H, t, J = 8.0 Hz, 1H), 6.51 (Ar-H, app t, J = 1.6 Hz, 1H), 6.47 (Ar-H, dd, J = 8.0, 0.8 Hz, 1H), 6.51 (Ar-H, app t, J = 8.0, 1.2 Hz, 1H). 13C NMR (101 MHz, DMSO) δC 158.0 (C), 151.9, 143.2, 139.3, 137.4, 135.7, 134.6, 132.5, 129.9, 128.9, 126.1, 123.1, 111.2, 110.8, 107.2 (Ar–C).
C), 1267 (stretching, S:O) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.94 (NH, 1H, brs), 9.31 (OH, 1H, brs), 9.13 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.51 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.34 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.26 (Ar-H, dd, J = 8.0, 1.5 Hz, 1H), 7.73–7.69 (Ar-H, m, 2H), 6.83 (Ar-H, t, J = 8.0 Hz, 1H), 6.51 (Ar-H, app t, J = 1.6 Hz, 1H), 6.47 (Ar-H, dd, J = 8.0, 0.8 Hz, 1H), 6.51 (Ar-H, app t, J = 8.0, 1.2 Hz, 1H). 13C NMR (101 MHz, DMSO) δC 158.0 (C), 151.9, 143.2, 139.3, 137.4, 135.7, 134.6, 132.5, 129.9, 128.9, 126.1, 123.1, 111.2, 110.8, 107.2 (Ar–C).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 260–262 °C, FTIR (ν cm−1), 3262 (stretching, NH), 1586 (stretching, C
3), M.P: 260–262 °C, FTIR (ν cm−1), 3262 (stretching, NH), 1586 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1324 (stretching, S:O) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 9.11, (dd, J = 4.23, 1.79, 1H), 8.51, (dd, J = 8.40, 1.79 Hz) 8.36, (dd, J = 7.33, 1.44 Hz, 1H), 8.27, (dd, J = 8.29, 1.42), 7.81, (t, J = 6.48, 1H), 7.71, (m, 2H), 7.42 (d, J = 8.79, 2H), 6.87, (d, J = 8.43 Hz, 2H), 4.09 (d, J = 6.53 Hz, 2H). 13C NMR (100 MHz, DMSO) δC 151.8, 142.8, 142.7, 137.3, 135.0, 134.9, 133.6, 132.9, 128.7, 125.9, 123.0, 119.0, 118.4 (Ar-Cs), 47.6 (CH2).
C), 1324 (stretching, S:O) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 9.11, (dd, J = 4.23, 1.79, 1H), 8.51, (dd, J = 8.40, 1.79 Hz) 8.36, (dd, J = 7.33, 1.44 Hz, 1H), 8.27, (dd, J = 8.29, 1.42), 7.81, (t, J = 6.48, 1H), 7.71, (m, 2H), 7.42 (d, J = 8.79, 2H), 6.87, (d, J = 8.43 Hz, 2H), 4.09 (d, J = 6.53 Hz, 2H). 13C NMR (100 MHz, DMSO) δC 151.8, 142.8, 142.7, 137.3, 135.0, 134.9, 133.6, 132.9, 128.7, 125.9, 123.0, 119.0, 118.4 (Ar-Cs), 47.6 (CH2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 250–252 °C, FTIR (ν cm−1), 3411 (stretching, NH), 2954 (stretching, CH), 1687 (stretching, C
3), M.P: 250–252 °C, FTIR (ν cm−1), 3411 (stretching, NH), 2954 (stretching, CH), 1687 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1367 (stretching, S:O), 703 (stretching, C–F). 1H NMR (400 MHz, DMSO-d6) δH 9.03 (Ar-H, app dd, J = 4.0, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.25–8.16 (Ar-H, 2H, m), 7.81 (NH, app t, J = 6.0 Hz, 1H), 7.72–7.61 (Ar-H, 2H, m), 7.09–6.96 (Ar-H, 4H, m), 4.08 (CH2, d, J = 6.0 1.4 Hz, 2H), 13C NMR (101 MHz, DMSO) δC 151.6, 143.0, 137.34/137.28, 136.9, 133.8, 131.8, 130.9, 129.8, 128.8, 127.8, 126.0, 122.8 (Ar-C), 46.4 (CH2). Anal. calculated for C16H13FN2O2S, %: C, 60.75; H, 4.14; N, 8.86; found, %: C, 60.88; H, 4.34; N, 8.95.
C), 1367 (stretching, S:O), 703 (stretching, C–F). 1H NMR (400 MHz, DMSO-d6) δH 9.03 (Ar-H, app dd, J = 4.0, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.25–8.16 (Ar-H, 2H, m), 7.81 (NH, app t, J = 6.0 Hz, 1H), 7.72–7.61 (Ar-H, 2H, m), 7.09–6.96 (Ar-H, 4H, m), 4.08 (CH2, d, J = 6.0 1.4 Hz, 2H), 13C NMR (101 MHz, DMSO) δC 151.6, 143.0, 137.34/137.28, 136.9, 133.8, 131.8, 130.9, 129.8, 128.8, 127.8, 126.0, 122.8 (Ar-C), 46.4 (CH2). Anal. calculated for C16H13FN2O2S, %: C, 60.75; H, 4.14; N, 8.86; found, %: C, 60.88; H, 4.34; N, 8.95.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 258–260 °C, FTIR (ν cm−1), 3282 (stretching, NH), 1658 (stretching, C
3), M.P: 258–260 °C, FTIR (ν cm−1), 3282 (stretching, NH), 1658 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1334 (stretching, S:O), 746 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.03 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.21 (Ar-H, dd, J = 8.0, 1.4 Hz, 2H), 7.78 (NH, app t, J = 8.0 Hz, 1H), 7.69–7.64 (Ar-H, 2H, m), 7.06 (Ar-H, dd, J = 8.0, 3.2 Hz, 2H), 6.65 (Ar-H, app t, J = 8.0 Hz, 2H), 4.08 (Ar-H, d, J = 8.0 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 160.2/160.2, 151.6, 142.9, 137.4/137.3, 133.8, 130.8, 129.99/129.91, 128.8, 126.0, 122.9, 114.7/114.5 (Ar-C), 46.4 (CH2). Anal. calculated for C16H13ClN2O2S, %: C, 53.13; H, 3.15; N, 7.29; found, %: C, 53.28; H, 3.43; N, 7.48.
C), 1334 (stretching, S:O), 746 (stretching, C–F) cm−1. 1H NMR (400 MHz, DMSO-d6) δH 9.03 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.47 (Ar-H, dd, J = 8.0, 1.8 Hz, 1H), 8.21 (Ar-H, dd, J = 8.0, 1.4 Hz, 2H), 7.78 (NH, app t, J = 8.0 Hz, 1H), 7.69–7.64 (Ar-H, 2H, m), 7.06 (Ar-H, dd, J = 8.0, 3.2 Hz, 2H), 6.65 (Ar-H, app t, J = 8.0 Hz, 2H), 4.08 (Ar-H, d, J = 8.0 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 160.2/160.2, 151.6, 142.9, 137.4/137.3, 133.8, 130.8, 129.99/129.91, 128.8, 126.0, 122.9, 114.7/114.5 (Ar-C), 46.4 (CH2). Anal. calculated for C16H13ClN2O2S, %: C, 53.13; H, 3.15; N, 7.29; found, %: C, 53.28; H, 3.43; N, 7.48.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 264–266 °C, FTIR (ν cm−1), 3273 (stretching, NH), 1610 (stretching, C
3), M.P: 264–266 °C, FTIR (ν cm−1), 3273 (stretching, NH), 1610 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C),1311 (stretching, S:O) cm−1. 1H (400 MHz, DMSO-d6) δH, 9.19 (dd, J = 4.3, 1.7 Hz, 1H, Ar-H), 8.62 (dd, J = 8.3, 1.7 Hz, 1H, Ar-H), 8.42–8.27 (m, 2H, Ar-H), 7.92–7.78 (m, 2H, Ar-H), 7.24 (t, J = 6.4 Hz, NH, 1H), 6.94 (d, J = 8.2 Hz, 1H, Ar-H), 6.70 (m, 2H, Ar-H), 4.16 (d, J = 6.3 Hz, 2H, CH2). 13C NMR (100 MHz, DMSO) δ 161.32, 158.79, 152.46, 143.93, 138.41, 137.93, 134.82, 131.65, 131.41, 129.80, 127.06, 123.84, 117.54, 105.03, 98.62 (Ar Cs), 43.15 (CH2).
C),1311 (stretching, S:O) cm−1. 1H (400 MHz, DMSO-d6) δH, 9.19 (dd, J = 4.3, 1.7 Hz, 1H, Ar-H), 8.62 (dd, J = 8.3, 1.7 Hz, 1H, Ar-H), 8.42–8.27 (m, 2H, Ar-H), 7.92–7.78 (m, 2H, Ar-H), 7.24 (t, J = 6.4 Hz, NH, 1H), 6.94 (d, J = 8.2 Hz, 1H, Ar-H), 6.70 (m, 2H, Ar-H), 4.16 (d, J = 6.3 Hz, 2H, CH2). 13C NMR (100 MHz, DMSO) δ 161.32, 158.79, 152.46, 143.93, 138.41, 137.93, 134.82, 131.65, 131.41, 129.80, 127.06, 123.84, 117.54, 105.03, 98.62 (Ar Cs), 43.15 (CH2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 262–263 °C, FTIR (ν cm−1), 3300 (stretching, NH), 1613 (stretching, C
3), M.P: 262–263 °C, FTIR (ν cm−1), 3300 (stretching, NH), 1613 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1326 (stretching, S:O) cm−1. 1H (400 MHz, DMSO-d6) δH, 9.28 (dd, J = 4.3, 1.8 Hz, 1H, Ar-H), 8.75 (dd, J = 8.4, 1.8 Hz, 1H, Ar-H), 8.51 (m, 2H, Ar-H), 7.92 (m, 3H, Ar-H), 7.65 (t, J = 6.55, NH 1H) 7.36 (m, 1H, Ar-H), 7.29 (dd, J = 8.1, 1.6 Hz, 1H, Ar-H), 6.99 (td, J = 7.6, 1.7 Hz, 1H, Ar-H), 4.23 (d, J = 6.52, 2H, Ar-H). 1H NMR (400 MHz, DMSO-d6) δ 9.28 (dd, J = 4.3, 1.8 Hz, 1H), 8.75 (dd, J = 8.4, 1.8 Hz, 1H), 8.51 (m, 2H), 7.92 (m, 3H), 7.65 (t, J = 6.55, 1H) 7.36 (m, 1H), 7.29 (dd, J = 8.1, 1.6 Hz, 1H), 6.99 (td, J = 7.6, 1.7 Hz, 1H), 4.23 (d, J = 6.52, 2H). 13C NMR (100 MHz, DMSO) δ 152.24, 142.84, 139.96, 138.92, 137.75, 135.76, 134.94, 133.80, 131.68, 129.52, 129.02, 127.49, 126.21, 123.32, 122.69 (Ar-C), 45.89 (CH2).
C), 1326 (stretching, S:O) cm−1. 1H (400 MHz, DMSO-d6) δH, 9.28 (dd, J = 4.3, 1.8 Hz, 1H, Ar-H), 8.75 (dd, J = 8.4, 1.8 Hz, 1H, Ar-H), 8.51 (m, 2H, Ar-H), 7.92 (m, 3H, Ar-H), 7.65 (t, J = 6.55, NH 1H) 7.36 (m, 1H, Ar-H), 7.29 (dd, J = 8.1, 1.6 Hz, 1H, Ar-H), 6.99 (td, J = 7.6, 1.7 Hz, 1H, Ar-H), 4.23 (d, J = 6.52, 2H, Ar-H). 1H NMR (400 MHz, DMSO-d6) δ 9.28 (dd, J = 4.3, 1.8 Hz, 1H), 8.75 (dd, J = 8.4, 1.8 Hz, 1H), 8.51 (m, 2H), 7.92 (m, 3H), 7.65 (t, J = 6.55, 1H) 7.36 (m, 1H), 7.29 (dd, J = 8.1, 1.6 Hz, 1H), 6.99 (td, J = 7.6, 1.7 Hz, 1H), 4.23 (d, J = 6.52, 2H). 13C NMR (100 MHz, DMSO) δ 152.24, 142.84, 139.96, 138.92, 137.75, 135.76, 134.94, 133.80, 131.68, 129.52, 129.02, 127.49, 126.21, 123.32, 122.69 (Ar-C), 45.89 (CH2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), M.P: 266–268, FTIR (ν cm−1), 3402 (stretching, OH), 3170 (stretching, NH), 1618 (stretching, C
3), M.P: 266–268, FTIR (ν cm−1), 3402 (stretching, OH), 3170 (stretching, NH), 1618 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1361 (stretching, S:O) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 10.13 (NH, 1H, brs), 9.89 (OH, 1H, brs), 9.14 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.53 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 8.34 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.29 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 7.75–7.70 (Ar-H, m, 2H), 6.98 (Ar-H, app s, 2H). 13C NMR (100 MHz, DMSO) δC 152.0 (C), 146.2, 143.0, 137.6, 135.1, 135.0, 132.7, 130.9, 128.8, 126.2, 123.2, 122.7, 120.9 (Ar-C).
C), 1361 (stretching, S:O) cm−1. 1H NMR (400 MHz, DMSO-d6) δH, 10.13 (NH, 1H, brs), 9.89 (OH, 1H, brs), 9.14 (Ar-H, dd, J = 4.0, 1.8 Hz, 1H), 8.53 (Ar-H, dd, J = 8.0, 4.0 Hz, 1H), 8.34 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 8.29 (Ar-H, dd, J = 8.0, 1.4 Hz, 1H), 7.75–7.70 (Ar-H, m, 2H), 6.98 (Ar-H, app s, 2H). 13C NMR (100 MHz, DMSO) δC 152.0 (C), 146.2, 143.0, 137.6, 135.1, 135.0, 132.7, 130.9, 128.8, 126.2, 123.2, 122.7, 120.9 (Ar-C).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) M.P: 265–268 °C, FTIR (ν cm−1), 3240 (stretching, NH), 1710 (stretching, C
3) M.P: 265–268 °C, FTIR (ν cm−1), 3240 (stretching, NH), 1710 (stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1324 (stretching, S:O) cm−1. 1H NMR (DMSO-d6) δH 9.03 (dd, J = 4.2, 1.8 Hz, 1H, Ar-H), 8.47 (dd, J = 8.3, 1.8 Hz, 1H), 8.21 (m, 2H, Ar-H), 7.81 (t, J = 6.5 Hz, 1H, Ar-H), 7.67 (m, 2H, Ar-H), 7.02 (m, 4H, Ar-H), 4.08 (d, J = 6.5 Hz, 2H, CH2), 3.57 (s, 3H, CH3). 13C NMR (100 MHz, DMSO) δC 151.58, 143.00, 137.34, 137.28, 136.92, 133.84, 131.79, 130.95, 129.76, 128.81, 127.84, 126.02, 122.87 (Ar-Cs), 58.48 (CH2), 46.36 (CH3).
C), 1324 (stretching, S:O) cm−1. 1H NMR (DMSO-d6) δH 9.03 (dd, J = 4.2, 1.8 Hz, 1H, Ar-H), 8.47 (dd, J = 8.3, 1.8 Hz, 1H), 8.21 (m, 2H, Ar-H), 7.81 (t, J = 6.5 Hz, 1H, Ar-H), 7.67 (m, 2H, Ar-H), 7.02 (m, 4H, Ar-H), 4.08 (d, J = 6.5 Hz, 2H, CH2), 3.57 (s, 3H, CH3). 13C NMR (100 MHz, DMSO) δC 151.58, 143.00, 137.34, 137.28, 136.92, 133.84, 131.79, 130.95, 129.76, 128.81, 127.84, 126.02, 122.87 (Ar-Cs), 58.48 (CH2), 46.36 (CH3).3.2. Biological activates (in vitro enzyme assay)
3.3. In silico studies
The MOE 2019 software package was used to conduct an in silico study on MAO-A (PDB ID: 2Z5Y), MOA-B (PDB ID: 2V5Z), AChE (PDB ID: 4BDT), and BChE (PDB ID: 4TPK). Re-docking of the cognate ligand occurred immediately after the protein was prepared.26 An RMSD of 1.8 were found, indicating that the docking protocol was appropriate.39 The re-docking process also showed that the ligand was in the same binding pocket as the original one. The binding affinity was found to be in a range of −7.9 to −10.76 kcal mol−1. This confirmed that the docking protocol was successful and yielded the expected results.403.4. Enzyme extraction
Mice livers from male albinos (between 250 and 300 g) were collected to obtain mitochondrial-enriched sections. In the first step, liver (20–30 g) was crushed into small pieces in an ice cold Hepes buffer (5.0 mM, pH 7.4) consisting of sucrose 70 mM, mannitol 210 mM, and ethylene glycol tetraacetic acid (EGTA) in a 50 mL beaker. The mixture was homogenized at 1100 rpm, and transferred into a 45 mL falcon and centrifuged at 1000 rpm for 30 min and the upper layer (supernatant) was collected and further centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min at 0 °C. repeated the same step twice. The pellet was re-suspended in 50 mM of buffer (NaHPO4, pH 7.4), transferred into small Eppen doffs and stored at −80 °C until further use.37
000 rpm for 30 min at 0 °C. repeated the same step twice. The pellet was re-suspended in 50 mM of buffer (NaHPO4, pH 7.4), transferred into small Eppen doffs and stored at −80 °C until further use.37
3.5. Docking protocol
3.6. In silico ADME analysis
Physicochemical and pharmacokinetic properties (Absorption, Distribution, Metabolism and Excretion) of all the compounds (a1–18) were assessed by SwissADME web server. The SMILES format of each synthetic derivative was obtained from ChemDraw and put on the web server to predict the pharmacokinetic profile.354 Conclusion
In summary, a new library of quinoline-based sulfonamide derivatives was synthesized and explored against MAO-A, MAO-B, AChE and BChE. Among them compounds a5, a12, a11, and a6 were found to be the most potent having IC50 values of 0.59 ± 0.04, 0.47 ± 0.03, 0.58 ± 0.05 and 1.10 ± 0.77 μM respectively. As compared to other enzymes, compounds with the m-chloro substitution displayed 2-fold enhanced potency against MAO-A. In contrast, the o-fluoro substitution exhibited a 2-fold higher potency against MAO-B than other enzymes. o-Hydroxy had a 1-fold enhanced potency toward AChE than other enzymes. The kinetic studies of the most potent compound showed competitive inhibition. In addition, in silico analysis revealed ligand–protein interactions such as hydrogen bonding, π–alkyl, and π–sulfur with various catalytic amino acid residues. The importance of this target in AD and PD pathogenesis suggests that these compounds may be useful for developing multitarget-directed ligands. Based on our results, compound a5, a6, a11, a12, a15, and a18 is a promising “one-compound-multi-target” candidate for AD treatment and needs further study.Author contributions
Saquib Jalil: synthesis, methodology, formal analysis, validation investigation (bioactivity), writing – original draft preparation (bioactivity part), software. Saquib Jalil and Zahid Hussain: synthesis, methodology, formal analysis, validation. Syed Mubashir Ali Abid: in silico studies (computational). Abdul Hameed: manuscript writing and spectral data. Jamshed Iqbal: supervision, resources (synthesis), data curation, writing– original draft reviewing and editing, supervision, resources (bioactivity and docking), funding acquisition.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors gratefully acknowledge the financial support for this research provided by the Higher Education Commission of Pakistan (HEC) via NRPU Project No. 20-15846/NRPU/R&D/HEC/2021, German-Pakistani Research Collaboration Programme and Equipment Grant funded by DAAD, Germany.References
- M. M. Gonzales, et al., Biological aging processes underlying cognitive decline and neurodegenerative disease, J. Clin. Invest., 2022, 132(10), e158453 CrossRef CAS PubMed.
- E. Connell, et al., Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia, Mol. Neurodegener., 2022, 17(1), 43 CrossRef CAS PubMed.
- M. Nasb, W. Tao and N. Chen, Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses, Aging Dis., 2023, 15(2), 43–73 Search PubMed.
- N. I. Bohnen, et al., Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia, Curr. Geriatr. Rep., 2018, 7, 1–11 CrossRef PubMed.
- N. Kumar, et al., Advancements in the development of multi-target directed ligands for the treatment of Alzheimer's disease, Bioorg. Med. Chem., 2022, 116742 CrossRef CAS PubMed.
- A. U. Syed, et al., Comparison of Monoamine Oxidase-A, Aβ Plaques, Tau, and Translocator Protein Levels in Postmortem Human Alzheimer's Disease Brain, Int. J. Mol. Sci., 2023, 24(13), 10808 CrossRef CAS PubMed.
- Z.-R. Chen, et al., Role of cholinergic signaling in Alzheimer's disease, Molecules, 2022, 27(6), 1816 CrossRef CAS PubMed.
- S. Manzoor and N. Hoda, A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer’s disease agents: A review, Eur. J. Med. Chem., 2020, 206, 112787 CrossRef CAS PubMed.
- M. B. Youdim, Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases, J. Neural Transm., 2018, 125(11), 1719–1733 CrossRef CAS PubMed.
- I. Vecchio, et al., The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer's disease, J. Cent. Nerv. Syst. Dis., 2021, 13, 11795735211029113 Search PubMed.
- B. S. Matada, R. Pattanashettar and N. G. Yernale, A comprehensive review on the biological interest of quinoline and its derivatives, Bioorg. Med. Chem., 2021, 32, 115973 CrossRef CAS PubMed.
- D. J. Kucharski, M. K. Jaszczak and P. Boratyński, A review of modifications of quinoline antimalarials: mefloquine and (hydroxy) chloroquine, Molecules, 2022, 27(3), 1003 CrossRef CAS PubMed.
- K. B. Patel and P. Kumari, A review: Structure-activity relationship and antibacterial activities of Quinoline based hybrids, J. Mol. Struct., 2022, 1268, 133634 CrossRef CAS.
- T.-H. Qin, et al., Synthesis and biological evaluation of new 2-substituted-4-amino-quinolines and-quinazoline as potential antifungal agents, Bioorg. Med. Chem. Lett., 2022, 72, 128877 CrossRef CAS PubMed.
- M. Jeleń, B. Morak-Młodawska and R. Korlacki, Anticancer activities of tetra-, penta-, and hexacyclic phenothiazines modified with quinoline moiety, Mol. Struct., 2023, 135700 CrossRef.
- H. B. Shivaji and K. R. Rajendra, Structure based drug discovery, docking modelling, synthesis and anticonvulsant pharmacological activity of new quinoline derivatives, Int. J. Health Sci., 2022,(II), 9534–9548 Search PubMed.
- A. M. Ghanim, et al., Design and synthesis of ibuprofen-quinoline conjugates as potential anti-inflammatory and analgesic drug candidates, Bioorg. Chem., 2022, 119, 105557 CrossRef CAS PubMed.
- V. Snehi, et al., An Extensive Review on Biological Interest of Quinoline and Its Analogues, Int. J. Sci. Healthcare Res., 2023, 8, 45–66 CAS.
- M. S. S. Chauhan, T. Umar and M. K. Aulakh, Quinolines: Privileged Scaffolds for Developing New Anti-neurodegenerative Agents, ChemistrySelect, 2023, 8(14), e202204960 CrossRef CAS.
- A. Marella, et al., Quinoline: A versatile heterocyclic, Saudi Pharm. J., 2013, 21(1), 1–12 CrossRef PubMed.
- A. Irfan, et al., Coumarin sulfonamide derivatives: An emerging class of therapeutic agents, Heterocycl. Commun., 2020, 26(1), 46–59 CrossRef CAS.
- B. Mavroidi, et al., The prophylactic and multimodal activity of two isatin thiosemicarbazones against Alzheimer's disease in vitro, Brain Sci., 2022, 12(6), 806 CrossRef CAS PubMed.
- S. Kumar, et al., Exploration of the detailed structure–activity relationships of isatin and their isomers as monoamine oxidase inhibitors, ACS Omega, 2022, 7(19), 16244–16259 CrossRef CAS PubMed.
- A. A. Shetnev, et al., Investigation of Novel Thiazole Derivatives Bearing the Benzenesulfonamide Moiety as MAO Inhibitors with a Promising Activity Profiles. Preprint, 2023 Search PubMed.
- Z. H. Li, L. Q. Yin, D. H. Zhao, L. H. Jin, Y. J. Sun and C. Tan, SAR studies of quinoline and derivatives as potential treatments for Alzheimer's disease, Arabian J. Chem., 2023, 16(2), 104502 CrossRef CAS.
- H. Chen, et al., Design, synthesis and evaluation of quinoline-O-carbamate derivatives as multifunctional agents for the treatment of Alzheimer's disease, J. Enzyme Inhib. Med. Chem., 2023, 38(1), 2169682 CrossRef PubMed.
- M. B. Rosenthal, Novel Alzheimer disease treatments and reconsideration of us pharmaceutical reimbursement policy, J. Am. Med. Assoc., 2023, 303(6), 505–506 CrossRef PubMed.
- M. M. Martins, P. S. Branco and L. M. Ferreira, Enhancing the Therapeutic Effect in Alzheimer's Disease Drugs: The role of Polypharmacology and Cholinesterase inhibitors, ChemistrySelect, 2023, 8(10), e202300461 CrossRef CAS.
- A. A. Njan, et al., Identification of neurotherapeutic constituents in Ocimum gratissimum with cholinesterase and mono amine oxidase inhibitory activities, using GC-MS analysis, in vitro, and in silico approaches, Inf. Med. Unlocked, 2023, 101261 CrossRef.
- Q.-H. Jin, et al., (S)-N-Benzyl-1-phenyl-3, 4-dihydroisoqunoline-2 (1 H)-carboxamide Derivatives, Multi-Target Inhibitors of Monoamine Oxidase and Cholinesterase: Design, Synthesis, and Biological Activity, Molecules, 2023, 28(4), 1654 CrossRef CAS PubMed.
- K. Jangid, et al., Virtual screening and molecular dynamics simulation approach for the identification of potential multi-target directed ligands for the treatment of Alzheimer's disease, J. Biomol. Struct. Dyn., 2023, 1–19 Search PubMed.
- S. Kumar and S. R. Ayyannan, Identification of new small molecule monoamine oxidase-B inhibitors through pharmacophore-based virtual screening, molecular docking and molecular dynamics simulation studies, J. Biomol. Struct. Dyn., 2022, 1–22 Search PubMed.
- A. Daina, O. Michielin and V. Zoete, SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci. Rep., 2017, 7(1), 42717 CrossRef PubMed.
- A. Daina and V. Zoete, A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules, ChemMedChem, 2016, 11(11), 1117–1121 CrossRef CAS PubMed.
- C. A. Lipinski, et al., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Delivery Rev., 1997, 23(1–3), 3–25 CrossRef CAS.
- J. B. Baell and G. A. Holloway, New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays, J. Med. Chem., 2010, 53(7), 2719–2740 CrossRef CAS PubMed.
- N. T. Tzvetkov, et al., Indazole- and Indole-5-carboxamides: Selective and Reversible Monoamine Oxidase B Inhibitors with Subnanomolar Potency, J. Med. Chem., 2014, 57(15), 6679–6703 CrossRef CAS PubMed.
- P. George and M. Abernethy, Improved Ellman procedure for erythrocyte cholinesterase, Clin. Chem., 1983, 29(2), 365–368 CrossRef CAS.
- N. T. Tzvetkov, et al., Indazole-and indole-5-carboxamides: Selective and reversible monoamine oxidase B inhibitors with subnanomolar potency, J. Med. Chem., 2014, 57(15), 6679–6703 CrossRef CAS PubMed.
- P. C. Tomy and C. G. Mohan, Chemical space navigation by machine learning models for discovering selective MAO-B enzyme inhibitors for Parkinson's disease, Artif. Intell. Chem., 2023, 1(2), 100012 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05501a |
| This journal is © The Royal Society of Chemistry 2024 |