Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reductive activation of arenes by potassium metal with potassium salts†
Giuseppe
Nocera
a,
Iain
Robb
a,
Kenneth F.
Clark
 a,
Thomas M.
McGuire
b,
Laura
Evans
b,
Shunsuke
Chiba
a,
Thomas M.
McGuire
b,
Laura
Evans
b,
Shunsuke
Chiba
 c and
John A.
Murphy
c and
John A.
Murphy
 *a
*a
aDepartment of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL, UK. E-mail: john.murphy@strath.ac.uk
bMedicinal Chemistry, Research and Early Development, Oncology R&D, AstraZeneca, Cambridge CB10 1XL, UK
cSchool of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore, 637371 Singapore
First published on 23rd July 2024
Abstract
Benzene is routinely dried by refluxing over potassium or other alkali metals and is inert to the metal. However, dramatic chemistry occurs when potassium salts are added. At 150 °C, dimerisation occurs to afford biphenyl as the product. In the absence of salt, no reaction occurs. We propose that the process is initiated by activation of the arene by the salt followed by electron transfer from potassium. In support of this, within the added salt, systematic alteration of (i) the anion and (ii) the cation shows that the cation is the important component; thus, K+ is effective but Na+ and Li+ are not. Studies with a mixture of benzene and benzene-d6 show facile transfer of H−/D− ions between molecules during the reaction. Extension of the study to other arene hydrocarbons shows the generality of the chemistry.
Introduction
Cation–π interactions have been recognised as an important phenomenon in organic chemistry for many years. Their role in biological processes is widespread,1,2 and their effects in synthetic organic chemistry are also important.3 In this paper, we report the use of arene–K+ interactions to activate arene hydrocarbons. Recent papers have highlighted fundamental differences between the effects of potassium salts and their sodium counterparts in triggering organic reactions and this paper extends those observations into electron transfer reductions of arenes.4This study arose from our investigations of the initiation of the coupling of aryl halides to arenes in the presence of KOtBu, the ‘Base-promoted homolytic aromatic substitution’ (BHAS) reaction.5 Our results had shown that the reactions could be initiated by organic electron donors in the presence of KOtBu. We went on to study potassium metal as an inorganic electron donor in the presence of KOtBu and found that system also worked. We then performed a ‘blank’ reaction, where the aryl halide was omitted, and simply K, KOtBu and benzene were present. Although no biphenyl was expected as a product, it was formed, as reported here. This unexpected result led us to examine the effects of potassium metal alone and potassium metal + potassium salts, resulting in the range of experiments described in this paper.
Results and discussion
Potassium and sodium metals are widely recommended for the drying of liquid arene solvents, such as benzene and toluene.6 Potassium is particularly convenient in this regard, as its melting point (63 °C) is below the boiling points of these solvents at atmospheric pressure, and this allows a fresh liquid surface of the metal to be constantly exposed. The solvent is dried but is otherwise unaffected by exposure to the metal. However, when benzene 1, was treated with potassium tert-butoxide (KOtBu) in the presence of potassium metal (150 °C, in a pressure tube, 21 h), then the reaction mixture turned black. During the reaction, the molten potassium disappeared as the metal was transformed. Workup revealed a considerable amount of biphenyl 2 (Table 1, entry 1) together with traces of dihydrobiphenyls (3 and 4), identified by GCMS and 1H NMR spectroscopy.7| Entry | Reactantsb | 2 | 3 | 4 |
|---|---|---|---|---|
| Throughout this paper, all reactions were worked up by addition of water (following removal of any unreacted potassium metal for separate work up in t-BuOH – Caution!).a Compound 5 was not detected in these experiments but was detected in some later experiments and so is shown here in inset.b In 5 mL (56 mmol) benzene.c Identified from integration of alkene signal (4 H) at δ 5.86–5.81 ppm.d Identified from integration of alkene signal (1 H) at δ 6.41–6.38 ppm.e Conducted at room temperature. | ||||
| 1 | K, (1.5 mmol), KOtBu (1.5 mmol) | 122 mg | 12 mgc | 3 mgd |
| 2 | K (1.5 mmol) | 0 | 0 | 0 |
| 3 | KOtBu (1.5 mmol) | 0 | 0 | 0 |
| 4 | K (0.1 mmol), KOtBu (1.5 mmol) | 1 mg | 0 | 0 |
| 5 | K (0.5 mmol), KOtBu (0.1 mmol) | 30 mg | 0 | 0 |
| 6e | K (0.5 mmol), KOtBu (0.1 mmol) | 0 | 0 | 0 |
Blank reactions showed that when potassium metal was heated under the same conditions with benzene, but in the absence of KOtBu (entry 2), there was no reaction; the mixture stayed colourless and the sphere of molten potassium did not dissolve or dissipate. Similarly, heating benzene with KOtBu alone (entry 3), also gave no reaction.
Firstly, we investigated how varying the quantities of the two reactants altered the outcome of the reaction. Comparison of entry 4 with entry 5 shows that the effect of the stoichiometry of the potassium metal is much more significant than the stoichiometry of KOtBu. This underscores the fact that a reductive activation of the arene is needed, with potassium as the source of electrons and with the activation being assisted by the salt. As seen here, if the electrons in K metal are available, then the reductive activation can occur with much less than stoichiometric salt, but if the amount of K is curtailed, then the progress of the reaction is limited. Entry 6 shows that the reaction does not occur at room temperature.
The investigation now progressed to vary the nature of the metal salt and the metal (Table 2). Potassium iodide (entry 1) and potassium bromide (entry 2) both afforded similar quantities of biphenyl, but about half of the amount that had resulted from potassium tert-butoxide. Both additives, but particularly the KBr, afforded notable quantities of dihydrobiphenyls 3 and 4. With KF as additive (entry 3), the yield of biphenyl 2 dropped. Moving to a much less tightly bound potassium cation in KBF4, (entry 4) significantly increased the yield of biphenyl,8 although small amounts of dihydrobiphenyls 3 and 4 were still produced.
| Entry | Reactantsa | 2 | 3 | 4 |
|---|---|---|---|---|
| a 150 °C, 21 h, benzene [5 mL (56 mmol)] for reactions with 1.5 mmol K; [2 mL (22 mmol)] for reactions with 0.5 mmol. | ||||
| 1 | K (1.5 mmol), KI (1.5 mmol) | 54 mg | Traces | Traces |
| 2 | K (1.5 mmol), KBr (1.5 mmol) | 47 mg | 17 mg | 5 mg |
| 3 | K (1.5 mmol), KF (1.5 mmol) | 14 mg | 11 mg | Traces |
| 4 | K (1.5 mmol), KBF4 (1.5 mmol) | 96 mg | 11 mg | 5 mg |
| 5 | K (1.5 mmol), Me4NCI (1.5 mmol) | 0 | 0 | 0 |
| 6 | K (1.5 mmol), NaI (1.5 mmol) | 15 mg | 6 mg | Traces |
| 7 | K (1.5 mmol), NaBF4 (1.5 mmol) | 0 | 5 mg | 0 |
| 8 | K (1.5 mmol), NaOtBu (1.5 mmol) | 0 | 0 | 0 |
| 9 | K (1.5 mmol), LiI (1.5 mmol) | 0 | 0 | 0 |
| 10 | Na (0.5 mmol), KOtBu (0.5 mmol) | 0 | 0 | 0 |
| 11 | K (1.5 mmol), MgI2 (1.5 mmol) | 0 | 0 | 0 |
| 12 | K (1.5 mmol), MgBr2 (1.5 mmol) | 0 | 0 | 0 |
| 13 | K (1.5 mmol), CaI2 (1.5 mmol) | 44 mg | Traces | Traces |
| 14 | K (0.5 mmol), SrI2 (0.5 mmol) | 5 mg | 0 | 0 |
| 15 | K (1.5 mmol), RbI (1.5 mmol) | 42 mg | 41 mg | 0 |
| 16 | K (1.5 mmol), CsI (1.5 mmol) | 18 mg | 43 mg | 25 mg |
Tetramethylammonium chloride was next used (entry 5) and gave no conversion to biphenyl. Entries 6–9 examined the effectiveness of sodium and lithium salts in place of the potassium salts.4h NaOtBu showed no conversion, NaBF4 afforded a very low yield of one isomer of a dihydrobiphenyl, but no biphenyl itself, while Nal afforded a low yield of biphenyl and of dihydro analogue 3. In all cases, it is seen that the activity is an order of magnitude lower than for the corresponding potassium salt. Extending the study, LiI (entry 9) gave no reaction. Next, changing the metal from potassium to sodium and conducting the reaction in the presence of KOtBu (entry 10) led to no biphenyl being formed. Entries 11–14 tested respectively MgI2, MgBr2, CaI2, SrI2. Of these, only CaI2 showed good ability to activate benzene. Finally, RbI and CsI were tested with K metal.3c In both cases, reductive coupling was seen, with CsI affording significant amounts of the dihydrobiphenyls 3 and 4 in addition to biphenyl 2.
The experiments suggest possible mechanisms (Scheme 1). Coordination of the potassium cation to a benzene ring activates the ring as 6 to electron transfer from potassium metal to form radical anion salt 7. The benzene is present in vast excess, and so reduction to the radical anion is likely, rather than further reduction to the antiaromatic benzene dianion. Arene radical anions are quite unreactive towards adding to unactivated arenes, but two radical anions could dimerise to form the dianionic bis-cyclohexadienyl intermediate 8. If this material were directly quenched on workup, it would afford a tetrahydrobiphenyl, but that was never observed. Accordingly, at least one ring in 8 must transfer an H entity. Loss of KH would afford anion 9. Quenching of this species on workup with water would give dihydrobiphenyl 3 and isomers. Alternatively, expulsion of another KH would give biphenyl 2, which could be reduced to radical anion 10 (to give biphenyl on workup), or dianion 11 (to give dihydrobiphenyls on workup).
To investigate the chemistry further, the reaction was repeated, but with benzene-d6 (1-d6) rather than benzene. This afforded biphenyl 2-d10 together with three reduced derivatives, which were identified by GCMS. Each was a dihydrobiphenyl, i.e. with the formula C12H2D10. The fact that these products bore two H atoms on workup with H2O suggests that they arose from workup of the dianion of the deuterated biphenyl, 11-d10. Picking up two H atoms allowed us to identify dihydro isomers 3-d10, 4-d10 and 5-d10 from the 1H NMR chemical shifts.
Our next experiment treated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of C6H6 and C6D6 with K + KOtBu. This afforded biphenyl 2, but the deuteration pattern was interesting. Instead of d0-, d5- and d10-biphenyl, the mass spectrum of the product isotopologues gave a statistical bell-shaped deuteration pattern from d0 (m/z 154) to d10 (m/z 164), with m/z 158 and 159 as the major contributors (Fig. 1). Thus, H and D entities are being readily exchanged between molecules in the reaction mixture. We considered the possibility of hydride ions and/or H atoms being transferred. In support of hydride ions being exchanged, Chiba et al. have observed regioselective addition of KH para to a cyano group in a cyanonaphthalene,9 and isotopic H/D exchange of benzene by alkaline earth hydrides has been reported by Harder et al.10 Further evidence for hydride ion transfer emerges later in this paper that shows that arene radical anions and arene dianions are resistant to such transfers, as would be expected on coulombic grounds.
1 mixture of C6H6 and C6D6 with K + KOtBu. This afforded biphenyl 2, but the deuteration pattern was interesting. Instead of d0-, d5- and d10-biphenyl, the mass spectrum of the product isotopologues gave a statistical bell-shaped deuteration pattern from d0 (m/z 154) to d10 (m/z 164), with m/z 158 and 159 as the major contributors (Fig. 1). Thus, H and D entities are being readily exchanged between molecules in the reaction mixture. We considered the possibility of hydride ions and/or H atoms being transferred. In support of hydride ions being exchanged, Chiba et al. have observed regioselective addition of KH para to a cyano group in a cyanonaphthalene,9 and isotopic H/D exchange of benzene by alkaline earth hydrides has been reported by Harder et al.10 Further evidence for hydride ion transfer emerges later in this paper that shows that arene radical anions and arene dianions are resistant to such transfers, as would be expected on coulombic grounds.
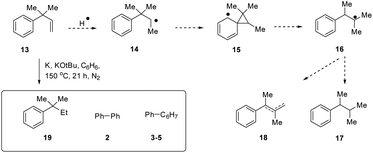
Additionally, we prepared a substrate 13 (m/z 146) that had the potential to indicate involvement of H atom transfer (HAT) (Scheme 2).11 If HAT occurred, this should give radical 14, which would undergo a rapid neophyl rearrangement12via15 to give tertiary radical 16 from which 17 and/or 18 are the likely products. However, no trace of rearrangement was observed in the actual reaction, and, besides biphenyl 2 and dihydrobiphenyls, the reduced product 19 (m/z 148) was the only product.13 The fragmentation (EI ionisation) pattern of 19 showed ions (M-Me)+ 133.1 and (M-Et)+, 119.1, [but no (M-iPr)+] which confirmed that rearrangement had not occurred. Accordingly, in the absence of evidence for H atom transfer (i.e. a radical transfer process), we propose that hydride ions from KH are the species that are exchanged.
To check that benzene was the sole source of H-transfer, i.e. that KOtBu was not involved, an experiment was carried out in C6H6, but using KOtBu-d9 in place of KOtBu. This afforded biphenyl 2 as predominant product, with no evidence of deuteration, thereby indicating that KOtBu plays no role in H-transfer.
An interesting outcome of these experiments was the detection of biphenyl, but not of terphenyl or tetraphenyl, etc. And so, to see if biphenyl would be converted into terphenyl or tetraphenyl, the next reaction involved C6D6 as solvent, but with biphenyl 2, C12H10 (1.5 mmol) (m/z 154) as an additive. However, this experiment did not afford terphenyl or tetraphenyl. Instead, this gave biphenyl 2 together with dihydrobiphenyls 3–5. The deuteration patterns of these compounds were interesting and will now be discussed (Scheme 3A). The product biphenyl mass spectrum showed both (i) statistically deuterated isotopologues of biphenyl with the major contributor as m/z 160, and (ii) an intense undeuterated biphenyl peak at m/z 154 (see Fig. 2). The statistically deuterated product must have involved build up from C6D6, but with liberal incorporation of H atoms from the added biphenyl, C12H10. Likewise, those C12H10 molecules would likely form part of the statistical mixture of isotopologues of biphenyl following the transfer of H and D atoms. But the fact that a strong and distinctive peak for biphenyl, m/z 154, was also present implied that a subset of biphenyl molecules was protected from deuteration, and this is likely because they were present as the radical anion 10 or the dianion 11. Being anionic species, it is easy to understand that they would be resistant to addition of hydride ions.
 | ||
| Fig. 2 GCMS traces for biphenyl-d0 together with biphenyl-d0 → d10 formed when biphenyl C12H10 was an additive in C6D6. | ||
Workup of the dianion 11 would give dihydro-derivatives of biphenyl, but workup of the radical anion 10 would afford biphenyl 2 following SET to air. So, the biphenyl peak at m/z 154 indicates that the radical anion was protected from H/D exchange. Evidence to support the proposal that the dianion 11 was also protected from H/D exchange came from the mass spectra of the dihydro products 3–5, which showed that these compounds were undeuterated. Accordingly, the biphenyl C12H10 that was added to this reaction either becomes involved in the exchange of H atoms with the C6D6 as the deuterated benzene gets converted into dimeric products, or the biphenyl gets rapidly reduced to radical anion or dianion, which are resistant to hydride ion exchange with C6D6 or compounds derived therefrom. It was again noted that no terphenyl or tetraphenyl were detected. It is easy to rationalise that the dianion 11 has no tendency to undergo reaction to form a terphenyl or tetraphenyl and instead only reacts by quenching during workup, but the unreactivity of the radical anion 10 is less expected. It is likely that the delocalisation of this radical anion is so great that intermolecular reactions are slow or reversible (see below for comparative reactivity of radical anions of tBuPh and tBuC6H4C6H4tBu towards expulsion of tBu radicals).
We next tested our reaction on a substituted benzene, tert-butylbenzene, 20 and saw the black colour develop, indicating a successful reaction (Scheme 3B). The GCMS pattern was complex and indicated isomers of tBuC6H4C6H4tBu 21 as well as dihydro-derivatives 22. tBuC6H4tBu 23 was also identified in trace amounts. The latter compound likely arose by liberation of a tert-butyl radical from the radical anion of tBuPh; the tert-butyl radical would then add to tBuPh in a base-promoted homolytic aromatic substitution (BHAS) reaction.12
Di-tert-butylbiphenyl 21 was next reacted in benzene with K + KOtBu (Scheme 3C). This compound has been widely used in electron transfer reactions in the literature.13 Work-up afforded biphenyl 2 and di-tert-butylbiphenyl 21. There was no evidence of liberation of tert-butyl radicals from the di-tert-butylbiphenyl, as this should have led to tBu-C6H4-Ph among other products, but none was observed. This can reflect the relative stability of a biaryl radical anion compared to the radical anion of a simple benzene.
The experiment was repeated in C6D6 as solvent and led to predominantly biphenyl-d10 (2-d10, m/z 164). However, some exchange of D/H atoms had taken place in the reaction, as the predominant peak for the recovered di-tert-butylbiphenyl in the mass spectrum was m/z 270, corresponding to di-tert-butylbiphenyl-d4 and the d5 isotopologue (m/z 271) was also clearly present. No dihydro derivatives of di-tert-butylbiphenyl were detected.
The next substrate examined was tetraphenylmethane 24 (481 mg) which was treated in benzene (5 mL) under the conditions of the reaction.14,15 Crude product (126 mg) was subjected to chromatography, giving biphenyl 2 (11 mg), residual tetraphenylmethane 24 (4 mg) and an inseparable mixture (57 mg) of products (25–29) (Scheme 4). From GCMS, Fig. 3 below shows the GC trace together with the mass spectrum of 27 (m/z = 242).
 | ||
| Fig. 3 From GCMS, the GC trace for reaction of tetraphenylmethane 24, together with the mass spectrum of 27 (m/z = 242). | ||
Possible routes to compounds 25–29 are shown in Scheme 5. Electron transfer to tetraphenylmethane 24 affords the radical anion 30. This can undergo fragmentation to phenyl radical 31 and triphenylmethylpotassium 32, and/or to phenylpotassium 33 and triphenylmethyl radical 34.
Formation of biphenyl 2 can of course occur by the route described in Scheme 1 but, in this case, it could also occur from phenyl radicals 31 through a base-promoted homolytic aromatic substitution (BHAS) reaction with benzene.5a Thus, phenyl radical 31 adds to benzene to form phenylcyclohexadienyl radical 35 which is deprotonated to afford radical anion 10; the latter is transformed into biphenyl 2 through loss of an electron, either during the reaction or on workup.
Alternatively, phenylpotassium (33) can add to benzene16 to afford phenylcyclohexadienyl potassium 9 which evolves to biphenyl 2 as in Scheme 1.
The route to diphenylmethane 25 likely starts with tritylpotassium 32 or with tiphenylmethyl radical 34. Considering 32, this can deprotonate tBuOH to form triphenylmethane 26. Electron transfer from potassium metal affords radical anion 36 which undergoes mesolytic fragmentation. If this affords diphenylmethyl anion 37, then a further proton transfer yields diphenylmethane 25. Fluorenes 27–29 were also formed through intramolecular reactions which may arise in a similar way to the intermolecular dimerisation of benzene to biphenyl in Scheme 1.
The reaction was then repeated with 24 (481 mg) but using C6D6 as solvent in order to observe the extent of any H/D exchange. Separation of 2 (d0 → d10) (13 mg) left a mixture (176 mg) of 24 (d0 → d6), 25 (d0 → d12) and 27 (d0 → d14) (Scheme 6) as shown in the GC trace below, which also shows the mass spectrum of 27 (d0 → d14). Chromatography only partially isolated the mixture allowing isolation of small quantities of pure 25 (d0 → d12) as well as an inseparable mixture (98 mg) for which GC is shown in Fig. 4.
 | ||
| Fig. 4 GCMS data from reaction of tetraphenylmethane 24 in C6D6. GC trace together with mass spectrum of 27 (d0 → d14). | ||
Naphthalene 39 was the final substrate that was examined. It is known to react with alkali metals even in the absence of added salts,17 so it was of interest to determine its reactivity under our conditions, when conducted in C6D6. Firstly, naphthalene 39 was subjected to the standard conditions as an additive to investigate its reactivity in benzene (Scheme 7).
This reaction led to a mixture of products (127 mg) where the main components were 40 and 2 as well as recovered 39. 1- and 2-phenylnaphthalene (41 and 46, respectively) along with three isomers of 1-phenyl-dihydronaphthalene (42–44) and 1-phenyl-1,2,3,4-tetrahydronaphthalene (45) were also detected in trace amounts. Purification by chromatography led to partial resolution of the mixture allowing isolation of pure samples of 2 (5 mg) and 1-phenylnaphthalene 41, (2 mg). The formation of 41–46 all show that naphthalene 39 and benzene can be coupled under these conditions. The reduced compound 1,4-dihydronaphthalene (40) was also observed, thus indicating the involvement of the naphthalene dianion which, upon aqueous work-up, receives two protons to afford 40. Products 42–44 could all result from the dianions of 41, which upon work-up give the corresponding phenyl dihydronaphthalene isomers.
As with the previous additives, the reaction was repeated in benzene-d6 to investigate the outcome of H/D exchange (Scheme 8). This led to a mixture of products (102 mg) – see ESI† for GCMS data of the mixture. Once again, high levels of H/D exchange were observed in the reaction products 41-d0→11, 42-d0→11 and 43-d0→11 or 44-d0→11. 1,4-Dihydronaphthalene (40) was again produced in this reaction and detected by 1H NMR spectroscopy, where no H/D exchange was evident. Residual naphthalene was also detected where the dominant peak showed undeuterated naphthalene 39 (m/z 128) starting material. Mono-di-and trideuterated isotopologues were also present, but the level of deuteration again implies some level of protection from D− ions. The extent of protection of substrates from the H/D exchange reaction likely depends on the kinetics of the reactions. Rapid electron transfer from potassium metal to some substrate molecules at the start of the reaction may occur before the H/D exchange reaction – the rate of such electron transfers is likely to vary with the substrate and the conditions.
Conclusions
Potassium salts facilitate reaction of benzene with potassium metal in benzene as solvent at 150 °C leading to formation of biphenyl and dihydrobiphenyls. The reactions are selective for potassium + potassium salts. No evidence for terphenyls was found and, even when biphenyl was added to the reaction, no terphenyls or tetraphenyls were detected. Reactions of arenes with K + KOtBu in C6D6 show that H−/D− transfers occur readily between molecules and a mechanism is proposed. In competition with this reactivity, some substrates undergo rapid electron transfer to a portion of the arene substrate leading to formation of radical anions and dianions which are protected against the hydride ion transfer process.Author contributions
GN, IR and KC performed the experiments and analysed the results. TMcG, LE, SC and JAM supervised the research, and analysed the results. JAM conceived the project. JAM and IR drafted the paper. All authors approved the paper.Data availability
Spectroscopic data supporting compound assignments are provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of Strathclyde, AstraZeneca and the Royal Society for funding. Additionally, we thank Craig Irving, Patricia Keating, Dr Graeme Anderson and Dr Jessica Bame for NMR and mass spectrometry assistance.References
- (a) R. A. Kumpf and D. A. Dougherty, A Mechanism for Ion Selectivity in Potassium Channels: Computational Studies of Cation-π Interactions, Science, 1993, 261, 1708–1710 CrossRef CAS PubMed; (b) M. R. Davis and D. A. Dougherty, Cation-π interactions: computational analyses of the aromatic box motif and the fluorination strategy for experimental evaluation, Phys. Chem. Chem. Phys., 2015, 17, 29262–29270 RSC.
- A. S. Mahadevi and G. N. Sastry, Cation−π Interaction: Its Role and Relevance in Chemistry, Biology, and Material Science, Chem. Rev., 2013, 113, 2100–2138 CrossRef CAS PubMed.
- (a) S. Yamada, Cation−π Interactions in Organic Synthesis, Chem. Rev., 2018, 118, 11353–11432 CrossRef CAS PubMed; (b) G. W. Gokel, S. L. De Wall and E. S. Meadows, Experimental Evidence for Alkali Metal Cation-π Interactions, Eur. J. Org. Chem., 2000, 2967–2978 CrossRef CAS; (c) S. Tsuzuki, T. Uchimaru and M. Mikami, Is the Cation/π Interaction in Alkaline-Earth-Metal Dication /Benzene Complexes a Covalent Interaction?, J. Phys. Chem. A, 2003, 107, 10414–10418 CrossRef CAS.
- (a) E. Masson and M. Schlosser, π-Arene/Metal Binding: An Issue Not Only of Structure but Also of Reactivity, Org. Lett., 2005, 7, 1923–1925 CrossRef CAS PubMed; (b) A. J. Wooten, P. J. Carroll and P. J. Walsh, Impact of Na− and K−C π-Interactions on the Structure and Binding of M3(sol)n(BINOLate)3Ln Catalysts, Org. Lett., 2007, 9, 3359–3362 CrossRef CAS PubMed; (c) C.-L. Sun, H. Li, D.-G. Yu, M. Yu, X. Zhou, X.-Y. Lu, K. Huang, S.-F. Zheng, B.-J. Li and Z.-J. Shi, An Efficient Organocatalytic Method for Constructing Biaryls through Aromatic C−H Activation, Nat. Chem., 2010, 2, 1044–1049 CrossRef CAS PubMed; (d) X. Peng, B. M. K. Tong, H. Hirao and S. Chiba, S. Inorganic-Base-Mediated Hydroamination of Alkenyl Oximes for the Synthesis of Cyclic Nitrones, Angew. Chem., Int. Ed., 2014, 53, 1959–1962 CrossRef CAS PubMed; (e) S. Zhou, E. Doni, G. M. Anderson, R. G. Kane, S. W. MacDougall, V. M. Ironmonger, T. Tuttle and J. A. Murphy, Identifying the Roles of Amino Acids, Alcohols and 1,2-Diamines as Mediators in Coupling of Haloarenes to Arenes, J. Am. Chem. Soc., 2014, 136, 17818–17826 CrossRef CAS PubMed; (f) A. Kaga, X. Peng, H. Hirao and S. Chiba, S. Diastereodivergent Synthesis of Saturated Azaheterocycles Enabled by tBuOK-Mediated Hydroamination of Alkenyl Hydrazones, Chem. – Eur. J., 2015, 21, 19112–19118 CrossRef CAS PubMed; (g) A. A. Toutov, W. B. Liu, K. N. Betz, A. Fedorov, B. M. Stoltz and R. H. Grubbs, Silylation of C-H Bonds in Aromatic Heterocycles by an Earth-Abundant Metal Catalyst, Nature, 2015, 518, 80–84 CrossRef CAS PubMed; (h) S. Banerjee, Y. F. Yang, I. D. Jenkins, Y. Liang, A. A. Toutov, W. B. Liu, D. P. Schuman, R. H. Grubbs, B. M. Stoltz, E. H. Krenske, K. N. Houk and R. N. Zare, Ionic and Neutral Mechanisms for C−H Bond Silylation of Aromatic Heterocycles Catalyzed by Potassium tert-Butoxide, J. Am. Chem. Soc., 2017, 139, 6880–6887 CrossRef CAS PubMed; (i) G. Nocera, A. Young, F. Palumbo, K. J. Emery, G. Coulthard, T. M. McGuire, T. Tuttle and J. A. Murphy, Electron Transfer Reactions: KOtBu (but not NaOtBu) Photoreduces Benzophenone under Activation by Visible Light, J. Am. Chem. Soc., 2018, 140, 9751–9757 CrossRef CAS PubMed; (j) P. Asgari, Y. Hua, C. Thiamsiri, W. Prasitwatcharakorn, A. Karedath, X. Chen, S. Sardar, K. Yum, G. Leem, B. S. Pierce, K. Nam, J. Gao and J. Jeon, Catalytic hydrogen atom transfer from hydrosilanes to vinylarenes for hydrosilylation and polymerization, Nat. Catal., 2019, 2, 164–173 CrossRef CAS PubMed; (k) K. F. Clark, S. Tyerman, L. Evans, C. M. Robertson and J. A. Murphy, An assay for aryl radicals using BHAS coupling, Org. Biomol. Chem., 2024, 22, 1018–1022 RSC.
- (a) A. Studer and D. P. Curran, Organocatalysis and C-H Activation Meet Radical- and Electron-Transfer, Angew. Chem., Int. Ed., 2011, 50, 5018–5022 CrossRef CAS PubMed; (b) S. Zhou, G. M. Anderson, B. Mondal, E. Doni, V. Ironmonger, M. Kranz, T. Tuttle and J. A. Murphy, Organic super-electron-donors: initiators in transition metal-free haloarene–arene coupling, Chem. Sci., 2014, 5, 476–482 RSC; (c) G. Nocera and J. A. Murphy, Ground State Cross-Coupling of Haloarenes with Arenes Initiated by Organic Electron Donors, Formed in situ: An Overview, Synthesis, 2020, 52, 327–336 CrossRef CAS.
- (a) K. M. Kadish, X. Mu and J. E. Anderson, Recommended methods for the Purification of Solvents and Tests for Impurities-Benzene and Toluene, Pure Appl. Chem., 1989, 61, 1823–1828 CrossRef CAS; (b) D. R. Burfield, K.-H. Lee and R. H. Smither, Desiccant Efficiency in Solvent Drying. A Reappraisal by Application of a Novel Method for Solvent Water Assay, J. Org. Chem., 1977, 42, 3060–3065 CrossRef CAS.
- Some later samples of potassium reduced substrates without the need for added salts. We attribute this to traces of potassium salts present in the potassium. All experiments used potassium freshly cut in a glovebox under nitrogen.
- For effect of anionic counterions on complexation, see S. Bartoli and S. Roelens, Binding of Acetylcholine and Tetramethylammonium to a Cyclophane Receptor: Anion's Contribution to the Cation-π Interaction, J. Am. Chem. Soc., 2002, 124, 8307–8315 CrossRef CAS PubMed In general, looser binding between the cation and anions of the salt enhances complexation of the cation to the arene.
- (a) For addition of KH to cyanonaphthalenes, see Y. Sekiguchi, J. H. Pang, J. S. Ng, J. Chen, K. Watanabe, R. Takita and S. Chiba, Base-Induced Dehydrogenative and Dearomative Transformation of 1-Naphthylmethylamines to 1,4-Dihydronaphthalene-1-carbonitriles, JACS Au, 2022, 2, 2758–2764 CrossRef CAS PubMed.
- (a) J. Martin, J. Eyselein, S. Grams and S. Harder, Hydrogen Isotope Exchange with Superbulky Alkaline Earth Amide complexes, ACS Catal., 2020, 10, 7792–7799 CrossRef CAS; (b) B. Roesch, T. X. Gentner, H. Elsen, C. A. Fischer, J. Langer, M. Wiesinger and S. Harder, Nucleophilic Aromatic Substitution at Benzene with Powerful Strontium Hydride and Alkyl Complexes, Angew. Chem., Int. Ed., 2019, 58, 5396–5401 CrossRef CAS PubMed.
- (a) A. J. Smith, D. Dimitrova, J. N. Arokianathar, K. F. Clark, D. L. Poole, S. G. Leach and J. A. Murphy, Et3SiH + KOtBu provide multiple reactive intermediates that compete in the reactions and rearrangements of benzyl nitriles and indolenines, Chem. Sci., 2020, 11, 12364–12370 RSC; (b) O. J. Turner, D. J. Hirst and J. A. Murphy, Hydrogen Atom Transfer-Mediated Domino Cyclisation Reaction to Access (Spiro)Quinazolinones, Chem. – Eur. J., 2020, 26, 3026–3029 CrossRef CAS PubMed; (c) M. Saladrigas, C. Bosch, G. V. Saborit, J. Bonjoch and B. Bradshaw, Radical Cyclization of Alkene-Tethered Ketones Initiated by Hydrogen-Atom Transfer, Angew. Chem., Int. Ed., 2018, 57, 182–186 CrossRef CAS PubMed; (d) J. C. Lo, D. Kim, C.-M. Pan, J. T. Edwards, Y. Tabr, J. Gui, T. Qin, S. Guttierez, J. Giacoboni, M. W. Smith, P. L. Holland and P. S. Baran, Fe-Catalyzed C–C Bond Construction from Olefins via Radicals, J. Am. Chem. Soc., 2017, 139, 2484–2503 CrossRef CAS PubMed; (e) S. W. M. Crossley, C. Obradors, R. M. Martinez and R. A. Shenvi, Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins, Chem. Rev., 2016, 116, 8912–9000 CrossRef CAS PubMed.
- Z.-M. Chen, X.-M. Zhang and Y.-Q. Tu, Radical aryl migration reactions and synthetic applications, Chem. Soc. Rev., 2015, 44, 5220–5245 RSC.
- Although we are not aware of KH addition to unactivated alkenes, alkaline earth metal hydrides are known to add to unactivated alkenes; for review, see K. Watanabe, J. H. Pang, R. Takita and S. Chiba, Chem. Sci., 2022, 13, 27–28 RSC.
- (a) P. K. Freeman and L. L. Hutchinson, Alkyllithium reagents from alkyl halides and lithium radical anions, J. Org. Chem., 1980, 45, 1924–1930 CrossRef CAS; (b) T. J. Donohoe and D. House, Ammonia Free Partial Reduction of Aromatic Compounds Using Lithium Di-tert-butylbiphenyl (LiDBB), J. Org. Chem., 2002, 67, 5015–5018 CrossRef CAS PubMed; (c) M. Yus, Arene-catalysed Lithiation Reactions, Chem. Soc. Rev., 1996, 155–161 RSC.
- P. P. Schorygin and I. W. Matschinskaya, Russ. J. Gen. Chem., 1939, 9, 1546–1558 Search PubMed For abstract, see: Investigations in the field of Tetraarylmethane: about decomposition using potassium-sodium alloy, Chem. Zentralbl., 1940, 111(5), 703.
- A. Morton and E. J. Lanpher, An Addition-Metalation Reaction of Benzene with Phenylpotassium, J. Org. Chem., 1958, 23, 1639–1642 CrossRef CAS.
- M. Yus, R. P. Herrera and A. Guijarro, On the Mechanism of Arene-Catalyzed Lithiation: The Role of Arene Dianions—Naphthalene Radical Anion versus Naphthalene Dianion, Chem. Eur. J., 2002, 8, 2574–2584 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectra. See DOI: https://doi.org/10.1039/d4qo01027b |
| This journal is © the Partner Organisations 2024 |