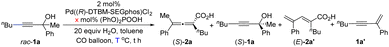Open Access Article
Open Access ArticlePd((R)-DTBM-SEGphos)Cl2-catalyzed kinetic resolution of tertiary propargylic alcohols†
Jie
Wang‡
 a,
Wei-Feng
Zheng‡
a,
Yuling
Li
b,
Yin-Long
Guo
a,
Wei-Feng
Zheng‡
a,
Yuling
Li
b,
Yin-Long
Guo
 *b,
Hui
Qian
*b,
Hui
Qian
 *a and
Shengming
Ma
*a and
Shengming
Ma
 ab
ab
aResearch Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai 200433, P. R. China. E-mail: qian_hui@fudan.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: ylguo@sioc.ac.cn
First published on 1st March 2024
Abstract
We report here an asymmetric carboxylation reaction based on kinetic resolution of tertiary propargylic alcohols by identifying Pd((R)-DTBM-SEGphos)Cl2 as the pre-catalyst. A variety of optically active tertiary propargylic alcohols and tetrasubstituted 2,3-allenoic acids were obtained in good yields with excellent enantioselectivities. The salient features of this report include the use of readily available substrates, a readily available precatalyst, mild reaction conditions, remarkable functional group tolerance, gram-scale synthesis, and versatile synthetic transformations. Mass spectrometry experiments trapped some key intermediates, which revealed the mechanism.
Introduction
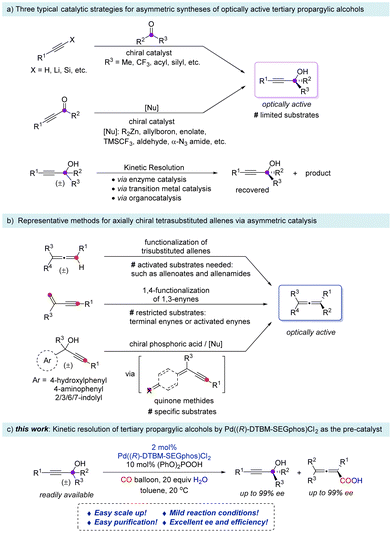
Optically active tertiary propargylic alcohols are useful building blocks in organic synthesis.1 Typically, three catalytic strategies have been developed for asymmetric synthesis of tertiary propargylic alcohols (Scheme 1a):2–6 (a) enantioselective alkynylation of methyl ketones,2b,c,e,g,h trifluoromethyl ketones,3b–f α-carbonyl ketones,4 and acyl silanes5 with terminal alkynes or 1-alkynyl trimethylsilanes4b in >90% ee; (b) enantioselective addition of nucleophiles (including Me2Zn, Et2Zn, TMSCF3, aldehydes, α-N3 amides, etc.) with 4-phenylbut-3-yn-2-one,6a pyridin-2-yl 1-alkynyl ketones,6dtert-butyl-substituted ethynyl ketones,6e propargylic ketoesters,6f or trifluoromethyl 1-alkynyl ketones6g,h in >90% ee; (c) catalytic kinetic resolution of racemic tertiary propargylic alcohols:7 In 2019, Oestreich and coworkers reported the kinetic resolution by the enantioselective Si–O coupling catalyzed by MesCu/(R,R)-Ph-BPE affording tertiary 1-phenyl-1-(n-butyl)- or 1-cyclohexyl (or N-Boc-piperidinyl-4-yl)-1-methyl-2-alkynols in 92–96% ee;7c in 2021, Li and coworkers realized the kinetic resolution via chiral Rh(III)-catalyzed allenylation of benzamides affording tertiary 1-aryl-1-bulky alkyl (tert-butyl, adamantyl, cyclohexyl, isopropyl)-2-alkynols in >90% ee;7d in the same year, Zhou and coworkers demonstrated the kinetic resolution via Cu(I)-catalyzed azide–alkyne cycloaddition affording tertiary 1-aryl-1-bis(cyclohexyloxy)methyl (or fluoroalkyl)-2-alkynols in >90% ee.7e On the other hand, due to the axial allenes serving as versatile precursors in organic transformations and material science,8 development of expeditious paths for constructing optically active tetrasubstituted allenes has been receiving increasing attention in the synthetic community. Representative catalytic methods for axially chiral tetrasubstituted allenes9,10 are as follows (Scheme 1b): (a) direct asymmetric functionalization of trisubstituted allenes.9f–i These reported strategies are generally based on the formation of allenic carbanion analogues through the deprotonation of trisubstituted allenes to react with electrophiles, which demand the potential acidity of trisubstituted allenes such as trisubstituted allenoates and allenamides. (b) Asymmetric 1,4-functionalization of 1,3-enynes.9j–o It is restricted to terminal enynes or activated enynes. (c) Chiral phosphoric acid (CPA) catalyzed conjugate addition to quinone methides9p–r formed from specific substrates including 4-hydroxylphenyl, 4-aminophenyl, or 2/3/6/7-indolyl substituted propargylic alcohols. Therefore, catalytic asymmetric formation of tetrasubstituted allenes, especially from readily available chemicals, remains challenging. Recently, with the help of the supporting ligand PPh3, we reported a Pd-catalyzed kinetic resolution carboxylation reaction of tertiary propargylic alcohols for a series of chiral 2,3-allenoic acids11 and chiral tertiary propargylic alcohols12 under different reaction conditions. Here we wish to report the identification of pre-prepared Pd((R)-DTBM-SEGphos)Cl2 as the pre-catalyst, and both optically active tertiary propargylic alcohols and tetrasubstituted 2,3-allenoic acids could be easily accessed under mild reaction conditions with high efficiency and enantioselectivities via a kinetic resolution process. Furthermore, the synthetic potential of the current method has been showcased by scale-up reactions and derivatization reactions of optically active products.Results and discussion
Optimization of reaction conditions
With Pd((R)-DTBM-SEGphos)Cl2 as the pre-catalyst, the reactions of 2-phenyloct-3-yn-2-ol rac-1a were conducted and some of the typical results are shown in Table 1. First of all, two sets of control experiments were conducted by using Pd((R)-DTBM-SEGphos)Cl2 as the catalyst instead of PdCl2 and a chiral phosphine ligand under our previous optimal conditions (entries 1 and 2):11,12 no products were observed at −5 °C (entry 1), and the reaction only delivered (S)-2a in 23% NMR yield at 25 °C (entry 2). Interestingly, the reaction exhibited a moderate efficiency at 15 °C and provided (S)-2a in 17% NMR yield with 93% ee in the absence of the supporting ligand, which suggested that the catalytic species involved in the current Pd-complex-catalyzed reaction may be different from that of the former protocols (entry 3). By prolonging the reaction time to 36 hours, the yield of (S)-2a was slightly improved with a higher yield of the enyne product (entry 4). To our delight, 44% yield of (S)-1a with 90% ee was observed when the reaction was carried out at 20 °C (entry 5). By applying 10 mol% of (PhO)2POOH, the desired product (S)-1a was formed in 46% yield with 98% ee (entries 6–10). Thus, the optimal reaction conditions of this Pd((R)-DTBM-SEGphos)Cl2-catalyzed kinetic resolution carboxylation reaction for the optically active tertiary propargylic alcohols have been identified as shown in entry 7 of Table 1. Under the same conditions, optically active tetrasubstitued 2,3-allenoic acid (S)-2a could also be smoothly obtained in 45% yield with 91% ee by just shortening the reaction time to 12 hours (entry 8).| Entry | x | T (°C) | t (h) | (S)-2a | (S)-1a | (E)-2a′ | 1a′ |
|---|---|---|---|---|---|---|---|
| Yield,b eec (%) | Recovery,b eec (%) | Yieldb (%) | Yieldb (%) | ||||
| a Reaction conditions: rac-1a (0.2 mmol), Pd((R)-DTBM-SEGphos)Cl2 (2 mol%), (PhO)2POOH (x mol%), and H2O (20 equiv.) in toluene (1 mL) at T °C with a CO balloon unless otherwise noted. b Determined by 1H NMR analysis using dibromomethane as the internal standard. c Determined by HPLC analysis. d 20 mol% PPh3 was added. | |||||||
| 1d | 20 | −5 | 18 | 0, — | 100, — | — | — |
| 2d | 2 | 25 | 18 | 23, 90 | 78, 30 | — | — |
| 3 | 20 | 15 | 18 | 17, 93 | 78, 16 | — | 5 |
| 4 | 20 | 15 | 36 | 20, 94 | 65, 19 | — | 13 |
| 5 | 20 | 20 | 18 | 51, 85 | 44, 90 | 2 | 4 |
| 6 | 15 | 20 | 18 | 51, 84 | 41, 93 | 2 | 4 |
| 7 | 10 | 20 | 18 | 51, 82 | 46, 98 | 2 | 2 |
| 8 | 10 | 20 | 12 | 45, 91 | 54, 70 | — | — |
| 9 | 5 | 20 | 18 | 55, 82 | 45, 96 | — | — |
| 10 | 2.5 | 20 | 18 | 51, 85 | 51, 91 | — | — |
Substrate scope
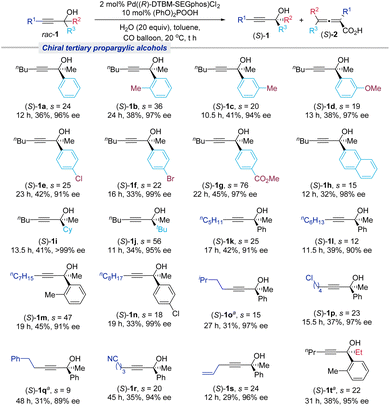
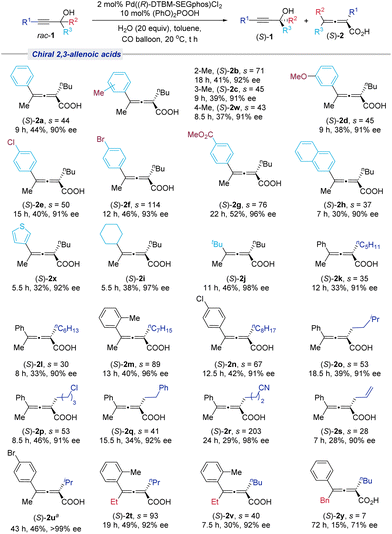
With the optimized reaction conditions in hand, the generality of this Pd((R)-DTBM-SEGphos)Cl2-catalyzed carboxylation reaction was investigated. As shown in Scheme 2, a range of tertiary propargylic alcohols containing electron-donating groups (1b–1d) or electron-withdrawing (1e–1g) on the phenyl ring furnished the corresponding products in good yields (33%–45%) with excellent ee (91%–99%). Naphthyl-substituted tertiary propargylic alcohol (1h) was compatible with the current system. Moreover, the substrates employing aliphatic substituents (Cy and tBu) were also successfully resolved to afford the desired products (S)-1i and (S)-1j in good yields with excellent enantioselectivities. Besides nBu substitution at the R1 position, a series of tertiary propargylic alcohols containing different carbon chains ranging from C3 to C8 and versatile functional groups, such as the halogen atom (Cl), cyano, and allyl, were all suitable, affording the corresponding optically active tertiary propargylic alcohol products (S)-1k–(S)-1s in 29–45% yields with up to 99% ee. For the R2 group, the methyl substituent may also be replaced with ethyl to recover (S)-1t in 38% yield with 95% ee.Next, we turned our attention to the substrate scope for the formation of chiral 2,3-allenoic acids (Scheme 3). No obvious steric effect was observed since the substrates containing the methyl group at the 2-, 3- or 4-position of the phenyl group provided the targeted products (S)-2b, (S)-2c and (S)-2w in good yields (37%–41%) with high ee (91%–92%). The substrates bearing the functional groups OMe, Cl, Br, and CO2Me on the phenyl ring also underwent the carboxylation reaction efficiently, affording the corresponding products (S)-2d–(S)-2g in good yields with more than 90% ee. 2-Naphthyl substituted and 3-thienyl substituted propargylic alcohols were also well tolerated (1h and 1x). Notably, the alkyl substituted substrates at R3 could also be converted to the desired products (S)-2i and (S)-2j smoothly. R1 with different carbon chains bearing a variety of different functional groups (halide, cyano, allyl) afforded the desired products (S)-2k–(S)-2s and (S)-2u in good yields with no less than 90% ee. Furthermore, when R2 was an ethyl group, the reaction also formed the chiral 2,3-allenoic acids (S)-2t and (S)-2v with high enantioselectivities. However, when R2 was Bn, the reaction was slow, affording 15% of (S)-2y with 71% ee after 72 hours.
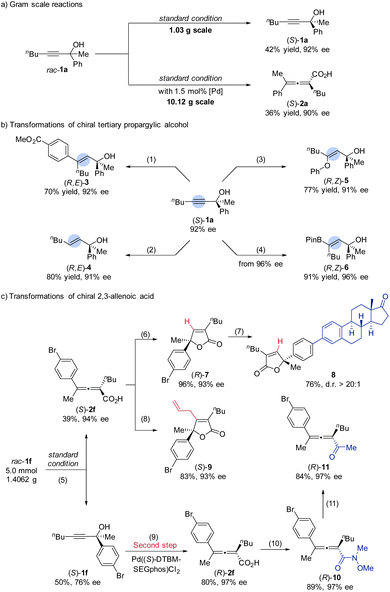
Gram scale reactions and synthetic applications
A gram-scale carboxylation reaction worked smoothly, delivering the corresponding chiral product (S)-1a in 42% yield with 92% ee (Scheme 4a); the 50 mmol scale reaction of rac-1a afforded 4.24 g of (S)-2a in 36% isolated yield and 90% ee. To exhibit the synthetic utility, several transformations of (S)-1a have been carried out as shown in Scheme 4b: rhodium catalyzed highly regioselective hydroarylation of (S)-1a with boronic acid afforded the desired product (R,E)-3 in good yields without erosion of ee;13 the reaction of (S)-1a with red-Al afforded the allylic alcohol (R,E)-4 in 80% yield with 91% ee;14 (S)-1a could be selectively transformed to phenyl enol ether (R,Z)-5 in 77% yield with 91% ee under gold catalysis.15 Moreover, the copper-catalyzed hydroboration of (S)-1a delivered the useful intermediate (R,Z)-6 in 91% yield.16 On the other hand, 1.4 g of rac-1f was smoothly converted to (S)-2f in 39% yield with 94% ee and 50% of (S)-1f was recovered in 76% ee under the standard conditions (Scheme 4c). Subsequently, a successful successive kinetic resolution of the recovered (S)-1f to afford (R)-2f in higher yield (80%) and ee (97%) was realized with Pd((S)-DTBM-SEGphos)Cl2. Employing this pair of enantiomeric allenoic acids (S)-2f and (R)-2f, a series of transformations were investigated. A CuCl-catalyzed cycloisomerization reaction was realized affording (R)-7 in excellent yield without the loss of ee.17 Furthermore, a Suzuki coupling reaction with the estrone-derived boronic acid afforded 8 in good yield and dr (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).18 A cyclization reaction of (S)-2f catalyzed by PdCl2 in the presence of allyl bromide afforded allyl furanone (S)-9 in 83% yield with 93% ee.19 The reaction of (R)-2f with methyl methoxylamine hydrochloride led to the formation of Weinreb amide (R)-10 in 89% yield with 97% ee.20 In addition, the treatment of (R)-10 with MeMgBr could afford chiral allenone (R)-11 in 84% yield and with 97% ee.
1).18 A cyclization reaction of (S)-2f catalyzed by PdCl2 in the presence of allyl bromide afforded allyl furanone (S)-9 in 83% yield with 93% ee.19 The reaction of (R)-2f with methyl methoxylamine hydrochloride led to the formation of Weinreb amide (R)-10 in 89% yield with 97% ee.20 In addition, the treatment of (R)-10 with MeMgBr could afford chiral allenone (R)-11 in 84% yield and with 97% ee.
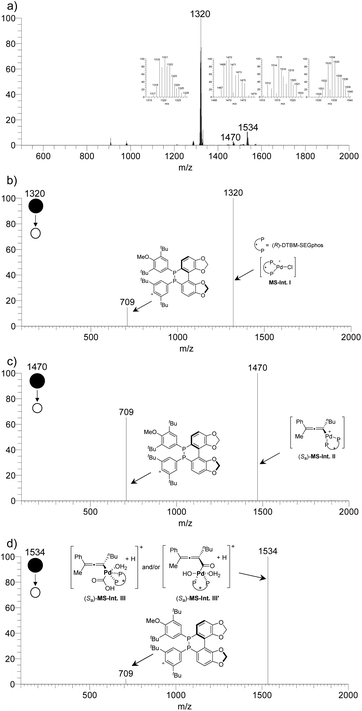
SAESI-MS studies
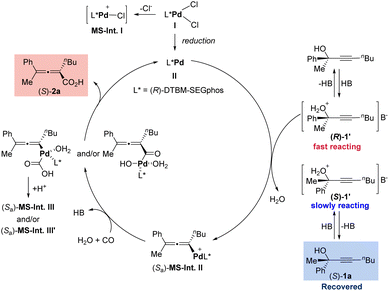
To further reveal the process of this Pd((R)-DTBM-SEGphos)Cl2-catalyzed reaction, solvent-assisted electrospray ionization mass spectrometry (SAESI-MS) studies were carried out (Scheme 5).21 Under standard conditions, the resulting mixture was analyzed after stirring for 10 min. A signal at m/z 1320 was observed, which matched the m/z of the intermediate [Pd((R)-DTBM-SEGphos)Cl]+ (calcd for C74H10035ClO8P2106Pd+: 1319.5611) MS-Int. I (Scheme 5b). The reaction of the catalyst with the H+-activated tertiary propargylic alcohols, H2O, and CO could afford the allenylpalladium intermediate (Sa)-MS-Int. II (Scheme 5c, m/z 1470). Moreover, the carboxylation intermediate (Sa)-MS-Int. III and/or (Sa)-MS-Int. III′ (Scheme 5d, m/z 1534) was also detected.Combining the 1H NMR monitoring experiment (for details see ESI Table 1†) and mass spectrometric studies, a catalytic cycle was proposed as shown in Scheme 6. First, Pd((R)-DTBM-SEGphos)Cl2I would be reduced in situ to form the catalytically active species Pd(0)((R)-DTBM-SEGphos) II. Then II would react with the configuration-matched H+-activated propargylic alcohol (R)-1′ to afford the allenylpalladium intermediate (Sa)-MS-Int. IIvia stereo-defined anti-SN2′-type oxidative addition. The subsequent reaction of (Sa)-MS-Int. II with CO and H2O delivered the carboxylation intermediate (Sa)-MS-Int. III and/or (Sa)-MS-Int. III′, which generated the product 2,3-allenoic acid (S)-2avia reductive elimination. Moreover, the slowly reacting propargylic alcohol (S)-1a could be recovered in excellent ee.
Conclusions
In summary, a Pd((R)-DTBM-SEGphos)Cl2-catalyzed carboxylative kinetic resolution reaction of racemic tertiary propargylic alcohols has been developed. Under this set of mild reaction conditions, a variety of enantioenriched tertiary propargylic alcohols and optically active tetrasubstituted 2,3-allenoic acids were obtained in good yields with excellent ee (up to >99%). Gram-scale reactions were easily realized and the optically active tertiary propargylic alcohols and 2,3-allenoic acids could be converted to a series of optically active functionalized products indicating the generality and practicality of this strategy. Mass spectrometry experiments revealed the catalytic process. Further studies in this topic are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Key R&D Program of China (2022YFA1503200 for S. M.), the National Natural Science Foundation of China (21988101 for S. M. and 22171048 for H. Q.), and the Shanghai Rising-Star Program (23QA1400400 for H. Q.) is greatly appreciated. We thank Dr Yizhan Zhai in this group for reproducing the results of (S)-1h, (S)-1j and (S)-2l, presented in Schemes 2 and 3.References
- For selected reviews, see: (a) O. Riant and J. Hannedouche, Asymmetric catalysis for the construction of quaternary carbon centres: nucleophilic addition on ketones and ketimines, Org. Biomol. Chem., 2007, 5, 873–888 RSC; (b) P. G. Cozzi, R. Hilgraf and N. Zimmermann, Enantioselective catalytic formation of quaternary stereogenic centers, Eur. J. Org. Chem., 2007, 5969–5994 CrossRef CAS; (c) M. Shibasaki and M. Kanai, Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C−C bond formation to ketones and ketimines, Chem. Rev., 2008, 108, 2853–2873 CrossRef CAS PubMed; (d) B. M. Trost and A. H. Weiss, The enantioselective addition of alkyne nucleophiles to carbonyl groups, Adv. Synth. Catal., 2009, 351, 963–983 CrossRef CAS PubMed; (e) T. Ohshima, H. Morimoto and K. Morisaki, Catalytic Asymmetric 1,2-Alkynylation, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2015 (an update of Comprehensive Chirality, 2012, Vol. 4, pp. 355–377); Search PubMed; (f) V. Bisai and V. K. Singh, Recent developments in asymmetric alkynylations, Tetrahedron Lett., 2016, 57, 4771–4784 CrossRef CAS; (g) Y.-L. Liu and X.-T. Lin, Recent advances in catalytic asymmetric synthesis of tertiary alcohols via nucleophilic addition to ketones, Adv. Synth. Catal., 2019, 361, 876–918 CrossRef CAS.
- For examples of enantioselective alkynylations of methyl ketones, see: (a) P. G. Cozzi, Enantioselective alkynylation of ketones catalyzed by Zn(Salen) complexes, Angew. Chem., Int. Ed., 2003, 42, 2895–2898 CrossRef CAS; (b) G. Lu, X. Li, X. Jia, W. L. Chan and A. S. C. Chan, Enantioselective alkynylation of aromatic ketones catalyzed by chiral camphorsulfonamide ligands, Angew. Chem., Int. Ed., 2003, 42, 5057–5058 CrossRef CAS PubMed; (c) Y. Zhou, R. Wang, Z. Xu, W. Yan, L. Liu, Y. Kang and Z. Han, Highly enantioselective phenylacetylene additions to ketones catalyzed by (S)-BINOL-Ti complex, Org. Lett., 2004, 6, 4147–4149 CrossRef CAS PubMed; (d) L. Liu, R. Wang, Y.-F. Kang, C. Chen, Z.-Q. Xu, Y.-F. Zhou, M. Ni, H.-Q. Cai and M.-Z. Gong, Highly enantioselective phenylacetylene addition to aromatic ketones catalyzed by cinchona alkaloid-aluminum complexes, J. Org. Chem., 2005, 70, 1084–1086 CrossRef CAS PubMed; (e) C. Chen, L. Hong, Z.-Q. Xu, L. Liu and R. Wang, Low ligand loading, highly enantioselective addition of phenylacetylene to aromatic ketones catalyzed by Schiff-base amino alcohols, Org. Lett., 2006, 8, 2277–2280 CrossRef CAS PubMed; (f) T. Ueda, K. Tanaka, T. Ichibakase, Y. Orito and M. Nakajima, Enantioselective alkynylation of carbonyl compounds with trimethoxysilylalkynes catalyzed by lithium binaphtholate, Tetrahedron, 2010, 66, 7726–7731 CrossRef CAS; (g) S. Kotani, K. Kukita, K. Tanaka, T. Ichibakase and M. Nakajima, Lithium binaphtholate-catalyzed asymmetric addition of lithium acetylides to carbonyl compounds, J. Org. Chem., 2014, 79, 4817–4825 CrossRef CAS PubMed; (h) K. Yamashita, Y. Tabata, K. Yamakawa, T. Mochizuki, K. Matsui, M. Hatano and K. Ishihara, Chiral macrocyclic catalysts for the enantioselective addition of lithium acetylides to ketones, J. Am. Chem. Soc., 2023, 145, 26238–26248 CrossRef CAS PubMed.
- For examples of enantioselective alkynylations of trifluoromethyl ketones, see: (a) R. Motoki, M. Kanai and M. Shibasaki, Copper(I) alkoxide-catalyzed alkynylation of trifluoromethyl ketones, Org. Lett., 2007, 9, 2997–3000 CrossRef CAS PubMed; (b) G.-W. Zhang, W. Meng, H. Ma, J. Nie, W.-Q. Zhang and J.-A. Ma, Catalytic enantioselective alkynylation of trifluoromethyl ketones: pronounced metal fluoride effects and implications of zinc-to-titanium transmetallation, Angew. Chem., Int. Ed., 2011, 50, 3538–3542 CrossRef CAS PubMed; (c) A. M. Cook and C. Wolf, Efficient access to multifunctional trifluoromethyl alcohols through base-free catalytic asymmetric C−C bond formation with terminal ynamides, Angew. Chem., Int. Ed., 2016, 55, 2929–2933 CrossRef CAS PubMed; (d) J.-i. Ito, S. Ubukata, S. Muraoka and H. Nishiyama, Enantioselective direct alkynylation of ketones catalyzed by chiral CCN pincer RhIII complexes, Chem., – Eur. J., 2016, 22, 16801–16804 CrossRef CAS PubMed; (e) Y. Zheng, Y. Tan, K. Harms, M. Marsch, R. Riedel, L. Zhang and E. Meggers, Octahedral ruthenium complex with exclusive metal-centered chirality for highly effective asymmetric catalysis, J. Am. Chem. Soc., 2017, 139, 4322–4325 CrossRef CAS PubMed; (f) D. Park, C. I. Jette, J. Kim, W.-O. Jung, Y. Lee, J. Park, S. Kang, M. S. Han, B. M. Stoltz and S. Hong, Enantioselective alkynylation of trifluoromethyl ketones catalyzed by cation-binding salen nickel complexes, Angew. Chem., Int. Ed., 2020, 59, 775–779 CrossRef CAS PubMed.
- For examples of enantioselective alkynylations of α-carbonyl ketones, see: (a) B. Jiang, Z. Chen and X. Tang, Highly enantioselective alkynylation of α-keto ester: an efficient method for constructing a chiral tertiary carbon center, Org. Lett., 2002, 4, 3451–3453 CrossRef CAS PubMed; (b) K. Aikawa, Y. Hioki and K. Mikami, Highly enantioselective alkynylation of trifluoropyruvate with alkynylsilanes catalyzed by the BINAP-Pd complex: access to α-trifluoromethyl-substituted tertiary alcohols, Org. Lett., 2010, 12, 5716–5719 CrossRef CAS PubMed; (c) T. Ohshima, T. Kawabata, Y. Takeuchi, T. Kakinuma, T. Iwasaki, T. Yonezawa, H. Murakami, H. Nishiyama and K. Mashima, C 1-symmetric Rh/Phebox-catalyzed asymmetric alkynylation of α-ketoesters, Angew. Chem., Int. Ed., 2011, 50, 6296–6300 CrossRef CAS PubMed; (d) R. Infante, J. M. Martin-Alvarez, C. Andrés and J. Nieto, Dimethylzinc-mediated addition of phenylacetylene to α-diketones catalyzed by chiral perhydro-1,3-benzoxazines, Org. Lett., 2017, 19, 1516–1519 CrossRef CAS PubMed; (e) B. P. Zavesky and J. S. Johnson, Direct zinc(II)-catalyzed enantioconvergent additions of terminal alkynes to α-keto esters, Angew. Chem., Int. Ed., 2017, 56, 8805–8808 CrossRef CAS PubMed; (f) M. C. Schwarzer, A. Fujioka, T. Ishii, H. Ohmiya, S. Mori and M. Sawamura, Enantiocontrol by assembled attractive interactions in copper-catalyzed asymmetric direct alkynylation of α-ketoesters with terminal alkynes: OH⋯O/sp3-CH⋯O two-point hydrogen bonding combined with dispersive attractions, Chem. Sci., 2018, 9, 3484–3493 RSC.
- For examples of terminal alkyne asymmetric addition to acyl silanes, see: (a) P. Smirnov, J. Mathew, A. Nijs, E. Katan, M. Karni, C. Bolm, Y. Apeloig and I. Marek, One-pot zinc-promoted asymmetric alkynylation/Brook-type rearrangement/ene-allene cyclization: highly selective formation of three new bonds and two stereocenters in acyclic systems, Angew. Chem., Int. Ed., 2013, 52, 13717–13721 CrossRef CAS PubMed; (b) P. Smirnov, E. Katan, J. Mathew, A. Kostenko, M. Karni, A. Nijs, C. Bolm, Y. Apeloig and I. Marek, Formation of three new bonds and two stereocenters in acyclic systems by zinc-mediated enantioselective alkynylation of acylsilanes, Brook rearrangement, and ene-allene carbocyclization reactions, J. Org. Chem., 2014, 79, 12122–12135 CrossRef CAS PubMed.
- For examples of enantioselective addition to ynones, see: (a) M. Yus, D. J. Ramón and O. Prieto, Highly enantioselective addition of dialkylzinc reagents to ketones promoted by titanium tetraisopropoxide, Tetrahedron: Asymmetry, 2002, 13, 2291–2293 CrossRef CAS; (b) S. E. Denmark and Y. Fan, Catalytic, enantioselective Aldol additions to ketones, J. Am. Chem. Soc., 2002, 124, 4233–4235 CrossRef CAS PubMed; (c) S. Lou, P. N. Moquist and S. E. Schaus, Asymmetric allylboration of ketones catalyzed by chiral diols, J. Am. Chem. Soc., 2006, 128, 12660–12661 CrossRef CAS PubMed; (d) D. K. Friel, M. L. Snapper and A. H. Hoveyda, Aluminum-catalyzed asymmetric alkylations of pyridyl-substituted alkynyl ketones with dialkylzinc reagents, J. Am. Chem. Soc., 2008, 130, 9942–9951 CrossRef CAS PubMed; (e) H. Kawai, K. Tachi, E. Tokunaga, M. Shiro and N. Shibata, Cinchona alkaloid-catalyzed asymmetric trifluoromethylation of alkynyl ketones with trimethylsilyl trifluoromethane, Org. Lett., 2010, 12, 5104–5107 CrossRef CAS PubMed; (f) J. M. García, J. M. Odriozola, J. Razkin, I. Lapuerta, A. Odriozola, I. Urruzuno, S. Vera, M. Oiarbide and C. Palomo, Catalytic enantioselective quick route to Aldol-tethered 1,6- and 1,7-enynes from ω-unsaturated aldehydes, Chem. – Eur. J., 2014, 20, 15543–15554 CrossRef PubMed; (g) E. Sánchez-Díez, M. Fernández, U. Uria, E. Reyes, L. Carrillo and J. L. Vicario, Enantioselective synthesis of tertiary propargylic alcohols under N-heterocyclic carbene catalysis, Chem. – Eur. J., 2015, 21, 8384–8388 CrossRef PubMed; (h) H. Noda, F. Amemiya, K. Weidner, N. Kumagai and M. Shibasaki, Catalytic asymmetric synthesis of CF3-substituted tertiary propargylic alcohols via direct Aldol reaction of α-N3 amide, Chem. Sci., 2017, 8, 3260–3269 RSC; (i) F. W. van der Mei, C. Qin, R. J. Morrison and A. H. Hoveyda, Practical, broadly applicable, α-selective, Z-selective, diastereoselective, and enantioselective addition of allylboron compounds to mono-, di-, tri-, and polyfluoroalkyl ketones, J. Am. Chem. Soc., 2017, 139, 9053–9065 CrossRef CAS PubMed.
- For examples of kinetic resolution of racemic tertiary propargylic alcohols, for enzyme catalysis, see: (a) S. Bartsch, R. Kourist and U. T. Bornscheuer, Complete inversion of enantioselectivity towards acetylated tertiary alcohols by a double mutant of a bacillus subtilis esterase, Angew. Chem., Int. Ed., 2008, 47, 1508–1511 CrossRef PubMed; For transition metal catalysis, see: (b) Z. Li, V. Boyarskikh, J. H. Hansen, J. Autschbach, D. G. Musaev and H. M. L. Davies, Scope and mechanistic analysis of the enantioselective synthesis of allenes by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and propargylic alcohols, J. Am. Chem. Soc., 2012, 134, 15497–15504 CrossRef CAS PubMed; (c) J. Seliger, X. Dong and M. Oestreich, Kinetic resolution of tertiary propargylic alcohols by enantioselective Cu–H-catalyzed Si–O coupling, Angew. Chem., Int. Ed., 2019, 58, 1970–1974 CrossRef CAS PubMed; (d) R. Mao, Y. Zhao, X. Zhu, F. Wang, W.-Q. Deng and X. Li, Rhodium-catalyzed and chiral zinc carboxylate-assisted allenylation of benzamides via kinetic resolution, Org. Lett., 2021, 23, 7038–7043 CrossRef CAS; (e) K. Liao, Y. Gong, R.-Y. Zhu, C. Wang, F. Zhou and J. Zhou, Highly enantioselective CuAAC of functional tertiary alcohols featuring an ethynyl group and their kinetic resolution, Angew. Chem., Int. Ed., 2021, 60, 8488–8493 CrossRef CAS; For organocatalysis, see: (f) S. Niu, H. Zhang, W. Xu, P. R. Bagdi, G. Zhang, J. Liu, S. Yang and X. Fang, Access to enantioenriched compounds bearing challenging tetrasubstituted stereocenters via kinetic resolution of auxiliary adjacent alcohols, Nat. Commun., 2021, 12, 3735 CrossRef CAS PubMed; (g) T. Desrues, X. Liu, J.-M. Pons, V. Monnier, J.-A. Amalian, L. Charles, A. Quintard and C. Bressy, Indirect tertiary alcohol enantiocontrol by acylative organocatalytic kinetic resolution, Org. Lett., 2021, 23, 4332–4336 CrossRef CAS PubMed; (h) G. Wang, L. Li, Y. Jiang, X. Zhao, X. Ban, T. Shao, Y. Yin and Z. Jiang, Kinetic resolution of azaarylethynyl tertiary alcohols by chiral Brønsted acid catalysed phosphine-mediated deoxygenation, Angew. Chem., Int. Ed., 2023, 62, e202214838 CrossRef CAS PubMed; (i) S. Xie, X. Gao, F. Zhou, H. Wu and J. Zhou, Enantioselective carboxylative cyclization of propargylic alcohol with carbon dioxide under mild conditions, Chin. Chem. Lett., 2020, 31, 324–328 CrossRef CAS.
- (a) R. Zimmer, C. U. Dinesh, E. Nandanan and F. A. Khan, Palladium-catalyzed reactions of allenes, Chem. Rev., 2000, 100, 3067–3125 CrossRef CAS PubMed; (b) S. Ma, Transition metal-catalyzed/mediated reaction of allenes with a nucleophilic functionality connected to the α-carbon atom, Acc. Chem. Res., 2003, 36, 701–712 CrossRef CAS PubMed; (c) S. Ma, Some typical advances in the synthetic applications of allenes, Chem. Rev., 2005, 105, 2829–2871 CrossRef PubMed; (d) S. Ma, Electrophilic addition and cyclization reactions of allenes, Acc. Chem. Res., 2009, 42, 1679–1688 CrossRef CAS PubMed; (e) S. Yu and S. Ma, Allenes in catalytic asymmetric synthesis and natural product syntheses, Angew. Chem., Int. Ed., 2012, 51, 3074–3112 CrossRef CAS; (f) P. Rivera-Fuentes and F. Diederich, Allenes in molecular materials, Angew. Chem., Int. Ed., 2012, 51, 2818–2828 CrossRef CAS PubMed; (g) J. Ye and S. Ma, Palladium-catalyzed cyclization reactions of allenes in the presence of unsaturated carbon-carbon bonds, Acc. Chem. Res., 2014, 47, 989–1000 CrossRef CAS PubMed; (h) M. P. Muñoz, Silver and platinum-catalysed addition of O-H and N-H bonds to allenes, Chem. Soc. Rev., 2014, 43, 3164–3183 RSC; (i) J.-Y. Wang, W.-J. Hao, S.-J. Tu and B. Jiang, Engaging yne-allenes in cycloaddition reactions: recent developments, Chin. J. Chem., 2022, 40, 1224–1242 CrossRef CAS.
- Selected reviews for the synthesis of axially chiral allenes: (a) S. Yu and S. Ma, How easy are the syntheses of allenes?, Chem. Commun., 2011, 47, 5384–5418 RSC; (b) W.-D. Chu, Y. Zhang and J. Wang, Recent advances in catalytic asymmetric synthesis of allenes, Catal. Sci. Technol., 2017, 7, 4570–4579 RSC; (c) W. Xiao and J. Wu, Recent advances in the metal-catalyzed asymmetric synthesis of chiral allenes, Org. Chem. Front., 2022, 9, 5053–5073 RSC; (d) X. Wang, X. Chen, W. Lin, P. Li and W. Li, Recent advances in organocatalytic enantioselective synthesis of axially chiral allenes, Adv. Synth. Catal., 2022, 364, 1212–1222 CrossRef CAS; (e) T. T. Nguyen, Organocatalytic synthesis of axially chiral tetrasubstituted allenes, Org. Biomol. Chem., 2023, 21, 252–272 RSC; For asymmetric functionalization of trisubstituted allenes, see: (f) T. Hashimoto, K. Sakata, F. Tamakuni, M. J. Dutton and K. Maruoka, Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes, Nat. Chem., 2013, 5, 240–244 CrossRef CAS PubMed; (g) C. T. Mbofana and S. J. Miller, Diastereo- and enantioselective addition of anilide-functionalized allenoates to N-acylimines catalyzed by a pyridylalanine-based peptide, J. Am. Chem. Soc., 2014, 136, 3285–3292 CrossRef CAS PubMed; (h) G. Wang, X. Liu, Y. Chen, J. Yang, J. Li, L. Lin and X. Feng, Diastereoselective and enantioselective alleno-Aldol reaction of allenoates with isatins to synthesis of carbinol allenoates catalyzed by gold, ACS Catal., 2016, 6, 2482–2486 CrossRef CAS; (i) Y. Hu, W. Shi, B. Zheng, J. Liao, W. Wang, Y. Wu and H. Guo, Organocatalytic asymmetric C(sp2)-H allylic alkylation: enantioselective synthesis of tetrasubstituted allenoates, Angew. Chem., Int. Ed., 2020, 59, 19820–19824 CrossRef CAS PubMed; For asymmetric 1,4-functionalization of 1,3-enynes, see: (j) A. Tap, A. Blond, V. N. Wakchaure and B. List, Chiral allenes via alkynylogous Mukaiyama Aldol reaction, Angew. Chem., Int. Ed., 2016, 55, 8962–8965 CrossRef CAS PubMed; (k) Y. Liao, X. Yin, X. Wang, W. Yu, D. Fang, L. Hu, M. Wang and J. Liao, Enantioselective synthesis of multisubstituted allenes by cooperative Cu/Pd-catalyzed 1,4-arylboration of 1,3-enynes, Angew. Chem., Int. Ed., 2020, 59, 1176–1180 CrossRef CAS PubMed; (l) Y. Zeng, M.-F. Chiou, X. Zhu, J. Cao, D. Lv, W. Jian, Y. Li, X. Zhang and H. Bao, Copper-catalyzed enantioselective radical 1,4-difunctionalization of 1,3-enynes, J. Am. Chem. Soc., 2020, 142, 18014–18021 CrossRef CAS PubMed; (m) X.-Y. Dong, T.-Y. Zhan, S.-P. Jiang, X.-D. Liu, L. Ye, Z.-L. Li, Q.-S. Gu and X.-Y. Liu, Copper-catalyzed asymmetric coupling of allenyl radicals with terminal alkynes to access tetrasubstituted allenes, Angew. Chem., Int. Ed., 2021, 60, 2160–2164 CrossRef CAS PubMed; (n) H. Huang, H. Zhang, Q. Wang, Y. Sun, L. Su, W. Xu, Y. Ma, S. Kong, G. Zhang and R. Guo, Copper-catalyzed asymmetric 1,4-aryl/alkynylation of 1,3-enynes to access axially chiral tetrasubstituted allenes, ChemCatChem, 2023, 15, e202300697 CrossRef CAS; (o) Y. Zhang, J. Wu, L. Ning, Q. Chen, X. Feng and X. Liu, Enantioselective synthesis of tetrasubstituted allenes via addition/arylation tandem reaction of 2-activated 1,3-enynes, Sci. China: Chem., 2023, 66, 526–533 CrossRef CAS; For chiral phosphoric acid (CPA) catalyzed conjugate addition to quinone methides formed from propargylic alcohols, see: (p) D. Qian, L. Wu, Z. Lin and J. Sun, Organocatalytic synthesis of chiral tetrasubstituted allenes from racemic propargylic alcohols, Nat. Commun., 2017, 8, 567 CrossRef PubMed; (q) P. Zhang, Q. Huang, Y. Cheng, R. Li, P. Li and W. Li, Remote stereocontrolled construction of vicinal axially chiral tetrasubstituted allenes and heteroatom-functionalized quaternary carbon stereocenters, Org. Lett., 2019, 21, 503–507 CrossRef CAS PubMed; (r) W.-R. Zhu, Q. Su, H.-J. Diao, E.-X. Wang, F. Wu, Y.-L. Zhao, J. Weng and G. Lu, Enantioselective dehydrative γ-arylation of α-indolyl propargylic alcohols with phenols: access to chiral tetrasubstituted allenes and naphthopyrans, Org. Lett., 2020, 22, 6873–6878 CrossRef CAS PubMed; Selected other examples: (s) T. Hayashi, N. Tokunaga and K. Inoue, Rhodium-catalyzed asymmetric 1,6-addition of aryltitanates to enynones giving axially chiral allenes, Org. Lett., 2004, 6, 305–307 CrossRef CAS PubMed; (t) M. Hammel and J. Deska, Enantioselective synthesis of axially chiral tetrasubstituted allenes via lipase-catalyzed desymmetrization, Synthesis, 2012, 44, 3789–3796 CrossRef CAS; (u) Y. Tang, J. Xu, J. Yang, L. Lin, X. Feng and X. Liu, Asymmetric three-component reaction for the synthesis of tetrasubstituted allenoates via allenoate-copper intermediates, Chem, 2018, 4, 1658–1672 CrossRef CAS; (v) B. Shi, J.-B. Liu, Z.-T. Wang, L. Wang, Y. Lan, L.-Q. Lu and W.-J. Xiao, Synthesis of chiral endocyclic allenes by palladium-catalyzed asymmetric annulation followed by Cope rearrangement, Angew. Chem., Int. Ed., 2022, 61, e202117215 CrossRef CAS PubMed; (w) J. Wang, W.-F. Zheng, X. Zhang, H. Qian and S. Ma, Stereoselectivity control in Rh-catalyzed β-OH elimination for chiral allene formation, Nat. Commun., 2023, 14, 7399 CrossRef PubMed.
- Selected examples for heteroatom-substituted chiral tetrasubstituted allenes: (a) M. Wang, Z.-L. Liu, X. Zhang, P.-P. Tian, Y.-H. Xu and T.-P. Loh, Synthesis of highly substituted racemic and enantioenriched allenylsilanes via copper-catalyzed hydrosilylation of (Z,)-2-alken-4-ynoates with silylboronate, J. Am. Chem. Soc., 2015, 137, 14830–14833 CrossRef CAS PubMed; (b) J. Yang, Z. Wang, Z. He, G. Li, L. Hong, W. Sun and R. Wang, Organocatalytic enantioselective synthesis of tetrasubstituted α-amino allenoates by dearomative γ-addition of 2,3-disubstituted indoles to β,γ-alkynyl-α-imino esters, Angew. Chem., Int. Ed., 2020, 59, 642–647 CrossRef CAS PubMed; (c) A. G. Woldegiorgis, Z. Han and X. Lin, Organocatalytic asymmetric dearomatization reaction for the synthesis of axial chiral allene-derived naphthalenones bearing quaternary stereocenters, Org. Lett., 2021, 23, 6606–6611 CrossRef CAS PubMed; (d) F. Li, S. Liang, Y. Luan, X. Chen, H. Zhao, A. Huang, P. Li and W. Li, Organocatalytic regio-, diastereo- and enantioselective γ-additions of isoxazol-5(4H)-ones to β,γ-alkynyl-α-imino esters for the synthesis of axially chiral tetrasubstituted α-amino allenoates, Org. Chem. Front., 2021, 8, 1243–1248 RSC; (e) C. Sheng, Z. Ling, J. Xiao, K. Yang, F. Xie, S. Ma and W. Zhang, Enantio- and diastereoselective synthesis of chiral tetrasubstituted α-amino allenoates bearing a vicinal all-carbon quaternary stereocenter with dual-copper-catalysis, Angew. Chem., Int. Ed., 2023, 62, e202305680 CrossRef PubMed; (f) T. J. O'Connor, B. K. Mai, J. Nafie, P. Liu and F. D. Toste, Generation of axially chiral fluoroallenes through a copper-catalyzed enantioselective β-fluoride elimination, J. Am. Chem. Soc., 2021, 143, 13759–13768 CrossRef PubMed; (g) J. S. Ng and T. Hayashi, Asymmetric synthesis of fluorinated allenes by rhodium-catalyzed enantioselective alkylation/defluorination of propargyl difluorides with alkylzincs, Angew. Chem., Int. Ed., 2021, 60, 20771–20775 CrossRef CAS PubMed.
- W.-F. Zheng, W. Zhang, C. Huang, P. Wu, H. Qian, L. Wang, Y.-L. Guo and S. Ma, Tetrasubstituted allenes via the palladium-catalysed kinetic resolution of propargylic alcohols using a supporting ligand, Nat. Catal., 2019, 2, 997–1005 CrossRef CAS.
- J. Wang, W. Zhang, P. Wu, C. Huang, Y. Zheng, W.-F. Zheng, H. Qian and S. Ma, Chiral tertiary propargylic alcohols via Pd-catalyzed carboxylative kinetic resolution, Org. Chem. Front., 2020, 7, 3907–3911 RSC.
- W. Wang, H. Qian and S. Ma, Rh-catalyzed reaction of propargylic alcohols with aryl boronic acids-switch from β-OH elimination to protodemetalation, Chin. J. Chem., 2020, 38, 331–345 CrossRef CAS.
- W. Zhang and S. Ma, Palladium/H+-cocatalyzed kinetic resolution of tertiary propargylic alcohols, Chem. Commun., 2018, 54, 6064–6067 RSC.
- V. Laserna, C. J. Rojas and T. D. Sheppard, Gold-catalyzed hydrophenoxylation of propargylic alcohols and amines: synthesis of phenyl enol ethers, Org. Lett., 2019, 21, 4443–4447 CrossRef CAS PubMed.
- A. L. Moure, R. G. Arrayás, D. J. Cárdenas, I. Alonso and J. C. Carretero, Regiocontrolled CuI-catalyzed borylation of propargylic-functionalized internal alkynes, J. Am. Chem. Soc., 2012, 134, 7219–7222 CrossRef CAS PubMed.
- S. Ma, Z. Yu and S. Wu, CuCl-catalyzed cycloisomerization reaction of 1,2-allenyl carboxylic acids. A cost-effective synthesis of β-unsubstituted butenolides, Tetrahedron, 2001, 57, 1585–1588 CrossRef CAS.
- N. Miyaura, T. Yanagi and A. Suzuki, The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases, Synth. Commun., 1981, 11, 513–519 CrossRef CAS.
- S. Ma and Z. Yu, Pd(II)-catalyzed coupling cyclization of 2,3-allenoic acids with allylic halides. An efficient methodology for the synthesis of β-allylic butenolides, J. Org. Chem., 2003, 68, 6149–6152 CrossRef CAS PubMed.
- K. P. Melnykov, D. S. Granat, D. M. Volochnyuk, S. V. Ryabukhin and O. O. Grygorenko, Multigram synthesis of C4/C5 3,3-difluorocyclobutyl-substituted building blocks, Synthesis, 2018, 50, 4949–4957 CrossRef CAS.
- J.-T. Zhang, H.-Y. Wang, W. Zhu, T.-T. Cai and Y.-L. Guo, Solvent-assisted electrospray ionization for direct analysis of various compounds (complex) from low/nonpolar solvents and eluents, Anal. Chem., 2014, 86, 8937–8942 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qo00082j |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2024 |