Open Access Article
Open Access ArticleSynthesis, conformational properties, and molecular recognition abilities of novel prism[5]arenes with branched and bulky alkyl groups†
Rocco
Del Regno
 a,
Paolo
Della Sala
a,
Paolo
Della Sala
 a,
Ivan
Vollono
a,
Ivan
Vollono
 a,
Carmen
Talotta
a,
Carmen
Talotta
 a,
Placido
Neri
a,
Placido
Neri
 a,
Neal
Hickey
a,
Neal
Hickey
 b,
Siddharth
Joshi
b,
Siddharth
Joshi
 b,
Silvano
Geremia
b,
Silvano
Geremia
 *b and
Carmine
Gaeta
*b and
Carmine
Gaeta
 *a
*a
aDipartimento di Chimica e Biologia “A. Zambelli”. Università di Salerno, Via Giovanni Paolo II, I-84084, Fisciano, Salerno, Italy. E-mail: cgaeta@unisa.it
bCentro di Eccellenza in Biocristallografia, Dipartimento di Scienze Chimiche e Farmaceutiche, Università di Trieste, Via L. Giorgieri 1, I-34127, Trieste, Italy. E-mail: sgeremia@units.it
First published on 21st February 2024
Abstract
The direct macrocyclization to prism[5]arenes of 2,6-dialkoxynaphthalenes with branched and bulky alkyl groups has been obtained in good yields in the presence of 1,4-dihexyl-DABCO template. These novel prism[5]arenes exhibit typical D5-symmetry and DFT calculations indicate that the homochiral all-pS (all-pR) conformation is the most stable of all the possible conformations. Prism[5]arenes crystallize as racemic mixtures (all-pS/all-pR) in centrosymmetric space groups. The eight crystal structures show C2 point symmetry of the macrorings, with a larger opening of the cavity observed for PrS[5]iBu, PrS[5]MeCy and the β-form of PrS[5]PrCy. The dynamic stereochemical inversion (from pR to pS and vice versa) of prism[5]arenes was examined by 1H VT NMR experiments. The racemization of prism[5]arene derivatives occurs by through the-annulus-rotation of the naphthalene units. With increasing length of linear alkyl substituents from C2 to C9, the chains tend to pack at both rims, thereby diminishing the conformational freedom of the naphthalene units. The presence of branched alkyl groups at both rims of the prism[5]arenes increases the energy barrier to racemization and consequently lowers the rate of the racemization process. Prism[5]arenes bearing branched or bulky alkyl groups at both rims can form endo-cavity complexes with 1,4-dihexyl-DABCO 22+ and piperazonium 32+ guests. Two hosts with branched alkyl groups, PrS[5]iPr and PrS[5]iBu, show guest binding affinities higher than those calculated for the analogous linear PrS[5]nPr and PrS[5]nBu prism[5]arenes. This result can be justified on the basis of the greater conformational rigidity and preorganization of prism[5]arenes bearing branched groups.
Introduction
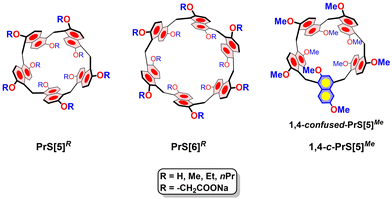
The first generation of macrocyclic hosts, such as cryptands,1 crown ethers,2 and spherands,3 were introduced in the 1960s and 1970s by Lehn, Pedersen and Cram. These hosts featured cavities decorated with oxygen atoms and were specifically designed to host spherical alkali-cations. Subsequently, between the 1980s and 1990s, calixarenes,4 resorcinarenes,5 and cucurbiturils6 gained significant popularity due to their facile chemical functionalisation and conformational versatility. In contrast to the first generation of hosts, calixarenes and resorcinarenes exhibit large aromatic cavities capable of hosting small organic molecules.4,5 In 2008, Ogoshi introduced pillararenes,7 macrocycles formed by 1,4-dialkoxybenzene units bridged by methylene groups, marking the advent of the third generation of macrocyclic hosts. Unlike calix[4]arene, which adopts a cone-shaped structure, pillararenes feature a distinctive pillar-shaped cavity. The rigid structure of pillararenes, along with their unique supramolecular properties, paved the way for the design of a plethora of novel macrocyclic structures, including biphenarenes,8 oxatubarenes,9 saucerarenes,10 pagodarenes,11 calix[2]naphth[2]arene,12 and naphthotubes,13 all characterized by biomimetic deep aromatic cavities.In this regard, in 2020 we reported a novel class of macrocyclic hosts named prism[n]arenes14PrS[n]R (n = 5 and 6, in Fig. 1). These prismarenes are constituted by 1,5-methylene bridged 2,6-dialkoxynaphthalene units (Fig. 1). Prismarenes exhibit a deep π-electron rich aromatic cavity14–19 and can form endo-cavity complexes with ammonium guests, both in organic14–17 and aqueous media. These complexes are stabilized by cation⋯π and C–H⋯π interactions.18
As a result of these appealing properties, prismarenes have attracted significant interest in the scientific community over the last three years.20–26 An intriguing aspect of prismarenes pertains to their chirality. Like pillararenes,27–29 prism[n]arenes exhibit planar chirality (Fig. 2). The pS and pR conformational isomers of the permethylated prismarenes can undergo interconversion via oxygen-through-the-annulus passage of the naphthalene units, a process observed to be fast on the NMR time scale (Fig. 2).24 To isolate pS- and pR-enantiomeric forms of prism[5]arenes, it becomes necessary to impede the rotational motion of the naphthalene units. Ogoshi and coworkers demonstrated27,28 that the presence of bulky and branched groups on both rims of pillar[5]arene is essential to prevent racemization.27 Their findings revealed that the size and structure of alkyl chains on the rims of pillararenes play a crucial role in this regard. More recently, the chiral and conformational features of prism[5]arenes have been exploited for the chiral recognition of aminoacids.24 The complexation with chiral guests resulted in strong circular dichroism (CD) signals at the absorption band of prism[5]arenes. Notably, the CD signals induced by the chiral complexation varied with the length of the alkyl chains on the prism[5]arene rims.24
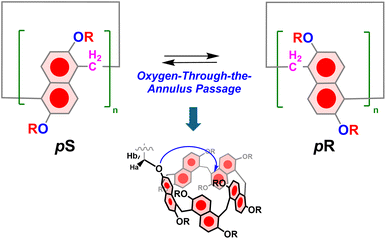 | ||
| Fig. 2 Planar chirality of prismarenes and racemization process by oxygen-through-the-annulus passage of naphthalene units. | ||
Regarding the synthesis of prismarenes, we demonstrated that the direct macrocyclization of 2,6-dimethoxynaphthalene in the presence of 1,4-dihexyl-DABCO as a template yielded PrS[5]Me (Fig. 1) as the major product (47%).14 In contrast, when attempting direct macrocyclization starting from 2,6-diethoxynaphthalene or 2,6-dipropoxynaphthalene in the presence of 1,4-dihexyl-DABCO 22+, a mixture of PrS[6]R/PrS[5]R (R = Et or nPr) was formed.15 Differently, in the absence of 1,4-dihexyl-DABCO 22+, the direct macrocyclization delivered the hexamer in very high yields (60–80%) and in short reaction times (30–40 min) in various solvents (cyclohexane, toluene, decaline, chlorocyclohexane).15 Thus, we concluded that the direct macrocyclization of 2,6-diethoxy or 2,6-dipropoxynaphthalene was driven by an intramolecular thermodynamic self-templating effect,15 resulting from the self-filling of the internal cavity of the hexamer with ethyl or propyl chains. Both PrS[6]Et and PrS[6]nPr assumed, in solution and in the solid state, a conformation in which four alkyl chains occupied the cavity of the macrocycle.15 This self-templating effect drives the direct macrocyclization toward the hexamer, even in the presence of 22+. These considerations clearly demonstrate that the length of the alkyl chain plays a pivotal role in prismarene synthesis. Consequently, the question arises as to whether direct macrocyclization to prism[5]arene is again favoured in the presence of 1,4-dihexyl-DABCO 22+ when starting from monomers bearing linear C4–C9 alkyl chains (1a–e in Scheme 1) or α–δ branched (1f–h) groups or even more bulky cyclic substituents (1j–q).
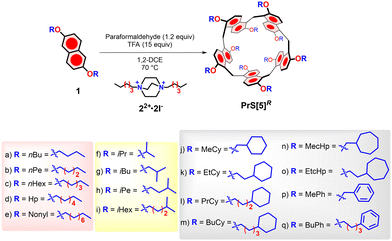 | ||
| Scheme 1 Direct macrocyclization of 2,6-dialkoxynaphthalene monomers to give prism[5]arene derivatives: 1,2-DCE (5 mM), TFA (15 equiv.), paraformaldehyde (1.2 equiv.), 70 °C, 22+·2I− (1.0 equiv.). | ||
In the present study, we report on the direct macrocyclization of 2,6-dialkoxynaphthalenes with alkyl groups of different lengths and structures, resulting in novel prism[5]arene derivatives. Additionally, their conformational properties and molecular recognition abilities have been investigated through NMR studies and X-ray crystallography.
Results and discussion
Synthesis of prism[5]arenes
Initially, we investigated the direct macrocyclization of 2,6-dipentoxynaphthalenes, 1b, to prism[5]arene (Scheme 1). The reaction utilized 1,4-dihexyl-DABCO 22+ as a template and 1,2-dichloroethane as the solvent. Under the conditions previously reported by us,14,15 a mixture of 2,6-dipentoxynaphthalene 1b, 1,4-dihexyl-DABCO 22+ (1.0 equiv.), paraformaldehyde (1.2 equiv.) and trifluoroacetic acid (TFA, 15 equiv.) was stirred in 1,2-dichloroethane (1,2-DCE) at 70 °C for 22 h. After purification by column chromatography, the isolated product was identified as 2,6-bis(pentoxy)prism[5]arene, PrS[5]nPe, in a yield of 25%. Notably, extending the reaction time to 72 h at 70 °C increased the yield to 35%, and further optimization resulted in a yield of 45% after 120 hours. The structure of PrS[5]nPe was confirmed through 1D and 2D NMR analysis (ESI†), high-resolution mass spectrometry and single crystal X-ray diffraction (ESI† and vide infra). The 1D and 2D NMR spectra (CD2Cl2, 298 K, 600 MHz) were consistent with the D5 symmetry previously observed for PrS[5]Me. Specifically, the 1H NMR spectrum revealed an AX system at 8.31 and 6.95 ppm, corresponding to the aromatic H-atoms of the macrocycle, with a singlet at 4.68 ppm attributable to the methylene-bridges. Encouraged by these results, we extended our investigations to the systematic study of direct macrocyclization of 2,6-dialkoxynaphthalene monomers as a function of alkyl chain length, adding butyl 1a, hexyl 1c, heptyl 1d, and nonyl 1e.As indicated in Table 1 (entries 1–4), the direct macrocyclization of 1a, 1c, 1d and 1e to prism[5]arenes occurred in good yields in the presence of the template 1,4-dihexyl-DABCO 22+ cation. The NMR spectral features of all these prism[5]arenes are consistent with the D5 symmetry previously observed for other peralkylated prism[5]arene derivatives. Subsequently, a series of experiments were conducted to investigate the effects of branched alkyl groups on the efficiency of the macrocyclization outlined in Scheme 1 (Table 1, entries 6–15). Starting with 2,6-diisopropoxynaphthalene 1f, the 2,6-bis(isopropoxy)prism[5]arene, PrS[5]iPr, was obtained in 48% yield after 24 h. In this case, the formation of the hexamer 2,6-bis(isopropoxy)prism[6]arene, PrS[6]iPr,17 was significant, at a 36% yield even in presence of the template 1,4-dihexyl-DABCO 22+ cation. In fact, the PrS[6]iPr was formed as major product (60%) after 120 h.17 As recently reported by us,17 the isopropyl groups can stabilize the cuboid D2-conformation of the 2,6-bis(isopropoxy)prism[6]arene, PrS[6]iPr, through a self-filling effect.17 Starting with 2,6-diisobutoxynaphthalene 1g, a PrS[5]iBu/PrS[6]iBu ratio of 3/1 was observed with an overall lower yield. The formation of hexameric prismarene may be attributed to a competitive self-templated effect of the isobutyl chains. However, the situation becomes more complex in this case, as indicated by the crystallographic analysis, revealing that two methyl groups of the isobutyl chains are deeply inserted into the cavity of the pentameric prismarene (see below). The macrocyclization to prism[5]arene was selective with 2,6-diisopentoxynaphthalene 1h (Table 1, entry 8) and 2,6-diisohexyloxynaphthalene 1i (Table 1, entry 9), resulting in the formation of the respective prism[5]arene derivatives in 37% and 43% yields, respectively, with no evidence of hexamer in their crude reaction mixtures. Longer isopentyl and isohexyl chains are hypothesized to be less effective in filling the internal cavity of the prism[6]arene, allowing the template effect of 22+ to prevail. Interestingly, the direct macrocyclization of 1j, bearing cyclohexylmethyl groups, delivered the prism[5]arene PrS[5]MeCy in a 25% yield (Table 1, entry 10), while higher yields were obtained after the direct macrocyclization of 1k (Table 1, entry 11) and 1l (Table 1, entry 12) bearing cyclohexylethyl (44%) and cyclohexylpropyl (43%) groups, respectively. These results indicate that, as the cyclohexyl unit gets closer to the rims, the yield of macrocyclization drops significantly (1k, 44% → 1j, 25%). Analogous results were observed for the direct macrocyclization of 1n (R = cycloheptylmethyl, Table 1 entry 14) and 1o (R = cycloheptylethyl, Table 1, entry 15), which gave PrS[5]MecHp and PrS[5]EtcHp in 30% and 45% yields, respectively. Significantly, the direct macrocyclization of 1p leads to the formation of 2,6-bis(benzyloxy)prism[5]arene PrS[5]MePh in 20% yield (Table 1, entry 16). This prismarene can be considered as a useful precursor of perhydroxylated prism[5]arene.19 In all the reactions explored in this study (Table 1), the formation of prismarenes with naphthalene units linked at positions 1,4 (confused-prism[5]arenes) was not observed. Typically, the confused-prism[5]arene skeleton adopts a conformation where the 1,4-bridged naphthalene ring is oriented inside the cavity. It is likely that in the presence of long and/or branched alkyl groups, confused-prism[5]arenes cannot be formed, primarily due to the significant steric hindrance caused by these groups within the cavity. Interestingly, the prism[5]arenes bearing long C6–C9 alkyl chains or bulky α−ε branched alkyl groups at both rims, exhibit typical D5 NMR-patterns. Similar results were observed for prism[5]arene derivatives bearing cyclic units at both rims. Specifically, percyclohexylmethyl-prism[5]arene PrS[5]MeCy shows an aromatic AX system at 8.24 and 6.92 ppm with a Δδ value of 1.32 ppm, a value significantly higher than that observed for the 2,6-bis(methoxy)prism[5]arene PrS[5]Me, which has a Δδ of 1.04 ppm. Similarly, a wide aromatic spacing of 1.44 ppm was observed between the doublets of the 2,6-bis(isobutoxy)prism[5]arene PrS[5]iBu. Prism[5]arenes bearing larger cycloheptylmethyl and cycloheptylethyl groups, show aromatic AX systems with a Δδ value of 1.30 and 1.35 ppm, respectively. Also noteworthy is the Δδ value of 1.41 ppm observed between the aromatic doublets of 2,6-bis(cyclohexylpropyl)prism[5]arene PrS[5]PrCy. As previously reported by us,15 an aromatic Δδ value of 1.30–1.50 ppm suggests that the prism[5]arene skeleton adopts an open conformation15 in which the aromatic walls exhibit canting angle averaged values close to 90°. Consequently, these results indicate that, in solution, a greater opening of the cavity is observed for PrS[5]iBu, PrS[5]MeCy and PrS[5]PrCy. In this open prismatic conformation, the aromatic H atoms move away from the cavity and experience a down-field shifting with respect to the closed conformation of PrS[5]Me, in which a naphthalene ring is folded inside the cavity.
| Entry | PrS[5]R | R | Time | Yield (%) |
|---|---|---|---|---|
| 1 | PrS[5]nBu | n-Butyl | 120 h | 46 |
| 2 | PrS[5]nPe | n-Pentyl | 120 h | 45 |
| 3 | PrS[5]nHex | n-Hexyl | 120 h | 48 |
| 4 | PrS[5]Hp | n-Heptyl | 120 h | 35 |
| 5 | PrS[5]Nonyl | n-Nonyl | 120 h | 46 |
| 6 | PrS[5]iPr | Isopropyl | 24 h | 48 |
| 7 | PrS[5]iBu | Isobutyl | 120 h | 30 |
| 8 | PrS[5]iPe | Isopentyl | 120 h | 37 |
| 9 | PrS[5]iHex | Isohexyl | 120 h | 43 |
| 10 | PrS[5]MeCy | Cyclohexylmethyl | 120 h | 25 |
| 11 | PrS[5]EtCy | Cyclohexylethyl | 120 h | 44 |
| 12 | PrS[5]PrCy | Cyclohexylpropyl | 120 h | 43 |
| 13 | PrS[5]BuCy | Cyclohexylbutyl | 120 h | 12 |
| 14 | PrS[5]MecHp | Cycloheptylmethyl | 120 h | 30 |
| 15 | PrS[5]EtcHp | Cycloheptylethyl | 120 h | 45 |
| 16 | PrS[5]MePh | Benzyl | 120 h | 20 |
| 17 | PrS[5]BuPh | Phenylbutyl | 120 h | 27 |
Structural properties of prism[5]arenes in the solid state: the role of the alkyl chains
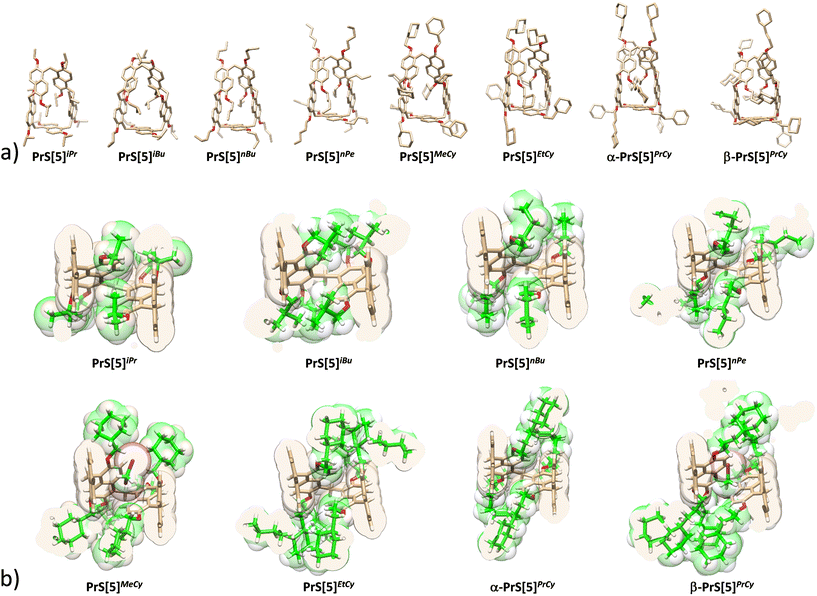
The crystallographic structures of PrS[5]iPr, PrS[5]nBu, PrS[5]iBu, PrS[5]nPe, PrS[5]MeCy, PrS[5]EtCy, and PrS[5]PrCy (α- and β- forms) were determined (Fig. 3a). Details of data collection, structure refinements and geometrical features are reported in the ESI.† All molecules crystallize as racemic mixtures of inherent chiral pairs in centrosymmetric space groups. All prismarene molecules exhibit a similar macrocycle conformation, analogous to the one observed for PrS[5]Me, PrS[5]Et and PrS[5]nPr.15 The PrS[5]iBu, PrS[5]EtCy and the α form of PrS[5]PrCy show a crystallographic twofold axis passing through the methylene bridge connecting the C and C′ rings and the middle of the opposite A naphthalene moiety (Fig. S135†). The crystal structures of the other molecules show a pseudo C2 point symmetry for the PrS[5]R scaffold. The canting angles reported in Table S9† illustrate this situation: the dihedral angles of the B/B′ and C/C′ couples (see Fig. S135†) are supplementary angles with mean values of 68(2)°/113(2)° for B/B′ and 136(4)°/44(5)° for C/C′; while the A angle is approximately 90° with a mean value of 89(1)°. The standard deviations reported in parentheses indicate relatively larger variability in the conformation of the C/C′ couple with the most fixed position for the A naphthalene. In agreement with NMR studies in solution, a greater opening of the cavity is observed for PrS[5]iBu, PrS[5]MeCy and the β-form of PrS[5]PrCy, where the C/C′ angles decrease and increase by about 6°, respectively, in comparison to the other structures (Table S9†). This greater opening is also reflected in the C/C′ dihedral angles. A mean value of 60(2)° is observed for PrS[5]iBu, PrS[5]MeCy and β-PrS[5]PrCy, while a mean value of 49(1)° is observed for all the other structures. The opening observed for PrS[5]MeCy and β-PrS[5]PrCy can be attributed to the presence of a dichloromethane solvent molecule hosted in the prismarene cavity (Fig. 3b). In the case of PrS[5]iBu, a self-filling of two symmetry-related methyl groups of the alkoxy chains, one from each C and C′ naphthalene moiety, is observed (Fig. 3b). The analogous PrS[5]iPr molecule shows a more closed conformation, with the self-filling of only one methyl group (Fig. 3b). These structural differences in intramolecular interactions could be connected to the differences observed for the product distribution in the synthesis. The other structures all show comparable closed conformations, with the alkoxy chains outside the cavity (Fig. 3b).Conformational dynamics of prism[5]arenes PrS[5]R
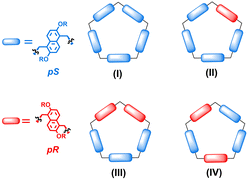
Analogously to pillararene macrocycles, prism[5]arenes exhibit planar chirality (Fig. 2 and 4). The pentamers PrS[5]R can adopt 8 conformations of different planar chirality corresponding to 4 enantiomeric pairs (I–IV in Fig. 4).As reported for pillararenes,27–29 the chirality in these conformational isomers of prism[5]arenes can be described using the pR and pS nomenclature (Fig. 4).
The stability of the conformational isomers of peralkylated prism[5]arene derivatives was evaluated by DFT calculations at the B97D3/SVP/SVPFIT level of theory (ESI†).30 The calculations for PrS[5]nPr,15 indicate conformation I, all-pS (all-pR) (Fig. 4), as more stable than all the other possible conformations II, III, and IV by 11.8, 14.7, and 8.1 kcal mol−1 (ESI†), respectively.
Analogous results were obtained for the percyclohexylethyl-prism[5]arene PrS[5]EtCy: DFT calculations indicate conformation I as more stable compared to conformations II, III and IV, by 22.6, 22.9 and 4.4 kcal mol−1, respectively. Similarly, for PrS[5]iPr, the all-pS (all-pR) (Fig. 4), was calculated as more stable than II, III, and IV by 10.0, 11.5, and 6.5 kcal mol−1 (ESI†), respectively. The conformational features of the peralkylated prism[5]arene derivatives PrS[5]R in Scheme 1 were investigated by variable temperature 1H NMR experiments. The 1D and 2D NMR spectra (25 °C) of the PrS[5]R derivatives are in agreement with the D5 symmetry of their all-pR (or all-pS) conformation I. The presence of diastereotopic resonances attributable to OCH2 groups in the 1H NMR spectra of 2,6-bis(ethoxy)prism[5]arene (Fig. 5a) and 2,6-bis(propoxy)prism[5]arene at 298 K (300 MHz, Toluene-d8, ESI†) confirms their planar chirality. As reported in literature for pillar[5]arenes,27,29,31 the separation of the OCH2 diastereotopic resonances is a useful probe to study whether racemization of planar chiral macrocycles (Fig. 2 and 5) takes place on the NMR time scale. Racemization processes (Fig. 2) become faster on the NMR time scale as the temperature increases, leading to the coalescence of diastereotopic OCH2 resonances due to the rapidly averaged chemical environment around OCHaHb protons.27,29,31 Above the coalescence temperature (Tc), the –OCH2– proton signals generally converge into a sharp singlet (Fig. 5a and b). The presence of diastereotopic resonances attributable to OCH2 groups of PrS[5]R (R = Et, n-Pr) indicates that the inversion process between their pR and pS enantiomeric forms is slow on the NMR time scale, at room temperature. On heating, the diastereotopic OCH2 signals of PrS[5]Et and PrS[5]nPr coalesced at 338 K (Fig. 5a) and 348 K (Fig. 5b) (in toluene-d8), respectively. From these values, energy barriers32 values of 16.8 and 17.4 kcal mol−1 were calculated for the racemization process of PrS[5]Et and PrS[5]nPr, respectively. Table 2 shows the coalescence temperatures (Tc) and the energy barrier ΔG‡ values for the racemization of prism[5]arene derivatives in Scheme 1. The data in Table 2 clearly indicate an increase in the energy barrier ΔG‡ with the increase of the n-alkyl chain length from C3 to C9 and thus decreasing the conformational freedom of the macrocycles. Thus, 2,6-bis(nonyloxy)prism[5]arene PrS[5]Nonyl showed a broadening of the OCH2 signals at 383 K, in toluene-d8 (ESI†) suggesting that rotations of the 2,6-bis(nonyloxy)naphthalene units still occur more slowly than the NMR time scale at this temperature. It seems likely that, with an increase in the length of alkyl substituents, the linear chains pack on both rims, consequently reducing the conformational freedom of the naphthalene units within these prism[5]arenes. The presence of branched alkyl groups at both rims of the prism[5]arenes increases the energy barrier to racemization (Fig. 2) and consequently lowers the rate of the process. In detail, in 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr the diastereotopic (CH3)aCH(CH3)b signals didn't change their shape even on heating (ESI†). This result clearly indicates that the rotation of the 2,6-bis(isopropoxy)naphthalene units in PrS[5]iPr did not take place on the NMR time scale in the temperature range investigated (298–383 K). In contrast, rotation of 2,6-bis(propoxy)naphthalene units in PrS[5]nPr is considerably faster. On heating, the diastereotopic OCH2 signals of PrS[5]nPr show a coalescence temperature of 348 K (in toluene-d8), and an energy barrier of 17.4 kcal mol−1 to the racemization process. Similar results were observed for PrS[5]iBu and PrS[5]iPe, which show coalescence temperatures of 373 K and energy barriers of 18.9 and 18.3 kcal mol−1, respectively, higher than that observed for PrS[5]nBu and PrS[5]nPe(see Table 2). We have previously shown17 that 2,6-bis(isopropoxy)prism[6]arene PrS[6]iPr exhibits an energy barrier to the racemization process significantly higher than that measured for the isomer 2,6-bis(propoxy)prism[6]arene PrS[6]nPr.15 These results clearly indicate that the presence of branched groups at the rims of prismarene macrocycles increase the energy barrier to the racemization process to a greater extent than the respective isomeric linear groups.
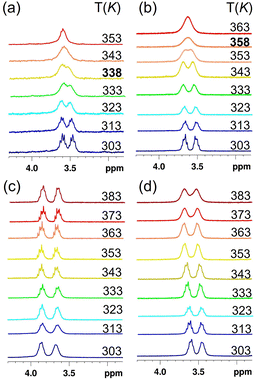 | ||
| Fig. 5 Partial DNMR spectra of OCH2 protons: (a) PrS[5]Et, (b) PrS[5]nPe, (c) PrS[5]EtCy and (d) PrS[5]BuPh in toluene-d8. | ||
| Entry | PrS[5]R | R | T C (K) | ΔG‡ (Kcal mol−1) |
|---|---|---|---|---|
| a A broadening of the OCH2 diastereotopic signals was observed at 383 K. b No evidence of coalescence was observed in the temperature range investigated (298–383 K) in toluene-d8. c Deuterated chlorobenzene was used as solvent due to the accidental isochronous appearance of OCH2 signals in deuterated toluene. In chlorobenzene-d5 no evidence of coalescence was observed in the temperature range investigated (298–383 K). | ||||
| 1 | PrS[5]Et | Ethyl | 338 | 16.8 |
| 2 | PrS[5]nPr | n-Propyl | 348 | 17.4 |
| 3 | PrS[5]nBu | n-Butyl | 353 | 17.8 |
| 4 | PrS[5]nPe | n-Pentyl | 358 | 18.0 |
| 5 | PrS[5]nHex | n-Hexyl | 363 | 18.5 |
| 6 | PrS[5]Hp | n-Heptyl | 373 | 18.6 |
| 7 | PrS[5]Nonyl | n-Nonyl | >19.2 | |
| 8 | PrS[5]iPr | Isopropyl | — | |
| 9 | PrS[5]iBu | Isobutyl | 373 | 18.9 |
| 10 | PrS[5]iPe | Isopentyl | 373 | 18.3 |
| 11 | PrS[5]iHex | Isohexyl | 383 | 19.2 |
| 12 | PrS[5]MeCy | Cyclohexylmethyl | — | |
| 13 | PrS[5]EtCy | Cyclohexylethyl | — | |
| 14 | PrS[5]PrCy | Cyclohexylpropyl | — | |
| 15 | PrS[5]BuCy | Cyclohexylbutyl | — | |
| 16 | PrS[5]MecHp | Cycloheptylmethyl | — | |
| 17 | PrS[5]EtcHp | Cycloheptylethyl | — | |
| 18 | PrS[5]BuPh | Phenylbutyl | — | |
Prompted by these observations, we have investigated the conformational dynamics of prism[5]arenes bearing cyclohexylmethyl (PrS[5]MeCy), cyclohexylethyl (PrS[5]EtCy), cyclohexylpropyl (PrS[5]PrCy), cyclohexylbutyl (PrS[5]BuCy), cycloheptylethyl (PrS[5]EtcHp), and phenylbutyl groups (PrS[5]BuPh) at both rims. In all these cases, the rotation of the respective 2,6-dialkoxynaphthalene units is blocked on the NMR time scale. On heating, the diastereotopic OCH2 signals of these derivatives didn't show any evidence of broadening in the temperature range investigated (298–383 K, Fig. 5c and d).
To gain insights into the aspect of chiral stability, chiral HPLC studies were carried out for percyclohexylethyl (and percyclohexylpropyl) substituted prism[5]arenes (ESI†). Upon injection of PrS[5]EtCy onto a chiral HPLC column, two peaks of equal area were observed in the chromatogram (ESI†). The two enantiomers were collected and reinjected separately. Unfortunately, racemization was again observed after HPLC runs. Analogous results were observed for PrS[5]PrCy. Therefore, the chiral prism[5]arene enantiomers are not stable at room temperature, however the chiral separation was observed. This observation is in agreement with the conclusion that the rotation of the 2,6-dicyclohexylethyloxynaphthalene and 2,6-dicyclohexylpropoxynaphthalene units is blocked on the NMR time scale.
Molecular recognition studies
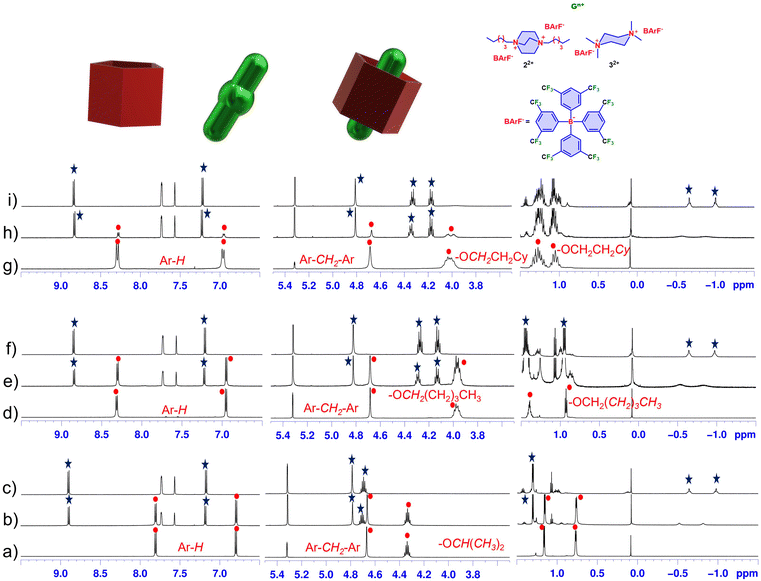
As previously reported by us and others,11–26 prismarenes form endo-cavity supramolecular complexes stabilized by secondary interactions such as cation⋯π, C–H⋯π, van der Waals and the hydrophobic effect. Specifically, the PrS[5]R (R = Et, nPr)15 show a high binding affinity toward 1,4-dihexyl-DABCO 22+ and N,N,N′,N’-tetramethylpiperazonium 32+ as barfate salts (Table 3 and Fig. 6). Based on these results, we have explored the recognition abilities of some prism[5]arenes obtained in this work. Our aim was to study how the presence of long alkyl chains or branched/bulky groups in PrS[5]R can affect the complexation abilities of these new derivatives. When 1,4-dihexyl-DABCO 22+ as barfate salt33,34 was mixed with 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr in CD2Cl2 solution, the 1H NMR spectrum of the host showed significant changes (Fig. 6a–c). The 1H NMR titration experiment in Fig. 6a–c is indicative of an endo-cavity complexation process in which chemical exchange between free and complexed species is slow compared to the NMR time scale. In fact, when PrS[5]iPr and 22+ were mixed in a 1/0.5 ratio in CD2Cl2 solution, the 1H NMR spectrum (Fig. 6b) showed the presence of both, complexed and uncomplexed species. Upon addition of 1 equiv. of 22+ as a barfate salt to a CD2Cl2 solution of PrS[5]iPr the 1H NMR spectrum (Fig. 6c) showed the presence of the 22+@PrS[5]iPr complex alone, while no indication of uncomplexed species was observed. Thus, the signals at negative values of chemical shifts (−0.64 and −0.97 ppm) in Fig. 6c are attributable to 22+ hosted inside the cavity of PrS[5]iPr. In addition, the 1H NMR spectrum of 22+@PrS[5]iPr (Fig. 6c) shows a singlet at 4.79 ppm attributable to ArCH2Ar groups (4.67 ppm for the free host), a signal at 4.70 ppm for the OCH(CH3)2 groups and two diastereotopic doublets at 1.59 and 1.30 ppm attributable to methyl groups of OCH(CH3)2, (1.16 and 0.77 ppm, for the free host). Calculation of the binding constant of the 22+@PrS[5]iPr pseudorotaxane complex, by direct peak integration was not possible as the 1H NMR signals (Fig. 6c) of the host PrS[5]iPr and guest 22+ in the unbound forms were undetectable. Following a procedure previously reported by us and other groups, binding constants assessment was carried out by means of a competition experiment in which 1 equiv. of 22+ as a barfate salt was added to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr and 2,6-bis(propoxy)prism[5]arene PrS[5]nPr (in CD2Cl2). The 22+@PrS[5]iPr complex was preferentially formed (ESI†), with a percentage of formation of 81% versus 19% for 22+@PrS[5]nPr. From these data and knowing the binding constant value of the 22+@PrS[5]nPr complex in CD2Cl2, an association constant value of 3.3 × 109 M−1 was calculated (Table 3), significantly higher than that observed for the formation of the 22+@PrS[5]nPr complex of 1.7 × 108 M−1. This result can be justified on the basis of the greater conformational rigidity and preorganization of the 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr macrocycle compared to PrS[5]nPr. Interestingly, the 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr showed a greater binding affinity toward N,N,N′,N’-tetramethylpiperazonium 32+ guest than 22+, with a 32+@PrS[5]iPr/22+@PrS[5]iPrselectivity ratio of 3.6 (Table 3).
1 mixture of 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr and 2,6-bis(propoxy)prism[5]arene PrS[5]nPr (in CD2Cl2). The 22+@PrS[5]iPr complex was preferentially formed (ESI†), with a percentage of formation of 81% versus 19% for 22+@PrS[5]nPr. From these data and knowing the binding constant value of the 22+@PrS[5]nPr complex in CD2Cl2, an association constant value of 3.3 × 109 M−1 was calculated (Table 3), significantly higher than that observed for the formation of the 22+@PrS[5]nPr complex of 1.7 × 108 M−1. This result can be justified on the basis of the greater conformational rigidity and preorganization of the 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr macrocycle compared to PrS[5]nPr. Interestingly, the 2,6-bis(isopropoxy)prism[5]arene PrS[5]iPr showed a greater binding affinity toward N,N,N′,N’-tetramethylpiperazonium 32+ guest than 22+, with a 32+@PrS[5]iPr/22+@PrS[5]iPrselectivity ratio of 3.6 (Table 3).
| PrS[5]R | 22+ (Kass, M−1) | 32+ (Kass, M−1) |
|---|---|---|
| a See ref. 15. b The binding constant obtained by NMR competition experiment is in perfect agreement with the value determined through fluorescence titration experiment. See ESI,† pages S170–173, for further details. | ||
| PrS[5]Et | 1.4 ± 0.2 × 108 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
2.8 ± 0.4 × 108 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| PrS[5]nPr | 1.7 ± 0.3 × 108 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
1.4 ± 0.2 × 109 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
| PrS[5]nBu | 4.6 ± 0.6 × 108 M−1 | 1.1 ± 0.2 × 109 M−1 |
| PrS[5]nPe | 4.3 ± 0.6 × 108 M−1 | 7.4 ± 0.9 × 108 M−1 |
| PrS[5]iPr | 3.3 ± 0.4 × 109 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
1.2 ± 0.2 × 1010 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| PrS[5]iBu | 9.6 ± 0.9 × 108 M−1 | 4.3 ± 0.6 × 109 M−1 |
| PrS[5]iPe | 2.7 ± 0.4 × 108 M−1 | 5.8 ± 0.8 × 108 M−1 |
| PrS[5]EtCy | 2.1 ± 0.3 × 108 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
6.8 ± 0.9 × 108 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
| PrS[5]EtcHp | 2.1 ± 0.3 × 108 M−1 | 2.8 ± 0.4 × 108 M−1 |
Analogously, the 2,6-bis(isobutoxy)prism[5]arene PrS[5]iBu gives endo-cavity complexations in the presence of 22+ and 32+ as barfate salt, with binding affinities higher than those observed for the analogous complexation with 2,6-bis(butoxy)prism[5]arene PrS[5]nBu (Table 3). The structure of the endo-cavity complex 22+@PrS[5]iBu, was investigated by a 2D NOESY experiment (ESI†), revealing diagnostic dipolar couplings between the methylene signals of 22+ shielded inside the cavity of PrS[5]iBu at −0.54/−0.85 ppm and the ArH signals of PrS[5]iBu at 8.86 and 7.21 ppm (Fig. S105, ESI†), confirming the formation of the endo-cavity 22+@PrS[5]iBu complex. High binding affinity (≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108 M−1, Table 3) toward 22+ and 32+ was also observed in the presence of bulky cyclohexylethyl and cycloheptylethyl groups at both rims of PrS[5]EtCy (Fig. 6g–i†) and PrS[5]EtcHp (ESI†), respectively (Table 3).
108 M−1, Table 3) toward 22+ and 32+ was also observed in the presence of bulky cyclohexylethyl and cycloheptylethyl groups at both rims of PrS[5]EtCy (Fig. 6g–i†) and PrS[5]EtcHp (ESI†), respectively (Table 3).
Conclusions
In this work we report the direct macrocyclization to prism[5]arenes of 2,6-dialkoxynaphthalenes bearing branched and bulky alkyl groups. Utilizing the 1,4-dihexyl-DABCO template, we successfully accomplish the direct macrocyclization of 2,6-dialkoxynaphthalene monomers into prism[5]arene with good yields. This process is effective in the presence of both long and branched alkyl chains. The NMR spectral characteristics of all the synthesized prism[5]arenes, are in accordance with the chiral D5 symmetry that has been previously reported for other prism[5]arene derivatives. In all cases, DFT calculations indicate that the homochiral all-pS (all-pR) conformation of PrS[5]R as more stable than all the other possible conformations.The solid state structures of PrS[5]iPr, PrS[5]nBu, PrS[5]iBu, PrS[5]nPe, PrS[5]MeCy, PrS[5]EtCy, and PrS[5]PrCy (α- and β- forms) were described. In the solid state, a large opening of the cavity is observed for PrS[5]iBu, PrS[5]MeCy and the β-form of PrS[5]PrCy. The opening observed for PrS[5]MeCy and β-PrS[5]PrCy can be attributed to the presence of a dichloromethane solvent molecule hosted in the prismarene cavity. In the case of PrS[5]iBu, a self-filling effect involving two methyl groups of the alkoxy chains is observed. All molecules crystallize as racemic mixtures of inherent chiral pairs (all-pS/all-pR) in centrosymmetric space groups. The dynamic stereochemical inversion (from pR to pS and vice versa) behaviour of prism[5]arenes was examined by variable temperature NMR experiments. These studies suggest that the racemization of prism[5]arene derivatives occurs by through-the-annulus rotation of the naphthalene units. With increasing length of linear alkyl substituents from C2 to C9, the chains tend to pack on both rims, thereby diminishing the conformational freedom of the naphthalene units.
The presence of branched alkyl groups at both rims of the prism[5]arenes increases the energy barrier to racemization and consequently lowers the rate of the process. As a result, PrS[5]iPr and PrS[5]iBu exhibit significantly higher energy barriers to racemization compared to those found for their analogous linear isomers PrS[5]nPr and PrS[5]nBu. Finally, in prism[5]arenes bearing cyclic units on both rims such as cyclohexylmethyl and cyclohexylethyl, the rotation of the respective 2,6-dialkoxynaphthalene units is blocked on the NMR time scale.
Prism[5]arene derivatives bearing branched or bulky alkyl groups on both rims can form endo-cavity complexes with 1,4-dihexyl-DABCO 22+ and piperazonium 32+ guests. PrS[5]iPr and PrS[5]iBu hosts show higher relative binding affinities toward 22+ and 32+ than those calculated for their linear analogues PrS[5]nPr and PrS[5]nBu. This result can be justified on the basis of the greater conformational rigidity and preorganization of PrS[5]iPr and PrS[5]iBu compared to PrS[5]nPr and PrS[5]nBu.
The knowledge acquired from this study, encompassing the synthesis, structural and conformational properties, as well as the molecular recognition abilities of prism[5]arenes featuring branched and bulky groups at both rims, has the potential to lay the foundation for the development of innovative functional systems based on prismarenes.
Author contributions
The manuscript was written through contributions of all authors.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
C. G. thanks the Italian Ministero dell'Università e della Ricerca (MUR) (PRIN_PNRR P2022XHLTX_PrismaSens) for financial support.References
- J.-M. Lehn, Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices, Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef.
- C. J. Pedersen, Cyclic Polyethers and Their Complexes with Metal Salts, J. Am. Chem. Soc., 1967, 89, 7017–7036 CrossRef CAS.
- D. J. Cram, The Design of Molecular Hosts, Guests, and Their Complexes, Angew. Chem., Int. Ed. Engl., 1988, 27, 1009–1020 CrossRef.
- P. Neri, J. L. Sessler and M.-X. Wang, Calixarenes and Beyond, Springer Int Publishing, 2016 Search PubMed.
- D. J. Cram and J. M. Cram, Container Molecules and Their Guests, The Royal Society of Chemistry, Cambridge, 1994 Search PubMed.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, The Cucurbit[n]uril Family, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS PubMed.
- T. Ogoshi, Pillararenes, The Royal Society of Chemistry, Cambridge, 2015 Search PubMed.
- H. Chen, J. Fan, X. Hu, J. Ma, S. Wang, J. Li, Y. Yu, X. Jia and C. Li, Biphen[n]arenes, Chem. Sci., 2015, 6, 197–202 RSC.
- F. Jia, Z. He, L.-P. Yang, Z.-S. Pan, M. Yi, R.-W. Jiang and W. Jiang, Oxatub[4]arene: A Smart Macrocyclic Receptor With Multiple Interconvertible Cavities, Chem. Sci., 2015, 6, 6731–6738 RSC.
- J. Li, H.-Y. Zhou, Y. Han and C.-F. Chen, Saucer[n]arenes: Synthesis, Structure, Complexation, and Guest-Induced Circularly Polarized Luminescence Property, Angew. Chem., Int. Ed., 2021, 60, 21927–21933 CrossRef CAS PubMed.
- (a) X.-N. Han, Y. Han and C.-F. Chen, Pagoda[4]arene and i-Pagoda[4]arene, J. Am. Chem. Soc., 2020, 142(18), 8262–8269 CrossRef CAS PubMed; (b) X.-N. Han, Y. Han and C.-F. Chen, Recent advances in the synthesis and applications of macrocyclic arenes, Chem. Soc. Rev., 2023, 52, 3265–3298 RSC; (c) X.-N. Han, Q.-S. Zong, Y. Han and C.-F. Chen, Pagoda[5]arene with Large and Rigid Cavity for the Formation of 1:2 Host–Guest Complexes and Acid/Base-Responsive Crystalline Vapochromic Properties, CCS Chem., 2022, 4, 318–330 CrossRef CAS.
- R. Del Regno, P. Della Sala, A. Spinella, C. Talotta, D. Iannone, S. Geremia, N. Hickey, P. Neri and C. Gaeta, Calix[2]naphth[2]arene: A Class of Naphthalene–Phenol Hybrid Macrocyclic Hosts, Org. Lett., 2020, 22, 6166–6170 CrossRef CAS PubMed.
- L.-P. Yang, X. Wang, H. Yao and W. Jiang, Naphthotubes: Macrocyclic Hosts with a Biomimetic Cavity Feature, Acc. Chem. Res., 2020, 53, 198–208 CrossRef CAS PubMed.
- P. Della Sala, R. Del Regno, C. Talotta, A. Capobianco, N. Hickey, S. Geremia, M. De Rosa, A. Spinella, A. Soriente, A. Spinella, P. Neri and C. Gaeta, Prismarenes: A New Class of Macrocyclic Hosts Obtained by Templation in a Thermodynamically Controlled Synthesis, J. Am. Chem. Soc., 2020, 142, 1752–1756 CrossRef CAS PubMed.
- P. Della Sala, R. Del Regno, L. Di Marino, C. Calabrese, C. Palo, S. Geremia, C. Talotta, S. Geremia, N. Hickey, A. Capobianco, P. Neri and C. Gaeta, An intramolecularly self-templated synthesis of macrocycles: self-filling effects on the formation of prismarenes, Chem. Sci., 2021, 12, 9952–9961 RSC.
- P. Della Sala, R. Del Regno, V. Iuliano, A. Capobianco, C. Talotta, S. Geremia, N. Hickey, P. Neri and C. Gaeta, Confused-Prism[5]arene: a Conformationally Adaptive Host by Stereoselective Opening of the 1,4-Bridged Naphthalene Flap, Chem. – Eur. J., 2023, 29, e202203030 CrossRef CAS PubMed.
- R. Del Regno, P. Della Sala, N. Hickey, S. Geremia, C. Talotta, P. Neri and C. Gaeta, Insights into the Self-Filling Effects of Branched Isopropyl Groups on the Conformational and Supramolecular Properties of Isopropoxyprism[6]arene, Eur. J. Org. Chem., 2023, e202300608 CrossRef CAS.
- R. Del Regno, G. D. G. Santonoceta, P. Della Sala, M. De Rosa, A. Soriente, C. Talotta, A. Spinella, P. Neri, C. Sgarlata and C. Gaeta, Molecular Recognition in an Aqueous Medium Using Water-Soluble Prismarene Hosts, Org. Lett., 2022, 24, 2711–2715 CrossRef CAS PubMed.
- R. Del Regno, P. Della Sala, D. Picariello, C. Talotta, A. Spinella, P. Neri and C. Gaeta, per-Hydroxylated Prism[n]arenes: Supramolecularly Assisted Demethylation of Methoxy-Prism[5]arene, Org. Lett., 2021, 23, 8143–8146 CrossRef CAS PubMed.
- C. Zhai and L. Isaacs, New Synthetic Route to Water-Soluble Prism[5]arene Hosts and Their Molecular Recognition Properties, Chem. – Eur. J., 2022, 28, e202201743 CrossRef CAS PubMed.
- Q. Song, K. Shang, T. Xue, Z. Wang, D. Pei, S. Zhao, J. Nie and Y. Chang, Macrocyclic Photoinitiator Based on Prism[5]arene Matching LEDs Light with Low Migration, Macromol. Rapid Commun., 2021, 42, 2100299 CrossRef CAS PubMed.
- Q. Song, K. Zhao, T. Xue, S. Zhao, D. Pei, J. Nie and Y. Chang, Nondiffusion-Controlled Photoelectron Transfer Induced by Host–Guest Complexes to Initiate Cationic Photopolymerization, Macromolecules, 2021, 54, 8314–8320 CrossRef CAS.
- D. Pei, W. Guo, P. Liu, T. Xue, X. Meng, X. Shu, J. Nie and Y. Chang, Prism[5]arene-based nonporous adaptive crystals for the capture and detection of aromatic volatile organic compounds, J. Chem. Eng., 2022, 433, 134463 CrossRef CAS.
- X. Liang, Y. Shen, D. Zhou, J. Ji, H. Wang, T. Zhao, T. Mori, W. Wu and C. Yang, Chiroptical induction with prism[5]arene alkoxy-homologs, Chem. Commun., 2022, 58, 13584–13587 RSC.
- G. Zhang, Z. Li, Z. Pan, D. Zhao and C. Han, A facile and efficient preparation of prism[6]arene and its dual responsive complexation with 1-adamantane ammonium tetrakis[3,5-bis(trifluoromethyl)-phenyl]borate, New J. Chem., 2023, 47, 18910–18913 RSC.
- J. Xie, Z. Xi, Y. Yang, X. Zhang, Z. Yang and M. He, Computational investigation of the structures, properties, and host-guest chemistry of prism[n]arenes, Int. J. Quantum Chem., 2023, e27253 Search PubMed.
- T. Ogoshi, K. Masaki, R. Shiga, K. Kitajima and T.-A. Yamagishi, Planar-Chiral Macrocyclic Host Pillar[5]arene: No Rotation of Units and Isolation of Enantiomers by Introducing Bulky Substituents, Org. Lett., 2011, 13, 1264–1266 CrossRef CAS PubMed.
- N. L. Strutt, H. Zhang and J. F. Stoddart, Enantiopure pillar[5]arene active domains within a homochiral metal–organic framework, Chem. Commun., 2014, 50, 7455–7458 RSC.
- T. Ogoshi, K. Kitajima, T. Aoki, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, Synthesis and Conformational Characteristics of Alkyl-Substituted Pillar[5]arenes, J. Org. Chem., 2010, 75, 3268–3273 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2019 Search PubMed.
- K. Du, P. Demay-Drouhard, K. Samanta, S. Li, T. U. Thikekar, H. Wang, M. Guo, B. van Lagen, H. Zuilhof and A. C.-H. Sue, Stereochemical Inversion of Rim-Differentiated Pillar[5]arene Molecular Swings, J. Org. Chem., 2020, 85, 11368–11374 CrossRef CAS PubMed.
- R. J. Kurland, M. B. Rubin and M. B. Wise, Inversion Barrier in Singly Bridged Biphenyls, J. Chem. Phys., 1964, 40, 2426–2427 CrossRef CAS.
- P. Della Sala, C. Talotta, T. Caruso, M. De Rosa, A. Soriente, P. Neri and C. Gaeta, Tuning Cycloparaphenylene Host Properties by Chemical Modification, J. Org. Chem., 2017, 82, 9885–9889 CrossRef CAS PubMed.
- C. Gaeta, C. Talotta, L. Margarucci, A. Casapullo and P. Neri, Through-the-annulus threading of the larger calix[8]arene macrocycle, J. Org. Chem., 2013, 78, 7627–7638 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2251128 and 2308578–2308584. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo02139d |
| This journal is © the Partner Organisations 2024 |