Open Access Article
Open Access ArticleMechanochromic hydrogen-bonded cocrystals with a salient effect upon heating†
Armando
Navarro-Huerta
 a,
Antonio
Juárez-Calixto
a,
María Eugenia
Sandoval-Salinas
b,
Yoarhy A.
Amador-Sánchez
a,
Antonio
Juárez-Calixto
a,
María Eugenia
Sandoval-Salinas
b,
Yoarhy A.
Amador-Sánchez
 c,
Joelis
Rodríguez-Hernández
c,
Joelis
Rodríguez-Hernández
 d,
Alejandra
Núñez-Pineda
ae,
Mario
Rodríguez
f,
Rachel
Crespo-Otero
d,
Alejandra
Núñez-Pineda
ae,
Mario
Rodríguez
f,
Rachel
Crespo-Otero
 b and
Braulio
Rodríguez-Molina
b and
Braulio
Rodríguez-Molina
 *a
*a
aInstituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior S/N Ciudad Universitaria, Coyoacan 04510, Mexico City, Mexico. E-mail: brodriguez@iquimica.unam.mx
bUCL Department of Chemistry, London WC1H 0AJ, UK
cLaboratorio de Fisicoquímica y Reactividad de Superficies (LaFReS), Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior S/N Ciudad Universitaria, Coyoacán 04510, Mexico City, Mexico
dCentro de Investigación en Química Aplicada (CIQA), Boulevard Enrique Reyna Hermosillo No. 140, Saltillo 25294, Coahuila, Mexico
eCentro Conjunto de Investigación en Química Sustentable (CCIQS) UAEM-UNAM, Carretera Toluca-Atlacomulco Km 14.5, Toluca, Estado de México, Mexico
fResearch Group of Optical Properties of Materials (GPOM), Centro de Investigaciones en Óptica, CIO, Apdo. Postal 1-948, 37000 León, Guanajuato, Mexico
First published on 15th August 2024
Abstract
Herein, we report the characterization of two hydrogen-bonded cocrystals, termed AC-TFTA and AC-PFBA, with mechanofluorochromism upon grinding. The heating of single crystals of AC-TFTA promotes microscopic displacements due to a decarboxylation process, which was studied through calorimetry-based techniques, gas chromatography/mass spectrometry, and hot-stage microscopy. Using solid-state nuclear magnetic resonance (ssNMR), powder X-ray diffraction (PXRD), and UV-Vis/fluorescence spectroscopy, we demonstrated that AC-TFTA displays sharper photophysical changes than its analog AC-PFBA, attributable to differences in the energies of their non-covalent interactions. Furthermore, the reversibility of the amorphous phase in both cocrystals was explored. However, fatigue tests led us to conclude that AC-TFTA displays potential for application as an anticounterfeiting agent, in contrast with the more robust crystalline AC-PFBA. This work emphasizes the importance of cocrystallization in generating accessible and functional mechanofluorochromic materials.
1. Introduction
The quest for smart materials capable of dynamically responding to an external stimulus has led to significant advancements in materials science and engineering. Among these responsive materials, mechanofluorochromic (MFC) compounds have emerged as promising candidates due to their ability to undergo reversible changes in their fluorescence emission under mechanical stress.1,2 Their expanding array of applications, ranging from anticounterfeiting to ref. 3 encoding,4 sensing,5 and other emissive applications,6 has attracted considerable interest in recent years.Frequently reported mechanofluorochromic compounds are based on boranyls,7,8 tetraphenylethylene compounds,9–11 benzimidazole-like skeletons12,13 and cyanovinylethylene acceptors.14 In addition, gold-containing cyano-based small molecules and other inorganic materials can display these attractive properties.15–20
A typical methodology for developing MFC materials involves the amalgamation of electron-donating and electron-withdrawing functional groups in one molecule via covalent bonds. Such a combination allows for specific modulation of the resulting frontier molecular orbitals (FMOs) and causes a red-shift in the photoluminescence (PL) properties.21
The use of bulky and twisted conjugated components also helps to obtain MFC compounds because the grinding process tends to modify the dihedral angles of the moieties with respect to the backbone, thus provoking changes in the π-conjugation and impacting the optical properties of the materials.9 When the structural variations are drastic, they may lead to changes from one solid form to another, whether in a single-crystal to single-crystal (SCSC) transition,22 or from a crystalline to an amorphous material,23 among other possibilities.
Including the mentioned features in a single molecule could be synthetically challenging and potentially lead to low yields, quenched emission, or minimal solubility of the resulting compounds. To overcome the cited limitations, several groups have relied on the principles of crystal engineering and supramolecular chemistry using cocrystallization as the preferred approach to enable modifications of the photoluminescence of solids.24–26 Molecular cocrystals are multicomponent solids composed of neutral, crystalline molecules in specific stoichiometric ratios, termed coformers.27 Its development has enhanced the control of physicochemical properties, for instance solubility, thermal/photo stability and its photophysical properties, owing to the availability of synthetic methods and commercial coformers.28
Interestingly, the research of crystalline MFC materials based on cocrystallization is scarce.29–31 In this context, the hydrogen bond, a directional, strong, non-covalent interaction, has been a pivotal area of study due to the robustness that it can provide to crystalline solids. Notable examples of these compounds are the works of Ito,32 Xie,33 and Dar,34,35 who have successfully developed crystalline materials with high responsiveness and multicolor emission.
The multi-responsiveness of crystalline materials is not limited to the stress-induced stimuli but includes the thermal treatment of crystalline samples that can lead to macroscopic displacements due to the release of molecules, such as carbon dioxide.36 In the context of cocrystals, Gong et al. reported the jumping mechanism of a coronene-TFBQ cocrystal triggered by the expelling of the acceptor molecule in a thermal stimulus.37 More recently, Das et al. reported a salt formed by piperazine-trifluoroacetic acid where the crystals released TFA molecules upon bending.38
Considering the above, we envisioned using a fused carbazole-acridine fragment as the hydrogen bond acceptor due to its straightforward synthesis, thermal robustness, and photoluminescent properties. Complementarily, we draw on perfluorobenzoic and tetrafluoroterephthalic acids to facilitate new crystalline forms and possible responsiveness inspired by previous reports.33,39,40 It is important to note that additional non-classic hydrogen bonds may also enable interesting properties both in the strength and lattice responsiveness of the crystalline packing.5,41
Here, we report the systematic characterization of two hydrogen-bonded cocrystals with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (AC-PFBA) or 2
1 (AC-PFBA) or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (AC-TFTA) stoichiometries. The properties of each solid were thoroughly explored and characterized by single crystal and powder X-ray diffraction, fluorescence, UV spectroscopy, and variable temperature solid-state NMR. Remarkably, the heating process of AC-TFTA is translated into jumping of its single crystals, which were successfully correlated with a decarboxylation of the TFTA moiety. Further experiments with this cocrystal revealed its potential application in anticounterfeiting due to the remarkable color change between ground and crystalline phases upon heating and vapor exposure.
1 (AC-TFTA) stoichiometries. The properties of each solid were thoroughly explored and characterized by single crystal and powder X-ray diffraction, fluorescence, UV spectroscopy, and variable temperature solid-state NMR. Remarkably, the heating process of AC-TFTA is translated into jumping of its single crystals, which were successfully correlated with a decarboxylation of the TFTA moiety. Further experiments with this cocrystal revealed its potential application in anticounterfeiting due to the remarkable color change between ground and crystalline phases upon heating and vapor exposure.
2. Experimental section
Reagents and solvents were purchased from the company Sigma-Aldrich®. Reactions were monitored through TLC using silica gel plates 60 F254 purchased from Merck®. Spots were detected by UV-light absorption. Reactions were carried out in an inert atmosphere using nitrogen (N2). Solution 1H and 13C experiments were recorded at room temperature using a Jeol Eclipse 270. The spectroscopic data is referenced to CDCl3 (1H: δ = 7.26 ppm, s; 13C: δ = 77.0 ppm). High-resolution mass spectrometry was obtained in a Jeol JMS-AccuTOF JMS-T100LC spectrometer, ionization mode: direct analysis in real time (DART). FTIR-ATR spectra experiments were recorded with Bruker ATR equipped with a diamond tip in the spectral window from 4000 to 500 cm−1. Unless otherwise noted, uncorrected melting points were determined in a Fisher-Johns melting point apparatus. Optical microscopy images were acquired using an Olympus BX43 microscope in a 4× zoom lens with a Samsung SM-A525 M (F1.8, 0.0 eV, 1/60, 24 mm, and ISO from 80 to 2000). In contrast, Hot-stage microscopy images were captured using a hot-stage Linkam LTS420E with a ramp program of 100 °C min−1. Photoluminescence spectra and Fluorescence quantum yields (PLQY) in the solid state were measured on an Edimburg Instruments FS5 spectrofluorometer equipped with an integrating sphere (SC-30) at room temperature. Fluorescence lifetimes were measured at room temperature using the standard time-correlated single-photon counting (TCSPC) technique using a picosecond pulsed laser diode (EPL-405) at 405 nm from Edinburgh Instruments (EPL-405). Large single crystals were grown according to the procedures described below to obtain pristine powdered samples of the cocrystals. Then, the obtained crystals were slightly crushed to obtain smaller crystals with no preferred orientation confirmed through PXRD.2.1. Synthesis of cocrystal AC-TFTA
21 mg of AC (0.06 mmol, 1 eq.) and 14 mg of TFTA (0.06 mmol, 1 eq.) were placed in a 10 mL vial. Afterward, 5 mL of dichloromethane/methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was poured into the vial, resulting in a red solution. The system was sealed and heated at 70 °C until no solids were observed. If solid matter would remain, the solution was filtered through a Pasteur pipette plugged with a small piece of cotton into another 10 mL dry vial. The cap is loosened, and the solution evaporates for 4 to 5 days to obtain yellow prismatic-shaped single crystals. They were washed with a small amount of cold methanol to dry the crystals and vacuum-filtered using a Hirsh funnel. Yield: 24 mg (85%). Elemental analysis for AC-TFTA (C33H18F4N2O4): calc. C: 75.16, H: 3.70, N: 6.04; found C: 75.11, H: 4.04, N: 6.41.
1) was poured into the vial, resulting in a red solution. The system was sealed and heated at 70 °C until no solids were observed. If solid matter would remain, the solution was filtered through a Pasteur pipette plugged with a small piece of cotton into another 10 mL dry vial. The cap is loosened, and the solution evaporates for 4 to 5 days to obtain yellow prismatic-shaped single crystals. They were washed with a small amount of cold methanol to dry the crystals and vacuum-filtered using a Hirsh funnel. Yield: 24 mg (85%). Elemental analysis for AC-TFTA (C33H18F4N2O4): calc. C: 75.16, H: 3.70, N: 6.04; found C: 75.11, H: 4.04, N: 6.41.
2.2. Synthesis of cocrystal AC-PFBA
34 mg of AC (0.10 mmol, 1 eq.) and 21 mg of PFBA (0.10 mmol, 1 eq.) were placed in a 20 mL vial. Afterward, 10 mL of dichloromethane/hexane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was poured into the vial, forming a red solution. The heating and cooling process is the same as employed for AC-PFBA. The solution is allowed to evaporate for three days to obtain yellow needle-shaped single crystals. They were washed with a small amount of cold hexane to dry the crystals and vacuum-filtered using a Hirsh funnel. Yield: 45 mg (81%). Elemental analysis for AC-PFBA (C32H17F5N2O2): calc. C: 69.07, H: 3.08, N: 5.03; found C: 68.12, H: 3.26, N: 5.29.
1) was poured into the vial, forming a red solution. The heating and cooling process is the same as employed for AC-PFBA. The solution is allowed to evaporate for three days to obtain yellow needle-shaped single crystals. They were washed with a small amount of cold hexane to dry the crystals and vacuum-filtered using a Hirsh funnel. Yield: 45 mg (81%). Elemental analysis for AC-PFBA (C32H17F5N2O2): calc. C: 69.07, H: 3.08, N: 5.03; found C: 68.12, H: 3.26, N: 5.29.
2.3. Cocrystal grinding
Mechanical grinding of cocrystals was performed in a planetary ball miller Fritsch® Pulverisette 7. Two grinding bowls of zirconium oxide (20 mL) with steel casing and sealing silicone rings were employed simultaneously, each with 160 mg of cocrystal (AC-TFTA or AC-PFBA). Additionally, each container was charged with 5 zirconium-oxide grinding balls (diameter 10 mm). The process was carried out using the neat-grinding method for 60 minutes at 850 rpm.3. Results and discussion
3.1. Synthesis of cocrystals and SC XRD structural determination
The carbazole-acridine fused molecule, 9-(9H-carbazol-9-yl)acridine, abbreviated ‘AC,’ consisting of an Ullmann-type coupling using Cu(I) as catalyst (Scheme S1, ESI†) was previously reported by Zeghada42 and reproduced for this work. The title compound was characterized through 1H and 13C solution NMR spectroscopy (Fig. S1 and S2, ESI†), high-resolution mass spectrometry, and Fourier-transformed infrared spectroscopy, with excellent agreement with the mentioned reference. During the purification steps, we realized that saturated methanol/THF and toluene mixtures could produce yellow, prismatic-shaped crystals as solvated forms. Also, an acetonitrile solution produced the solvent-free form, as reported by Koshima.43 Through all these structures, a twisted structure with a pronounced dihedral angle between the carbazole and the acridine fragment was visualized, likely resulting from the steric hindrance of the protons of positions 1 and 8.The combination of AC and tetrafluoroterephthalic (TFTA) or perfluorobenzoic (PFBA) acids led to the formation of cocrystals AC-TFTA and AC-PFBA as pristine solids, which were obtained from saturated mixtures of dichloromethane/methanol and dichloromethane/hexane, respectively, suitable to be studied using single crystal X-ray diffraction (SC XRD). The collection and solution of the structures allowed us to elucidate a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group for AC-TFTA, while a monoclinic system P21/c space group was determined for AC-PFBA. The colors and habits of their structures can be visualized in Fig. 1.
space group for AC-TFTA, while a monoclinic system P21/c space group was determined for AC-PFBA. The colors and habits of their structures can be visualized in Fig. 1.
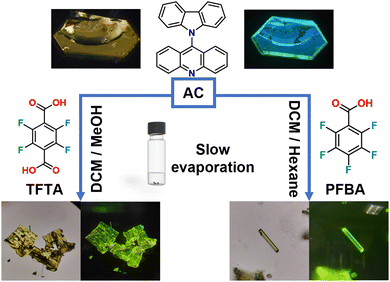 | ||
| Fig. 1 Combination of AC with coformers TFTA or PFBA to form new hydrogen-bonded cocrystals. The resulting single crystals are shown under natural light and under UV light. | ||
The asymmetric unit of AC-TFTA comprises two molecules of AC and one molecule of TFTA. However, the two molecules of AC and two complete molecules of TFTA are non-equivalent. Due to this feature, one of the TFTA molecules establishes two hydrogen bond interactions, described by a distance (Odonor⋯Nacceptor) of 2.592(1) Å, while the angle ∠NAC⋯H–OTFTA was 168(2)°. Different parameters were observed for the other TFTA molecule, consisting of a 2.609(1) Å distance and an angle of 173(2)°. All these interactions have been represented in Fig. 2a. Regarding AC-PFBA (Fig. 2b), this cocrystal comprises one molecule of both donor and acceptor of hydrogen bonds in the asymmetric unit. The associated parameters for this interaction are composed of a distance of 2.653(3) Å and an ∠NAC⋯H-OPFBA angle of 167(2)°.
3.2. Spectroscopic features of pristine solids
The association of the corresponding carboxylic acids with the AC molecules could be visualized and further analyzed using Fourier-transform infrared spectroscopy (FTIR). Both cases illustrated in Fig. S3 (ESI†) emphasize the presence of the O–H stretching band of starting materials, which typically appears in the region between 3000–2500 cm−1 with a notable broadening due to the formation of dimers between the carboxylic acid molecules.44 Contrastingly, when the cocrystallization occurs, the O–H stretching is resolved and shifted towards higher frequencies as barely perceivable bands with respect to the sole carboxylic acids. An equally visible change between the spectra is the decrease in the intensity and position of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carbonyl towards higher frequencies when the cocrystals are formed, from 1698 to 1720 cm−1 for the TFTA, and from 1711 to 1714 cm−1 for the PFBA.45
O stretching of the carbonyl towards higher frequencies when the cocrystals are formed, from 1698 to 1720 cm−1 for the TFTA, and from 1711 to 1714 cm−1 for the PFBA.45
The presence of a hydrogen-bond donor in the cocrystals led to interesting crystalline packings and very different photoluminescence (PL) properties compared to the starting emissive material AC, as seen in photographs from Fig. 1. Fresh samples of pristine powders were prepared to assess and compare this property. The experimental diffractograms of these solids were obtained using powder X-ray diffraction and compared against the calculated powder diffractograms from the crystallographic information files to ensure the correct phase was obtained. These are depicted in Fig. S4 (ESI†).
Next, room-temperature fluorescence spectroscopy was employed to characterize the resulting solids’ photoluminescence. The PL spectra of both AC and pristine cocrystals are depicted in Fig. 3a, illustrating an emission maximum at 507 nm for the starting material AC. As for the cocrystals, their PL spectra display two emission maxima at 550 and 563 nm, corresponding to AC-PFBA and AC-TFTA, respectively. Despite the closeness in the emission wavelength of the new solids, the measured photoluminescence quantum yields (PLQY, ΦF) are very different. At a λex of 405 nm, the pristine AC showed a ΦF of 74.2%, while the value for AC-PFBA was 26.4% and 15.4% for AC-TFTA.
Moreover, the time-resolved fluorescence decay experiments at room temperature allowed us to determine the lifetimes (τ) of the pristine samples. Firstly, an exponential fit with three fluorescence lifetimes was proposed for the pristine AC as a solvent-free crystalline powder (Fig. 3b). These times were 9.7 ns for τ1, 28.3 ns for τ2, and 48.8 ns for τ3. These values are slightly higher for a small fluorophore in comparison with other solid-state carbazole derivatives;46,47 which could be attributed to the rigidity of the crystal as well as lack of vibrations, owing to the π-conjugated system and strong intermolecular interactions between AC molecules.29 On the other side, the fluorescence lifetimes of the pristine cocrystals were determined through a biexponential fit. For AC-TFTA, the τ1 and τ2 values were 7.1 and 20.3 ns, respectively, whereas for AC-PFBA, these were 8.3 and 21.6.
The reduction in the fluorescence lifetimes and PLQY of the cocrystals compared to AC could be attributed to the vibrational relaxation of the proton at the carboxylic acid, reducing the fluorescence in the cocrystals.48 Another detrimental factor for fluorescence emission is the presence of π-stacking in both cocrystals, depicted in Fig. S5 (ESI†), since associated energy transfer, non-radiative processes can be involved due to orbital overlap between fragments.49 For comparison purposes, the relevant PL data is displayed in Table 1.
| Sample | Treatment | λ em (nm) | Φ F (%) | τ n (n = 1,2,3…) (ns) | ||
|---|---|---|---|---|---|---|
| a λ ex = 405 nm. | ||||||
| AC | Pristine | 507 | 74.2 | 9.7 | 28.3 | 48.8 |
| AC-TFTA | Pristine | 563 | 15.4 | 7.1 | 20.3 | |
| Ground | 656 | 0.6 | 5.6 | 15.1 | 32.5 | |
| Fumed | 595 | 2.1 | 6.1 | 15.9 | 31.4 | |
| AC-PFBA | Pristine | 550 | 26.4 | 8.3 | 21.6 | |
| Ground | 567 | 5.6 | 6.6 | 18.4 | 44.3 | |
| Fumed | 553 | 22.5 | 8.9 | 19.8 | 47.9 | |
To generate a deeper understanding of the photophysics of the generated solids, UV-Vis spectroscopy experiments using the diffuse reflectance technique were carried out on polycrystalline samples. From the spectra shown in Fig. 3c, a red shift in the absorption bands of the cocrystals is visualized compared to the AC powder. AC displays its maximum at ca. 292 nm, whereas AC-TFTA depicts an absorption maximum at 424 nm, and AC-PFBA does it at 419 nm. Moreover, through the conversion of diffuse reflectance data into Tauc plots, it was determined the optical gaps in the cocrystals, which correspond to the energy of the lowest electronic transition accessible via absorption of a single photon.50 For the case of AC-TFTA, this energy corresponds to 2.32 eV; for AC-PFBA, it is 2.36 eV. The comparative plot can be visualized in Fig. S6 (ESI†).
3.3. Theoretical calculations based on the SC-XRD structures of cocrystals
We investigated the electronic transitions behind the absorption and emission spectra in the crystalline phase for pure AC crystal and both cocrystals. First, the crystals were refined using periodic DFT (see Computational details in the ESI†). Then, we considered embedded quantum mechanics (QM: QM′) cluster models implemented in the fromage package to analyze the excited states.51,52 Considering a molecular-centered mechanism, as previously done for similar carbazole-based crystalline systems53,54 the QM region was defined to be a monomer (AC), dyad (AC-PFBA), or triad (AC-TFTA) and was described as the TD-CAM-B3LYP-D3/6-311++G(d,p) level of theory. The QM’ region consists of a semi-spherical radius cluster ∼22 Å and was described by the GFN2 extended tight binding (GFN2-xTB) Hamiltonian. Further details are provided in the ESI.†The S1 of AC is a transition mainly located over the acridine moiety (Fig. 3d) with absorption at 352 nm (3.52 eV), slightly red-shifted to the experimental maximum absorption peak at 292 nm. Upon cocrystallization, the S1 state of the dyad and triads remains with the same local excitation character over the AC molecule. The energy of the S0 → S1 transition is 3.45 eV for AC-PFBA with an oscillator strength of 0.1423, which is very close to the computed values for the AC crystal (Fig. 3e). In the case of the asymmetric triads observed in the AC-TFTA, S1, and S2 are quasi-degenerated, resulting from the in-phase (out-phase) electronic transition dipole moment interaction of the S0 → S1 transition of each AC fragment.55 While S1 (Fig. 3f and g) is the brighter state, whose f is around twice the value computed for AC and AC-PFBA, the S2 of AC-TFTA-a(b) is a dark state with excitation energy of 3.50 eV (3.47 eV). Due to the degree of localization of the orbitals, the energies of the frontier orbitals of the cocrystals are closer to the ones of the pure AC crystal, as can be observed in Fig. S7 (ESI†).
To simulate the photoluminescence properties of the cocrystals, we obtained the optimized geometry for the S1 state using the same ONIOM QM: QM′ embedded scheme described before. The predicted emission energy of the pristine AC crystal is 2.99 eV, which overestimates the experimental maximum (507 nm, 2.45 eV) by around 0.5 eV. The predicted values for AC-PFBA and AC-TFTA are slightly red-shifted (around 0.1 eV) for both crystals, which agrees with the experimental results for both the absorption maxima and the optical gaps. In both cocrystals, the PL originated from the S1min geometry, localized on the AC molecule with almost no influence of the acid. This explains the very close emission of AC-PFBA and AC-TFTA with AC. The generation of orbitals and associated energies are visualized in Fig. S8 (ESI†).
3.4. Decarboxylation-mediated thermosalient effect of AC-TFTA
As we described in the introduction of this work, TFTA and PFBA are coformers used in the synthesis of cocrystals with photosalient and thermosalient effects. Driven by these precedents, we decided to test the capabilities of our cocrystals toward thermal responsiveness. Regarding AC-TFTA, it was observed that when single crystals of this solid (as a pristine sample) were heated using a Fisher-Johns apparatus, these started to jump out of the plate at ca. 265 °C, as visualized in the progressive images from Fig. S9 and Movie S1 (ESI†).To gather more detailed information on the macroscopic phenomena, we used Hot-Stage microscopy with a heating ramp setup of 100 °C min−1, coupled with a high-speed camera. The resulting cut frames from the original movie (Movie S2, ESI†) display that when the temperature approaches 260 °C, the material starts to lose some crystallinity, as seen from the darkening of the prisms under polarized light. Afterward, a swift collapse of the crystals occurs in a direction towards the center of the prisms along the significant diagonal, as described in the arrows of Fig. 4a.
Surprised by our observations, a fresh sample of pristine AC-TFTA was studied through coupled differential scanning calorimetry and thermogravimetric analyses (DSC/TGA). It was observed that the DSC curve highlights an endothermal peak from 245 up to 270 °C, with a maximum of 262 °C (Fig. 4b). Interestingly, the interval of temperatures matches with a 27% loss of mass from the initial sample from the TGA curve, which could be initially associated with the loss of CO2 molecules.
To confirm the identity of the molecules released during the heating process, freshly grown single crystals of AC-TFTA, covered by a thin layer of Parabar 10312 (an oil used for crystallographic purposes), were placed on the Fisher-Johns melting apparatus. Towards 260 °C, it was observed that a large amount of bubbles emanated from the oil, followed by the melting of the remaining material (Fig. 4c). This process was recorded in Movie S3 (ESI†). Subsequently, we relied on the characterization of the volatile compounds through gas chromatography (GC) coupled to mass spectrometry. A sealed vial, with an initial quantity of the pristine AC-TFTA, was loaded and purged with N2, then heated towards 270 °C, and the volatile compounds generated were injected into the GC. The resulting chromatogram is shown in Fig. S10a (ESI†), which displays the resolution of several peaks. The detection of each peak and its associated compound is depicted in the spectrograms of Fig. S10b–h (ESI†). A series of fragmentations of the AC and the TFTA molecules could be identified, especially carbon dioxide and tetrafluorobenzene, due to the decomposition of TFTA. At longer retention times, the presence of acridine, carbazole, and AC molecules was also detected.
After submitting a sample of AC-TFTA under complete decarboxylation, we gathered information on the identity of the remaining solid. The residual yellow powder after the heating process was characterized using PXRD and fluorescence spectroscopy in the solid state. From Fig. S11a (ESI†), the PXRD diffractogram shows a series of broad reflections that barely agree with any of the diffractograms for AC pristine or AC-TFTA. On the other hand, Fig. S11b (ESI†) displays the PL spectra of the compound with an emission maximum of 468 nm for the recovered solid. The DSC/TGA curve of pristine AC is shown in Fig. S12 (ESI†), which displays the melting of the sole compound at 255 °C. The data shows that a new crystalline phase of residual AC is present after the dramatic heating of the cocrystals towards the thermosalient effect and further cooling at room temperature.
Overall, the joint evidence led us to postulate that a decarboxylation of the TFTA coformer inside the crystalline network was responsible for the thermosalient effect, followed closely by the melting of AC. Contrastingly, we did not observe the decarboxylation effect on crystalline TFTA on its own. To corroborate this, crystals of TFTA were studied through DSC/TGA (Fig. S13, ESI†). The curves display the sublimation of TFTA starting from 250 °C up to an endothermal peak at 283 °C.
Similar tests were carried out for the single crystals of AC-PFBA. The heating of freshly grown samples in the melting apparatus did not show the described jumping behavior. A recording of the visual changes under UV light (254 nm) through the heating interval can be recovered from Movie S4 (ESI†). Furthermore, crystals of AC-PFBA were placed, covered with Parabar, and heated up to 260 °C. In Movie S5 (ESI†), a slow release of bubbles was observed, starting at ca. 180 °C and continuing up to 230 °C. More information was obtained through the DSC/TGA curves of pristine AC-PFBA (Fig. S14, ESI†), which show an endothermal peak at 177 °C, in agreement with a mass loss of 37.5%, corresponding to the release of complete PFBA molecules, instead of a decarboxylation process. As the temperature increases, the DSC profile shows a new endotherm at 267 °C, which can be associated with the melting of AC.
The significantly different thermal responses in both samples led us to find a feasible explanation through the analysis of their non-covalent energy interactions for each crystalline packing. For this matter, we performed theoretical calculations using the CE-B3LYP56 6-31G(d,p) functional implemented in CrystalExplorer 21.5.57 The resulting energy values suggest that the hydrogen bond interactions in AC-TFTA (ca. −58 to −61 kJ mol−1) are stronger than in AC-PFBA (ca. −53 kJ mol−1). Both values are in agreement with some reports of the association of carboxylic acids and acridines through hydrogen bonds in the solid state.58–61
Despite this fact, the distribution of weaker non-covalent interactions, such as C–H⋯π, is somewhat different in both cocrystals. For instance, it was observed that the non-covalent interactions around the TFTA molecules in AC-TFTA range from −7 to −9 kJ mol−1. In sharp contrast, inside AC-PFBA, the non-covalent interactions of the PFBA molecules with neighboring molecules are stronger and range between −15 to −29 kJ mol−1 for adjacent PFBA molecules and −32 kJ mol−1 for AC fragments.
The contrasting energy differences between both cocrystals indicate a higher anisotropy in the crystalline packing of AC-TFTA against the more uniform AC-PFBA moiety. As described recently by Geerts and coworkers, the anisotropy derived from different non-covalent interactions can result in dramatic effects in the mechanical and responsive properties of crystals, also providing a methodology for the slow or fast release of organic molecules or gases with thermosalient effects.38 For illustration purposes, Fig. 5a and b highlights the strongest interactions around fluorinated molecules in each cocrystal. To complement the analyses, Hirsfeld surfaces of the fragments are depicted as insets in the corresponding fingerprint plots (Fig. S15 and S16, ESI†), where the principal interactions of these cocrystals are displayed.
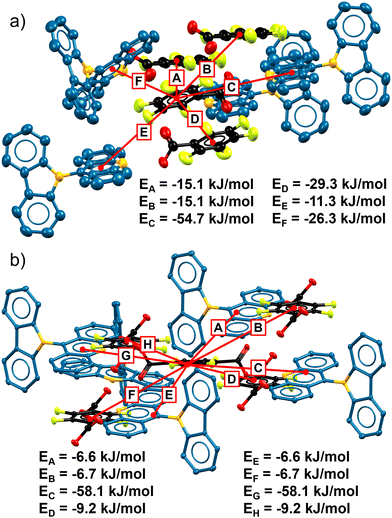 | ||
| Fig. 5 Distribution of non-covalent interaction energies in (a) AC-PFBA and (b) AC-TFTA, obtained from calculations at the CE-B3LYP 6-31G(d,p) level of theory in CrystalExplorer 21.5. | ||
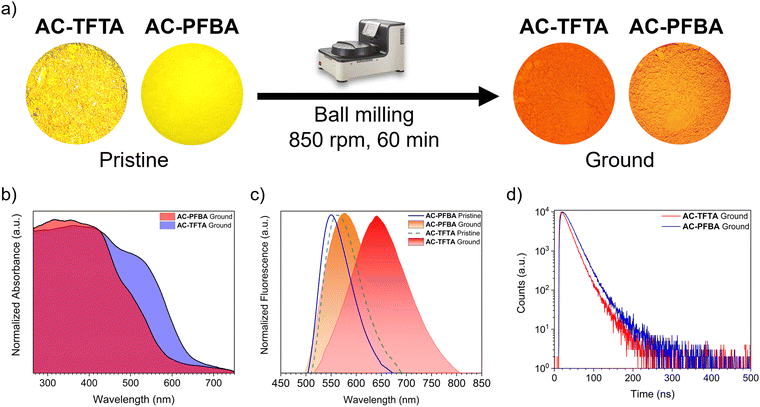
3.5. Mechanical induction of photophysical and structural changes in cocrystals
The presence of different degrees of anisotropy in the cocrystals herein described motivated us to explore changes in the photophysical properties of the pristine solids via mechanical stress through the grinding of microcrystalline samples. For this purpose, a planetary ball-miller was employed, as described in the Experimental section. After one hour of grinding at 850 rpm, the two solids featured abrupt changes in their physical appearances. Fig. 6a depicts a visual comparison of the powders before and after the grinding. AC-TFTA displayed a deep-red color upon grinding, whereas AC-PFBA resulted in a faint orange color.The characterization of the crystallinity, performed through PXRD at room temperature, showed remarkable differences between both powders. Firstly, the ground powder of AC-TFTA is significantly amorphized compared to ground AC-PFBA under the same conditions, as shown in Fig. S17 (ESI†). A LeBail analysis of the experimental diffractograms helped us determine the percentage of crystalline material remaining after the milling of the solids. While AC-TFTA displayed a 20% of crystalline material remaining, AC-PFBA contained 52% of crystalline powder under the same grinding conditions.
To quantitatively compare the absorption of the new powders due to the grinding, Fig. 6b portrays the UV-Vis profiles of both cocrystals after the grinding process. It can be visualized that AC-TFTA, after grinding, displays a significantly broader band, extended up to ca. 600 nm, with an absorption maximum of 365 nm. On the other hand, the broadening of the absorption in AC-PFBA is not as dramatic as in the previous cocrystal, extended towards 520 nm. Similarly to the pristine solids, the data from diffuse reflectance was employed in calculating the energy optical gaps, through the Tauc plots displayed in Fig. S18 (ESI†). The obtained values were considerably lower than those for pristine solids, being 2.02 eV for AC-TFTA against 2.22 eV for AC-PFBA.
Regarding the PL of the ground solids, the spectra are shown in Fig. 6c. It highlights an emission maximum of 656 nm for AC-TFTA, while for AC-PFBA, it is 567 nm. In comparison with the pristine samples, AC-TFTA showed a bathochromic shift of 93 nm, and AC-PFBA only showed a 17 nm red-shift. The ground samples showed a dramatic quenching in the fluorescence; in the case of AC-TFTA, the PLQY went from 15.4% to 0.6%, and for AC-PFBA, the value changed from 26.4% to 5.6%. The smaller values of quantum yields agree well with a higher contribution of amorphous solid component, with a rise in the degree of freedom of the molecules, promoting more effective vibrational relaxations.62 Additionally, the time-resolved fluorescence decay profiles were determined for these samples (Fig. 6d). The concentrated data is displayed in Table 1, which shows that for both AC-TFTA and AC-PFBA, the decay was fitted as a three-component exponential.
3.6. ssNMR experiments of pristine and ground powders
To provide a deeper understanding of the structural changes of the transformation from grinding, we used solid-state nuclear magnetic resonance (ssNMR) through the cross-polarization (CP) magic-angle spinning (MAS) for 13C and direct pulse with MAS for 19F. Fig. 7a shows that for the case of crystalline AC-TFTA, a 13C CP MAS spectrum with six signals between 100 and 165 ppm is presented. When this spectrum is compared with the ground powder, a broadening of signals is evident, especially with the pair of signals centered at 123 and 119 ppm. This effect is attributed to the different orientations of the 13C carbon atoms due to the amorphization of the sample. A less pronounced broadening is observed for the signals in the AC-PFBA spectra from Fig. 7b.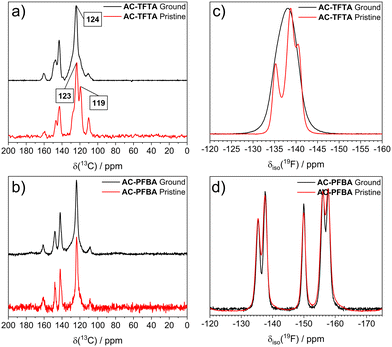 | ||
| Fig. 7 Characterization of pristine and ground powders of cocrystals through ssNMR. 13C MAS spectra with cross-polarization (125.8 MHz, Spin Rate = 20 kHz, d1 = 20 s) of (a) AC-TFTA, and (b) AC-PFBA19F MAS spectra (470.6 MHz, Spin Rate = 20 kHz, d1 = 10 s) of (c) AC-TFTA, and (d) AC-PFBA. In these spectra, only the central signals are shown. For the full spectra, please visualize the ESI† file. | ||
Even more interestingly, significant changes are observed in the ssNMR 19F MAS spectra of both cocrystals. The comparison of ground and pristine AC-TFTA at room temperature is shown in Fig. 7c. A set of signals between −130 and −145 ppm correspond to the fluorine atoms of the TFTA when the periodic arrangement is intact. On the other hand, upon amorphization of the sample, the resolution of the signals is notoriously diminished, resulting in the coalescence into a broadened signal centered at −143 ppm. Additional experiments at variable temperatures (Fig. S19, ESI†) were conducted to evaluate possible changes in the chemical shift of the signals. However, no noticeable effects were observed through the temperature interval between 248 and 308 K. For the case of AC-PFBA, even after the thorough grinding process, the 19F spectra associated with both pristine and ground solids remain essentially unaltered both at room (Fig. 7d) and low temperatures (248 K), as can be seen from Fig. S20 (ESI†).
3.7. Regeneration of crystallinity through thermal and fuming stimuli
A practical yet not fully understood feature of mechanofluorochromic materials is their capability to regenerate their crystalline structure through the application of external stimuli, such as solvent vapors or temperature. We were curious to unveil whether these cocrystals portrayed this type of behavior. For this matter, we placed a portion of each ground powder in a holder and then immersed it in a beaker previously saturated with dichloromethane vapors. After 10 minutes of fuming for both powders, there was a noticeable change in the colors, with AC-TFTA the most notable return from the red coloration to a faint yellow. In contrast, the faint orange of AC-PFBA was quickly returned to its original yellow.The color changes were recorded and can be visualized in Movie S6 (ESI†). The vapor-treated powders were characterized through PXRD to examine the degree of crystallinity after the whole process. The resulting data, presented in Fig. 8a and b, allowed us to visualize the regeneration of the crystalline solid with good agreement between the reflections of the experimental diffractogram and the calculated from SC XRD structures. Moreover, the photophysical parameters for these solids were determined. The Fig. S21 (ESI†) displays the solid-state fluorescence spectra of the fumed solids, depicting a λem of 595 nm for AC-TFTA, different from the 563 nm determined for the pristine sample. Meanwhile, for AC-PFBA, the maximum appears at 553 nm, which is extremely close to the initial 550 nm of the pristine sample.
Furthermore, the quantum yields of the fumed samples were measured. It was clear that for AC-TFTA, not only was the emission maximum drastically changed, but it also suffered a quenching in the emission of the powder with a 2.1% yield. The determined fluorescence lifetimes of this fumed sample were 6.1, 15.9, and 31.4 ns, which closely resemble the lifetimes of the ground powder. Contrastingly, the fumed powder of AC-PFBA showed a remarkable 22.5% yield against the 26.4% of the pristine sample, while the fitted fluorescence lifetimes were determined to be 8.9, 19.8, and 47.9 ns. The decay curves are shown in Fig. 8c.
Due to the contrasting results in the recovery, we decided to perform fatigue tests on powdered samples of both cocrystals. For this purpose, three grinding-recovery (fuming) cycles were carried out. Fluorescence spectroscopy was employed to monitor the regeneration of the crystalline phase. The reduced spectral data is displayed in Fig. 8d, and the progressive solid-state spectra are depicted in Fig. S22 and S23 (ESI†). From the fuming of AC-TFTA in the first cycle, this powder showed clear signs of fatigue, as seen in the ever-smaller differences between the emission wavelength of the ground and the fumed sample. By the end of the third cycle, the difference between emission maxima was only 21 nm (from 600 to 579 nm), in contrast to the first grinding-fuming with a difference of 61 nm. On the other side, AC-PFBA showed remarkable recovery properties under fuming since the most significant difference between the ground and the fumed samples was only 21 nm and occurred during the second cycle.
Afterward, we tested the thermal-induced regenerating capabilities of the ground powders. First, we decided to gather information on the thermal stability and behavior of ground powders. The resulting calorimetric and thermogravimetric analysis can be visualized in Fig. 9a. AC-TFTA, in its ground form, showed notable changes in the DSC curve in comparison with the pristine solid. From 78 °C and up to 245 °C, the DSC displays a progressive, increasing heat flow, which can be associated with the gradual migration of the amorphous solid to regenerate the crystalline arrangement. Afterward, an exotherm at 252 °C, which is aligned with a mass loss of 22% (from the TGA curve), could be related to the progressive decomposition of TFTA, expelling CO2. Finally, an endothermal peak towards 267 °C features the melting of AC coformer.
A similar case is exposed for AC-PFBA ground, which displays a slow but continuous heat entry until an endotherm at 166 °C is evident, in agreement with the expulsion of PFBA and a 35% loss of starting mass. Afterward, at ca. 266 °C, as happened in AC-TFTA, an endotherm is presented, which could also be associated with the melting of AC.
To obtain visual evidence of the recovery of crystallinity under thermal stimuli, amounts of both ground cocrystals were placed on a glass holder and put on a hot plate at 80 °C for 10 minutes. The transition was recorded in Movie S7 (ESI†), and the characterization of the thermal-treated powders was carried out through PXRD. The diffractograms are exposed in Fig. 9b and c, with insets of the appearance of the resulting solids after the thermal treatment. Despite the recovery of some of the initial crystallinity, especially in AC-TFTA, it is notorious that the crystalline phase is not fully recovered under the conditions employed, with the presence of a higher amorphous component in comparison with the PXRD obtained for the fuming procedure.
The regeneration of the crystalline phases due to external stimuli is an ongoing field of study. So far, the evidence collected not only in this manuscript but also in previous works suggests that the presence of highly twisted, rigid fragments plays a role in the change in the photophysical properties during the grinding process, overall, changing the dihedral angles between fragments, modifying the π-conjugation across the system, with a subsequent change in the absorption of the material, as visualized from Fig. 6.9,46,63 A synergistic effect between the amorphization of the crystalline structure and the torsional changes of molecules give rise to a metastable phase. This phase, overall, in an amorphous state, should contain seeds of remaining microcrystals capable of inducing the regeneration of the crystalline solid when the external stimuli are applied. The degree of regeneration of these materials can vary with the anisotropy of the crystalline packing.33 If the distribution of the non-covalent interaction energies around the molecules is similar, as in the case of AC-PFBA herein presented, the recovery of the material should occur with a high probability. On the contrary, if some energies are remarkably different from the rest, as in AC-TFTA, the regeneration of the crystalline phase will rely primarily on the recovery of these non-covalent interactions, with a lower probability of recovery and a high tendency towards fatigue.
3.8. Potential of AC-TFTA towards application in anticounterfeiting
Finally, due to the dramatic change of color upon grinding and to qualitatively show the possible use of AC-TFTA towards anticounterfeiting applications, a filter paper was soaked in a DCM/MeOH solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing AC and TFTA. The paper was left to slowly dry at room temperature, generating a yellow canvas in which, by applying mechanical pressure with a mortar pestle, we first drew the silhouette of a flat-bottom flask, as depicted in Fig. 9d. Then, the paper was treated in a chamber containing DCM vapors for 10 minutes. It was observed that the first figure was erased, and the initial yellow canvas was left almost intact. On the same paper, we proceeded to draw the letters ‘IQ’, which stands for “Instituto de Química,” then the paper was heated on a hot surface at ca. 80 °C for 10 minutes, which produced the same erasing effect. This experiment led us to corroborate that the regeneration of the photophysical properties of AC-TFTA not only occurs in the microcrystalline solid but also when it is supported on a paper surface.
1) containing AC and TFTA. The paper was left to slowly dry at room temperature, generating a yellow canvas in which, by applying mechanical pressure with a mortar pestle, we first drew the silhouette of a flat-bottom flask, as depicted in Fig. 9d. Then, the paper was treated in a chamber containing DCM vapors for 10 minutes. It was observed that the first figure was erased, and the initial yellow canvas was left almost intact. On the same paper, we proceeded to draw the letters ‘IQ’, which stands for “Instituto de Química,” then the paper was heated on a hot surface at ca. 80 °C for 10 minutes, which produced the same erasing effect. This experiment led us to corroborate that the regeneration of the photophysical properties of AC-TFTA not only occurs in the microcrystalline solid but also when it is supported on a paper surface.
4. Conclusions
In this manuscript, we have taken advantage of the cocrystallization technique through the usage of an acridine/carbazole hydrogen-bond acceptor and thoughtfully combined it with two fluorinated carboxylic acids. Despite the apparent similarities in the structures of the acids, we found very different non-covalent interactions, leading to varying behaviors of the cocrystals upon mechanical grinding. Even more interestingly, we have obtained insights into the structural and spectroscopic features of the pristine and ground cocrystals, as well as described the regeneration processes of their crystallinity under solvent and thermal stimuli, provided feasible origins of the photoluminescence in both structures, and characterized both facets of these solids via solid-state NMR techniques, UV-Vis and fluorescence spectroscopy, and differential scanning calorimetry. Our results show that the cocrystal AC-TFTA features a more fragile crystalline packing than its analogue AC-PFBA, which is translated into more dramatic changes in photoluminescence, UV-Vis absorption, and thermal stability. Interestingly, one of the cocrystals showed a high-temperature jumping effect due to decarboxylation. The work herein reported provides deep insights into the development of stimuli-responsive materials with additional jumping capabilities through the release of gas as a driving force.Data availability
The data supporting this article has been included as part of the ESI.† Further information can be provided upon reasonable request from the corresponding author. E-mail: brodriguez@iquimica.unam.mx.Conflicts of interest
The authors declare no competing interests.Acknowledgements
DGAPA PAPIIT IN207222 and CONAHCYT Ciencia de Frontera 1715644 financially supported this work. A. N.-H. Thanks, CONAHCYT, for a PhD scholarship (957838). Y. A. A.-S. acknowledges the support from the DGAPA-UNAM postdoctoral fellowship. We acknowledge the technical assistance from Mayra León-Santiago MSc. (GC-MS, Laboratorio Nacional de Ciencias para la Investigación y Conservación del Patrimonio Cultural, LANCIC), Dr Ma. C. García-González and Dr J. F. Pérez-Flores (MS); Dr M. E. García-Aguilera, Ma. Angeles Peña-González BSc, Dr B. Quiroz, Dr I. Chávez, E. Huerta-Salazar MSc and Dr R. Gaviño (solution NMR); Dr U. Hernández, M. Tapia MSc (PXRD); Dr A. Romo-Pérez (FTIR); Dr D. Martínez-Otero (SCXRD) and MSc. Virginia Gomez-Vidales (EPR). We thank Dr P. Guadarrama, Dr Diego Solis-Ibarra and N.C. Cabrera-Quiñones MSc for instrumentation access. We are also thankful for computer time LANCAD-UNAM-DGTIC-392. R. C. O. and M. E. S. S. acknowledge the use of the UCL High Performance Computing Facilities Myriad@UCL and Kathleen@UCL and funding by the UK Research and Innovation under the UK government's Horizon Europe funding guarantee (grant number EP/X020908/2).Notes and references
- F. Khan, A. Ekbote, G. Singh and R. Misra, Mechanochromic luminogens with hypsochromically shifted emission switching property: recent advances and perspectives, J. Mater. Chem. C, 2022, 10, 5024–5064 RSC.
- J. Guo, C. Hu, J. Liu, Y. Wang and L. Ma, Mechanochromism, tunable pure organic room temperature phosphorescence, single-molecule near-white emission, digital encryption, and anti-counterfeiting, Dyes Pigm., 2024, 221, 111760 CrossRef CAS.
- Y. Yin, Q. Guan, Z. Chen, D. Deng, S. Liu, Y. Sun and S. H. Liu, Force-triggered hypso- and bathochromic bidirectional fluorescence switching beyond 120 nm and its anticounterfeiting applications, Sci. Adv., 2024, 10, eadk5444 CrossRef CAS PubMed.
- W. Shi, B. Rong, S. Li, Y. Lin, Y. Liao, C. Cao, S. Pan and B. Wei, Mechanochromic properties of acridine heterocyclic derivatives with a donor-acceptor configuration, J. Mol. Struct., 2024, 1308, 138050 CrossRef CAS.
- P. Gayathri, S. Ravi, P. Nantheeswaran, M. Mariappan, S. Karthikeyan, M. Pannipara, A. G. Al-Sehemi, D. Moon and S. P. Anthony, CF3 H-bonding locked aromatic stacking of picric acid with mechanofluorochromic fluorophores: highly selective reusable sensor and rewritable fluorescence platform, Mol. Syst. Des. Eng., 2022, 7, 1277–1286 RSC.
- P. Gayathri, P. Nantheeswaran, M. Mariappan, S. Karthikeyan, M. Pannipara, A. G. Al-Sehemi, D. Moon and S. P. Anthony, Methoxy substituent facilitated wide solvatofluorochromism, white light emission, polymorphism and stimuli-responsive fluorescence switching in donor-π-acceptor, Spectrochim. Acta, Part A, 2023, 286, 121989 CrossRef CAS PubMed.
- Y. Wang, W. Liu, L. Ren and G. Ge, Deep insights into polymorphism initiated by exploring multicolor conversion materials, Mater. Chem. Front., 2019, 3, 1661–1670 RSC.
- S. Saotome, K. Suenaga, K. Tanaka and Y. Chujo, Design for multi-step mechanochromic luminescence property by enhancement of environmental sensitivity in a solid-state emissive boron complex, Mater. Chem. Front., 2020, 4, 1781–1788 RSC.
- M. C. García-González, A. Navarro-Huerta, F. C. Rodríguez-Muñoz, E. G. Vera-Alvízar, M. A. Vera Ramírez, J. Rodríguez-Hernández, M. Rodríguez and B. Rodríguez-Molina, The design of dihalogenated TPE monoboronate complexes as mechanofluorochromic crystals, CrystEngComm, 2021, 23, 5908–5917 RSC.
- J. Xiong, K. Wang, Z. Yao, B. Zou, J. Xu and X.-H. Bu, Multi-Stimuli-Responsive Fluorescence Switching from a Pyridine-Functionalized Tetraphenylethene AIEgen, ACS Appl. Mater. Interfaces, 2018, 10, 5819–5827 CrossRef CAS PubMed.
- T. Sun, D. Cheng, Y. Chai, J. Gong, M. Sun and F. Zhao, High contrast mechanofluorochromic behavior of new tetraphenylethene-based Schiff base derivatives, Dyes Pigm., 2019, 170, 107619 CrossRef CAS.
- S. Takahashi, S. Nagai, M. Asami and S. Ito, Two types of two-step mechanochromic luminescence of phenanthroimidazolylbenzothiadiazoles, Mater. Adv., 2020, 1, 708–719 RSC.
- R. Kubota, Y. Yuan, R. Yoshida, T. Tachikawa and S. Ito, Tunable mechanochromic luminescence via surface protonation of pyridyl-substituted imidazole crystals, Mater. Adv., 2022, 3, 5826–5835 RSC.
- W. Wang, R. Li, S. Xiao, Q. Xing, X. Yan, J. Zhang, X. Zhang, H. Lan and T. Yi, Design of High-Contrast Mechanochromic Materials Based on Aggregation-Induced Emissive Pyran Derivatives Guided by Polymorph Predictions, CCS Chem., 2022, 4, 899–909 CrossRef CAS.
- T. Seki, Y. Takamatsu and H. Ito, A Screening Approach for the Discovery of Mechanochromic Gold(I) Isocyanide Complexes with Crystal-to-Crystal Phase Transitions, J. Am. Chem. Soc., 2016, 138, 6252–6260 CrossRef CAS PubMed.
- R. Zhang, J.-W. Liu, W.-Y. Zhong, J.-L. Chen, F. Zhao, S.-J. Liu and H.-R. Wen, Mechanochromic and Selective Vapochromic Solid-State Luminescence of a Dinuclear Cuprous Complex, Inorg. Chem., 2023, 62, 11510–11517 CrossRef CAS PubMed.
- P. Yu, D. Peng, L.-H. He, J.-L. Chen, J.-Y. Wang, S.-J. Liu and H.-R. Wen, A Mechanochromic and Vapochromic Luminescent Cuprous Complex Based on a Switchable Intramolecular π⋯π Interaction, Inorg. Chem., 2022, 61, 254–264 CrossRef CAS PubMed.
- S. Cheng, Z. Chen, Y. Yin, Y. Sun and S. Liu, Progress in mechanochromic luminescence of gold(I) complexes, Chin. Chem. Lett., 2021, 32, 3718–3732 CrossRef CAS.
- C. Xing, Z. Qi, B. Zhou, D. Yan and W.-H. Fang, Solid-State Photochemical Cascade Process Boosting Smart Ultralong Room-Temperature Phosphorescence in Bismuth Halides, Angew. Chem., Int. Ed., 2024, 63, e202402634 CrossRef CAS PubMed.
- Y.-J. Ma, G. Xiao, X. Fang, T. Chen and D. Yan, Leveraging Crystalline and Amorphous States of a Metal-Organic Complex for Transformation of the Photosalient Effect and Positive-Negative Photochromism, Angew. Chem., Int. Ed., 2023, 62, e202217054 CrossRef CAS PubMed.
- D. K. Sivadas, P. Gayathri, S. Ravi, S. Karthikeyan, M. Pannipara, A. G. Al-Sehemi, D. Moon, S. P. Anthony and V. Madhu, Distinct fluorescence state, mechanofluorochromism of terpyridine conjugated fluorophores and the reusable sensing of nitroaromatics in aqueous medium, New J. Chem., 2023, 47, 12770–12778 RSC.
- H.-W. Liang, T. Jia, X. Yong, J.-K. Yu, Y. Yi, S. Lu, L. Wang and H. Wang, Photoinduced Single-Crystal to Single-Crystal Transformation via Conformational Change with Turn-On Fluorescence, Cryst. Growth Des., 2022, 22, 2082–2086 CrossRef CAS.
- S. Ito, Recent Advances in Mechanochromic Luminescence of Organic Crystalline Compounds, Chem. Lett., 2021, 50, 649–660 CrossRef CAS.
- L. Sun, W. Zhu, X. Zhang, L. Li, H. Dong and W. Hu, Creating Organic Functional Materials beyond Chemical Bond Synthesis by Organic Cocrystal Engineering, J. Am. Chem. Soc., 2021, 143, 19243–19256 CrossRef CAS PubMed.
- L. Sun, Y. Wang, F. Yang, X. Zhang and W. Hu, Cocrystal Engineering: A Collaborative Strategy toward Functional Materials, Adv. Mater., 2019, 31, 1902328 CrossRef PubMed.
- L. Sun, W. Zhu, F. Yang, B. Li, X. Ren, X. Zhang and W. Hu, Molecular cocrystals: Design, charge-transfer and optoelectronic functionality, Phys. Chem. Chem. Phys., 2018, 20, 6009–6023 RSC.
- D. Yan, A. Delori, G. O. Lloyd, T. Friščić, G. M. Day, W. Jones, J. Lu, M. Wei, D. G. Evans and X. Duan, A cocrystal strategy to tune the luminescent properties of stilbene-type organic solid-state materials, Angew. Chem., Int. Ed., 2011, 50, 12483–12486 CrossRef CAS PubMed.
- B. Zhou, Q. Zhao, L. Tang and D. Yan, Tunable room temperature phosphorescence and energy transfer in ratiometric co-crystals, Chem. Commun., 2020, 56, 7698–7701 RSC.
- Q. Chen, X. Chen, Y. Han, T. Zhang, C.-P. Li, J. Mu, J. Zhang, J. Hao and P. Xue, Multistimuli-Responsive Fluorescent Switches Based on Reversible Decomposition and Regeneration of charge-transfer Complexes, Cryst. Growth Des., 2022, 22, 693–702 CrossRef CAS.
- Y. Liu, A. Li, S. Xu, W. Xu, Y. Liu, W. Tian and B. Xu, Reversible Luminescent Switching in an Organic Cocrystal: Multi-Stimuli-Induced Crystal-to-Crystal Phase Transformation, Angew. Chem., Int. Ed., 2020, 59, 15098–15103 CrossRef CAS PubMed.
- S. Wang, W. Xiang, C. Pan, J. Chen, W. Li, J. Zhang, J. Zhao and G. Liu, Changes in piezochromic luminescence of a charge transfer complex subjected to grinding and isotropic compression, CrystEngComm, 2023, 25, 3861–3865 RSC.
- S. Ito, C. Nishimoto and S. Nagai, Sequential halochromic/mechanochromic luminescence of pyridyl-substituted solid-state emissive dyes: thermally controlled stepwise recovery of the original emission color, CrystEngComm, 2019, 21, 5699–5706 RSC.
- Y. Sun, Y. Ye, X. Hu, Y. Bai and C. Xie, Mechano-/seeding-triggered crystal-to-crystal phase transition in luminescent switching cocrystals, Dyes Pigm., 2023, 215, 111275 CrossRef CAS.
- A. A. Ganie and A. A. Dar, Water Switched Reversible Thermochromism in Organic Salt of Sulfonated Anil, Cryst. Growth Des., 2021, 21, 3014–3023 CrossRef CAS.
- I. Ahmad and A. A. Dar, Switching the Solid-State Emission of Organic Crystals through Coformer Choice and Vapochromism, J. Phys. Chem. C, 2023, 127, 18684–18693 CrossRef CAS.
- J. S. Zambounis, Z. Hao and A. Iqbal, Latent pigments activated by heat, Nature, 1997, 388, 131–132 CrossRef CAS.
- Y. Chen, J. Li and J. Gong, Jumping Crystal Based on an Organic Charge Transfer Complex with Reversible ON/OFF Switching of Luminescence by External Thermal Stimuli, ACS Mater. Lett., 2021, 3, 275–281 CrossRef CAS.
- S. Das, L. Catalano and Y. Geerts, Gas Release as an Efficient Strategy to Tune Mechanical Properties and Thermoresponsiveness of Dynamic Molecular Crystals, Small, 2024, 2401317 CrossRef PubMed.
- S. Li and D. Yan, Tuning Light-Driven Motion and Bending in Macroscale-Flexible Molecular Crystals Based on a Cocrystal Approach, ACS Appl. Mater. Interfaces, 2018, 10, 22703–22710 CrossRef CAS PubMed.
- S. Li, B. Lu, X. Fang and D. Yan, Manipulating Light-Induced Dynamic Macro-Movement and Static Photonic Properties within 1D Isostructural Hydrogen-Bonded Molecular Cocrystals, Angew. Chem., Int. Ed., 2020, 59, 22623–22630 CrossRef CAS PubMed.
- A. Navarro-Huerta, M. J. Jellen, J. Arcudia, S. J. Teat, R. A. Toscano, G. Merino and B. Rodríguez-Molina, Tailoring the cavities of hydrogen-bonded amphidynamic crystals using weak contacts: towards faster molecular machines, Chem. Sci., 2021, 12, 2181–2188 RSC.
- S. Zeghada, G. Bentabed-Ababsa, O. Mongin, W. Erb, L. Picot, V. Thiéry, T. Roisnel, V. Dorcet and F. Mongin, 2-Aminobenzaldehyde, a common precursor to acridines and acridones endowed with bioactivities, Tetrahedron, 2020, 76, 131435 CrossRef CAS.
- H. Koshima, Solid State Bimolecular Photoreaction in a Simple Polycrystalline Mixture of Acridine and Carbazole, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 2001, 356, 483–486 CrossRef CAS.
- U. Garg, Y. Azim, M. Alam, A. Kar and C. P. Pradeep, Extensive Analyses on Expanding the Scope of Acid–Aminopyrimidine Synthons for the Design of Molecular Solids, Cryst. Growth Des., 2022, 22, 4316–4331 CrossRef CAS.
- L. Bian, H. Zhao, H. Hao, Q. Yin, S. Wu, J. Gong and W. Dong, Novel Glutaric Acid Cocrystal Formation via Cogrinding and Solution Crystallization, Chem. Eng. Technol., 2013, 36, 1292–1299 CrossRef CAS.
- H. Zhu, S. Weng, H. Zhang, H. Yu, L. Kong, Y. Zhong, Y. Tian and J. Yang, A novel carbazole derivative containing fluorobenzene unit: aggregation-induced fluorescence emission, polymorphism, mechanochromism and non-reversible thermo-stimulus fluorescence, CrystEngComm, 2018, 20, 2772–2779 RSC.
- Y. Shen, J. Hou, Y. Liu, X. Wan, M. Bai, X. Jiang, W. Duan and F. Yan, Deep insight into the charge-transfer cocrystals: Decreasing structural overlap induced bathochromically shift emission, Dyes Pigm., 2023, 215, 111277 CrossRef CAS.
- E. A. Hernández-Morales, A. Colin-Molina, J. Arcudia, F. J. Hernández, M. Rodríguez, R. A. Toscano, R. Crespo-Otero, G. Merino and B. Rodríguez-Molina, Indolocarbazole as a Platform for Concatenated Crystalline Rotors, Cryst. Growth Des., 2023, 23, 6785–6794 CrossRef.
- Q. Li and Z. Li, The Strong Light-Emission Materials in the Aggregated State: What Happens from a Single Molecule to the Collective Group, Adv. Sci., 2017, 4, 1600484 CrossRef PubMed.
- J.-L. Bredas, Mind the gap!, Mater. Horiz., 2014, 1, 17–19 RSC.
- M. Rivera, M. Dommett and R. Crespo-Otero, ONIOM(QM:QM′) Electrostatic Embedding Schemes for Photochemistry in Molecular Crystals, J. Chem. Theory Comput., 2019, 15, 2504–2516 CrossRef CAS PubMed.
- M. Rivera, M. Dommett, A. Sidat, W. Rahim and R. Crespo-Otero, fromage: A library for the study of molecular crystal excited states at the aggregate scale, J. Comput. Chem., 2020, 41, 1045–1058 CrossRef CAS PubMed.
- F. J. Hernández and R. Crespo-Otero, Excited state mechanisms in crystalline carbazole: the role of aggregation and isomeric defects, J. Mater. Chem. C, 2021, 9, 11882–11892 RSC.
- L. A. Rodríguez-Cortés, F. J. Hernández, M. Rodríguez, R. A. Toscano, A. Jiménez-Sánchez, R. Crespo-Otero and B. Rodríguez-Molina, Conformational emissive states in dual-state emitters with benzotriazole acceptors, Matter, 2023, 6, 1140–1159 CrossRef.
- S. Ma, S. Du, G. Pan, S. Dai, B. Xu and W. Tian, Organic molecular aggregates: From aggregation structure to emission property, Aggregate, 2021, 2, e96 CrossRef CAS.
- C. F. Mackenzie, P. R. Spackman, D. Jayatilaka and M. A. Spackman, CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems, IUCrJ, 2017, 4, 575–587 CrossRef CAS PubMed.
- M. A. Spackman, P. R. Spackman and S. P. Thomas, in Complementary Bonding Analysis, ed. S. Grabowsky, D. Gruyter, 2021, pp. 329–352 Search PubMed.
- A. Lemmerer, D. A. Adsmond, C. Esterhuysen and J. Bernstein, Polymorphic Co-crystals from Polymorphic Co-crystal Formers: Competition between Carboxylic Acid⋯Pyridine and Phenol⋯Pyridine Hydrogen Bonds, Cryst. Growth Des., 2013, 13, 3935–3952 CrossRef CAS.
- P. P. Mazzeo, S. Canossa, C. Carraro, P. Pelagatti and A. Bacchi, Systematic coformer contribution to cocrystal stabilization: energy and packing trends, CrystEngComm, 2020, 22, 7341–7349 RSC.
- M. Mirzaei, F. Sadeghi, K. Molčanov, J. K. Zarȩba, R. M. Gomila and A. Frontera, Recurrent Supramolecular Motifs in a Series of Acid-Base Adducts Based on Pyridine-2,5-Dicarboxylic Acid N-Oxide and Organic Bases: Inter- And Intramolecular Hydrogen Bonding, Cryst. Growth Des., 2020, 20, 1738–1751 CrossRef CAS.
- N. Bedeković, V. Stilinović and T. Piteša, Aromatic versus Aliphatic Carboxyl Group as a Hydrogen Bond Donor in Salts and Cocrystals of an Asymmetric Diacid and Pyridine Derivatives, Cryst. Growth Des., 2017, 17, 5732–5743 CrossRef.
- A. Navarro-Huerta, D. A. Hall, J. Blahut, V. Gómez-Vidales, S. J. Teat, J. M. Marmolejo-Tejada, M. Dračínský, M. A. Mosquera and B. Rodríguez-Molina, Influence of Internal Molecular Motions in the Photothermal Conversion Effect of Charge-Transfer Cocrystals, Chem. Mater., 2023, 35, 10009–10017 CrossRef CAS.
- P. Gayathri, M. Pannipara, A. G. Al-Sehemi and S. P. Anthony, Triphenylamine-based stimuli-responsive solid state fluorescent materials, New J. Chem., 2020, 44, 8680–8696 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and experimental procedures, spectroscopic data (NMR, FTIR, HRMS), VT ssNMR spectra, final crystallographic refinement parameters, PXRD diffractograms, Hirshfeld surface maps, DFT computational details and ESR spectra. CCDC 2351862–2351865. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qm00421c |
| This journal is © the Partner Organisations 2024 |