 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Piano-stool metal complexes as inhibitors of amyloid-β aggregation in vitro and in vivo†
Gloria
Vigueras
 a,
Raimon
Sabate
bc,
Leoní A.
Barrios
a,
Raimon
Sabate
bc,
Leoní A.
Barrios
 cd,
Ana B.
Caballero
*cd,
Samanta
Hernández-García
cd,
Ana B.
Caballero
*cd,
Samanta
Hernández-García
 f,
Pau
Bayón
g,
Fernando
Gandía-Herrero
f,
Pau
Bayón
g,
Fernando
Gandía-Herrero
 *f,
José
Ruiz
*f,
José
Ruiz
 *a and
Patrick
Gamez
*a and
Patrick
Gamez
 cde
cde
aDepartamento de Química Inorgánica, Universidad de Murcia, Biomedical Research Institute of Murcia (IMIB-Arrixaca), E-30100 Murcia, Spain. E-mail: jruiz@um.es
bDepartament de Farmàcia, Tecnologia Farmacèutica i Fisicoquímica, Secció Fisicoquímica, Facultat de Farmàcia i Ciències de l'Alimentació, Joan XXIII 27-31 08020-Barcelona, Joan XXIII 27-31, E-08020 Barcelona, Spain
cInstitute of Nanoscience and Nanotechnology (IN2UB), Universitat de Barcelona, 08028 Barcelona, Spain
dDepartament de Química Inorgànica i Orgànica, Secció Química Inorgànica, Facultat de Química, Universitat de Barcelona, Martí i Franquès 1-11, E-08028 Barcelona, Spain. E-mail: ana.caballero@ub.edu
eCatalan Institution for Research and Advanced Studies (ICREA), Passeig Lluís Companys 23, 08010 Barcelona, Spain
fDepartamento de Bioquímica y Biología Molecular A, Unidad Docente de Biología, Facultad de Veterinaria. Regional Campus of International Excellence “Campus Mare Nostrum”. Universidad de Murcia, E-30100 Murcia, Spain. E-mail: fgandia@um.es
gDepartament de Química, Facultat de Ciències, Universitat Autònoma de Barcelona, Campus Bellaterra, 08193 Cerdanyola del Vallès, Barcelona, Spain
First published on 30th July 2024
Abstract
Alzheimer's disease (AD) is the most common form of dementia worldwide. There is currently no cure for this neurodegenerative disorder, and the available therapies only temporarily lessen some symptoms rather than stopping the disease's progression. One of the pathological hallmarks in AD brains is the formation of amyloid-β plaques. Transition-metal complexes have aroused great interest as potential chemical modulators of Aβ aggregation thanks to intrinsic features such as the metal oxidation state or the coordination geometry. Four related piano-stool complexes with different metal ions, namely Ru(II), Os(II), Ir(III) and Rh(III), were prepared and their inhibition properties of Aβ aggregation were investigated. It was found that all of them favour an alternative folding pathway, impeding the formation of mature fibres. Moreover, the metal centre seems to play a crucial role in their inhibiting activities. Ru(II) (1), Ir(III) (3) and Rh(III) (4) compounds were remarkable inhibitors of Aβ aggregation in vitro, most particularly 4, and they appear to share the inhibitory mechanism. However Os(II) complex 2 acts differently on the Aβ aggregation process. In vivo studies using a Caenorhabditis elegans animal model of AD revealed the potential of complexes 2 and 4, with 2 exhibiting better inhibition potential than 4, thus illustrating the possible occurrence of additional variables to the aggregation process when moving from in vitro experiments to a living organism. To the best of our knowledge, 2 is the first osmium complex reported as an effective inhibitor of amyloid-β aggregation both in vitro and in vivo.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative cerebral disease giving rise to synaptic reduction and cognitive decline, eventually leading to death.1 AD is characterized by the presence of extracellular plaques of amyloid-β (Aβ) peptide and intracellular neurofibrillary tangles of hyperphosphorylated tau protein in the brain of patients.2Targeting Aβ overproduction, aggregation and toxicity represents an active area of AD research in chemistry.3–7 The roles of these pathological events in AD progression,8,9 including the involvement of biometals like zinc and copper,10,11 have been investigated thoroughly,5,12 and they are still considered to be potential therapeutic targets.13 Several anti-aggregation agents,14,15 metal chelators16,17 and antioxidants18,19 have been developed in the last three decades.20 However, the track record of success in AD clinical trials so far has been very poor. For that reason, the use of in vivo models of the disease is important to evaluate the potential of novel therapeutic approaches.21,22
The use of metal complexes to tackle neurological issues induced by Aβ is a research topic of growing interest.23–26 Both AD therapy and diagnosis (e.g., imaging) with transition-metal complexes are currently being investigated.27 For instance, 64Cu, 68Ga and 89Zr complexes have been developed for PET imaging.28 Fluorescent probes based on Ru(II), Re(I), Ir(III) and Pt(II) have been reported for the detection of Aβ aggregates.2999mTc and 111In coordination compounds have been used for SPECT imaging,28 and Gd-based contrast agents have been designed for MRI applications.27,30 Various transition metals, including Co(III), Ni(II), V(V), Rh(III), Ir(III), Ru(II), Ru(III) and Pt(II) have been studied as potential anti-AD agents.31,32 A number of metal complexes have also been reported for theranostic applications, for example, some Ru(II),33,34 Ir(III)35 and Gd(III)36 coordination species.6,27
In the last years, Ru(II) complexes have gained interest in the field of Aβ aggregation after reports on the use of polypyridyl-type37,38 or NAMI-A-like39,40 compounds. Hence, Ru(II)41,42 and Ru(III)43–45 complexes are increasingly described in the literature. Comparatively, Ir(III)46 and Rh(III)47 complexes to modulate Aβ aggregation are much less reported.
Piano-stool metal complexes have been reported to exhibit interesting inhibitory activities against Aβ aggregation,48 and some benzimidazole scaffolds have been described to have anti-AD properties.49,50
Herein, the quinoline benzimidazole-based ligand L1 (Chart 1) was used for the preparation of four piano-stool complexes with four different transition metals and two distinct π-arene rings: [RuCl(η6-p-cym)L1]PF6 (1), [OsCl(η6-p-cym)L1]PF6 (2), [IrCl(η5-Cp*)L1]PF6 (3) and [RhCl(η5-Cp*)L1]PF6 (4) (with p-cym = p-cymene and Cp* = pentamethylcyclopentadienyl). The effect of these complexes on the aggregation of Aβ in vitro was subsequently investigated by using thioflavin T (ThT) fluorescence, dynamic light scattering (DLS), transmission electron microscopy (TEM) and ESI-MS. Subsequently, in vivo assays were performed with Caenorhabditis elegans mutant strains expressing human amyloid peptide either in their body muscle or in their neurons. To the best of our knowledge, the first Os(II) complex acting as an inhibitor of amyloid-β aggregation both in vitro and in vivo has been identified.
Results and discussion
Preparation and characterization of compounds 1–4
Complexes 1–4 were typically prepared by reaction of the corresponding [(π-arene)MCl2]2 dimer and L1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 in methanol at room temperature, as depicted in Scheme 1 (see Experimental section for details). The dimer precursors [(η6-p-cymene)RuCl2]2,52 [(η6-p-cymene)OsCl2]2,53 [(η5-C5Me5)IrCl2]2
51 in methanol at room temperature, as depicted in Scheme 1 (see Experimental section for details). The dimer precursors [(η6-p-cymene)RuCl2]2,52 [(η6-p-cymene)OsCl2]2,53 [(η5-C5Me5)IrCl2]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 and [(η5-C5Me5)RhCl2]2
54 and [(η5-C5Me5)RhCl2]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 were prepared as reported in the literature. The complexes were obtained with yields ranging from 42 to 87%. The compounds were characterized by NMR, UV-Vis spectroscopy, ESI-MS and elemental analysis.
54 were prepared as reported in the literature. The complexes were obtained with yields ranging from 42 to 87%. The compounds were characterized by NMR, UV-Vis spectroscopy, ESI-MS and elemental analysis.
The stability of the four complexes was evaluated in phosphate-buffered saline (PBS) containing 5% of DMSO ([complex] = 10 μM) for 24 hours at 37 °C, using UV-Vis spectroscopy (Fig. S1†). No spectral variations were observed for all compounds after one day, indicating that they remained unchanged in solution, under the conditions used. The decrease of the absorbance intensity observed for 4 suggests a gradual precipitation of the complex in PBS/5% DMSO.
Single crystals suitable for X-ray diffraction analysis were obtained from the slow diffusion of hexane into a saturated solution of complex 4 in CH2Cl2. This compound crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and exhibits the typical “three-legged piano-stool” geometry with bond lengths ranging from 2.105(2) to 2.397(1) Å (Table S1†) and a π-ring centroid⋯Rh distance of 1.780(1) Å (Table S2†). A representation of the solid-state structure of 4 is given in Fig. 1. The coordination angles, including those involving the π-ring centroid, vary from 75.07(7) to 128.85(6)°. Bond lengths and angles are similar to those for other comparable organorhodium(III) complexes.55,56 The full crystal data and structure refinement parameters are listed in Table S1,† and selected bond distances and angles are given in Table S2.†
, and exhibits the typical “three-legged piano-stool” geometry with bond lengths ranging from 2.105(2) to 2.397(1) Å (Table S1†) and a π-ring centroid⋯Rh distance of 1.780(1) Å (Table S2†). A representation of the solid-state structure of 4 is given in Fig. 1. The coordination angles, including those involving the π-ring centroid, vary from 75.07(7) to 128.85(6)°. Bond lengths and angles are similar to those for other comparable organorhodium(III) complexes.55,56 The full crystal data and structure refinement parameters are listed in Table S1,† and selected bond distances and angles are given in Table S2.†
In vitro evaluation of the anti-amyloid properties of 1–4 against Aβ40 peptide
The effect of complexes 1–4 on the aggregation of Aβ40 was investigated using thioflavin T (ThT), which gives strong fluorescence upon binding to amyloid fibrils characterized by the presence of β sheets (λexc = 450 nm; λem = 482 nm).57 First, we verified that the fluorescence emission of 1–4 was falling out of that of ThT. Actually, all the compounds emit in the range 403–422 nm, which is far enough from the strong emission of amyloid-bound ThT at 482 nm (Fig. S2†).The complexes were first incubated with monomeric Aβ40 at a 5-fold excess, and the protein aggregation was monitored in PBS at 37 °C (Fig. S3†). All the complexes completely inhibited Aβ40 aggregation as ThT fluorescence was not detected, even after 16 h (data not shown).
Given the efficacy shown by the four compounds at the tested complex-to-Aβ ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, aggregation experiments were then carried out at a lower molar ratio, namely 1
1, aggregation experiments were then carried out at a lower molar ratio, namely 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2). Under these conditions, distinct behaviours were observed for the different metal-containing compounds. To better compare their anti-amyloid behaviour, the aggregation curves were fitted to a two-step autocatalytic process (Scheme 2).58 The calculated lag time (t0), half aggregation time (t1/2) and end time of the aggregation (t1), are listed in Table 1. Ir(III) complex 3 and Rh(III) complex 4 drastically hinder amyloid aggregation, displaying nearly complete inhibition (Fig. 2). Thus, after 24 hours, 89 and 95% inhibition of Aβ40 aggregation were achieved respectively for 3 and 4 (Table 1). Ru(II) complex 1 and Os(II) complex 2 exhibited more modest inhibitory properties than 3 and 4. Interestingly, while the inhibition by complex 1 shows an unequivocal increase in t0, t1/2 and t1 (Table 1), these time values remain practically unaltered in the presence of 2, suggesting different inhibition pathways. As shown in Fig. 2, complexes 3 and 4 increase the lag time (t0) to 250–250 minutes (ThT fluorescence remains practically unchanged within these times), suggesting that these complexes might display a similar inhibitory mechanism to complex 1.
1 (Fig. 2). Under these conditions, distinct behaviours were observed for the different metal-containing compounds. To better compare their anti-amyloid behaviour, the aggregation curves were fitted to a two-step autocatalytic process (Scheme 2).58 The calculated lag time (t0), half aggregation time (t1/2) and end time of the aggregation (t1), are listed in Table 1. Ir(III) complex 3 and Rh(III) complex 4 drastically hinder amyloid aggregation, displaying nearly complete inhibition (Fig. 2). Thus, after 24 hours, 89 and 95% inhibition of Aβ40 aggregation were achieved respectively for 3 and 4 (Table 1). Ru(II) complex 1 and Os(II) complex 2 exhibited more modest inhibitory properties than 3 and 4. Interestingly, while the inhibition by complex 1 shows an unequivocal increase in t0, t1/2 and t1 (Table 1), these time values remain practically unaltered in the presence of 2, suggesting different inhibition pathways. As shown in Fig. 2, complexes 3 and 4 increase the lag time (t0) to 250–250 minutes (ThT fluorescence remains practically unchanged within these times), suggesting that these complexes might display a similar inhibitory mechanism to complex 1.
 | ||
| Fig. 2 Aggregation of Aβ40 in the presence of equimolar amounts of complexes 1–4. [Aβ40] = 10 μM, PBS. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Control | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| a [Aβ40] = 10 μM; [complex] = 10 μM. Standard error (SD) < 5%. | |||||
| t 0 (min) | 34.1 | 53.4 | 39.0 | — | — |
| t 1/2 (min) | 47.4 | 98.4 | 53.7 | — | — |
| t 1 (min) | 60.8 | 143.4 | 68.4 | — | — |
| Inhibition (%) | 0.0 | 81.0 | 77.7 | 89.4 | 95.1 |
Isostructural complexes 1 and 2 (with metals of group 8) led to analogous overall inhibition of amyloid aggregation but different pathways are suggested. Ru(II) compound 1 inhibits aggregation by delaying both the formation of nuclei and the subsequent fibrillation. In the case of isostructural 3 and 4 (with metals of group 9), hardly any differences in anti-aggregation activity were observed between them at equimolar concentrations to that of the protein. Unlike 1 and 2, complexes 3 and 4 inhibited Aβ40 aggregation strongly, Rh(III) complex 4 being slightly stronger than Ir(III) compound 3. Overall, neither the nature of the π-arene ligand nor the oxidation state of the metal ion seems to have a strong influence on the inhibitory properties of the complex; the variations noticed are clearly due to the nature of the metal ion.
At the end of each aggregation process (i.e., after 24 h), the resulting suspensions of Aβ40 aggregates were analysed by dynamic light scattering (DLS, Fig. S5†) and transmission electron microscopy (TEM, Fig. 3). Complexes 3 and 4, which exhibit analogous anti-aggregation properties, give rise to the generation of species with hydrodynamic diameter below 1.1 μm, which is the Z-average size of the aggregates formed by non-treated Aβ40. Very small species (in the 30–100 nm range) are also found in the presence of compound 4. TEM images show these aggregates as short non-canonical fibrils and potential oligomeric species of protein, and their sizes agree with the hydrodynamic size measurements. These fibrils are ThT-silent because they lack amyloid mature structure. Compounds 1 and 2 led to comparatively bigger aggregates (over 1.1 μm) but their macroscopic morphology in TEM is rather different. Ribbon-like structures with appearance of long flexible fibrils are formed in the presence of 1, while different fibril-like structures were found exclusively when the protein was treated with 2. It should be noted that such fibrillated species formed with compound 2 were hardly found along the grid, which indicates qualitatively their lower amount. In concordance with the kinetic profiles, amyloid-like species shown by TEM in the presence of complexes 3 and 4 display similar macroscopic structures to those obtained in the presence of complex 1, but they are shorter, particularly in the presence of complex 4. This again suggests a shared inhibitory mechanism for complexes 1, 3, and 4. Compiled data suggest that all compounds favour an alternative folding pathway, leading to aggregation in a non-canonical amyloid conformation hampering the formation of mature fibres.
 | ||
| Fig. 3 TEM images of Aβ40 samples incubated for 24 h (a) without added compound, and with equimolar amounts of compounds 1–4 (b–e), respectively. [Aβ] = 10 μM; [complex] = 10 μM. Scale bar 100 nm. | ||
To provide further insights on the mechanism of action of compounds 1–4, samples of Aβ40 incubated with equimolar amounts of each compound for 24 h were analysed by ESI-MS (Fig. S7†). For compounds 1, 3 and 4, the MS spectra display a peak corresponding to the respective 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 protein adduct with (η6-p-cymene)Ru, (η5-C5Me5)Ir and (η5-C5Me5)Rh. No metal-Aβ adducts were detected when the peptide was treated with 2. These results suggest that the metal ions of compounds 1, 3 and 4 bind the peptide upon losing both L1 ligand and the labile chloride. Previously, Ma and coworkers had described the loss of a C^N-ligand in a Rh(III) complex to bind Aβ40, which resulted in a strong inhibition of the aggregation.35 Strikingly, no evidence of such interaction was found for the Os(II) complex 2 in ESI-MS spectra. Hence, it is not clear how this compound acts beyond a suggested non-covalent binding.
1 protein adduct with (η6-p-cymene)Ru, (η5-C5Me5)Ir and (η5-C5Me5)Rh. No metal-Aβ adducts were detected when the peptide was treated with 2. These results suggest that the metal ions of compounds 1, 3 and 4 bind the peptide upon losing both L1 ligand and the labile chloride. Previously, Ma and coworkers had described the loss of a C^N-ligand in a Rh(III) complex to bind Aβ40, which resulted in a strong inhibition of the aggregation.35 Strikingly, no evidence of such interaction was found for the Os(II) complex 2 in ESI-MS spectra. Hence, it is not clear how this compound acts beyond a suggested non-covalent binding.
For comparison, the four compounds were tested with the more aggregation-prone Aβ42 peptide, using the same concentration (10 μM) and conditions as with Aβ40. All of them, even compounds 1 and 2, inhibited completely the amyloid aggregation in this case (Fig. S4†). Since Aβ42 is known to form a higher number of oligomers, the compounds might be more active because they act mainly in the nucleation phase. Further studies, which are out of the scope of this work, would be necessary to confirm this hypothesis.59
Since two alternatively mechanisms of inhibition have been suggested, we next investigated the effect of the concentration of complexes of the heaviest metals, i.e.2 and 4, which have shown very distinct behaviours. To compare their behaviour in more detail, the aggregation curves were fitted to a two-step autocatalytic process (Scheme 2).58 Complex concentrations of 0.1, 1.0, 2.5 and 5 μM were used with a 10 μM solution of Aβ40. The resulting aggregation plots are shown in Fig. 4, and the calculated kinetic parameters, namely the nucleation rate constant (kn), the elongation rate constant (ke), the lag time (t0), the half aggregation time (t1/2) and the end time of the aggregation (t1), are listed in Tables 2 and 3. As expected, a very different concentration-dependent behaviour was observed between both compounds.
 | ||
| Fig. 4 Aggregation of Aβ40 (10 μM) in the presence of increasing concentrations of (a) complex 2 and (b) complex 4. | ||
| Control | 0.1 μM | 1.0 μM | 2.5 μM | 5.0 μM | |
|---|---|---|---|---|---|
| Standard error (SD) < 5%. | |||||
| k n (10−6·min−1) | 271 | 505 | 482 | 495 | 992 |
| k e (M−1·min−1) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 210 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
| t 0 (min) | 23.9 | 23.3 | 22.8 | 23.0 | 21.3 |
| t 1/2 (min) | 36.8 | 36.9 | 34.4 | 34.9 | 34.5 |
| t 1 (min) | 49.6 | 50.5 | 46.0 | 46.7 | 47.6 |
| Inhibition (%) | 0.0 | 19.1 | 29.2 | 45.7 | 52.8 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
| Control | 0.1 μM | 1.0 μM | 2.5 μM | 5.0 μM | |
|---|---|---|---|---|---|
| Standard error (SD) < 5%. | |||||
| k n (10−6·min−1) | 271 | 292 | 200 | 164 | 129 |
| k e (M−1·min−1) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080 |
6317 | 2392 | 864 |
| t 0 (min) | 23.9 | 22.4 | 50.6 | 61.9 | 199.8 |
| t 1/2 (min) | 36.8 | 35.4 | 91.6 | 138.8 | 493.9 |
| t 1 (min) | 49.6 | 48.5 | 132.5 | 215.8 | 788.1 |
| Inhibition (%) | 0.0 | 37.2 | 52.9 | 80.4 | 90.2 |
The data suggest that compound 2 primarily accelerates the nucleation phase in a concentration-dependent manner (Table 2). At 5.0 μM, the nucleation constant increases four-fold. In contrast, the elongation constant is slightly reduced, indicating a slowdown in fibril elongation once the nuclei are formed. The accelerated nucleation may lead to the formation of non-canonical nuclei (i.e., bad template nuclei) or amorphous species, impeding the formation of normal amyloid fibrils. In the presence of compound 2, the final kinetic curves at all concentrations are comparable to those without the complex, as it occurred at equimolar concentrations (Fig. 2). Thus, because of the opposite effects on the nucleation constant (kn) and the elongation constant (ke), all the plots obtained appear to have the same shape as that observed for free Aβ40; only the plateaus -i.e. the final amount of fibres- are concentration-dependent and decrease with increasing concentration (Fig. 4a). We could hypothesize that the formation of non-canonical nuclei upon the interaction with the metal complex hinders the formation of normal amyloid fibrils, slowing down the fibril elongation.
The plots obtained for compound 4 (Fig. 4b) differ significantly from those for compound 2. Complex 4 (which appear to share the behaviour of compounds 1 and 3) reduces both nucleation and elongation processes, leading to an increase in all kinetic times (t0, t1/2, t1) that is dependent on concentration (Table 3). In this case, the shape of the aggregation curve is strongly influenced by the concentration of compound 4. Both the lag and elongation times are significantly altered as the complex-to-Aβ40 ratio increases. The formation of aggregates slows down as the concentration of compound 4 increases, and their final amount is significantly reduced; for example, nearly 80% inhibition of aggregation is achieved with 5 μM of 4 (Table 3). Thus, compound 4 acts as a very efficient kinetic inhibitor of aggregation, drastically reducing both nucleation and elongation processes. In summary, the data suggest that the coordination binding of this compound to Aβ40 occurs in the soluble species or during the initial nucleation events, resulting in the formation of non-productive nuclei and, consequently, impeding fibril elongation and the eventual formation of mature fibres. The effectiveness of the metal-to-protein binding is clearly demonstrated by the corresponding half-maximal inhibitory concentrations (IC50), which are 4.50 μM and 0.36 μM for complex 2 and compound 4, respectively—over 10 times lower for compound 4 (Fig. S6†).
Effects of the metal complexes in vivo
Given their distinct in vitro behaviour, complexes 2 and 4 were selected to carry out in vivo experiments with several Caenorhabditis elegans strains model of AD. Despite its simplicity, the human genome and the C. elegans genome share about 44% of the genes, including many genes and processes that cause human disorders. Therefore, such nematode can be used as an animal model for molecular mechanism research and in vivo drug testing. Moreover, gene editing has been simplified for this species to generate new phenotypes, including AD models.60The CL4176 C. elegans strain contains a temperature-inducible transgene which produces human amyloid protein in the animal's muscle walls once the temperature is shifted from 16 °C to 25 °C.61 Consequently, the worms suffer fast and irreversible paralysis due to the accumulation of Aβ aggregates in the body's walls. As a result, the nematodes are unable to move and feed, hence resulting in premature death. Treatment with 2 delayed the onset of paralysis by up to 7 hours (Fig. 5). Moreover, 2 led to an increase in the PT50 (Paralysis Time 50%) value (viz., the mean time interval at which 50% of the worms were paralyzed), a key parameter to evaluate the nematode's motility.62 The PT50 value for control animals was 45.6 ± 0.3 hours, whereas it increased up to 47.5 ± 0.3 hours with worms supplemented with complex 2. Complex 4 did not increase the paralysis time in a significant manner (Fig. 5).
However, both complexes were able to significantly reduce the number of amyloid aggregates in the body wall muscle of the nematodes (Fig. 6). The CL2331 strain63 contains a temperature-inducible transgene that produces human Aβ fused with GFP upon induction. Hence, the protein aggregates can easily be visualized under a microscope (Fig. 6a).64 Complex 2 reduced by 30% (Fig. 6b and d) the formation of human Aβ aggregates in the animal's body, while 4 reduced the protein plaques by 25% (Fig. 6c and d). Moreover, complex 2 also protected neurons against Aβ-induced toxicity, as shown by the chemotaxis assays performed with the mutant strain CL2355 (Fig. 7). This strain expresses human Aβ in the nematode's neurons that give rise to an alteration of the chemotaxis behaviour induced by the protein toxicity.65 The non-treated CL2322 nematodes, which were used as a control strain, had a chemotaxis index (C.I.) of 0.5 ± 0.1, while the CL2355 strain had a C.I. of 0.18 ± 0.07, showing how impaired the strain is due to amyloid accumulation in neurons. Treatment with complex 2 protected the neurons from Aβ toxicity, as illustrated by the C.I. of 0.52 ± 0.03 of the treated CL2355 animals.
The differences between the in vitro results, in which the amyloid protein is free, and those of a complete organism such as C. elegans, where the protein is expressed inside a tissue, could be due to the higher biological complexity of the nematodes and differences in the experimental conditions. Often, a combination of in vitro and in vivo studies is necessary to understand biological processes or the effects of a treatment fully. This is why we have used both approaches in this comprehensive study.
The discrepancy noted between C. elegans strains has been reported before. Gioran et al. reported that verbascoside reduced the paralysis of the animals, but no reduction in Aβ aggregate formation was shown.66 The authors discussed that the apparent discrepancy could be due to the different strains used or that the mechanism of action did not necessarily involve only the reduction in Aβ aggregation. Nevertheless, in our study, complex 4 did not improve the PT50 but was able to prolong the paralysis time of the last nematodes, as shown in the paralysis curves (Fig. 5b). The maximum paralysis time, understood as the time in which the last worm was paralyzed, for complex 4 was 65.47 hours, while the maximum time for control nematodes was 60.34 hours. Thus, a tendency to improve the paralysis of the animals is shown for complex 4, though complex 2 performed better in the biological assays for all the tested strains.
Experimental
Materials and methods
Common chemicals and solvents (HPLC grade or reagent grade quality) were purchased from commercial sources and used without further purification. Deuterated solvents were purchased from Euriso-top. 1H and 13C{1H} NMR spectra were carried out on a Bruker AV 400 or Bruker AV 600 NMR spectrometer and chemical shifts are reported in ppm and cited relative to SiMe4 and using the residual proton impurities in the solvents for 1H and 13C{1H} NMR spectroscopy. Peak multiplicities are abbreviated as: s = singlet, m = multiplet, d = doublet, t = triplet, dd = doublet of doublet.The C, H, and N analyses were performed with a Carlo Erba model EA 1108 microanalyzer, with an EAGER 200 software. The combustion of the samples was carried out in the presence of V2O5 and MgO as additives. The duration of the analysis was 15 minutes.
ESI-MS spectra of Aβ-containing samples were recorded on a Bruker timsTOF Pro2 instrument, equipped with an HPLC Bruker pump for sample injection, and calibrated with ESI-L Low Concentration Tuning Mix (Agilent Technologies). The measuring conditions were: 10 μL of sample injected at 50 μL·min−1 at 3.5–5.0 kV capillary-counter voltage, 90–110 °C of desolvation temperature, and dry gas at 6 L·min−1. The liquid carrier was a mixture of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (90
methanol (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to avoid interaction with conventional ammonia buffers. Samples were prepared in PBS.
10) to avoid interaction with conventional ammonia buffers. Samples were prepared in PBS.
The UV-Vis spectra were registered using a PerkinElmer Lambda 750 S spectrometer. Fluorescence spectra were registered at room temperature with a HORIBA Jobin–Yvon iHR320 spectrofluorometer. The instrument excitation and emission slits were both set at 5 nm.
Synthesis of complexes 1–4
X-ray studies
Data for compound 4 was collected on a Bruker APEX II QUAZAR diffractometer equipped with a microfocus multilayer monochromator with Mo Kα radiation (λ = 0.71073 Å). Data reduction and absorption corrections were performed by using SAINT and SADABS, respectively.67,68 The structure was solved using SHELXT and refined with full-matrix least squares on F2 by using SHELXL.69 Details can be found in CCDC 2359733† which contains the supplementary crystallographic data for this structure.In vitro evaluation of the anti-amyloid properties
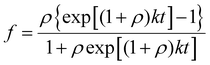 | (1) |
In vivo experiments
All the worms used in the experiments were age-synchronized. The eggs were left at 16 °C to hatch over 24 hours. Once hatched, 25–30 L1 larvae were transferred to 50 mm analysis plates (3–6 plates per condition), containing 8 mL of NGM agar, the metal complexes at 10 μM and 100 μL of E. coli OP50. The animals were maintained at 16 °C for 36 hours, and then the temperature was increased to 25 °C for transgene induction.
The phenotypes were verified with the visible gene marker rol-6 for the strains CL2331 and CL4176 and the fluorescent constitutive mtl-2:GFP for the CL2355 and CL2122 strains.
The chemotaxis plates were prepared following the Margie, Palmer, and Chin-Sang's protocol.73 Then, the worms were placed in the center of the plate in a drop of buffer, and the plates were left in the dark at 25 °C for one hour. This procedure was done in triplicate for both strains treated with the metal complexes and DMSO. Two independent experiments were performed.
After one hour at 25 °C, the animals in each quadrant were counted, and the chemotaxis index was calculated using the eqn (2) obtained from established protocols:
 | (2) |
Conclusions
Four piano-stool complexes containing Ru(II) (1), Os(II) (2), Ir(III) (3) and Rh(III) (4) were prepared from the same ligand L1. All the structurally related organometallic compounds were stable in PBS solution and the crystal structure of the Rh(III) compound confirmed the typical “three-legged piano-stool” geometry for this type of metal complexes.Investigation of the effect of 1–4 on the aggregation of Aβ40 in vitro suggested that all of them favour an alternative folding pathway, impeding the formation of mature fibres. However, the metal centre seems to play a crucial role in their inhibiting activities. Group 8 metal complexes 1 and 2 led to analogous overall inhibition of amyloid aggregation (ca. 70%), but following distinct mechanisms of action: Ru(II) compound 1 delayed both the nucleation and the fibril elongation steps, while the Os(II) complex 2 acted by accelerating the nucleation process but slowing down the fibril elongation. Group 9 metal complexes 3 and 4 inhibited almost completely the aggregation of Aβ40, Rh(III) complex 4 being slightly stronger than Ir(III) compound 3. Consequently, the aggregates formed with 3 and 4 were remarkably smaller than those formed with 1 and 2. ESI-MS analysis revealed that Aβ40 displaces chloride and L1 ligand to bind the metal centre of compounds 1, 3 and 4, but no evidence of such interaction was found for 2.
In vivo studies revealed that 2 and 4 were able to significantly reduce the number of amyloid aggregates in the body muscle wall of C. elegans nematodes. It can be noted that 2 was more efficient than 4in vivo, in contrast to the in vitro studies. For instance, treatment of the CL4176 strain of C. elegans with 2 delayed the paralysis onset by up to 7 hours while 4 did not rescue this paralysis phenotype. Remarkably, complex 2 also protected the nematode's neurons against Aβ-induced toxicity, as shown by the chemotaxis assays performed with the mutant strain CL2355. It can be stressed here that the activity differences observed between the in vitro and in vivo experiments should encourage carrying out both types of studies when investigating the activity of a potential drug.
To the best of our knowledge, 2 is the first reported osmium complex showing high efficacy in the inhibition of Aβ aggregation, both in vitro and in vivo. The promising data achieved with this organoosmium compound indicate that such metal complexes represent potential candidates for the development of potential anti-AD agents targeting Aβ.
Author contributions
Conceptualization: G. V., J. R., A. B. C., F. G.-H., P. G. Data curation: G. V., R. S., L. A. B., S. H.-G., P. B. Funding acquisition: J. R., F. G.-H., P. G. Investigation: G. V., R. S., L. A. B., A. B. C., S. H.-G., P. B. Methodology: G. V., R. S., L. A. B., A. B. C., S. H.-G. Project administration: G. V., J. R., A. B. C., F. G.-H., P. G. Resources: J. R., A. B. C., F. G.-H., P. G. Software: G. V., R. S., L. A. B., S. H.-G. Supervision: J. R., A. B. C., F. G.-H., P. G. Validation: G. V., J. R., A. B. C., F. G.-H., P. G. Writing – original draft: G. V., A. B. C. Writing – review & editing: G. V., J. R., A. B. C., S. H.-G., F. G.-H., P. G.Data availability
Crystallographic data for complex 4 has been deposited at CCDC 2359733. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d4qi01460j.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Spanish Ministerio de Ciencia e Innovación-Agencia Estatal de Investigación (Projects PID2020-115537RB-I00, PCI2021-122027-2B, PID2021-122850NB-I0, PID2021-127863OB-I00 and PID2021-122896NB-I00; MCIU/AEI/10.13039/501100011033) and from European Union NextGenerationEU/PRTR is kindly acknowledged. P. G. thanks the Catalan Institution for Research and Advanced Studies (ICREA).References
- D. S. Knopman, H. Amieva, R. C. Petersen, G. Chetelat, D. M. Holtzman, B. T. Hyman, R. A. Nixon and D. T. Jones, Alzheimer disease, Nat. Rev. Dis. Primers, 2021, 7, 21 CrossRef.
- M. P. Murphy and H. LeVine, Alzheimer's Disease and the Amyloid-beta Peptide, J. Alzheimer's Dis., 2010, 19, 311–323 Search PubMed.
- P. V. Fish, D. Steadman, E. D. Bayle and P. Whiting, New approaches for the treatment of Alzheimer's disease, Bioorg. Med. Chem. Lett., 2019, 29, 125–133 CrossRef CAS PubMed.
- J. L. Cummings, T. Morstorf and K. Zhong, Alzheimer's disease drug-development pipeline: few candidates, frequent failures, Alzheimers Res. Ther., 2014, 6, 7 CrossRef PubMed.
- Y. L. Yi and M. H. Lim, Current understanding of metal-dependent amyloid-beta aggregation and toxicity, RSC Chem. Biol., 2023, 4, 121–131 RSC.
- X. H. Wang, X. Y. Wang and Z. J. Guo, Metal-involved theranostics: An emerging strategy for fighting Alzheimer's disease, Coord. Chem. Rev., 2018, 362, 72–84 CrossRef CAS.
- L. Grcic, G. Leech, K. Kwan and T. Storr, Targeting misfolding and aggregation of the amyloid-beta peptide and mutant p53 protein using multifunctional molecules, Chem. Commun., 2024, 60, 1372–1388 RSC.
- A. Rauk, The chemistry of Alzheimer's disease, Chem. Soc. Rev., 2009, 38, 2698–2715 RSC.
- D. J. Selkoe, The molecular pathology of alzheimers-disease, Neuron, 1991, 6, 487–498 CrossRef CAS.
- A. I. Bush and R. E. Tanzi, Therapeutics for Alzheimer's disease based on the Metal Hypothesis, Neurotherapeutics, 2008, 5, 421–432 CrossRef CAS.
- N. Puentes-Diaz, D. Chaparro, D. Morales-Morales, A. Flores-Gaspar and J. Ali-Torres, Role of Metal Cations of Copper, Iron, and Aluminum and Multifunctional Ligands in Alzheimer’s Disease: Experimental and Computational Insights, ACS Omega, 2023, 8(5), 4508–4526 CrossRef CAS.
- P. Faller and C. Hureau, Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-beta peptide, Dalton Trans., 2009, 1080–1094, 10.1039/b813398k.
- C. Haass and D. Selkoe, If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline?, PLoS Biol., 2022, 20, 15 CrossRef.
- A. Pasieka, D. Panek, N. Szalaj, A. Espargaro, A. Wieckowska, B. Malawska, R. Sabate and M. Bajda, Dual Inhibitors of Amyloid-beta and Tau Aggregation with Amyloid-beta Disaggregating Properties: Extended In Cellulo, In Silico, and Kinetic Studies of Multifunctional Anti-Alzheimer's Agents, ACS Chem. Neurosci., 2021, 12, 2057–2068 CrossRef CAS PubMed.
- X. Shao, C. R. Yan, C. Wang, C. L. Wang, Y. Cao, Y. Zhou, P. Guan, X. L. Hu, W. L. Zhu and S. C. Ding, Advanced nanomaterials for modulating Alzheimer's related amyloid aggregation, Nanoscale Adv., 2022, 5, 46–80 RSC.
- K. D. Fasae, A. O. Abolaji, T. R. Faloye, A. Y. Odunsi, B. O. Oyetayo, J. I. Enya, J. A. Rotimi, R. O. Akinyemi, A. J. Whitworth and M. Aschner, Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer's disease: Limitations, and current and future perspectives, J. Trace Elem. Med. Biol., 2021, 67, 21 CrossRef.
- S. Ben-Shushan and Y. Miller, Neuropeptides: roles and activities as metal chelators in neurodegenerative diseases, J. Phys. Chem. B, 2021, 125, 2796–2811 CrossRef CAS.
- G. Veurink, G. Perry and S. K. Singh, Role of antioxidants and a nutrient rich diet in Alzheimer's disease, Open Biol., 2020, 10, 200084 CrossRef CAS PubMed.
- M. Simunkova, S. H. Alwasel, I. M. Alhazza, K. Jomova, V. Kollar, M. Rusko and M. Valko, Management of oxidative stress and other pathologies in Alzheimer's disease, Arch. Toxicol., 2019, 93, 2491–2513 CrossRef CAS.
- S. Laurent, M. R. Ejtehadi, M. Rezaei, P. G. Kehoe and M. Mahmoudi, Interdisciplinary challenges and promising theranostic effects of nanoscience in Alzheimer's disease, RSC Adv., 2012, 2, 5008–5033 RSC.
- Y. Lian, Y. J. Jia, J. Wong, X. F. Zhou, W. Song, J. Guo, C. L. Masters and Y. J. Wang, Clarity on the blazing trail: clearing the way for amyloid-removing therapies for Alzheimer’s disease, Mol. Psychiatry, 2024, 29, 297–305 CrossRef CAS.
- E. Drummond and T. Wisniewski, Alzheimer's disease: experimental models and reality, Acta Neuropathol., 2017, 133, 155–175 CrossRef CAS PubMed.
- J. M. Suh, G. Kim, J. Kang and M. H. Lim, Strategies Employing Transition Metal Complexes To Modulate Amyloid-beta Aggregation, Inorg. Chem., 2019, 58, 8–17 CrossRef CAS.
- D. J. Hayne, S. Lim and P. S. Donnelly, Metal complexes designed to bind to amyloid-beta for the diagnosis and treatment of Alzheimer's disease, Chem. Soc. Rev., 2014, 43, 6701–6715 RSC.
- T. Storr, Multifunctional compounds for the treatment of Alzheimer's disease, Can. J. Chem., 2021, 99, 1–9 CrossRef CAS.
- L. M. F. Gomes, J. C. Bataglioli and T. Storr, Metal complexes that bind to the amyloid-beta peptide of relevance to Alzheimer's disease, Coord. Chem. Rev., 2020, 412, 15 CrossRef.
- H. Liu, Y. W. Qu and X. H. Wang, Amyloid beta-targeted metal complexes for potential applications in Alzheimer's disease, Future Med. Chem., 2018, 10, 679–701 CrossRef CAS PubMed.
- K. H. Chen and M. C. Cui, Recent progress in the development of metal complexes as beta-amyloid imaging probes in the brain, MedChemComm, 2017, 8, 1393–1407 RSC.
- B. Jiang and A. A. Marti, Probing Amyloid Nanostructures using Photoluminescent Metal Complexes, Eur. J. Inorg. Chem., 2021, 2021, 4408–4424 CrossRef CAS.
- G. Bort, S. Catoen, H. Borderies, A. Kebsi, S. Ballet, G. Louin, M. Port and C. Ferroud, Gadolinium-based contrast agents targeted to amyloid aggregates for the early diagnosis of Alzheimer's disease by MRI, Eur. J. Med. Chem., 2014, 87, 843–861 CrossRef CAS PubMed.
- T. G. Chan, C. L. Ruehl, S. V. Morse, M. Simon, V. Rakers, H. Watts, F. A. Aprile, J. J. Choi and R. Vilar, Modulation of amyloid-beta aggregation by metal complexes with a dual binding mode and their delivery across the blood-brain barrier using focused ultrasound, Chem. Sci., 2021, 12, 9485–9493 RSC.
- L. He, X. S. Wang, D. S. Zhu, C. Zhao and W. H. Du, Methionine oxidation of amyloid peptides by peroxovanadium complexes: inhibition of fibril formation through a distinct mechanism, Metallomics, 2015, 7, 1562–1572 CrossRef CAS PubMed.
- D. E. S. Silva, M. P. Cali, W. M. Pazin, E. Carlos-Lima, M. T. S. Trevisan, T. Venancio, M. Arcisio-Miranda, A. S. Ito and R. M. Carlos, Luminescent Ru(II) Phenanthroline Complexes as a Probe for Real-Time Imaging of A beta Self-Aggregation and Therapeutic Applications in Alzheimer's Disease, J. Med. Chem., 2016, 59, 9215–9227 CrossRef CAS PubMed.
- E. Babu, P. M. Mareeswaran, V. Sathish, S. Singaravadivel and S. Rajagopal, Sensing and inhibition of amyloid-beta based on the simple luminescent aptamer-ruthenium complex system, Talanta, 2015, 134, 348–353 CrossRef CAS PubMed.
- B. Y. W. Man, H. M. Chan, C. H. Leung, D. S. H. Chan, L. P. Bai, Z. H. Jiang, H. W. Li and D. L. Ma, Group 9 metal-based inhibitors of beta-amyloid (1-40) fibrillation as potential therapeutic agents for Alzheimer's disease, Chem. Sci., 2011, 2, 917–921 RSC.
- A. F. Martins, A. C. Oliveira, J. F. Morfin, D. V. Laurents, E. Toth and C. Geraldes, Associating a negatively charged GdDOTA-derivative to the Pittsburgh compound B for targeting A beta amyloid aggregates, J. Biol. Inorg. Chem., 2016, 21, 83–99 CrossRef CAS PubMed.
- N. A. Vyas, S. S. Bhat, A. S. Kumbhar, U. B. Sonawane, V. Jani, R. R. Joshi, S. N. Ramteke, P. P. Kulkarni and B. Joshi, Ruthenium(II) polypyridyl complex as inhibitor of acetylcholinesterase and A beta aggregation, Eur. J. Med. Chem., 2014, 75, 375–381 CrossRef CAS PubMed.
- N. A. Vyas, S. N. Ramteke, A. S. Kumbhar, P. P. Kulkarni, V. Jani, U. B. Sonawane, R. R. Joshi, B. Joshi and A. Erxleben, Ruthenium(II) polypyridyl complexes with hydrophobic ancillary ligand as A beta aggregation inhibitors, Eur. J. Med. Chem., 2016, 121, 793–802 CrossRef CAS PubMed.
- L. Messori, M. Camarri, T. Ferraro, C. Gabbiani and D. Franceschini, Promising in Vitro anti-Alzheimer Properties for a Ruthenium(III) Complex, ACS Med. Chem. Lett., 2013, 4, 329–332 CrossRef CAS.
- X. S. Wang, D. S. Zhu, C. Zhao, L. He and W. H. Du, Inhibitory effects of NAMI-A-like ruthenium complexes on prion neuropeptide fibril formation, Metallomics, 2015, 7, 837–846 CrossRef CAS.
- M. P. Cali, L. M. B. Pereira, M. D. Teodoro, T. A. Sellani, E. G. Rodrigues and R. M. Carlos, Comparison of A beta (1-40, 1-28, 11-22, and 29-40) aggregation processes and inhibition of toxic species generated in early stages of aggregation by a water-soluble ruthenium complex, J. Inorg. Biochem., 2021, 215, 6 CrossRef.
- J. C. Bataglioli, L. M. F. Gomes, C. Maunoir, J. R. Smith, H. D. Cole, J. McCain, T. Sainuddin, C. G. Cameron, S. A. McFarland and T. Storr, Modification of amyloid-beta peptide aggregation via photoactivation of strained Ru(ii) polypyridyl complexes, Chem. Sci., 2021, 12, 7510–7520 RSC.
- S. E. Huffman, G. K. Yawson, S. S. Fisher, P. J. Bothwell, D. C. Platt, M. A. Jones, C. G. Hamaker and M. I. Webb, Ruthenium(III) complexes containing thiazole-based ligands that modulate amyloid-beta aggregation, Metallomics, 2020, 12, 491–503 CrossRef CAS PubMed.
- G. K. Yawson, M. F. Will, S. E. Huffman, E. T. Strandquist, P. J. Bothwell, E. B. Oliver, C. F. Apuzzo, D. C. Platt, C. S. Weitzel, M. A. Jones, G. M. Ferrence, C. G. Hamaker and M. I. Webb, A Dual-Pronged Approach: A Ruthenium(III) Complex That Modulates Amyloid-beta Aggregation and Disrupts Its Formed Aggregates, Inorg. Chem., 2022, 61, 2733–2744 CrossRef CAS.
- B. J. Wall, M. F. Will, G. K. Yawson, P. J. Bothwell, D. C. Platt, C. F. Apuzzo, M. A. Jones, G. M. Ferrence and M. I. Webb, Importance of Hydrogen Bonding: Structure-Activity Relationships of Ruthenium(III) Complexes with Pyridine-Based Ligands for Alzheimer's Disease Therapy, J. Med. Chem., 2021, 64, 10124–10138 CrossRef CAS PubMed.
- J. Kang, J. S. Nam, H. J. Lee, G. Nam, H. W. Rhee, T. H. Kwon and M. H. Lim, Chemical strategies to modify amyloidogenic peptides using iridium(III) complexes: coordination and photo-induced oxidation, Chem. Sci., 2019, 10, 6855–6862 RSC.
- D. L. Ma, D. S. H. Chan and C. H. Leung, Group 9 Organometallic Compounds for Therapeutic and Bioanalytical Applications, Acc. Chem. Res., 2014, 47, 3614–3631 CrossRef CAS.
- G. S. Yellol, J. G. Yellol, V. B. Kenche, X. M. Liu, K. J. Barnham, A. Donaire, C. Janiak and J. Ruiz, Synthesis of 2-pyridyl-benzimidazole iridium(III), ruthenium(II), and platinum(II) complexes. study of the activity as inhibitors of amyloid-beta aggregation and neurotoxicity evaluation, Inorg. Chem., 2015, 54, 470–475 CrossRef CAS.
- H. O. Gulcan, A. Mavideniz, M. F. Sahin and I. E. Orhan, Benzimidazole-derived Compounds Designed for Different Targets of Alzheimer's Disease, Curr. Med. Chem., 2019, 26, 3260–3278 CrossRef CAS.
- S. Ali, M. Asad, S. Maity, W. Zada, A. A. Rizvanov, J. Iqbal, B. Babak and I. Hussain, Fluoro-benzimidazole derivatives to cure Alzheimer's disease: In-silico studies, synthesis, structure-activity relationship and in vivo evaluation for beta secretase enzyme inhibition, Bioorg. Chem., 2019, 88, 102936 CrossRef CAS PubMed.
- J. Yellol, S. A. Perez, G. Yellol, J. Zajac, A. Donaire, G. Vigueras, V. Novohradsky, C. Janiak, V. Brabec and J. Ruiz, Highly potent extranuclear-targeted luminescent iridium(III) antitumor agents containing benzimidazole-based ligands with a handle for functionalization, Chem. Commun., 2016, 52, 14165–14168 RSC.
- M. A. Bennett and A. K. Smith, Arene ruthenium(II) complexes formed by dehydrogenation of cyclohexadienes with ruthenium(III) trichloride, J. Chem. Soc., Dalton Trans., 1974, 233–241, 10.1039/dt9740000233.
- W. A. Kiel, R. G. Ball and W. A. G. Graham, Carbonyl-eta-hexamethylbenzene complexes of osmium - carbon-hydrogen activation by (eta-C6Me6)Os(CO)(H)2, J. Organomet. Chem., 1990, 383, 481–496 CrossRef CAS.
- C. White, A. Yates and P. M. Maitlis, in Transition Metal Organometallics and Ligands, ed. R. N. Grimes, Inorganic Syntheses, Inc., 1992, ch. 53, vol. 29, pp. 228–234 Search PubMed.
- Y. Kashiwame, M. Watanabe, K. Araki, S. Kuwata and T. Ikariya, Synthesis, Structure, and Proton-Transfer Reactions of Bronsted Acidic Pyridylpyrazole Complexes of Ruthenium, Bull. Chem. Soc. Jpn., 2011, 84, 251–258 CrossRef CAS.
- Q. F. Zhang, R. D. Adams and W. H. Leung, Syntheses, spectroscopic properties and crystal structures of two organoruthenium(II) complexes of bipyridines: {Cp*Ru(2,2′-bipy)}(2)(mu-Cl) PF6 and {(eta(6)-p-cymene)Ru}(2)(mu-OH)(2)(4,4′-bipy) (2) BF4 (4), Inorg. Chim. Acta, 2006, 359, 978–983 CrossRef CAS.
- H. Naiki, K. Higuchi, M. Hosokawa and T. Takeda, Fluorometric-determination of amyloid fibrils invitro using the fluorescent dye, thioflavine-T, Anal. Biochem., 1989, 177, 244–249 CrossRef CAS.
- R. Sabaté, M. Gallardo and J. Estelrich, An autocatalytic reaction as a model for the kinetics of the aggregation of β-amyloid, Biopolymers, 2003, 71, 190–195 CrossRef.
- M. Iljina, G. A. Garcia, A. J. Dear, J. Flint, P. Narayan, T. C. T. Michaels, C. M. Dobson, D. Frenkel, T. P. J. Knowles and D. Klenerman, Quantitative analysis of co-oligomer formation by amyloid-beta peptide isoforms, Sci. Rep., 2016, 6, 28658 CrossRef CAS PubMed.
- C. D. Link, Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 9368–9372 CrossRef CAS PubMed.
- J. Drake, C. D. Link and D. A. Butterfield, Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid β-peptide (1-42) in a transgenic Caenorhabditis elegans model, Neurobiol. Aging, 2003, 24, 415–420 CrossRef CAS PubMed.
- L. Diomede, S. Rigacci, M. Romeo, M. Stefani and M. Salmona, Oleuropein Aglycone Protects Transgenic C. elegans Strains Expressing Aβ42 by Reducing Plaque Load and Motor Deficit, PLoS One, 2013, 8, e58893 Search PubMed.
- C. D. Link, V. Fonte, C. M. Roberts, B. Hiester, M. A. Silverman and G. H. Stein, The β amyloid peptide can act as a modular aggregation domain, Neurobiol. Dis., 2008, 32, 420–425 CrossRef CAS PubMed.
- A. Gea-González, S. Hernández-García, P. Henarejos-Escudero, P. Martínez-Rodríguez, F. García-Carmona and F. Gandía-Herrero, Polyphenols from traditional Chinese medicine and Mediterranean diet are effective against Aβ toxicity in vitro and in vivo in Caenorhabditis elegans, Food Funct., 2022, 13, 1206–1217 RSC.
- L. E. Dosanjh, M. K. Brown, G. Rao, C. D. Link and Y. Luo, Behavioral Phenotyping of a Transgenic Caenorhabditis elegans Expressing Neuronal Amyloid-β, J. Alzheimer's Dis., 2010, 19, 681–690 CAS.
- A. Gioran, Y. Paikopoulos, E. Panagiotidou, A. E. I. Rizou, G. I. Nasi, V. D. Dimaki, K. D. Vraila, D. S. Bezantakou, P. M. Spatharas, N. C. Papandreou, V. Magafa, F. N. Lamari, V. A. Iconomidou and N. Chondrogianni, Beneficial Effects of Sideritis clandestina Extracts and Sideridiol against Amyloid β Toxicity, Antioxidants, 2024, 13, 261 CrossRef CAS PubMed.
- G. M. Sheldrick, SAINT and SADABS, 2012 Search PubMed.
- G. M. Sheldrick, SHELXT - integrated space-group and crystal-structure determination, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 Search PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- A. Espargaró, A. Medina, O. Di Pietro, D. Muñoz-Torrero and R. Sabate, Ultra rapid in vivo screening for anti-Alzheimer anti-amyloid drugs, Sci. Rep., 2016, 6, 23349 CrossRef PubMed.
- N. Stroustrup, B. E. Ulmschneider, Z. M. Nash, I. F. López-Moyado, J. Apfeld and W. Fontana, The Caenorhabditis elegans Lifespan Machine, Nat. Methods, 2013, 10, 665–670 CrossRef CAS PubMed.
- M. A. Guerrero-Rubio, S. Hernández-García, F. García-Carmona and F. Gandía-Herrero, Extension of life-span using a RNAi model and in vivo antioxidant effect of Opuntia fruit extracts and pure betalains in Caenorhabditis elegans, Food Chem., 2019, 274, 840–847 CrossRef CAS PubMed.
- O. Margie, C. Palmer and I. Chin-Sang, C. elegans Chemotaxis Assay, J. Visualized Exp., 2013, e50069, DOI:10.3791/50069.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2359733. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi01460j |
| This journal is © the Partner Organisations 2024 |







