Open Access Article
Open Access ArticleThermal cleavage of hydrogen bond-induced LCST-type phase separation of PHEMA and related poly(hydroxyalkyl (meth)acrylate)s in mixed organic solvents†
Natsuki
Inaba‡
a,
Koki
Takasu‡
b,
Keitaro
Matsuoka
 *ac and
Kazuki
Sada
*ac and
Kazuki
Sada
 *ac
*ac
aGraduate School of Chemical Sciences and Engineering, Hokkaido University, Sapporo 060-0810, Japan. E-mail: sadatcm@sci.hokudai.ac.jp; ma2oka@sci.hokudai.ac.jp
bDepartment of Chemistry, School of Science, Hokkaido University, Sapporo 060-0810, Japan
cDepartment of Chemistry, Faculty of Science, Hokkaido University, Sapporo 060-0810, Japan
First published on 7th May 2024
Abstract
Molecular design exhibiting LCST-type phase separation in water has been widely accepted as desolvation of water-soluble amphiphilic polymers with a small alkyl group triggered by heat. However, for organic media, molecular design is not yet achievable due to the difficulty in designing the entropy-driven exothermic desolvation. In this report, we demonstrate LCST-type phase separations of poly(2-hydroxyethyl methacrylate) (PHEMA) and related poly(hydroxyalkyl (meth)acrylate)s in an organic medium at ambient temperature. Our design is based on the utility of a binary solvent mixture of hydrogen-bonding solvents for solvation and non-polar solvents for desolvation. The thermal cleavage of the hydrogen bond between the hydroxy group of the polymer chain and the hydrogen-bonding group of the solvent easily induced the LCST-type phase separation. We revealed a correlation between the critical molar ratio for LCST-type phase separation and the length of the alkyl groups both in the polymer side chain and 1-alcohol good solvents. These findings led to the development of a reliable molecular design strategy that could facilitate the development of smart materials in organic solvents.
Introduction
Since the inception of polymer chemistry nearly a century ago, a vast array of polymers have been synthesized and their properties explored, particularly in the context of applications such as fibers, plastics, and rubbers. Among these, numerous vinyl polymers, including polyacrylates and polymethacrylates, exhibit flexible polymer chains, rendering them soluble in various media, such as organic solvents and water.1,2 The solubility is contingent upon the substituent group as the side chain, dictating the compatibility between the media and the polymer chain. The application of these flexible and soluble polymers, especially thermo-responsive polymers3–7 and related stimuli-responsive polymers8–16 in solution, has garnered significant attention due to their pronounced conformational changes in response to temperature fluctuations. In particular, lower critical solution temperature (LCST)-type thermo-responsive polymers exhibit collapse of the polymer chain above a certain temperature, known as the cloud point, and subsequently dissolve upon cooling below it. In aqueous solutions, a multitude of flexible amphiphilic polymers and their copolymers have been extensively investigated,17–23 particularly for biomedical applications, given the adjustability of the cloud point to match body temperature.24–26 Thermodynamic studies have unveiled that LCST-type phase separation is an entropy-driven endothermic process. This phase separation, characterized by positive entropy and positive enthalpy, can be elucidated by the dehydration of water molecules from the polymer chain, i.e. the dissociation of hydrogen bonds between them.5Compared to the extensive studies in aqueous solutions, LCST-type thermo-responsive polymers in non-aqueous solvents and their applications, such as in organogels27 and polymer micelles,28 remain relatively unexplored.29 Most of the early examples are vinyl polymers in hydrocarbons at a temperature near the liquid–vapor critical point of the solvent, induced by the compressibility effect of the polymer chain by extreme heating.19,30–35 Other instances of LCST-type thermo-responsive polymers in specific media, such as organic solvents36–48 and ionic liquids,49–61 have been reported, but they were largely discovered serendipitously or through screening polymer-solvent combinations in a library, due to the difficulty in controlling the desolvation of polymer chains at ambient temperatures.
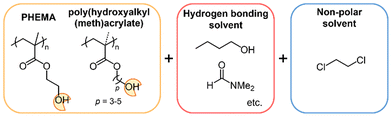
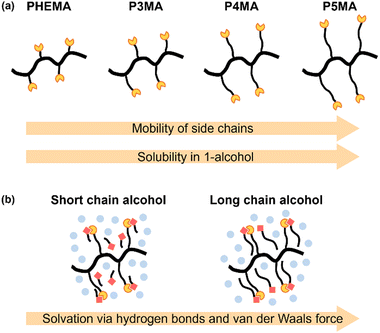
As part of a strategy to achieve predictable LCST-type phase separations in water, Ritter and coworkers reported on an aqueous solution of a polymer containing an adamantyl moiety in the presence of methylated β-cyclodextrins (β-CDs).62,63 The LCST-type phase separations were induced by the dissociation of β-CD from the polymer complex upon heating. Inspired by this supramolecular design, we have demonstrated the LCST-type phase separations of ternary polymer solutions consisting of a flexible polymer and a pseudo-solvating molecule called an effector in a poor solvent at ambient temperature.64–69 Effectors can stabilize the extended coil state of the polymer chain through the formation of hydrogen bonds64–67 or a charge transfer complex68,69 in a poor solvent. For instance, the acrylate polymers with urea64 or TADDOL66 as the hydrogen-bonding functional groups in the side chain exhibited LCST-type phase separations in the presence of hydrogen-bonding effectors. Additionally, we recently reported that poly(4-hydroxy styrene) (PHS), a commodity plastic, undergoes LCST-type phase separations in binary solvent mixtures of common hydrogen-bonding solvents and a non-polar solvent.70 The phase separations are induced readily by heating, due to the dissociation of the polymer complexes as pseudo-desolvation. In particular, the thermal cleavage of the hydrogen bonds between a hydrogen-bonding functional group in the polymer chain and a hydrogen-bonding solvent plays a pivotal role in designing the desolvation of the polymer chain in the entropy-driven LCST-type phase separation. In this study, to demonstrate the versatility of this molecular design based on supramolecular interactions, we report on the rational design of LCST-type phase separations in polymer solutions of poly(2-hydroxyethyl methacrylate) (PHEMA) and related poly(ω-hydroxyalkyl (meth)acrylate)s (P3MA, P4MA, P5MA, P3A, and P4A) (Fig. 1). Since they are flexible vinyl polymers with a hydroxy group at the terminal of the side chain, our strategy should be easily applicable in mixtures of hydrogen bonding good solvents and a poor solvent. Moreover, the effects of the alkyl spacer in the polymer side chain and the alkyl length of 1-alcohol good solvents on the solvent conditions for LCST-type phase separation were investigated systematically to get an insight for more reliable molecular design, especially predictable solvent critical molar ratios in binary solvent mixtures that are currently unpredictable.
Due to the water-soluble nature of the polymer chain, PHEMA has frequently been employed as a non-ionic hydrophilic component in copolymers with LCST-type thermo-responsive polymers such as poly(N-isopropylacrylamide)71–73 to adjust the cloud point. More recently, Armes and coworkers reported that PHEMA with a degree of polymerization between 20 and 45 exhibited LCST-type phase separations in water.74 In contrast to these findings, there have been no reports attempting to induce LCST-type phase separations of PHEMA in non-aqueous media. Only upper critical solution temperature (UCST)-type phase separation was observed in a series of pure aliphatic alcohols, namely, 1-propanol, 1-butanol, 2-methyl-1-propanol, 2-butanol, and glycerol.75 Other polymers in this study have never been reported for thermal phase separations in any organic solvent mixture.
Results and discussion
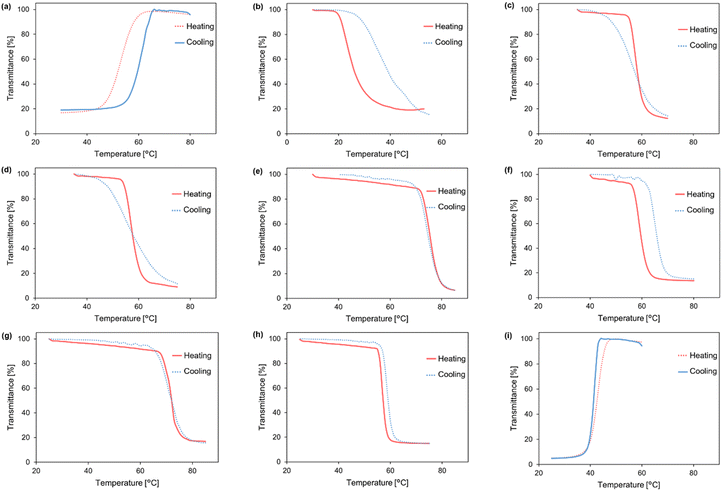
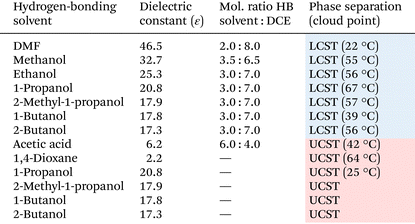
We initially investigated the solubility of commercially available PHEMA (Sigma-Aldrich, Mv = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) in various pure solvents (ca. 2.5 mg in 100 μL) (Table S1†). At low temperatures (0 °C), room temperature (ca. 25 °C), and elevated temperature (ca. 100 °C), PHEMA was soluble in DMSO, DMF, methanol, ethanol, and acetic acid, while being practically insoluble in acetonitrile, 2-butanone, 1-octanol, THF, ethyl acetate, toluene, DCE and cyclohexane. In comparison to PHS in our previous study,70PHEMA exhibits poor solubility in solvents with moderate polarity such as THF and acetonitrile. The relatively weak hydrogen bond acceptors could not solubilize the polymer chain due to the association of hydroxy groups between the polymer chains. We confirmed UCST-type phase separations in a series of aliphatic alcohols such as 1-propanol, 1-butanol, 2-butanol, and 2-methyl-1-propanol (Table S2†), consistent with the previous studies.75 Additionally, we observed UCST-type phase separation in 1,4-dioxane. The temperature-dependent transmittance changes at 800 nm revealed a cloud point at 63 °C (24 mg mL−1 in 1,4-dioxane) (Fig. 2a). Polar solvents with hydrogen bond acceptors can readily solubilize the polymer chain of PHEMA by breaking the hydrogen bonds of the hydroxy groups between the polymer chain and forming new hydrogen bonds with the solvent molecules as solvation.
000) in various pure solvents (ca. 2.5 mg in 100 μL) (Table S1†). At low temperatures (0 °C), room temperature (ca. 25 °C), and elevated temperature (ca. 100 °C), PHEMA was soluble in DMSO, DMF, methanol, ethanol, and acetic acid, while being practically insoluble in acetonitrile, 2-butanone, 1-octanol, THF, ethyl acetate, toluene, DCE and cyclohexane. In comparison to PHS in our previous study,70PHEMA exhibits poor solubility in solvents with moderate polarity such as THF and acetonitrile. The relatively weak hydrogen bond acceptors could not solubilize the polymer chain due to the association of hydroxy groups between the polymer chains. We confirmed UCST-type phase separations in a series of aliphatic alcohols such as 1-propanol, 1-butanol, 2-butanol, and 2-methyl-1-propanol (Table S2†), consistent with the previous studies.75 Additionally, we observed UCST-type phase separation in 1,4-dioxane. The temperature-dependent transmittance changes at 800 nm revealed a cloud point at 63 °C (24 mg mL−1 in 1,4-dioxane) (Fig. 2a). Polar solvents with hydrogen bond acceptors can readily solubilize the polymer chain of PHEMA by breaking the hydrogen bonds of the hydroxy groups between the polymer chain and forming new hydrogen bonds with the solvent molecules as solvation.
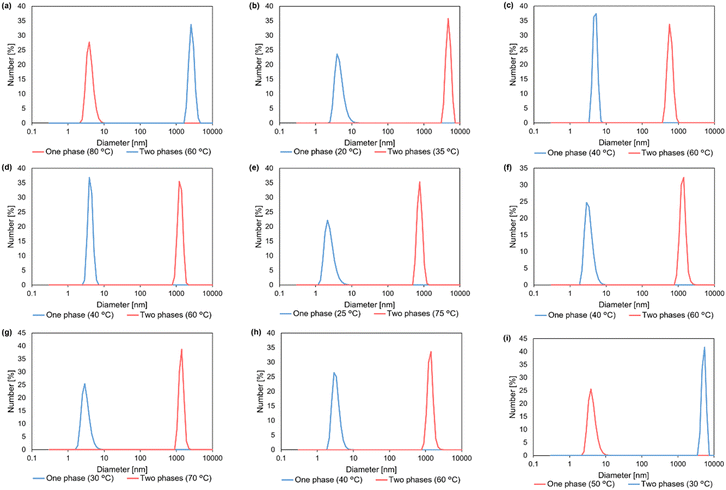
To induce LCST-type phase separation, we examined the temperature-dependent solubility of PHEMA in various solvent mixtures comprising hydrogen-bonding good solvents and non-polar poor solvents with varying ratios. According to our previous studies, we selected DCE as a poor solvent due to its moderate polarity and low solubility for many polymethacrylates bearing a hydrogen bonding group. At specific molar ratios, diverse hydrogen-bonding good solvents, including alcohols and DMF, exhibited LCST-type phase separations (Table 1 and Table S3†). The cloud point (Tcp) was determined as the temperature at which the transmittance reached 90% during both the heating process for LCST-type phase separation and the cooling process for UCST-type phase separation (Fig. 2b–i). In the absence of the hydrogen-bonding good solvents, DCE acted as a poor solvent to promote the hydrogen bonding between the polymer chains. Consequently, the polymer chain could be solvated through hydrogen bonds in the presence of alcohols and DMF at lower temperatures, whereas at higher temperatures, the loss of hydrogen bonds substantially reduced the solubility, leading to LCST-type phase separation. It is noteworthy that while pure alcohols resulted in UCST-type phase separation, the addition of DCE to polymer solutions of 1-propanol, 2-methyl-1-propanol, 1-butanol, or 2-butanol induced LCST-type phase separation. Conversely, UCST-type phase separation occurred in the mixed solvent of acetic acid, acting as a hydrogen-bonding solvent with DCE. PHEMA became soluble at elevated temperatures, confirmed by the transmittance change in the solution (Fig. 2i). In the solvent mixture, acetic acid might act as a weak hydrogen bonding acceptor, insufficient to form hydrogen bonds with the polymer chain at room temperature. An elevated temperature would be required for the solvation of PHEMA to induce UCST-type phase separation. In addition, the phase separation behaviour of PHEMA was validated through dynamic light scattering (DLS) measurements, observing differences in polymer particle size at temperatures below and above the cloud point (Fig. 3a–i). Diminished solvation ability above the cloud points for LCST-type phase separations and below them for UCST-type phase separations prompts polymer chain aggregation and particle growth. These solution behaviours induced by heating or cooling closely resemble those exhibited by PHS. To elucidate the aggregation process during the LCST-type phase separation, we conducted particle size measurements of PHEMA in 1-propanol/DCE = 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.0 (Tcp = 67 °C) at various temperatures (Fig. S7†). At lower temperatures (40, 50, and 60 °C), there were no significant changes observed in the particle size, remaining below 10 nm. However, the particle size increased to approximately 30 nm near the Tcp (65 °C) and dramatically rose to 1000 nm above the Tcp (70 and 75 °C). These findings suggest that even below the Tcp, PHEMA chains may gradually form nano-aggregates upon heating, eventually leading to the formation of larger aggregates. Additionally, the transmittance change, which gradually decreases from 100% to 95–90% with increasing temperature and significantly drops below 20% at Tcp, further supports the process of aggregation formation.
7.0 (Tcp = 67 °C) at various temperatures (Fig. S7†). At lower temperatures (40, 50, and 60 °C), there were no significant changes observed in the particle size, remaining below 10 nm. However, the particle size increased to approximately 30 nm near the Tcp (65 °C) and dramatically rose to 1000 nm above the Tcp (70 and 75 °C). These findings suggest that even below the Tcp, PHEMA chains may gradually form nano-aggregates upon heating, eventually leading to the formation of larger aggregates. Additionally, the transmittance change, which gradually decreases from 100% to 95–90% with increasing temperature and significantly drops below 20% at Tcp, further supports the process of aggregation formation.
| a The conditions: PHEMA (ca. 25 mg) in pure or mixed organic solvents (ca. 1.0 mL) from ca. 0 °C to ca. 100 °C. b Cloud point was determined as the temperature at which the transmittance reached 90% during both the heating process for LCST-type phase separation and the cooling process for UCST-type phase separation. |
|---|
|
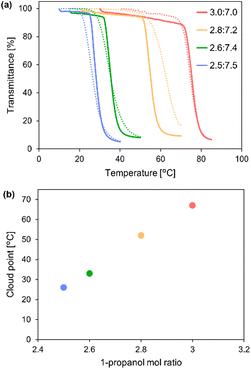
To precisely control the LCST-type phase separation behaviour of PHEMA and its cloud point, we investigated the variation of Tcp by slightly altering the mixing ratios of 1-propanol and DCE. As shown in Fig. 4a, the decrease of the 1-propanol ratio from 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.0 to 2.5
7.0 to 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 decreased Tcp from 67 °C to 26 °C. Moreover, the cloud point vs. the mol ratio of 1-propanol in the mixture linearly decreased, indicating the adjustability of the cloud point for LCST-type phase separation by simple control of the mixing ratios (Fig. 4b). The solubility of PHEMA reduced as the 1-propanol ratio decreased, and LCST-type phase separation easily occurred at lower temperatures through desolvation by the cleavage of the hydrogen bonds between the hydroxy groups in the polymer chain and the hydrogen bonding solvent. The cloud point could also be controlled by varying the polymer concentration from 10 to 75 mg mL−1 with fixed solvent molar ratios (1-propanol
7.5 decreased Tcp from 67 °C to 26 °C. Moreover, the cloud point vs. the mol ratio of 1-propanol in the mixture linearly decreased, indicating the adjustability of the cloud point for LCST-type phase separation by simple control of the mixing ratios (Fig. 4b). The solubility of PHEMA reduced as the 1-propanol ratio decreased, and LCST-type phase separation easily occurred at lower temperatures through desolvation by the cleavage of the hydrogen bonds between the hydroxy groups in the polymer chain and the hydrogen bonding solvent. The cloud point could also be controlled by varying the polymer concentration from 10 to 75 mg mL−1 with fixed solvent molar ratios (1-propanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCE = 2.8
DCE = 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2) (Fig. S8†). As the concentration increased, the cloud point decreased due to a decrease in solubility. For polymers with different molecular weights, a small adjustment in the molar ratio of 1-propanol
7.2) (Fig. S8†). As the concentration increased, the cloud point decreased due to a decrease in solubility. For polymers with different molecular weights, a small adjustment in the molar ratio of 1-propanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCE resulted in LCST-type phase separation at a similar cloud point (Table S9†).
DCE resulted in LCST-type phase separation at a similar cloud point (Table S9†).
To expand our strategy for LCST-type phase separation, we further investigated the solubility and thermal behaviour of poly(ω-hydroxyalkyl methacrylate)s (P3MA, P4MA, and P5MA) and acrylates (P3A and P4A) with different lengths of the alkyl spacer. First, we synthesized them via controlled radical polymerization. The polymerization reaction of the corresponding methacrylic or acrylic monomers proceeded at 80 °C for 24 h in the presence of AIBN as an initiator and an appropriate RAFT agent in a degassed solution of DMAc (for detailed reaction conditions, see the ESI†). After quenching the reaction mixture, the solution was diluted with methanol and reprecipitated dropwise into diethyl ether. The resulting yellow solid was subjected to 1H NMR and size exclusion chromatography (SEC) measurements, and the results are summarized in Table 2. The number average molecular weights of the resulting polymers ranged from 7.05 × 103 to 1.03 × 104, and the polydispersity index (PDI) was found to be 1.04–1.35.
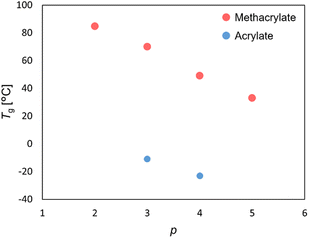
Since it is widely accepted that the flexibility of the polymer chain plays a significant role in the solubility of the polymer chain, we initially conducted thermal analysis using differential scanning calorimetry (DSC) in the bulk state (Fig. 5). The glass transition temperature (Tg) of a series of poly(methacrylates) and poly(acrylates) decreased with an increase in the number of carbon atoms: PHEMA > P3MA > P4MA > P5MA, and P3A > P4A. These results indicated that the extended alkyl length between the hydroxy terminal and the polymer backbone results in a lower Tg due to the facile mobility of the side chain. Compared to poly(methacrylate) and poly(acrylate) with the same alkyl chain length, poly(acrylate) had a lower Tg than poly(methacrylate). It is clear that poly(acrylate) typically has a lower Tg because of the increased mobility in the main chain.
As a result of preliminary screening, DCE, toluene, and cyclohexane acted as non-polar poor solvents for a series of poly(methacrylate)s and poly(acrylate)s. Thus, we examined the solubility of a series of primary aliphatic alcohols to identify hydrogen-bonding good solvents (Tables S10 and 11†). Compared with a series of poly(methacrylate)s, the solubility appeared to correlate with the length of the alkyl chain to the terminal hydroxyl group. The polymers with a shorter alkyl chain (PHEMA and P3MA) were dissolved only in alcohols with fewer carbon atoms, such as methanol and ethanol, and the solubility decreased as the alkyl chain length of the alcohol increased. In contrast, polymers with a longer alkyl chain (P5MA and P4MA) had high solubility in most of the alcohols. Focusing on the differences between the alcohols, methanol dissolved all the polymers, while 1-octanol was unable to solubilize PHEMA, P3MA, and P4MA with a shorter alkyl chain and could only dissolve P5MA. As both the polymers and the solvents have a hydroxyl group that serves as a hydrogen bonding donor and acceptor, the solubility of the polymers is affected by the length of the alkyl chain, that is, the number of carbon atoms in each molecule. In other words, the van der Waals interaction of the alkyl groups between the polymer chain and the solvent could also play a crucial role in solubility during solvation. It is interesting to note that the solubility trends of poly(methacrylate)s (P3MA and P4MA) and poly(acrylate)s (P3A and P4A) with the same alkyl chain length agreed rather well, despite the significant difference in Tg. The enhanced mobility in the poly(acrylate)s does not correlate with the solubility in alcohols, and the variations in solubility may be attributed to the length of the alkyl groups in the side chain for solvation.
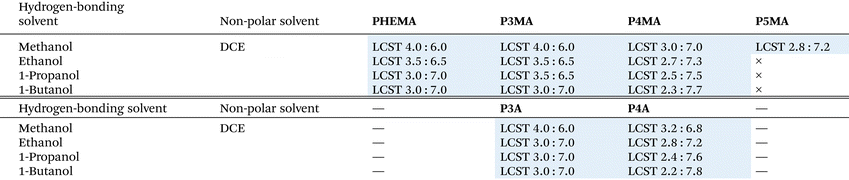
To elucidate the factors influencing the LCST-type phase separation in a series of poly(ω-hydroxyalkyl methacrylate)s (P3MA, P4MA, and P5MA) and acrylates (P3A and P4A), we examined the temperature-dependent solubility change in binary mixtures with four primary alcohols (methanol, ethanol, 1-propanol, and 1-butanol) as a good solvent and DCE as a poor solvent at a concentration of approximately 50 mg mL−1 due to the relatively lower Mw of these polymers. As shown in Table 3, a series of poly(methacrylate)s (P3MA and P4MA) exhibited distinctive LCST-type phase separation in all mixed solvents, with the critical molar ratio depending on the length of the alkyl group in both the polymer chain and the 1-alcohols. Notably, P5MA displayed LCST-type phase separation solely in the mixed solvent with methanol due to its high solubility in other alcohols. Similar thermal behaviours were observed in poly(acrylate)s (P3A and P4A) with comparable alkyl chain lengths. The critical molar ratios of DCE in the solvent mixtures were correlated well with both the alkyl spacer in the polymer side chain and the alkyl length of the 1-alcohols. For methanol as a good solvent, the molar ratio of DCE increased from 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 for PHEMA to 2.8
6.0 for PHEMA to 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.2 for P5MA, and this increasing trend persisted across various 1-alcohols. The increased mobility of the side chains allows easier access to 1-alcohols and increases the solubility of the polymer chains, resulting in a decrease in the molar ratio of the 1-alcohol and an increase in that of DCE for the LCST-type phase separations (Fig. 6a). For P4MA as a polymer chain, the molar ratio of DCE also increased with increasing the lengths of the 1-alcohols, varying from 3.0
7.2 for P5MA, and this increasing trend persisted across various 1-alcohols. The increased mobility of the side chains allows easier access to 1-alcohols and increases the solubility of the polymer chains, resulting in a decrease in the molar ratio of the 1-alcohol and an increase in that of DCE for the LCST-type phase separations (Fig. 6a). For P4MA as a polymer chain, the molar ratio of DCE also increased with increasing the lengths of the 1-alcohols, varying from 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.0 for methanol to 2.3
7.0 for methanol to 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.7 for 1-butanol, and this trend also persisted across the polymers. The longer alkyl groups either in the polymer side chain or in the solvent molecule would contribute more efficiently to hydrogen bonds between the hydroxy group in the polymer side chain and 1-alcohol as the solvation of the polymer chains in DCE mixtures. Although the hydrogen bonds between the polymer chain and the solvent molecules play a key role in the LCST-type phase separations due to adjusting the solubility, the alkyl groups contributed subsidiary to the solvation of the polymer chain by van der Waals force (Fig. 6b). As a result, both in the polymer side chain and the good solvents, the elongation of the alkyl group was associated with increasing trends of the molar ratios of DCE at the LCST-type phase separation, indicating that the alkyl lengths were crucial determinants for the critical molar ratios in these systems. In all combinations of the polymer and 1-alcohol, the highest and lowest molar ratios of DCE were observed in P4MA/1-butanol (2.3
7.7 for 1-butanol, and this trend also persisted across the polymers. The longer alkyl groups either in the polymer side chain or in the solvent molecule would contribute more efficiently to hydrogen bonds between the hydroxy group in the polymer side chain and 1-alcohol as the solvation of the polymer chains in DCE mixtures. Although the hydrogen bonds between the polymer chain and the solvent molecules play a key role in the LCST-type phase separations due to adjusting the solubility, the alkyl groups contributed subsidiary to the solvation of the polymer chain by van der Waals force (Fig. 6b). As a result, both in the polymer side chain and the good solvents, the elongation of the alkyl group was associated with increasing trends of the molar ratios of DCE at the LCST-type phase separation, indicating that the alkyl lengths were crucial determinants for the critical molar ratios in these systems. In all combinations of the polymer and 1-alcohol, the highest and lowest molar ratios of DCE were observed in P4MA/1-butanol (2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.7) and PHEMA/methanol (4.0
7.7) and PHEMA/methanol (4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0), respectively. Therefore, they might be predictable from the size of the non-OH groups for the polymer chains as well as the hydrogen-bonding solvents for LCST-type phase separations of a series of poly(hydroxyalkyl (meth)acrylate)s.
6.0), respectively. Therefore, they might be predictable from the size of the non-OH groups for the polymer chains as well as the hydrogen-bonding solvents for LCST-type phase separations of a series of poly(hydroxyalkyl (meth)acrylate)s.
| a Conditions: Poly(hydroxyalkyl (meth)acrylate)s (ca. 50 mg) in mixed organic solvents (ca. 1.0 mL) with the appropriate molar ratio at ca. 0 °C to ca. 100 °C. |
|---|
|
Conclusions
We demonstrated LCST-type phase separations of PHEMA and its congeners, namely poly(ω-hydroxyalkyl (meth)acrylate)s (P3MA, P4MA, P5MA, P3A, and P4A), in suitable binary solvent mixtures. These mixtures comprise hydrogen-bonding solvents such as alcohols and DCE as a typical non-solvent at a polymer concentration of approximately 25–50 mg mL−1 at ambient temperature. At the critical molar ratio, the hydrogen bonds facilitating solvation between the polymer chain and the solvents readily dissociate under thermal treatment, prompting the collapse of the polymer chain and, consequently, inducing LCST-type phase separation. The critical molar ratio is correlated with the length of the alkyl group in both the polymer side chain and the 1-alcohols, suggesting that the critical molar ratios for these polymers would be predictable from the size of the non-hydroxy groups in the polymer chain and the hydrogen bonding solvents. In the realm of polymer physics, the importance of molecular weight and its distribution concerning LCST-type phase separation has been well documented. However, our focus is on developing novel LCST-type thermo-responsive polymers designed from both synthetic polymer chemistry and supramolecular chemistry. In this study, we investigated LCST-type phase separation by heating the polymer solution after adjusting the solubility through the addition of DCE as the non-solvent. Therefore, as long as the molecular weights of the polymers allow them to dissolve in good solvents, LCST-type phase separations are anticipated to be exhibited using this methodology.Author contributions
N. I.: investigation, visualisation. K. T.: investigation, visualisation, validation. K. M.: supervision, project administration, visualisation, writing – review & editing. K. S.: conceptualization, funding acquisition, supervision, project administration, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Prof. K. Konishi in HU for DLS measurement and Prof. Y. Kageyama in HU for DSC measurement. This work was supported in part by JSPS KAKENHI Grant Number JP21H01980 for K. S.References
- U. Ali, K. J. Bt. A. Karim and N. A. Buang, Polym. Rev., 2015, 55, 678 CrossRef CAS.
- G. Vancoillie, D. Frank and R. Hoogenboom, Prog. Polym. Sci., 2014, 39, 1074 CrossRef CAS.
- V. Aseyev, H. Tenhu and F. M. Winnik, Adv. Polym. Sci., 2011, 242, 29 CrossRef CAS.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214 RSC.
- C. Zhao, Z. Ma and X. X. Zhu, Prog. Polym. Sci., 2019, 90, 269 CrossRef CAS.
- Q. Zhang and R. Hoogenboom, Prog. Polym. Sci., 2015, 48, 122 CrossRef CAS.
- J. Seuring and S. Agarwal, ACS Macro Lett., 2013, 2, 597 CrossRef CAS.
- M. Irie and D. Kungwatchakun, Proc. Jpn. Acad., Ser. B, 1992, 68, 127 CrossRef CAS.
- M. Irie, Adv. Polym. Sci., 1993, 110, 49 CrossRef CAS.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Prog. Polym. Sci., 2010, 35, 278 CrossRef CAS.
- J. M. Korde and B. Kandasubramanian, Ind. Eng. Chem. Res., 2019, 58, 9709 CrossRef CAS.
- F. Liu and M. W. Urban, Prog. Polym. Sci., 2010, 35, 3 CrossRef CAS.
- S. Wang, Q. Liu, L. Li and M. W. Urban, Macromol. Rapid Commun., 2021, 42, 1 Search PubMed.
- Y. F. Gao, M. L. Wei, X. Li, W. W. Xu, A. Ahiabu, J. Perdiz, Z. N. Liu and M. J. Serpe, Macromol. Res., 2017, 25, 513 CrossRef CAS.
- F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468 RSC.
- P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25 RSC.
- A. Halperin, M. Kröger and F. M. Winnik, Angew. Chem., Int. Ed., 2015, 54, 15342 CrossRef CAS.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163 CrossRef CAS.
- S. Saeki, N. Kuwahara, M. Nakata and M. Kaneko, Polymer, 1976, 17, 685 CrossRef CAS.
- C. Weber, R. Hoogenboom and U. S. Schubert, Prog. Polym. Sci., 2012, 37, 686 CrossRef CAS.
- F. A. Plamper, A. Schmalz, M. Ballauff and A. H. E. Müller, J. Am. Chem. Soc., 2007, 129, 14538 CrossRef CAS.
- J. F. Lutz, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3459 CrossRef CAS.
- J. F. Lutz, Ö. Akdemir and A. Hoth, J. Am. Chem. Soc., 2006, 128, 13046 CrossRef CAS PubMed.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101 CrossRef PubMed.
- M. Wei, Y. Gao, X. Li and M. J. Serpe, Polym. Chem., 2017, 8, 127 RSC.
- F. Doberenz, K. Zeng, C. Willems, K. Zhang and T. Groth, J. Mater. Chem. B, 2020, 8, 607 RSC.
- S. Okada and E. Sato, Polymers, 2021, 13, 329 CrossRef CAS PubMed.
- S. Tamura, T. Ueki, K. Ueno, K. Kodama and M. Watanabe, Macromolecules, 2009, 42, 6239 CrossRef CAS.
- M. Concilio, V. P. Beyer and C. R. Becer, Polym. Chem., 2022, 13, 6423 RSC.
- S. Saeki, N. Kuwahara, S. Konno and M. Kaneko, Macromolecules, 1973, 6, 246 CrossRef CAS.
- S. Saeki, N. Kuwahara and S. Konno, Macromolecules, 1973, 6, 589 CrossRef CAS.
- A. Imre and W. A. vanHook, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 751 CrossRef CAS.
- B. Folie and M. Radosz, Ind. Eng. Chem. Res., 1995, 34, 1501 CrossRef CAS.
- N. Kuwahara, S. Saeki, T. Chiba and M. Kaneko, Polymer, 1974, 15, 777 CrossRef CAS.
- M. Schnell, S. Stryuk and B. A. Wolf, Ind. Eng. Chem. Res., 2004, 43, 2852 CrossRef CAS.
- Z. Liu, Y. Guo and K. Inomata, Polym. J., 2011, 43, 676 CrossRef CAS.
- Y. Guo, Z. Liu and K. Inomata, Fluid Phase Equilib., 2012, 315, 29 CrossRef CAS.
- K. Grygiel, W. Zhang, C. Detrembleur and J. Yuan, RSC Adv., 2016, 6, 57117 RSC.
- S. Matsumura, A. R. Hlil, C. Lepiller, J. Gaudet, D. Guay, Z. Shi, S. Holdcroft and A. S. Hay, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7207 CrossRef.
- K. Wang, Q. Liu, G. Liu and Y. Zeng, Polymer, 2020, 203, 122746 CrossRef CAS.
- S. Li, L. Feng, H. Lu and S. Feng, New J. Chem., 2017, 41, 1997 RSC.
- S. Jana, M. Anas, T. Maji, S. Banerjee and T. K. Mandal, Polym. Chem., 2019, 10, 526 RSC.
- M. Ouchi, M. Nakano, T. Nakanishi and M. Sawamoto, Angew. Chem., Int. Ed., 2016, 55, 14584 CrossRef CAS PubMed.
- E. Cazares-Cortes, B. C. Baker, K. Nishimori, M. Ouchi and F. Tournilhac, Macromolecules, 2019, 52, 5995 CrossRef CAS.
- J. Lee, B. Lee, J. Park, J. Oh, T. Kim, M. Seo and S. Y. Kim, Polymer, 2018, 153, 430 CrossRef CAS.
- K. Pagonis and G. Bokias, Polymer, 2004, 45, 2149 CrossRef CAS.
- N. Metz and P. Theato, Eur. Polym. J., 2007, 43, 1202 CrossRef CAS.
- E. Sato, Y. Masuda, J. Kadota, T. Nishiyama and H. Horibe, Eur. Polym. J., 2015, 69, 605 CrossRef CAS.
- T. Ueki and M. Watanabe, Bull. Chem. Soc. Jpn., 2012, 85, 33 CrossRef CAS.
- T. Ueki, Polym. J., 2014, 46, 646 CrossRef CAS.
- T. Ueki, A. A. Arai, K. Kodama, S. Kaino, N. Takada, T. Morita, K. Nishikawa and M. Watanabe, Pure Appl. Chem., 2009, 81, 1829 CrossRef CAS.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Polym. Chem., 2015, 6, 2163 RSC.
- Y. Kobayashi, Y. Kitazawa, K. Hashimoto, T. Ueki, H. Kokubo and M. Watanabe, Langmuir, 2017, 33, 14105 CrossRef CAS PubMed.
- W. Li and P. Wu, Polym. Chem., 2014, 5, 5578 RSC.
- H. Rodríguez and R. D. Rogers, Fluid Phase Equilib., 2010, 294, 7 CrossRef.
- Y. Biswas, P. Banerjee and T. K. Mandal, Macromolecules, 2019, 52, 945 CrossRef CAS.
- D. Yokota, A. Kanazawa and S. Aoshima, Macromolecules, 2019, 52, 6241 CrossRef CAS.
- A. Kharel, C. Hall, P. Černoch, P. Stepanek and T. P. Lodge, Macromolecules, 2020, 53, 885 CrossRef CAS.
- H. N. Lee and T. P. Lodge, J. Phys. Chem. Lett., 2010, 1, 1962 CrossRef CAS.
- H. N. Lee and T. P. Lodge, J. Phys. Chem. B, 2011, 115, 1971 CrossRef CAS.
- W. Li and P. Wu, Polym. Chem., 2014, 5, 761 RSC.
- S. Schmitz and H. Ritter, Angew. Chem., Int. Ed., 2005, 44, 5658 CrossRef CAS PubMed.
- O. Kretschmann, C. Steffens and H. Ritter, Angew. Chem., Int. Ed., 2007, 46, 2708 CrossRef CAS PubMed.
- S. Amemori, K. Kokado and K. Sada, J. Am. Chem. Soc., 2012, 134, 8344 CrossRef CAS.
- S. Amemori, K. Iseda, S. Anan, T. Ono, K. Kokado and K. Sada, Polym. Chem., 2017, 8, 3921 RSC.
- M. Naya, K. Kokado and K. Sada, ACS Appl. Polym. Mater., 2020, 2, 4415 CrossRef CAS.
- M. Naya, K. Kokado, K. B. Landenberger, S. Kanaoka, S. Aoshima and K. Sada, Macromol. Chem. Phys., 2020, 221, 1900455 CrossRef CAS.
- S. Amemori, K. Kokado and K. Sada, Angew. Chem., Int. Ed., 2013, 52, 4174 CrossRef CAS PubMed.
- D. H. Gharib, S. Amemori, M. Naya, K. Kokado and K. Sada, RSC Adv., 2015, 5, 89319 RSC.
- N. Inaba, K. Hashimoto, M. Kubota, K. Matsuoka and K. Sada, Mol. Syst. Des. Eng., 2023, 8, 79 RSC.
- Z. Cao, W. Liu, G. Ye, X. Zhao, X. Lin, P. Gao and K. Yao, Macromol. Chem. Phys., 2006, 207, 2329 CrossRef CAS.
- Z. Shen, K. Terao, Y. Maki, T. Dobashi, G. Ma and T. Yamamoto, Colloid Polym. Sci., 2006, 284, 1001 CrossRef CAS.
- X. Xu, K. Shibata and M. Ouchi, Polym. Chem., 2023, 14, 55 RSC.
- J. V. M. Weaver, I. Bannister, K. L. Robinson, X. Bories-Azeau and S. P. Armes, Macromolecules, 2004, 37, 2395 CrossRef CAS.
- K. Dušek, Collect. Czech. Chem. Commun., 1969, 34, 3309 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py00250d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |