Open Access Article
Open Access ArticleNano-steps in altered opioid pharmacokinetics: a perspective on potential drug delivery post-bariatric surgery applications
A. E.
Avanu
 a,
A. M.
Ciubotariu
a,
A. M.
Ciornei
b,
A. D.
Cozmîncă
b and
G.
Dodi
a,
A. M.
Ciubotariu
a,
A. M.
Ciornei
b,
A. D.
Cozmîncă
b and
G.
Dodi
 *c
*c
aFaculty of Medicine, Grigore T. Popa University of Medicine and Pharmacy of Iasi, 700115 Iasi, Romania. E-mail: alexandra-elisabeta-avanu@students.umfiasi.ro; alexandra.ciubotariu99@gmail.com
bFaculty of Medicine, Carol Davila University of Medicine and Pharmacy of Bucharest, 020021, Romania. E-mail: ciornei.alexandramariuca@yahoo.com; cozminca.anca@yahoo.com
cFaculty of Medical Bioengineering, Grigore T. Popa University of Medicine and Pharmacy of Iasi, 700115 Iasi, Romania. E-mail: gianina.dodi@umfiasi.ro
First published on 23rd September 2024
Abstract
Despite being a transformative intervention in treating obesity, bariatric surgery, encompassing procedures like Roux-en-Y gastric bypass and vertical sleeve gastrectomy, presents unique challenges in postoperative pain management due to altered pharmacokinetics in both adult and pediatric populations. Opioid medication, while being effective, poses risks of addiction and life-threatening side effects, thus, inviting alternative therapeutic approaches. Nanotechnology holds promise as it provides targeted solutions via nano-drug delivery systems, thereby reducing adverse effects and enhancing efficacy in an altered gastrointestinal system. Different methods, including subcutaneous and nasal delivery systems, prolong drug release, offer potential alternatives for patients with modified drug absorption and metabolism, as demonstrated by in vivo and in vitro studies investigating tramadol, ketamine, fentanyl, buprenorphine and others. Currently, safety issues associated with nanocarriers hinder their clinical deployment. This review prompts a new perspective on nano-controlled release methods and their applications in opioid analgesia, indicating that nanotechnology could address the pharmacokinetic challenges in pain management post-bariatric surgery. Alternative strategies, including the use of endogenous neuropeptides, are discussed for mitigating opioid-related complications and improving pain management outcomes.
1. Introduction
Obesity is linked to over 120 million adult person-years lost annually to non-communicable diseases such as diabetes, stroke, coronary heart disease and cancer.1 While developing nations bear the heaviest burden, countries such as the United States, Malta, New Zealand, Australia and Canada also rank among the top 10 grappling with this issue. By 2035, it is expected that the number of adults with a high body mass index (BMI) ≥ 30 kg m-2 will surge to approximately 1.77 billion, marking a notable 47% increase compared with 2020. A similar projection indicates a 33% rise in obesity in the pediatric population, with an anticipated impact on two out of every five children aged 5 to 19 years.Bariatric surgery, a transformative and underused intervention2 for severe obesity, offers sustained weight loss3 and reduces related comorbidities such as hypertension and diabetes,4–6 consequently improving the quality of life, while diminishing the financial burden associated with obesity-related health issues.7,8 It is also claimed that it decreases the relative risk of mortality by 89%.6 The two most commonly performed techniques are Vertical Sleeve Gastrectomy (VSG) and Roux-en-Y Gastric Bypass (RYGB).7 VSG has become the top choice for all ages with severe obesity due to its effectiveness in weight reduction, improved comorbidity management,9 fewer surgical revisions, enhanced nutrient absorption8 and increased quality-of-life.9
However, bariatric surgery comes with its own risks and downsides, including new-onset depression, anxiety, disability, a relative need for repeat interventions5,10 and long waiting lists.11 Another documented risk is developing chronic pain, found in up to 61.4% of adult patients.12–22 The suspected causes of pain are neuropathic and nociceptive in nature,23 including musculoskeletal pain,4 heightened pain sensitivity,24 dietary factors leading to overdistension, GI (gastrointestinal) disorders and psychological distress20,24 (Fig. 1), currently under investigation.
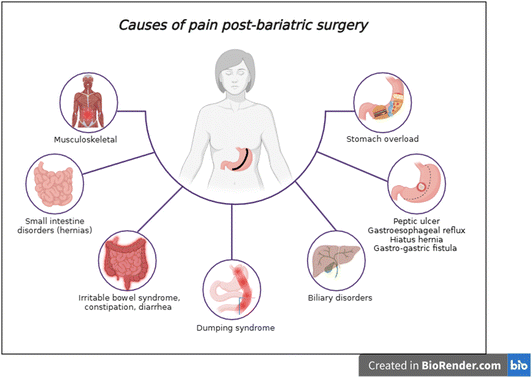 | ||
| Fig. 1 Probable sources of pain, ranging from pre-existing to postoperative conditions. Musculoskeletal pain was identified as the most commonly encountered form. | ||
The GI side-effects of non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen and insufficient analgesia often prompt the bypassing of the World Health Organization's analgesic ladder,25 advancing directly to the second or third step, specifically resorting to opioids.4,26 A significant repercussion is the development of new persistent opioid use (NPOU).27 This condition involves the initiation of opioid usage more than 90 days post-surgery,27 with rates ranging from 3.6 to 9%,4,5,28–32 notably overrepresented in substance use treatment facilities31 and significantly more common than those in non-surgical scenarios (0.4%).5,28 Key factors associated with NPOU include pre-existing mental disorders, prior non-opioid substance use (specifically, tobacco) and, uniquely characteristic to the US, public health insurance.27
Chronic use is only advisable after the patient has not responded to any other therapy.33,34 Due to the scarcity of standardized guidelines30,35–37 and the absence of a threshold for clinically relevant opioid use,31 patients often receive moderate to high doses of medication, resulting in a surplus, part of which leads to drug abuse, immediately or in the longer term, directly or indirectly, by sharing with others.22 Recognizing this issue, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program introduced the Bariatric Surgery Targeting Opioid Prescriptions (BSTOP) protocol in 2019, with the hope of minimizing opioid use, while effectively managing pain.22 Efforts to develop opioids with antagonists or abuse-deterrent features have also been made, but there is still a critical need for new agonists offering sustained pain relief while reducing abuse potential.38
Gradually, guidelines such as Enhanced Recovery After Surgery (ERAS) are being developed for children, not just for adults,39–41 due to evidence indicating reduced opioid requirements and shorter hospital stays.40 Multimodal pain management, particularly in pediatric surgery, remains crucial due to opioid risks, with efforts to reduce opioid overprescription, currently being investigated in clinical trials.42,43 Additionally, nanotechnology, already in use in pediatric and adult oncology, as well as other fields, offers targeted delivery with minimal toxicity.44,45 It has revolutionized the next generation of pain treatment by employing new or well-known nanoparticles (NPs) and nanomaterials (NMs) as drug carriers to form nano-drug delivery systems (NDDSs) that offer enhanced efficacy with lower doses and prolonged analgesia.38 They could potentially be safer, particularly for drugs with narrow therapeutic indices such as opioids.46
Overall, nanomedicine's application to pain management in general and in bariatric surgery in particular has been limited by the complexity of the biological barriers to pain management,47 the intractable nature of chronic pain,38 safety worries about the carrier itself and the influence of different types of bariatric surgery on drug pharmacokinetics (PKs).3 The latter is the culprit that drives the good, the bad and all the other outcomes in-between in bariatric surgery.48
Only a handful of recent studies employ nanotechnology for opioid delivery. While these “nano” advancements may suggest a futuristic approach, the pressing issue of opioid misuse renders this exploration long overdue. In this review we investigate changes in PKs post-bariatric surgery, challenges and recent progress in nanotechnology-driven strategies for pain management, opioids and alternative solutions. We examine the potential application of nanotechnology in opioid delivery post-bariatric surgery, narrowing down from its broader range of uses.
2. Methods
A qualitative literature search spanning from 2017 to February 2024 was conducted, using Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OVID), EMBASE (OVID), Google Scholar (first 200 relevant results) and PubMed. No language filter was applied and we cross-referenced relevant papers to ensure comprehensive coverage of all in vivo, in vitro and clinical studies focusing on nanotechnology's applications in pain management that could possibly be administered post-bariatric surgery. Multiple subject headings (MeSH) related to bariatric surgery, pain, opioids, and pharmacokinetic (PK) alterations were employed in the search.3. Results
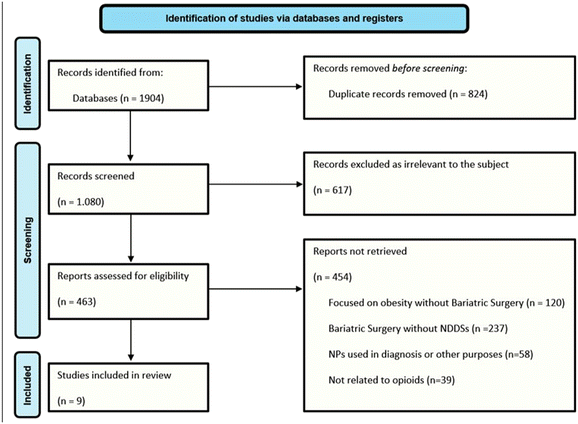
We retrieved a total of 9 studies that meet the inclusion criteria as depicted in Fig. 2. All studies were screened by title, abstract and full article review. They were then analysed by specific clinical indications and appropriate data were presented based on critical analysis of those articles.4. PK considerations in bariatric surgery
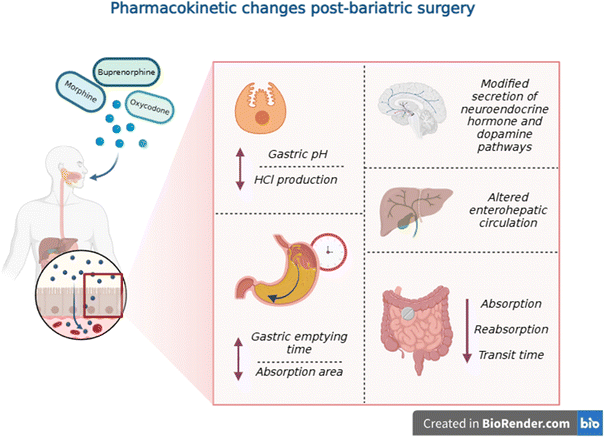
PKs examine drug–body interactions, including absorption, distribution, metabolism and excretion49 for purposes such as dose adjustment, bioavailability and toxicity studies.46 PK studies are crucial for understanding nanotechnology's potency,50 even more, in the case of an altered GI system, post-bariatric surgery (Fig. 3). Changes in GI anatomy likely impact oral drug PKs,7 underscoring the importance of taking into account patient characteristics, drug formulation and interactions in treatment decisions.27,51 In a 2016 study, RYGB surgery in rats led to increased motivation for morphine self-administration, compared to control groups, suggesting that alterations in GI function, nutrient absorption and opioid PKs impact the reward system and pain management.52 Additionally, the reduction in high-fat diet intake post-surgery could potentially reverse the diminished reward system seen in obese rats, leading to increased opioid sensitivity. Furthermore, the surgery might exacerbate a pre-existing ‘reward deficiency syndrome,’ where disruptions in dopamine signaling, common in both obesity and addiction, could heighten vulnerability to substance use. In this context, NDDSs should be explored as a potential viable solution.When using NDDSs, PK studies must include the concentrations of free and loaded drugs, carrier materials and drug-loaded particles in the blood, in order to gather valuable information on drug release kinetics.53
The approved nanodrugs in clinical trials highlight their possible nontoxic carrier nature; however, they can interact with the immune system and impact metabolism, demanding careful consideration. Both the properties of NDDSs, such as surface charge, particle size,53 and increased surface area,54 which extend the half-life of the drug53 and the changes in gastric pH could significantly influence drug absorption.7,51 NPs face some level of barrier in GI absorption due to the epithelium and mucous layer, but because they are absorbed through Peyer's patches and intestinal enterocytes, generally they enhance drug delivery46 and improve the therapeutic impact on the target.53
In the case of opioids, there is a scarcity of their study in the literature, but the available information indicates potential short- and long-term effects on PKs.55 Notably, significant gaps exist in pediatric PK research,56 where using adult standard doses may not suffice and optimal dosing remains uncertain.57 Simulations of methadone dosing highlight the need to account for the genotype associated with obesity, as some patients may require half of the standard dose.58
The European Association for the Study of Obesity recommends careful review of potential shifts in drug absorption caused by bariatric procedures.59 While initial data showed no significant changes in absorption and bioavailability of free morphine,7,60 later studies revealed that these can increase as much as four times,51,61 which is concerning given morphine's narrow therapeutic index.20,51 Lloret-Linares et al. (2014) observed extensive alterations in oral morphine's peak plasma concentration time (tmax), with levels being two-fold lower at 1–2 weeks and 7.5 times higher at 6 months, compared to earlier observations, accompanied by increases in maximum plasma concentration (Cmax) at both time points.51 Moreover, the average oral morphine area under the plasma concentration–time curve (AUC) showed a notable increase from pre-surgery to 6 months post-bariatric surgery. Similar findings were observed for oxycodone.51,62,63
Each type of bariatric surgery has a different impact, with RYGB being more likely to affect overall absorption, on account of the loss of mucosal exposure and reduced bile-salt mixing.7 In RYGB and VSG, the reduction in parietal cells results in elevated gastric pH due to low hydrochloric acid production,7,51 which impacts opioids sensitive to pH variations.64,65
The magnitude of these effects is unclear because the stomach exhibits a much lower surface-area-to-volume ratio than the small intestine,7 which is where absorption occurs predominantly. Bypassing the proximal small intestine in RYGB leads to increased drug absorption in the distal portion, which has a smaller surface area, because of the slower transit time. The properties of NDDS carriers could be optimized to enhance absorption at these new sites.53
Different formulations – lipid-based or water-swellable – did not show differences in PKs.51 While changing the route of administration showed variations, it alone is not sufficient to prevent the alteration of absorption, as described in a case study of a patient who was treated for many years with sublingual buprenorphine, a synthetic opioid, in an opioid maintenance treatment program.66 A week after VSG, the patient exhibited symptoms of withdrawal. Systemic exposure decreased to 43% after one month, accompanied by increased clearance, which remained constant throughout the year. Alterations in salivary pH towards lower values can modify absorption due to a greater proportion of the drug being ionized, alongside lowered saliva production experienced by patients, post-bariatric surgery and other factors whose mechanisms are not yet fully elucidated.
Besides absorption, drug distribution and metabolism are also critical factors influenced by bariatric surgery and NDDSs. Drug distribution governs the amount of drug reaching target sites compared to the rest of the body and thus plays an important role in drug efficacy and toxicity.53 The distribution of NDDSs in tissues and organs depends on the physicochemical and surface properties of the drug-loaded particles. It is also affected by other factors, such as protein binding, hemodynamics of tissues and organs and vascular morphology.
Regarding metabolism, RYGB appears to induce the most notable alterations, attributed to bypassing of the cytochrome P450 enzymes and drug transporters expressed in the duodenum.20 The complex interplay between NDDS properties, drug metabolism and altered physiological conditions post-bariatric surgery, underscores the need for tailored drug delivery strategies to optimize therapeutic outcomes.
The PK and toxicological profiles of nanocarriers cannot be generalized.53 Current assessment methods are inadequate for accurately measuring their effectiveness, emphasizing the need for new tools. The physiologically based PK model presents as a promising solution, serving as a mathematical tool that explores the interconnection between physiology and drugs in clinical scenarios, encompassing various types of NPs. By employing computer simulations, it holds the potential to replace in vivo and clinical studies, providing comprehensive insights into PK outcomes. Nonetheless, its effective integration into nanomedicine mandates interdisciplinary collaboration spanning materials science, pharmacology and mathematical modelling, aimed at optimizing nanoformulation design for optimal PKs.
5. Nano-opioid delivery systems with possible applications in bariatric surgery
Advancements in nanotechnology regarding opioids are undeniable, from extraction of morphine, codeine67 or buprenorphine68–70 to sensing70–77 and abuse-deterrent opioid approaches,78,79 all the way to the more recent steps towards NDDSs. Numerous NDDSs remain unutilized in clinics, largely due to concerns regarding the efficacy and safety of nanocarriers.80 The Food and Drug Administration (FDA) has approved just a few nanomedicines for opioid analgesia. Notable examples are: morphine sulfate extended-release formulations for post-operative pain such as Avinza,81 released in 2002, with nanocrystal carriers, DepoDur,82 in 2004, with liposome carriers and more recent, liposomal bupivacaine for post-hemorroidectomy and bunionectomy83 and an extended-release formulation of oxycodone encapsulated in tamper-resistant beads.84 Another instance is Zalviso (Sufentanil NanoTab), which was recently withdrawn in 2022.85,86The current delivery routes for opioid drugs carry substantial health risks, including abuse, addiction, respiratory depression and even death.87 However, these risks could be mitigated through the controlled release of opioids at therapeutic levels over extended periods. Although oral drug administration has the greatest patient adherence, it also has the major drawbacks of first pass metabolism and rapid clearance.88 Bariatric surgery through aforementioned PK mechanisms augments this effect.
NDDSs enable the integration of various methods into a singular platform,54 improving drug efficacy with prolonged circulation time, avoidance of drug resistance53 and minimum dosage for minimal side-effects.38,89–91 This is achieved through specific targeting and safety measures by integrating drugs in biocompatible nanocarriers,38,92,93 using GI elements to their advantage.94 When one or more drugs are loaded on nanocarriers they could target pain receptors through the blood–brain barrier (BBB), without the risk of addiction.38 NDDSs feature customizable parameters like size, shape, surface charge and cargo dose for enhanced specificity.89,90
Nanotechnology-based approaches not only increase drug surface area but also modify the physiochemical properties of active pharmaceutical ingredients.46 In recent years, there has been a significant focus among biomedical researchers on developing smart polymeric drug delivery systems. NPs made from biocompatible materials like phospholipids, polylactic-co-glycolic acid (PLGA) and natural polymers are generally free from adverse effects. Strategic fabrication of polymeric NPs, often coated with substances like polyethylene glycol (PEG), dextran and citrate, enhances their biodistribution. From a financial perspective, utilizing active compounds that have already undergone investigation and approval by relevant agencies results in cost savings.95
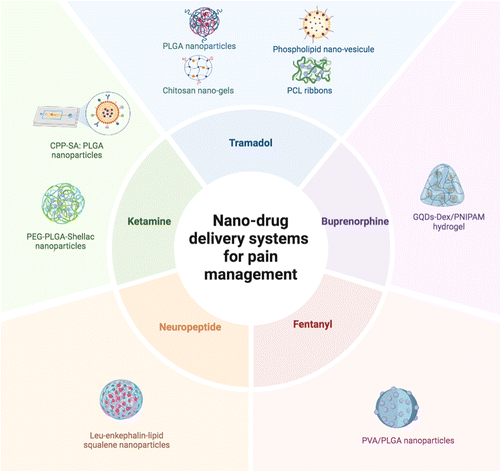
The most commonly used drugs after bariatric surgery are hydrocodone, tramadol, oxycodone and hydromorphone.31,96 Despite the absence of clinical trials, studies employing in vivo and in vitro models have explored various NDDSs, focusing on some of these drugs, and explored new directions, as depicted in Fig. 4. Most studies included both in vivo and in vitro experiments, except for three that were limited to in vitro experiments only, namely in vitro drug release profiles of tramadol containing chitosan-based pro-nanogels using Franz cells,97 an in vitro dialysis bag method for tramadol loaded PLGA nanoparticles34 and tramadol release in PBS from PCL ribbons.98
5.1 Tramadol
Tramadol, a synthetic opioid used for moderate to severe pain,96,99 offers extended-release formulations with a half-life of 48 h maximum.97,98 However, these standard formulations are suboptimal for most patients, leading to reduced compliance and, as we emphasized, for bariatric surgery patients in particular. While oral tramadol reaches peak effectiveness within 2–4 h, only intravenous (IV) administration ensures efficient analgesia with a relatively short onset of action.Barati et al. (2018) explored a chitosan (CS) based in situ gel as a delivery system for tramadol.97 CS, a natural polysaccharide, valued for its biocompatibility, biodegradability and low toxicity, is widely used in biomedical applications, though its pH-sensitive swelling limits mechanical stability.97,98 NPs are commonly derived from CS and are known for their ability to regulate drug release, making them well-suited for this purpose. In this study, the hydrogels (crosslinked with pentasodium triphosphate (TPP) or without TPP), intended for subcutaneous (SC) administration, were designed to address the need for a sustained-release formulation suitable for chronic pain management.97 By mixing the drug with an aqueous solvent, the gel encapsulating the drug forms in situ, presenting a novel concept of “pro-nanogels” for controlled drug delivery. The morphology exposed relative spherical nanocavities in the homogeneous gel structure, mainly due to the presence of TPP. Out of the eight conventional models used to describe the tramadol release from the gel formulations (in vitro, using Franz cells and a dialysis membrane), the most fitted model was Weibull for both of the pro-nanogels with TPP (R2 = 0.945) and without TPP (R2 = 0.936). The study demonstrated different prolonged drug releases over 8 h, namely nearly 30% for the formulation without TPP and 80% for the crosslinked one, due to the formed inner nanocavities. The pro-nanogels formed without such nanocavities offered the possibility to extend the release phenomenon even for days when injected subcutaneously.
Touitou et al. (2020) explored a different approach by investigating the use of phospholipid nanovesicles as a carrier for non-invasive nasal delivery of tramadol.99 Their objective was to circumvent the hepatic first pass metabolism, ultimately facilitating direct delivery to the brain. The mouse model demonstrated a notably faster onset of action and enhanced analgesic efficacy compared to conventional nasal and oral delivery methods. The maximum possible effect (MPE) reached 68.7% at 10 minutes and 62.3% at 180 minutes, in contrast to the less than 40% with oral administration. Pain relief was further affirmed in a rat model, correlating with sustained tramadol levels in plasma and brain tissue, with immediate and consistent onset of action, alongside over two-fold higher penetration through the BBB compared to oral administration and superior integration of the nanocarrier, with no influence on the vesicle shape, aggregation, or crystallization, ensuring stability. The nasal nanovesicular system led to faster, higher and more extensive absorption of tramadol in both the plasma and brain.
Moreover, the PK crossover study in the sheep model demonstrated good bioavailability. In plasma, Tmax values indicated that the nasal nanovesicular system reached the maximum concentration six times faster, leading to a much quicker onset of action in the brain. The AUC from 0 to 240 minutes for the nasal nanovesicular system was much larger, which signifies a higher overall exposure to tramadol in the plasma when administered nasally, which could imply more effective central nervous system delivery.
The PK parameters of tramadol and its metabolite M1 in plasma and cerebrospinal fluid (CSF) following nasal versus IV injection in sheep revealed a shorter half-life in plasma but a significantly longer half-life in CSF. The bioavailability values for nasal administration were 1.09 in plasma and 0.87 in CSF. For M1, the nasal route showed a slightly longer half-life and Tmax, with higher Cmax and AUC0–last (area under the concentration–time curve from time zero to the last measurable concentration) and AUC0–∞ (area under the curve from time 0 extrapolated to infinite time) compared to IV. These differences highlight the potential advantages of the nasal nanovesicular system for achieving quicker and more effective drug delivery to the brain, which could be particularly beneficial for conditions requiring rapid onset of analgesia.
Another research aligning with the pursuit of alternative delivery systems for tramadol, akin to prior investigations, had a different approach.34 By encapsulating tramadol within PLGA NPs, one of the most widely used polymeric carriers for sustained release formulations, Yildirim et al. (2023) elucidated that higher polymer concentrations led to larger NPs with a spherical shape and a smooth surface, while increased homogenizer speed and stabilizer ratio enhanced encapsulation efficiency. The drug release analysis showed a burst release around 90% within the first 2 h for free tramadol and 15–35% within the first hour for loaded NPs. Afterwards, the release rate was decreased and the formulations exhibited a sustained release for at least 30 h due to the entrapment of tramadol into PLGA nanoparticles. Notably in this study, the NPs exhibited a slower drug release rate compared to conventional drug solutions, underscoring their potential for sustained therapeutic effects.
In another study, Mabrouk et al. (2018) incorporated tramadol into PCL (poly ε-caprolactone) ribbons, an aliphatic polyester,98 followed or not by PVA and β-cyclodextrin coating. The drug loading efficiency was high (85–97% depending on the ribbon coating) and in vitro release studies demonstrated controlled drug release up to 45 days, effectively avoiding an initial burst release, which would result in reduced treatment efficacy due to the drug being lost in an uncontrolled and unpredictable manner. Tramadol showed a high release percentage from uncoated ribbons up to 94.14% and a decreased initial release percentage (26.90 and 38.40%) from the PVA–β-cyclodextrin coated ribbons. pH variation analyses indicated higher values in coated ribbons and a stable pH environment over time. Other PK parameters were not assessed.
5.2 Ketamine
Motivated by ketamine's short half-life, Han et al. (2020) introduced a novel method to achieve high ketamine loading (41.8%) within polymeric NPs, designed for sustained release.100 They enhanced the PK profile, with prolonged half-life, increased systemic exposure and reduced clearance, addressing challenges commonly encountered by the bariatric surgery patients. Ketamine has a short half-life of 0.60 hours, while the ketamine-loaded NPs showed significantly prolonged half-lives of 103.10 hours and 79.70 hours for PEG–PLGA nanoparticles and shellac (SH) nanoparticles, respectively. The released ketamine from the PEG–PLGA:SH and PEG–PLGA NPs reached 81.9% and 56.6%, respectively, on day 21 following the in vitro release dialysis membrane model. Their sustained release over a 3-week period was due to the unique drug–core polymer–shell structure, enabling controlled drug diffusion through the polymer matrix. This approach could offer a solution to optimize pain management in both cancer, which was the author's focus, and bariatric surgery, as we assess, while maintaining a favorable safety profile. Ketamine-loaded NPs exhibited increased AUC0–last values in in vivo studies (C57BL/6J male mice), compared to free ketamine, suggesting enhanced drug exposure and prolonged circulation in the bloodstream. Similarly, the AUC0–∞ values for ketamine-loaded NPs were significantly higher, indicating prolonged drug exposure beyond the last measured time point.Sometimes, when high doses do not alleviate pain, invasive routes like intrathecal administration are used. The in vitro release profiles of hydromorphone/ketamine from a polymer mixture of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 CPP-SA
50 CPP-SA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLGA NPs fabricated by a two-stage microfluidic method were evaluated over 28 days showing a drug loading of around 35%.94 Furthermore, in a rat model the drugs provided pain relief within 3 h, lasting steadily for 78 h, after single intrathecal injection on a rat model of peripheral neuropathic pain. Overall, lipid NPs fabricated with the CPP-SA polymer demonstrated more sustained release compared to PLGA, but the efficacy and duration of hydromorphone-loaded NPs alone matched those of added ketamine.
PLGA NPs fabricated by a two-stage microfluidic method were evaluated over 28 days showing a drug loading of around 35%.94 Furthermore, in a rat model the drugs provided pain relief within 3 h, lasting steadily for 78 h, after single intrathecal injection on a rat model of peripheral neuropathic pain. Overall, lipid NPs fabricated with the CPP-SA polymer demonstrated more sustained release compared to PLGA, but the efficacy and duration of hydromorphone-loaded NPs alone matched those of added ketamine.
5.3 Buprenorphine
Taking another route, Yue et al. (2019) developed a thermo-responsive graphene quantum dot (GQD) loaded dextran/poly(N-isopropylacrylamide) (Dex/PNIPAM) copolymeric matrix as a sustained implantation drug delivery system for buprenorphine.101 Thermoresponsive polymeric nanomedicine, comprising nanomicelles, nanocapsules and NPs has garnered considerable attention over the past decades for various drug delivery applications. These systems aim to minimize side effects, reduce dosage requirements, extend in vivo circulation time, protect drugs from biological degradation and enhance therapeutic efficacy. This innovative approach offers minimally invasive administration and self-administration through skin permeability enhanced by temperature changes. Such thermoresponsive polymers, often termed smart polymers, find extensive applications in pain management. PNIPAM, in particular, is widely used in various biological fields due to its ability to respond to changes in temperature, making it a versatile material for drug delivery, tissue engineering, sensing and cell culture applications. After the ionic liquid mediated synthesis of GQDs, the loaded composite hydrogel was synthesized by an in situ dispersion polymerization method, obtaining a porous morphology copolymeric matrix with uniform distribution and monodisperse ultra-small GQDs. The in vitro release profile of the buprenorphine loaded GQDs–Dex/PNIPAM thermo-responsive hydrogel matrix, assessed for 7 days at below (25 °C and 32 °C) and above the low critical solution temperature (39 °C), respectively, exhibited sustained and enhanced drug release percentages dependent on temperature and time. The tissue feasibility analysis performed on isolated animals’ sciatic nerve and adjacent tissue responses of implantations after 7 days post-surgery demonstrated that the buprenorphine loaded composite was responsible for the pain management, improving the anti-inflammatory efficiency.5.4 Fentanyl
A particular challenge is fentanyl dosing in obese adolescents which necessitates careful consideration of weight descriptors57 to prevent variability and higher peak plasma concentrations.102 Kovaliov et al. (2017) developed fentanyl-bearing polylactide and polyglycolide NPs (Fen-PLA/PLGA NPs) for SC administration in a mouse model, demonstrating sustained drug release for up to six days with a single administration.28 Non-covalent encapsulation of drugs into polymer matrices sometimes presents drawbacks such as burst release and low drug loading percentages. The authors successfully avoided the use of ring-opening polymerization (ROP). The study underscored that polymer composition and NP size play crucial roles in achieving controlled release, which is essential for preventing abuse and euphoria. The hot plate nociceptive assay revealed a 50% MPE on day 1 and approximately 40% MPE on day 6, with a diminishing therapeutic effect by day 14, indicating NP-mediated extended-release for sustained pain relief. However, the current SC delivery method might result in unintended NP absorption and clearance before therapeutic opioid release.5.5 Alternatives – neuropeptides
Recent research has explored alternatives to opioids, acknowledging their PK limitations. Feng et al. (2019) introduced a novel concept demonstrating the pharmacological efficacy of the neuropeptide Leu-enkephalin (LENK) when conjugated with the lipid squalene (SQ).103 Despite the complexities of peptide–lipid chemistry, the authors synthesized LENK-SQ bioconjugates with different chemical linkers (dioxycarbonyl, diglycolic, and amide) to control LENK release after formulation into NPs with sizes varying from 60 to 120 nm (in water). In a rat model, this innovative SQ-based nanoformulation effectively prevented rapid plasma degradation of LENK and showed prolonged analgesic effects compared to morphine. Biodistribution studies and opioid receptor antagonist tests suggested that LENK-SQ NPs act through peripheral opioid receptors, particularly δ-opioid receptors, with reduced abuse potential.104 This study introduced an encouraging approach for targeted delivery of LENK neuropeptide to inflamed tissues for pain management, maintaining stable serum concentrations for over 48 hours.102 Injection of LENK-SQ NPs in rats resulted in a substantial reduction in thermal hyperalgesia, as indicated by a notable increase in the respective AUC values compared to rats treated with either free LENK peptide or blank SQ NPs. The effect was significant within 2 h and lasted for 10 minutes, demonstrating efficacy with no observed toxicity.5.6 Translational level
These findings underscore the potential of nanotechnology to optimize pain management potentially in bariatric surgery patients and highlight the need for further research to bridge the gap between preclinical evidence and clinical application. But, in order to reach the path to clinics, the first key after efficiency is evaluation of the developed supports from a biological point of view, mainly regarding the biocompatibility of the new painkillers and their components. Even if a variety of materials known for their biocompatibility have been used to incorporate/encapsulate pain drug molecules, only a few studies pointed their research also on compatibility with cells/tissues either in vitro or in vivo. The authors presented below an overview of the compatibility analysis on the included studies.Local safety of the phospholipid nanocarrier and tramadol system tested in rats indicated no irritation and toxicity of the nasal mucosa after administration, according to the histopathological images of different regions of the nasal cavity (cartilage and turbinate bone, lamina propria and submucosa, mucosal epithelium and lumen).99
The safety profile of the carrier matrix of ketamine (PEG–PLGA:SH) was also analysed, this time using haematological data.
The leukocyte, erythrocyte and thrombocyte parameters, assessed at study completion on day 5 post-dosing were within the normal ranges for mice.100
A more comprehensive biocompatibility investigation was performed for the thermo-responsive buprenorphine loaded composite (GQDs–Dex/PNIPAM copolymeric matrix), using both in vitro cytocompatibility (according to ISO 10993-5) and in vivo skin irritation tests (according to ISO 10993-23) after 7th day post-surgery. The in vitro cytocompatibility investigated using MTT assay onto the mouse fibroblast cell line (L929) demonstrated 96% cell viability percentage for the in situ drug carriers. The histological observations at the implantation site established that the hydrogel was biocompatible with the nerves and surrounding tissues, with no inflammation and acute toxicity.101
In the study by Kovaliov et al. (2017) novel biohybrids based on Fen-PLA/PLGA NPs were evaluated only in vitro for their biocompatibility. The ATP assay performed by incubating SHSY5Y neuroblastoma cells for 48 h with fentanyl-polymer NPs showed a 100% survival rate (ATP level% from the control) in the entire tested concentration range (0.15–333 μg mL−1).28
Toxicity study (according to ISO 10993-11) was performed on adult male Sprague-Dawley rats injected with LENK-SQ-Am NPs (20 mg kg−1). The aspartate transaminase (AST) and alanine transaminase (ALT) levels in plasma and histopathological examination of the liver, kidneys, spleen, heart and lungs were determined at 24 or 48 h after intravenous injection. Normal levels of transaminases and histology of vital organs confirmed the safety of the therapeutic dose of 20 mg kg−1 LENK-SQ NPs upon intravenous administration.103
Future research endeavours should focus on refining NP formulations, optimizing administration routes and conducting clinical trials to validate the efficacy and safety of these innovative drug delivery systems. For an overview of all NDDSs discussed previously, see Table 1. Fig. 5 describes the available nano-opioid delivery systems with possible applications in bariatric surgery based on the delivery routes for the nanocarriers and the analysed release profile time of the incorporated opioid drugs.
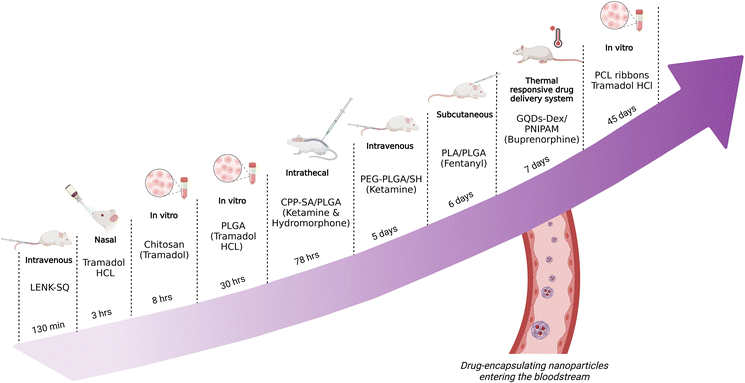 | ||
| Fig. 5 Design of NDDSs, delivery routes and the analysed release time of the incorporated opioid drugs. | ||
| NPs | Size of particles (nm) | Preparation method | Ref. |
|---|---|---|---|
| Abbreviations: NP – nanoparticle; HCL – hydrochloric acid; PLGA – polylactic-co-glycolic acid; W/O/W – double emulsification solvent evaporation; PCL – poly ε-caprolactone; PEG – polyethylene glycol; SH – shellac; CPP – 1,3-bis(p-carboxyphenoxy)propane; SA – sebacic acid; GQD – graphene quantum dots; Dex – dextran; PNIPAM – poly(N-isopropylacrylamide); PLA – polylactide; and LENK-SQ – Leu-enkephalin-squalene. | |||
| Chitosan | 162.1 | Ionic gelation | Barati et al. (2018)97 |
| Phospholipid nanovesicles | ∼200 | Thin-film hydration | Touitou et al. (2020)99 |
| PLGA | 237.2–348.6 | Double emulsification solvent evaporation method | Yildirim et al. (2023)34 |
| PCL ribbons (uncoated and β-cyclodextrin coated) | 2–5 | Slip casting solvent evaporation | Mabrouk et al. (2018)98 |
| PEG–PLGA/SH | 98.8–107.4 | Sequential nanoprecipitation | Han et al. (2020)100 |
| CPP-SA/PLGA | 50–500 | Microfluidic | Zhu et al. (2020)95 |
| GQDs–Dex/PNIPAM | 2–12 | Dispersion polymerization | Yue et al. (2019)101 |
| PLA/PLGA | 362–508 | Ring-opening polymerization | Kovaliov et al. (2017)28 |
| LENK-SQ | 60–120 | Nanoprecipitation | Feng et al. (2019)103 |
6. Discussion: uncharted nanotechnology territory
NDDS strategies, including encapsulating and entrapping bioactive materials, particle size reduction and surface modification, point to enhanced pain relief by improving PKs and bioavailability. They present opportunities for addressing drug abuse concerns while maintaining therapeutic efficacy, a benefit relevant to both adult and pediatric populations.44 As applications diversify and advance, medical professionals must acknowledge their increased capacity to improve patient care.Despite varying age-related considerations, such as developmental stages and physiological differences,105 both groups encounter similar challenges in the management of obesity and postoperative pain with insufficient and inconsistent literature. For instance, studies in adults undergoing bariatric surgery reveal mixed outcomes regarding opioid use post-surgery, with some experiencing an increase5,31 while others a decrease.6,96 Specifically, a retrospective Swedish cohort study found that after surgery, most of the younger demographic of high consumers increased their dosage.5 In another notable example, the opioid use prevalence rose by 8.4% between six months and seven years post-surgery.31 In the same cohort, NPOU more than doubled after six years. On the other hand, Crémieux et al. found a significant decrease in pain medication use within four months post-surgery, although with no improvement beyond that period,6 aligning with the trend of opioid usage increasing after an initial decline post-surgery in other studies. Also interestingly, in a Sweden cohort, the rate of NPOU was lower than those in other previous studies and one explanation could be the different classifications of high opioid consumers,4 for which clear protocols could be of great value.
Similarly, a retrospective cohort analysis focused on postoperative pain intensity and opioid use showed only a mild association with the BMI in pediatric patients, challenging assumptions.106 On the pediatric side, early results on nano-medicine indicate promising avenues for innovative solutions in treating postoperative pain management,44 in conditions like cystic fibrosis, offering hope for improved patient outcomes. Additionally, developments such as nano-patches for transdermal drug delivery could be an alternative, providing steady release of lipophilic drugs like buprenorphine, while reducing side effects.44,107 However, safety evaluations remain paramount, particularly concerning opioid risks, highlighting the delicate balance between addressing pain effectively and minimizing potential harm, a critical consideration in pediatric population.
Other challenges, such as manufacturing scale-up, long-term nanoparticle toxicity and high costs for clinical trials, hinder widespread implementation. Overcoming these barriers requires ongoing translational research efforts in collaboration with regulatory agencies. Together with early referral for surgery and a more aggressive management of excess weight, nanotechnology could have a real impact on opioid consumption.96
Despite ongoing efforts, understanding of nanoparticle PKs remains fragmented, with a need for studies focusing on both drug and nanoparticle materials. This deeper understanding is crucial for optimizing NP fate and improving nanomedicine outcomes.46 An important challenge hindering the clinical advancement of NDDSs is the inadequate understanding of their internal behavior.
The paradigm is changing towards personalized therapeutic approaches, providing a potential solution to address these shared concerns. Thus, while the specific clinical contexts may differ, the overarching goal of improving patient outcomes through innovative approaches remains consistent.
The question was raised whether glucagon-like peptide-1 receptor agonists, such as semaglutide and liraglutide, could act as an alternative to bariatric surgery. Studies showed that patients are withdrawing from surgery lists because of their efficiency on weight loss,107,108 although, concerns persist over long-term complications such as weight regain, cardiometabolic changes,109 hypoglycemia110 and gastrointestinal issues,111 alongside increased long-term costs.2
A key limitation of the current paper is that the NDDSs investigated do not specifically consider the use and impact of controlled-release formulations in an altered GI system because of a lack of data. However, their favorable characteristics and parameters indicate their potential. Additional studies are required to understand how these delivery vehicles perform under these unique conditions.
Continued research efforts are essential to harness nanotechnology's full potential in both adult and pediatric medicine. Future studies should concentrate on understanding the mechanisms of post-bariatric surgery drug malabsorption and designing tailored drug delivery. The ultimate goal is to achieve maximum pain reduction with minimal adverse effects, incorporating alternative pain management methods as well.
7. Conclusion
Nanotechnology represents a burgeoning field with immense potential for the future, captivating researchers as they strive toward significant breakthroughs. While it offers significant potential in pain management, challenges persist, including the need for comprehensive understanding of NP behaviour for successful clinical translation of NDDSs. Understanding the mechanisms of post-bariatric surgery drug malabsorption and designing tailored drug delivery systems to enhance bioavailability will drive us to the ultimate goal to achieve maximum pain reduction with minimal adverse effects, incorporating alternative pain management methods as well.Abbreviations
| AUC0–∞ | Area under the curve from time 0 extrapolated to infinite time |
| AUC0–last | Area under the concentration–time curve from time zero to the last measurable concentration |
| AUC | Plasma concentration–time curve |
| BBB | Blood–brain barrier |
| BMI | Body mass index |
| BSTOP | Bariatric surgery targeting opioid prescriptions |
| C max | Maximum plasma concentration |
| ERAS | Enhanced recovery after surgery |
| FDA | Food and Drug Administration |
| GI | Gastrointestinal |
| IV | Intravenous |
| LENK | Leu-enkephalin |
| MeSH | Multiple subject headings |
| MPE | Maximum possible effect |
| MTT | (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
| NDDSs | Nano-drug delivery systems |
| NMs | Nanomaterials |
| NPOU | New persistent opioid use |
| NPs | Nanoparticles |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PCL | Poly ε-caprolactone |
| PK | Pharmacokinetic |
| PKs | Pharmacokinetics |
| PLA | Polylactide |
| PLGA | Polylactic-co-glycolic acid |
| PNIPAM | Poly(N-isopropylacrylamide) |
| ROP | Ring-opening polymerization |
| RYGB | Roux-en-Y gastric bypass |
| SQ | Lipid squalene |
| SC | Subcutaneous |
| t max | Peak plasma concentration time |
| VSG | Vertical sleeve gastrectomy |
| W/O/W | Double emulsification solvent evaporation |
Author contributions
Conceptualization, A. E. A., A. M. C. and G. D.; data curation, A. E. A., A. M. C., A. D. C. and A. M. C.; formal analysis, A. E. A. and G. D.; investigation, A. E. A., A. D. C. and A. M. C.; methodology, A. E. A. and G. D.; project administration, G. D. and A. E. A.; resources, G. D.; software, A. E. A. and A. M. C.; supervision, G. D.; visualization, A. E. A., A. M. C. and G. D.; writing – review, editing and revision, A. E. A. and G. D. All authors have read and agreed to the published version of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review. This study was carried out using publicly available data according to the reference list.Conflicts of interest
There are no conflicts to declare.References
- World Obesity Day Atlases|Obesity Atlas 2024, World Obesity Federation Global Obesity Observatory, accessed June 25, 2024, https://data.worldobesity.org/publications/?cat=22.
- M. Haseeb, J. Chhatwal, J. Xiao, P. Jirapinyo and C. C. Thompson, Semaglutide vs Endoscopic Sleeve Gastroplasty for Weight Loss, JAMA Netw. Open, 2024, 7(4), e246221, DOI:10.1001/jamanetworkopen.2024.6221.
- T. Girolamo and R. Allin, Bariatric surgery and medicines: from first principles to practice, Aust. Prescr., 2022, 45(5), 162–166, DOI:10.18773/austprescr.2022.053.
- S. Wallén, E. Szabo, M. Palmetun-Ekbäck and I. Näslund, Use of Opioid Analgesics Before and After Gastric Bypass Surgery in Sweden: a Population-Based Study, Obes. Surg., 2018, 28(11), 3518–3523, DOI:10.1007/s11695-018-3377-7.
- G. S. Jakobsen, M. C. Småstuen and R. Sandbu, et al., Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities, J. Am. Med. Assoc., 2018, 319(3), 291–301, DOI:10.1001/jama.2017.21055.
- P. Y. Crémieux, S. Ledoux, C. Clerici, F. Cremieux and M. Buessing, The impact of bariatric surgery on comorbidities and medication use among obese patients, Obes. Surg., 2010, 20(7), 861–870, DOI:10.1007/s11695-010-0163-6.
- C. Huang and F. Master, Bariatric surgery and the effects on drug pharmacokinetics, The Pharmaceutical Journal, published March 10, 2016, accessed March 9, 2024, https://pharmaceutical-journal.com/article/ld/bariatric-surgery-and-the-effects-on-drug-pharmacokinetics.
- V. Calcaterra, H. Cena and G. Pelizzo, et al., Bariatric Surgery in Adolescents: To Do or Not to Do?, Children, 2021, 8(6), 453, DOI:10.3390/children8060453.
- R. Schauer Philip, L. Bhatt Deepak and P. Kirwan John, et al., Bariatric Surgery versus Intensive Medical Therapy for Diabetes – 5-Year Outcomes, N. Engl. J. Med., 2017, 376(7), 641–651, DOI:10.1056/NEJMoa1600869.
- J. A. Davis, R. L. Robinson, T. K. Le and J. Xie, Incidence and impact of pain conditions and comorbid illnesses, J. Pain Res., 2011, 4, 331–345, DOI:10.2147/JPR.S24170.
- M. A. Rubio-Herrera, S. Mera-Carreiro, A. Sánchez-Pernaute and A. M. Ramos-Levi, Impact of Treatment with GLP1 Receptor Agonists, Liraglutide 3.0 mg and Semaglutide 1.0 mg, While on a Waiting List for Bariatric Surgery, Biomedicines, 2023, 11(10), 2785, DOI:10.3390/biomedicines11102785.
- I. K. Høgestøl, M. Chahal-Kummen and I. Eribe, et al., Chronic Abdominal Pain and Symptoms 5 Years After Gastric Bypass for Morbid Obesity, Obes. Surg., 2017, 27(6), 1438–1445, DOI:10.1007/s11695-016-2499-z.
- S. B. Gribsholt, E. Svensson, B. Richelsen, U. Raundahl, H. T. Sørensen and R. W. Thomsen, Rate of Acute Hospital Admissions Before and After Roux-en-Y Gastric Bypass Surgery: A Population-based Cohort Study, Ann. Surg., 2018, 267(2), 319, DOI:10.1097/SLA.0000000000002113.
- J. Saunders, G. H. Ballantyne and S. Belsley, et al., One-year Readmission Rates at a High Volume Bariatric Surgery Center: Laparoscopic Adjustable Gastric Banding, Laparoscopic Gastric Bypass and Vertical Banded Gastroplasty-Roux-en-Y Gastric Bypass, Obes. Surg., 2008, 18(10), 1233–1240, DOI:10.1007/s11695-008-9517-8.
- I. K. Blom-HØgestØl, A. Stubhaug, J. A. Kristinsson and T. Mala, Diagnosis and treatment of chronic abdominal pain 5 years after Roux-en-Y gastric bypass, Surg. Obes. Relat. Dis., 2018, 14(10), 1544–1551 CrossRef PubMed.
- M. S. Altieri, J. Yang and D. Groves, et al., Sleeve Gastrectomy: the first 3 Years: evaluation of emergency department visits, readmissions and reoperations for 14,080 patients in New York State, Surg. Endosc., 2018, 32(3), 1209–1214, DOI:10.1007/s00464-017-5793-5.
- T. Mala and I. H∅gest∅l, Abdominal Pain After Roux-En-Y Gastric Bypass for Morbid Obesity, Scand. J. Surg., 2018, 107(4), 277–284, DOI:10.1177/1457496918772360.
- A. S. Pierik, U. K. Coblijn, C. A. L. de Raaff, R. N. van Veen, W. F. van Tets and B. A. van Wagensveld, Unexplained abdominal pain in morbidly obese patients after bariatric surgery, Surg. Obes. Relat. Dis., 2017, 13(10), 1743–1751, DOI:10.1016/j.soard.2017.05.027.
- L. I. M. Pernar, R. Lockridge and C. McCormack, et al., An Effort to Develop an Algorithm to Target Abdominal CT Scans for Patients After Gastric Bypass, Obes. Surg., 2016, 26(10), 2543–2546, DOI:10.1007/s11695-016-2324-8.
- A. H. Simoni, L. Ladebo, L. L. Christrup, A. M. Drewes, S. P. Johnsen and A. E. Olesen, Chronic abdominal pain and persistent opioid use after bariatric surgery, Scand. J. Pain, 2020, 20(2), 239–251, DOI:10.1515/sjpain-2019-0092.
- S. B. Gribsholt, A. M. Pedersen, E. Svensson, R. W. Thomsen and B. Richelsen, Prevalence of Self-reported Symptoms After Gastric Bypass Surgery for Obesity, JAMA Surg., 2016, 151(6), 504–511, DOI:10.1001/jamasurg.2015.5110.
- R. Seu, X. Pereira and P. Goriacko, et al., Effectiveness of Bariatric Surgery Targeting Opioid Prescriptions (BSTOP) protocol on postoperative pain control, Surg. Endosc., 2023, 37(6), 4902–4909, DOI:10.1007/s00464-022-09646-4.
- J. R. Berger, The Neurological Complications of Bariatric Surgery, Arch. Neurol., 2004, 61(8), 1185–1189, DOI:10.1001/archneur.61.8.1185.
- L. J. Heinberg, L. Pudalov, H. Alameddin and K. Steffen, Opioids and bariatric surgery: A review and suggested recommendations for assessment and risk reduction, Surg. Obes. Relat. Dis., 2019, 15(2), 314–321, DOI:10.1016/j.soard.2018.11.019.
- J. Yang, B. A. Bauer, D. L. Wahner-Roedler, T. Y. Chon and L. Xiao, The Modified WHO Analgesic Ladder: Is It Appropriate for Chronic Non-Cancer Pain?, J. Pain Res., 2020, 13, 411–417, DOI:10.2147/JPR.S244173.
- J. M. Hagedorn, World Health Organization Analgesic Ladder, in Anesthesiology In-Training Exam Review: Regional Anesthesia and Chronic Pain, ed. R. K. Banik, Springer International Publishing, 2022, pp. 351–354. DOI:10.1007/978-3-030-87266-3_67.
- K. Nasser, K. Verhoeff and V. Mocanu, et al., New persistent opioid use after bariatric surgery: a systematic review and pooled proportion meta-analysis, Surg. Endosc., 2023, 37(1), 703–714, DOI:10.1007/s00464-022-09291-x.
- M. Kovaliov, S. Li and E. Korkmaz, et al., Extended-release of opioids using fentanyl-based polymeric nanoparticles for enhanced pain management, RSC Adv., 2017, 7(76), 47904–47912, 10.1039/C7RA08450A.
- C. M. Brummett, J. F. Waljee and J. Goesling, et al., New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults, JAMA Surg., 2017, 152(6), e170504, DOI:10.1001/jamasurg.2017.0504.
- M. E. Smith, J. S. Lee and A. Bonham, et al., Effect of new persistent opioid use on physiologic and psychologic outcomes following bariatric surgery, Surg. Endosc., 2019, 33(8), 2649–2656, DOI:10.1007/s00464-018-6542-0.
- W. C. King, J. Y. Chen and S. H. Belle, et al., Use of prescribed opioids before and after bariatric surgery: prospective evidence from a U.S. multicenter cohort study, Surg. Obes. Relat. Dis., 2017, 13(8), 1337–1346, DOI:10.1016/j.soard.2017.04.003.
- C. A. L. de Raaff, N. de Vries and B. A. van Wagensveld, Obstructive sleep apnea and bariatric surgical guidelines: summary and update, Curr. Opin. Anaesthesiol., 2018, 31(1), 104, DOI:10.1097/ACO.0000000000000542.
- C. Martin, A. De Baerdemaeker, J. Poelaert, A. Madder, R. Hoogenboom and S. Ballet, Controlled-release of opioids for improved pain management, Mater. Today, 2016, 19(9), 491–502, DOI:10.1016/j.mattod.2016.01.016.
- N. Yildirim, A. Savaser, O. Esim, G. R. Topal, C. Kose Ozkan and Y. Ozkan, Definition of Design Space for Preparation and Stability of Tramadol Hydrochloride Loaded Nanoparticles Using OFAT Experiments for Infusion in Pain Management, J. Cluster Sci., 2024, 35(6), 1–11, DOI:10.1007/s10876-024-02620-1.
- E. Blay, M. J. Nooromid and K. Y. Bilimoria, et al., Variation in Post-Discharge Opioid Prescriptions Among Members of a Surgical Team, Am. J. Surg., 2018, 216(1), 25–30, DOI:10.1016/j.amjsurg.2017.10.035.
- A. Boloori, B. B. Arnetz, F. Viens, T. Maiti and J. E. Arnetz, Misalignment of Stakeholder Incentives in the Opioid Crisis, Int. J. Environ. Res. Public Health, 2020, 17(20), 7535, DOI:10.3390/ijerph17207535.
- Abuse NI on D. Drug Overdose Death Rates|National Institute on Drug Abuse (NIDA), published May 14, 2024, accessed June 25, 2024, https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates.
- D. Bhansali, S. L. Teng, C. S. Lee, B. L. Schmidt, N. W. Bunnett and K. W. Leong, Nanotechnology for pain management: Current and future therapeutic interventions, Nano Today, 2021, 39, 101223, DOI:10.1016/j.nantod.2021.101223.
- A. Thorell, A. D. MacCormick, S. Awad, N. Reynolds, D. Roulin, N. Demartines, M. Vignaud, A. Alvarez, P. M. Singh and D. N. Lobo, Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations, World J. Surg., 2016, 40(9), 2065–2083, DOI:10.1007/s00268-016-3492-3.
- W. J. Svetanoff, K. Diefenbach and B. Hall, et al., Utilization of an Enhanced Recovery After Surgery (ERAS) protocol for pediatric metabolic and bariatric surgery, J. Pediatr. Surg., 2023, 58(4), 695–701, DOI:10.1016/j.jpedsurg.2022.12.014.
- Y. J. Huh and D. J. Kim, Enhanced Recovery after Surgery in Bariatric Surgery, J. Metab. Bariatr. Surg., 2021, 10(2), 47–54, DOI:10.17476/jmbs.2021.10.2.47.
- Göteborg University, AMOS2 (Adolescent Morbid Obesity Surgery), clinicaltrials.gov, 2022, accessed January 1, 2024, https://clinicaltrials.gov/study/NCT02378259.
- S. Belle, Longitudinal Assessment of Bariatric Surgery (LABS-2), clinicaltrials.gov, 2016, accessed January 1, 2024, https://clinicaltrials.gov/study/NCT00465829.
- H. Omidian and K. Mfoafo, Exploring the Potential of Nanotechnology in Pediatric Healthcare: Advances, Challenges and Future Directions, Pharmaceutics, 2023, 15(6), 1583, DOI:10.3390/pharmaceutics15061583.
- S. Sindhwani and W. C. W. Chan, Nanotechnology for modern medicine: next step towards clinical translation, J. Intern. Med., 2021, 290(3), 486–498, DOI:10.1111/joim.13254.
- K. Raza, P. Kumar, N. Kumar and R. Malik, Pharmacokinetics and biodistribution of the nanoparticles, Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids, 2017, ch. 9. DOI:10.1016/B978-0-08-100557-6.00009-2.
- Advances in Biomaterials for Drug Delivery – Fenton – 2018 – Advanced Materials – Wiley Online Library, Accessed June 25, 2024, https://onlinelibrary-wiley-com.dbproxy.umfiasi.ro/doi/10.1002/adma.201705328.
- T. H. Huang, C. J. Chen, H. C. A. Lin, C. H. Chen and J. Y. Fang, Self-Nanoemulsifying Drug Delivery System-Containing the Poorly Absorbed Drug – Valsartan in Post-Bariatric Surgery, Int. J. Nanomed., 2023, 18, 2647–2658, DOI:10.2147/IJN.S394624.
- Basic Principles of Pharmacokinetics – Leslie Z. Benet, Parnian Zia-Amirhosseini, 1995, accessed June 25, 2024, https://journals.sagepub.com/doi/10.1177/019262339502300203.
- V. Kumar, R. Parveen and S. Ahmad, Pharmacokinetics of nanomedicine, In Nanotechnology Principles in Drug Targeting and Diagnosis. Micro and Nano Technologies, ed. M. Rai, S. Khan and A. Belgamwar, Elsevier, 2023, pp. 127–142. DOI:10.1016/B978-0-323-91763-6.00008-4.
- L. Ladebo, A. Y. Abuhelwa, D. J. R. Foster, J. P. Kroustrup, G. J. Pacyk, K. T. Kongstad, A. M. Drewes, L. L. Christrup and A. E. Olesen, Effect of Roux–en–Y gastric bypass on the pharmacokinetic–pharmacodynamic relationships of liquid and controlled-release formulations of oxycodone, Basic Clin. Pharmacol. Toxicol., 2021, 129(3), 232–245, DOI:10.1111/bcpt.13634.
- J. M. Biegler, C. S. Freet, N. Horvath, A. M. Rogers and A. Hajnal, Increased intravenous morphine self-administration following Roux-en-Y gastric bypass in dietary obese rats, Brain Res. Bull., 2016, 123, 47–52, DOI:10.1016/j.brainresbull.2015.08.003.
- X. Shao, C. Shi, S. Wu, F. Wang and W. Li, Review of the pharmacokinetics of nanodrugs, Nanotechnol. Rev., 2023, 12, 20220525, DOI:10.1515/ntrev-2022-0525.
- A. E. Avanu, A. M. Ciubotariu and G. Dodi, Can Nano Yield Big Insights? Oligonucleotide-Based Biosensors in Early Diagnosis of Gastric Cancer, Chemosensors, 2024, 12(3), 44, DOI:10.3390/chemosensors12030044.
- C. Lau, C. van Kesteren, R. Smeenk, A. Huitema and C. A. J. Knibbe, Impact of Bariatric Surgery in the Short and Long Term: A Need for Time-Dependent Dosing of Drugs, Obes. Surg., 2023, 33(10), 3266–3302, DOI:10.1007/s11695-023-06770-5.
- J. G. Kendrick, R. R. Carr and M. H. Ensom, Pediatric Obesity: Pharmacokinetics and Implications for Drug Dosing, Clin. Ther., 2015, 37(9), 1897–1923, DOI:10.1016/j.clinthera.2015.05.495.
- J. D. Vaughns, V. C. Ziesenitz and E. F. Williams, et al., Use of Fentanyl in Adolescents with Clinically Severe Obesity Undergoing Bariatric Surgery: A Pilot Study, Paediatr. Drugs, 2017, 19(3), 251–257, DOI:10.1007/s40272-017-0216-6.
- J. G. Gerhart, F. O. Carreño and J. L. Ford, et al., Use of physiologically-based pharmacokinetic modeling to inform dosing of the opioid analgesics fentanyl and methadone in children with obesity, CPT: Pharmacometrics Syst. Pharmacol., 2022, 11(6), 778–791, DOI:10.1002/psp4.12793.
- L. Busetto, D. Dicker and C. Azran, et al., Obesity Management Task Force of the European Association for the Study of Obesity Released “Practical Recommendations for the Post-Bariatric Surgery Medical Management”, Obes. Surg., 2018, 28(7), 2117–2121, DOI:10.1007/s11695-018-3283-z.
- A. Edwards and M. H. Ensom, Pharmacokinetic Effects of Bariatric Surgery, Ann. Pharmacother., 2012, 46(1), 130–136, DOI:10.1345/aph.1Q414.
- P. C. Angeles, I. Robertsen, L. T. Seeberg, V. Krogstad, J. Skattebu, R. Sandbu, A. Asberg and J. Hjelmesaeth, The influence of bariatric surgery on oral drug bioavailability in patients with obesity: A systematic review, Obes. Rev., 2019, 20(9), 1299–1311, DOI:10.1111/obr.12869.
- G. Mikus and R. Klimas, Contribution of oxycodone and its metabolites to the analgesic effect, Br. J. Anaesth., 2014, 112(5), 944–945, DOI:10.1093/bja/aeu123.
- B. Lalovic, E. Kharasch, C. Hoffer, L. Risler, L. Y. Liu-Chen and D. D. Shen, Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites, Clin. Pharmacol. Ther., 2006, 79(5), 461–479, DOI:10.1016/j.clpt.2006.01.009.
- N. Ghosh, K. Kesh, S. Ramakrishnan and S. Roy, Opioid Use in Murine Model Results in Severe Gastric Pathology that May Be Attenuated by Proton Pump Inhibition, Am. J. Pathol., 2022, 192(8), 1136–1150, DOI:10.1016/j.ajpath.2022.04.005.
- B. B. Osinaike, P. S. Ogunro, E. O. Oyebamiji and T. O. Ogungbamigbe, Effects of varying doses of tramadol on gastric pH, Anesth. Essays Res., 2013, 7(1), 25–28, DOI:10.4103/0259-1162.113982.
- H. M. Krabseth, M. Strømmen, O. Spigset and A. Helland, Effect of Sleeve Gastrectomy on Buprenorphine Pharmacokinetics: A Planned Case Observation, Clin. Ther., 2020, 42(11), 2232–2237, DOI:10.1016/j.clinthera.2020.08.016.
- H. Abdolmohammad-Zadeh, A. Zamani and Z. Shamsi, Preconcentration of morphine and codeine using a magnetite/reduced graphene oxide/silver nano-composite and their determination by high-performance liquid chromatography, J. Chromatogr., A, 2019, 1590, 2–9, DOI:10.1016/j.chroma.2018.12.064.
- T. Alizadeh, F. Atashi, M. Akhoundian and M. R. Ganjali, Highly selective extraction and voltammetric determination of the opioid drug buprenorphine via a carbon paste electrode impregnated with nano-sized molecularly imprinted polymer, Mikrochim. Acta, 2019, 186(9), 654, DOI:10.1007/s00604-019-3736-7.
- F. Ganjavi, M. Ansari, M. Kazemipour and L. Zeidabadinejad, Computational design, synthesis and utilization of a magnetic molecularly imprinted polymer on graphene oxide nanosheets for highly selective extraction and determination of buprenorphine in biological fluids and tablets, Anal. Methods, 2018, 10(43), 5214–5226, 10.1039/C8AY01757C.
- F. Kharazmi, F. S. Hosseini and H. Ebrahimzadeh, Synthesis, characterization of MOF NiCoZn-LDH@GO on carbon cloth as sensitive and novel nanocomposite applied to electrospun nanofibers network as thin-film microextraction sorbent for detection trace amount of opioid and analgesic drugs from biological fluids, Talanta, 2024, 267, 125241, DOI:10.1016/j.talanta.2023.125241.
- L. Liu, F. Grillo and F. Canfarotta, et al., Carboxyl-fentanyl detection using optical fibre grating-based sensors functionalised with molecularly imprinted nanoparticles, Biosens. Bioelectron., 2021, 177, 113002, DOI:10.1016/j.bios.2021.113002.
- M. Khanlari, B. Daraei, L. Torkian, M. Shekarchi and M. R. Manafi, Application of the oxycodone templated molecular imprinted polymer in adsorption of the drug from human blood plasma as the real biological environment; a joint experimental and density functional theory study, Front. Chem., 2022, 10, 1045552, DOI:10.3389/fchem.2022.1045552.
- S. S. Alterary, Construction of novel potentiometric sensors modified with biogenically synthesized metal oxide nanoparticles for sensitive detection of the opioid agonist-antagonist nalbuphine hydrochloride in its injection, Heliyon, 2023, 9(10), e20510, DOI:10.1016/j.heliyon.2023.e20510.
- S. R. Ahmed, R. Chand, S. Kumar, N. Mittal, S. Srinivasan and A. R. Rajabzadeh, Recent biosensing advances in the rapid detection of illicit drugs, TrAC, Trends Anal. Chem., 2020, 131, 116006, DOI:10.1016/j.trac.2020.116006.
- H. Bagheri, A. Shirzadmehr, M. Rezaei and H. Khoshsafar, Determination of tramadol in pharmaceutical products and biological samples using a new nanocomposite carbon paste sensor based on decorated nanographene/tramadol-imprinted polymer nanoparticles/ionic liquid, Ionics, 2018, 24(3), 833–843, DOI:10.1007/s11581-017-2252-1.
- F. Grillo, Development of a Novel Assay for Drugs of Abuse Based on Molecularly Imprinted Polymers as Synthetic Antibodies, MPhil thesis, University of Leicester, 2018, accessed June 25, 2024, https://figshare.le.ac.uk/articles/thesis/Development_of_a_novel_assay_for_drugs_of_abuse_based_on_Molecularly_Imprinted_Polymers_as_synthetic_antibodies/10196048/1 Search PubMed.
- W. Chen, L. Yang and C. Yan, et al., Surface-Confined Building of Au@Pt-Centered and Multi-G-Quadruplex/Hemin Wire-Surrounded Electroactive Super-nanostructures for Ultrasensitive Monitoring of Morphine, ACS Sens., 2020, 5(8), 2644–2651, DOI:10.1021/acssensors.0c01230.
- A. Soni, A. Paprikar and S. Lin, Effect of alkalizing agent on abuse deterrent potential of multiple-unit ingestion of bilayer abuse-deterrent extended-release tablets using propranolol as model drug for opioids overdose crisis, Int. J. Pharm., 2021, 600, 120480, DOI:10.1016/j.ijpharm.2021.120480.
- M. Murshed, M. Salim and B. J. Boyd, Existing and emerging mitigation strategies for the prevention of accidental overdose from oral pharmaceutical products, Eur. J. Pharm. Biopharm., 2022, 180, 201–211, DOI:10.1016/j.ejpb.2022.10.002.
- F. Molavi, M. Barzegar-Jalali and H. Hamishehkar, Polyester based polymeric nano and microparticles for pharmaceutical purposes: A review on formulation approaches, J. Controlled Release, 2020, 320, 265–282, DOI:10.1016/j.jconrel.2020.01.028.
- AVINZA-morphine sulfate capsule, extended release Pfizer Laboratories Div Pfizer Inc., accessed June 25, 2024, https://labeling.pfizer.com/showlabeling.aspx?id=876.
- D. J. Cada, T. Levien and D. E. Baker, Morphine Sulfate Extended-Release Liposome Injection, Hosp. Pharm., 2004, 39(9), 871–879, DOI:10.1177/001857870403900910.
- Y. He, L. Qin, Y. Huang and C. Ma, Advances of Nano-Structured Extended-Release Local Anesthetics, Nanoscale Res. Lett., 2020, 15(1), 13, DOI:10.1186/s11671-019-3241-2.
- L. L. Mazaleuskaya, V. R. Muzykantov and G. A. FitzGerald, Nanotherapeutic-directed approaches to analgesia, Trends Pharmacol. Sci., 2021, 42(7), 527–550, DOI:10.1016/j.tips.2021.03.007.
- NCT01539642, A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of the Sufentanil NanoTab PCA System/15 mcg (Zalviso™) for the Treatment of Post-Operative Pain in Patients After Open Abdominal Surgery, Published online May 31, 2018, https://clinicaltrials.gov/show/NCT01539642. DOI:10.1002/central/CN-01535763.
- Zalviso|European Medicines Agency, Accessed June 25, 2024, https://www.ema.europa.eu/en/medicines/human/EPAR/zalviso.
- A. M. Young, J. R. Havens and C. G. Leukefeld, Route of administration for illicit prescription opioids: a comparison of rural and urban drug users, Harm, Reduct. J., 2010, 7, 24, DOI:10.1186/1477-7517-7-24.
- D. A. Subramanian, R. Langer and G. Traverso, Mucus interaction to improve gastrointestinal retention and pharmacokinetics of orally administered nano-drug delivery systems, J. Nanobiotechnol., 2022, 20(1), 362, DOI:10.1186/s12951-022-01539-x.
- J. P. M. Almeida, A. L. Chen, A. Foster and R. Drezek, In vivo biodistribution of nanoparticles, Nanomedicine, 2011, 6(5), 815–835, DOI:10.2217/nnm.11.79.
- E. Blanco, H. Shen and M. Ferrari, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat. Bitechnol., 2015, 33(9), 941–951, DOI:10.1038/nbt.3330.
- T. Miyazawa, M. Itaya, G. C. Burdeos, K. Nakagawa and T. Miyazawa, A Critical Review of the Use of Surfactant-Coated Nanoparticles in, Nanomedicine and Food Nanotechnology, Int. J. Nanomedicine, 2021, 16, 3937–3999, DOI:10.2147/IJN.S298606.
- S. Su and P. M. Kang, Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems, Pharmaceutics, 2020, 12(9), 837, DOI:10.3390/pharmaceutics12090837.
- N. Mamidi, R. M. Velasco Delgadillo, E. V. Barrera, S. Ramakrishna and N. Annabi, Carbonaceous nanomaterials incorporated biomaterials: The present and future of the flourishing field, Composites, Part B, 2022, 243, 110150, DOI:10.1016/j.compositesb.2022.110150.
- O. Almeanazel, F. Alanazi and I. Alsarra, et al., Nanotechnology as a tool to overcome the bariatric surgery malabsorption, Saudi Pharm. J., 2020, 28(5), 565–573, DOI:10.1016/j.jsps.2020.03.008.
- M. Zhu, A. K. Whittaker and X. Jiang, et al., Use of Microfluidics to Fabricate Bioerodable Lipid Hybrid Nanoparticles Containing Hydromorphone or Ketamine for the Relief of Intractable Pain, Pharm. Res., 2020, 37(10), 211, DOI:10.1007/s11095-020-02939-0.
- P. Iranmanash, K. Barlow and M. Anvari, The effect of bariatric surgery on opioid consumption in patients with obesity: a registry-based cohort study, Surg. Obes. Relat. Dis., 2023, 19(9), 952–961, DOI:10.1016/j.soard.2023.03.004.
- M. Barati, S. Mohammadi Samani, L. Pourtalebi Jahromi, H. Ashrafi and A. Azadi, Controlled-release in-situ gel forming formulation of tramadol containing chitosan-based pro-nanogels, Int. J. Biol. Macromol., 2018, 118, 1449–1454, DOI:10.1016/j.ijbiomac.2018.06.152.
- M. Mabrouk, H. H. Beherei, S. ElShebiney and M. Tanaka, Newly developed controlled release subcutaneous formulation for tramadol hydrochloride, Saudi Pharm. J., 2018, 26(4), 585–592, DOI:10.1016/j.jsps.2018.01.014.
- E. Touitou, S. Duchi and H. Natsheh, A new nanovesicular system for nasal drug administration, Int. J. Pharm., 2020, 580, 119243, DOI:10.1016/j.ijpharm.2020.119243.
- F. Y. Han, Y. Liu and V. Kumar, et al., Sustained-release ketamine-loaded nanoparticles fabricated by sequential nanoprecipitation, Int. J. Pharm., 2020, 581, 119291, DOI:10.1016/j.ijpharm.2020.119291.
- J. Yue, L. He, Y. Tang, L. Yang, B. Wu and J. Ni, Facile design and development of photoluminescent graphene quantum dots grafted dextran/glycol-polymeric hydrogel for thermoresponsive triggered delivery of buprenorphine on pain management in tissue implantation, J. Photochem. Photobiol., B, 2019, 197, 111530, DOI:10.1016/j.jphotobiol.2019.111530.
- C. R. Okada, T. K. Henthorn and J. Zuk, et al., Population Pharmacokinetics of Single Bolus Dose Fentanyl in Obese Children, Anesth. Analg., 2024, 138(1), 99, DOI:10.1213/ANE.0000000000006554.
- J. Feng, S. Lepetre-Mouelhi and A. Gautier, et al., A new painkiller nanomedicine to bypass the blood-brain barrier and the use of morphine, Sci. Adv., 2019, 5(2), eaau5148, DOI:10.1126/sciadv.aau5148.
- C. Contet, B. L. Kieffer and K. Befort, Mu opioid receptor: a gateway to drug addiction, Curr. Opin. Neurobiol., 2004, 14(3), 370–378, DOI:10.1016/j.conb.2004.05.005.
- C. Filisetti, M. D. Gregori, M. Allegri, D. Bugada, L. Cobianchi and G. Riccipetitoni, Pain Management in Pediatric Surgery: New Horizons, J. Pain Relief, 2016, 5(2), 240, DOI:10.4172/2167-0846.1000240.
- B. Cohen, M. A. Tanios, O. Koyuncu, H. O. Yilmaz, S. Raza, J. Mukhdomi, A. S. Artis, J. Seif, S. Chhabada and A. Turan, Association between higher BMI and postoperative pain and opioid consumption in pediatric inpatients – A retrospective cohort study, J. Clin. Anesth., 2020, 62, 109729, DOI:10.1016/j.jclinane.2020.109729.
- S. Mastrangelo, M. A. Capozza and S. Triarico, et al., Opioid transdermal delivery system: a useful method for pain management in children, Ann. Transl. Med., 2021, 9(2), 185–185, DOI:10.21037/atm-20-2619.
- M. A. Rubio-Herrera, S. Mera-Carreiro, A. Sánchez-Pernaute and A. M. Ramos-Levi, Impact of Treatment with GLP1 Receptor Agonists, Liraglutide 3.0 mg and Semaglutide 1.0 mg, While on a Waiting List for Bariatric Surgery, Biomedicines, 2023, 11(10), 2785, DOI:10.3390/biomedicines11102785.
- N. Klair, U. Patel, A. Saxena, D. Patel, I. E. Ayesha, N. R. Monson and S. Ramphall, What Is Best for Weight Loss? A Comparative Review of the Safety and Efficacy of Bariatric Surgery Versus Glucagon-Like Peptide-1 Analogue, Cureus, 2023, 15(9), e46197, DOI:10.7759/cureus.46197.
- Z. Xie, S. Yang, W. Deng, J. Li and J. Chen, Efficacy and Safety of Liraglutide and Semaglutide on Weight Loss in People with Obesity or Overweight: A Systematic Review, Clin. Epidemiol., 2022, 14, 1463–1476, DOI:10.2147/CLEP.S391819.
- M. Sodhi, R. Rezaeianzadeh, A. Kezouh and M. Etminan, Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss, J. Am. Med. Assoc., 2023, 330(18), 1795–1797, DOI:10.1001/jama.2023.19574.
| This journal is © The Royal Society of Chemistry 2024 |