Open Access Article
Open Access ArticleA model binary system for the evaluation of novel ion pair formulations of diclofenac
Mignon
Cristofoli
 *a,
Jonathan
Hadgraft
b,
Majella E.
Lane
b and
Bruno C.
Sil
a
*a,
Jonathan
Hadgraft
b,
Majella E.
Lane
b and
Bruno C.
Sil
a
aSchool of Human Sciences, London Metropolitan University, 166-220 Holloway Road, London N7 8DB, UK. E-mail: mic0501@my.londonmet.ac.uk
bSchool of Pharmacy, University College London, 29-39 Brunswick Square, London WC1N 1AX, UK
First published on 9th April 2024
Abstract
Diclofenac (DF) is well established as a topical treatment option for conditions such as osteoarthritis. In investigating novel DF ion pairs for topical delivery, studies to determine the impact of various amino acids on the distribution of DF between octanol and aqueous environments were conducted. These studies identified the amino acid L-histidine hydrochloride monohydrate (LHSS) as an ion pair candidate for diclofenac sodium (DNa). Preliminary porcine skin permeation studies indicated that the addition of LHSS to DNa solutions increased the amount of DF that permeated through porcine skin. With increasing amounts of LHSS added, greater amounts of DF precipitated out of solution. In the present work, the solubility of DNa in various solvents was assessed, with the intention of identifying solvents in which DNa was most soluble. Binary systems comprising water and selected solvents were tested for both miscibility and the solubility of DNa and LHSS. The model system selected to evaluate novel ion pair formulations using porcine skin in vitro permeation studies under finite dose (10 μL) conditions comprised Transcutol® (TC) and water. The tested formulations contained DNa at concentrations of 5, 7.5 and 10 mg mL−1. Higher LHSS concentrations were possible when the DNa concentrations were lower, and ranged from 10–25 mg mL−1. However, increasing the DNa concentration to 10 mg mL−1, without adding LHSS, resulted in a significant reduction in the amount of DF that partitioned and permeated, relative to formulations that contained either 5 mg mL−1 DNa in combination with LHSS (at 12.5 or 25 mg mL−1), or 7.5 mg mL−1 DNa together with 12.5 mg mL−1 LHSS. The current work confirms previous investigations, suggesting that the addition of LHSS to DNa in a formulation may increase the partition and permeation of DF.
Introduction
Osteoarthritis (OA) is a painful and degenerative condition of the joints, affecting the hips, knees and hands. According to the Global Burden of Disease study, the condition affects approximately 7% of the world's population, amounting to more than 500 million people.1 Direct costs associated with OA are estimated at 1–2% of the Gross National Product of countries with established market economies, including the UK, the USA, Canada and Australia.2 Indirect costs such as the loss of productivity and early retirement, serve to exacerbate the already substantial economic implications.Various organisations worldwide have published guidelines relating to the treatment of OA.3,4 In the UK, topical non-steroidal anti-inflammatory drugs (NSAIDs) are considered first-line pharmacological treatment options for OA, due to the adverse drug reactions associated with other options such as opioids and oral NSAIDs.5 The European Society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases have strongly recommended the use of topical NSAIDs, particularly where so-called symptomatic slow-acting drugs such as chondroitin sulfate and prescription crystalline glucosamine sulfate, in conjunction with paracetamol, have not relieved the symptoms of OA.6 In the US, the use of topical NSAIDs for the treatment of OA has been endorsed by the American College of Rheumatology in conjunction with the Arthritis Foundation7 as well as the American Academy of Orthopaedic Surgeons.8 The global organisation, Osteoarthritis Research Society International, have also strongly recommended the use of topical NSAIDs as a treatment option for OA of the knee.9 As the most prescribed NSAID worldwide,10 it is unsurprising therefore that topical formulations using diclofenac (DF) are widely recognised as effective treatment options for OA.11 Unfortunately, due to the efficacy of the barrier properties of the stratum corneum, only a small percentage of topically applied pharmaceutical salt preparations partition into the skin. Consequently, much of the applied pharmaceutical product never reaches its target site. Rational formulation design of topical DF products offers the potential for both economic savings as well as an opportunity to demonstrate commitment to reducing the environmental consequences of conscious formulation choices. This is consistent with the policies of many large pharmaceutical companies (such as Astra Zeneca,12 Novartis13 and Roche14,15) who are committed to reducing, where possible, the presence of pharmaceuticals in the environment.
Strategies to overcome the skin barrier are frequently categorised into two groups. The first comprises active or physical methods16,17 such as ionotophoresis,18–20 sonophoresis,21 microneedles,22–25 magnetophoresis26 and electroporation.27 The second consists of passive techniques that focus specifically on the formulation. Examples include increasing the thermodynamic activity of the active pharmaceutical ingredient,28–31 the inclusion of various excipients as skin penetration enhancers28,32–35 and the use of ion pairs to address ionised drug molecules.36
Previously the amino acid L-histidine hydrochloride monohydrate (LHSS) was identified as an ion pair candidate for diclofenac sodium (DNa).37 This determination resulted from studies performed to investigate the impact of LHSS on the distribution of DF between octanol and aqueous environments. Experiments comprised DNa and LHSS in various ratios, namely 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5; 1
0.5; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The results suggested that increasing the quantity of LHSS relative to DNa, would result in an increase in the amount of DF that partitioned into an organic medium. Preliminary porcine skin permeation studies confirmed that the addition of LHSS to DNa aqueous solutions also increased the amount of DF that permeated through porcine skin. The formulations used comprised DF at 100 μg mL−1 and 350 μg mL−1. LHSS was either not included, for the purposes of a control (1
50. The results suggested that increasing the quantity of LHSS relative to DNa, would result in an increase in the amount of DF that partitioned into an organic medium. Preliminary porcine skin permeation studies confirmed that the addition of LHSS to DNa aqueous solutions also increased the amount of DF that permeated through porcine skin. The formulations used comprised DF at 100 μg mL−1 and 350 μg mL−1. LHSS was either not included, for the purposes of a control (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) or added at 1
0) or added at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 molar ratios. The more LHSS that was added, however, the more DF precipitated out of solution. This was particularly evident at the higher concentration of DF.37 As LHSS is only soluble in water and DNa has very low solubility in water, a binary solvent system was developed. The aims of the present study, therefore, were to build upon the previous investigations37 with two main objectives: (i) to address the issue of the solubility of both DNa and LHSS and (ii) to develop a model binary system to evaluate novel DNa
50 molar ratios. The more LHSS that was added, however, the more DF precipitated out of solution. This was particularly evident at the higher concentration of DF.37 As LHSS is only soluble in water and DNa has very low solubility in water, a binary solvent system was developed. The aims of the present study, therefore, were to build upon the previous investigations37 with two main objectives: (i) to address the issue of the solubility of both DNa and LHSS and (ii) to develop a model binary system to evaluate novel DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS ion pair formulations, using porcine skin in vitro permeation studies (IVPT) under finite dose (10 μL) conditions.
LHSS ion pair formulations, using porcine skin in vitro permeation studies (IVPT) under finite dose (10 μL) conditions.
Materials and methods
Materials
DNa 98% and the amino acid salt, LHSS, were supplied by VWR (Leicestershire, UK). High vacuum grease was obtained from Dow Corning (Seneffe, Belgium). Oxoid™ phosphate buffered saline (PBS) tablets were purchased from Thermo Fisher Scientific (Lancashire, UK). Filter paper, 150 mm diameter, as well as HPLC grade acetonitrile (ACN) and trifluoroacetic acid (TFA), were purchased from Fisher Scientific (Lancashire, UK). Propylene glycol was supplied by Merck Life Sciences (Poole, UK). Hexylene glycol, butylene glycol and dipropylene glycol were supplied by VWR (Leicestershire, UK). Isopropyl alcohol was purchased from Honeywell (Berkshire, UK). Dimethyl isosorbide, isopropyl myristate and mineral oil were obtained from Thermo Fisher Scientific (Lancashire, UK). Diethylene glycol monoethyl ether (Transcutol®), propylene glycol monocaprylate type II (Capryol 90®), propylene glycol monolaurate type 1 (Lauroglycol 90®) and medium chain triglycerides (Labrafac Lipophile W1349®) were kind donations from Gattefosse (St Priest, France).HPLC analysis
The detection and quantification of DF was performed using the method previously reported. This method was validated in accordance with ICH (2005) guidelines (International Conference on Harmonisation Expert Working Group, 2005) for linearity, accuracy, precision, robustness, limit of detection (LOD) and limit of quantification (LOQ).37 The mobile phase was made up of acetonitrile (ACN)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1% trifluoroacetic acid in water (70
0.1% trifluoroacetic acid in water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Calibration curves for the detection of diclofenac were prepared using DNa. They ranged from 0.05 to 100 μg mL−1. The LOD was 0.03 μg mL−1 and LOQ was 0.10 μg mL−1.37
30). Calibration curves for the detection of diclofenac were prepared using DNa. They ranged from 0.05 to 100 μg mL−1. The LOD was 0.03 μg mL−1 and LOQ was 0.10 μg mL−1.37
Solubility studies, solubility parameters (SP) of solvents, miscibility studies and stability studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, at a temperature of 32 ± 1 °C. The pipette tips and centrifuge tubes used to perform these tasks were maintained at 32 ± 1 °C in an oven for at least 30 min prior to use. Samples were diluted where required and analysed by HPLC.
000 rpm, at a temperature of 32 ± 1 °C. The pipette tips and centrifuge tubes used to perform these tasks were maintained at 32 ± 1 °C in an oven for at least 30 min prior to use. Samples were diluted where required and analysed by HPLC.
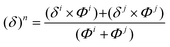 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 30
80, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, 40
70, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 50
60, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70
40, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 80
30, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 90
20 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v). The non-aqueous solvent options were identified through the single solvent solubility studies mentioned above. As shown in Fig. 1, DNa was determined to be most soluble in Transcutol® (TC), dipropylene glycol (DiPG) and propylene glycol (PG), which were selected for this study. Methylene blue was added to all samples to confirm miscibility. These studies were carried out using DNa at fixed concentrations of 1.00%, 0.75% and 0.50% (w/v). Stock solutions containing 50 mg mL−1 and 25 mg mL−1 of LHSS in water were prepared. In contrast to the fixed concentrations of DNa, the concentration of LHSS in the samples increased or decreased relative to the volume of LHSS stock solution contained in the sample. Samples were sealed with Parafilm® and shaken for 24 h using an orbital shaker (VWR, Leicestershire, UK) set to 32 °C and 800 rpm. The samples were then left at room temperature and evaluated at 24 h and 72 h.
10 (v/v). The non-aqueous solvent options were identified through the single solvent solubility studies mentioned above. As shown in Fig. 1, DNa was determined to be most soluble in Transcutol® (TC), dipropylene glycol (DiPG) and propylene glycol (PG), which were selected for this study. Methylene blue was added to all samples to confirm miscibility. These studies were carried out using DNa at fixed concentrations of 1.00%, 0.75% and 0.50% (w/v). Stock solutions containing 50 mg mL−1 and 25 mg mL−1 of LHSS in water were prepared. In contrast to the fixed concentrations of DNa, the concentration of LHSS in the samples increased or decreased relative to the volume of LHSS stock solution contained in the sample. Samples were sealed with Parafilm® and shaken for 24 h using an orbital shaker (VWR, Leicestershire, UK) set to 32 °C and 800 rpm. The samples were then left at room temperature and evaluated at 24 h and 72 h.
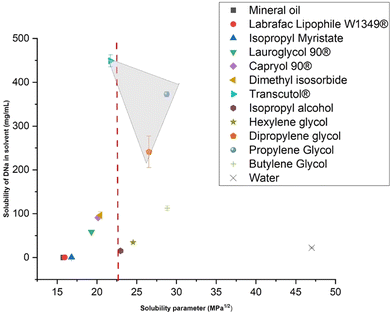 | ||
| Fig. 1 The results of the saturated solubility of DNa in individual solvents are plotted against their SPs (n ≥ 3; mean ± SD). The dashed red line represents the SP of DNa determined by Barra et al.44 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v/v), was used due the increased solubility of DNa.41
15 v/v), was used due the increased solubility of DNa.41
Results and discussion
Solubility studies, SP of solvents, drug-loaded miscibility studies and stability studies
As a result of the single solvent solubility studies, which indicated that DNa exhibited the highest solubility in TC, PG and DiPG, these solvents were chosen for the subsequent phase of formulation development. They are shown alongside water and DNa in Table 1 together with their CAS numbers, chemical structures, molecular weights, dielectric constant values at 25 °C and SPs. The solvents, selected primarily to maximise the solubility of DNa, are reported to function as permeation enhancers,33 and are also commonly used as excipients in topically applied pharmaceutical formulations. One such example is the inclusion of PG in the commercial formulation, Voltaren® 1% gel (GSK Consumer Health, New Jersey, USA). As such, they appear in the FDA Inactive Ingredients Database. Currently the maximum daily exposure (MDE) for TC (CAS 111-90-0) in topical applied gels is 1500 mg and the maximum potency per unit dose (MPPUD) for transdermal systems is 430 mg. PG (CAS 57-55-6) has a MDE for topically applied creams of 6113 mg and a MPPUD of 65% (w/w) for topical ointments. DiPG (CAS 25265-71-8) has a 296 mg MDE for extended-release films for transdermal use, while general transdermal systems are limited to 6 mg. No MPPUD is currently listed for DiPG contained in transdermal systems.
| Compound name | CAS | Chemical structure | Molar mass (g mol−1) | Dielectric constant of solvent (ε) at 25 °C | Solubility parameter (MPa1/2) of solvents |
|---|---|---|---|---|---|
| DNa | 15307-79-6 |
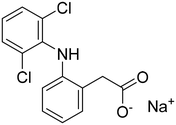
|
318.13 | n/a | n/a |
| DiPG | 25265-71-8 |
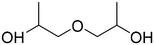
|
134.17 | 19.80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 53 |
26.54 |
| PG | 57-55-6 |
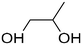
|
76.09 | 28.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51–30.20 51–30.20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
28.78 |
| TC | 111-90-0 |
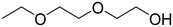
|
134.18 | 14.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
21.72 |
| Water | 7732-18-5 |
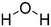
|
18.02 | 78.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 54 |
47.00 |
While these solvents were chosen specifically due to their efficacy as solubilisers of DNa, the work by Minghetti et al. revealed the need for caution when focusing primarily on solubility. It was ascertained that DNa was far more soluble in PG (567 ± 31 μg mL−1) and TC (660 ± 70 μg mL−1) than oleic acid (25 ± 10 μg mL−1) or water (37 ± 10 μg mL−1). Despite the application of saturated solutions, the flux from water (2.29 ± 0.37 μg cm−2 h−1) and oleic acid (1.84 ± 0.18 μg cm−2 h−1) was greater than the flux from PG (1.21 ± 0.06 μg cm−2 h−1) and TC (0.06 ± 0.01 μg cm−2 h−1).46 The study demonstrated that the assumption of equivalent thermodynamic activity for saturated solutions is negated when the activity coefficients of the solute in the solvents vary.46 This was addressed by Higuchi, who explained that a high affinity between solute and vehicle translates into low activity coefficients. This in turn results in reduced rates of partition of the solute from the vehicle into the membrane.47 Minghetti described this affinity as a very small difference between the SP of the active pharmaceutical ingredient (API) and the solvents, PG and TC, which reduced the ability of the API to partition into the membrane.46 This study indicated that a similarity in SPs could cause a reduction in the activity coefficient and therefore the thermodynamic activity of the active in the formulation. While this would suggest potential challenges for single solvent systems, or combinations of the solvents selected for maximum DNa solubility, the inclusion of water should mitigate any such concerns. The SP of water (47.00 MPa1/2) is distinct from that of PG (28.78 Mpa1/2), DiPG (26.54 Mpa1/2) and TC (21.72 Mpa1/2), and therefore should result in a higher activity coefficient, thermodynamic activity and ability to partition into the membrane. The dielectric constants of solvents and solvent systems should also be considered due to their bearing on the stability of ion pairs. In general at lower dielectric constants, the association between ion pairs increases, while the converse is true for higher dielectric constants.48,49 The three solvents TC, PG and DiPG have dielectric constants of 14.1,50 28.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51–30.2
51–30.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 and 19.8
52 and 19.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 respectively, at 25 °C. These values are lower than that of water which exhibits a dielectric constant of 78.3 at the same temperature.54 Thus, the addition of any of the selected solvents would result in a reduction in the dielectric constant and polarity of water alone. As the organic component of the formulation increases, the electrostatic attraction generated by the solvent system diminishes in relation to the ions. This reduction leads to decreased interference in the electrostatic attraction between the ion pairs.55
53 respectively, at 25 °C. These values are lower than that of water which exhibits a dielectric constant of 78.3 at the same temperature.54 Thus, the addition of any of the selected solvents would result in a reduction in the dielectric constant and polarity of water alone. As the organic component of the formulation increases, the electrostatic attraction generated by the solvent system diminishes in relation to the ions. This reduction leads to decreased interference in the electrostatic attraction between the ion pairs.55
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 20
90, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 30
80, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, 40
70, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 50
60, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70
40, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 80
30, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 90
20 and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v). DNa was included in fixed concentrations of 1.00%, 0.75% and 0.50% (w/v). The concentration of LHSS varied according to the volume of 50 mg mL−1 or 25 mg mL−1 LHSS solution added to the sample. Methylene blue was used to confirm miscibility. Table 2 indicates all miscible solvent combinations, at specific concentrations of DNa and LHSS that showed no apparent precipitation.
10 (v/v). DNa was included in fixed concentrations of 1.00%, 0.75% and 0.50% (w/v). The concentration of LHSS varied according to the volume of 50 mg mL−1 or 25 mg mL−1 LHSS solution added to the sample. Methylene blue was used to confirm miscibility. Table 2 indicates all miscible solvent combinations, at specific concentrations of DNa and LHSS that showed no apparent precipitation.
| % DNa | Solvent% (v/v) | Mols LHSS/mol DNa | %DNa | Solvent% (v/v) | Mols LHSS/mol DNa | %DNa | Solvent% (v/v) | Mols LHSS/mol DNa | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TC | Water | DiPG | Water | PG | Water | ||||||
| LHSS solution 50 mg mL −1 | |||||||||||
| 0.75 | 50 | 50 | 4.71 | 1.00 | 50 | 50 | 3.53 | 0.50 | 60 | 40 | 5.65 |
| 0.50 | 50 | 50 | 7.06 | 0.75 | 50 | 50 | 4.71 | 0.50 | 80 | 20 | 2.83 |
| 0.50 | 40 | 60 | 8.48 | 0.50 | 50 | 50 | 7.06 | 0.50 | 90 | 10 | 1.41 |
| 0.50 | 40 | 60 | 8.48 | ||||||||
| LHSS solution 25 mg mL −1 | |||||||||||
| 1.00 | 50 | 50 | 1.77 | 1.00 | 60 | 40 | 1.41 | 1.00 | 70 | 30 | 1.06 |
| 1.00 | 60 | 40 | 1.41 | 1.00 | 70 | 30 | 1.06 | 1.00 | 80 | 20 | 0.71 |
| 1.00 | 70 | 30 | 1.06 | 1.00 | 80 | 20 | 0.71 | 1.00 | 90 | 10 | 0.35 |
| 0.75 | 50 | 50 | 2.35 | 1.00 | 90 | 10 | 0.35 | 0.50 | 60 | 40 | 2.83 |
| 0.75 | 60 | 40 | 1.88 | 0.75 | 70 | 30 | 1.41 | 0.50 | 70 | 30 | 2.12 |
| 0.75 | 70 | 30 | 1.41 | 0.75 | 80 | 20 | 0.94 | 0.50 | 80 | 20 | 1.41 |
| 0.50 | 40 | 60 | 4.24 | 0.75 | 90 | 10 | 0.47 | 0.50 | 90 | 10 | 0.71 |
| 0.50 | 50 | 50 | 3.53 | 0.50 | 50 | 50 | 3.53 | ||||
| 0.50 | 60 | 40 | 2.83 | 0.50 | 60 | 40 | 2.83 | ||||
| 0.50 | 70 | 30 | 2.12 | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water was selected as a model binary system as it facilitated comparisons where concentrations of DF, as well as solvent ratios, remained constant while the concentration of the counter ion was varied. This system also contained stable combinations of increased concentrations of DF at the same and different solvent ratios, as shown in Table 2. DiPG
water was selected as a model binary system as it facilitated comparisons where concentrations of DF, as well as solvent ratios, remained constant while the concentration of the counter ion was varied. This system also contained stable combinations of increased concentrations of DF at the same and different solvent ratios, as shown in Table 2. DiPG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water systems were not selected, as they did not comprise a sufficient number of stable formulations appropriate for comparative purposes. This was particularly relevant in relation to formulations containing an aqueous content of 50% (v/v), where the concentration of LHSS would be maximised. PG
water systems were not selected, as they did not comprise a sufficient number of stable formulations appropriate for comparative purposes. This was particularly relevant in relation to formulations containing an aqueous content of 50% (v/v), where the concentration of LHSS would be maximised. PG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water systems were not considered due to their consistently low aqueous content, limiting the quantity of LHSS. Formulations shown in bold were selected for IVPT as they appeared to be stable after 72 h and were therefore suitable for comparative purposes.
water systems were not considered due to their consistently low aqueous content, limiting the quantity of LHSS. Formulations shown in bold were selected for IVPT as they appeared to be stable after 72 h and were therefore suitable for comparative purposes.
Results of finite dose (10 μL) binary IVPT and mass balance studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 50 v/v), containing 5 mg mL−1 DNa and 25 mg mL−1 LHSS (5DL25), 12.5 mg mL−1 LHSS (5DL12.5) or 0 mg mL−1 LHSS (5DL0).
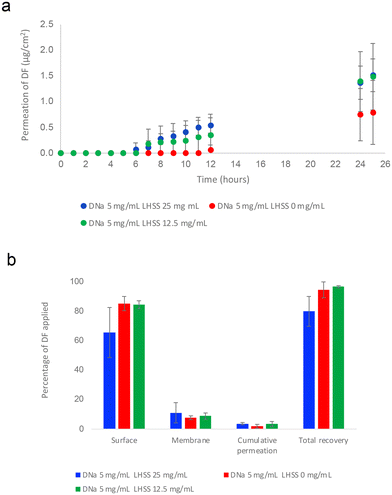
The data observed in Table 3 and Fig. 2(a) suggests that the addition of LHSS enhanced the permeation of DF across porcine skin at 25 h, relative to the control formulation containing no LHSS. The variations, however, were not statistically significant (p > 0.05). Cumulative permeation of DF at 25 h ranged from 0.79 ± 0.62 μg cm−2, (5DL0), to comparable amounts of 1.48 ± 0.65 μg cm−2 and 1.52 ± 0.32 μg cm−2 for the 5DL12.5 and 5DL25 formulations, respectively (p > 0.05). The percentage value of DF that permeated followed the same order, amounting to between 1.76 ± 1.37% for the control sample increasing to 3.47 ± 1.56% (5DL12.5) and 3.49 ± 0.73% (5DL25) (p > 0.05). All formulations resulted in comparable percentages of DF being extracted from the membrane, varying from 7.60 ± 1.19%–11.00 ± 7.21% (p > 0.05). To obtain a clearer picture of the total drug compound partitioning and permeating, the values for membrane retention and permeation were combined. Again, the results for all formulations were comparable, with the total percentages of DF amounting to 9.36 ± 2.49% (5DL0), 12.26 ± 3.06% (5DL12.5) and 14.49 ± 7.76% (5DL25, p > 0). Despite these amounts representing increases of approximately 55% (5DL25) and 30% (5DL12.5) relative to the control, they were not statistically significant (p > 0.05).
50 v/v), containing 5 mg mL−1 DNa and 25 mg mL−1 LHSS (5DL25), 12.5 mg mL−1 LHSS (5DL12.5) or 0 mg mL−1 LHSS (5DL0).
The data observed in Table 3 and Fig. 2(a) suggests that the addition of LHSS enhanced the permeation of DF across porcine skin at 25 h, relative to the control formulation containing no LHSS. The variations, however, were not statistically significant (p > 0.05). Cumulative permeation of DF at 25 h ranged from 0.79 ± 0.62 μg cm−2, (5DL0), to comparable amounts of 1.48 ± 0.65 μg cm−2 and 1.52 ± 0.32 μg cm−2 for the 5DL12.5 and 5DL25 formulations, respectively (p > 0.05). The percentage value of DF that permeated followed the same order, amounting to between 1.76 ± 1.37% for the control sample increasing to 3.47 ± 1.56% (5DL12.5) and 3.49 ± 0.73% (5DL25) (p > 0.05). All formulations resulted in comparable percentages of DF being extracted from the membrane, varying from 7.60 ± 1.19%–11.00 ± 7.21% (p > 0.05). To obtain a clearer picture of the total drug compound partitioning and permeating, the values for membrane retention and permeation were combined. Again, the results for all formulations were comparable, with the total percentages of DF amounting to 9.36 ± 2.49% (5DL0), 12.26 ± 3.06% (5DL12.5) and 14.49 ± 7.76% (5DL25, p > 0). Despite these amounts representing increases of approximately 55% (5DL25) and 30% (5DL12.5) relative to the control, they were not statistically significant (p > 0.05).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), containing 5 mg mL−1 DNa and 0, 12.5 or 25 mg mL−1 LHSS. The table shows (i) cumulative permeation of DF (μg cm−2) at 25 h as well as the percentages of DF applied that (ii) permeated, (iii) remained on the skin surface, (iv) remained in the membrane, (v) permeated plus remained in the membrane and (vi) were recovered. In addition, the table contains a reference to the molar ratio of LHSS relative DNa, that was applied (4 ≤ n ≤ 5; mean ± SD)
50 v/v), containing 5 mg mL−1 DNa and 0, 12.5 or 25 mg mL−1 LHSS. The table shows (i) cumulative permeation of DF (μg cm−2) at 25 h as well as the percentages of DF applied that (ii) permeated, (iii) remained on the skin surface, (iv) remained in the membrane, (v) permeated plus remained in the membrane and (vi) were recovered. In addition, the table contains a reference to the molar ratio of LHSS relative DNa, that was applied (4 ≤ n ≤ 5; mean ± SD)
| Amount DF partitioned and permeated | DNa 5 mg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS 25 mg mL−1 (5DL25) LHSS 25 mg mL−1 (5DL25) |
DNa 5 mg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS 12.5 mg mL−1 (5DL12.5) LHSS 12.5 mg mL−1 (5DL12.5) |
DNa 5 mg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS 0 mg mL−1 (5DL0) LHSS 0 mg mL−1 (5DL0) |
|---|---|---|---|
| Cumulative permeation μg cm−2 at 25 h | 1.52 ± 0.32 | 1.48 ± 0.65 | 0.79 ± 0.62 |
| Permeated 25 h % | 3.49 ± 0.73 | 3.47 ± 1.56 | 1.76 ± 1.37 |
| Retained on the skin surface % | 65.53 ± 17.57 | 84.28 ± 2.90 | 85.02 ± 5.83 |
| Retained in the membrane % | 11.00 ± 7.21 | 8.79 ± 2.05 | 7.60 ± 1.19 |
| Retained in membrane plus permeated % | 14.49 ± 7.76 | 12.26 ± 3.06 | 9.36 ± 2.49 |
| Recovery % | 80.02 ± 11.39 | 96.54 ± 1.81 | 94.38 ± 6.01 |
DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS molar ratio LHSS molar ratio |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.1 7.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
As mentioned previously, TC was selected due to its proficiency as a solubiliser, particularly in relation to compounds exhibiting poor water-solubility.56–58 Despite its capacity to partition into and permeate through human skin as a neat solvent59 high solubility of active ingredients in TC has not always resulted in high permeation values.46,60,61 The incorporation of water to create binary solvent systems, however, has frequently served to increase the permeation of the active compound.56,62 This has been corroborated by investigations concerning the solubility and thermodynamic activity of various low water-soluble compounds in TC, water and binary combinations thereof.40,63–67 In these studies, the compounds exhibited high solubility in TC, and had SPs that closely aligned with that of TC. A clear relationship emerged with the introduction of water, whereby an increase in the mole fraction of water corresponded to an elevated activity coefficient of the compound in the solvent system. As both the experimental and calculated SP44,45 of DNa is reported to be similar to that of TC, the addition of water increases the thermodynamic activity of the active in the formulation.67 Thus, the selection of a binary solvent system comprising a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) ratio of TC
50 (v/v) ratio of TC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, balances the requirement of solubility for both DNa and LHSS while addressing the issue of the thermodynamic activity of DNa in the formulation.
water, balances the requirement of solubility for both DNa and LHSS while addressing the issue of the thermodynamic activity of DNa in the formulation.
Recovery of DF was 94.38 ± 6.01% where no LHSS was used (5DL0), increasing to 96.54 ± 1.81% for the formulation containing 12.5 mg mL−1 LHSS (5DL12.5). Significantly less DF (80.02 ± 11.39% p < 0.05) was recovered from the final formulation, L5DL25.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 50 v/v), containing 7.5 mg mL−1 DNa and 0 mg mL−1 LHSS (7.5DL0) or 12.5 mg mL−1 LHSS (7.5DL12.5).
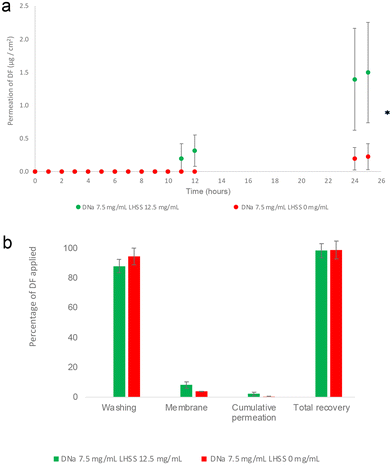
The results of the permeation study, as shown in Table 4 and Fig. 3(a) & (b), indicate that the addition of 12.5 mg mL−1 LHSS to a higher concentration of DNa (7.5 mg mL−1), significantly increased the permeation of DF at 25 h relative to the control. Permeation values for the LHSS-containing formulation (7.5DL12.5) amounted to 1.49 ± 0.76 μg cm−2 while the control (7.5DL0) was 0.22 ± 0.19 μg cm−2 (p < 0.05).
50 v/v), containing 7.5 mg mL−1 DNa and 0 mg mL−1 LHSS (7.5DL0) or 12.5 mg mL−1 LHSS (7.5DL12.5).
The results of the permeation study, as shown in Table 4 and Fig. 3(a) & (b), indicate that the addition of 12.5 mg mL−1 LHSS to a higher concentration of DNa (7.5 mg mL−1), significantly increased the permeation of DF at 25 h relative to the control. Permeation values for the LHSS-containing formulation (7.5DL12.5) amounted to 1.49 ± 0.76 μg cm−2 while the control (7.5DL0) was 0.22 ± 0.19 μg cm−2 (p < 0.05).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), containing 7.5 mg mL−1 DNa and 0 or 12.5 mg mL−1 LHSS. The table shows (i) cumulative permeation of DF (μg cm−2) at 25 h as well as the percentages of DF applied that (ii) permeated, (iii) remained on the skin surface, (iv) remained in the membrane, (v) permeated plus remained in the membrane and (vi) were recovered. In addition, the table contains a reference to the molar ratio of LHSS relative to DNa, that was applied (3 ≤ n ≤ 4; mean ± SD)
50 v/v), containing 7.5 mg mL−1 DNa and 0 or 12.5 mg mL−1 LHSS. The table shows (i) cumulative permeation of DF (μg cm−2) at 25 h as well as the percentages of DF applied that (ii) permeated, (iii) remained on the skin surface, (iv) remained in the membrane, (v) permeated plus remained in the membrane and (vi) were recovered. In addition, the table contains a reference to the molar ratio of LHSS relative to DNa, that was applied (3 ≤ n ≤ 4; mean ± SD)
| Amount DF partitioned and permeated | DNa 7.5 mg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS 12.5 mg mL−1 (7.5DL12.5) LHSS 12.5 mg mL−1 (7.5DL12.5) |
DNa 7.5 mg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS 0 mg mL−1 (7.5DL0) LHSS 0 mg mL−1 (7.5DL0) |
|---|---|---|
| Cumulative permeation μg cm−2 at 25 h | 1.49 ± 0.76 | 0.22 ± 0.19 |
| Permeated 25 h % | 2.24 ± 1.15 | 0.35 ± 0.30 |
| Retained on the skin surface % | 88.18 ± 4.41 | 94.64 ± 5.66 |
| Retained in the membrane % | 8.14 ± 2.24 | 3.95 ± 0.12 |
| Retained in membrane plus permeated % | 10.38 ± 2.49 | 4.30 ± 0.42 |
| Recovery % | 98.56 ± 4.89 | 98.94 ± 6.08 |
DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS molar ratio LHSS molar ratio |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.35 2.35 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Although one of the previous formulations tested (5DL12.5 shown in Table 3) contained an equivalent quantity of LHSS (12.5 mg mL−1 LHSS), the increase in the concentration of DNa from 5–7.5 mg mL−1, resulted in a change to the DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS molar ratio. Previously (5DL12.5) this ratio was 1
LHSS molar ratio. Previously (5DL12.5) this ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5, reducing to 1
3.5, reducing to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.35 (7.5DL12.5), as a result of the increase in DNa concentration. These changes appeared to have no significant impact on the cumulative permeation of DF from the 7.5DL12.5 formulation (1.49 ± 0.76 μg cm−2) relative to the 5DL12.5 experiment (1.48 ± 0.65 μg cm−2, p > 0.05). When viewed as a percentage of the DF applied, the amount reduced from 3.47 ± 1.56% (5DL12.5) to 2.24 ± 1.15% (7.5DL12.5), however this was not considered statistically significant (p > 0.05). When considering the quantity of DF in the membrane, the addition of LHSS in the current experiment (7.5 mg mL−1 DNa) resulted in a significantly higher percentage being extracted (8.14 ± 2.24%) when compared to the control (3.95 ± 0.12%, p < 0.05). This remained consistent when the percentage of DF that permeated was added to that extracted from the membrane, with values of 10.38 ± 2.49% for the LHSS formulation and 4.30 ± 0.42% for the control (p < 0.05).
2.35 (7.5DL12.5), as a result of the increase in DNa concentration. These changes appeared to have no significant impact on the cumulative permeation of DF from the 7.5DL12.5 formulation (1.49 ± 0.76 μg cm−2) relative to the 5DL12.5 experiment (1.48 ± 0.65 μg cm−2, p > 0.05). When viewed as a percentage of the DF applied, the amount reduced from 3.47 ± 1.56% (5DL12.5) to 2.24 ± 1.15% (7.5DL12.5), however this was not considered statistically significant (p > 0.05). When considering the quantity of DF in the membrane, the addition of LHSS in the current experiment (7.5 mg mL−1 DNa) resulted in a significantly higher percentage being extracted (8.14 ± 2.24%) when compared to the control (3.95 ± 0.12%, p < 0.05). This remained consistent when the percentage of DF that permeated was added to that extracted from the membrane, with values of 10.38 ± 2.49% for the LHSS formulation and 4.30 ± 0.42% for the control (p < 0.05).
A comparison of the percentage of DF extracted from the membrane for the 5DL12.5 samples (8.79 ± 2.05%) with the results of the 7.5DL12.5 samples (8.14 ± 2.24%), showed no significant differences (p > 0.05). Moreover, the combination of the amount of DF that permeated with that extracted from the membrane amounted to 12.26 ± 3.06% for the 5DL12.5 formulation, which was comparable to 10.38 ± 2.29% for the 7.5DL12.5 preparation (p > 0.05). Recovery of DF was approximately 98% for both the 7.5 mg mL−1 DNa formulation containing LHSS as well as the control. This was consistent with the range recommended by the OECD guidelines.68
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 40 v/v), containing 10 mg mL−1 DNa and 0 mg mL−1 LHSS (10DL0) or 10 mg mL−1 LHSS (10DL10).
To ascertain the impact of increasing the concentration of DNa to 10 mg mL−1 while reducing LHSS to an equivalent amount, further IVPT were performed. In addition to changes in the amounts of DNa and LHSS, the solvent ratios of TC
40 v/v), containing 10 mg mL−1 DNa and 0 mg mL−1 LHSS (10DL0) or 10 mg mL−1 LHSS (10DL10).
To ascertain the impact of increasing the concentration of DNa to 10 mg mL−1 while reducing LHSS to an equivalent amount, further IVPT were performed. In addition to changes in the amounts of DNa and LHSS, the solvent ratios of TC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water were modified from 50
water were modified from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) to 60
50 (v/v) to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
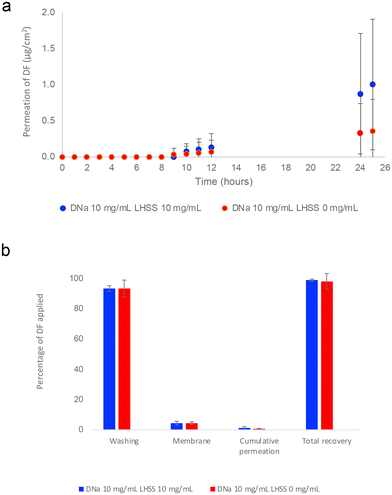
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v). Although cumulative permeation profiles shown in Fig. 4(a) suggest enhanced permeation of DF from the LHSS-containing vehicle at 25 h, differences were not statistically significant (p > 0.05). As shown in Table 5, permeation of DF at 25 h from the control (10DL0) was 0.36 ± 0.44 μg cm−2, equivalent to 0.41 ± 0.49% of the DF applied. This was consistent with the values of the LHSS formulation (10DL10), where permeation of DF was 1.01 ± 0.91 μg cm−2 or 1.10 ± 0.97% of the DF applied (p > 0.05).
40 (v/v). Although cumulative permeation profiles shown in Fig. 4(a) suggest enhanced permeation of DF from the LHSS-containing vehicle at 25 h, differences were not statistically significant (p > 0.05). As shown in Table 5, permeation of DF at 25 h from the control (10DL0) was 0.36 ± 0.44 μg cm−2, equivalent to 0.41 ± 0.49% of the DF applied. This was consistent with the values of the LHSS formulation (10DL10), where permeation of DF was 1.01 ± 0.91 μg cm−2 or 1.10 ± 0.97% of the DF applied (p > 0.05).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v), containing 10 mg mL−1 DNa and 0 or 10 mg mL−1 LHSS. The table shows (i) cumulative permeation of DF (μg cm−2) at 25 h as well as the percentages of DF applied that (ii) permeated, (iii) remained on the skin surface, (iv) remained in the membrane, (v) permeated plus remained in the membrane and (vi) were recovered. In addition, the table contains a reference to the molar ratio of LHSS relative DNa, that was applied (n = 5; mean ± SD)
40 v/v), containing 10 mg mL−1 DNa and 0 or 10 mg mL−1 LHSS. The table shows (i) cumulative permeation of DF (μg cm−2) at 25 h as well as the percentages of DF applied that (ii) permeated, (iii) remained on the skin surface, (iv) remained in the membrane, (v) permeated plus remained in the membrane and (vi) were recovered. In addition, the table contains a reference to the molar ratio of LHSS relative DNa, that was applied (n = 5; mean ± SD)
| Amount DF partitioned and permeated | DNa 10 mg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS 10 mg mL−1 (10DL10) LHSS 10 mg mL−1 (10DL10) |
DNa 10 mg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS 0 mg mL−1 (10DL0) LHSS 0 mg mL−1 (10DL0) |
|---|---|---|
| Cumulative permeation μg cm−2 at 25 h | 1.01 ± 0.91 | 0.36 ± 0.44 |
| Permeated 25 h % | 1.10 ± 0.98 | 0.41 ± 0.49 |
| Retained on the skin surface % | 93.55 ± 1.90 | 93.43 ± 5.49 |
| Retained in the membrane % | 4.31 ± 1.34 | 4.39 ± 0.95 |
| Retained in membrane plus permeated % | 5.41 ± 2.21 | 4.80 ± 1.08 |
| Recovery % | 98.96 ± 0.86 | 98.23 ± 5.13 |
DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS molar ratio LHSS molar ratio |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.41 1.41 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Furthermore, the percentage and actual amounts (μg cm−2) of DF that permeated from 10DL10 and 10DL0 were comparable to both 7.5 mg DNa formulations as well as the 5 mg DNa formulation control formulations (p > 0.05). However, percentages of DF that permeated from the 5 mg mL−1 formulations, 5DL12.5 (3.47 ± 1.56%) and 5DL25 (3.49 ± 0.73%), were significantly greater than the 1.10 ± 0.98% that permeated from 10DL10 (p < 0.05).
Analysis of the percentage of DF retained within the skin for the 10 mg mL−1 DNa formulations, revealed no significant differences between 10DL10 (4.31 ± 1.34%) and 10DL0 (4.39 ± 0.95%, p > 0.05). Furthermore, the combined values of DF extracted from the membrane and permeating, amounted to 5.41 ± 2.21% (10DL10) and 4.80 ± 1.08% (10DL0), were not significantly different (p > 0.05). This suggests that the molar ratio of DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS (1
LHSS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.41), did not impact either partition into the skin or permeation in this solvent system. Comparisons of the amounts of DF retained in the membrane for 7.5 and 10 mg mL−1 DNa formulations did, however, reveal significant differences when LHSS was included. The reduction of LHSS (12.5–10 mg mL−1), while simultaneously varying the solvent ratio (TC
1.41), did not impact either partition into the skin or permeation in this solvent system. Comparisons of the amounts of DF retained in the membrane for 7.5 and 10 mg mL−1 DNa formulations did, however, reveal significant differences when LHSS was included. The reduction of LHSS (12.5–10 mg mL−1), while simultaneously varying the solvent ratio (TC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water from 50
water from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50–60
50–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40), caused a significant decrease in the percentage of DF retained in the membrane. This amount reduced from 8.14 ± 2.24% (7.5DL12.5) to 4.31 ± 1.34% (10DL10) despite the increase in DNa concentration (7.5–10 mg mL−1, p < 0.05). This was not the case, however in relation to the 7.5DL0, where the DF retained in the membrane was comparable to that of the 10DL10 formulation (p > 0.05). As mentioned previously, this could indicate that the molar quantity of LHSS was not high enough relative to that of DNa, to result in an increase in penetration of the active. The total percentage of DF that partitioned and permeated reflected a similar pattern, significantly decreasing from 10.38 ± 2.49% (7.5DL12.5) to 5.41 ± 2.21% (10DL10) (p < 0.05). There was no significant difference in the percentages that partitioned and permeated from the 7.5DL0 (4.30 ± 0.42%) and 10DL10 (5.41 ± 2.21%) formulations (p > 0.05).
40), caused a significant decrease in the percentage of DF retained in the membrane. This amount reduced from 8.14 ± 2.24% (7.5DL12.5) to 4.31 ± 1.34% (10DL10) despite the increase in DNa concentration (7.5–10 mg mL−1, p < 0.05). This was not the case, however in relation to the 7.5DL0, where the DF retained in the membrane was comparable to that of the 10DL10 formulation (p > 0.05). As mentioned previously, this could indicate that the molar quantity of LHSS was not high enough relative to that of DNa, to result in an increase in penetration of the active. The total percentage of DF that partitioned and permeated reflected a similar pattern, significantly decreasing from 10.38 ± 2.49% (7.5DL12.5) to 5.41 ± 2.21% (10DL10) (p < 0.05). There was no significant difference in the percentages that partitioned and permeated from the 7.5DL0 (4.30 ± 0.42%) and 10DL10 (5.41 ± 2.21%) formulations (p > 0.05).
Differences in the amounts of DF retained in the membrane between the two 5 mg mL−1 preparations and the 10 mg mL−1 formulation containing LHSS, were also statistically different (p < 0.05). Notwithstanding the increase in the concentration of DNa (5–10 mg mL−1), the quantity of DF extracted from the membrane reduced from 8.79 ± 2.05% (5DL12.5) and 11.00 ± 7.21% (5DL25) to 4.31 ± 1.34% for the 10DL10 formulation. When the amount of DF permeating was added to that recovered from the skin, the results followed the same pattern. Values reduced from 12.26 ± 3.06% (5DL12.5) and 14.49 ± 7.76% (5DL25) when the concentration of DNa applied was 5 mg mL−1 to 5.41 ± 2.21% (10DL10) when the concentration of DNa increased to 10 mg mL−1 (p < 0.05). Values of DF for 5DL0 (9.35 ± 2.49%) and 10DL10 (5.41 ± 2.21%) were comparable (p > 0.05).
The observed changes can be partially attributed to the adjustment of the TC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solvent ratio from 50
water solvent ratio from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 to 60
50 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v). This modification directly impacts the SP of the solvent system,65,66 reducing it from 34.36 to 31.83 MPa1/2, bringing it closer to the SPs of TC and DNa. The thermodynamic consequences of increasing the TC fraction in binary TC
40 (v/v). This modification directly impacts the SP of the solvent system,65,66 reducing it from 34.36 to 31.83 MPa1/2, bringing it closer to the SPs of TC and DNa. The thermodynamic consequences of increasing the TC fraction in binary TC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water systems, where the permeant is sparingly soluble in water, but freely soluble in TC, were investigated using paracetamol,69 DNa67,69 and various other active ingredients.40,63–66 It was shown that increasing TC relative to water decreased the thermodynamic activity of the active ingredients within the solvent systems. This effect was demonstrated by Bialik et al. with IVPT using ibuprofen.62 Due to its low solubility in water (0.021 mg mL−1) relative to TC (400 mg mL−1),56 ibuprofen permeation decreased with increasing TC concentration, due to the alteration of the permeant's thermodynamic activity in the vehicle.62
water systems, where the permeant is sparingly soluble in water, but freely soluble in TC, were investigated using paracetamol,69 DNa67,69 and various other active ingredients.40,63–66 It was shown that increasing TC relative to water decreased the thermodynamic activity of the active ingredients within the solvent systems. This effect was demonstrated by Bialik et al. with IVPT using ibuprofen.62 Due to its low solubility in water (0.021 mg mL−1) relative to TC (400 mg mL−1),56 ibuprofen permeation decreased with increasing TC concentration, due to the alteration of the permeant's thermodynamic activity in the vehicle.62
Apart from the alteration in solvent ratio, the reduction in the DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS molar ratio to (1
LHSS molar ratio to (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.41) could have contributed to a decrease in DF partitioning and permeation. This may indicate that a minimum amount of LHSS is required to achieve any increased partition and permeation results. Prior studies have indicated a correlation between DF partitioning and increased LHSS counter ion concentration.37
1.41) could have contributed to a decrease in DF partitioning and permeation. This may indicate that a minimum amount of LHSS is required to achieve any increased partition and permeation results. Prior studies have indicated a correlation between DF partitioning and increased LHSS counter ion concentration.37
Finally, as shown in Table 5, recovery of the DF applied exceeded 98% for both the 10DL10 and the control sample sets, satisfying the guidelines set out by the OECD.68
Conclusion
Building on previous research, the current study has addressed challenges pertaining to solubility and identified a binary solvent model comprising TC and water to evaluate DNa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LHSS ion pairs. The tested formulations included (i) fixed concentrations of DNa (5 mg mL−1) and solvent ratios (50
LHSS ion pairs. The tested formulations included (i) fixed concentrations of DNa (5 mg mL−1) and solvent ratios (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v)) while varying the counter ion concentration (12.5 or 25 mg mL−1), (ii) an increased concentration of DNa (7.5 mg mL−1) at fixed solvent ratios (50
50 (v/v)) while varying the counter ion concentration (12.5 or 25 mg mL−1), (ii) an increased concentration of DNa (7.5 mg mL−1) at fixed solvent ratios (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v)) and counter ion concentrations (12.5 mg mL−1), and (iii) increasing the concentration of DNa (10 mg mL−1) while varying the TC
50 (v/v)) and counter ion concentrations (12.5 mg mL−1), and (iii) increasing the concentration of DNa (10 mg mL−1) while varying the TC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solvent ratio (60
water solvent ratio (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v)) and decreasing the counter ion concentration (10 mg mL−1). All formulations complied with the MDE and MPPUD for TC outlined in the FDA's Inactive Ingredient Database.
40 (v/v)) and decreasing the counter ion concentration (10 mg mL−1). All formulations complied with the MDE and MPPUD for TC outlined in the FDA's Inactive Ingredient Database.
The selection of TC, a solvent with a SP similar to that reported for DNa, resulted in a large increase in the solubility of the active when compared to our previous work. Challenges associated with this choice, such as a reduction in the activity coefficient of DNa in the solvent system and its ability to partition out of the formulation and into the membrane, were addressed by the inclusion of water. The effect of reducing the water content was demonstrated by the alteration of the TC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio from 50
water ratio from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 to 60
50 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (v/v). Although the increase in TC enabled the DNa concentration to be doubled (5–10 mg mL−1), this had no significant effect on the actual amount of DF partitioning and permeating from the 10DL0 system, relative to any of the other control samples. Furthermore, the reduction in the dielectric constant of the solvent system attributable to the increase in the TC fraction, was not able to offset the drop in the quantity of LHSS from 25 mg mL−1 and 12.5 mg mL−1 to 10 mg mL−1. This was evidenced by the significant reduction the amount of DF partitioning and permeating from the 10DL0 formulation relative to the 5DL12.5, 5DL25 and 7.5DL12.5 samples.
40 (v/v). Although the increase in TC enabled the DNa concentration to be doubled (5–10 mg mL−1), this had no significant effect on the actual amount of DF partitioning and permeating from the 10DL0 system, relative to any of the other control samples. Furthermore, the reduction in the dielectric constant of the solvent system attributable to the increase in the TC fraction, was not able to offset the drop in the quantity of LHSS from 25 mg mL−1 and 12.5 mg mL−1 to 10 mg mL−1. This was evidenced by the significant reduction the amount of DF partitioning and permeating from the 10DL0 formulation relative to the 5DL12.5, 5DL25 and 7.5DL12.5 samples.
The studies showed that while the inclusion of LHSS at 5 mg mL−1 increased the partition and permeation of DF by approximately 30% (5DL12.5) and 55% (5DL25) relative to the control; this was not statistically significant. However, when the concentration of DNa was increased to 7.5 mg mL−1 (7.5DL12.5), the inclusion of LHSS significantly enhanced the amount of DF that partitioned and permeated (approximately 145%), when compared to the control formulation. The increase in the amount of DNa from 5–7.5 mg mL−1 had no significant effect on the partition and permeation of DF, when the quantity of LHSS remained constant at 12.5 mg mL−1.
In accordance with our previous investigations, the current work suggests that the inclusion of LHSS with DNa in a formulation may increase the partition and permeation of DF. This represents a further step in the development of an ion pair formulation where less DNa may be required within the preparation to achieve a therapeutic result. In continuing this process, the solubility of the active and the counter ion require further consideration, particularly in relation to the ratio in which they are most effective. Additionally, the importance of the activity coefficient of the active in the formulation should be balanced with the potential to stabilise the ion pairs, by increasing the use of solvents with a lower dielectric constant than water. Further work has already commenced exploring the implications of substituting TC with an alternative solvent, DiPG, as the DNa-solubiliser. This substitution should enable the impact of a solvent change on IVPT results to be determined. Additional investigations will incorporate more than one solvent into the DNa-solubilising fraction. These ternary systems will be tested via IVPT to further optimize the ion pair formulation.
Author contributions
Mignon Cristofoli: conceptualization, methodology, validation, investigation, formal analysis, visualization, writing – original draft. Jonathan Hadgraft: conceptualization. Majella E. Lane: conceptualization, resources, supervision, writing – review & editing. Bruno C. Sil: conceptualization, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.References
- D. J. Hunter, L. March and M. Chew, Lancet, 2020, 396, 1711–1712 CrossRef PubMed.
- V. P. Leifer, J. N. Katz and E. Losina, Osteoarthritis Cartilage, 2022, 30, 10–16 CrossRef CAS PubMed.
- D. Primorac, V. Molnar, V. Matišić, D. Hudetz, Ž. Jeleč, E. Rod, F. Čukelj, D. Vidović, T. Vrdoljak and B. Dobričić, Pharmaceuticals, 2021, 14, 205 CrossRef CAS PubMed.
- F. Rannou, J.-P. Pelletier and J. Martel-Pelletier, Semin. Arthritis Rheum., 2016, 45, S18–S21 CrossRef CAS PubMed.
- The National Institute for Health and Care Excellence (NICE), Osteoarthritis in over 16s: diagnosis and management, report NG226, 2022.
- O. Bruyère, G. Honvo, N. Veronese, N. K. Arden, J. Branco, E. M. Curtis, N. M. Al-Daghri, G. Herrero-Beaumont, J. Martel-Pelletier and J.-P. Pelletier, Semin. Arthritis Rheum., 2019, 49, 337–350 CrossRef PubMed.
- S. L. Kolasinski, T. Neogi, M. C. Hochberg, C. Oatis, G. Guyatt, J. Block, L. Callahan, C. Copenhaver, C. Dodge and D. Felson, Arthritis Rheumatol., 2020, 72, 220–233 CrossRef PubMed.
- G. A. Brown, J. Am. Acad. Orthop. Surg., 2013, 21(9), 577–579 Search PubMed.
- R. R. Bannuru, M. Osani, E. Vaysbrot, N. Arden, K. Bennell, S. Bierma-Zeinstra, V. Kraus, L. S. Lohmander, J. Abbott and M. Bhandari, Osteoarthritis Cartilage, 2019, 27, 1578–1589 CrossRef CAS PubMed.
- P. McGettigan and D. Henry, PLoS Med., 2013, 10, e1001388 CrossRef PubMed.
- F. Bariguian Revel, M. Fayet and M. Hagen, Rheumatol. Ther., 2020, 7, 217–236 CrossRef PubMed.
- A. Zeneca, Pharmaceuticals in the Environment Statement, Cambridge, U.K., 2024 Search PubMed.
- A. G. Novartis, Novartis Position on Pharmaceuticals in the Environment, Basel, Switzerland, 2023 Search PubMed.
- F. Hoffmann-La Roche AG, Roche Position on Pharmaceuticals in the Environment (PiE), Basel, Switzerland, 2011 Search PubMed.
- F. Hoffmann-La Roche AG, Roche Position on Product Stewardship, Basel, Switzerland, 2017 Search PubMed.
- Y. Gao, L. Du, Q. Li, Q. Li, L. Zhu, M. Yang, X. Wang, B. Zhao and S. Ma, Medicine, 2022, 101(26), e29314, DOI:10.1097/MD.0000000000029314.
- S. Coulman, C. Allender and J. Birchall, Crit. Rev. Ther. Drug Carrier Syst., 2006, 23(3), 205–258 CrossRef CAS PubMed.
- Y. Wang, R. Thakur, Q. Fan and B. Michniak, Eur. J. Pharm. Biopharm., 2005, 60, 179–191 CrossRef CAS PubMed.
- N. Dixit, V. Bali, S. Baboota, A. Ahuja and J. Ali, Curr. Drug Delivery, 2007, 4, 1–10 CAS.
- M. B. Delgado-Charro and R. H. Guy, in Transdermal Drug Delivery Systems, CRC Press, 2002, pp. 199–224 Search PubMed.
- E. Vranić, Bosnian J. Basic Med. Sci., 2004, 4, 25 CrossRef PubMed.
- S. Mitragotri, Adv. Drug Delivery Rev., 2013, 65, 100–103 CrossRef CAS PubMed.
- J. H. Jung and S. G. Jin, J. Pharm. Invest., 2021, 1–15 Search PubMed.
- T.-M. Tuan-Mahmood, M. T. McCrudden, B. M. Torrisi, E. McAlister, M. J. Garland, T. R. R. Singh and R. F. Donnelly, Eur. J. Pharm. Sci., 2013, 50, 623–637 CrossRef CAS PubMed.
- K. Ita, Pharmaceutics, 2015, 7, 90–105 CrossRef CAS PubMed.
- S. N. Murthy, S. M. Sammeta and C. Bowers, J. Controlled Release, 2010, 148, 197–203 CrossRef CAS PubMed.
- X. Chen, L. Zhu, R. Li, L. Pang, S. Zhu, J. Ma, L. Du and Y. Jin, Eur. J. Pharm. Sci., 2020, 151, 105410 CrossRef CAS PubMed.
- J. Hadgraft, Int. J. Pharm., 1999, 184, 1–6 CrossRef CAS PubMed.
- P. Santos, A. C. Watkinson, J. Hadgraft and M. E. Lane, Int. J. Pharm., 2012, 439, 260–268 CrossRef CAS PubMed.
- P. Santos, A. Watkinson, J. Hadgraft and M. Lane, Int. J. Pharm., 2011, 416, 155–159 CAS.
- P. Tapfumaneyi, M. Imran, S. E. Alavi and Y. Mohammed, Drug Discovery Today, 2023, 28, 103521–103521 CrossRef CAS PubMed.
- M. E. Lane, P. Santos, A. C. Watkinson and J. Hadgraft, Passive skin permeation enhancement, Topical and transdermal drug delivery, John Wiley & Sons, Inc, Hoboken, NJ, USA, 2012, pp. 23–42, DOI:10.1002/9781118140505.ch2.
- M. E. Lane, Int. J. Pharm., 2013, 447, 12–21 CrossRef CAS PubMed.
- A. C. Williams and B. W. Barry, Adv. Drug Delivery Rev., 2004, 56, 603–618 CrossRef CAS PubMed.
- H. Marwah, T. Garg, A. K. Goyal and G. Rath, Drug Delivery, 2016, 23, 564–578 CrossRef CAS PubMed.
- M. Cristofoli, C.-P. Kung, J. Hadgraft, M. E. Lane and B. C. Sil, Pharmaceutics, 2021, 13, 909 CrossRef CAS PubMed.
- M. Cristofoli, J. Hadgraft, M. E. Lane and B. C. Sil, Int. J. Pharm., 2022, 623, 121906–121906 CrossRef CAS PubMed.
- A. F. Barton, CRC Handbook of Solubility Parameters and Other Cohesion Parameters, CRC Press, 1991 Search PubMed.
- M. J. Louwerse, A. Maldonado, S. Rousseau, C. Moreau-Masselon, B. Roux and G. Rothenberg, ChemPhysChem, 2017, 18, 2999–3006 CrossRef CAS PubMed.
- S. Alshehri and F. Shakeel, J. Mol. Liq., 2020, 307, 112970 CrossRef CAS.
- A. A. Saei, F. Jabbaribar, M. A. A. Fakhree, W. E. Acree and A. Jouyban, J. Drug Delivery Sci. Technol., 2008, 18, 149–151 CrossRef CAS.
- C. M. Hansen, Hansen solubility parameters: a user's handbook, CRC press, 2007 Search PubMed.
- B. C. Hancock, P. York and R. C. Rowe, Int. J. Pharm., 1997, 148, 1–21 CrossRef CAS.
- J. Barra, M.-A. Peña and P. Bustamante, Eur. J. Pharm. Sci., 2000, 10, 153–161 CrossRef CAS PubMed.
- P. Bustamante, M. A. Peña and J. Barra, J. Pharm. Pharmacol., 1998, 50, 975–982 CrossRef CAS PubMed.
- P. Minghetti, F. Cilurzo, A. Casiraghi, L. Montanari and A. Fini, J. Pharm. Sci., 2007, 96, 814–823 CrossRef CAS PubMed.
- T. Higuchi, J. Soc. Cosmet. Chem., 1960, 11, 85–97 Search PubMed.
- N. Bjerrum, Untersuchungen über Ionenassoziation, AF Høst, 1926 Search PubMed.
- C. A. Kraus, J. Phys. Chem., 1956, 60, 129–141 CrossRef CAS.
- Gattefossé SAS, Transcutol® P for efficient drug solubilization and skin penetration, 2018 Search PubMed.
- T. Vishwam, S. Shihab, V. K. Murthy, H. S. Tiong and S. S. Sastry, Spectrochim. Acta, Part A, 2017, 179, 74–82 CrossRef CAS PubMed.
- R. Sengwa, J. Mol. Liq., 2003, 108, 47–60 CrossRef CAS.
- E. Ikada, J. Phys. Chem., 1971, 75, 1240–1246 CrossRef CAS.
- C. Malmberg and A. Maryott, J. Res. Natl. Bur. Stand. (U. S.), 1956, 56, 1–8 CrossRef CAS.
- I. N. Levine, Physical chemistry McGraw-Hill Higher Education, London, U.K., 6th edn, International edn., 2009 Search PubMed.
- D. W. Osborne and J. Musakhanian, AAPS PharmSciTech, 2018, 19, 3512–3533 CrossRef CAS PubMed.
- E.-S. Ha, S.-K. Lee, D. H. Choi, S. H. Jeong, S.-J. Hwang and M.-S. Kim, J. Pharm. Invest., 2020, 50, 231–250 CrossRef CAS.
- N. Hashemzadeh and A. Jouyban, J. Pharm. Pharm. Sci., 2022, 25, 340–353 Search PubMed.
- P. H. Dugard, M. Walker, S. J. Mawdsley and R. C. Scott, Environ. Health Perspect., 1984, 57, 193–197 CrossRef CAS PubMed.
- L. Kikwai, N. Kanikkannan, R. Babu and M. Singh, J. Controlled Release, 2002, 83, 307–311 CrossRef CAS PubMed.
- E. Csizmazia, G. Erős, O. Berkesi, S. Berkó, P. Szabó-Révész and E. Csányi, J. Drug Delivery Sci. Technol., 2011, 21, 411–415 CrossRef CAS.
- W. Bialik, K. Walters, K. Brain and J. Hadgraft, Int. J. Pharm., 1993, 92, 219–223 CrossRef CAS.
- F. Shakeel and S. Alshehri, Processes, 2020, 8, 1204 CrossRef CAS.
- F. Shakeel, N. Haq, F. K. Alanazi, S. A. Alanazi and I. A. Alsarra, J. Therm. Anal. Calorim., 2020, 142, 1437–1446 CrossRef CAS.
- F. Shakeel, M. Kazi, F. K. Alanazi and P. Alam, Molecules, 2021, 26, 7052 CrossRef CAS PubMed.
- F. Shakeel, N. Haq and S. Alshehri, Molecules, 2020, 25, 2743 CrossRef CAS PubMed.
- G. Shazly, N. Haq and F. Shakeel, Pharmazie, 2014, 69, 335–339 CAS.
- OECD, OECD Test Guideline 428: Skin absorption: in vitro Method, 2004, 1–8, DOI:10.1787/9789264071087-en.
- F. Shakeel, F. K. Alanazi, I. A. Alsarra and N. Haq, J. Chem. Eng. Data, 2013, 58, 3551–3556 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |