Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cerium oxide particles: coating with charged polysaccharides for limiting the aggregation state in biological media and potential application for antibiotic delivery†
Cléa
Chesneau
a,
André
Pawlak
bc,
Séna
Hamadi
a,
Eric
Leroy
a and
Sabrina
Belbekhouche
 *a
*a
aUniversité Paris Est Creteil, CNRS, Institut Chimie et Matériaux Paris Est, UMR 7182, 2 Rue Henri Dunant, 94320 Thiais, France. E-mail: sabrina.belbekhouche@cnrs.fr; Tel: + 331 4978 1149
bInstitut National de la Santé et de la Recherche Médicale (INSERM), IMRB U955, Créteil, F-94010, France
cUniv Paris Est Creteil, Faculté de Médecine, UMRS 955, Créteil, F-94010 France
First published on 19th February 2024
Abstract
Bacterial resistance to antibiotics has emerged as a major health issue. Developing new antibacterial systems is crucial. We propose to exploit cerium oxide particles which present interesting physicochemical and biological properties. We demonstrated by zeta potential measurement that according to the pH, cerium oxide particles present either negatively or positively charged surfaces (isoelectric point determined around 8). We then take advantage of this property for modifying the particle surfaces with charged polysaccharides (dextran derivative to limit aggregation in aqueous media). The surface modification of particles has been examined by FT-IR, DRX and TGA measurements. The physicochemical properties of the resulting dispersion have been investigated as the size, dispersity and potential zeta value in physiological media. A fluorescent probe (Nile red) has then been loaded as a model of hydrophobic cargo, and then a hydrophobic antibiotic has been loaded (e.g. ciprofloxacin). Finally, the inhibitory effect on bacterial growth of the resulting antibiotic-loaded particles has been evaluated against antibiotic-resistant bacteria, namely spectinomycin-resistant Escherichia coli. These findings demonstrated the potential of the particles to be employed as an antimicrobial material, more specifically those resistant to antibiotic therapy.
1. Introduction
Antibiotic use is often required to treat intracellular bacterial infections and resistance in pathogenic microbes, but because of their poor stability or water solubility, a step of vectorization is often needed for their efficient administration.1 An efficient and safe antibiotic delivery system is needed for delivering therapeutic agents at an adequate concentration within the intracellular medium to prevent antibacterial resistance.2,3Antibiotic-loaded particles provide a compelling alternative to antibiotics because they rely on entirely different mechanisms of antibacterial activity than those of antibiotics. The mode of action of particles is mainly based on direct contact with the wall of the bacterial cell, inducing damage to the bacterial cell.4 In this sense, several works have reported on the increased antibacterial activity of antibiotic-conjugated particles.5–9 Porous particles are highly appropriate materials for antibiotic delivery application. Among them, we may cite silica, calcium phosphate and calcium carbonate particles to name but a few.10 For more insight into the fabrication of porous materials, the reader may refer to the recently published review articles.11,12 Most of these particles can be safely excreted by the human body via the kidneys and can contain and then transport a high amount of therapeutic bioactives with few side effects.13
Among the different carriers, we focus on cerium oxide particles (CeO2) which are a rare earth oxide material employed in several technological applications.14 In the last decade, cerium oxide particles have gained increased interest in the biomedical field, because of their self-regenerating antioxidant properties, e.g. are promising antioxidants for healing several untreatable oxidative-stress-related diseases, as discussed in previous works.15–17 Cerium oxide nanoparticles certainly scavenge each ROS and RNS inclusive of the hydroxyl radical18 and nitric oxide19 whilst mimicking the antioxidant enzymes superoxide dismutase and catalase.20 Interestingly, the antioxidant activity of such particles has been related to a shielding effect now not most effective on neurons,21 but additionally on endothelial cells.22 In this sens, cerium oxide nanoparticles had been evidenced to be useful for numerous pathologies which include most cancers,23 cardiovascular injuries24 or diseases of the principal worried system inclusive of Alzheimer's disease.25
A very important issue related to the application of nanoparticles in biological media is the need for a stable dispersion, especially at physiological pH. Unfortunately, cerium oxide particles are known to be prone to aggregation in aqueous media. In this sense, several pathways developed for stabilizing cerium oxide particles in aqueous media can be found in the literature.26,27 For instance, Jiménez-Rojo et al. showed that lipid-based liposomes could stabilize suspensions of metal oxide nanoparticles.26 Another promising approach is the stabilization of such particles by the adsorption of polyelectrolytes.28 Polyelectrolytes are macromolecular chains consisting of linked charged or chargeable groups. The interactions of polyelectrolytes with particles have been examined in detail by Skirtach et al.29,30 for example on a suspension of gold particles. They evidenced that polyelectrolytes can be efficiently used for improving the aggregation of gold nanoparticles.
Motivated by the aforementioned results, we decided to explore the interactions between ceria nanoparticles and an anionic polysaccharide to reduce the aggregation state. Polysaccharides are one of the biopolymer classes that are promising because of their weak toxicity toward mammalian cells.31 Among them, we focus on dextran sulfate, selected herein because of its ability to retain its charge in a broad pH range.
Ciprofloxacin(1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid) is a fluoroquinolone antibiotic classically employed to treat numerous bacterial infections such as lung infections.32 Unfortunately, its potential benefits are limited by low bioavailability, poor water solubility (≥1 mg mL−1 in water) and a short half-life. These shortcomings may be limited by administering the antibiotic through an alternative approach.
Research has been reported on the incorporation of ciprofloxacin into particles and their antibacterial activity has been evaluated.33–35 Although many researchers have fabricated ciprofloxacin-loaded particles for different biomedical applications, to the best of our knowledge, cerium oxide particles have not been hitherto loaded with ciprofloxacin for use against spectinomycin-resistant Escherichia coli (which is an antibiotic-resistant bacteria). Therefore, in the present paper, cerium oxide particles loaded with ciprofloxacin have been fabricated towards this aim.
2. Materials and methods
2.1. Materials
Cerium oxide particles, dextran sulfate (dex−, Mn ∼2.104 g mol−1, Đ ∼1.15 determined by size exclusion chromatography; see Fig. S1†), Nile red and ciprofloxacin were purchased from Sigma-Aldrich. Lysogeny broth (LB) culture medium was purchased from Fisher Scientific. Water at 18 MΩ was produced using a Milli-Q system (Millipore).2.2. Isoelectric point (pI) of cerium oxide particles suspended in aqueous media
The isoelectric point (pI) of the suspension of cerium oxide particles was determined by preparing a suspension of cerium oxide particles (0.1 g L−1) at different pH values (ranging from 2 to 12). Each sample was analyzed in triplicate using a Zetasizer.2.3. Coating of cerium oxide particles by negatively charged dextran
The surface modification of negatively charged cerium oxide particles was done via the saturation method. In the literature, this approach is widely described.36–38 This elegant approach proposes the possibility of directly incorporating the required amount of polyelectrolyte to coat efficiently all cerium oxide particle surfaces and then does not require a purification process. The saturation concentration of dex- has to be empirically determined over zeta potential measurements. A suspension of positively charged cerium oxide particles (0.025 g l−1 at pH 2) was progressively covered with a polyanion, namely dex- (10 g L−1 solubilized in water). The adsorption process of this negatively charged biopolymer was monitored by zeta potential measurements. The detailed conditions of the preparation of the nanomaterial suspension will be determined and then discussed in the text.2.4. Hydrophobic cargo-loaded cerium oxide particles: investigation of Nile red and ciprofloxacin
A hydrophobic solution (Nile red or ciprofloxacin) at a concentration of 1 g L−1 in ethanol was mixed with a suspension of cerium oxide particles and stirred for 24 h. The particles were then centrifuged and washed with ethanol to remove the free drug. This process was repeated twice. The loading with the fluorescent probe (Nile red) was examined with an optical fluorescence ZEISS microscope.2.5. Characterization of cerium oxide particles
2.6. Bactericidal activity of cerium oxide particles loaded with ciprofloxacin
For investigating the bactericidal activity of cerium oxide particles loaded with ciprofloxacin, to 10.5 mL of suspension of spectinomycin-resistant E. coli (first grown at 37 °C for 24 h in lysogeny broth medium), 500 μL (10 mg mL−1) of the suspension of particles were added and then incubated at 37 °C. Water was used as a reference. Bacterial growth was followed periodically by reading the optical density of bacterial suspensions using a UV-visible spectrophotometer at 620 nm.The percentage of bacterial growth inhibition was estimated using eqn (1):
| I(%): percentage of inhibition (I = 100 − ((ODsample/ODref) × 100)) | (1) |
2.7. Bacterial biofilm formation
A biofilm of E. coli was prepared on a polystyrene plate by using the protocol developed by Ashrit et al.40 The bacterial viability was investigated via an orange acridine test coupled with fluorescence microscopy observation.413. Results and discussion
The investigated strategy relied on the use of cerium oxide particles for (i) limiting particle aggregation via a polysaccharide coating and (ii) hydrophobic antibiotic loading. The antibacterial activity of the designed materials was examined on environmental bacteria, namely antibiotic-resistant Escherichia coli (E. coli) in a liquid LB medium. Note that E. coli is a Gram-negative bacterium and is an important component of the normal intestinal microflora of humans. However, it can be a highly versatile and frequently deadly pathogen.3.1. Coating of cerium oxide particles by dextran sulfate: impact on aggregation in aqueous media
In Fig. 1, the DRX peaks are indexed using JCPDS card no: 34-0394. The DRX spectra of CeO2 nanoparticles indicated that the fine crystalline and single phase of CeO2 could be indexed to the cubic structure with the lattice parameters a = b = c = 5.411 Å and α = β = γ = 90°. Diffraction peaks were observed at 28.41° (111), 32.99° (200), 47.37° (220), 56.26° (311), 59.03° (222), 69.27° (400), 76.60° (331) and 79.01° (420). The broadened peak showed nanometer-sized crystallites. The average crystallite size (D) of CeO2 nanoparticles has been estimated using the Debye–Scherer equation (eqn (2)):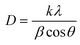 | (2) |
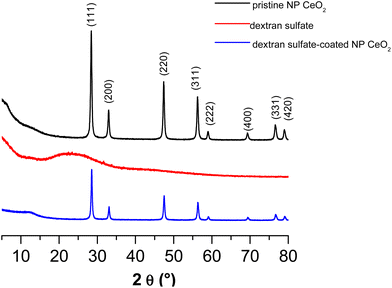 | ||
| Fig. 1 DRX spectra of pristine cerium oxide particles (—), dextran sulfate (—) and dextran sulfate-coated cerium oxide particles (—). | ||
After drying a suspension of cerium oxide particles prepared at 0.5 g L−1 in water at pH 6, images of such particles were obtained by Transmission Electron Microscopy. Fig. 2A clearly shows that pristine or “naked” cerium oxide particles present a high trend of aggregation in aqueous media. From TEM, we also notice the polydispersity, cuboidal shape and heterogeneity in particle characteristics. This result is difficult to explain as for this investigation, we use commercial powders with an unknown synthesis procedure. We supposed that the method used to prepare them does not take place in a controlled manner and leads to the observed characteristics.
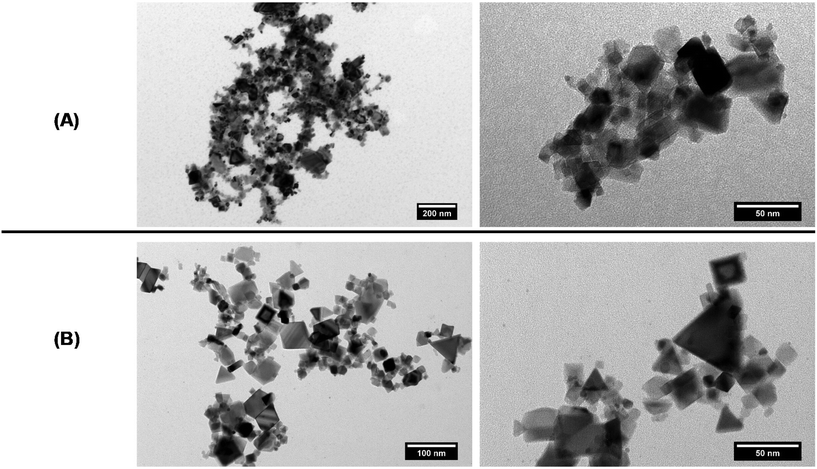 | ||
| Fig. 2 TEM pictures of (A) pristine cerium oxide particles and (B) dextran sulfate-coated cerium oxide particles. | ||
One of the prerequisites for examining the adsorption behavior of polyelectrolytes on metal oxide aqueous surfaces is to determine the isoelectric point (pI). This parameter was determined by measuring the zeta potential values of cerium oxide particle suspensions at 0.025 g L−1 CeO2 particles and with various pH values between 2 and 11. The zeta potential values as a function of pH are presented in Fig. 3. From linear interpolation, an isoelectric point of 8.3 was derived. This is in accordance with the results obtained by the work by Quik et al.42 and Van Hoecke et al.,43 who determined an isoelectric point of 8.0 and 7.9 respectively, for the same nature particles. Therefore pristine particles may not be suitable for biological pH and thus this is the reason why we have developed our strategy, i.e. coating such particles with dextran sulfate.
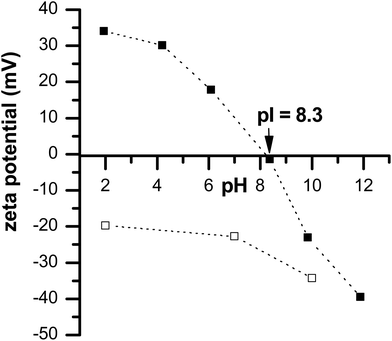 | ||
| Fig. 3 Zeta potential values of a suspension of pristine CeO2 particles (■) and dextran sulfate-coated cerium oxide particles (□)as a function of pH in deionized water. | ||
With the aim of limiting the aggregation of cerium oxide particles in aqueous media, surface modification of the charged cerium oxide particle nanoparticles was performed by a direct saturation method. This means neither washing nor purification steps. Considering the surface modification of charged particles by polyelectrolytes, the centrifugation process is one of the most used methods to separate adsorbed polyelectrolytes onto the particle surfaces from free polyelectrolytes in solution. However, this presents some drawbacks; for instance, it is time-consuming. The saturation method is a more attractive strategy, as this offers the great advantage of avoiding post-processing procedures, e.g. washing and centrifugation steps. The amount of polyelectrolytes needed to be adsorbed onto the particles was experimentally estimated by zeta-potential measurements.38,44
As the ceria nanoparticles are positively charged at pH = 2 (see Fig. 3), and dextran sulfate is negatively charged (Fig. 4A) in the entire pH range, the coating of ceria nanoparticles is supposed to occur at pH 2. The addition of dextran sulfate to a suspension of CeO2 particles induces a decrease of the potential zeta value from a positive to a negative value confirming the adsorption of this polyelectrolyte (in this case dex-) on the surface of metal oxide particles. The addition of dex- was stopped from the moment the measured zeta potential value of the suspension of the coated cerium oxide nanoparticles reached a value near to those obtained for the free dextran sulfate in solution (Fig. 4B). Thus, it can be supposed that the amount of free dextran sulfate in the suspension was very insignificant/low. The required volumes of stock dextran sulfate solution (10 g L−1) to form a stable first layer were 40 μl for cerium oxide particles.
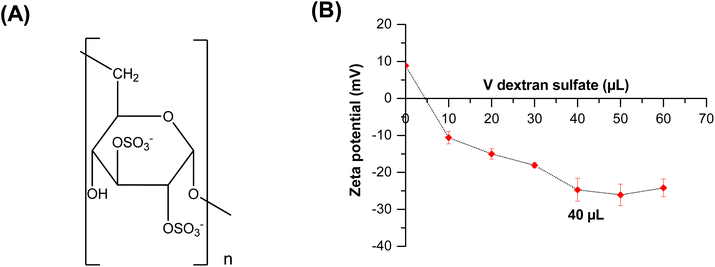 | ||
| Fig. 4 (A) Chemical structure of dextran sulfate, (B) determination of the saturation concentration of dextran sulfate over zeta potential measurements. | ||
As a first test, a visual observation (i.e. sedimentation test) of both dextran sulfate-coated and uncoated samples was done at various pH values. Sedimentation of uncoated CeO2 particles was observed at pH = 8 (Fig. 5); this was not seen with the dextran derivative coating. This result proved that stability is increased by coating the cerium oxide particles with dextran sulfate and it also served as a clear indicator that dextran sulfate coating occurs. In contrast to pristine cerium oxide particles, dextran sulfate coated-cerium oxide particles remain negatively charged regardless of the pH (see Fig. 3), and this ensures colloidal stability.
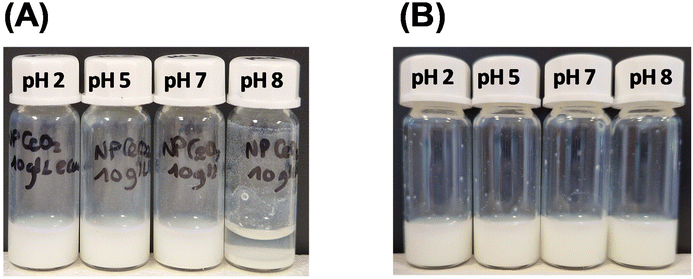 | ||
| Fig. 5 (A) Solution of pristine CeO2 particles dispersed in water at pH 2, 5, 7 and 8 and (B) solution of dextran sulfate-coated CeO2 particles dispersed in water at pH 2, 5, 7 and 8. | ||
From Fig. 6, in the FTIR spectrum of dextran sulfate the presence of the sulpho group (SO2) can be determined by the presence of absorption bands at about 1220 cm−1 and 979 cm−1 assigned to νas (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and νs (S
O) and νs (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibrations, respectively, as well as by the presence of absorption bands at 804 cm−1 and 580 cm−1 assigned to νas (O–S–O) and νs (O–S–O) vibrations, respectively.45 The spectral region from 1000 to 700 cm−1 is important for the structural analysis of polysaccharides and is assigned to C–CH, C–C–O, O–C–O and C–O–C. The band around 3470 cm−1 is seen and can be assigned to the O–H group. For the FTIR spectrum of cerium oxide particles, a band around 3460 cm−1 is present, which can be assigned to the vibrational tension mode of O–H46 due to residual water and/or hydroxyl groups. A small band at 1040 cm−1 is seen and may be attributed to the stretching vibrations of Ce–O–C.47 The O–C–O stretching band is also seen in the region 1300–1600 cm−1. The absorption band at around 1630 cm−1 is assigned to the bending vibration of absorbed molecular water. The band at 700 cm−1 can be assigned to Ce–O stretching. The FTIR spectrum of dextran sulfate-coated cerium oxide particles shows the characteristic peaks (cited below) of both dextran sulfate and cerium oxide particles.
O) vibrations, respectively, as well as by the presence of absorption bands at 804 cm−1 and 580 cm−1 assigned to νas (O–S–O) and νs (O–S–O) vibrations, respectively.45 The spectral region from 1000 to 700 cm−1 is important for the structural analysis of polysaccharides and is assigned to C–CH, C–C–O, O–C–O and C–O–C. The band around 3470 cm−1 is seen and can be assigned to the O–H group. For the FTIR spectrum of cerium oxide particles, a band around 3460 cm−1 is present, which can be assigned to the vibrational tension mode of O–H46 due to residual water and/or hydroxyl groups. A small band at 1040 cm−1 is seen and may be attributed to the stretching vibrations of Ce–O–C.47 The O–C–O stretching band is also seen in the region 1300–1600 cm−1. The absorption band at around 1630 cm−1 is assigned to the bending vibration of absorbed molecular water. The band at 700 cm−1 can be assigned to Ce–O stretching. The FTIR spectrum of dextran sulfate-coated cerium oxide particles shows the characteristic peaks (cited below) of both dextran sulfate and cerium oxide particles.
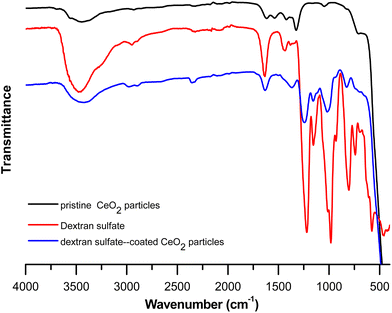 | ||
| Fig. 6 Fourier Transform Infrared (FTIR) spectra of pristine cerium oxide particles (—), dextran sulfate (—) and dextran sulfate-coated cerium oxide particles (—). | ||
In Fig. 1, the DRX spectrum of the dextran sulfate does not present any peak which is due to the amorphous structure of this polysaccharide. The DRX spectrum of dextran sulfate-coated cerium oxide particles presents the characteristic peak of pristine cerium oxide.
The influence of the size of the cerium oxide particles suspended at 0.025 g L−1 in water at pH = 2 was investigated. Table 1 shows sizes that vary from 1189 ± 64 nm to 277 ± 27 nm after dextran sulfate coating as well as a decrease in the polydispersity value (varies from 0.7 to 0.3). The dextran derivative coating allows for limiting the aggregation of cerium oxide particles and favors colloidal stability.
| Pristine CeO2 (pH 2) | Coated CeO2 particles with dex- (pH 2) | |
|---|---|---|
| Size (nm) | 1189 ± 64 | 277 ± 27 |
| Polydispersity | 0.7 | 0.3 |
After drying a suspension of dextran sulfate-coated cerium oxide particles, the surface morphology of the obtained dextran sulfate-coated cerium oxide particles examined by transmission electron microscopy (TEM) is shown in Fig. 1B and reveals that the particles are less aggregated than without the coating. This result confirmed the DLS measurement reported in Table 1 and previously discussed.
Thermogravimetric analysis of cerium dioxide nanoparticles, dextran sulfate and dextran sulfate-coated particles are shown in Fig. 7. Dextran sulfate started to decompose at around 100 °C until 700 °C (weight loss 30%). CeO2 particles show no significant weight loss which indicates good thermal stability. In contrast to pristine CeO2 particles, dextran sulfate-coated CeO2 particles present a weight loss of 5% in the temperature range studied which is in accordance with the dextran sulfate deposition of cerium oxide particles and the amount can then be estimated as 5% of dextran sulfate in the studied sample.
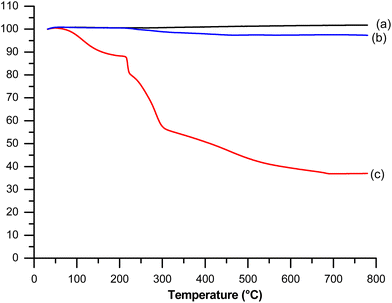 | ||
| Fig. 7 TGA curves of (a) pristine cerium oxide particles, (b) dextran sulfate-coated cerium oxide particles and (c) dextran sulfate. | ||
The experimental results obtained using multiple techniques confirm that adsorption of dextran sulfate takes place on the CeO2 particle surface under examined experimental conditions and allows limiting aggregation of the metal oxide when suspended in aqueous media.
3.2. Antibiotic loading inside cerium oxide particles
Cerium oxide particles were selected because of their non-toxic nature at low concentrations.15,16 Herein, we used commercial cerium oxide particles to load hydrophobic cargo.Loading was done by soaking cerium oxide particles into hydrophobic antibiotic solutions under the same conditions found for the loading of the hydrophobic Nile Red. The antibiotic remains loaded inside the particle for up to 15 days which significantly extends the persistence ability of ciprofloxacin.
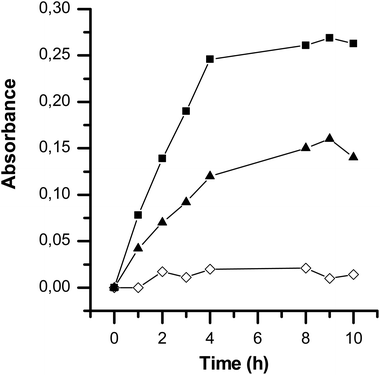
In Fig. 9, the growth curves of E. coli were determined based on the bacterial cell optical density. We distinguished two classical phases of growth: the exponential phase and the stationary phase.48 The stepwise variation in the growth rate could be assigned to the changes seen in expression profiles of genes coding for enzymes involved in biosynthesis or nutrient assimilation.49 In the microbial growth curve, the growth phases can also be defined in terms of the metabolic processes and physiological states occurring during growth, which have been directly correlated with the nutritional content of the growth media.50Fig. 9 presents the bacterial growth response according to exposure to the antibiotic-loaded particles over time. Bacterial growth was strongly impacted in the presence of cerium oxide particles loaded with ciprofloxacin. Indeed, the exposition of such particles to bacteria led to the direct inhibition of their growth (inhibition of up to 94%). This is of major interest as ciprofloxacin is almost insoluble in water and bacterial resistance to this antibiotic has emerged.51 For instance, Fantin et al. reported that increasing the concentration of this antibiotic induced a better bactericidal effect but induced resistance to ciprofloxacin in human commensal bacteria.51
The MIC value of ciprofloxacin is reported to be around 0.15–4 μg ml−1 against S. aureus and E. coli.52,53 The required amount of ciprofloxacin was then directly released when using ciprofloxacin-loaded cerium oxide particles.
On the other hand, as seen in Fig. 9, the addition of a solution of ciprofloxacin in the presence of the bacteria studied induced partial inhibition of the activity of this bacterium. This is due to the hydrophobicity of ciprofloxacin, which does not enter the bacteria sufficiently at this concentration. This clearly highlights the importance of vectoring this antibiotic into the particles studied.
Ciprofloxacin is an antibiotic that is known to be a DNA-targeting agent, and to interact with their target type II topoisomerases to enhance the generation of single- and double-strand DNA breaks associated with stalled or collapsed replication forks.54,55 The cerium oxide particles loaded with this antibiotic may first interact with bacterial surfaces. Then, the antibiotic is released, which causes bacterial cell wall damage.
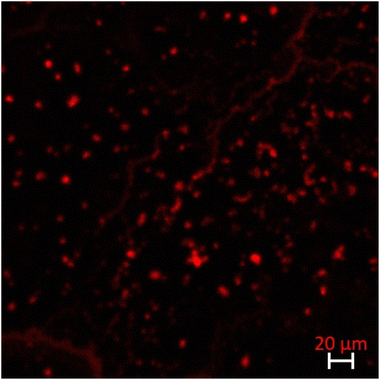
Note that we can combine the two approaches proposed in this work to generate a CeO2 delivery system with high solubility. Indeed, we have shown that after trapping the compound (part developed in section 3.2), it is still possible to modify the surface with the polyelectrolyte (part developed in section 3.1). These particles were then brought into contact with an E. coli biofilm prepared on the surface of a polystyrene plate. Polystyrene plates were selected as these materials are currently employed in medical materials which are embedded in the human body and microbial strains easily adhere to these materials and then form biofilms.56We show that compared to the uncoated particles those coated with dextran enhance their incorporation in the biofilm and induce bacterial death due to antibiotic release. Indeed, bacterial viability was examined with acridine orange which allows discriminating between dead or living bacteria.57 A red fluorescence was observed after staining bacteria (Fig. SI 2†), showing the death of bacterial cells due to exposure to dextran-coated particles.
4. Conclusion
We demonstrated (from TEM, DLS, electrophoretic mobility, FTIR and TGA measurements) that the adsorption of dextran sulfate on a suspension of ceria nanoparticles takes place under the examined experimental conditions. Under studied physicochemical conditions, after the adsorption of the biopolymer, the aggregation is limited in aqueous media.We then reported on the use of cerium oxide particles as good candidates for designing antibacterial systems loaded with a hydrophobic antibiotic namely ciprofloxacin (poor water solubility, ≥1 mg mL−1 in water). Our approach is simple and allows us to tackle the issue of poor solubility in aqueous media. The particles could be loaded with a sufficient amount of antibiotics to prevent the growth of spectinomycin-resistant E. coli. We showed that antibiotic-loaded particles were more effective than the antibiotic alone.
We then combined surface modification with a dextran derivative and antibiotic loading. We demonstrated the effectiveness of such a system on an E. coli biofilm prepared on the surface of a polystyrene plate. This plate has been selected as these materials are currently employed in medical materials which are embedded in the human body and microbial strains easily adhere to these materials and then form biofilms.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Agence Nationale de la Recherche (grants ANR-22-CE92-0086) for the financial support during this research project.References
- G. L. Mislin and I. J. Schalk, Siderophore-dependent iron uptake systems as gates for antibiotic Trojan horse strategies against Pseudomonas aeruginosa, Metallomics, 2014, 6(3), 408–420 CrossRef CAS.
- D. Zhao, R.-X. Zhuo and S.-X. Cheng, Modification of calcium carbonate based gene and drug delivery systems by a cell-penetrating peptide, Mol. BioSyst., 2012, 8(12), 3288–3294 RSC.
- R. Y. Pelgrift and A. J. Friedman, Nanotechnology as a therapeutic tool to combat microbial resistance, Adv. Drug Delivery Rev., 2013, 65(13–14), 1803–1815 CrossRef CAS PubMed.
- L. Wang, C. Hu and L. Shao, The antimicrobial activity of nanoparticles: present situation and prospects for the future, Int. J. Nanomed., 2017, 12, 1227 CrossRef CAS PubMed.
- V. Akbari, et al., Ciprofloxacin nano-niosomes for targeting intracellular infections: an in vitro evaluation, J. Nanopart. Res., 2013, 15(4), 1556 CrossRef.
- S. Chono, et al., Pharmacokinetic and pharmacodynamic efficacy of intrapulmonary administration of ciprofloxacin for the treatment of respiratory infections, Drug Metab. Pharmacokinet., 2007, 22(2), 88–95 CrossRef CAS.
- H. X. Ong, et al., Liposomal nanoparticles control the uptake of ciprofloxacin across respiratory epithelia, Pharm. Res., 2012, 29(12), 3335–3346 CrossRef CAS PubMed.
- L. Michely, et al., Easy way for fabricating calcium carbonate hybrid microparticles-supported carrier: Focus on the loading of several hydrosoluble cargos all at once, J. Drug Delivery Sci. Technol., 2022, 74, 103485 CrossRef CAS.
- F. A. Said, et al., Antibiotic loading and development of antibacterial capsules by using porous CaCO3 microparticles as starting material, Int. J. Pharm., 2020, 579, 119175 CrossRef.
- W. Wang, et al., Calcium carbonate-doxorubicin@ silica-indocyanine green nanospheres with photo-triggered drug delivery enhance cell killing in drug-resistant breast cancer cells, Nano Res., 2018, 11(6), 3385–3395 CrossRef CAS.
- X. Du, et al., Disulfide–bridged organosilica frameworks: designed, synthesis, redox–triggered biodegradation, and nanobiomedical applications, Adv. Funct. Mater., 2018, 28(26), 1707325 CrossRef.
- Y. Li and J. Shi, Hollow–structured mesoporous materials: chemical synthesis, functionalization and applications, Adv. Mater., 2014, 26(20), 3176–3205 CrossRef CAS PubMed.
- J. G. Croissant, et al., Syntheses and applications of periodic mesoporous organosilica nanoparticles, Nanoscale, 2015, 7(48), 20318–20334 RSC.
- C. Sun, H. Li and L. Chen, Nanostructured ceria-based materials: synthesis, properties, and applications, Energy Environ. Sci., 2012, 5(9), 8475–8505 RSC.
- I. Celardo, E. Traversa and L. Ghibelli, Cerium oxide nanoparticles: a promise for applications in therapy, J. Exp. Ther. Oncol., 2011, 9(1), 47–51 CAS.
- S. Das, et al., Cerium oxide nanoparticles: applications and prospects in nanomedicine, Nanomedicine, 2013, 8(9), 1483–1508 CrossRef CAS PubMed.
- F. Caputo, M. De Nicola and L. Ghibelli, Pharmacological potential of bioactive engineered nanomaterials, Biochem. Pharmacol., 2014, 92(1), 112–130 CrossRef CAS PubMed.
- Y. Xue, et al., Direct Evidence for Hydroxyl Radical Scavenging Activity of Cerium Oxide Nanoparticles, J. Phys. Chem. C, 2011, 115(11), 4433–4438 CrossRef CAS.
- J. M. Dowding, et al., Cerium oxide nanoparticles scavenge nitric oxide radical (˙NO), Chem. Commun., 2012, 48(40), 4896–4898 RSC.
- V. Baldim and N. Yadav, Polymer-Coated Cerium Oxide Nanoparticles as Oxidoreductase-like Catalysts, ACS Appl. Mater. Interfaces, 2020, 12(37), 42056–42066 CrossRef CAS PubMed.
- S. Nagarajan, et al., Novel biocompatible electrospun gelatin fiber mats with antibiotic drug delivery properties, J. Mater. Chem. B, 2016, 4(6), 1134–1141 RSC.
- Q. Bao, et al., Simultaneous Blood-Brain Barrier Crossing and Protection for Stroke Treatment Based on Edaravone-Loaded Ceria Nanoparticles, ACS Nano, 2018, 12(7), 6794–6805 CrossRef CAS.
- H. Ghaznavi, et al., Neuro-protective effects of cerium and yttrium oxide nanoparticles on high glucose-induced oxidative stress and apoptosis in undifferentiated PC12 cells, Neurol. Res., 2015, 37(7), 624–632 CrossRef CAS PubMed.
- F. Pagliari, et al., Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress, ACS Nano, 2012, 6(5), 3767–3775 CrossRef CAS.
- C.-C. Yu, et al., Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering, Mater. Lett., 2013, 93, 133–136 CrossRef CAS.
- N. Jiménez-Rojo, et al., Lipidic nanovesicles stabilize suspensions of metal oxide nanoparticles, Chem. Phys. Lipids, 2015, 191, 84–90 CrossRef.
- E. J. Salazar-Sandoval, M. K. G. Johansson and A. Ahniyaz, Aminopolycarboxylic acids as a versatile tool to stabilize ceria nanoparticles – a fundamental model experimentally demonstrated, RSC Adv., 2014, 4(18), 9048–9055 RSC.
- D. I. Gittins and F. Caruso, Tailoring the Polyelectrolyte Coating of Metal Nanoparticles, J. Phys. Chem. B, 2001, 105(29), 6846–6852 CrossRef CAS.
- A. G. Skirtach, et al., Nanoparticles Distribution Control by Polymers:Aggregates versus Nonaggregates, J. Phys. Chem. C, 2007, 111(2), 555–564 CrossRef CAS.
- B. V. Parakhonskiy, et al., Nanoparticles on Polyelectrolytes at Low Concentration: Controlling Concentration and Size, J. Phys. Chem. C, 2010, 114(5), 1996–2002 CrossRef CAS.
- S. Tokura, et al., Biological activities of biodegradable polysaccharide, in Macromolecular symposia, 1996, Wiley Online Library Search PubMed.
- B. Scully, et al., Ciprofloxacin therapy in cystic fibrosis, Am. J. Med., 1987, 82(4A), 196–201 CAS.
- A. Toncheva, et al., Antibacterial fluoroquinolone antibiotic-containing fibrous materials from poly(L-lactide-co-D,L-lactide) prepared by electrospinning, Eur. J. Pharm. Sci., 2012, 47(4), 642–651 CrossRef CAS PubMed.
- A. R. Unnithan, et al., Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl, Carbohydr. Polym., 2012, 90(4), 1786–1793 CrossRef CAS PubMed.
- S. P. Parwe, et al. Synthesis of ciprofloxacin-conjugated poly (L-lactic acid) polymer for nanofiber fabrication and antibacterial evaluation, Int. J. Nanomed., 2014, 9, 1463–1477, DOI:10.2147/ijn.s54971.
- U. Bazylinska, et al., Novel approach to long sustained multilayer nanocapsules: influence of surfactant head groups and polyelectrolyte layer number on the release of hydrophobic compounds, Soft Matter, 2011, 7(13), 6113–6124 RSC.
- U. Bazylinska, et al., Influence of dicephalic ionic surfactant interactions with oppositely charged polyelectrolyte upon the in vitro dye release from oil core nanocapsules, Bioelectrochemistry, 2012, 87, 147–153 CrossRef CAS PubMed.
- S. Belbekhouche, et al., Latex nanoparticles surface modified via the layer-by-layer technique for two drugs loading, Colloids Surf., A, 2017, 524, 28–34 CrossRef CAS.
- T. Misono, Dynamic Light Scattering (DLS), in Measurement Techniques and Practices of Colloid and Interface Phenomena, ed. M. Abe, Springer Singapore, Singapore, 2019, pp. 65–69 Search PubMed.
- P. Ashrit, et al., A microplate-based Response Surface Methodology model for growth optimization and biofilm formation on polystyrene polymeric material in a Candida albicans and Escherichia coli co-culture, Polym. Adv. Technol., 2022, 33(9), 2872–2885 CrossRef CAS.
- L. Boulos, et al., LIVE/DEAD® BacLight™: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water, J. Microbiol. Methods, 1999, 37(1), 77–86 CrossRef CAS.
- J. T. K. Quik, et al., Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water, Chemosphere, 2010, 81(6), 711–715 CrossRef CAS PubMed.
- K. Van Hoecke, et al., Aggregation and ecotoxicity of CeO2 nanoparticles in synthetic and natural waters with variable pH, organic matter concentration and ionic strength, Environ. Pollut., 2011, 159(4), 970–976 CrossRef CAS PubMed.
- A. Voigt, et al., Membrane filtration for microencapsulation and microcapsules fabrication by layer-by-layer polyelectrolyte adsorption, Ind. Eng. Chem. Res., 1999, 38(10), 4037–4043 CrossRef CAS.
- M. Cakić, et al., Synthesis, characterization and antimicrobial activity of dextran sulphate stabilized silver nanoparticles, J. Mol. Struct., 2016, 1110, 156–161 CrossRef.
- S. Phoka, et al., Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route, Mater. Chem. Phys., 2009, 115(1), 423–428 CrossRef CAS.
- D. Andreescu, E. Matijević and D. V. Goia, Formation of uniform colloidal ceria in polyol, Colloids Surf., A, 2006, 291(1), 93–100 CrossRef CAS.
- B. L. Wanner and M. R. Wilmes-Riesenberg, Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli, J. Bacteriol., 1992, 174(7), 2124–2130 CrossRef CAS PubMed.
- M. V. Baev, et al., Growth of Escherichia coli MG1655 on LB medium: monitoring utilization of sugars, alcohols, and organic acids with transcriptional microarrays, Appl. Microbiol. Biotechnol., 2006, 71(3), 310–316 CrossRef CAS PubMed.
- G. Sezonov, D. Joseleau-Petit and R. D'Ari, Escherichia coli physiology in Luria-Bertani broth, J. Bacteriol., 2007, 189(23), 8746–8749 CrossRef CAS PubMed.
- B. Fantin, et al., Ciprofloxacin dosage and emergence of resistance in human commensal bacteria, J. Infect. Dis., 2009, 200(3), 390–398 CrossRef CAS PubMed.
- A. K. Kidsley, et al., Antimicrobial Susceptibility of Escherichia coli and Salmonella spp. Isolates From Healthy Pigs in Australia: Results of a Pilot National Survey, Front. Microbiol., 2018, 9(1207) DOI:10.3389/fmicb.2018.01207.
- J.-P. Didier, et al., Impact of ciprofloxacin exposure on Staphylococcus aureus genomic alterations linked with emergence of rifampin resistance, Antimicrob. Agents Chemother., 2011, 55(5), 1946–1952 CrossRef CAS PubMed.
- K. Drlica, et al., Quinolone-mediated bacterial death, Antimicrob. Agents Chemother., 2008, 52(2), 385–392 CrossRef CAS PubMed.
- M. Malik, X. Zhao and K. Drlica, Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones, Mol. Microbiol., 2006, 61(3), 810–825 CrossRef CAS PubMed.
- A. Festas, A. Ramos and J. P. Davim, Medical devices biomaterials – A review, Proc. Inst. Mech. Eng., Part L, 2019, 234, 146442071988245 Search PubMed.
- D. E. Francisco, R. A. Mah and A. C. Rabin, Acridine orange-epifluorescence technique for counting bacteria in natural waters, Trans. Am. Microsc. Soc., 1973, 416–421 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3pm00081h |
| This journal is © The Royal Society of Chemistry 2024 |