 Open Access Article
Open Access ArticleC3-Chlorination of C2-substituted benzo[b]thiophene derivatives in the presence of sodium hypochlorite†
Vincent
Conrad Oppenheimer‡
,
Peter
Le‡
,
Cathy
Tran
,
Haobin
Wang
 * and
Marino J. E.
Resendiz
* and
Marino J. E.
Resendiz
 *
*
Department of Chemistry, University of Colorado Denver, Science Building 1151 Arapahoe St, Denver, CO 80204, USA. E-mail: marino.resendiz@ucdenver.edu; haobin.wang@ucdenver.edu
First published on 10th October 2024
Abstract
Benzo[b]thiophene rings are common synthons for the development of novel drugs and materials, and thus, the discovery of facile ways for their functionalization is of value. In this work, a new method for the C3-chlorination of C2-substituted benzothiophene derivatives is described. The chlorine source is sodium hypochlorite pentahydrate (NaOCl·5H2O), and optimal transformations occur in aqueous acetonitrile at 65–75 °C to provide the corresponding C3-halogenated products in variable yields (30–65%). The reaction occurs in the presence of vinyl and alkyl groups, while the presence of alcohols leads to competing oxidation reactions at the heterobenzylic position. The presence of a carbonyl group at the C2-position inhibited the halogenation reaction, while the use of benzofuran led to a highly exothermic reaction, presumably via the formation of a peroxide intermediate. Reactions carried out at lower temperatures led to side reactions associated with competing oxidative processes. To gain a better understanding of the mechanism of the reaction, DFT calculations were carried out, where the heteroatom enables the formation of a hypochlorous acidium ion that serves to generate a C2–C3 chloronium ion intermediate in a step-wise manner, which in turn leads to the formation of an S-stabilized C2-carbocation that undergoes re-aromatization to the corresponding C3-chlorinated products. To probe potential synthetic applications, a model C3-chloro derivative was coupled with phenylboronic acid using standard Suzuki–Miyaura coupling conditions.
Introduction
Thiophene and benzothiophene derivatives have been used as effective synthons for functional materials,1 as useful pharmacophores2,3 and drugs,4 and in the functionalization of natural products (Fig. 1A).5 Of particular importance to this work is the finding that chlorinated derivatives of benzothiophene have been studied as effective neurotoxin inhibitors6 or that synthetic methods for the preparation of 3-chlorobenzothiophene molecules have been developed,7 given their antimicrobial8 and potential biological activity.9 Importantly, these factors have garnered scientific interest with respect to their toxicity.10 Thus, expanding the synthetic toolkit by which this heterocycle can be functionalized, particularly under mild conditions, is of importance. While functionalization of benzo[b]thiophene at position C3 has been reported,11 this chemistry is underdeveloped,12 which has prompted the discovery of alternative methods, e.g., annulation reactions.13–15 In this regard, we report a facile, metal-free, and efficient C3-chlorination of benzo[b]thiophene rings in the presence of sodium hypochlorite pentahydrate, a novel procedure that will also serve to expand on the known reactivity of this reagent.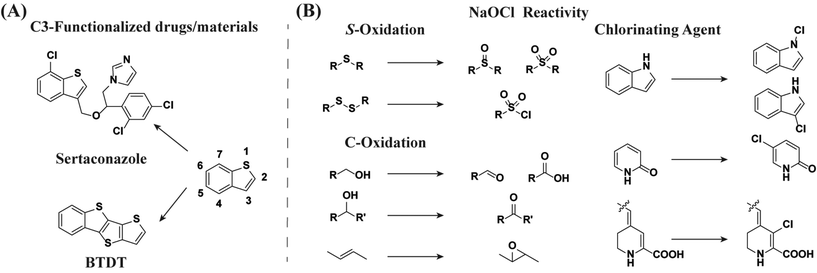 | ||
| Fig. 1 (A) The numbering system of benzo[b]thiophene along with a drug displaying C3-functionalization, as well as a functional derivative that can be obtained from C3-functionalization of benzo[b]thiophene.39 (B) Examples of the most common reactions of sodium hypochlorite pentahydrate as an oxidant or a chlorination agent, with references mentioned within the text. | ||
Sodium hypochlorite pentahydrate (NaOCl·5H2O) has been reported to be an efficient oxidant (Fig. 1B) for the oxidation of primary and secondary alcohols, in the presence16–18 and absence19,20 of additives, e.g., TEMPO or acetic acid, to their corresponding aldehydes/ketones. Other oxidation reactions include the esterification of aldehydes in the presence of an acid21 or tetrabutylammonium bromide,22 as well as the epoxidation of alkenes.23 Reactions of substituted benzothiophene rings in the presence of sodium hypochlorite have also been reported, where the oxidation of methylbenzothiophene24 or C3-acylated benzothiophene25 leads to the corresponding carboxylic acids. Furthermore, the reactivity of NaOCl with sulfides can result in oxidation reactions to the corresponding sulfonyl chlorides,26 sulfoxides or sulfones.27 Of particular importance to this work is the ability of NaOCl to function as a chlorinating agent. In this regard, reactions in the presence of other heteroaromatic cycles are also known, including the N-chlorination of pyridones28 or the C3-position of indole via Cl-rearrangement from the N-atom.29 Other salts such as calcium hypochlorite can oxidize methyl ethers to their corresponding ketones.30 NaOCl has also been used to generate other useful intermediates, such as ClN3,31 or as a source of chlorinium ions in the chlorination of alpha-carbons32 and olefins.29,33 In addition, sodium hypochlorite can also be used for the preparation of other oxidants such as t-butylhypochlorite, which has been used in the chlorination of benzothiophene, albeit with low selectivity and low yields.34 The described polyfunctional reactivity has made NaOCl a useful reagent, and its involvement in multistep synthesis has been reported.35–37 Thus, considering that synthetic ways to modify the C3-position of benzo[b]thiophene rings are less common than those to obtain the C2-regioisomer,38 the novel pathway reported herein is expected to be useful in the C3-functionalization of benzothiophene rings, broadly.
Results
Upon probing the reactivity/stability of benzothiophene-2-methanol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 with different sodium-based salt oxidants, an unexpected outcome was observed, in the presence of sodium hypochlorite and applied heat (65–75 °C). We noticed the formation of two less polar compounds (via TLC), which were characterized as aldehyde derivatives 2 and 3 (Scheme 1A). The characterization of the slower eluting fraction was consistent with the oxidation of the heterobenzylic alcohol to the corresponding aldehyde 3. On the other hand, features of the 1H-NMR spectra of the faster eluting fraction were highlighted by the disappearance of the C3-proton, along with the appearance of a peak displaying a heavier mass (+35 units, via GC-MS), consistent with benzothiophene derivative 2. We hypothesized that chlorination (via intermediate 4) was the first step, as prolonged reactions or increased amounts of sodium hypochlorite did not enhance the yields of chlorinated benzothiophene 2. This was corroborated by carrying out independent reactions in the presence of C2-formyl derivative 3, where the chlorinated material was not observed. In attempts to increase the yield, the reaction conditions were varied, revealing that (1) decreasing the temperature to <55° C led to the formation of aldehyde 3, as the major product; (2) reactions carried out in the absence of solvent, with applied heat, led to the desired chlorinated alcohol 4, along with aldehyde 2, albeit in low yields (28 and 10%, respectively); and (3) reactions carried out in different solvent mixtures (MeOH/H2O, acetone/H2O, THF/H2O, CH3CN, acetone, and MeOH) did not lead to enhanced yields or lacked reactivity. To compare this reactivity to that of other standard reagents, N-chlorosuccinimide (NCS) was used in the presence of alcohol 1, to observe the formation of the desired chlorinated derivative 4 as the major product, along with heterobenzylic oxidation to the corresponding aldehyde 3. To circumvent the competing oxidation reactions, alcohol 1 was protected with a TBDMS group, to yield silyl ether 5. Gratifyingly, reactions in the presence of sodium hypochlorite and heat yielded chlorinated product 6, in a higher yield (65%), corroborated via1H-NMR and GC-MS. Lastly, desilylation in the presence of hydrofluoride led to alcohol 4, also independently obtained from the reduction of aldehyde 2 in the presence of sodium borohydride.
40 with different sodium-based salt oxidants, an unexpected outcome was observed, in the presence of sodium hypochlorite and applied heat (65–75 °C). We noticed the formation of two less polar compounds (via TLC), which were characterized as aldehyde derivatives 2 and 3 (Scheme 1A). The characterization of the slower eluting fraction was consistent with the oxidation of the heterobenzylic alcohol to the corresponding aldehyde 3. On the other hand, features of the 1H-NMR spectra of the faster eluting fraction were highlighted by the disappearance of the C3-proton, along with the appearance of a peak displaying a heavier mass (+35 units, via GC-MS), consistent with benzothiophene derivative 2. We hypothesized that chlorination (via intermediate 4) was the first step, as prolonged reactions or increased amounts of sodium hypochlorite did not enhance the yields of chlorinated benzothiophene 2. This was corroborated by carrying out independent reactions in the presence of C2-formyl derivative 3, where the chlorinated material was not observed. In attempts to increase the yield, the reaction conditions were varied, revealing that (1) decreasing the temperature to <55° C led to the formation of aldehyde 3, as the major product; (2) reactions carried out in the absence of solvent, with applied heat, led to the desired chlorinated alcohol 4, along with aldehyde 2, albeit in low yields (28 and 10%, respectively); and (3) reactions carried out in different solvent mixtures (MeOH/H2O, acetone/H2O, THF/H2O, CH3CN, acetone, and MeOH) did not lead to enhanced yields or lacked reactivity. To compare this reactivity to that of other standard reagents, N-chlorosuccinimide (NCS) was used in the presence of alcohol 1, to observe the formation of the desired chlorinated derivative 4 as the major product, along with heterobenzylic oxidation to the corresponding aldehyde 3. To circumvent the competing oxidation reactions, alcohol 1 was protected with a TBDMS group, to yield silyl ether 5. Gratifyingly, reactions in the presence of sodium hypochlorite and heat yielded chlorinated product 6, in a higher yield (65%), corroborated via1H-NMR and GC-MS. Lastly, desilylation in the presence of hydrofluoride led to alcohol 4, also independently obtained from the reduction of aldehyde 2 in the presence of sodium borohydride.
We then decided to explore the scope of the reaction and functionalized the benzothiophene ring with other electron-rich substituents, specifically, methyl or allyl derivatives (Scheme 1B and C). Electron withdrawing groups were avoided, given the unwanted reactivity observed with aldehyde 3. Methylation at the C2-position was achieved by aryl deprotonation in the presence of n-butyllithium, followed by treatment with iodomethane, to yield benzothiophene derivative 7. Gratifyingly, chlorination reactions in the presence of sodium hypochlorite corroborated the previous observations, where reactions carried out at higher temperatures (65–75 °C) led to the C3-chlorination reaction, to yield derivative 8 as the major product. Unexpectedly, heterobenzylic chlorination occurred as a side reaction, to yield chlorinated derivative 9, where 1H-NMR spectra displayed the loss of a proton corresponding to the methyl signal, along with a downfield chemical shift. We then proceeded to probe the temperature effect, to find that reactions carried out at room temperature and extended periods of time (48 h) led to C3-chlorination product 8 in lower yield, along with a mixture of inseparable side-products, associated with S-oxidation, consistent with the appearance of sulfone 11 (this was the only side-product that was purified). Furthermore, reactions carried out in the absence of solvent (neat) led to low yields and a mixture of chlorinated and oxidized products (Scheme 1B, conditions iii). To gain a better understanding of the mechanism and establish the first step in the formation of derivative 11 (oxidation or halogenation), methylbenzothiophene 7 was oxidized in the presence of oxone. The obtained derivative 12 was then treated with sodium hypochlorite at rt or with applied heat; however, the starting material was recovered in ∼50% yield, along with an inseparable mixture of unidentified products, indicating that halogenation is the first step. To compare reactivity, reactions were carried out in the presence of NCS, to yield C3-chlorinated products in yields comparable to those obtained from the hypochlorite reaction. It is worth noting that bis-chlorinated product 10 was also obtained, possibly from the formation of two consecutive chloronium ion intermediates.
Next, we decided to probe the chlorination reaction in the presence of an olefin and prepared derivative 13 by treatment of benzothiophene in the presence of n-butyllithium and allyl bromide. Unfortunately, the corresponding product was obtained with impurities that were inseparable by column chromatography, or via fractional distillation (Scheme 1C). In agreement with previous results, chlorinated allyl derivative 14 was obtained upon treatment with sodium hypochlorite. It is worth noting that selective chlorination of the aromatic ring was observed, suggesting that the use of this reagent can be amenable to the presence of olefins. In this regard, the use of NCS resulted in competing vinyl halogenation, evident from the disappearance of peaks in the vinylic region (1H-NMR spectra) and the appearance of peaks in the 3–4 ppm region, associated with the formation of chlorinated hydrocarbon products. To investigate the role of the heteroatom, we examined the reaction between benzofuran 15 and NaOCl·5H2O (Scheme 1D). However, these conditions led to a highly exothermic reaction, along with poor mass balance recovery, presumably from the consumption of the hypochlorite and/or the formation of other unidentified byproducts. This reactivity is consistent with the formation of peroxides, which can be highly reactive (Video S1†). In fact, the synthesis of t-butyl hypochlorite, from NaOCl and t-BuOH, can lead to vigorous decomposition.41 To avoid competition from oxidation reactions, the hydroxyl group was methylated16 and treated with sodium hypochlorite; however, the same outcome was observed. Importantly, these results are in agreement with a benzothiophene chlorination mechanism that is assisted by the heteroatom.
To explore a potentially useful synthetic application of C3-chlorinated benzothiophene products, we employed their use in palladium couplings. We opted for the Suzuki–Miyaura coupling42 of methylbenzothiophene 8 and used phenyl boronic acid as a substrate, to this end (Scheme 1E). Gratifyingly, this chemistry yielded the corresponding coupling product 17 in modest yield, along with the corresponding reduced product 7, thus corroborating that chlorinated derivatives can effectively be used as synthons in the functionalization of benzothiophene.
Computational study
To gain a better understanding of the mechanism of the reaction, we carried out computational studies, using density functional theory (DFT) methodology. The calculations were performed using the quantum chemistry program package and Gaussian16.43 Geometry optimization of the minimum energy structures and transition states of various molecular intermediates reported in this paper was carried out employing the M06-2X functional44 and the basis sets 6-31+G* and 6-311++G**. The latter larger basis set was used to generate the results reported below. The solution environment was modeled by employing the polarizable continuum model (PCM) with water as the solvent. Standard normal mode analysis and frequency calculations were performed for each stationary structure (minimum or transition state) using the same level of theory and the 6-311++G** basis set. With respect to each transition state, a pre-reaction complex and a post-reaction complex were explored by first carrying out intrinsic reaction coordinate (IRC) calculations along both forward and backward directions using the 6-31+G* basis set. Afterwards, the two complexes around each transition state were reoptimized with the larger 6-311++G** basis set. To compare the energies of various reaction intermediates on the same footing, as listed in Fig. 2 below, the total number of atoms of each reaction step is balanced by adding appropriate small molecules or ions that participate in the reaction to the corresponding side, such as H2O, H3O+, HCl, and HOCl.For estimating the effective rate constant, only the relative free energy between the highest transition state and the reactant matters. This conclusion can be reached by employing canonical transition state theory and statistical analysis (see ESI S46 and S47† for more details). Furthermore, similar analysis using microcanonical transition state theory can also be done.45,46 The energy difference between a transition state and a nearby intermediate is largely irrelevant to the overall rate constant because the numbers cancel out in the expression for the branching ratio. In the context of variational transition state theory,47 a free energy barrier exists and serves as the transition state for the association reaction. This barrier is usually not high. In our case, it is likely lower than the transition state for the isomerization reaction, suggesting a two-step reaction mechanism. In that case, the effective barrier height equals the free energy difference between the second transition state (the saddle point identified in DFT calculations) and the reactants (see eqn (1)–(15) and S48†).
We probed C3-chlorination using C2-methylated derivative 7 as a model, where the mechanistic map of the energies supported the stepwise formation of a chloronium ion, via the formation of a hypochlorous acidium species assisted by an S-heteroatom, in a thermodynamically favoured process (Fig. 2, path M1 → M11). The mechanism involves three transition states (‡TS1–‡TS3) with a rate determining step at the second transition state, where (1) overcoming ‡TS1 (M1 → M5) allows for the association of an S–O bond between hypochlorous acid (HOCl) and the benzothiophene ring, followed by (2) transformation between intermediates M5 and M8 through ‡TS2, which is highlighted by the formation of a hypochlorous acidium ion (H2OCl) in M6, held in close proximity to the benzothiophene ring by an H–S close contact in M7, which (3) leads to chlorination of the C2 carbon in species M8 and (4) completion of the reaction via the formation of a chloronium ion (‡TS3, M9), bridge disassociation initiated by the sulfur atom (M10) and rearomatization to the corresponding product M11. We also considered S-oxidation as a competing process and explored the formation of the sulfone derivative (path M5 → M12 → M16), which occurred via a lower activation energy in ‡TS2a. This is in agreement with experimental results, where oxidation is favoured at room temperature, through a kinetically favoured process that results in the stepwise oxidation of the sulfur atom from hypochlorous acid.
It is interesting that, in the formation of the chloronium ion, our model shows attack by carbon-2 of the benzothiophene ring, instead of the presumably more electron rich C-3 position (via electron delocalization from the sulfide). This may be explained by the possible association of the sulfur atom with a water molecule and electron induction from the methyl group that results in the attack of the chlorine cation by the carbon atom at the C2-position. Furthermore, the formation of a chloronium ion bridge can be inferred thanks to the finding of transition structure ‡TS3, in the exploration of the potential energy surface. In this instance, the relevant structure displays equal bond distances within the C2–Cl–C3 bridge, in close agreement with distances reported elsewhere,48 followed by ring opening assisted by the sulfur atom (M10). Another important aspect is the formation of a hypochlorous acidium ion as the source for the chlorination reaction, which has been previously studied in the electrophilic aromatic substitution of anisole.49 Calculations were also carried out using acetonitrile as solvent, using the minimum amount of water or additional water molecules to obtain comparable values (see Tables S1 and S2†).
With this information in hand, we decided to use the same methodology to probe the reaction using benzothiophene derivative 3, containing a formyl group at the C2-position, which did not participate (experimentally) in the C3-chlorination process. As illustrated in Fig. S37,† unlike methylated derivative 8, the presence of the carbonyl group prevented the formation of the hypochlorous acidium ion, via H-bonding between the carbonyl oxygen of the aldehyde and the H2O+Cl− species, resulting in the formation of hypochlorite. It is plausible that this process also leads to other oxidation reactions at the carbonyl site. Although this model supports the experimental results, we were puzzled by the fact that alcohol derivative 1 proceeds to chlorination, suggesting that the hydroxyl group does not impede the formation of the acidium ion. To confirm this hypothesis, we carried out calculations using benzothiophene-2-methanol 1 as the starting material (Fig. S38†). Gratifyingly, analysis of the computed mechanism showed no interaction between the hydroxyl group and hypochlorous acid, which allowed for the formation of H2O+Cl− and eventual chlorination (as observed experimentally). Furthermore, the same result was obtained upon substitution of the hydroxyl group with a methyl ether (Fig. S39†), which suggests that the inhibition of the reactivity may occur when the oxygen atom is within a carbonyl group.
We then carried out the same analysis with the benzofuran analog of 8 (Fig. S40†), where a highly exothermic reaction was observed. In this case, the barrier to the formation of the first transition state was two times higher than that observed for the benzothiophene derivative, corroborating the high reactivity of this species, consistent with the high exothermicity of the reaction observed experimentally, along with the formation of a benzofuryl peroxy species with hypochlorite. Another interesting aspect was the formation of an unexpected species, with the oxidation of the aromatic ring, which is consistent with experimental results and the formation of unidentified side-products.
Discussion
The C3-chlorination of C2-substituted benzo[b]benzothiophene derivatives is reported, and the mechanism is rationalized using DFT. In addition, the scope of the reaction along with advantages/disadvantages over other chlorinating agents, namely NCS, was explored. The proposed mechanism (Scheme 2) involves the formation of a hydroxylated sulfide intermediate (I → II), followed by the formation of a hypochlorous acidium ion (II → III), which serves as the chloronium source in an electrophilic aromatic substitution (III → IV) that leads to the subsequent formation of a bridged chloronium ion intermediate, S-assisted migration of the chlorine atom to the C3-position (IV → V) and final rearomatization to the chlorinated product (V → VI). In contrast to our findings (albeit in oxidation reactions), most reports to date have proposed reaction mechanisms where the sulphur atom acts as a nucleophile on the chlorine atom of hypochlorous acid, which eventually results in S-oxidation26,27 or ketone formation via Cl attack by an oxygen atom within an ether group.20 However, the formation of a hypochlorous acidium ion is not unprecedented, where (1) chloronium delivery from H2OCl was proposed previously (although the chlorinium ion proposed therein is not likely to be formed)49,50 and (2) in a different reaction, an intermediate involving a tetrahedral intermediate with a hypochlorous acidium ion was observed in the chlorination of amine-boranes.51 Furthermore, a more recent report described the chlorination of betanin in the presence of hypochlorous acid via the formation of a hypochlorous acidium ion at pH 3–5, thus supporting the findings described herein.52 While sodium hypochlorite can lead to different species, hypochlorous acid is the active species in this work. The measured pH of the reactions that were carried out, with heat, was recorded to be around 4–5 (using pH-indicator paper), which is in the optimal range for the presence of HOCl as the predominant species.53 In addition, sodium hypochlorite at temperatures 50–60 °C has shown that <1% chlorite is generated,54 rendering this species irrelevant. In other instances, NaOCl was used as a chlorinating agent, where it was shown that chlorine (Cl2) is not the source.52With regard to the scope of the reaction, the ability of sodium hypochlorite to function as an effective oxidant poses limitations for its use as a chlorinating agent; the studies reported herein suggest that the presence of a benzylic alcohol (or other alcohols, based on previous reports) can result in their oxidation as a competing process. Chlorination reactions at the benzylic position (heterobenzylic position in this case) may also occur. The presence of a furan ring can lead to highly exothermic reactions, and the presence of a formyl group 2 atoms away from the sulfur can diminish the reactivity. On the other hand, unlike the observed reactivity with NCS, the C3-chlorination of benzothiophene can be selective in the presence of olefins. The best results were obtained with the allyl, methyl, methyl alcohol, and in particular the –OTBDMS derivative(s).
Conclusion
A novel way to chlorinate C2-functionalized derivatives of benzothiophene was discovered, and its corresponding plausible reaction mechanism was probed chemically and using DFT methods. The outcome of the reaction was compared to that of NCS to establish the advantages and disadvantages of both. This expands the reactivity of sodium hypochlorite pentahydrate and its potential use in the C3-chlorination of benzothiophene derivatives, which can in turn be of use broadly, e.g., in the synthesis of materials and pharmacophores.Experimental
General information
Pyridine, methylene chloride, and dimethyl formamide were distilled over calcium hydride. n-Butanol was distilled over magnesium sulfate and stored over molecular sieves (4 Å). Tetrahydrofuran was distilled over sodium and benzophenone. All other reagents were used as purchased without further purification. 1H NMR and 13C NMR spectra were recorded at 300 and 75 MHz, respectively. IR spectra were recorded on a diamond ATR sampler using powders or oils of pure materials. Gas chromatography-mass spectrometry (GC-MS) analyses were carried out using a Hewlett Packard 6890 series GC equipped with an HP mass selective detector using an Agilent J&W DB-5 ms Ultra Inert GC column (30 m, 0.25 mm, 0.25 µm, and 5 inch cage) with an injection volume of 1 μL, a solvent delay of 3 min, and an initial temperature of 40 °C with a ramp of 20 °C min−1 to a maximum of 260 °C using helium gas with a pressure of 16.08 psi. Analyses of all intermediates and compounds for HRMS were carried out on a Waters AP-GC-TOF instrument equipped with an atmospheric pressure source and ran in both dry and wet methanol infusion modes. Melting points were recorded in triplicate on a Digit Melt MPA160 (0–250 °C) or Mel-Temp 1101D (0–400 °C) apparatus. Experimental procedures and full characterization of all compounds reported in this work are available within the ESI.†(A) General procedure for chlorination in the presence of sodium hypochlorite pentahydrate. A solution of the desired benzothiophene in acetonitrile (0.5 M, 25 mL) was prepared and heat (70 °C) was applied, with stirring, followed by the addition of an aqueous solution of sodium hypochlorite pentahydrate (2.67 M, 10 mL). The solution was stirred for 20 min, followed by the addition of a second portion of an NaOCl·5H2O aqueous solution (2.5 M, 2.5 mL) with vigorous stirring. The biphasic solution was cooled to room temperature and partitioned in water (50 mL) and methylene chloride (50 mL). The aqueous layer was washed with methylene chloride (2 × 20 mL). The organic residues were combined to wash with brine (30 mL), dried over sodium sulfate, concentrated under reduced pressure and purified via column chromatography.
(B) General procedure for chlorination in the presence of sodium hypochlorite pentahydrate at room temperature. A solution of the desired benzothiophene was prepared in acetonitrile (0.5 M, 25 mL), with stirring, followed by the addition of an aqueous solution of sodium hypochlorite pentahydrate (2.67 M, 10 mL). The solution was stirred for 48 h and partitioned in water (50 mL) and methylene chloride (50 mL). The aqueous layer was washed with methylene chloride (2 × 20 mL). The organic residues were combined to wash with brine (30 mL), dried over sodium sulfate, concentrated under reduced pressure and purified via column chromatography.
(C) General procedure for chlorination in the presence of sodium hypochlorite pentahydrate in the absence of solvent (neat). A round bottom flask was charged with the desired benzothiophene derivative and 5 molar equivalents of sodium hypochlorite pentahydrate. The reaction flask was submerged in a pre-heated oil bath (75 °C), with stirring for 1 h, followed by the addition of 2 mol eq. of sodium hypochlorite pentahydrate and stirring of the oil for an additional 1 h. The flask was cooled to rt and partitioned in water (20 mL) and ethyl acetate (20 mL). The aqueous layer was extracted with ethyl acetate (2 × 10 mL). Organic fractions were combined, washed with brine (20 mL), dried over sodium sulfate, concentrated under reduced pressure and purified via flash column chromatography.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 (2 g, 12.2 mmol) was used, followed by purification via flash column chromatography using a gradient from hexanes to 2% ethyl acetate in hexanes. The first collected fraction yielded chlorinated benzothiophene 2 in the form of a colorless oil (0.72 g, 3.7 mmol, 30%). 1H NMR (400 MHz, CDCl3): δ 10.35 (s, 1H), 8.02 (d, J = 8 Hz, 1H), 7.87 (d, J = 8 Hz, 1H), 7.61–7.5 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 184.7, 143.4, 142.7, 138.6, 128.2, 126.3, 125.3, 123.4; IR (neat): 2954, 2921, 2868, 1705, 1665 cm−1; bp: 106–108 °C; GC-MS: 195, 168, 132, 89; HRMS calcd for C9H5ClOS, 195.9750, obtained 195.9737.
31 (2 g, 12.2 mmol) was used, followed by purification via flash column chromatography using a gradient from hexanes to 2% ethyl acetate in hexanes. The first collected fraction yielded chlorinated benzothiophene 2 in the form of a colorless oil (0.72 g, 3.7 mmol, 30%). 1H NMR (400 MHz, CDCl3): δ 10.35 (s, 1H), 8.02 (d, J = 8 Hz, 1H), 7.87 (d, J = 8 Hz, 1H), 7.61–7.5 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 184.7, 143.4, 142.7, 138.6, 128.2, 126.3, 125.3, 123.4; IR (neat): 2954, 2921, 2868, 1705, 1665 cm−1; bp: 106–108 °C; GC-MS: 195, 168, 132, 89; HRMS calcd for C9H5ClOS, 195.9750, obtained 195.9737.
Author contributions
V. C. O., P. L., and M. J. E. R. contributed to the experimental work. C. T., M. J. E. R., and H. W. contributed to the theoretical work. H. W. and M. J. E. R. wrote the manuscript.Data availability
The ESI† includes (1) experimental procedures and full characterization of all compounds reported in this work, (2) reaction coordinate maps for the hypochlorite reaction of derivatives 1, 3, and 7, and (3) XYZ coordinates of all structures of interest.Although the submitted materials contain all available data, additional pertinent data can be made readily available upon request.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. L. would like to acknowledge Organic Syntheses Corp. for support via a grant for primarily undergraduate institutions. The contributions of Mr John Pham, also supported by the Organic Syntheses Corp., as well as those of Mr Jacobo Guevara [supported via the RMSREE program (R25EB029342)], are acknowledged. M. J. E. R. acknowledges support from NIGMS via 2R15GM132816-02. This work was also supported by a Teacher-Scholar Award (M. J. E. R.), TH-21-028, from the Henry Dreyfus Foundation. HW acknowledges the support from the National Science Foundation CHE-1954639. This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number ACI-1548562.References
- S. Nagorny, M. Schewe, T. Weingartz, A. Eitzeroth, J. Adams, C. Rembe and A. Schmidt, Mater. Adv., 2024, 5, 159 RSC.
- R. S. Keri, K. Chand, S. Budagumpi, S. B. Somappa, S. A. Patil and B. M. Nagaraja, Eur. J. Med. Chem., 2017, 138, 1002 CrossRef CAS.
- H. Singh, A. Thirupathi, B. Das, M. Janni, R. Kumari, S. Singh, M. Rashid, M. Wahajuddin, M. M. Balamurali, K. Jagavelu and S. Peruncheralathan, J. Med. Chem., 2022, 65, 120 CrossRef CAS.
- S. Singh, S. Nerella, S. Pabbaraja and G. Mehta, J. Org. Chem., 2021, 86, 12093 CrossRef CAS.
- S. Hirazawa, Y. Saito, M. Sagano, M. Goto and K. Nakagawa-Goto, J. Nat. Prod., 2022, 85, 136 CrossRef CAS.
- P. Čapek, Y. Zhang, D. J. Barlow, K. L. Houseknecht, G. R. Smith and T. J. Dickerson, ACS Chem. Neurosci., 2011, 2, 288 CrossRef.
- P. J. Masih, T. Kesharwani, E. Rodriguez, M. A. Vertudez, M. L. Motakhaveri, T. K. Le, M. K. Tran, M. R. Cloyd, C. T. Kornman and A. M. Phillips, Pharmaceuticals, 2022, 15, 39 CrossRef CAS.
- G. Naganagowda and A. Petsom, J. Sulphur Chem., 2011, 32, 223 CrossRef CAS.
- M. C. Bagley, J. E. Dwyer, M. D. B. Molina, A. W. Rand, H. L. Rand and N. C. O. Tomkinson, Org. Biomol. Chem., 2015, 13, 6814 RSC.
- C. K. Jalakanki, N. Taxak, R. A. Varikoti and P. V. Bharatam, Chem. Res. Toxicol., 2015, 28, 2364 Search PubMed.
- J. A. Joule and K. Mills, in Heterocyclic Chemistry, Blackwell Publishing Ltd., 5th edn, 2010, ch. 21, and references therein Search PubMed.
- H. J. Shrives, J. A. Fernández-Salas, C. Hedtke, A. P. Pulis and D. J. Procter, Nat. Commun., 2017, 8, 14801 Search PubMed.
- R. Mancuso, S. Cuglietta, R. Strangis and B. Gabriele, J. Org. Chem., 2023, 88, 5180 CrossRef CAS.
- T. Matsuzawa, T. Hosoya and S. Yoshida, Chem. Sci., 2020, 11, 9691 RSC.
- B. Wu and N. Yoshikai, Org. Biomol. Chem., 2016, 14, 5402 RSC.
- T. Okada, T. Asawa, Y. Sugiyama, M. Kirihara, T. Iwai and Y. Kimura, Synlett, 2014, 25, 596 CrossRef CAS.
- T. Hirashita, Y. Sugihara, S. Ishikawa, Y. Naito, Y. Matsukawa and S. Araki, Synlett, 2018, 29, 2404 CrossRef CAS.
- Z. R. Bright, C. R. Luyeye, A. S. M. Morton, M. Sedenko, R. G. Landolt, M. J. Bronzi, K. M. Bohovic, M. W. A. Gonser, T. E. Lapainis and W. H. Hendrickson, J. Org. Chem., 2005, 70, 684 CrossRef CAS.
- N. Fukuda, T. Kajiwara, T. Katou, K. Majima and T. Ikemoto, Synlett, 2013, 24, 1438 CrossRef CAS.
- S. O. Nwaukwa and P. M. Keehn, Tetrahedron Lett., 1982, 23, 35 CrossRef CAS.
- R. V. Stevens and K. T. Chapman, Tetrahedron Lett., 1982, 23, 4647 CrossRef CAS.
- A. B. Leduc and T. F. Jamison, Org. Process Res. Dev., 2012, 16, 1082 CrossRef CAS.
- H. Veisi, Sodium Hypochlorite (NaOCl); Spotlight 215, Synlett, 2007, 2607 CrossRef CAS.
- M. W. Farrar and R. Levine, J. Am. Chem. Soc., 1950, 72, 4433 CrossRef CAS.
- S. Mitsumori, T. Tsuri, T. Honma, Y. Hiramatsu, T. Okada, H. Hashizume, S. Kida, M. Inagaki, A. Arimura, K. Yasui, F. Asanuma, J. Kishino and M. Ohtani, J. Med. Chem., 2003, 46, 2446 CrossRef CAS.
- M. Kirihara, S. Yamahara, T. Okada, H. Matsumuro, Y. Kinoshita, A. Kitajima, Y. Takamura, T. Odagiri, T. Asawa, Y. Sugiyama and Y. Kimura, Synthesis, 2022, 54, 4120 CrossRef CAS.
- M. Kirihara, T. Okada, Y. Sugiyama, M. Akiyoshi, T. Matsunaga and Y. Kimura, Org. Process Res. Dev., 2017, 21, 1925 CrossRef CAS.
- A. R. Katritsky, G. R. Khan and D. E. Leahy, J. Org. Chem., 1984, 49, 4784 CrossRef.
- N. Kimpe and N. Schamp, Org. Prep. Proced. Int., 1981, 13, 241 CrossRef.
- P. J. Gilissen, D. Blanco-Ania and F. P. J. T. Rutjes, J. Org. Chem., 2017, 82, 6671 CrossRef CAS.
- B. Leforestier and M. Vögtle, Synlett, 2016, 27, 1957 CrossRef CAS.
- I. Neogi, P. J. Das and F. Grynszpan, Synlett, 2018, 29, 1043 CrossRef CAS.
- M. De Rosa, J. Org. Chem., 1982, 47, 1008 CrossRef CAS.
- P. Geneste, J. L. Olivé and N. Ung, J. Heterocycl. Chem., 1977, 14, 449 CrossRef CAS.
- A. Eggert, K. T. Schuppe, H. L. S. Fuchs, M. Brönstrup and M. Kalesse, Org. Lett., 2024, 26, 2893 CrossRef CAS.
- E. Ruggles and R. E. Maleczka Jr., Org. Lett., 2002, 4, 3899 CrossRef CAS.
- S. Eiβler, A. Stoncius, M. Nahrwold and N. Sewald, Synthesis, 2006, 3747 Search PubMed.
- Y. Shi, B. Yang, Y. Yang, L. Yan, F. Yang, J. Liu, X. Liao, P. Yu, Z. Bin and J. You, J. Am. Chem. Soc., 2021, 143, 21066 CrossRef CAS.
- J. Youn, M.-C. Chen, Y.-J. Liang, H. Huang, R. P. Prtiz, C. Kim, C. Stern, T.-S. Hu, L.-H. Chen, J.-Y. Yan, A. Facchetti and T. J. Marks, Chem. Mater., 2010, 22, 5031 CrossRef CAS.
- C. O'Hara, C.-H. Yang, A. Francis, B. Newell, H. Wang and M. J. E. Resendiz, J. Org. Chem., 2019, 84, 9714 CrossRef.
- M. J. Mintz and C. Walling, Org. Synth., 1973, 5, 184 Search PubMed.
- C. A. Fleckenstein and H. Plenio, Chem. – Eur. J., 2008, 14, 4267 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 Search PubMed.
- H. Wang and W. L. Hase, J. Am. Chem. Soc., 1995, 117, 9347–9356 CrossRef CAS.
- H. Wang and W. L. Hase, J. Am. Chem. Soc., 1997, 119, 3093 CrossRef CAS.
- D. G. Truhlar and B. C. Garrett, Acc. Chem. Res., 1980, 13, 440 CrossRef CAS.
- E. S. Stoyanov and I. V. Stoyanova, Int. J. Mol. Sci., 2022, 23, 9111 CrossRef CAS.
- C. G. Swain and D. L. R. Crist, J. Am. Chem. Soc., 1972, 94, 3195 CrossRef CAS.
- P. W. G. Smith and A. R. Tatchell, VII - The Olefins, in Fundamental Aliphatic Chemistry, Pergamon, 1965, pp. 111–128. ISBN 9780080107462 Search PubMed.
- H. Kelly and S. C. Yasui, Inorg. Chem., 1984, 23, 2220 CrossRef CAS.
- S. Wybraniec, K. Starzak and Z. Pietrzkoski, J. Agric. Food Chem., 2016, 64, 2865 CrossRef CAS.
- R. K. B. Karlsson and A. Cornell, Chem. Rev., 2016, 116, 2982 CrossRef CAS.
- M. W. Lister, Can. J. Chem., 1956, 34, 465 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob01185f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |



