 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two-step access to bis-meso-perfluoroalkyl-corroles towards meso-perfluoroacyl-ABC-corroles†
Paul-Gabriel
Julliard
 a,
Simon
Pascal‡
a,
Simon
Pascal‡
 a,
Olivier
Siri
a,
Olivier
Siri
 a,
Michel
Giorgi
a,
Michel
Giorgi
 b,
Diego
Cortés-Arriagada
b,
Diego
Cortés-Arriagada
 c,
Luis
Sanhueza
*de and
Gabriel
Canard
c,
Luis
Sanhueza
*de and
Gabriel
Canard
 *a
*a
aAix Marseille Univ., CNRS, CINAM, UMR 7325, Centre Interdisciplinaire de Nanoscience de Marseille, Campus de Luminy, 13288 Marseille cedex 09, France. E-mail: gabriel.canard@univ-amu.fr
bAix Marseille Univ., CNRS, FSCM, Spectropole, Marseille, France
cInstituto Universitario de Investigación y Desarrollo Tecnologico, Universidad Tecnológica Metropolitana, Ignacio Valdivieso 2409, San Joaquín, Santiago, Chile
dDepartamento de Ciencias Biológicas y Químicas, Facultad de Recursos Naturales, Universidad Católica de Temuco, Temuco, Chile
eNúcleo de Investigación en Bioproductos y Materiales Avanzados (BioMA), Université de Nantes, CEISAM UMR 6230, CNRS, Nantes F-44000, France
First published on 7th February 2024
Abstract
A solventless and acid-catalyzed condensation of meso-perfluoroalkyl-dipyrromethanes with selected benzaldehydes was used to prepare ten different bilanes that were isolated before their oxidation into trans-A2B-corroles bearing two meso-perfluoroalkyl groups. Macrocycles bearing long chains (C3F7 or C7F15) are key precursors to afford ABC-corroles having a meso-acyl substituent when subjected to a mild and basic hydrolysis affecting one of the alkyl substituents.
Corrole, the contracted homologue of the porphyrin macrocycle, is a very popular member of the porphyrinoid family and its derivatives and/or metal complexes are now used in numerous fields including, for example, sensors,1 medicine,2,3 catalysis4 or the activation of small molecules.5,6 This growing interest in the last two decades relies on the description of efficient synthetic procedures that, in a few synthetic steps, produce corroles in good yields from commercially available reagents.7 Nevertheless, these efficient methods are mainly devoted to the preparation of corroles bearing aryl groups on their three meso-positions, which can be further functionalized and used to build simple to very sophisticated structures.8,9 In contrast, the access to such macrocycles having a free meso position or bearing either meso-alkyl or meso-functional groups is rarely described and it usually gives low cyclization yields.10 Because of their strong electron-withdrawing character, meso-perfluoroalkyl chains would be particularly attractive to increase the electro-catalytic activity of several metallocorroles whereas their lipophilicity may play a role when using such derivatives in biological studies.11 The first example of a corrole bearing such a kind of substitution was the rhenium(V) complex of corrole 1 which was obtained through a porphyrin ring contraction (Fig. 1).12 The access to the corresponding free base 1 was only described a decade later with a yield of 0.65%, which was increased recently and significantly up to 6% following a two-step procedure requiring the isolation of the intermediate bilane before the oxidation step.13 Only the three other free bases 2–4 complete the rare examples of meso-perfluoroalkyl-corroles bearing perfluoroalkyl chains. A one-pot procedure was used to obtain 1% yield of 2 bearing three meso-heptafluoroalkyl chains whereas syntheses starting from meso-trifluoromethyl dipyrromethane (meso-CF3-DPM) afforded 3 and 4 with respective yields of 5 and 3%.14
In the course of our research relying on the use of metallocorroles in electro-catalysis,15 we are focusing on the preparation of trans-A2B-corroles bearing two perfluoroalkyl chains on the 5,15 meso positions whereas the remaining one occupied by an aryl group is a potential platform for the introduction of grafting moieties and/or functional groups. Starting from meso-CF3-DPM and 4-cyano-benzaldehyde, most of the previously reported synthetic procedures of meso-substituted corroles failed to give us more than traces of the expected corrole. Inspired by the recently optimized access to corrole 1,13 and knowing that the bilane leading to corrole 3 was previously produced by a solventless procedure,14b we have investigated the solvent-free formation of bilane from meso-CF3-DPM16 and 4-cyano-benzaldehyde.
Different acids, temperatures and reaction times were used for the condensation of stoichiometric amounts of 4-cyano-benzaldehyde and meso-CF3-DPM 5a (Table S1, see the ESI†). Although the highest isolated yield of bilane 6a (30%) was produced using 0.5 equivalents of propionic acid overnight at room temperature (RT), we chose to replace it with trifluoroacetic acid because it leads to 6a with a similar yield (27%) in less than 5 minutes (Table S1, see the ESI†). Using these experimental conditions and starting from DPM 5a–c16,17 and four benzaldehyde derivatives, we obtained ten bilanes (6a–j) with isolated yields ranging from 11 to 40% (Table 1). Because the use of expensive PIFA as the oxidizing agent did not improve the cyclization yield of 7a (17%), the classical oxidation procedure (DDQ, DCM, RT) was applied to these intermediates and it produced 9 to 33% yields of the ten corroles 3 and 7a–i for which the meso-perfluoroalkyl moieties have one, three or seven carbon atoms (Table 1).
| DPM | Alk | Ar | Bilanes (yields) | Corroles (yields) |
|---|---|---|---|---|
| 5a | CF3 | p-CN-C6H4 | 6a (26%) | 7a (18%) |
| CF3 | p-CH3-C6H4 | 6b (19%) | 7b (13%) | |
| CF3 | p-CH3O-C6H4 | 6c (40%) | 7c (17%) | |
| 5b | C3F7 | p-CN-C6H4 | 6d (25%) | 7d (33%) |
| C3F7 | p-CH3-C6H4 | 6e (17%) | 7e (6%) | |
| C3F7 | p-CH3O-C6H4 | 6f (11%) | 7f (24%) | |
| 5c | C7F15 | p-CN-C6H4 | 6g (13%) | 7g (7%) |
| C7F15 | p-CH3-C6H4 | 6h (18%) | 7h (11%) | |
| C7F15 | p-CH3O-C6H4 | 6i (15%) | 7i (14%) | |
| 5a | CF3 | C6F5 | 6j (13%) | 3 (9%) |
It was previously described that hydrolysis of the meso-CF3 substituents of corrole 1 afforded the corresponding corrole bearing three meso-CO2H groups. No reaction occurred when mild experimental conditions (THF, aq. NaOH, 20 mM, RT, 12 h) were used to perform the hydrolysis of the meso-CF3 of 7a, whereas the ABC corroles 8a and 8b bearing a meso-keto group were produced starting from 7d and 7g bearing longer alkyl chains (Scheme 1). The modest 32–35% yields result from the slow reactivity of 7d and 7g which are still present in the mixture after 12 hours. The stronger conditions (THF, sat. aq. NaOH, reflux, 12 h) applied to 7a led to corrole 9 not only bearing the two expected meso-CO2H substituents but also an extra carboxylic group resulting from the hydrolysis of the cyano group of the aryl moiety (Scheme 1). A higher yield of corrole 9 (60%) was also obtained when trying to obtain meso-bis-keto corroles by applying strong experimental hydrolyzing conditions to corrole 7d.
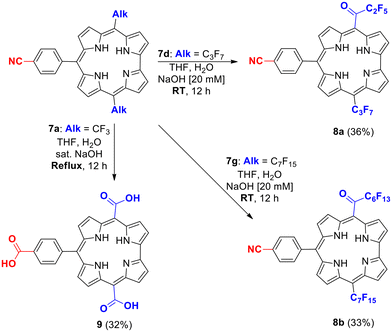 | ||
| Scheme 1 Hydrolysis of the perfluoroalkyl chains of corroles 7a, 7d and 7g leading to AB2 corrole 9 and ABC corroles 8a and 8b. | ||
The impact of the meso-perfluoro alkyl and meso-perfluoro acyl chains on the physico-chemical properties of corroles 3, 7a–i, 8a, 8b and 9 was first studied by single crystal X-ray diffraction and electronic absorption and emission spectroscopy supported by DFT and TD-DFT calculations. As was found for the previously reported single crystal X-ray diffraction structures of corroles 1,13a2,14a314b and 4,14c the ones of corroles 7a, 7b, 7c, 7d and 7i do not feature a particularly enhanced out of-plane distortion of the macrocycle brought by the meso-alkyl substituents regardless of their lengths (Fig. 2 and Fig. S1–S5 in the ESI†). As commonly observed in corrole free base structures, the deformation of the aromatic macrocycle is mainly due to the steric repulsion of the inner hydrogen atoms.
However, this distortion has a higher amplitude in the structure of 7d in which the inner hydrogen atoms are borne by the pyrrole rings A, B and D whereas the other tautomeric form is observed in the other structures. In the five structures, hydrogen bonds involving the inner hydrogen and nitrogen atoms assemble the macrocycles into dimers that are differently packed into columns depending on the length of the alkyl chains or the presence of solvent molecules.
As for corrole 3, the UV-visible absorption spectra of meso-perfluoroalkyl corroles 7a–i feature a split Soret band in the near UV and three Q bands in the visible range (Fig. 3 and Fig. S4–S8 in the ESI†). Excitation in the Q band domain (λ = 550 nm) produces a relatively intense and structured emission in the far-red region (Table 2, Fig. 3 and Fig. S4–S8 in the ESI†) with quantum yields of ca. 10%. Increasing the length of the meso-alkyl chain produces only a trifling bathochromic shift of some of the bands displayed in the absorption and emission spectra (Table 2). In contrast, after hydrolysis, the absorption and emission spectra of the meso-keto corroles 8a and 8b are significantly red-shifted compared to those of the starting compounds 7d and 7g (Fig. 3 and Fig. S2–S4 in the ESI†). This bathochromic shift is also accompanied by substantial quenching of their fluorescence (Table 2).
 | ||
| Fig. 3 Electronic absorption (black) and normalized corrected emission spectra (red) of corroles 7g (solid lines) and 8b (dashed lines) in aerated dichloromethane at room temperature. | ||
| Corroles | Absorption dataa | Fluorescence dataa | Electrochemical datab | |||
|---|---|---|---|---|---|---|
| Soret bands λmax (nm) (ε (10−4 M−1 cm−1)) | Q bands λmax (nm) (ε (10−4 M−1 cm−1)) | λ max (nm)c | Φ | Reduction (V) | Oxidation (V) | |
| a Recorded in air-equilibrated dichloromethane. b Peak potentials measured in dichloromethane containing 0.1 M [(nBu4N)PF6] (scan rate of 100 mV s−1). c λ max for the bands derived from corrected emission spectra. d Luminescence quantum yields obtained using tetraphenylporphyrin (TPP) in aerated acetonitrile as a standard (Φ = 0.15).20 e Recorded in air-equilibrated methanol. | ||||||
| 3 | 400 (14.74), 417 (13.55) | 547 (1.92), 563 (1.39), 611 (1.91) | 628, 681 | 0.09 | −0.64 | 1.07, 1.22, 1.45 |
| 7a | 402 (10.67), 420 (10.43) | 513 (1.00), 549 (1.71), 614 (1.34) | 639, 697 | 0.09 | −0.55 | 0.96, 1.20, 1.41 |
| 7b | 402 (13.25), 420 (12.12) | 516 (1.16), 550 (2.16), 617 (1.58) | 642, 696 | 0.09 | −0.76 | 0.86, 1.04, 1.29 |
| 7c | 403 (10.62), 420 (10.57) | 513 (1.10), 550 (1.74), 615 (1.45) | 644, 691 | 0.09 | −0.86 | 0.83, 1.02, 1.24 |
| 7d | 404 (12.34), 420 (12.53) | 514 (1.07), 551 (1.94), 617 (1.47) | 637, 695 | 0.12 | −0.73 | 0.98, 1.18, 1.44 |
| 7e | 404 (12.54), 419 (11.63) | 515 (1.33), 552 (2.36), 620 (1.73) | 641, 696 | 0.14 | −0.59 | 0.90, 1.03, 1.27 |
| 7f | 405 (13.50), 420 (12.10) | 514 (1.14), 552 (2.07), 620 (1.50) | 644, 699 | 0.14 | −0.80 | 0.87, 1.02, 1.21 |
| 7g | 406 (11.73), 420 (11.89) | 515 (1.16), 552 (1.96), 618 (1.50) | 637, 695 | 0.12 | −0.72 | 0.98, 1.19, 1.36 |
| 7h | 405 (11.01), 420 (10.20) | 516 (1.40), 553 (2.15), 621 (1.59) | 641, 695 | 0.13 | −0.65 | 0.94, 1.05, 1.27 |
| 7i | 407 (12.53), 420 (11.26) | 516 (1.40), 553 (2.15), 621 (1.59) | 644, 698 | 0.15 | −0.59 | 0.91, 1.01, 1.22 |
| 8a | 429 (9.37) | 589 (1.81), 665 (0.93) | 688 | 0.02 | −0.34 | 1.02, 1.22 |
| 8b | 428 (9.24) | 583 (1.84), 653 (0.90) | 693 | 0.03 | −0.65 | 1.04, 1.20, 1.37 |
| 9 | 414 (4.41)e | 574 (0.74), 639 (0.54)e | 669e | 0.02e | ||
These trends were confirmed by DFT calculations performed on corroles 7a, 7d, 7g, 8a and 8b (see the ESI†). Increasing the lengths of the meso-perfluoroalkyl chain slightly stabilizes the HOMO and the LUMO energy levels of 7a, 7d and 7g, which have comparable HOMO–LUMO gaps (Fig. S14 in the ESI†). These calculations reveal the role of the acyl moieties, which contribute to the distribution of the electronic density of the LUMO of 8a, 8b, inducing noticeably reduced HOMO–LUMO gaps compared to those of 7a, 7d and 7g (Fig. S14 in the ESI†). TD-DFT methods gave convoluted UV-visible absorption spectra of the five corroles, which are in good agreement with the experimental ones and confirmed the impact of the acyl group on the red-shifted absorption spectra of 8a, 8b compared to those of their parent chromophores 7d and 7g (Fig. S16–S21 in the ESI†).
Next, cyclic voltammetry experiments conducted on all the corroles also gave evidence of the respective electron-withdrawing strength of the meso-alkyl and meso-acyl chains (Fig. S9 and S10 in the ESI†). Cyclic voltammograms of 3, 7a–i and 8a, 8b feature one irreversible reduction process and up to three irreversible oxidation ones. It was shown that the irreversibility of these redox processes arises from the gain or loss of inner protons when oxidizing or reducing corrole free bases.18 Comparing the peak potentials of the first reduction of each corrole is problematic because it produces very large waves under our experimental conditions (DCM, RT, scan rate of 100 mV s−1).
In the anodic range, a slight shift of 20 mV affects the value of the first oxidation potential when increasing the length of the perfluoro-alkyl group from 7a to 7d and to 7g (Table 2). In the same way, corroles 8a and 8b are harder to oxidize by ca. 50 mV than their parent corroles 7d and 7g (Table 2). The amplitude of these shifts is small but still reflects the difference between Hammett constants of analogous perfluoro alkyl and acyl groups (σp(CF2CF3) = 0.52 < σp(COCF2CF2CF3) = 0.79 ∼ σp(COCF3) = 0.80).19
Metallocorroles are particularly efficient in the electrocatalytic reduction of small molecules, which requires the introduction of strongly electron withdrawing substituents on the macrocycle peripheral positions which decrease the associated activation potentials.6,21 To this purpose, we describe herein a two-step access to corroles displaying two meso-perfluoro-alkyl groups. The formation of the bilane was conducted under acidic neat conditions without any detectable acidolysis/recombination of the polypyrrolic oligomers. After their crucial isolation, the oxidation of these precursors led to the formation of ten corroles substituted with two meso-alkyl groups having one, three or seven carbon atoms. If longer alkyl chains do not tune significantly the physico-chemical properties of the macrocycles, they are of particular interest because their hydrolysis has led to unprecedented ABC-corroles bearing meso-perfluoro acyl groups. Two examples of such derivatives were prepared and characterized, highlighting the electron-accepting strength of such meso-acyl moieties, which are also responsible for red-shifted light absorption and emission properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This contribution comes within the framework of the ECOS Sud-Chile project no. C19E07 supported by the Chilean Agencia Nacional de Investigación y Desarrollo (ANID), the French Ministère de l'Enseignement Supérieur, de la Recherche et de l'Innovation (MESRI) and the French Ministère de l'Europe et des Affaires Étrangères (MEAE). This work was supported in France by the Centre National de la Recherche Scientifique (CNRS) and by the MESRI. PGJ acknowledges the MESRI (PhD grant). LS and DCA are thankful for the computational resources through the CONICYT-FONDEQUIP-EQM180180 and acknowledge ANID/FONDECYT projects 11181187 and 1210355, respectively.Notes and references
- C. Di Natale, C. P. Gros and R. Paolesse, Chem. Soc. Rev., 2022, 51, 1277–1335 RSC.
- R. D. Teo, J. Y. Hwang, J. Termini, Z. Gross and H. B. Gray, Chem. Rev., 2017, 117, 2711–2729 CrossRef CAS PubMed.
- S. M. M. Lopes, M. Pineiro and T. M. V. D. Pinho e Melo, Molecules, 2020, 25, 3450 CrossRef CAS PubMed.
- I. Aviv and Z. Gross, Chem. Commun., 2007, 1987–1999 RSC.
- V. A. Vaillard, P. D. Nieres, S. E. Vaillard, F. Doctorovich, B. Sarkar and N. I. Neuman, Eur. J. Inorg. Chem., 2022, e202100767 CrossRef CAS.
- Y. Li, N. Wang, H. Lei, X. Li, H. Zheng, H. Wang, W. Zhang and R. Cao, Coord. Chem. Rev., 2021, 442, 213996 CrossRef CAS.
- R. Orłowski, D. Gryko and D. T. Gryko, Chem. Rev., 2017, 117, 3102–3137 CrossRef PubMed.
- J. P. Collman and R. A. Decréau, Org. Lett., 2005, 7, 975–978 CrossRef CAS PubMed.
- L. Flamigni and D. T. Gryko, Chem. Soc. Rev., 2009, 38, 1635–1646 RSC.
- (a) J. Sankar, V. G. Anand, S. Venkatraman, H. Rath and T. K. Chandrashekar, Org. Lett., 2002, 4, 4233–4235 CrossRef CAS PubMed; (b) G. Canard, D. Gao, A. D'Aléo, M. Giorgi, F.-X. Dang and T. S. Balaban, Chem. – Eur. J., 2015, 21, 7760–7771 CrossRef CAS PubMed; (c) S. Ooi, T. Yoneda, T. Tanaka and A. Osuka, Chem. – Eur. J., 2015, 21, 7772–7779 CrossRef CAS PubMed; (d) A. Kumar, P. Yadav, M. Majdoub, I. Saltsman, N. Fridman, S. Kumar, A. Kumar, A. Mahammed and Z. Gross, Angew. Chem., Int. Ed., 2021, 60, 25097–25103 CrossRef CAS PubMed.
- (a) K. Sudhakar and P. K. Panda, ACS Appl. Energy Mater., 2022, 5, 13492–13500 CrossRef CAS; (b) H. C. Honig, A. Friedman, N. Zion and L. Elbaz, Chem. Commun., 2020, 56, 8627–8630 RSC; (c) B. E. Smart, J. Fluorine Chem., 2001, 109, 3–11 CrossRef CAS.
- M. K. Tse, Z. Zhang and K. S. Chan, Chem. Commun., 1998, 1199–1200 RSC.
- (a) C. K. E. Thomas, J. Conradie, L. K. Hansen and A. Ghosh, Inorg. Chem., 2011, 50, 3247–3251 CrossRef PubMed; (b) Q.-C. Chen, M. Soll, A. Mizrahi, I. Saltsman, N. Fridman, M. Saphier and Z. Gross, Angew. Chem., Int. Ed., 2018, 57, 1006–1010 CrossRef CAS PubMed; (c) P. Yadav, S. Khoury, A. Mahammed, M. Morales, S. C. Virgil, H. B. Gray and Z. Gross, Org. Lett., 2020, 22, 3119–3122 CrossRef CAS PubMed.
- (a) L. Simkhovich, I. Goldberg and Z. Gross, J. Inorg. Biochem., 2000, 80, 235–238 CrossRef CAS PubMed; (b) R. Goldschmidt, I. Goldberg, Y. Balazs and Z. Gross, J. Porphyrins Phthalocyanines, 2006, 10, 76–86 CrossRef CAS; (c) P. Yadav, S. Khoury, N. Fridman, V. K. Sharma, A. Kumar, M. Majdoub, A. Kumar, Y. Diskin-Posner, A. Mahammed and Z. Gross, Angew. Chem., Int. Ed., 2021, 60, 12829–12834 CrossRef CAS PubMed.
- P.-G. Julliard, M. Hanana, L. Alvarez, R. Cornut, B. Jousselme, G. Canard and S. Campidelli, Energy Fuels, 2023, 37, 684–692 CrossRef CAS.
- (a) W. Dmowski, K. Piasecka-Maciejewska and Z. Urbanczyk-Lipkowska, Synthesis, 2003, 841–844 CrossRef CAS; (b) W. Dmowski, K. Piasecka-Maciejewska and Z. Urbańczyk-Lipkowska, Kem. Ind., 2004, 53, 339–341 CAS.
- P.-G. Julliard, S. Pascal, O. Siri, D. Cortés-Arriagada, L. Sanhueza and G. Canard, C. R. Chim., 2021, 24, 27–45 CrossRef.
- J. Shen, J. Shao, Z. Ou, E. Wembo, B. Koszarna, D. T. Gryko and K. M. Kadish, Inorg. Chem., 2006, 45, 2251–2265 CrossRef CAS PubMed.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- A. M. Brouwer, Pure Appl. Chem., 2011, 83, 2213–2228 CrossRef CAS.
- H. Lei, X. Li, J. Meng, H. Zheng, W. Zhang and R. Cao, ACS Catal., 2019, 9, 4320–4344 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental protocols, 1H and 13C NMR spectra, theoretical details and further DFT/TD-DFT analyses. CCDC 2267223–2267227. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob00191e |
| ‡ Present address: Laboratoire CEISAM, CNRS UMR 6230, Université de Nantes, 2, rue de la Houssinière, 44322 Nantes, France. |
| This journal is © The Royal Society of Chemistry 2024 |



