Synthesis of trifluoroethoxy/aryloxy cinnolines, cinnolinones and indazoles from o-alkynylanilines via metal-free diazotization reagent†
Abstract
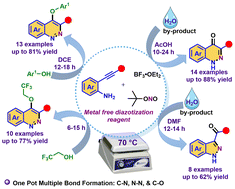
A facile and user-friendly protocol for the synthesis of trifluoroethoxy/aryloxy cinnolines, cinnolinones and indazoles from o-alkynylaniline in good-to-excellent yields has been developed using a metal-free diazotization reagent (a combination of BF3·OEt2 and TBN). The methodology has been further extended to construct bis-cinnolinones and for the chemoselective synthesis of N-propargylated cinnolinones.


 Please wait while we load your content...
Please wait while we load your content...