Open Access Article
Open Access ArticleTiF4-catalyzed direct amidation of carboxylic acids and amino acids with amines†
Abdulkhaliq A.
Alawaed
and
P. Veeraraghavan
Ramachandran
 *
*
Department of Chemistry, Purdue University, West Lafayette, Indiana 47907, USA. E-mail: chandran@purdue.edu
First published on 2nd February 2024
Abstract
Unlike other metal fluorides, catalytic titanium tetrafluoride enhances the direct amidation of aromatic and aliphatic carboxylic acids and N-protected amino acids in refluxing toluene. While aromatic acids were converted to amides with 10 mol% of the catalyst within 24 h, aliphatic acids underwent a faster reaction (12 h), with lower catalyst loading (5 mol%). This protocol is equally efficient with alkyl and aryl amines providing a variety of carboxamides and peptides in 60–99% yields.
Introduction
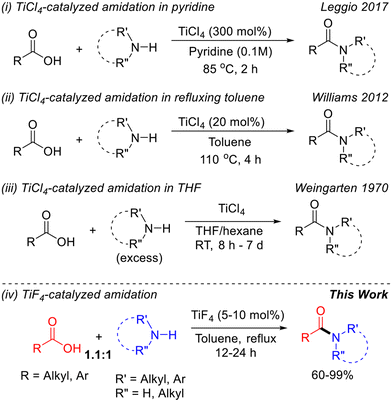
The prevalence of amides in organic molecules and biomolecules has made their preparation, structure, and reactivity a much-discussed subject. The direct amidation of carboxylic acids is the most straightforward protocol to access them, although it is a challenging reaction1 due to the formation of ammonium salt. Dehydration of the salt to an amide can be achieved at high temperatures. For milder reactions, the conversion of the acid to a derivative capable of undergoing facile nucleophilic acyl substitution, such as an acid chloride or anhydride, is opted for. Also, a variety of carbodiimide reagents, such as dicyclohexylcarbodiimide (DCC),2 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC),3 and 1,1′-carbonyldiimidazole (CDI)4 or phosphate agents, such as benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP),5 Castro's reagent (BOP),6 T3P,7 HBTU,8 HATU,9etc. have been developed for the dehydrative coupling of an acid with an amine.10,11 However, in addition to the reagent cost, the need for additional equivalents of amines and excess reagents adds to the difficulties in the separation of the amides from stoichiometric quantities of the side products. Leading the intense activity in developing alternate catalyzed direct amidation protocols12,13 are boron-14 and silicon-based15 catalysts as well as transition metal salts, particularly those of Ti,16–18 Mn,19 Fe,20 Zn,21,22 Zr,17,23,24 In,25 Hf,26 Ta,27,28etc. Indeed, some of these reactions are carried out in sealed tubes and are effective only for aliphatic acids and several of these protocols are inefficient for amidation with anilines.We have recently reported the effect of catalytic titanium tetrachloride (TiCl4) for the borane-amine mediated reduction of ketones,29 acids,30 amides,31 and nitriles,32 deoxygenation of esters to ethers33 and deoxyhalogenation of carbonyl compounds.34 During these projects, we undertook a systematic evaluation of the catalytic ability of several metal halides and established that the reaction depended on the Lewis acidity of these halides and TiCl4 is a critical and superior catalyst. Interested in TiCl4's applicability for amidation, a literature search was undertaken, which led to a recent, impractical process with 300 mol% of TiCl4 in pyridine as the solvent (Scheme 1(i)).18 A prior report comparing several Lewis acids for amidation reported that ZrCl4 is superior to TiCl4 (Scheme 1(ii)).17 The first report from half a century ago using large excess of amines is inconclusive (Scheme 1(iii)).16
Results and discussion
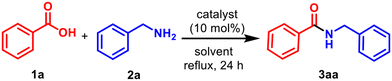
After attempting an amidation of benzoic acid (1a) with benzylamine (2a) without any catalyst in refluxing toluene (24 h, 15% yield; Table 1, entry 1), a similar reaction in the presence of 10 mol% TiCl4 provided 89% yield of the corresponding amide 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 (Table 1, entry 2). TiBr4 was equally effective (91% yield, entry 3). Other group 4 metal chlorides, such as ZrCl4 and HfCl4, were poor amidation catalysts (entries 4 and 5) under similar conditions. Mader and Helquist have reported that titanium isopropoxide (TIPO) is a poor catalyst for intermolecular amidation.36 However, Adolfsson and coworkers reported successful amidation in THF in a sealed tube with 1.5 equiv. of the acid and catalytic TIPO or Ti(n-BuO)4.37 Attempts to scale-up the reaction with TiCl4 and TiBr4 were unrealistic due to the corrosive nature of the reagents, so we turned our attention to TiF4.
35 (Table 1, entry 2). TiBr4 was equally effective (91% yield, entry 3). Other group 4 metal chlorides, such as ZrCl4 and HfCl4, were poor amidation catalysts (entries 4 and 5) under similar conditions. Mader and Helquist have reported that titanium isopropoxide (TIPO) is a poor catalyst for intermolecular amidation.36 However, Adolfsson and coworkers reported successful amidation in THF in a sealed tube with 1.5 equiv. of the acid and catalytic TIPO or Ti(n-BuO)4.37 Attempts to scale-up the reaction with TiCl4 and TiBr4 were unrealistic due to the corrosive nature of the reagents, so we turned our attention to TiF4.
| Entry | LA | Solvent | Yield,a % |
|---|---|---|---|
| a Isolated yields for reactions using 1.1 equiv. of 1a and 1.0 equiv. of 2a. b 1.0 equiv. of carboxylic acid. c 5 mol% of TiF4 used. d Reaction at 80 °C. e From ref. 43 in refluxing benzene (100 h) in the presence of Et3N. NR = No reaction. | |||
| 1 | None | Toluene | 15 |
| 2 | TiCl4 | Toluene | 89 |
| 3 | TiBr4 | Toluene | 91 |
| 4 | ZrCl4 | Toluene | 45 |
| 5 | HfCl4 | Toluene | 47 |
| 6 | TiF4 | Toluene | 77b |
| 7 | TiF4 | Toluene | 96 |
| 8 | TiF4 | Toluene | 28c |
| 9 | TiF4 | Toluene | NRd |
| 10 | TiF4 | Xylene | 69 |
| 11 | TiF4 | Benzene | NRc |
| 12 | TiF4 | Cyclohexane | NRc |
| 13 | TiF4 | THF | NRc |
| 14 | TiF4 | CH3CN | NRc |
| 15 | BF3-Et2O | Toluene | 79 (83)e |
| 16 | ZnF2 | Toluene | 43 |
| 17 | ZrF4 | Toluene | 41 |
We had observed during our earlier-mentioned deoxyhalogenation of carbonyls34 using TiCl4 and TiBr4 that the corresponding fluoride did not participate in the nucleophilic transfer of the fluoride. This led us to believe that TiF4 might be a stable catalyst for Lewis acid-mediated reactions. Surprisingly, there are only scarce reports on direct amidation using metal fluorides as catalysts. Metal fluoride salts, such as ZnF2 and AlF3, are ineffective compared to chlorides for the formylation of amines.21 Also, a recent report on the screening of different fluoride salts such as CsF, NaF, KF, AgSbF6, and KPF6 as promoters for a similar esterification produced poor results with all of them, except KPF6.38 The hexafluorophosphate was effective for amidation as well.38 Our long-time interest in the chemistry of fluorinated molecules stimulated us to examine TiF4 for the direct amidation of carboxylic acids. The literature suggested that unlike the chloride analog, TiF4 is easier to handle, stable in air and water,39 and has excellent solubility in dimethoxyethane and THF.40,41 Our concerns were the possible complexation of TiF4 with amines,42 which could be detrimental to their nucleophilicity. The results documented herein unequivocally confirm that 5–10% of TiF4 is a catalytic promoter for the direct amidation of both aliphatic and aromatic carboxylic acids with both classes of amines (Scheme 1(iv)).
A reaction of an equivalent each of 1a with 2a in refluxing toluene in the presence of 10 mol% TiF4 for 24 h provided 77% of 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 (entry 6), which was increased to 96% with an additional 10% of the acid (entry 7). A large scale 50 mmol reaction was carried out for the synthesis of N-benzylbenzamide (3aa), which resulted in a slightly reduced yield of 77%. We then explored the limitations of the process by varying the catalyst loading, solvent, reaction temperature, etc. (entries 8–14) and concluded that 10% catalyst with 1.1 equiv. of the acid provided the optimal yield. BF3-Et2O,43 ZnF2 and, ZrF4, were also compared (entries 15–17), which gave unsatisfactory results.
35 (entry 6), which was increased to 96% with an additional 10% of the acid (entry 7). A large scale 50 mmol reaction was carried out for the synthesis of N-benzylbenzamide (3aa), which resulted in a slightly reduced yield of 77%. We then explored the limitations of the process by varying the catalyst loading, solvent, reaction temperature, etc. (entries 8–14) and concluded that 10% catalyst with 1.1 equiv. of the acid provided the optimal yield. BF3-Et2O,43 ZnF2 and, ZrF4, were also compared (entries 15–17), which gave unsatisfactory results.
Having determined the optimal conditions for the amidation of 1a with 2a, the generality of the protocol was examined by varying the acids and amines (Fig. 1 and 2). In all cases, pure amides were isolated after a simple acid–base workup. Initially, we maintained the amine (benzylamine) and focused on the effect of the aromatic acid (Fig. 1). Substitutions on the benzene ring of 1a were examined with benzoic acid substituents bearing electron-withdrawing groups (EWGs) and electron-donating groups (EDGs) (1b–1j). All of the amides were obtained in good to excellent yields. However, moderate yield was realized with ortho-substituted benzoic acids 1b and 1g, probably due to the increased steric effects. Among those tested, 4-nitrobenzoic acid failed to undergo amidation. Cinnamic acid (1k) and nicotinic acid (1l) were also treated with 2a to yield the corresponding amides 3ka and 3la in 99% and 93% yields, respectively. Replacing benzylamine with cyclohexylamine (2c) resulted in 24% yield of the amide 3ac. However, increasing the catalyst to 50 mol% afforded 90% yield of the product.
Preparation of tertiary amides from aromatic acids was examined using morpholine (2d) as the representative secondary amine. Thus, benzoic acids with EWGs and EDGs were amidated with 2d in 90–98% yield, except 2-methoxybenzoic acid 1g, which, as with 2a, provided 3gd in 61% yield. Significantly, amidations of benzoic acids and anilines each bearing EDGs and EWGs were complete within 24 h, and the products were isolated in 77–96% yields. It is noteworthy that amidations with anilines are seldom reported for several of the procedures described in the literature. Under similar conditions, metoclopramide (3mh), an antiemetic and gut motility stimulator,44 was synthesized from 4-amino-5-chloro-2-methoxybenzoic acid (1m) and N,N-diethylethane-1,2-diamine (2h).
The direct amidation of aliphatic acids (4a–4r) required only 5 mol% TiF4 and were faster (12 h) (Fig. 2). Formic, acetic, cyclic, linear, and arylacetic acids reacted smoothly and the amides (5)35 were obtained in good to excellent yields (Fig. 2). The amide 5ia from adamantylcarboxylic acid (4i) and benzylamine (2a) was obtained in only 47% yield under the standard conditions. However, it improved to 83% with 50 mol% of the catalyst.
The reaction of the anti-inflammatory drug ibuprofen (4l) and 2a produced the corresponding amide 5la in 98% yield. A decreased yield of 47% was observed for amide 5ma from mandelic acid (4m) under the standard conditions, which was improved to 65% with 50 mol% of the catalyst. The need for excess catalyst could be due to the reaction of the hydroxyl group with the catalyst. No side reaction was observed with substrates having distal double (4p) and triple (4q) bonds in the carboxylic acids and amides 5pa and 5qa were obtained in 86% and 94% yields, respectively. Nevertheless, the yield of the amide 5ji was low (29%) when phenylacetic acid (4j) was reacted with a secondary amine (2i). An optically pure amine, (R)-(+)-α-methylbenzylamine (2j), was treated with 4j to examine the stereochemical retention during the amidation. The chiral amide 5jj, which was isolated in 57% yield, revealed an optical rotation comparable to the pure chiral amide reported in the literature,45 demonstrating the applicability of this process for chiral substrates.
The new amidation protocol was next examined to prepare peptides from amino acids. The amidation of the sterically hindered N-Fmoc and N-Boc protected glycine (6a and 6b) with benzylamine 2a (Fig. 3) yielded the target amides 7aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 and 7ba in 62% and 88% yields, respectively.
35 and 7ba in 62% and 88% yields, respectively.
More challenging di- and tripeptide couplings were also successfully achieved via the combination of N-Fmoc-Gly-OH (6a) and N-Boc-Gly-OH (6b), with ethyl α-amino esters of phenylalanine 2k under the described conditions when the amides 7ak and 7bk were obtained in excellent (99%) yields. Subsequently, dipeptide 7bk was deprotected to give the intermediate Gly-Phe-OEt, which was further reacted with N-Boc-Gly-OH (6b) to furnish the tripeptide N-Boc-Gly-Gly-Phe-OEt 7ck in 63% yield, demonstrating the capability of the reaction for sequential amidation reactions.
The TiF4-mediated amidation is believed to proceed via the coordination of the catalyst with the acid, which prepared the carbonyl for the nucleophilic attack by the amine, which was followed by a proton transfer, elimination of water and regeneration of the catalyst (Fig. 4). A similar mechanism proposed for the amidation of carboxylic acids with catalytic ZrCl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 forms the basis of our hypothesis. TiF4 is known to complex with amines.42 It is known to complex with ketones and carboxylic acids as well.41 However, the formation of an ammonium salt upon the mixing of the carboxylic acid and amine may preclude the formation of the amine–titanium complex. In refluxing toluene, the ammonium salt is probably cleaved, and the amine attacks the Ti-complexed carbonyl carbon.
46 forms the basis of our hypothesis. TiF4 is known to complex with amines.42 It is known to complex with ketones and carboxylic acids as well.41 However, the formation of an ammonium salt upon the mixing of the carboxylic acid and amine may preclude the formation of the amine–titanium complex. In refluxing toluene, the ammonium salt is probably cleaved, and the amine attacks the Ti-complexed carbonyl carbon.
Conclusion
In conclusion, we have described a direct amidation protocol using catalytic TiF4 (10 mol% for aromatic or 5 mol% for aliphatic acids) in refluxing toluene. The former is amidated within 24 h, while the latter requires only 12 h. This procedure can provide secondary and tertiary amides from both aliphatic and aromatic acids and amines in very high yields. This is applicable for the synthesis of peptides from amino esters and N-protected amino acids and has several advantages, such as: (i) ready availability and (ii) very low molecular weight of the catalyst, (iii) non-chromatographic purification, (iv) no requirement of removal of water using azeotropic/molecular sieves for small scale reactions, and (v) consistently high yields. We continue to explore ways to improve and extend this amidation procedure.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Hebert C. Brown Center for Borane Research for financial support and Henry J. Hamann of our group for helpful discussions.References
- D. G. Brown and J. Bostrom, J. Med. Chem., 2016, 59, 4443–4458 CrossRef CAS PubMed.
- J. C. Sheehan and G. P. Hess, J. Am. Chem. Soc., 1955, 77, 1067–1068 CrossRef CAS.
- N. Goyal, Synlett, 2010, 335–336 CAS.
- R. Paul and G. W. Anderson, J. Am. Chem. Soc., 1960, 82, 4596–4600 CrossRef CAS.
- J. Coste, D. Lenguyen and B. Castro, Tetrahedron Lett., 1990, 31, 205–208 CrossRef CAS.
- B. Castro, J. R. Dormoy, G. Evin and C. Selve, Tetrahedron Lett., 1975, 1219–1222 CrossRef CAS.
- H. Wissmann and H. J. Kleiner, Angew. Chem., Int. Ed. Engl., 1980, 19, 133–134 CrossRef.
- V. Dourtoglou, B. Gross, V. Lambropoulou and C. Zioudrou, Synthesis, 1984, 572–574 CrossRef CAS.
- L. A. Carpino, J. Am. Chem. Soc., 1993, 115, 4397–4398 CrossRef CAS.
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC.
- J. R. Dunetz, J. Magano and G. A. Weisenburger, Org. Process Res. Dev., 2016, 20, 140–177 CrossRef CAS.
- J. Magano, Org. Process Res. Dev., 2022, 26, 1562–1689 CrossRef CAS.
- A. S. Santos, A. M. S. Silva and M. M. B. Marques, Eur. J. Org. Chem., 2020, 2501–2516 CrossRef CAS.
- M. T. Sabatini, L. T. Boulton and T. D. Sheppard, Sci. Adv., 2017, 3, e1701028 CrossRef PubMed.
- J. J. Davies, D. C. Braddock and P. D. Lickiss, Org. Biomol. Chem., 2021, 19, 6746–6760 RSC.
- J. D. Wilson and H. Weingart, Can. J. Chem., 1970, 48, 983 CrossRef CAS.
- C. L. Allen, A. R. Chhatwal and J. M. J. Williams, Chem. Commun., 2012, 48, 666–668 RSC.
- A. Leggio, J. Bagalà, E. L. Belsito, A. Comande, M. Greco and A. Liguori, Chem. Cent. J., 2017, 11, 87 CrossRef PubMed.
- Z. Q. Fu, X. H. Wang, S. Tao, Q. Q. Bu, D. H. Wei and N. Liu, J. Org. Chem., 2021, 86, 2339–2358 CrossRef CAS PubMed.
- Basavaprabhu, K. Muniyappa, N. R. Panguluri, P. Veladi and V. V. Sureshbabu, New J. Chem., 2015, 39, 7746–7749 RSC.
- A. C. Shekhar, A. R. Kurnar, G. Sathaiah, V. L. Paul, M. Sridhar and P. S. Rao, Tetrahedron Lett., 2009, 50, 7099–7101 CrossRef CAS.
- J.-G. Kim and D. O. Jang, Bull. Korean Chem. Soc., 2010, 31, 2989–2991 CrossRef CAS.
- H. Lundberg, F. Tinnis, N. Selander and H. Adolfsson, Chem. Soc. Rev., 2014, 43, 2714–2742 RSC.
- H. Lundberg, F. Tinnis and H. Adolfsson, Chem. – Eur. J., 2012, 18, 3822–3826 CrossRef CAS PubMed.
- J.-G. Kim and D. O. Jang, Synlett, 2010, 1231–1234 CAS.
- M. Hong and G. Xiao, J. Fluorine Chem., 2013, 146, 11–14 CrossRef CAS.
- A. M. Gabdullin, R. N. Kadikova, O. S. Mozgovoj and I. R. Ramazanov, ChemistrySelect, 2023, 8, 7 CrossRef.
- W. Muramatsu and H. Yamamoto, J. Am. Chem. Soc., 2019, 141, 18926–18931 CrossRef CAS PubMed.
- P. V. Ramachandran, A. A. Alawaed and H. J. Hamann, J. Org. Chem., 2022, 87, 13259–13269 CrossRef CAS PubMed.
- P. V. Ramachandran, A. A. Alawaed and H. J. Hamann, Org. Lett., 2022, 24, 8481–8486 CrossRef CAS PubMed.
- P. V. Ramachandran, A. A. Alawaed and A. Singh, Molecules, 2023, 28, 4575 CrossRef CAS PubMed.
- P. V. Ramachandran and A. A. Alawaed, Molecules, 2023, 28, 60 CrossRef CAS PubMed.
- P. V. Ramachandran, A. A. Alawaed and H. J. Hamann, Org. Lett., 2023, 25, 6902–6906 CrossRef CAS PubMed.
- P. V. Ramachandran, A. A. Alawaed and H. J. Hamann, Org. Lett., 2023, 25, 4650–4655 CrossRef CAS PubMed.
- The first letter of the amide number refers to the acid and the second letter refers to the amine used.
- M. Mader and P. Helquist, Tetrahedron Lett., 1988, 29, 3049–3052 CrossRef CAS.
- H. Lundberg, F. Tinnis and H. Adolfsson, Synlett, 2012, 2201–2204 CAS.
- Sonam, V. N. Shinde and A. Kumar, J. Org. Chem., 2022, 87, 2651–2661 CrossRef CAS PubMed.
- E. Jorda, A. Tuel, R. Teissier and J. Kervennal, Zeolites, 1997, 19, 238–245 CrossRef CAS.
- G. B. Nikiforov, C. Knapp, J. Passmore and A. Decken, J. Fluorine Chem., 2006, 127, 1398–1404 CrossRef CAS.
- E. L. Muetterties, J. Am. Chem. Soc., 1960, 82, 1082–1087 CrossRef CAS.
- J. A. Chandler and R. S. Drago, Inorg. Chem., 1962, 1, 356 CrossRef CAS.
- J. Tani, T. Oine and I. Inoue, Synthesis, 1975, 714–715 CrossRef CAS.
- T. J. Shen, V. T. Hanh, T. Q. Nguyen, M. K. Jhan, M. R. Ho and C. F. Lin, Front. Cell. Infect. Microbiol., 2020, 10, 606743 CrossRef CAS PubMed.
- J. H. Dam;, G. Osztrovszky;, L. U. Nordstrøm; and R. Madsen, Chem. – Eur. J., 2010, 16, 6820–6827 CrossRef PubMed.
- H. Lundberg, F. Tinnis, J. J. Zhang, A. G. Algarra, F. Himo and H. Adolfsson, J. Am. Chem. Soc., 2017, 139, 2286–2295 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra of products. See DOI: https://doi.org/10.1039/d3ob01943h |
| This journal is © The Royal Society of Chemistry 2024 |