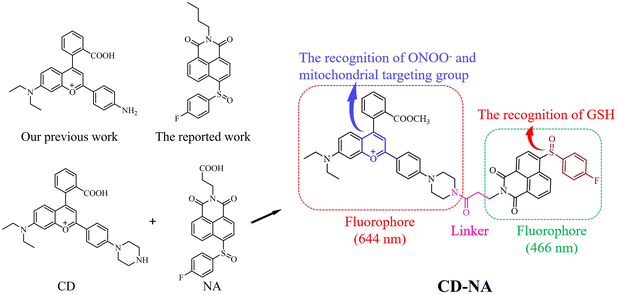
Visualization of peroxynitrite/GSH cross-talk in the oxidant–antioxidant balance by a dual-fluorophore and dual-site based mito-specific fluorescent probe†
Yu
Liu‡
,
Jinjin
Zhao‡
and
Yingzhe
Wang
 *
*
Laboratory for NanoMedical Photonics, School of Basic Medical Science, Henan University, Kaifeng 475004, P. R. China. E-mail: wangyzh@henu.edu.cn
First published on 24th November 2023
Abstract
Peroxynitrite (ONOO−) and glutathione (GSH) play mutually regulating roles in the oxidant–antioxidant balance of organisms, which has a profound relationship with people's health and disease. In this study, we designed a two-photon fluorescent probe CD-NA that could simultaneously detect ONOO− and GSH via dual-fluorophore and dual-site properties. CD-NA shows different fluorescence responses to ONOO− (annihilated red fluorescence) and GSH (enhanced green emission) with high specificity and sensitivity. Notably, the response of CD-NA to ONOO− was unaffected by GSH, and the reverse is also true. It allows the ONOO−/GSH cross-talk to be successfully imaged. Given these excellent properties, CD-NA has been favorably employed in detecting ONOO− and GSH in living cells with the ability to target mitochondria. Therefore, CD-NA offers an efficient method for understanding the oxidant–antioxidant balance and interrelated physiological functions of ONOO− and GSH in living systems, and provides a new strategy to sort out the complex relationships and roles of various analytes in complex physiological processes.
Introduction
Oxidation and anti-oxidation maintain the cells’ redox homeostasis, which is a necessary condition to preserve the life processes of cells and an essential factor in determining the biological state and the function of the cells.1–5 The evilest reactive oxygen species (ROS) is ONOO−, which can oxidize any organic molecules close to it, including proteins, DNA, and lipids.6–9 Studies have shown that diabetes, heart disease, muscular atrophy (spine) side sclerosis, Alzheimer's disease, and other sclerosis are all associated with the existence of ONOO−.10–12 Low-dose ONOO− has a meaningful impact on the signal transduction of nitrated tyrosine residues and immunogenic response against pathogen invasion. Moreover, ONOO− can regulate the expression of genes that play essential roles in signal transduction.13–15 However, high-dose ONOO− can cause oxidative stress, indirectly activate the antioxidant program, and increase the content of GSH.16–19 As the most commonly found small molecule thiol in most cells, GSH is a scavenger of ONOO− and considered as a cell protection molecule that participates in multiple cellular processes, such as differentiation, metabolism, and antioxidant.20–22 The combination of GSH with active oxygen and transformation from reduced GSH to oxidized GSSG play a vital role in the antioxidant process.23,24 The increased amount of GSH caused by oxidative stress will reduce excess ONOO− and maintain the cells’ redox balance. In short, ONOO− and GSH both restrain and promote each other, and participate in the maintenance of redox homeostasis in the cells at the same time.25–28 Therefore, it is imperative to image ONOO− and GSH simultaneously in a biological system.In recent years, due to the high sensitivity and specificity, non-destructive imaging, simple operation, and high-speed spatial and temporal analysis, fluorescent probes have been considered to be powerful tools for sensing and imaging in vivo.29–35 The necessary feature of a probe is its ability to specifically detect only one analyte.36–38 However, to clarify the relationships of various analytes in complex physiological processes, it is essential to detect multiple analytes simultaneously.39–41 Because the use of two or more fluorescent probes to detect dual or multi-analyte analytes will cause a series of problems, such as mutual interference among the various probes, different localization of different probes, and different photobleaching rates and metabolisms, the situation would be complicated and unsuitable for quantitative analysis.42 Thus, it is urgently needed to seek a new road for simultaneously imaging ONOO− and GSH.
Currently, there are only a few probes for simultaneously detecting ONOO− and GSH. In 2011, Han et al. reported on the probe Cy-PSe, which is initially quenched by the photoinduced electron transfer (PET) process. The oxidization of Cy-PSe by ONOO− leads to luminescence enhancement. Then, the reduction of GSH brings it back to the original state.43 In 2013, they improved the previous work by introducing 2-(phenyltellanyl)benzohydrazide (NTe) as a fluorescent modulator to protect the telluroenzyme mimic moiety from over-oxidation by ONOO−.44 In 2017, Sun et al. reported on the probe TP-Se to detect ONOO− and GSH.45 In 2018, James et al. proposed a probe (GSH-PF3) based on the “and” logic gates. The probe was initially non-fluorescent. After adding ONOO− or GSH, the fluorescence of GSH-PF3 is slightly enhanced. When both ONOO− and GSH are added, the fluorescence intensity of GSH-PF3 will markedly increase.27 Recently, a new probe (Mito-CM-CD) was reported. It could detect both ONOO− and GSH simultaneously, but the short excitation wavelength is still a severe problem.46 Reviewing the probes mentioned above, we found three flaws: (i) They cannot specifically target mitochondria, which is the central organelle for the ONOO− reaction and formation in the living cells, significantly reducing their sensitivity. (ii) With only one fluorophore or response group, it is too hard to distinguish the response between ONOO− and GSH. (iii) Compared with two-photon excitation, one-photon excitation has high background signal interference and a low signal-to-noise ratio. These defects make it difficult to monitor the roles of ONOO− and GSH in redox homeostasis in living cells. With these experiences, we believe that the mito-specific two-photon fluorescence probes based on the dual-fluorescence and dual-site properties will open up a new way for imaging closely related substances in living cells.
Here, we developed a mito-specific two-photon fluorescence probe (CD-NA) for detecting ONOO− and GSH through the two-fluorophore and two-site strategy. CD-NA exhibited high sensitivity (15 nM, 840 nM) and an ultrafast response towards ONOO− (18 s) and GSH (22 s) through two non-interference detection processes and emission channels. Furthermore, in the multiplex test system, various ROS (e.g., HOCl, H2O2, ˙OH, NO, NO2−, O2˙−) and RSS (e.g., H2S, Hcy, Cys, HSO3−, S2O32−, SO42−, His, Iso, Leu, Lys, Met, and Ser) did not affect the detection process. Moreover, CD-NA can efficiently target mitochondria, which is the prominent organelle of the ONOO− reaction and formation in living cells. CD-NA was successfully used for the simultaneous imaging of ONOO− and GSH by two-photon confocal fluorescence microscopy in living cells for the first time, and could monitor the dynamic change of ONOO− and GSH levels in a drug-induced acute liver injury model.
Results and discussion
Design rationale and synthesis of CD-NA
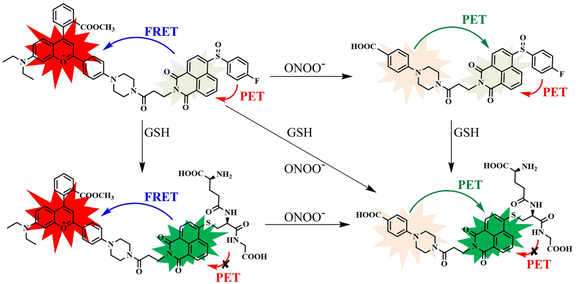
Since both ONOO− and GSH play essential roles in the redox balance in living cells, it is urgent to develop a probe that can simultaneously detect them. It has been reported that the sulfoxide structure (NA) can be easily reduced by GSH without being affected by other thiols.47 Happily, NA has excellent two-photon excitation (TPE) characteristics, which have many other remarkable properties aside from one-photon excitation, such as three-dimensional (3D) fluorescence imaging of biological samples, minor light damage to biological samples, low background fluorescence, and powerful tissue penetration. In addition, based on our previous work, the CD group showed excellent performance in detecting ONOO−.48,49 It can efficiently target mitochondria, which is the central organelle of the ONOO− reaction and formation in living cells. Furthermore, to enhance the cell membrane penetrability, CD was further embellished by an esterification reaction at the carboxyl group.50 Therefore, a new probe (CD-NA) was ultimately formed by combining two fluorophores, CD and NA (Scheme 1 and Fig. S1†). The outstanding properties of CD and NA provide the necessary conditions of the dual-fluorophore and dual-site two-photon probe, which facilitates the simultaneous detection of two or more substances that are closely related (Scheme 2). The possible response mechanisms of CD-NA to ONOO− and GSH are proved by the mass spectra in the ESI (Fig. S2–S6†).Spectral response of CD-NA to ONOO− and GSH
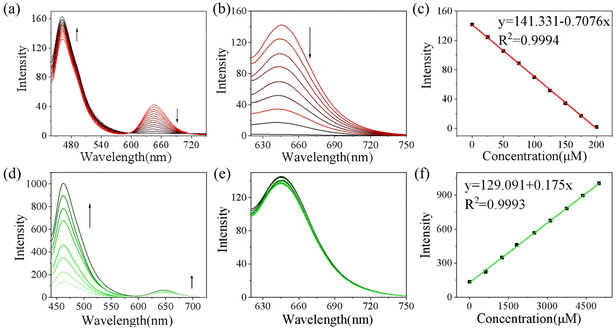
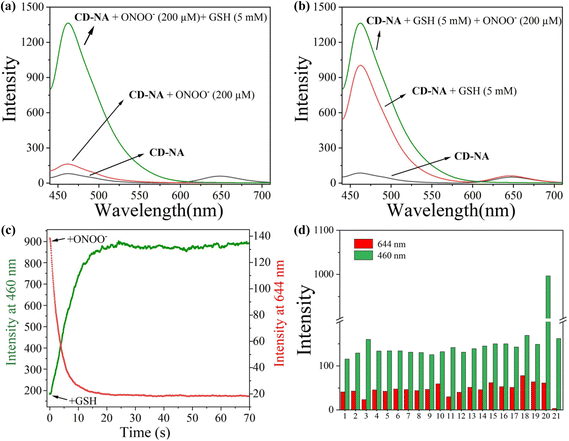
To monitor the sensing property of CD-NA for ONOO− and GSH in vitro, we measured its spectral properties in PBS (0.01 M, 20% EtOH, pH 7.4) under excitation at 405 and 600 nm (Fig. S7†). As shown in Fig. 1a, under excitation at 405 nm, when ONOO− (0–200 μM) was added to the solution of CD-NA (10 μM), the emission at 644 nm was entirely quenched. The emission band centered at 460 nm had a minor enhancement, which contributes to the fluorescence resonance energy transfer (FRET) process between CD and NA. The most substantial absorption peak of CD overlapped partially with the emission of NA (Fig. S7†). Thus, there was a weak FRET effect between CD and NA.51 When the fluorescence of CD was quenched, the FRET process was blocked, and the fluorescence of NA would be partially restored. Meanwhile, under the excitation at 600 nm, the fluorescence intensity of CD-NA (10 μM) at 644 nm gradually decreased by about 78-fold (Fig. 1b). The limit of detection (LOD) of CD-NA for ONOO− was calculated to be 15 nM, indicating that CD-NA is highly sensitive to ONOO− (Fig. 1c).52 Upon incubation with GSH (0–5 mM), the fluorescence at 460 nm was dramatically enhanced by 8 times, and the fluorescence at 644 nm almost slightly increased (Fig. 1d). This phenomenon also corresponds with the response mechanisms. The bond between an electron-deficient carbon and sulfur should be susceptible to nucleophilic attack. Upon the addition of GSH, the –SH of GSH attacks the bond and the sulfoxide derivatives were replaced. The 4-position of the 1,8-naphthalimide fluorophore (NA) transited from an electron-withdrawing sulfoxide derivative to an electron-donating sulfhydryl derivative. After reacting with GSH, the electronic structure of NA was converted from A–π–A to A–π–D. This blocked the PET capability, which further initiated the FRET process from NA-GSH to CD, thus enabling the fluorescence at 460 nm to be dramatically enhanced by 8 times and the fluorescence at 644 nm to have a slight enhancement at the same time. With the concentrations of GSH ranging between 0–5 mM, the superior linear fluorescence intensity of CD-NA at 460 nm showed a high correlation coefficient (R2 = 0.9993) (Fig. 1f). The LOD for GSH was calculated to be 840 nM. During treatment with GSH (5 mM) under excitation at 600 nm, the fluorescence intensity of CD-NA (10 μM) is impervious (Fig. 1e).Next, we further measured the ability of CD-NA to detect GSH in the presence of ONOO− under excitation at 405 nm (Fig. 2a). As described above, after adding ONOO− (200 μM) to the solution of CD-NA, the fluorescence at 644 nm was almost wholly quenched. The fluorescence at 460 nm was slightly enhanced. When GSH (5 mM) was added to the above solution, the fluorescence at 644 nm remained quenched and the fluorescence intensity at 460 nm was distinctly increased. This phenomenon indicated that the detection of GSH would not be affected by the existence of ONOO−. In view of ONOO− and NO being important reactive oxygen species (ROS) in living systems, we tested the probe response to NO and GSH as conditions of ONOO− and GSH (Fig. S9†). The test results show that NO cannot influence CD-NA and the detection of GSH, which strengthen the conclusion above. Correspondingly, we also measured the detection of ONOO− in the presence of GSH (Fig. 2b). It revealed that the detection of ONOO− would not be influenced by GSH either. All these experimental data provided the conditions for detecting ONOO− and GSH simultaneously.
Given that the reaction time is an important indicator to determine whether the probe is first-rate, the response time was measured. We found that the fluorescent signal at 644 nm achieved a plateau at 18 s upon the addition of ONOO− (200 μM), and the emission at 460 nm reached a plateau at 22 s upon the addition of GSH (5 mM) (Fig. 2c). These superior characteristics indicate that CD-NA is suitable for the real-time detection of ONOO− and GSH. Considering that other active substances in the complex physiological environment may affect the specific detection of ONOO− and GSH in vivo, we conducted the selective test of CD-NA. As shown in Fig. 2d, apart from ONOO− and GSH, other ROS/RSS (including ClO−, H2O2, ˙OH, NO, NO2−, O2˙−, H2S, Hcy, Cys, HSO3−, S2O32−, SO42−, His, Iso, Leu, Lys, Met, and Ser (200 μM)) could only trigger minimal fluorescence variation at 644 or 460 nm. These results confirmed that CD-NA had the capability to monitor ONOO− and GSH in vitro with high specificity and sensitivity.
The sensing mechanism study
To verify the proposed detection mechanism of this work, time-dependent density functional theory (TD-DFT) calculations were performed based on the optimized molecular structure, and the frontier molecular orbital and corresponding energies are shown in Fig. 3.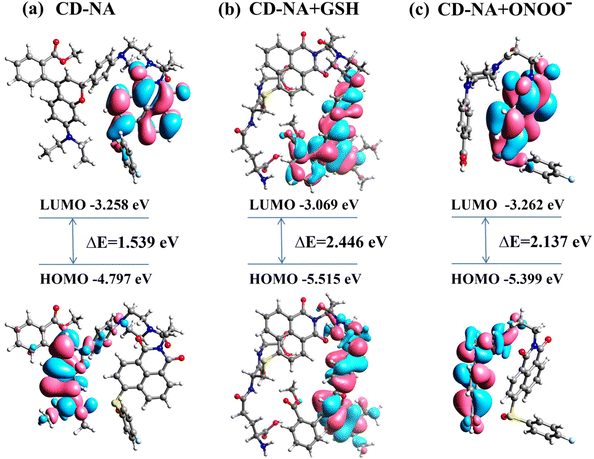 | ||
| Fig. 3 The molecular orbital plots (LUMO and HOMO) of probe CD-NA (a), compound CD-NA + GSH (b) and compound CD-NA + ONOO− (c). | ||
The results indicated that in CD-NA, the LUMO was distributed on the electron-donating naphthalimide part, whereas the HOMO was spread over the fluorophore CD moiety (Fig. 3a). Upon excitation, electrons transfer from the naphthalimide NA to the fluorophore CD, impeding the radiative relaxation and resulting in fluorescence quenching of the fluorophore CD moiety. However, after Probe CD-NA reacted with GSH, the HOMO and LUMO were both located at the fluorophore CD moiety (Fig. 3b). Therefore, there is no electron transfer from the naphthalimide NA moiety to the fluorophore CD moiety under excitation, and the fluorescence of CD is intensified upon reacting with GSH. Moreover, the LUMO of the probe CD-NA + ONOO− was located at the NA moiety and the HOMO was distributed on the CD skeleton destroyed by ONOO− (PBA), which suggested a possibility of a PET process from the PBA skeleton to the NA moiety, resulting in the quenching fluorescence of NA (Fig. 3c). In addition, the HOMO/LUMO energy gap of probe CD-NA, compound CD-NA + GSH and compound CD-NA + ONOO− were calculated to be 1.539 eV, 2.446 eV and 2.137 eV, respectively. These results showed that upon the reaction of CD-NA with GSH, the HOMO/LUMO energy gap was increased significantly, indicating that the reaction activity of CD-NA + GSH and CD-NA + ONOO− were poor. Thus, the formation of complex CD-NA + GSH and CD-NA + ONOO− stabilized the system.
Imaging of CD-NA with Endogenous ONOO− and GSH in living cells
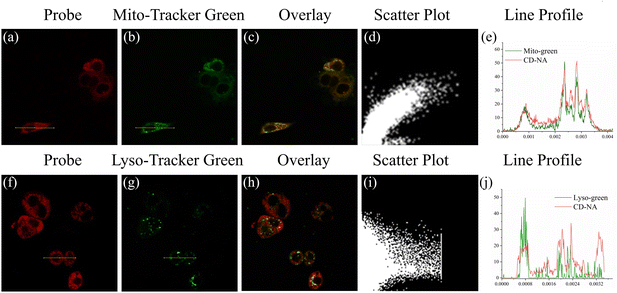
Encouraged by the excellent test results (fast response, excellent specificity and high sensitivity) in vitro, we evaluated the performance of CD-NA for detecting ONOO− and GSH by confocal fluorescence microscopy. The MTT assay of CD-NA was firstly studied in HepG2 cells (Fig. S9†). It can be seen clearly that the cell viability still exceeded 80% when incubating with CD-NA (20 μM) for 24 h. To study the distribution of CD-NA in subcellular organelles, the HepG2 cells were first pretreated with CD-NA (10 μM) for 20 min, and commercial dyes were then added for another 10 min (Fig. 4). Compared with Lyso-Tracker Green (Pearson coefficient 0.32), the luminescence of CD-NA in the red channel overlapped well with Mito-Tracker Green (Pearson coefficient 0.89) in the green channel. This result indicated that CD-NA possessed superb cell membrane permeability and could specifically target mitochondria, which is the main organelle for ONOO− formation and reaction. In summary, the above consequences indicated that CD-NA was very suitable for detecting ONOO− in living cells.Based on the above experimental results, the feasibility of CD-NA for detecting ONOO− and GSH was tested by two-photon confocal fluorescence microscopy. To find a suitable two-photon excitation wavelength, the two-photon action cross-section of CD-NA among 760–880 nm were all examined. As shown in Fig. S10,† the two-photon absorption spectra of CD-NA showed the maximum two-photon excitation action cross-section value at 850 nm, which is suitable for imaging in deep tissues and in vivo. For proving the advantages of two-photon excitation to the practical application, we compared the images by one-photon excitation and two-photon excitation (Fig. S11†). HepG2 cells were pretreated with CD-NA (5 μM) for 10 minutes and then imaged. It was clear that stronger fluorescence could be observed in both red and green channels by the excitation at 850 nm compared to 405 nm, demonstrating that CD-NA was well-suited for two-photon fluorescence imaging.
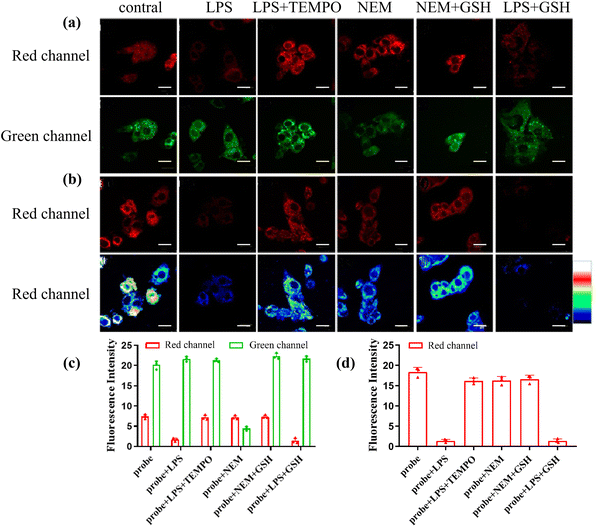
On account of these outstanding characteristics, we examined the ability of CD-NA for monitoring ONOO− and GSH by two-photon excitation. The experiments were divided into three groups for detecting ONOO−, GSH, and both ONOO− and GSH. In the first group, the cells incubated with CD-NA (10 μM, 30 min) showed strong fluorescence in both red and green channels. However, for the cells incubated with bacterial endotoxin lipopolysaccharide (LPS, which stimulates the production of endogenous ONOO−) (1 μg mL−1, 14 h) and then set with CD-NA (10 μM, 30 min), the result images exhibited bright fluorescence in the green channel and weak fluorescence in the red channel, confirming that the CD section was destroyed by ONOO− and NA was insusceptible.53 When the cells were pretreated with (TEMPO, a superoxide scavenger) (300 μM, 30 min) and further incubated with CD-NA and LPS, strong fluorescence in both green and red channels were observed again.54 This phenomenon was caused by the ONOO− produced by LPS-stimulated cells that were cleared by TEMPO. These data indicated that CD-NA could image exogenous ONOO− efficiently in living cells. In the second group, upon incubating N-ethylmaleimide (NEM, a thiol-blocking agent) (1 mM) for 30 min in advance, weaker fluorescence in the green channel could be observed, and the fluorescence in the red channel was constant.55 When GSH (3 mM) was added later, the fluorescence in the red channel remained unchanged, while the fluorescence in the green channel was significantly enhanced to the intensity level when the probe was incubated alone. This observation can be attributed to the cells themselves containing a lot of GSH. In the last group, we tried to exam the response of CD-NA in the presence of both ONOO− and GSH. When preincubated with LPS (1 μg mL−1, 14 h) and then incubated with CD-NA (10 μM, 30 min) and GSH (3 mM, 10 min), the cells displayed an increasing fluorescence in the green channel and a relatively attenuated fluorescence in the red channel, which corresponded to the in vitro experiment (Fig. 5a). The quantification of these groups by fluorescence intensity is shown in Fig. 4c. Moreover, we manufactured the above three groups of experiments under 559 nm excitation (Fig. 5b), and the quantified data are shown in Fig. 5d. The experimental results were consistent with the in vitro experimental data. Thus, we hypothesized that CD-NA was highly suitable for testing ONOO− and GSH in living cells.
Imaging of ONOO− and GSH in the acute liver injury model in living cells
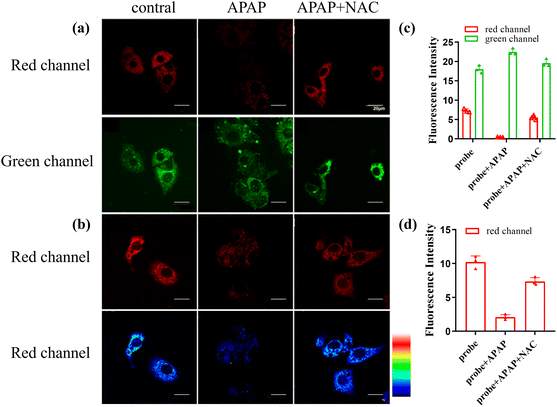
Drug-induced acute liver injury (DILI) is a common liver disease that affects many people around the world with increasing incidence each year. Excessive antipyretic Acetaminophen (APAP) could indirectly produce ONOO− by causing acute liver injury. Thus, we selected the cell model of DILI to study the performance of CD-NA.56 At the beginning of the test, the production of ONOO− stimulated with APAP in the HepG2 cells was evaluated. The cells incubated with CD-NA (10 μM, 30 min) showed strong emission in both red and green channels. When pre-stimulated with APAP (100 μM) for 30 min, the red emission was almost completely quenched, and the green emission had nearly no change. To better understand the features of CD-NA, N-acetyl cysteine (NAC, an antioxidant drug to scavenge ROS, which was approved by the U.S. Food and Medicine Administration (FDA) as medicine) was applied to the APAP-induced liver injury.57 After the addition of NAC (100 μM) into the cells, a noticeable fluorescence enhancement could be observed in the red channel and the green emission became stronger (Fig. 6a). The quantification of these groups by fluorescence intensity is shown in Fig. 6c. We also manufactured the above three groups of experiments under 559 nm excitation (Fig. 6b), and the quantified data are shown in Fig. 6d. It is evident that the signal change was mainly caused by the reaction of CD-NA with ONOO−, which illustrated the feasibility of CD-NA for monitoring ONOO− in living systems. In summary, these consequences strongly demonstrated the therapeutic effect as an antidote in the drug-induced cell injury, and the capacity of CD-NA for detecting and imaging ONOO− in living cells.Conclusion
In summary, by employing the dual-fluorophore and dual-site strategy, a two-photon mito-specific fluorescence probe CD-NA for sensitive, specific and simultaneous detection of ONOO− and GSH was successfully constructed. Due to the unique mechanism of the dual-fluorophore and dual-site probe, CD-NA could respond to ONOO− and GSH with different fluorescent signals. When stimulated with ONOO− and GSH simultaneously, the corresponding fluorescence responses in other channels would rule out luminous cross-interference, thus providing a powerful tool to visualize ONOO−/GSH cross-talk in living systems. It is worth noting that CD-NA can effectively detect endogenous ONOO− and GSH in the APAP-treated acute liver injury model. We believe that CD-NA would help explain the close relationship between ONOO− and GSH in vivo, and provide new clues to explain the changes in intracellular redox balance. It can also be expected that the novel dual-fluorophore and dual-site strategy may be a popular method to sort out the intricate connections and functions of diverse analytes in multiplex physiological processes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Yu Liu, Jinjin Zhao contributed equally to this work. This work was supported by the Key Research Project of Higher Education Institutions in Henan Province (No.: 23A150032) and the Natural Science Foundation of Henan Province (No.: 232300421368).References
- W. Dröge, Free Radicals in the Physiological Control of Cell Function, Physiol. Rev., 2002, 82, 47–95, DOI:10.1152/physrev.00018.2001.
- M. Jia, D. Qin, C. Zhao, L. Chai, Z. Yu and W. Wang, et al., Redox homeostasis maintained by GPX4 facilitates STING activation, Nat. Immunol., 2020, 21, 727–735, DOI:10.1038/s41590-020-0699-0.
- W. Łuczaj, A. Gęgotek and E. Skrzydlewska, Antioxidants and HNE in redox homeostasis, Free Radicals Biol. Med., 2017, 111, 87–101, DOI:10.1016/j.freeradbiomed.2016.11.033.
- S. Rapino, R. Marcu, A. Bigi, A. Soldà, M. Marcaccio and F. Paolucci, et al., Scanning electro-chemical microscopy reveals cancer cell redox state, Electrochim. Acta, 2015, 179, 65–73, DOI:10.1016/j.electacta.2015.04.053.
- J. Tian, L. Qin, D. Li, S. Qin, J. Han and W. Gao, et al., Carbofuran-imprinted sensor based on a modified electrode and prepared via combined multiple technologies: Preparation process, performance evaluation, and application, Electrochim. Acta, 2022, 404, 139600, DOI:10.1016/j.electacta.2021.139600.
- R. Radi, Peroxynitrite, a Stealthy Biological Oxidant, J. Biol. Chem., 2013, 288, 26464–26472, DOI:10.1074/jbc.R113.472936.
- J. S. Beckman, T. W. Beckman, J. Chen, P. A. Marshall and B. A. Freeman, Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 1620, DOI:10.1073/pnas.87.4.1620.
- J. S. Beckman, Oxidative Damage and Tyrosine Nitration from Peroxynitrite, Chem. Res. Toxicol., 1996, 9, 836–844, DOI:10.1021/tx9501445.
- R. E. Huie and S. Padmaja, The Reaction of no With Superoxide, Free Radical Res. Commun., 1993, 18, 195–199, DOI:10.3109/10715769309145868.
- R. Radi, J. S. Beckman, K. M. Bush and B. A. Freeman, Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide, J. Biol. Chem., 1991, 266, 4244–4250, DOI:10.1016/S0021-9258(20)64313-7.
- C. Ducrocq, B. Blanchard, B. Pignatelli and H. Ohshima, Peroxynitrite: an endogenous oxidizing and nitrating agent, Cell. Mol. Life Sci., 1999, 55, 1068–1077, DOI:10.1007/s000180050357.
- F. Wang, D. Liu, Y. Shen, J. Liu, D. Li and X. Tian, et al., A two-photon mitochondria-targeted fluorescent probe for the detection of pH fluctuation in tumor and living cells, Dyes Pigm., 2019, 166, 92–97, DOI:10.1016/j.dyepig.2019.03.033.
- G. Ferrersueta, N. Campolo, M. Trujillo, S. Bartesaghi, S. Carballal and N. Romero, et al., Biochemistry of Peroxynitrite and Protein Tyrosine Nitration, Chem. Rev., 2018, 118, 1338–1408, DOI:10.1021/acs.chemrev.7b00568.
- P. Pacher, J. S. Beckman and L. Liaudet, Nitric Oxide and Peroxynitrite in Health and Disease, Physiol. Rev., 2007, 87, 315–424, DOI:10.1152/physrev.00029.2006.
- D. Yang, Y. Tang, J. Chen, X. Wang, M. D. Bartberger and K. N. H. And, et al., Ketone-Catalyzed Decomposition of Peroxynitrite via Dioxirane Intermediates, J. Am. Chem. Soc., 1999, 121, 11976–11983, DOI:10.1021/ja984358v.
- M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Free radicals and antioxidants in normal physiological functions and human disease, Int. J. Biochem. Cell Biol., 2007, 39, 44–84, DOI:10.1016/j.biocel.2006.07.001.
- R. Mittler, Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, 7, 405–410, DOI:10.1016/S1360-1385(02)02312-9.
- L. Zhang, Y. Sun, Y. Jiang, Y. Li, G. Song and K. Huang, et al., Visual sensing of picric acid in 100% aqueous media based on supramolecular polythiophene assemblies with colorimetric and fluorescent dual response, Chin. Chem. Lett., 2020, 31, 2428–2432, DOI:10.1016/j.cclet.2020.04.003.
- T. Jin, M. Cui, D. Wu, W. Zhu, Y. Xu and X. Qian, NCL-based mitochondrial-targeting fluorescent probe for the detection of Glutathione in living cells, Chin. Chem. Lett., 2021, 32, 3899–3902, DOI:10.1016/j.cclet.2021.06.033.
- C. Prolo, N. Rios, L. Piacenza, M. N. Alvarez and R. Radi, Fluorescence and chemiluminescence approaches for peroxynitrite detection, Free Radicals Biol. Med., 2018, 128, 59–68, DOI:10.1016/j.freeradbiomed.2018.02.017.
- G. Ferrersueta and R. Radi, Chemical Biology of Peroxynitrite: Kinetics, Diffusion, and Radicals, ACS Chem. Biol., 2009, 4, 161–177, DOI:10.1021/cb800279q.
- M. Tian, M. Yang, Y. Liu and F.-L. Jiang, Rapid and Reversible Reaction-Based Ratiometric Fluorescent Probe for Imaging of Different Glutathione Levels in Living Cells, ACS Appl. Bio Mater., 2019, 2, 4503–4514, DOI:10.1021/acsabm.9b00642.
- I. Baeza, J. Fdeztresguerres, C. Ariznavarreta and M. D. La Fuente, Effects of growth hormone, melatonin, oestrogens and phytoestrogens on the oxidized glutathione (GSSG)/reduced glutathione (GSH) ratio and lipid peroxidation in aged ovariectomized rats, Biogerontology, 2010, 11, 687–701, DOI:10.1007/s10522-010-9282-7.
- Y. O. Mannery, T. R. Ziegler, Y. Park and D. P. Jones, Oxidation of plasma cysteine/cystine and GSH/GSSG redox potentials by acetaminophen and sulfur amino acid insufficiency in humans, J. Pharmacol. Exp. Ther., 2010, 333, 939–947, DOI:10.1124/jpet.110.166421.
- K. Srisook, C. Kim and Y. Cha, Cytotoxic and Cytoprotective Actions of O2− and NO (ONOO−) are Determined Both by Cellular GSH Level and HO Activity in Macrophages, Methods Enzymol., 2005, 396, 414–424, DOI:10.1016/S0076-6879(05)96035-7.
- Z. Cao and Y. Li, Protecting against peroxynitrite-mediated cytotoxicity in vascular smooth muscle cells via upregulating endogenous glutathione biosynthesis by 3H-1,2-dithiole-3-thione, Cardiovasc. Toxicol., 2004, 4, 339–353, DOI:10.1385/CT:4:4:339.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. He and T. D. James, The development of a novel AND logic based fluorescence probe for the detection of peroxynitrite and GSH, Chem. Sci., 2018, 9, 3672–3676, 10.1039/C8SC00733K.
- W. Zheng, Y. Zhao, X. Lin, Q. Zhang, K. Xiao and N. Cheng, et al., Rapid and selective detection of picric acid based on supramolecular self-assembly of a cationic perylene diimide in pure aqueous media, Dyes Pigm., 2022, 207, 110761, DOI:10.1016/j.dyepig.2022.110761.
- Q. Han, X. Liu, X. Wang, R. Yin, H. Jiang and J. Ru, et al., Rational design of a lysosomal-targeted ratiometric two-photon fluorescent probe for imaging hydrogen polysulfides in live cells, Dyes Pigm., 2020, 173, 107877, DOI:10.1016/j.dyepig.2019.107877.
- X. Li, X. Gao, W. Shi and H. Ma, Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes, Chem. Rev., 2014, 114, 590–659, DOI:10.1021/cr300508p.
- P. Gao, W. Pan, N. Li and B. Tang, Fluorescent probes for organelle-targeted bioactive species imaging, Chem. Sci., 2019, 10, 6035–6071, 10.1039/C9SC01652J.
- J. Du, M. Hu, J. Fan and X. Peng, Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media, Chem. Soc. Rev., 2012, 41, 4511–4535, 10.1039/C2CS00004K.
- W. Xu, Z. Zeng, J. Jiang, Y. Chang and L. Yuan, Discerning the Chemistry in Individual Organelles with Small–Molecule Fluorescent Probes, Angew. Chem., Int. Ed., 2016, 55, 13658–13699, DOI:10.1002/anie.201510721.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X. He and T. D. James, Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species, Acc. Chem. Res., 2019, 52, 2582–2597, DOI:10.1021/acs.accounts.9b00302.
- S. Li, D. Huang, J. Wan, S. Yan, J. Jiang and H. Xiao, A two-photon fluorescent probe derived from spirobifluorene for fast sensing of hypochlorite and mercury ions, Sens. Actuators, B, 2018, 275, 101–109, DOI:10.1016/j.snb.2018.08.035.
- Q. Zhai, C. Gao, J. Ding, Y. Zhang, B. Islam and W. Lan, et al., Selective recognition of c-MYC Pu22 G-quadruplex by a fluorescent probe, Nucleic Acids Res., 2019, 47, 2190–2204, DOI:10.1093/nar/gkz059.
- N. Trinh, K. A. Jolliffe and E. New, Dual-functionalisation of fluorophores for the preparation of targeted and selective probes, Angew. Chem., Int. Ed., 2020, 59, 20290, DOI:10.1002/anie.202007673.
- B. Wu, T. Xue and Y. He, Design of activatable red-emissive assay for cysteine detection in aqueous medium with aggregation induced emission characteristics, Chin. Chem. Lett., 2021, 32, 932–937, DOI:10.1016/j.cclet.2020.03.047.
- W. Wu, C. Zhang, T. W. Rees, X. Liao, X. Yan and Y. Chen, et al., Lysosome-Targeting Iridium(III) Probe with Near-Infrared Emission for the Visualization of NO/O2˙− Crosstalk via In Vivo Peroxynitrite Imaging, Anal. Chem., 2020, 92, 6003–6009, DOI:10.1021/acs.analchem.0c00259.
- W. Zhang, J. Liu, P. Li, X. Wang, S. Bi and J. Zhang, et al., In situ and real-time imaging of superoxide anion and peroxynitrite elucidating arginase 1 nitration aggravating hepatic ischemia-reperfusion injury, Biomaterials, 2019, 225, 119499, DOI:10.1016/j.biomaterials.2019.119499.
- X. Chen, L. Niu, N. Shao and Q. Yang, BODIPY-Based Fluorescent Probe for Dual-Channel Detection of Nitric Oxide and Glutathione: Visualization of Cross-Talk in Living Cells, Anal. Chem., 2019, 91, 4301–4306, DOI:10.1021/acs.analchem.9b00169.
- H. Komatsu, T. Miki, D. Citterio, T. Kubota, Y. Shindo and Y. Kitamura, et al., Single Molecular Multianalyte (Ca2+, Mg2+) Fluorescent Probe and Applications to Bioimaging, J. Am. Chem. Soc., 2005, 127, 10798–10799, DOI:10.1021/ja0528228.
- F. Yu, P. Li, G. Li, G. Zhao, T. Chu and K. Han, A Near-IR Reversible Fluorescent Probe Modulated by Selenium for Monitoring Peroxynitrite and Imaging in Living Cells, J. Am. Chem. Soc., 2011, 133, 11030–11033, DOI:10.1021/ja202582x.
- F. Yu, P. Li, B. Wang and K. Han, Reversible Near-Infrared Fluorescent Probe Introducing Tellurium to Mimetic Glutathione Peroxidase for Monitoring the Redox Cycles between Peroxynitrite and Glutathione in Vivo, J. Am. Chem. Soc., 2013, 135, 7674–7680, DOI:10.1021/ja401360a.
- C. Sun, W. Du, P. Wang, Y. Wu, B. Wang and J. Wang, et al., A novel mitochondria-targeted two-photon fluorescent probe for dynamic and reversible detection of the redox cycles between peroxynitrite and glutathione, Biochem. Biophys. Res. Commun., 2017, 494, 518–525, DOI:10.1016/j.bbrc.2017.10.123.
- C. Jing, Y. Wang, X. Song, X. Li, Y. Feng and M. Kou, et al., A dual-fluorophore and dual-site multifunctional fluorescent sensor for real-time visualization of mitochondrial ONOO−/GSH cross-talk in living cells, Sens. Actuators, B, 2022, 365, 131847, DOI:10.1016/j.snb.2022.131847.
- Y. Jiang, J. Cheng, C. Yang, Y. Hu, J. Li and Y. Han, et al., An ultrasensitive fluorogenic probe for revealing the role of glutathione in chemotherapy resistance, Chem. Sci., 2017, 8, 8012–8018, 10.1039/C7SC03338A.
- D. Cheng, Y. Pan, L. Wang, Z. Zeng, L. Yuan and X. Zhang, et al., Selective Visualization of the Endogenous Peroxynitrite in an Inflamed Mouse Model by a Mitochondria-Targetable Two-Photon Ratiometric Fluorescent Probe, J. Am. Chem. Soc., 2017, 139, 285–292, DOI:10.1021/jacs.6b10508.
- Y. Wang, B. Li, X. Song, R. Shen, D. Wang and Y. Yang, et al., Mito-Specific Ratiometric Terbium(III)-Complex-Based Luminescent Probe for Accurate Detection of Endogenous Peroxynitrite by Time-Resolved Luminescence Assay, Anal. Chem., 2019, 91, 12422–12427, DOI:10.1021/acs.analchem.9b03024.
- X. Gong, D. Cheng, W. Li, Y. Shen, R. Peng and L. Shi, et al., A highly selective ratiometric molecular probe for imaging peroxynitrite during drug-induced acute liver injury, J. Mater. Chem. B, 2021, 9, 8246–8252, 10.1039/D1TB01534F.
- X. Zhang, Y. Xiao and X. Qian, A ratiometric fluorescent probe based on FRET for imaging Hg2+ ions in living cells, Angew. Chem., Int. Ed., 2008, 47, 8025–8029, DOI:10.1002/anie.200803246.
- Y. Wang, Y. Yang, F. Qiu, Y. Feng, X. Song and G. Zhang, et al., A reversible and colorimetric fluorescence probe for highly sensitive detection of toxic BF3 in air, Sens. Actuators, B, 2018, 276, 166–172, DOI:10.1016/j.snb.2018.08.095.
- X. Jia, Q. Chen, Y. Yang, Y. Tang, R. Wang and Y. Xu, et al., FRET-Based Mito-Specific Fluorescent Probe for Ratiometric Detection and Imaging of Endogenous Peroxynitrite: Dyad of Cy3 and Cy5, J. Am. Chem. Soc., 2016, 138, 10778–10781, DOI:10.1021/jacs.6b06398.
- J. Miao, Y. Huo, Q. Liu, Z. Li, H. Shi and Y. Shi, et al., A new class of fast-response and highly selective fluorescent probes for visualizing peroxynitrite in live cells, subcellular organelles, and kidney tissue of diabetic rats, Biomaterials, 2016, 107, 33–43, DOI:10.1016/j.biomaterials.2016.08.032.
- M. Yang, J. Fan, W. Sun, J. Du and X. Peng, Mitochondria-Anchored Colorimetric and Ratiometric Fluorescent Chemosensor for Visualizing Cysteine/Homocysteine in Living Cells and Daphnia magna Model, Anal. Chem., 2019, 91, 12531–12537, DOI:10.1021/acs.analchem.9b03386.
- Y. Li, X. Xie, X. Yang, M. Li, X. Jiao and Y. Sun, et al., Two-photon fluorescent probe for revealing drug-induced hepatotoxicity via mapping fluctuation of peroxynitrite, Chem. Sci., 2017, 8, 4006–4011, 10.1039/C7SC00303J.
- J. Zhang, X. Zhen, P. K. Upputuri, M. Pramanik, P. Chen and K. Pu, Activatable Photoacoustic Nanoprobes for In Vivo Ratiometric Imaging of Peroxynitrite, Adv. Mater., 2017, 29, 1604764, DOI:10.1002/adma.201604764.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure, synthesis, characterization (1H NMR, 13C NMR and MS spectra), additional spectroscopic data and cell cytotoxicity. See DOI: https://doi.org/10.1039/d3ob00872j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |