Open Access Article
Open Access ArticleRecent advances in PEDOT/PProDOT-derived nano biosensors: engineering nano assemblies for fostering advanced detection platforms for biomolecule detection
Jayakrishnan
Aerathupalathu Janardhanan
 * and
Hsiao-hua
Yu
* and
Hsiao-hua
Yu
 *
*
Smart Organic Materials Laboratory (SOML), Institute of Chemistry, Academia Sinica No. 128, Sec. 2, Nankang District, Taipei City 115201, Taiwan. E-mail: jayakrishnanaj@gate.sinica.edu.tw; bruceyu@gate.sinica.edu.tw
First published on 30th July 2024
Abstract
With the recent unprecedented emergence of a global pandemic, unknown diseases and new metabolic patterns expressing serious health issues, the requirement to develop new diagnostic tools, therapeutic solutions, and healthcare and environmental monitoring systems are significantly higher in the present situation. Considering that high sensitivity, selectivity, stability and a low limit of detection (LOD) are inevitable requirements for an ideal biosensor, the class of conducting polymers of poly(3,4-ethylenedioxythiophene) (PEDOT) and recently poly(3,4-propylenedioxythiophene) (PProDOT) materials have been demonstrated to be promising candidates for designing sensor devices. Nanostructure engineering of these polymeric materials with tunable surface properties and side chain functionalization to enable sensor probe conjugation combined with signal amplification devices such as OECTs and OFETs can fulfil the requirements of next-generation smart nano-biosensors. In this review, we analyze recent reports on PEDOT/PProDOT nanostructures and nanocomposites for developing nano-biosensors and their application in the detection of different biomarkers, environmental, toxicology, marine and aquatic monitoring, forensic and illicit drug detection, etc. In addition, we discuss the challenges associated with the design of PEDOT/PProDOT nano-biosensors and future perspectives on the exploration of novel sensor platforms, particularly PProDOT derivatives for bioelectronics and novel design strategies for next-generation smart nano-biosensors.
1. Introduction
The field of electrically conducting polymers has been growing since the groundbreaking discovery by Alan Heeger, Hideki Shirakawa and Alan MacDiarmid reported that oxidized poly(acetylene) exhibits electrical conductivity. Their pioneering work exploring the transformation of insulating ‘plastic’ materials into electrically conducting building blocks unfolded a new era of electrically conducting polymers.1–4 The Nobel Prize in Chemistry was awarded to Heeger, Shirakawa, and MacDiarmid for the discovery of electrically conducting polymers, signaling the development of this research field. The perspective of organic chemists to tailor the intrinsic conducting properties of materials by exploring (hetero) aromatic rings created a new library of organic conducting polymeric materials made up of naphthalene, anthracene, pyrrole, thiophene and phenylene.5–7 Among the various conducting polymers reported to date, polydioxythiophene derivatives, particularly poly(3,4-ethylenedioxythiophene) (PEDOT) and recently poly(propylenedioxythiophene) (PProDOT)-derived materials, have attracted much more attention due to their unique properties such as low oxidation potential, ease of side chain functionalization, superior biocompatibility, soft nature and film-forming ability.8–23 Engineering nanostructures of PEDOT/PProDOT derivatives endow them with potential for diverse applications in materials, biotechnology, electronics, bio-sensing, tissue engineering, environmental monitoring, forensic science, etc. In this review, we focus on the molecular design strategy, diverse nanostructure engineering and applications of PEDOT and PProDOT and their composites for bio-sensing (Scheme 1).2. Poly(3,4-ethylenedioxythiophene) (PEDOT) – a versatile candidate with unbeatable properties
As one of the most successful conducting polymers with versatile properties both in fundamental and practical applications, PEDOT is considered a shining star among the reported conducting polymers with widespread applications in electronics, electric, anti-stacking, and electrode materials, inkjet printing, biomedical engineering, sensor development and many more.24–35 PEDOT was developed in the Bayer AG laboratories in the late 1980s from the corresponding EDOT monomer.36 Later, the modification of the PEDOT material with the water-soluble poly(styrene sulfonic acid, PSS) resulted in another novel polymeric material known as PEDOT:PSS, possessing excellent conductivity, stability, biocompatibility and transparency. To date, PEDOT:PSS has been widely applied in electronics, electrical and thermoelectric generators, photolithography, antistatic coatings, electrolyte in capacitors, batteries, solar cells, touch screens, organic light-emitting diodes, organic electrochemical transistors, etc.37–43 Furthermore, several PEDOT derivatives were designed by researchers considering their extraordinary properties, wide substrate scope to design novel molecular scaffolds and potential utility in diverse areas including electrode platforms, battery development, solar cells, electrochromic devices, organic electrochemical transistors (OECTs), field effect transistors (FETs), advanced bioelectronic platforms, wearable and flexible sensors, coatings, organic light-emitting diodes (OLEDs), and different display applications.20,44–51The conventional design of EDOT monomers mainly involves two methods, which can be found in many reports in the literature. Briefly, the first synthetic route involves the use of thiodiglycolic acid reagent and a cyclization reaction to produce a thiophene ring. Subsequently, EDOT derivatives are synthesized by either Mitsunobu reaction or base-catalyzed ring opening of epichlorohydrin derivatives. Finally, hydrolysis and reduction reactions result in the formation of the desired product. However, this old method has certain limitations including more synthetic steps compared to the second route, in which the Williamson etherification reaction of 3,4-dimethoxythiophene with 1,2-diols (EDOTs) or 1,3-diols (ProDOTs) can produce diverse EDOT/ProDOT derivatives (Scheme 2A). Although this route is expensive compared to the other route, a number of novel EDOT/ProDOT derivatives were synthesized using this approach.
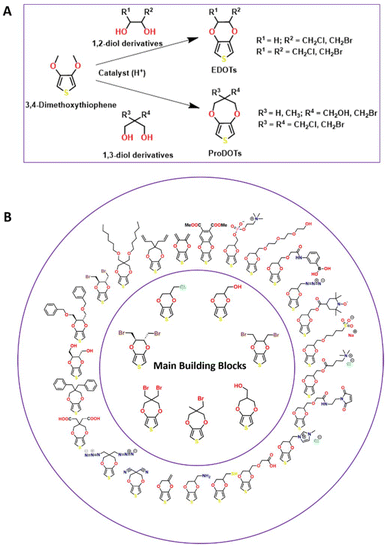 | ||
| Scheme 2 (A) Representative synthetic route for EDOTs and ProDOTs. (B) Examples of EDOT/ProDOT derivatives. | ||
The commercialized hydroxymethyl-appended EDOT (EDOT-OH, EDOT-MeOH or EDOT-M) is one of the most widely explored monomers in the EDOT family, highlighting the facile conversion of the hydroxyl group to other useful functional groups such as ester, carboxylic acid, alkyl, aromatic, alkyne, azide, amine, sulfonate, perfluoro, maleimide, phosphorylcholine, sulfobetaine, ethylene glycol, and boronic ester groups. Furthermore, chloromethyl- or bromomethyl-appended EDOT has also served as an interesting starting material to design more EDOT derivatives including ionic liquids such as imidazolium-incorporated EDOT, EDOT-SH, EDOT-azide, EDOT-amine, EDOT-acrylate, and EDOT-anthraquinone (Scheme 2B).
Poly(3,4-propylenedioxythiophene) (PProDOT, monomer denoted as ProDOT), another member of the PEDOT family, has not been explored as widely as many other PEDOTs. However, one of the most exciting features of PProDOT derivatives is the synthesis of regio-regular polymeric scaffolds and their superior electrochromic properties.52–55 Very recently, a set of C2-symmterical EDOTs were also developed by researchers, which are particularly interesting due to the presence of chiral centers in the molecular scaffolds.56 There are many reports discussing the chemistry of EDOT/ProDOT derivatives and their polymeric structures as well as their applications in different areas. Therefore, the detailed synthetic design strategies for EDOT/ProDOT monomer scaffolds and the chemistry of their corresponding polymer materials are beyond the scope of this review. Alternatively, our objective is to discuss the prospects of PEDOT and PProDOT derivatives for the design and development of nano-biosensors with particular interest in the engineering of different PEDOT/PProDOT polymeric nanostructures/nanocomposites by various polymerization techniques, which will be discussed in the following sections.
3. The bioelectronic interfaces (BEIs) – bridging material properties with biological events
Biological systems are some of the most complex systems, which confine the synergistic involvement of chemical, physical and many other genetic networks in multiple dimensions and timescales. The human body is composed of millions of components and its homeostasis is controlled through electrical impulses coordinated by the brain. Accordingly, there are many challenges associated with the incorporation of new electronic systems or external foreign materials in living organisms, human tissues, proteins, small biological entities and diverse cell environments because preserving their normal homeostasis and healthy environment is extremely important even after interfacing with electronics for sensing or regulating different biological functions in the human body. Therefore, a key in the design and development of novel bioelectronic interface materials is that they must satisfy many criteria.57–59Practically, it is not easy to incorporate readily available electronic components such as metals and inorganic semiconducting materials in the human body, even if they exhibit bio-compatibility. In this case, one of the biggest challenges in the incorporation of these materials as a human interface is their high rigidity despite their high electrical conductivity and mechanical stability. Given that human cells and tissues are composed of soft materials, the incorporation of soft materials as an interface can provide a better platform without severe mechanical rupturing, enabling the possibility of their long-term operation and lower interfacial capacitance. Thus, organic molecules with electrical conductivities similar to many semiconductor inorganic materials are promising candidates to serve bioelectronic interface (BEI) materials.60–62 The advantage of using organic conducting polymers as a bioelectronic interface mainly depends on the high biocompatibility of polymer materials and their tunable surface properties, such as surface roughness, surface morphology, higher flexibility to cope with soft tissues in the human body, and easy functionalization of their side chain to control their properties according to the requirements at the bioelectronic interfaces. However, the diverse biological platforms associated with human tissues make the development of bioelectronic interfaces a challenging task for researchers.
Presently, conducting polymers serve as one of the superior choices as bioelectronic interfaces due to their many chemical, physical, mechanical and biocompatibility advantages. Many studies have explored the utilization of PEDOT and its derivatives as some of the best bioelectronic interfaces. PEDOT derivatives with different functional groups and surface morphologies have been designed and used in various applications such as cell adhesion, bio-sensing, suitable materials to mimic cell membranes, capturing agents for circulating tumour cells and drug delivery. In this section, we focus on the strategic design of PEDOT/PProDOT derivatives to build unique bioelectronic candidates and their potential applications in bio-sensing, cell functions, drug delivery, tissue-engineering, electrode coatings, etc. The high biocompatibility and soft nature of PEDOT/PProDOT materials demand careful engineering of their molecular scaffolds to serve as bioelectronic platforms. Post-modification of the polymer chain or monomer side chain functionalization is widely used to design PEDOT/PProDOT bioelectronic interfaces. The design strategy and potential applications of PEDOT/PProDOT materials to serve as bioelectronic platforms are discussed below.
3.1 Bio-sensing applications
Functionalized PEDOTs have been widely used to develop high-efficiency sensor platforms. Recently, PProDOT derivatives have also emerged as versatile materials for bio-sensing applications, where different strategies have been developed for the design of these sensor platforms. One interesting approach involves the introduction of redox active species in the side chain of PEDOT/PProDOT polymer scaffolds or functionalization of monomers with redox molecules. In this case, the redox system can be either a biomolecule such as an enzyme molecule or molecules having redox characteristics such as ferrocene, Prussian blue, methylene blue, phenazine dye, and tris(bipyridine) ruthenium(II) [Ru(bpy)3]2 (best known for the development of electrochemiluminescence-based sensors).These sensor systems can monitor the changes in the biological environment during the interaction of biomolecules with their surface. Consequently, they have been applied in the development of wearable electronics for healthcare monitoring, detection of toxic chemicals such as heavy metal ions and volatile organic compounds (VOCs), drug detection, etc. For example, Saleh et al. reported the use of PEDOT:PSS-modified glucose oxidase (GOx) as the bio-recognition element and ferrocene (Fc) as the redox mediator. The enzymatic mechanism of sensing involves electrochemical reactions, where the analyte glucose interacts with GOx (antigen–antibody) and GOx is reduced with the generation of electrons, which shuttle by Fc from the enzyme center to PEDOT:PSS. The electron transfer leads to the reduction of PEDOT:PSS, which can be monitored through different electrochemical methods.63
Besides the use of redox probes directly in the sensor surface, PEDOT/PProDOT-based bioelectronic systems can be designed using other bio-recognition probes such as antibodies, aptamers, nucleic acids, cells, bacteria, viruses and polymers via the molecular imprinting technique (MIP). Hai et al. reported the sensing of human influenza virus (HIV) with PEDOT bearing sialic acid-terminated trisaccharides.64 The conjugation of 2,6-sialyllactose to oxylamine-bearing EDOT enabled the successful immobilization of the bio-recognition element. This sensor platform design highlights the ability to achieve mass production and point-of care testing of the influenza virus. Another virus sensor platform was developed by Ito and co-workers in which a PEDOT-based electrochemical peptide probe was designed to detect the influenza virus.65 The interesting application of PEDOT/ProDOT bioelectronics as promising sensor platforms was reported by Haya et al. Their work highlighted the exploration of PEDOT and ProDOT(COOH)2 conjugated with MUC1-specific aptamer as bioelectronic scaffolds to detect Mucin-1, an epithelial glycoprotein. The target analyte was a breast cancer biomarker.66
Furthermore, the design of novel sensor platforms for drug detection is highly demanding at present. Arvas et al. developed an electrochemical paracetamol sensor platform based on hybrid PEDOT and piperazine-substituted triazole-coumarin modified on a flexible highly oriented pyrolytic graphite electrode (HOPG).67 The sensing of organic pollutants and toxic chemicals such as organophosphates is an important application of PEDOT-derived materials. Nitrophenol (NP) is an organic pollutant that is released in large quantities from widespread industrial activities. Organophosphates such as diethyl 2,4-dinitrophenyl phosphate (DEDNPP) and diethyl 4-nitrophenyl phosphate (Paraoxon) are toxic and harmful to humans and the aquatic environment. Furthermore, these toxic chemicals and pesticides can disrupt the food chain. Moreover, organophosphates can be used as chemical weapons and are classified as potent contaminants as well as carcinogenic materials. Thus, the detection of these toxic materials and pollutants is important for the survival of organisms. Accordingly, an interesting sensor platform was developed by Hryniewicz et al. for the detection of nitrophenols and organophosphates utilizing PEDOT materials.68 Metal sensing is also another application of novel detection platforms. Research outcomes have shown that many promising sensor platforms have been developed for the sensing of heavy metal ions. From the perspective of both environmental concerns and human health, the detection of trace amounts of heavy metals is particularly important. Ngoensawat et al. reported the use of electrospun PEDOT and silver nanoparticles as a catalyst-free approach to engineer a conductive platform for the detection of heavy metals such as Zn(II), Cd(II), and Pb(II).69 Presently, the design and development of PEDOT/PProDOT-based materials for sensing applications have become hot research areas. The functionalization of polymer scaffolds to detect the target analytes, combining signal amplification devices such as OECT and OFET to enhance the sensitivity allows the detection of low-concentration analytes.
3.2 Applications in drug delivery
Novel materials for the site-specific delivery of therapeutics with reduced toxicity and patient tolerance with efficient pharmacology impacts are in high demand for the treatment of various diseases. In this case, polymeric materials have attracted attention from researchers for use as payloads to deliver drug molecules for chronic diseases. The site-specific delivery of drug molecules and the immune response towards foreign bodies when novel materials are implanted in the body are the most challenging issues in designing novel materials. Accordingly, PEDOT and its derivatives have been utilized as drug delivery systems. PEDOT can be functionalized with either drug molecules or nanoparticles, allowing the controlled release of therapeutic compounds, and some of the interesting reports are discussed here. In the work reported by Boehler et al., the anti-inflammatory drug dexamethasone was stored in PEDOT deposited on a flexible neural probe, enabling unrestricted neural recording.70 Moreover, the design allowed the controlled release of the drug molecules using the cyclic voltammetry (CV) technique. This drug delivery system has potential bioelectronic application in the fabrication of anti-inflammatory electrodes and long-term stable neural interfaces. Woeppel et al. reported the preparation of a hybrid system of PEDOT/silica nanoparticles for the controlled delivery of sodium nitroprusside, a vasodilator. The carbon fiber electrode with the drug delivery system was implanted in the cortex of a mouse, exhibiting potential applications in neural electrode–tissue interfaces with insight into neurovascular coupling.71 In another work, PEDOT and RGD-alginate hydrogel was used for coating a cochlear implant. The work reported by Chikar et al. demonstrated the successful release of brain-derived neurotrophic factor (BDNF), an important protein for the development and maintenance of the cochlea.72 Another application of a PEDOT bioelectronic platform for drug release was demonstrated by Hsiao et al. The novel electric cell–substrate impedance sensing (ECIS) consisted of PEDOT:PSS, polyethylene oxide (PEO) and (3-glycidyloxypropyl) trimethoxysilane (GOPS) materials to encapsulate antitumor chemotherapeutic agents such as doxorubicin (DOX), docetaxel (DTX), and combination of DOX/DTX. The drug release and subsequent monitoring of HeLa cells and MCF7 cells were performed based on the impedance responses. This drug delivery bioelectronic platform is a promising tool for drug-screening applications.73 PEDOT hydrogels have been used as promising platforms for drug delivery. In a report by Sakunpongpitiporn et al., they demonstrated the use of PEDOT:PSS-silk fibroin hydrogels as payloads to deliver insulin through iontophoresis.74 Tremendous efforts are still on-going to design novel bioelectronic systems based on PEDOT/PProDOT materials to serve as drug delivery payloads. As mentioned before, site-specific drug delivery with low-toxicity and high biocompatibility are key considerations for novel drug delivery systems, where novel material design strategies need to be adapted.This method is beneficial for applications in tissue engineering, regenerative medicine, or implanted drug delivery systems given that it allows localized drug distribution, accurate dosing, and sustained release profiles.
3.3 PEDOT/PProDOT materials as cell interface bioelectronics
Bioelectronic materials have unique properties to combine with cell interfaces. Given that the introduction of a foreign body in living cells induces an immune response and high possibility of rejection, the design of novel bioelectronic materials should have significant cell-interface properties. PEDOT materials have been proven as an excellent choice as cell–material interface platforms to modulate cell functions. There are many reports in which PEDOT/PProDOT materials have been explored as cell interface platforms. Hsiao et al. developed a 3D-PEDOT-based bioelectronic interface platform for capturing circulating tumor cells (CTC). The large-scale 3D micro/nanorod arrays engineered from carboxylic acid-appended PEDOT exhibited high biocompatibility and efficiency to capture CTCs. This bioelectronic interface platform is a promising candidate for use as a tumor cell-capturing agent.29 Polymer brushes are interesting choice in cell interface studies. Zhao et al. engineered polymer brush-grafted PEDOT films for protein absorption and cell adhesion. An EDOT-Br and EDOT mixture was utilized for atom transfer radical reaction (ATRP), which was further modified to a polymer brush through the grafting of poly((oligo-ethylene glycol) methacrylate), poly(OEGMA), and zwitterionic poly([2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide), poly(SBMA) brushes. These types of bioelectronics have potential applications in biometallic implants, which are essential parts of biomedical engineering.31 Another interesting application of PEDOT-based bioelectronic platforms was reported by Sauvage et al. for the design of a cardiac patch using a PEDOT:PSS and polyvinyl alcohol (PVA) hydrogel. The hydrogel material was bio-functionalized with an N-cadherin mimic peptide to mediate strong cell–scaffold adhesion. It was observed that the modification offered enhanced adherence and proliferation of cardiac fibroblasts (CFbs). Moreover, the design reduced the formation of bacterial biofilms, particularly against Staphylococcus aureus, highlighting a promising material that can be used to prevent surgical site infections (SSIs).75 The development of these innovative implant materials using PEDOT bioelectronic platforms as cell interfaces offers promising applications in health monitoring and wearable electronics. Furthermore, the promising application of PEDOT bioelectronic platforms as cell interface materials in the design of wound healing materials has been reported. For example, a mussel-inspired PEDOT gelatin-based hydrogel was developed by Li et al. for in vitro wound healing. The flexible, biodegradable hydrogel was prepared via the in situ polymerization of gelatin-polydopamine in EDOT in the presence of poly(ethylene glycol) diglycidylether (PEGDE). The newly designed material showed fibroblast adhesion, proliferation and migration, highlighting the use of this material as a promising candidate for wound dressing.764. Nanostructures of PEDOT/PProDOT derivatives – a promising strategy to tune the material properties
The extraordinary properties of materials with dimensions on the nanoscale have attracted attention from researchers to construct novel PEDOT derivatives/nanocomposites, and recently PProDOT derivatives have been utilized in diverse areas such as bio-sensing, biomedical engineering, cell-based chip applications, optical device development, wearable gadgets for healthcare monitoring, electrochromic devices, battery development, solar cells, supercapacitors, antistatic coatings, ink-jet printing, environmental, marine and aquatic monitoring, forensic and toxicology, hydrogen storage, OLEDs, and OPVs.33,48,77,78 Due to the excellent biocompatibility of PEDOT/PProDOT materials, these materials can be reshaped for use as bioelectronic interface platforms and biosensors for disease monitoring, a prospect in high demand, especially in the COVID-19 post-pandemic era. Briefly looking into the chemistry of PEDOT/PProDOT nanomaterials, their high surface area can enhance signal amplification in the design of biosensor platforms.79–81 Furthermore, their high surface area facilitates improved charge transport, making them ideal for applications such as organic solar cells, organic field-effect transistors, and supercapacitors. In addition, a higher surface area also promotes interactions with other materials, enabling the fabrication of hybrid nanocomposites with improved material properties.One of the promising applications of PEDOT-nanomaterials has been highlighted by the exploration of polymeric inks for ink-jet printing. Given that printing technology is one of the most widely explored industries globally, the design of novel materials with extraordinary properties is in high demand. In addition, for the design of flexible electronics, wearable sensors and organic photovoltaics, also printing techniques are essential. Thus, efforts have been devoted to developing novel PEDOT nanomaterial-based inks for printing, where Chang-Jian et al. reported the preparation of a PEDOT:PSS and WO3 nanoparticle electrochromic ink that can be easily processed through spray-coating or ink-jet printing to design novel electrochromic windows.82 The WO3/PEDOT:PSS ink was used to design a composite film by inkjet printing, which possessed a crack-free and uniform surface. Similarly, Pillai et al. designed conductive ink using PEDOT:PSS and MWCNT for the design of an energy-efficient all-printed flexible heater. This work highlights the design of PEDOT-MWCNT conductive ink with room temperature curable capability.83 These materials can be used effectively in the fabrication of flexible electronics. An interesting 3D printing method was reported by Su et al. using PEDOT:PSS and Ag nanoparticles for the 3D-printing of flexible OLED displays. This work highlighted the use of the 3D printing method without any microfabrication process.84 Cui et al. prepared a PEDOT:PSS/Ag nanoparticle composite via an in situ polymerization technique and used the ink-jet printing technique to coat on cotton fabric with polydopamine to enhance the adhesion properties.85 The design of these materials is highly useful for the fabrication of durable conductive textiles for healthcare, energy storage, sensing, etc. Cheng et al. reported the fabrication of an inkjet-printed flexible storage device based on a PEDOT:PSS/Ag grid electrode. Polyethylene terephthalate substrates were used for ink-jet printing, and subsequently flexible transparent all-solid state supercapacitors were engineered.86
Several reports focused on the design of novel conductive materials for ink-jet printing. The advantage of PEDOT-based conductive material-based ink-jet printing includes its easy fabrication and design on flexible substrates for wearable electronics, healthcare monitoring, energy storage, supercapacitors, flexible batteries, and diverse electrode platforms for bio-sensing applications. However, there are challenges associated with the development of novel polymeric materials for ink-jet printing. The chemical and physical properties of inks, such as surface tension, solute particle size, and density, used for printing should be considered in the development of novel ink materials. For example, clogging is one of the main problems observed during ink-jet printing using PEDOT:PSS inks, where the aggregation of solutes and their subsequent blocking of the nozzle tip during printing may damage the product. Also, satellite drop formation is another challenge in which undesired ink drops are observed on the printing substrate. Another challenge associated with the use of PEDOT:PSS materials for printing is the dilution properties of the ink. Given that commercial PEDOT:PSS is a water-based conductive material, the polymer ink composition with co-solvents must carefully be adjusted. Layer formation on the nozzle tip and adhesion properties on the printing surface are also challenges associated with PEDOT material-based inks. However, the careful design of PEDOT materials can solve these problems to a certain extent. For example, the addition of high-boiling point co-solvents such as glycerol and ethylene glycol can help avoid clogging and satellite drop formation to some extent. Also, the incorporation of nanoparticles to create PEDOT-derived hybrid nanocomposites is good choice to design thermostable and flexible electronics. Another strategy is the careful composition of materials to enhance the adhesion behavior of conductive inks to bypass cracking and irregular printing problems. The ultimate goal of printing technology is the mass production in a cost-effective method in greener way.
Furthermore, incorporating nanostructured building blocks in polymer scaffolds can enhance the flexibility, stretchability, and mechanical stability of polymers, which are useful to develop flexible electronics, wearable devices, and bioelectronics, where the materials need to withstand mechanical deformation without losing their electrical properties.87,88 In addition, the attachment of biomolecules such as antibodies, enzymes, proteins, aptamers, bacteria, viruses, and DNA as sensor probes is significantly higher in nanostructured polymeric materials due to their large surface area with nanoscale dimensions, which can significantly enhance the sensitivity of biosensor platforms. Several research outcomes have shown that engineering nanostructures as electrode platforms significantly reduces the impedance at the electrode–electrolyte interface.89–91 It was also reported that a nano-patterned conducting polymer layer (PEDOT:PSS) showed a decrease in solution resistance with an increase in nano-patterning due to the increase in surface area. In the investigation by Sanaur and co-workers, the impedance of the nano-patterned PEDOT:PSS/Au electrode was lower compared to the impedance exhibited by flat PEDOT:PSS/Au electrodes.92 Theoretical aspects of a decrease in solution resistance are associated with an increase in surface area.93 Furthermore, the interaction of nanomaterials with living cells had an impact on cell physiology, particularly gold nanoparticles (AuNP) have been extensively studied for medical applications.
4.1 Engineering strategy of PEDOT nanostructures
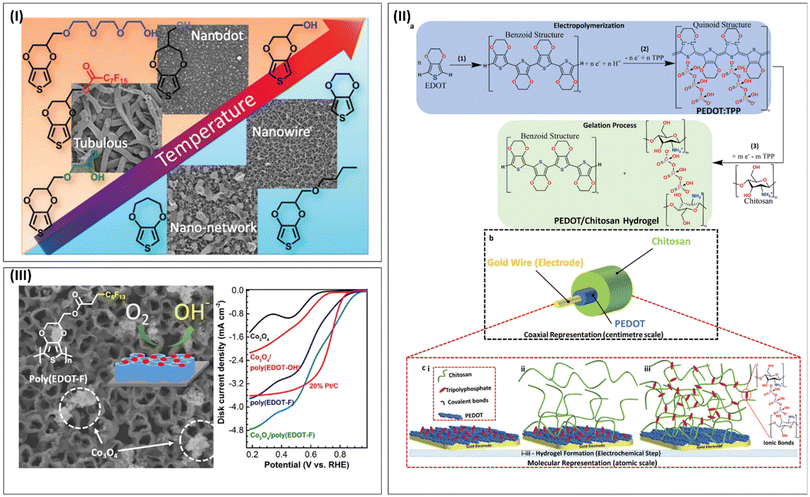
Nanostructured PEDOT derivatives or PEDOT/nanocomposites can be engineered through methods such as chemical oxidative polymerization, electrochemical polymerization, vapour phase polymerization, self-assembly and incorporation of metal nanoparticles, metal oxides, hybrid conducting polymeric scaffolds, carbon materials/polymeric composites, and conducting polymer–metal–organic frameworks (MOF). Briefly, the chemical polymerization of EDOT/ProDOT monomers is the basic method for the synthesis of PEDOT/PProDOT derivatives. The commonly used oxidants include iron(III) chloride (FeCl3) and iron(III) tosylate (Fe(OTs)3), which react with the monomers to form the corresponding polymers. The detailed chemistry of the polymerization mechanism can be found in many reports in the literature. Most of the recent reports to engineer nanostructures of PEDOT/PProDOT through chemical oxidative polymerization involved the use of templates (DNA, micro emulsions, micelles, and surfactants as soft templates or hard templates such as anodized aluminium oxide-AAO), micro-emulsions and block copolymers as well as polymer hybrid nanocomposite methods.Yu and co-workers developed functionalized PEDOT nano/microrod structures using the AAO method (Fig. 1-I). The AAO membrane was coated with a PEDOT precursor solution and polymerization was carried out inside the nanopores of the membrane. After polymerization, the template was dissolved, leaving behind PEDOT nanostructures.94 A modified self-assembled micellar soft-template method was used by Guo et al. to engineer PEDOT nanowires (NWs). Subsequently, flexible and self-supporting WSe2–nanosheet/PEDOT-NW composite films were prepared through the vacuum filtration method (Fig. 1-II). This report highlighted the advantage of nano sheets composed of inorganic materials/organic nanowire composites, which can improve the thermoelectric properties of conducting polymers.95 Raghavan et al. reported the interesting engineering of poly(3,4propylenedioxythiophene)-derivative (PProDOT-IPBz2) thin nanobelts constructed by chemical polymerization involving the reverse microemulsion technique96 (Fig. 1-III). Zhang and coworkers reported the preparation of a nanostructured polythiophene derivative for the fabrication of a large-size electrochromic device, in which the advantage of in situ chemical oxidative polymerization of EDOT without any template was employed to obtain the PEDOT material, followed by spray coating, forming PEDOT nanoparticles.97
 | ||
| Fig. 1 Different approaches to engineer PEDOT/PProDOT-derived nanostructures. (I) Schematic representation of functionalized PEDOT nano/microrod array engineering using AAO method. Reproduced from ref. 94 with permission. Copyright 2013, Wiley. (II) Schematic representation of PEDOT nanowire designed through self-assembled micellar soft-template method. Reproduced from ref. 95 under Creative Commons Attribution https://creativecommons.org/licenses/by/4.0/ licence. (III) Schematic representation of reverse microemulsion technique to engineer PProDOT-IPBz2 nanobelts. Reproduced from ref. 96 with permission. Copyright 2017, Elsevier. (IV) Template-free electropolymerization of ProDTM to obtain PProDTM nanowire structure: SEM images and graphical representation. Reproduced from ref. 98 with permission. Copyright 2018, Elsevier. | ||
A ProDOT derivative named (3,4-dihydro-2H-thieno [3,4-b] [1,4]dioxepin-3-yl)methanol (ProDTM) nanowire was reported by Niu et al. (Fig. 1-IV). The nanowires were engineered through the template-free electropolymerization method, which showed excellent redox property and stability for use in future supercapacitor applications.98 Recently, Pawula et al. reported an interesting work on the nanostructure engineering of a PEDOT-Tos thin film using the block copolymer lithography technique (Fig. 2-I). Self-assembled poly(styrene)-block-poly(methyl methacrylate) (PS-b-PMMA) layers were used as a mask to obtain the nanostructured polymeric material.99 Lee et al. reported the highly ordered nano-confinement effect from an in situ-polymerized EDOT derivative and PEO-b-PPO-b-PEO block copolymers using iron tosylate as an oxidant (Fe(Tos3))100 (Fig. 2-II). Chowdhury et al. reported the nanostructure engineering of PEDOT-Cl using oxidative chemical vapor deposition (oCVD) with antimony pentachloride (SbCl5) as the liquid oxidant and EDOT as the monomer. The polymerization technique resulted in the formation of a thin film of PEDOT-Cl polymer with excellent optoelectronic properties, precise control of the nanostructure formation, and good stability (Fig. 2-III). This material was used as a hole-transporting material in perovskite solar cells.101
 | ||
| Fig. 2 Different approaches to engineer PEDOT/PProDOT derived nanostructures (continue.) (I) Schematic presentation of Block copolymer lithography technique on PEDOT-Tos for nanostructures engineering. Reproduced from ref. 99 with permission. Copyright 2023 Elsevier. (II) In situ polymerized EDOT derivative and PEO-b-PPO-b-PEO block copolymers using (Fe(Tos3)) to obtain highly ordered nano confinement. Reproduced from ref. 100 with permission. Copyright 2017 ACS. (III) Oxidative chemical vapor deposition (oCVD) of EDOT with antimony pentachloride (SbCl5) as a liquid oxidant to obtain PEDOT-Cl nanostructures. Reproduced from ref. 101 with permission. Copyright 2024 ACS. | ||
As a member of the PEDOT family, ProDOT and its derivatives were synthesized and employed to engineer nanostructures using different methods. However, due to the solubility concerns of the polymer as well as its processability in the commonly used solvents, the surface tuning of ProDOT derivatives in chemical oxidative polymerization was significantly limited. In addition, there are few reports on the engineering of ProDOT-based nanostructures/nanocomposites. Deshagani et al. reported the fabrication of a supercapacitor composed of an NiMoO4@NiMnCo2O4 composite grown directly on Ni-foam and coated with PProDOT and combined with flaky carbon derived from groundnut shells, which was deployed as the cathode and anode in an asymmetric supercapacitor. PProDOT enhanced the overall electrical conductivity of the cathode, besides furnishing extra electroactive sites, acting as a high performance supercapacitor for electrochromic devices.102 A high-surface-area hybrid nanomaterial for efficient bioelectronic application using PEDOT:PSS and nanowire template 3D fuzzy graphene was reported by Garg et al. This material could be used for miniaturized microelectrodes for bioelectronic applications given that it showed excellent stability and electrochemical performance on continuous operation.103
Besides the extensive investigations on PEDOT, PProDOT and their derivatives to form nanostructures, poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) has also been used in combination with other nanoparticles to design PEDOT:PSS nanocomposites. In the usual procedure, these materials are developed by incorporating different nanoparticles such as gold nanoparticles (AuNP), silver nanoparticles (AgNP), quantum dots, carbon nanotubes, nanostructured graphene, transition metal nanoparticles, and nano-dispersions.104–106
4.2 Electrochemical polymerization
Electrochemical polymerization involves the deposition of polymeric materials on the surface of electrodes by the oxidation of monomers through cationic radicals. The applied potential, polymerization time, nature and composition of the monomers, electrode material, etc. influence the formation of the polymer and its surface morphology, nanostructure assembly and electrical properties.47,107 However, electrochemical polymerization leads to the formation of a small amount of polymer compared to the product formed during chemical polymerization.One of the interesting works reported for the electropolymerization of PEDOT/PProDOT by Yu and coworkers highlights the easiest way to make diverse nanostructures of PEDOT/PProDOT derivatives without the assistance of any templates. Controlling the electropolymerization conditions and modulating the functional groups at the polymer side chain resulted in the formation of nanotubes, nanodots, nanowires and nanofibers on the electrode platform.47 This report highlights an efficient and simple method to design a diverse library of PEDOT/PProDOT derivatives tailored with useful functional groups for bio-conjugation (Fig. 3-I). Zubair et al. reported an interesting work on the synthesis of PEDOT nanofibers using poly(vinyl alcohol) (PVA)-graphene and PEDOT by combining the electrospinning and electropolymerization techniques. The nanofibers of PVA-GO were prepared by electrospinning, and later PEDOT was electropolymerized on them through the template-free electropolymerization technique.108 Recently, Silva et al. prepared a PEDOT-chitosan hydrogel assembly by utilizing PEDOT materials on the electrode surface by the electropolymerization technique. The novel platform was explored due to the unique electroactivity of PEDOT materials, which can be utilized for bioelectronic applications109 (Fig. 3-II). Another interesting work on engineering nanostructures was reported by Mansoure et al. This report involved the use of PEDOT, PEDOT-OH and perfluoro-functionalized PEDOT (PEDOT-F), which were electropolymerized to obtain nanostructures. The new system was used as a catalyst for the oxidation–reduction reaction110 (Fig. 3-III). Luo and coworkers recently reported the interesting nanostructure engineering of zwitterionic phosphoryl choline (PC)-appended EDOT and EDOT-OH with different feed ratios through the electropolymerization method without any templates. This newly engineered material was used to understand the adsorption behavior of biomolecules111 (Fig. 4-I). Darmanin et al. prepared nanostructures of an EDOT derivative, 3,4-phenylenedioxythiophenes under simple electropolymerization conditions. A series of different 3,4-phenylenedioxythiophene monomers with different substituents allowed the formation of polymer surfaces with different surface morphologies46 (Fig. 4-II). The galvanostatic deposition of PEDOT nanostructures using an EDOT/aqueous solution of sodium dodecyl sulfate solution was reported by Nguyen et al. The deposition strategy involved the use of a carboxylated polystyrene template monolayer self-assembled on ITO. The interesting honeycomb-shaped nanostructures were observed by scanning electron microscopy112 (Fig. 4-III). Also, other useful methods such as soft lithography technique, nanolithography, laser patterning, and solution-processed nanostructure engineering have been employed to construct diverse PEDOT nanostructures.
 | ||
| Fig. 3 Different approaches to engineer PEDOT/PProDOT-derived nanostructures (continue.) (I) Diverse nanostructures of PEDOT and PProDOT derivatives through template-free electrochemical polymerization. Reproduced from ref. 47 with permission. Copyright 2012, ACS. (II) PEDOT-chitosan hydrogel through electropolymerization of PEDOT on the electrode surface for bioelectronic applications. Reproduced from ref. 109 under Creative Commons CC BY licence. (III) PEDOT, PEDOT-OH and perfluoro-functionalized PEDOT (PEDOT-F) electropolymerized to obtain nanostructures. Reproduced from ref. 110 with permission. Copyright 2020, ACS. | ||
 | ||
| Fig. 4 Different approaches to engineer PEDOT/PProDOT-derived nanostructures (continued). (I) Nanostructures of zwitterionic phosphoryl choline (PC)-appended EDOT and EDOT-OH with different feed ratios were prepared through the electropolymerization method without any templates. Reproduced from ref. 111 with permission. Copyright 2023, ACS. (II) Series of different 3,4-phenylenedioxythiophene monomers with different substituents afforded diverse nanostructures through electropolymerization. Reproduced from ref. 46 with permission. Copyright 2019, ACS. (III) PEDOT nanostructures were obtained through galvanostatic deposition of EDOT/aqueous solution of sodium dodecyl sulfate solution. Reproduced from ref. 112 with permission. Copyright 2015, ACS. | ||
5. PEDOT/PProDOT-based nano-biosensors
Biosensors are analytical devices used to detect chemicals or biological substances through a physiochemical detector, which can convert biological signals into readable electrical signals. The key component of a biosensor is the sensor probe, which is mostly a biological component such as antibodies, enzymes, microorganisms, cell receptors, nucleic acids, aptamers and biologically derived components that can interact, bind or recognize the particular analyte of consideration.113,114 The pioneering work on the first biosensor device was reported by Leland Charles Clark Jr. in 1956 (for this discovery, Prof. Clark is known as the father of biosensors).115 The working mechanism of modern-day glucose sensors is based on the discovery by Leland Clark's biosensor device. Presently, millions of people use glucose sensors to monitor their diabetic condition. The evolution of biosensors started after Clark's discovery and continued with promising discoveries including the most advanced wearable biosensors for continuous healthcare monitoring from a home-setting approach. Most of these biosensors have been developed based on the following: (i) different immobilized bio-receptors, (ii) different transducers used and (iii) implementation of different detection methods.The incorporation of diverse nanostructures in the electrode platform to increase the sensitivity and efficiency of biosensor devices is considered one of the effective design strategies. The key design strategy for the development of nano-biosensors significantly depends on modulating the size and surface morphology of sensor platforms, enhancing the biosensor detection efficiency and sensitivity. The typical design of nano-biosensor platforms is shown in Scheme 3. The considerable difference between conventional biosensors and nano-biosensor platforms is that the key-components are at the nanoscale dimensions in nano-biosensors. The basic steps for the development of a nano-biosensor involves:
• Design and selection of detection probes: bio-recognition elements such as antibodies, enzymes, aptamers, peptides, and molecularly imprinted polymers (MIPs) are the primary choice as sensor probes, which can specifically interact with the target analyte.
• Nanostructured materials: when nanostructured materials are introduced in biosensor platforms, their large surface area can offer significant conjugation sites for the recognition elements. This modification can enhance the sensitivity of the detection system.
• Immobilization of recognition elements: to ensure the stability and functionality of sensor probes, different immobilization techniques such as physical adsorption, covalent binding using functional groups such as –COOH, –NH2, N3, –SH, and –OH, and self-assembly have been employed.
• Transduction mechanism: this core part of a biosensor platform is its ability to convert the analyte–sensor probe interaction events into a measurable signal. Various transduction mechanisms such as optical, electrochemical, piezoelectric, and magnetic methods can be utilized.
• Signal readout and data analysis: the output signal from sensor platforms is usually collected and analyzed by advanced instrumentation systems involving spectrophotometers, potentiostats, or impedance analyzers, and then the final analysis and target analyte quantification are conducted.
• Integration of the sensor platform with next-generation smart devices: integrating bio-sensors into devices for the real-time detection of various analytes for biomedical diagnostics, environmental monitoring, food safety, and point-of-care testing should be the ultimate goal.
Blending interdisciplinary research areas to design novel nano-biosensor platforms is essential for the development of next-generation smart sensors. Here, we discuss the recent advancements of PEDOT/PProODT nano-biosensors for fostering the detection of diverse biomolecules/analytes.
5.1 Cancer biomarkers
Cancer biomarkers are signature molecules or events in the body that can pass information about the risk or occurrence of cancer. Many molecules have been identified as cancer biomarkers, which can be detected in body fluids through various imaging techniques including computerized tomography (CT) scan, positron emission tomography (PET) scan, ultrasound and X-ray techniques, magnetic resonance imaging (MRI) techniques and bone analysis, blood test, cancer metastasis monitoring, angiogenesis, early detection of proteins, genes and other molecules. Thus, significant efforts have been devoted to developing nano-biosensors for the early detection and diagnosis of cancer biomarkers owing to the idea of a home-setting platform. Shen et al. reported an interesting work on the detection of circulating tumor cells (CTCs) from blood samples of patients suffering from prostate cancer using PEDOT NanoVelcro chips.116 The nano biosensor platform design consisted of 3D nanostructures of phenylboronic acid (PBA)-grafted PEDOT NanoVelcro chips having an interfacial layer of poly(EDOT-PBA-co-EDOT-EG3), enhancing the CTC capturing efficiency and specificity to capture CTC (Fig. 5-I). The newly developed sensor platform was not only able to capture CTC cells but could simultaneously gently release the captured cells through competitive binding with sorbitol. Phenylboronic acid was used to conjugate the antibody and ethylene glycol (EG3) side chain used as an antifouling material to reduce the non-specific binding. The working mechanism for the capture and release of CTC proceeds through the conjugation of the antibody through the boronic acid-grafted nano-surface, followed by the specific capture of circulating tumor cells. The captured tumor cells were released through a competitive binding mechanism when glycan was introduced in the sensor system. The high binding affinity of glycan to sorbitol enabled the release of CTC cells. The feasibility of the novel device platform highlights the measurement of RNA signals from various diseases through the determination of prostate cancer-specific biomarkers in CTCs such as AR-FL, AR-V7, KLK3, FOLH1, and SChLAP1. Recently, Peng and co-workers developed AuNP-PEDOT coated silicon carbide nanowires and PdPtB nano-enhancer for the detection of beta amyloid oligomers. This strategy involved to use of the electro-chemiluminescence technique to detect the biomarker for the early diagnosis of Alzheimer's disease.117 The developed immunosensor showed a linear detection range of 20 pM to 20 nM with a limit of detection (LOD) 10 pM (Fig. 5-II). Sweety et al. reported the fabrication of a paper-based electrochemical biosensor for the detection of the EpCAM biomarker. This sensor was composed of the PEDOT:PSS and CuS nanocomposite system. EpCAM antibodies were immobilized in the polymer nanocomposite and the sensor exhibited a sensitivity of 104.31 µA pg−1 with a linear concentration range of 0.01 pg mL−1 to 1000 ng mL−1.118 In 2023, Kotagiri and co-workers reported the fabrication of an electrochemical strip sensor for the continuous monitoring of the chemotherapy drug dacarbazine using a graphene–Au–PEDOT nanocomposite (Fig. 5-III). This sensor system was developed using a screen-printed electrode, which was modified with an rGO–Au–PEDOT nanocomposite by the drop-casting method. The sensor system was characterized by the electrochemical method and the target analyte was detected by the square-wave voltammetry (SWV) and chronoamperometry (CA) methods. The new detection platform displayed a wide detection range of analyte concentration in human serum of 2.5 nM to 1500 nM with an LOD of 0.09 nM.119 | ||
| Fig. 5 Detection of cancer biomarkers using PEDOT-derived nanostructures. (I) 3D nanostructures of phenylboronic acid (PBA)-grafted PEDOT NanoVelcro chips having an interfacial layer of poly(EDOT-PBA-co-EDOT-EG3) for circulating tumor cell (CTC) detection. SEM images of the nanorods prepared showing scale bar 5 and 1 µm (enlarged), respectively. Adapted from ref. 116 with permission. Copyright 2017, Wiley. (II) Electrochemiluminescence (ECL) immunosensor design by PdPtB nano-enhancer and SiC@Au-PEDOT nanowires (NWs) for the specific and ultrasensitive detection of β-amyloid oligomers (AβOs). Reproduced from ref. 117 with permission. Copyright 2023, ACS. (III) AuNPs-PEDOT/rGO nanocomposite engineering for the electrochemical detection of dacarbazine (DTIC) on a screen-printed carbon electrode. Reproduced from ref. 119 with permission. Copyright 2023, ACS. | ||
Soares et al. developed an interesting nano-biosensor utilizing PEDOT nanotube-decorated Au nanoparticles for sensing folate binding protein (FBP), an important cancer biomarker.120 The sensor design consisted of the electropolymerization of EDOT on stainless steel mesh, which resulted the formation of PEDOT nanotubes. AuNPs were electrodeposited on the surface of PEDOT nanotubes and FBP immobilized on the electrode surface. The sensor exhibited an excellent LOD of 4.5 pmol L−1. The nanostructure engineering of the polymer surface and subsequent AuNP deposition enhanced the sensitivity of the electrode platform by decreasing its charge-transfer resistance and enhancing its surface area (Fig. 6-I). Wang et al. reported the development of a low fouling electrochemical biosensor for the detection of the breast cancer biomarker BRCA1. The sensor design consisted of a nanocomposite of PEDOT doped with a polypeptide sequence electrodeposited on a glassy carbon electrode (GCE). The polymer/nanocomposite surface displayed a 3D-microporous structure having a large surface area and antifouling properties. The surface was modified with the BRAC1 complimentary oligonucleotide, displaying a detection limit of 0.0034 pM for the target analyte121 (Fig. 6-II).
 | ||
| Fig. 6 Detection of cancer biomarkers using PEDOT-derived nanostructures (continued). (I) PEDOT nanotube-decorated Au nanoparticles for folate binding protein (FBP). Sensor surface modification highlighted PEDOT-NT-AuNP functionalized with MPA to introduce carboxylic acid, which was activated by EDC-NHS solution to immobilize the biotinylated FBP antibody. Adapted from ref. 120 with permission. Copyright 2021, ACS. (II) Schematic representation of nano biosensor design for the detection of the breast cancer marker BRAC1. The design steps involve the deposition of nanocomposite of PEDOT doped with a polypeptide followed by incubation in DNA (C1) solution. Then methylene blue (MB) redox indicator was immobilized and the target DNA was detected. Reproduced from ref. 121 with permission. Copyright 2020, Elsevier. | ||
Recently, an interesting biosensor platform based on a glassy carbon electrode was reported by Pandey et al., which could detect a cancer biomarker, soluble cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) at an attomole concentration (Fig. 7). The ultrasensitive sensor platform was developed through the combination of PEDOT:PSS and an AuNP nanocomposite, which enhanced the sensitivity of the sensor platform through its large surface area. The sensor probe used in this report consisted of a thiolated-anti CTLA-4 nano-antibody. The sensor platform showed excellent sensitivity and selectivity for target analyte in the presence of other interfering compounds with high accuracy. The wide detection range of analyte concentration of 100 ag mL−1–500 μg mL−1, LOD of 1.19 ag mL−1, high reproducibility and shelf-life of 2 weeks showed that this is a promising biosensor platform for clinical applications.122
 | ||
| Fig. 7 Detection of cancer biomarkers using PEDOT-derived nanostructures (continued). An ultrasensitive sensor platform was developed through the combination of PEDOT:PSS and an AuNP nanocomposite for the detection of the soluble cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) in attomole concentration using a GCE platform. Thiolated anti-CTLA-4 antibody was conjugated through Au-thiol coupling chemistry. (A)–(D) SEM images showing the electrode surface morphology after each modification. (A) ITO/PEDOT:PSS, (B) ITO/AuNPs, (C) ITO/PEDOT:PSS/AuNPs (D) ITO/PEDOT:PSS/AuNPs/NAb. Reproduced from ref. 122 with permission. Copyright 2023, Elsevier. | ||
5.2 Glucose nano biosensors
Elevated glucose levels in the blood cause severe damage to many organs including the eyes, kidneys, liver, nerves and heart. Abnormal glucose levels leads to diabetes, a chronic disease currently affecting millions of people. Glucose sensors are the second most successful and commercialized biosensor devices after pregnancy test kits. Tremendous efforts are on-going to develop non-invasive sensors for the detection of glucose in the urine, sweat, saliva and tears given that these body fluids offer painless extraction without lancet puncture to collect the sample, easy availability and can be stored as samples.GhavamiNejad et al. reported the fabrication of a non-enzymatic conductive hydrogel microneedle-based electrochemical sensor platform for continuous glucose detection using a PEDOT:PSS–Ag–Pt nanoparticle system123 (Fig. 8). The continuous glucose meter (CGM) was developed using a hydrogel synthesized using swellable-dopamine (DA)–hyaluronic acid (HA), followed by the synthesis of Ag and Pt nanoparticles inside the 3D porous hydrogel. The use of the PEDOT:PSS conducting polymer enhanced the electrical properties of the sensor patch system, making it suitable as a working electrode. The hydrogel microneedle (HMN) system with 3D conductive porous structure had in situ, continuous, real-time monitoring ability for glucose analyte. The novel conductive hydrogel system with nanoparticles and PEDOT:PSS conductive system (HMN-CGM) showed excellent stability for enzyme-less glucose sensing. The in situ reduction of Pt ions in the presence of catechol moieties in the dopamine system and Ag nanoparticles worked as an electrocatalyst to oxidize glucose, which was later detected electrochemically using CV and chronoamperometry. The in vivo experiments involved the testing of the developed sensor platform using a streptozotocin (STZ)-induced diabetic rat (T1D). In the rat model with type-1 diabetes, its decreasing glucose levels were measured by the novel sensor platform. The measurements were using the developed device were compared with that from a conventional glucose meter, which showed a good match, highlighting the excellent clinical practicality of the HMN-CGM platform for continuous glucose monitoring.
 | ||
| Fig. 8 Overview of PEDOT/PProDOT-derived nano-biosensor design for glucose detection. Schematic illustration of glucose sensor developed through non-enzymatic conductive hydrogel microneedle using PEDOT: PSS–Ag–Pt nanoparticle system. SEM images showing (Top) porosity of different composite hydrogels with DA conjugation. Scale bar 50 µm. Hydrogel microneedle fabrication processSEM images of glucose sensor patches fabricated with DA-HA, DHP and DHP-Ag–Pt (Scale bars 300 µm), different properties and glucose detection studies of newly developed sensor platform were displayed. Adapted from Ref. 123 with permission. Copyright 2023, Wiley. | ||
The recent report by Zhang et al. highlighted the development of an organic electrochemical transistor (OECT) device with enhanced transconductance properties through doping with plasmonic gold nanoparticles. The doping consisted of a solution-based process and photo-annealing techniques. The doping process increased the conductivity and volumetric capacitance of the channel layer, while photo-annealing was responsible for the improved crystallinity of the PEDOT:PSS channel. The strong bonding between PEDOT and AuNP significantly increased the stability of the OECT device. The gate electrode of the novel OECT device was modified with glucose oxidase (GOx) as the sensor probe through drop-casting method. The OECT device showed glucose detection capability in a wide range of glucose concentrations from 10 nM–1 mM (Fig. 9-I). The developed high transconductance OECT with excellent stability can be used for the detection of many biomarkers and analytes having low concentrations.124
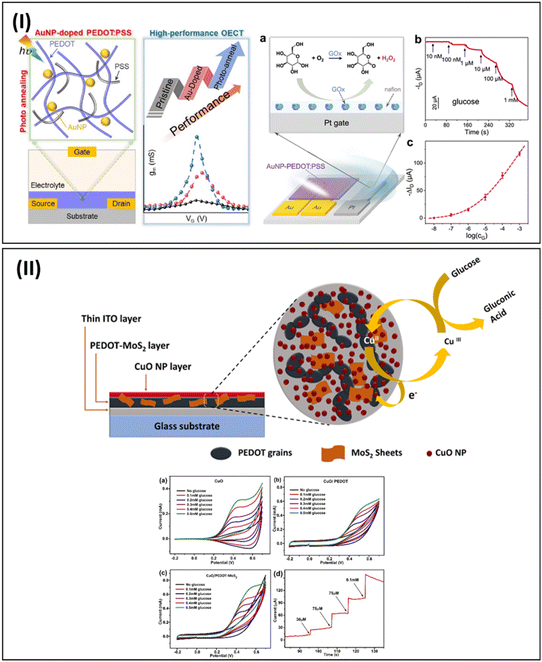 | ||
| Fig. 9 Overview of PEDOT/PProDOT-derived nano-biosensor design for glucose detection (continue). (I) OECT sensor platform having PEDOT:PSS doped with plasmonic gold nanoparticles. The plasmonic AuNP doped through solution process and photo annealing improved the OECT transconductance and channel crystallinity. Adapted from ref. 124 with permission. Copyright 2023, ACS. (II) Schematic representation of glucose sensor developed using CuO nanoparticles, PEDOT and nanoscale MoS2. SEM images showing electrode surface morphology after each modification steps with corresponding EDX spectra. Reproduced from ref. 126 under Creative Commons Attribution 4.0 License. | ||
In 2022, Murugan et al. developed an electrochemical glucose immunosensor based on a PEDOT:4-sulfocalix [4]arene/MXene nanocomposite using a multistep modification process.125 Specifically, EDOT monomers with 4-sulfocalix [4] arene (SCX) as a dopant were polymerized through chemical polymerization. Then, a PEDOT:SCX/MXene film was prepared through the dispersion of MXene in PEDOT:SCX by an ultra-sonication process. The sensor probe used to detect the analyte was glucose oxidase (GOx), which was immobilized on the PEDOT:SCX/MXene platform through chitosan as a binder. The sensor device showed a linear response upon the addition of glucose from 0.5 mM to 8 mM. The sensor could detect 22.5 μM of the glucose analyte in PBS buffer solution. Also, this new detection platform exhibited good stability, selectivity and sensitivity.
A non-enzymatic glucose sensor from a nanoscale CuO/PEDOT-MoS2 sensor platform was developed by Medhi et al. The sensor platform design consisted of CuO nanoparticles deposited on PEDOT infiltrated with nanoscale MoS2. Different characterization techniques were used to confirm the successful design of the electrode platform such as XRD, FE-SEM, EDX, FT-IR CV, and EIS. The hybrid nano-electrode platform exhibited good redox activity, electron transfer kinetics and large surface area. This sensor showed an LOD of 0.046 μM in the range of 303 μM–1.06 mM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 (Fig. 9-II).
126 (Fig. 9-II).
Another interesting glucose sensor using phenylboronic acid-functionalized poly(3,4-ethylenedioxythiophene) (PEDOT-PBA) nanotubes was designed by Huang et al.81 This work is interesting in the aspects of engineering nanotubular functionalized PEDOT structures without any templates through simple electropolymerization techniques. The core part of the sensor platform is phenylboronic acid, which worked as the probe to detect glucose analyte. The influence of the nanostructures on the electrode surface was confirmed through comparison with the glucose detection ability of the sensor platform having a smooth surface (Fig. 10-I). The nanotube-modified electrode platform showed enhanced sensitivity and different adsorption process for bovine serum albumin. Quartz crystal microbalance (QCM) and electrochemical impedance spectroscopy (EIS) were used to determine the glucose levels.
 | ||
| Fig. 10 PEDOT/PProDOT derived nano-biosensor design for glucose detection (continue). (I) Schematic representation of phenylboronic acid-functionalized PEDOT (PEDOT-PBA) nanotube through template-free electropolymerization. Reproduced from ref. 81 with permission. Copyright 2018 ACS. (II) PEDOT or polypyrrole (PPy) coated with polyacrylonitrile (PAN) – multiwall carbon nanotubes nanofibers (PAN-MWCNTs NFs). SEM image showing different percentage of MWCNT/PAN with PEDOT or PPy polymer. Adapted from ref. 129 with permission. Copyright 2023 Elsevier. | ||
In 2023, Dehbali et al. designed a portable smart glucometer to detect glucose in blood samples. The device design consisted of a Cu/Au/rGO/PEDOT:PSS-deposited IDE electrode platform. The sensor platform was modified with GOx enzyme as the sensor probe through the drop-casting method. The developed platform showed a detection limit of 1 µM glucose in a wide concentration range of 0 to 100 mM with excellent selectivity, sensitivity and stability. This device can be used for point-of-care (PoC) applications with further improvement.127 Very recently, Siufert et al. reported the development of a cotton-polyester (Cot-Pol) string electrode designed from stretchable nanofiber felt (NF-felt). The method involved the coating of non-conductive NF-felt with PEDOT:PSS, followed by a GOx incubation process. The newly designed electrode platform exhibited good electron transfer, diffusion of ions, etc. due to the nanostructure of the NF-felt string. The NF-felt sample showed a good LOD of 3.3 mM. Moreover, the felt string electrode showed excellent flexibility and stretchability, which can be used in diverse fields such as medical, military and athletic health monitoring.128 Cetin et al. constructed a high-performance glucose sensor designed from PEDOT or polypyrrole (PPy) coated with polyacrylonitrile (PAN)-multiwall carbon nanotube nanofibers (PAN-MWCNT NFs). The nanofibers of polyacrylonitrile-multiwall carbon nanotubes were obtained by the electrospinning technique.129 The nanofibers provided a high loading and stabilization of the biomolecule and MWCNT fillers were used for mechanical reinforcement. As reported by various groups, the conducting polymer layer provided high electron transfer and conductivity. The glucose detection probe was immobilized through the drop-casting of GOx and detection of the target analyte was confirmed by the amperometric method. This report highlighted the performance of two different sensor platforms with different conducting polymers, PEDOT and PPy, and concluded that the PEDOT-PPy-based sensor platform with MWCNTs exhibited slightly higher performance than that without MWCNTs (Fig. 10-II). Paul et al. developed a PEDOT:PSS graphene oxide (rGO)-titanium oxide (TiO2) nanohybrid paper sensor for glucose detection. The sensor platform consisted of Whatman filter paper modified with PEDOT:PSS by dip-coating.130 Following that, the paper was dipped in an rGO-TiO2 nanohybrid-PEDOT:PSS solution. The sensor platform was modified with GOx enzyme in PBS buffer. The paper sensor showed a linear range of detection of 0.01 to 3 mM of glucose with an LOD of 0.01 mM via the chronoamperometry method. It also showed good stability, selectivity and reproducibility (Fig. 11).
 | ||
| Fig. 11 Schematic representation of glucose sensor development with PEDOT:PSS graphene oxide (rGO)-titanium oxide (TiO2) nano-hybrid paper electrode. Glucose detection, interference study and electrode surface morphology. Adapted from ref. 130 with permission. Copyright 2021, Wiley. | ||
5.3 Lactate nano-biosensor
Lactate is an important molecule to regulate many body functions, including the immune system, inflammatory responses, and angiogenesis.131–133 Accordingly, elevated concentrations of lactate in body fluids indicate abnormal body functions and weak mental health. Thus, research efforts have been devoted to developing various biosensors to monitor the lactate concentrations in body fluids.Recently Meng et al. reported the fabrication of a sweat-based non-invasive flexible skin patch sensor for lactate detection. The sensor system was developed using a conducting polymer-reinforced laser-irradiated graphene (LIG) network as a 3D transducer platform. PEDOT was nano-deposited on the flexible system to reinforce it as a binder for enhanced stability and electrochemical kinetics. The pores present in the reinforced PEDOT/LIG worked as a 3D matrix to immobilize Prussian blue and lactate oxidase (Lox) enzyme. The novel nano-biosensor system could detect lactate in the range of 10–1350 µM in artificial sweat with a detection limit of 6.0 μM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134 (Fig. 12-I). Another study by Meng et al. highlighted the development of 3D nanofibrous biofunctionalized PEDOT with carboxylic acid side chains as a nano biosensor platform for the detection of lactate135 (Fig. 12-II). The sensor design consisted of EDOT monomer appended with carboxylic acid groups for efficient conjugation of the sensor probe. The monomers were copolymerized with EDOT and tetrabutylammonium perchlorate (TBAP) as a soft-template to generate a 3D nanofibrous structure with enhanced catalytic activities and low charge transfer resistance. The carboxylic side chains were utilized to anchor lactate dehydrogenase through EDC/S-NHS chemistry. The fabricated sensor device was utilized for the detection of lactate samples in the linear range of 0.05–1.8 mM with a sensitivity of 20.26 μA mM−1 cm−2.
134 (Fig. 12-I). Another study by Meng et al. highlighted the development of 3D nanofibrous biofunctionalized PEDOT with carboxylic acid side chains as a nano biosensor platform for the detection of lactate135 (Fig. 12-II). The sensor design consisted of EDOT monomer appended with carboxylic acid groups for efficient conjugation of the sensor probe. The monomers were copolymerized with EDOT and tetrabutylammonium perchlorate (TBAP) as a soft-template to generate a 3D nanofibrous structure with enhanced catalytic activities and low charge transfer resistance. The carboxylic side chains were utilized to anchor lactate dehydrogenase through EDC/S-NHS chemistry. The fabricated sensor device was utilized for the detection of lactate samples in the linear range of 0.05–1.8 mM with a sensitivity of 20.26 μA mM−1 cm−2.
 | ||
| Fig. 12 Different PEDOT nano-biosensors developed for the detection of lactate. (I) Non-invasive flexible skin patch sensor for sweat lactate detection through polymer-reinforced laser-irradiated graphene (LIG) network as a 3D transducer consisting of PEDOT nano deposition. Adapted from ref. 134 with https://creativecommons.org/licenses/by/4.0/ license. (II) Template-free nanofiber synthesis of PEDOT carboxylic acid-modified sensor platform for lactate detection. Reproduced from ref. 135 with permission. Copyright 2020, Elsevier. | ||
Hao et al. presented the development of a sweat biosensor inspired from the photosensitive stamp (PS) method. The vacuum filtration-transfer method (PS-VFTP) was used to design the flexible electrode array consisting of the SWCNT material. This method offers a simple, eco-friendly, approach. The patterned PS-VFTP system with SWCNT displayed flexibility, high reproducibility, precision, uniformity, conductivity and mechanical stability.136 This flexible sensor platform with many promising properties was further modified with Pt nanoparticles through the electrodeposition method for enhanced sensitivity and conductivity. Again, the sensor platform was modified by depositing Prussian blue or PEDOT layers to increase its electrocatalytic performance for glucose or lactates (Fig. 13).
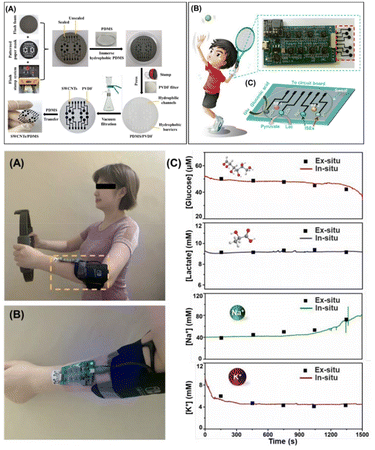 | ||
| Fig. 13 Different PEDOT nano-biosensors developed for the detection of lactate. Flexible electrode array consists of SWCNT material, Pt nanoparticles and PEDOT layers. Schematic illustration of PS-inspired flexible sensor array design, signal acquisition system ( (A)-(C) top images) and real-time monitoring of sweat markers in a non-invasive way( (A)–(C) bottom images). Adapted from ref. 136 with permission. Copyright 2022, ACS. | ||
5.4 PEDOT/ProDOT nano-biosensors for other metabolite sensing
The continuous monitoring of different metabolites in body fluids can facilitate early patient diagnosis and maximum healthcare can be provided to save their life. The development of bio-sensors to monitor metabolites can provide information on physiological events, health status, physical and mental health, disease monitoring, etc. The continuous monitoring of the well-being of individuals holds promise to expand a strong and healthy community. The continuous process of metabolite sensing using novel sensor platforms can help people detect diseases at the earliest, and consequently the diagnosis procedures can be decided at the earliest.137,138Chen et al. developed a dopamine sensor based on a porphyrin-metal organic framework (MOF525)-PEDOT nanocomposite. Two types of monomers, EDOT-OH and methylbenzoate-appended EDOT (EDOT-Ph-COOH), were used as monomers to design the polymer on the electrode surface, followed by the adsorption of MOF525. The ready-to-use microelectrode system was employed for dopamine detection (Fig. 14-I). The differential pulse voltammetry (DPV) technique was used to detect dopamine in a wide detection range of 4–100 μM with a sensitivity of 11 nA μM−1. Furthermore, this electrode platform showed high sensitivity and selectivity toward dopamine against interfering compounds such as ascorbic acid and uric acid.139
 | ||
| Fig. 14 Overview of the detection of different metabolites using PEDOT-derived nano-biosensors. (I) Dopamine detection by porphyrin-metal organic framework (MOF525)-PEDOT nanocomposite. Adapted from ref. 139 under Creative Commons Attribution (CC BY) license 4.0. (II) Schematic representation of peptide-imprinted PEDOT-OH nanotube deposited sensor platform for the detection α-synuclein (SNCA) in human brain organoids. Reproduced from ref. 140 with permission. Copyright 2020, ACS. (III) Schematic illustration of organic electrochemical transistor (OECT)/SERS (surface enhanced Raman spectroscopy) biosensor platform for the detection of dopamine. Nanostructured PEDOT-SH layer and AuNP were deposited on the channel layer of OECT, which enhanced the sensitivity of the device. Reproduced from ref. 141 under Creative Commons Attribution License (CC BY). | ||
Recently, Lee et al. designed an interesting nano biosensor platform for the detection of α-synuclein (SNCA) in human brain organoids using peptide-imprinted PEDOT-OH nanotubes140 (Fig. 14-II). α-Synuclein is an important biomarker for Parkinson's disease. The sensor design and development involved the electropolymerization of EDOT and EDOT-OH on ITO substrate with SNCA as the template. The electropolymerization involved the formation of nanotube structures. The SNCA peptide-imprinted PEDOT-OH nanotubes were used to detect SNCA analyte molecules and the sensor platform exhibited a detection range of 0.065 pM to 65 nM with a limit of detection of 4.0 pM. The clinical practicality of the sensor platform was confirmed through the detection of elevated levels of SNCA in a culture medium of human Parkinson's disease patient specific midbrain organoids.
An organic electrochemical transistor (OECT)/SERS (surface enhanced Raman spectroscopy) biosensor platform for the detection of dopamine was designed by Chou et al. This sensor platform consisted of a 3D OECT device having PEDOT:PSS as the active channel layer and nanostructured PEDOT-SH as the top layer, which was then modified with AuNPs. The nanoparticle deposition as well as the high signal amplification property of the OECT sensor device allowed the highly sensitive detection of dopamine (Fig. 14-III). The sensor platform could detect dopamine with excellent selectivity among other interfering compounds such as ascorbic acid and uric acid with a linear detection range of 50 nM–100 μM and detection limit of 37 nM. In addition, the sensor platform was used to detect p-cresol using the SERS technique.141
Maintaining the mental health of individuals is equally important as their physical health. Prolonged triggering of stress over a period of time can cause serious health issues including depression, cardiac problems, anxiety disorders, suicide attempts, lack of concentration, and weak immune response. Cortisol is one of the most significant biomarkers in the body to evaluate the stress level and the mental health of individuals. Cortisol is an adrenocorticosteroid hormone normally known as the stress hormone, which is associated with the circadian rhythm. Thus, abnormal levels of cortisol in body fluids indicate the early possibility of a weak health system, which must be monitored accurately. Presently, significant progress has been achieved in the development of different biosensor systems for the detection of cortisol.
Recently, Aerathupalathu Janardhanan et al. developed an OECT nano-biosensor for the sensitive detection of sweat cortisol79 (Fig. 15-I). This nano-biosensor platform consisted of poly(EDOT-COOH–co–EDOT-EG3) nanotubes engineered on the channel layer of an OECT device through a template free electropolymerization technique. The molecular design consisted of carboxylic acid-appended side chain to immobilize the antibody and ethylene glycol-appended PEDOT was used as an antifouling material. Subsequently, the polymer surface was modified with cortisol antibody through EDC/sulfo-NHS coupling chemistry. The OECT nano-biosensor platform could detect cortisol analyte in PBS buffer in the range of 1 fg mL−1 to 1 µg mL−1 with an excellent detection limit of 0.0088 fg mL−1. The nanostructure effect of the sensor platform was also investigated through the design of an OECT device without any nanostructures on its channel layer, which showed lower binding of the cortisol analyte compared to the OECT sensor platform with nanostructures. The device showed excellent reproducibility, selectivity and shelf life. Importantly, the clinical practicality of the OECT nano-biosensor platform was confirmed through an artificial cortisol-spiked artificial sweat.
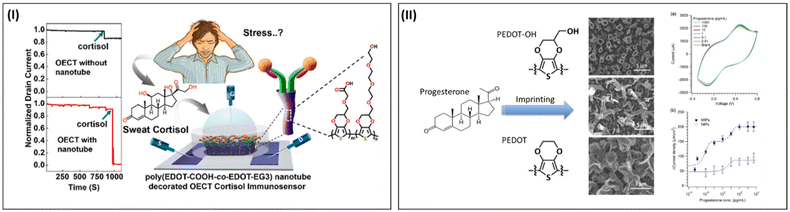 | ||
| Fig. 15 Overview of the detection of different metabolites using PEDOT-derived nano-biosensor. (I) Schematic illustration of an OECT nano-biosensor for the sensitive detection of sweat cortisol using poly(EDOT-COOH–co–EDOT-EG3) nanotubes decorated on the channel layer. The nanostructures were engineered through template-free electropolymerization. Reproduced from Ref. 79 with permission. Copyright 2022, ACS. (II) Schematic representation and analyte detection of molecularly imprinted polymer (MIP) technique for sensing urinary progesterone using PEDOT-OH nanostructures. Compared to the progesterone non-imprinted polymer, the MIP polymer showed greater target sensitivity. Adapted from ref. 142 with permission. Copyright 2017, Wiley. | ||
Luo et al. reported an interesting sensor platform to detect urinary progesterone using nanostructured PEDOT-OH. The highlight of this report is that the sensor platform did not have any detection probe such as antibodies and aptamers to sense the target molecule. Molecularly imprinted polymers have the advantages of cost-effective and easy fabrication protocols without the conjugation of any recognition elements. The biosensor platform was developed through the electro-co-polymerization of EDOT and EDOT-OH with progesterone as the template molecule. Different nanostructures were obtained such as porous, belt and tubular structures (Fig. 15-II). The detection of the progesterone molecule was conducted through the cyclic voltammetry technique. This sensor exhibited an LOD of less than 1 fg mL−1 with a linear range of 0.1 fg mL−1 to 0.1 ng mL−1. In addition, the sensor platform exhibited excellent selectivity to progesterone among other interfering compounds such as 17β estradiol, testosterone, urea and creatinine.142
Recently, An et al. designed a wearable cortisol aptasensor from nanofibers of polyacrylonitrile (PAN-NF) through the electrospinning method. Subsequently, the nanofibers were modified with carboxylated PEDOT through vapor deposition (Fig. 16).143 The sensor probe used in this design was the cortisol aptamer molecule with the sequence 5′-ATG GGC AAT GCG GGG TGG AGA ATG GTT GCC GCA CTT CGG C/3AmMO/-3′, which was immobilized through the coupling reagent DMT-MM with the carboxyl group of PEDOT-PAN NF. The sensing of the target cortisol was undertaken through a liquid–ion gated field effect transistor (FET) on a polyester (polyethylene terephthalate) (PET) substrate with PEDOT-PAN NF as the channel layer. The sensor platform showed the superior sensitivity of 10 pM and selectivity. The clinical practicality of the sensor was also tested by transplanting on a swab and human skin as well as on the perspiration of a moving person. The light-weight sensor platform designed on a silk substrate highlights a green approach with negligible burden on the environment.
 | ||
| Fig. 16 Overview of the detection of different metabolites using PEDOT-derived nano-biosensors (continued). (A) Cortisol aptasensor design through polyacrylonitrile (PAN-NF) by electrospinning method followed by modification with carboxylated PEDOT through vapor deposition.SEM images of the (B) pristine PAN template and (C) polymerized PEDOT coated on the PAN NF surface. Adapted from ref. 143 with permission. Copyright 2022, ACS. | ||
5.5 PEDOT/ProDOT nano biosensors for environmental monitoring, toxicology, forensic, marine and aquatic monitoring
Volatile organic compounds (VOCs) are some of the most dangerous substances for both living organisms and environment. Serious health issues on continuous exposure to VOCs have been reported including damage to the eyes, throat, lungs, kidneys, liver, nervous system, etc.144,145 In addition, the toxic chemicals, bacteria, poisonous gases present in the outdoor and indoor environment, food items, agriculture crops, soil, etc. must be detected and analyzed properly in the field of healthcare and environmental protection. Similarly, the detection of narcotics, illicit drugs, and explosives is one of the main objectives of forensic applications.146 Bypassing the use of highly sophisticated instrumentation and difficult sample collection from crime scenes and criminal investigations through the use of portable and miniaturized biosensors reflects the high demand of new sensor designs with promising properties. Furthermore, marine and aquatic monitoring including the quality of water, toxic living organisms, pH, chemical and metal ion contents can be detected by designing novel biosensor platforms. Accordingly, PEDOT/PProDOT nanostructures or their nanocomposites have been widely used to develop detection platforms for the target analytes in these applications.Aerathupalathu Janardhanan et al. Developed a chiral nano biosensor based on hydroxymethyl EDOT (EDOT-OH) for the detection of fetal bovine serum, RGD peptide, insulin and mandelic acid (MA) enantiomers. The sensor platform consisted of (R)-EDOH-OH and (S)-EDOT-OH monomers electropolymerized on a quartz crystal microbalance chip to detect different analytes (Fig. 17-I). The polymerization conditions were tuned to endow the polymer surface with different surface morphologies such as nanotubes and smooth films. The chiral recognition and detection of analytes highlight the synergistic effect of surface morphology and chirality. Compared to the senor platform having a smooth surface morphology, the nanotube-decorated sensor platform exhibited enhanced chiral discrimination of mandelic acid enantiomers. Density functional theory (DFT) and ab initio molecular dynamics (AIMD) techniques revealed the underlying sensing mechanism for the chiral discrimination of mandelic acid enantiomers and chiral sensor platforms.80
 | ||
| Fig. 17 Overview of PEDOT/PProDOT nano-biosensors for environmental, marine, forensic, toxicology, etc. (I) Schematic representation of chiral PEDOT-OH nano-biosensor for the detection of enantiomers. Nanostructured and smooth films of PEDOT-OH were deposited through template-free electropolymerization on QCM sensor chip for enantio-recognition. Reproduced from ref. 80 with permission. Copyright 2021, RSC. (II) Schematic illustration of PProDOT hollow nanofiber for electrochemical sensing of paracetamol. The nanostructures of PProDOT-MeSH2 hollow nanofiber material was prepared through cetyltrimethyl ammonium bromide (CTAB) surfactant-assisted polymerization method. Reproduced from ref. 147 with permission. Copyright 2019, Elsevier. (III) Schematic representation of PEDOT:PSS/poly(p-anisidine) nanocomposite platform for detection of CO gas. The chemiresistive sensing mechanism allowed enhanced CO detection through high surface area of electrode platform through nanostructures. Reproduced from ref. 148 under Creative Commons Attribution https://creativecommons.org/licenses/by-nc-nd/4.0/ licence. | ||
An interesting report by Zhang et al. highlighted the development of PProDOT hollow nanofibers for the electrochemical sensing of paracetamol. The sensor platform was fabricated with the novel polymer scaffold of PProDOT with pendant-grafted thiol group (PProDOT-MeSH2) hollow nanofiber material.147 The polymeric material was prepared through the cetyltrimethyl ammonium bromide (CTAB) surfactant-assisted polymerization method. Thiol grafted PProDOT hollow nanofiber material significantly improved the electrochemical sensing through its excellent electrocatalytic properties and molecular recognition abilities (Fig. 17-II).
Recently Farea et al. designed a high-performance carbon monoxide (CO) gas sensor platform using a PEDOT:PSS/poly(p-anisidine) nanocomposite.148 The sensor platform design involved the in situ polymerization of p-anisidine in the presence of PEDOT:PSS with sodium dodecyl sulfate (SDS) as the anionic surfactant. The nanocomposite was drop-casted on the interdigitated copper electrode platform based on the principle of chemi-resistive sensing (Fig. 17-III). The enhanced sensitivity of the sensor platform towards CO detection was attributed to the higher surface area provide by the nanocomposite. The sensor platform exhibited CO detection in the range of 50–300 ppm with a response time of 58 s and recovery time of 61 s for 100 ppm CO gas.
Gupta et al. constructed a PEDOT:PSS/Au-nanoparticle/nano flower-modified cost-effective, disposable and ultrasensitive copper electrode for the detection of methylene blue (MB) dye in water. MB was used as a model contaminant, which was detected using the newly designed sensor through the electrochemical detection method (Fig. 18-I). The 3D anisotropic nanoflower-like morphology provided approximately 6-times higher signal sensitivity compared to the sensor platform modified with gold nanoparticles. The analyte detection was performed using the new detection platform, which exhibited sensitivity in the range of 0.01–0.1 μM with an LOD of 0.0022 μM, which was 0.0052 μM for the gold-nanoparticle decorated sensor platform.149
 | ||
| Fig. 18 Overview of PEDOT/PProDOT nano-biosensors for environmental, marine, forensic, toxicology, etc. (continued). (I) Schematic illustration of sensor platform for the detection of methylene blue (MB) dye in water using PEDOT:PSS/Au-nanoparticle/nanoflower-modified, cost-effective, disposable and ultrasensitive copper electrode. Reproduced from ref. 149 with permission. Copyright 2020, ACS. (II) Sensor design for the detection of different VOCs including acetone, ethanol and formaldehyde using TiO2 nanoparticles and different polymers including PEDOT:PSS, polypyrrole and polystyrene sulfonate. Reproduced from ref. 150 with permission. Copyright 2021, Elsevier. (III) Schematic illustration of nanostructure PEDOT derivatives for the detection of paracetamol. Reproduced from ref. 156 with permission. Copyright 2020, RSC. | ||
An electronic nose based on PEDOT:TiO2 hybrid nanostructures was reported by Conti et al. The TiO2 nanofibers were prepared through electro-spinning and TiO2 nanoparticles were prepared through the sol–gel method. The materials were combined with different polymers including PEDOT:PSS, polypyrrole and polystyrene sulfonate. The impedimetric technique was used to identify and discriminate different VOCs including acetone, ethanol and formaldehyde vapor150 (Fig. 18-II). A recent report by Jin et al. presented a PEDOT:PSS MoS2 quantum dot sensor for the detection of acetaldehyde at room temperature. Acetaldehyde is a primary carcinogen and toxic chemical upon long-term exposure. The sensor was developed by doping MoS2 quantum dots with PEDOT:PSS through the in situ polymerization technique. The highest response value of 78.8% against 100 ppm acetaldehyde with an LOD of 1 ppm was observed for the nano-sensor. The high sensitivity of this sensor platform was possibly due to the large reaction sites on MOS2 and charge transfer between PEDOT:PSS and MOS2-quantum dots. The sensor platform showed excellent stability for three months.151 Dehsari et al. designed an ammonia sensor based on a copper(II) phthalocyanine-supported 3D-nitrogen-doped graphene/PEDOT-PSS nanocomposite. The high uniformity and large surface area of the sensor platform resulted in high sensitivity for the detection of ammonia gas in a wide concentration range of 1 ppm–1000 ppm at room temperature. The response time of the sensor was 2.5 min, while its recovery time was 1 min.152
Machhindra et al. developed a Prussian blue (PB)-PEDOT loaded laser scribed graphene glassy carbon electrode platform for the detection of Cd.2+ The heavy metal sensing platform composed of a conducting polymer nanocomposite having a large surface area could detect Cd2+ in a wide concentration range of 1 nM–10 μM with a promising detection limit of 0.85 nM through the differential pulse voltammetry (DPV) technique. The senor platform exhibited good stability, reproducibility and long-term stability, which was used to detect Cd2+ in packaged drinking water, highlighting the practical applications of the developed sensor platform.153
Wu et al. reported the fabrication of a nanostructured PEDOT-OH-modified electrode platform for the determination of the anticancer herbal drug shikonin. The nanostructures on the glassy carbon electrode surface were designed through a simple electropolymerization technique. The electrode modified with nanostructures possessed a large surface area and numerous active sites for the catalytic redox reaction, enhancing its electrocatalytic activity. The sensor platform showed a linear detection range of 1.0 nM to 10 μM with a good detection limit of 0.3 nM.154
Lin et al. reported the development of a nitrite sensor platform based on PEDOT doped with AuNP. This sensor platform was developed through the electropolymerization of EDOT monomer in a solution containing colloidal AuNP. The nanocomposite exhibited good conductivity and excellent electrocatalytic activity towards nitrite oxidation by amperometric detection. The sensor platform exhibited a wide concentration range for the detection of nitrite of 0.2 μM to 1400 μM and LOD of 60 nM.155
Zhang et al. developed different functional pendant group-grafted sensor platforms for the detection of paracetamol. EDOTCH2NH2, EDOTCH2OH and EDOTCH2SH monomers were selected and the corresponding polymers were obtained through the chemical oxidative polymerization technique. The amine-functionalized PEDOT exhibited a nanofiber morphology, while hydroxyl and thiol functional PEDOT exhibited sponge-like structures. The self-assembly properties of the polymers were studied in detail and the sensor platform was used to detect paracetamol as the target analyte. Among the polymer-modified electrodes, the thiol-functionalized PEDOT showed the highest electrochemical response to paracetamol analyte156 (Fig. 18-III).
Tian et al. designed a biosensor for the detection of environmental contaminants such as hydroquinone (HQ), catechol (CC), resorcinol (RC), and nitrite using poly(3,4-ethylenedioxythiophene)-graphene composites. The process involved the in situ electro-polymerization of EDOT monomer on graphene nanosheets. SEM experiments confirmed the formation of one-dimensional PEDOT structures. The sensor platform exhibited excellent electrocatalytic activity on dihydroxybenzene isomers and NO2− ions. The high electron transfer ability, conductivity, sensitivity, selectivity, organic compatibility, etc. were achieved through the PEDOT layer.157
Wang et al. reported the fabrication of an electrochemical sensor based on PEDOT-AuNF (gold nanoflower) supported on graphene oxide (GO) sponge for the sensitive detection of ochratoxin-A carcinogenic substance. This sensor consisted of a 3D sponge-like porous graphene oxide structure, which exhibited high electrical conductivity and a large surface area. PEDOT-AuNF was synthesized via an ionic liquid-assisted one-pot method and adhered on GO sponge through π–π packing interaction. The sensor probe to detect the target analyte was ochratoxin aptamer, which was conjugated on the surface of GO/PEDOT-AuNF through thiol–Au coupling. The developed sensor showed a linear detection range of 0.01–20 ng L−1 ochratoxin with an LOD of 1.4 pg L−1. The high signal amplification from GO and AuNF structures enhanced the sensitivity of the sensor platform. This sensor exhibited high sensitivity, selectivity and stability.158
Jyoti et al. developed PEDOT swaddled CoWO4 nano-capsules for the detection of phenacetin, which is an antipyretic, non-opioid analgesic drug and adulterant in narcotic drugs. The senor platform consisted of an MIP design having PEDOT wrapped with CoWO4 for the highly sensitive detection of the target analyte. CoWO4 was prepared through the hydrothermal technique, which was wrapped by PEDOT through the chemical polymerization of the corresponding monomers. The electrostatic force between thiophene sulfur present in PEDOT and metal atoms was the driving force for the formation of the polymer and molecularly imprinted metal nanocomposites. The nanostructure design of the newly developed MIP platform with many imprinted sites for the binding of phenacetin provided an excellent sensor sensitivity of 0.14 µA nM−1 cm−2 and detection limit of 0.091 nM.159
6 Conclusions, challenges and future perspectives
Since the development of thiophene as a conducting polymer for diverse applications, seamless efforts have been devoted to designing derivatives of thiophene with the aim to achieve extra stability and exciting material properties. Among them, 3,4-(ethylenedioxythiophene), and recently 3,4-(propylenedioxythiophene) are the most promising molecules with tunable properties. The most exciting properties of PEDOT and PProDOT materials highlight their superior biocompatibility, which make these materials a shining star in the field of biosensors, tissue engineering, cell-mimicking platforms, wearable healthcare devices, biotechnology, etc. The fine tuning of the material properties can be achieved mainly through (i) introducing novel functional groups such as carboxylic acids, hydroxyl groups, amines, thiols, alkenes, etc. and (ii) surface tuning of the material with different polymerization methods to impart novel nanostructures. Engineering nanostructures of conducting polymers with different functional groups can modulate the conjugation of biomolecules such as antibodies, aptamers, enzymes, cell receptors, and tissues, enabling the novel material to communicate with the surrounding biological environment, which is a design termed bio-sensors. The design of these biosensors with nanoscale dimensions endows them with extraordinary properties such as excellent electron transfer between the senor probe and transducer and large surface area, which helps to immobilize more recognition elements, enhancing the sensor sensitivity. Combining all these features in the surface allows the unique properties of materials to be employed in novel nano biosensor designs. PEDOT and its derivatives, recently PProDOT derivatives with nanostructured assembly or nanocomposite forms were used for the development of nano-biosensors.According to our analysis of the various research outcomes reported in the past few years on the design, development and applications of PEDOT/PProDOT nano-biosensors, we concludes that many efforts have been devoted to designing novel nano-biosensors with exceptional properties. In this case, it is necessary for biosensors to possess the features of sensitivity, selectivity, stability and reproducibility. Great efforts have been devoted to designing novel PEDOT derivatives with side chain functionalization as one of the attractive approaches utilized by researchers. This functionalization of the PEDOT side chain can bypass the solubility issues of polymeric materials, while enabling their deposition on the sensor substrate platform. Also, the access to bio-conjugation by exploring synthetic organic chemistry imparts an excellent opportunity for functionalized PEDOT materials. In addition, as soft semi-conductive materials, exploiting all the possibilities of the electropolymerization technique with or without templates can provide diverse nanostructures in different dimensions such as nanotubes, nanodots, nanowires, nanoflakes and nanofibers. However, it can be observed that the majority of nano-biosensors developed during the past few years were based on nanocomposites of PEDOT derivatives, particularly PEDOT nanocomposites and PEDOT:PSS/nanocomposites. These nano-biosensors possess the electrocatalytic properties of the conducting polymer and electron transfer and high surface area of different nanomaterials. The booming development of novel biosensors with promising sensitivity, selectivity and excellent limit of detection has been observed since the surge of the COVID-19 pandemic recently. These biosensors include different conducting polymers, particularly PEDOT nanomaterials/polymer nanocomposites, sensor probe conjugation, novel detection techniques, signal amplification devices such as OECT, OFET, and FET, and wearable electronics for the continuous monitoring of diseases. These devices are highly promising for the detection of cancer biomarkers, glucose, lactate, and cortisol for the early diagnosis of diseases, toxic gases, pollutants, drugs and poisonous chemicals for environmental monitoring, toxicology, marine and aquatic monitoring.
The design and development of diverse nano-biosensors with PEDOT/PProDOT nanostructures/nanocomposites in the past years, especially the thriving progress since the COVID-19 pandemic demonstrate the high demand for novel sensor platforms for the detection of biomolecules. However, our analysis on recent research works published on PEDOT/PProDOT nano-biosensors showed that there are still challenges associated with the development and design of nano-biosensor platforms. We observed that ProDOT derivatives or PProDOT/nanocomposites have not been sufficiently explored for the development of biosensors. As a new member of the thiophene family, PProDOT and its derivatives have only recently started to be explored for various applications. Alternatively, novel ProDOT materials with different functional groups on their side chains can be used to conjugate various recognition probes. Furthermore, the introduction of polar groups on the two sides can bypass the insolubility issues of the monomer, enabling it to be dissolved easily in different solvents for the polymerization process. Very recently, many synthetic strategies have been used to design novel ProDOT scaffolds through tailoring different functional groups. Miniaturization of nano-biosensor platforms for the continuous monitoring of biomarkers is another challenge in the development of wearable bioelectronics. Most of the reported wearable biosensor platforms explored body fluids such as perspiration, saliva and tears to extract the target analytes. Non-invasive sample collection is one of the attractive design strategies used to build body mountable biosensor platforms for real-time disease monitoring. However, despite the high biocompatibility and soft nature of PEDOT/PProDOT materials, they are not always suitable candidates for the fabrication of wearable bioelectronic platforms. In this case, nanomaterials of PEDOT/PProDOT derivatives must be stable in the high concentrations of body fluids, which may contain many other interfering salts and compounds. These difficulties need to be overcome through the design of PEDOT/PProDOT molecular scaffolds with side chain functionalization or post-functionalization strategies. Besides the molecular design of PEDOT/PProDOT derivatives for wearable applications, there are also challenges associated with these nano biosensors in environmental, toxicology, forensic and aquatic applications. These applications require the stability of the polymer platform with the recognition probe such as antibodies, aptamers, enzymes, tissues and cells to be addressed in different environments. The bio-recognition probes immobilized on the polymer scaffolds should possess high binding affinity to ensure the stability of the sensor platform. High temperature, humidity, acidic, basic or high salt concentrations with highly reactive interfering chemicals are the real obstacles in the design of biosensors. These challenges also need to be addressed through the careful functionalization of the polymer side chain or novel conjugation methods for the sensor probe.
Intriguing approaches to address these challenges for the development of PEDOT/PProDOT nano-biosensors mainly focus on the monomer design strategies, polymerization to create nanostructures, conjugation methods involving strong binding between the recognition element and polymer surface as well as the substrate used to deposit polymer material. Molecular design is the art of creating novel materials with tunable properties. In this case, considering PEDOT as a champion material for the development of nano-biosensors, many promising design strategies have already been used to design sensor platforms. Tailoring diverse functional groups in the side chain can solve the solubility issues in both aqueous and non-aqueous media together with facilitating the sensor probe conjugation. The sensor probe conjugation strategy is another perspective in the future that should be explored to increase the sensitivity of the sensor device. This includes the strong covalent attachment of the recognition element on nanostructured/nanocomposite platforms. Besides the classical conjugation methods such as EDC/sulfo-NHS coupling and isothiocyanate methods, novel methods can be developed based on the green chemistry approach, pericyclic reaction strategies, click chemistry, photoreactions or even the possibility of sonochemistry. Another future aspect anticipated for the development of nano-biosensor platforms is to explore PProDOT and its derivatives. PProDOT derivatives with regio-regular structural design can impart enhanced detection capabilities. Another strategy for the design of PEDOT/PProDOT nano-biosensors is the exploration of polymerization methods. Electrochemical polymerization techniques are interesting methods that produce engineering nanostructures with/without templates using EDOT/ProDOT monomers. Fine tuning of the electrochemical polymerization conditions together with diverse PEDOT/PProDOT side chain cues can impart exciting nanostructures to enhance the sensor sensitivity.
Another strategy to explore the design of nano-biosensors is the use of cost-effective platforms as the sensor substrate. For example, the use of paper as the electrode platform presents an easily available, inexpensive, flexible and lightweight material to design wearable nano-biosensors. Other flexible platforms include PET films, polyimides, fabric materials, threads, plant leaves, tattoos, gloves, face masks and contact lens. These platforms with nanostructures of PEDOT/PProDOT have potential for use as wearable nano-biosensors with novel molecular designs, diverse nanostructures and the possibility of multiplex sensing.
Finally, we look forward to the miniaturization aspects of PEDOT/PProDOT nano-biosensors for wearable electronics as well as environmental monitoring. Similar to the use of different flexible and cost effective substrates, miniaturized devices can help monitor diseases and different analytes without requiring sophisticated instrumentation and sampling methods. The exploration of these miniaturized devices with high signal amplification electronic platforms such as OECT, OFET, and FET can enhance the sensitivity and selectivity of sensors to the next level. The ultra-sensitivity of these devices through high signal amplification from the nanostructures and transistor devices can achieve extremely low limits of detection. These nano-biosensor platforms with multiplex detection strategies will be the next generation of smart nano-biosensors for the continuous monitoring of disease biomarkers from home setting functions and environmental, forensic and aquatic monitoring.
Author contributions
Jayakrishnan Aerathupalathu Janardhanan: conceptualization, writing – original draft, writing – review and editing. Hsiao-hua Yu: conceptualization, writing – review and editing, supervision, project administration, funding acquisition.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Academia Sinica (Grand Challenge Program, No: AS-GC-M05) and National Science & Technology Council (NSTC) Taiwan, (Grand No: MOST 111-2124-M-038-001) for the financial support.References
- H. Shirakawa, E. J. Louis, A. G. Macdiarmid, C. K. Chiang and A. J. Heeger, J. Chem. Soc., Chem. Commun., 1977, 578–580, 10.1039/c39770000578.
- C. K. Chiang, C. R. Fincher, Y. W. Park, A. J. Heeger, H. Shirakawa, E. J. Louis, S. C. Gau and A. G. Macdiarmid, Phys. Rev. Lett., 1977, 39, 1098–1101 CrossRef CAS.
- S. C. Rasmussen, Angew. Chem., Int. Ed., 2021, 60, 8012–8016 CrossRef CAS PubMed.
- T. Nezakati, A. Seifalian, A. Tan and A. M. Seifalian, Chem. Rev., 2018, 118, 6766–6843 CrossRef CAS PubMed.
- S. C. Rasmussen, ChemPlusChem, 2020, 85, 1412–1429 CrossRef CAS PubMed.
- N. Hall, Chem. Commun., 2003, 1–4 Search PubMed.
- X. Guo and A. Facchetti, Nat. Mater., 2020, 19, 922–928 CrossRef CAS PubMed.
- I. Bargigia, L. R. Savagian, A. M. Österholm, J. R. Reynolds and C. Silva, J. Am. Chem. Soc., 2021, 143, 294–308 CrossRef CAS PubMed.
- G. E. Fenoy, R. Hasler, F. Quartinello, W. A. Marmisollé, C. Lorenz, O. Azzaroni, P. Bäuerle and W. Knoll, J. Am. Chem. Soc. Au, 2023, 3, 275–275 CAS.
- P. Das, C. Z. Salamat, B. Zayat, R. Elizalde-Segovia, Y. F. Wang, X. D. Gu, S. R. Narayan, S. H. Tolbert and B. C. Thompson, Chem. Mater., 2022, 34, 2672–2686 CrossRef CAS.
- J. H. Wang, L. Zhang, Y. X. Ma, C. J. Zhou, J. Li, W. J. Li and C. Zhang, Electrochim. Acta, 2024, 477, 143783 CrossRef CAS.
- L. R. Savagian, A. M. Österholm, J. F. Ponder, K. J. Barth, J. Rivnay and J. R. Reynolds, Adv. Mater., 2018, 30, 1804647 CrossRef PubMed.
- M. T. Otley, F. A. Alamer, Y. M. Zhu, A. Singhayiranon, X. Z. Zhang, M. F. Li, A. Kumar and G. A. Sotzing, ACS Appl. Mater. Interfaces, 2014, 6, 1734–1739 CrossRef CAS PubMed.
- D. Mantione, N. Casado, A. Sanchez-Sanchez, H. Sardon and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2721–2724 CrossRef CAS.
- A. Schultheiss, A. Carella, S. Pouget, J. Faure-Vincent, R. Demadrille, A. Revaux and J. P. Simonato, J. Mater. Chem. C, 2020, 8, 17254–17260 RSC.
- J. F. Ponder, A. M. Österholm and J. R. Reynolds, Chem. Mater., 2017, 29, 4385–4392 CrossRef CAS.
- M. N. Gueye, A. Carella, N. Massonnet, E. Yvenou, S. Brenet, J. Faure-Vincent, S. Pouget, F. Rieutord, H. Okuno, A. Benayad, R. Demadrille and J. P. Simonato, Chem. Mater., 2016, 28, 3462–3468 CrossRef CAS.
- N. Kim, I. Petsagkourakis, S. Z. Chen, M. Berggren, X. Crispin, M. P. Jonsson and I. Zozoulenko, Conjugated Polymers: Properties, Processing, and Applications, Vol 1, 4th Edition, 2019, pp. 45–128 Search PubMed.
- K. Zhang, J. J. Qiu and S. R. Wang, Nanoscale, 2016, 8, 8033–8041 RSC.
- I. Zozoulenko, J. F. Franco-Gonzalez, V. Gueskine, A. Mehandzhiyski, M. Modarresi, N. Rolland and K. Tybrandt, Macromolecules, 2021, 54, 5915–5934 CrossRef CAS.
- M. J. Donahue, A. Sanchez-Sanchez, S. Inal, J. Qu, R. M. Owens, D. Mecerreyes, G. G. Malliaras and D. C. Martin, Mater. Sci. Eng., R, 2020, 140, 100546 CrossRef.
- M. N. Gueye, A. Carella, J. Faure-Vincent, R. Demadrille and J. P. Simonato, Prog. Mater. Sci., 2020, 108, 100616 CrossRef CAS.
- M. M. Durbin, A. H. Balzer, J. R. Reynolds, E. L. Ratcliff, N. Stingelin and A. M. Österholm, Chem. Mater., 2024, 36(6), 2634–2641 CrossRef CAS PubMed.
- D. Minudri, D. Mantione, A. Dominguez-Alfaro, S. Moya, E. Maza, C. Bellacanzone, M. R. Antognazza and D. Mecerreyes, Adv. Electron. Mater., 2020, 6, 2000510 CrossRef CAS.
- A. Zhuang, Q. C. Pan, Y. Qian, S. N. Fan, X. Yao, L. J. Song, B. Zhu and Y. P. Zhang, ACS Biomater. Sci. Eng., 2021, 7, 1202–1215 CrossRef CAS PubMed.
- M. Y. Shen, S. Yuran, Y. Aviv, H. Ayalew, C. H. Luo, Y. H. Tsai, M. Reches, H. H. Yu and R. Shenhar, ACS Appl. Mater. Interfaces, 2019, 11, 1201–1208 CrossRef CAS PubMed.
- B. C. Zhu, E. W. C. Chan, S. Y. Li, X. Sun and J. Travas-Sejdic, J. Mater. Chem. C, 2022, 10, 14882–14891 RSC.
- M. Y. Shen, T. Y. Huang, C. H. Luo, Y. C. Huang, Y. H. Tsai, T. L. Wang and H. H. Yu, J. Chin. Chem. Soc., 2018, 65, 149–155 CrossRef CAS.
- Y. S. Hsiao, S. C. Luo, S. Hou, B. Zhu, J. Sekine, C. W. Kuo, D. Y. Chueh, H. H. Yu, H. R. Tseng and P. L. Chen, Small, 2014, 10, 3012–3017 CrossRef CAS PubMed.
- B. Zhu, S. C. Luo, H. C. Zhao, H. A. Lin, J. Sekine, A. Nakao, C. Chen, Y. Yamashita and H. H. Yu, Nat. Commun., 2014, 5, 4523 CrossRef CAS PubMed.
- H. C. Zhao, B. Zhu, S. C. Luo, H. A. Lin, A. Nakao, Y. Yamashita and H. H. Yu, ACS Appl. Mater. Interfaces, 2013, 5, 4536–4543 CrossRef CAS.
- S. C. Luo, E. A. B. Kantchev, B. Zhu, Y. W. Siang and H. H. Yu, Chem. Commun., 2012, 48, 6942–6944 RSC.
- J. G. Wu, J. H. Chen, K. T. Liu and S. C. Luo, ACS Appl. Mater. Interfaces, 2019, 11, 21294–21307 CrossRef CAS PubMed.
- H. H. Lin, C. H. Lin and S. C. Luo, ACS Appl. Mater. Interfaces, 2023, 15, 29214–29223 CrossRef CAS PubMed.
- D. M. Nguyen, Y. H. Wu, A. Nolin, C. Y. Lo, T. Z. Guo, C. Dhong, D. C. Martin and L. V. Kayser, Adv. Eng. Mater., 2022, 24, 2200280 CrossRef CAS.
- F. Jonas and L. Schrader, Synth. Met., 1991, 41, 831–836 CrossRef CAS.
- M. Seiti, A. Giuri, C. E. Corcione and E. Ferraris, Biomater. Adv., 2023, 154, 213655 CrossRef CAS.
- N. Sharma, A. M. Koshy, G. R. Kandregula, K. Ramanujam, D. Ray and P. Swaminathan, ACS Appl. Energy Mater., 2023, 7, 363–372 CrossRef.
- M. G. Tadesse, N. Simon and J. F. Lübben, Adv. Energy Sustainability Res., 2024, 202400006 Search PubMed.
- Y. Wang, S. S. Jia and Z. T. Zhang, J. Mater. Chem. C, 2023, 11, 10435–10454 RSC.
- Y. L. Yang, Y. Q. Yao, Y. Li, X. S. Zhao, W. Cheng, B. H. Chen, L. J. Chen, P. Li and S. H. Tang, J. Mater. Chem. C, 2023, 11, 13814–13823 RSC.
- O. Faruk and B. Adak, Synth. Met., 2023, 297, 117384 CrossRef CAS.
- S. T. Keene, T. P. A. van der Pol, D. Zakhidov, C. H. L. Weijtens, R. A. J. Janssen, A. Salleo and Y. van de Burgt, Adv. Mater., 2020, 32, 2000270 CrossRef CAS.
- B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef.
- H. A. Lin, B. Zhu, Y. W. Wu, J. Sekine, A. Nakao, S. C. Luo, Y. Yamashita and H. H. Yu, Adv. Funct. Mater., 2018, 28, 201703890 Search PubMed.
- T. Darmanin, E. L. Klimareva, I. Schewtschenko, F. Guittard and I. F. Perepichka, Macromolecules, 2019, 52, 8088–8102 CrossRef CAS.
- S. C. Luo, J. Sekine, B. Zhu, H. Zhao, A. Nakao and H. H. Yu, ACS Nano, 2012, 6, 3018–3026 CrossRef CAS PubMed.
- D. Mantione, I. Del Agua, A. Sanchez-Sanchez and D. Mecerreyes, Polymers, 2017, 9(8), 354 CrossRef PubMed.
- A. Patra, M. Bendikov and S. Chand, Acc. Chem. Res., 2014, 47, 1465–1474 CrossRef CAS PubMed.
- T. M. Pappenfus, A. J. Helmin, W. D. Wilcox, S. M. Severson and D. E. Janzen, J. Org. Chem., 2019, 84, 11253–11257 CrossRef CAS PubMed.
- L. Q. Ouyang, B. Wei, C. C. Kuo, S. Pathak, B. Farrell and D. C. Martin, Sci. Adv., 2017, 3, 1600448 CrossRef PubMed.
- M. Ouyang, C. Kong, J. Zhang, L. Zhang, Q. Hua, B. Tao, X. Lv, D. Jiang, R. Bai and C. Zhang, Eur. Polym. J., 2024, 208, 112857 CrossRef CAS.
- L. Zhang, J. Li, J. H. Wang, Y. D. Zhao, Y. X. Tang, W. J. Li, M. Ouyang and C. Zhang, Dyes Pigm., 2024, 221, 111779 CrossRef CAS.
- K. C. Chen, C. Y. Hsu, C. W. Hu and K. C. Ho, Sol. Energy Mater. Sol. Cells, 2011, 95, 2238–2245 CrossRef CAS.
- Y. L. Zhang, L. Q. Kong, Y. Zhang, H. M. Du, J. S. Zhao, S. Chen, Y. Xie and Y. Wang, Org. Electron., 2020, 81, 105685 CrossRef CAS.
- A. Martinelli, A. Nitti, R. Po and D. Pasini, J. Org. Chem., 2024, 89, 4237–4243 CrossRef CAS PubMed.
- C. Sun, Z. Cheng, J. Abu-Halimah and B. Tian, iScience, 2023, 26, 106715 CrossRef CAS PubMed.
- J. Park, Y. Lee, T. Y. Kim, S. Hwang and J. Seo, ACS Appl. Electron. Mater., 2022, 4, 1449–1468 CrossRef CAS.
- D. Ohayon and S. Inal, Adv. Mater., 2020, 32, 2001439 CrossRef CAS PubMed.
- O. Bettucci, G. M. Matrone and F. Santoro, Adv. Mater. Technol., 2022, 7, 2100293 CrossRef CAS.
- D. M. Cox-Pridmore, F. A. Castro, S. R. P. Silva, P. Camelliti and Y. L. Zhao, Small, 2022, 18, 2105281 CrossRef CAS PubMed.
- E. M. Song, J. H. Li, S. M. Won, W. B. Bai and J. A. Rogers, Nat. Mater., 2020, 19, 590–603 CrossRef CAS PubMed.
- A. Saleh, S. Wustoni, L. Salvigni, A. Koklu, V. Druet, J. Surgailis, P. D. Nayak and S. Inal, Adv. Sens. Res., 2024, 3, 2300188 CrossRef CAS.
- W. Hai, T. Goda, H. Takeuchi, S. Yamaoka, Y. Horiguchi, A. Matsumoto and Y. Miyahara, ACS Appl. Mater. Interfaces, 2017, 9, 14162–14170 CrossRef CAS PubMed.
- T. Bahadur K.C., S. Tada, L. Zhu, T. Uzawa, N. Minagawa, S. C. Luo, H. Zhao, H. H. Yu, T. Aigaki and Y. Ito, Chem. Commun., 2018, 54, 5201–5204 RSC.
- G. Haya, D. O. Runsewe, M. U. Otakpor, G. E. Pohlman, A. Towne, T. Betancourt and J. A. Irvin, ACS Appl. Polym. Mater., 2023, 5, 1208–1218 CrossRef CAS.
- B. Arvas, S. Yazar, M. B. Arvas, S. Eglence-Bakır, M. Sahin and C. Yolacan, ECS J. Solid State Sci. Technol., 2023, 12, 077001 CrossRef.
- B. M. Hryniewicz, E. S. Orth and M. Vidotti, Sens. Actuators, B, 2018, 257, 570–578 CrossRef CAS.
- U. Ngoensawat, T. Pisuchpen, Y. Sritana-anant, N. Rodthongkum and V. P. Hoven, Talanta, 2022, 241, 123253 CrossRef CAS PubMed.
- C. Boehler, C. Kleber, N. Martini, Y. Xie, I. Dryg, T. Stieglitz, U. G. Hofmann and M. Asplund, Biomaterials, 2017, 129, 176–187 CrossRef CAS PubMed.
- K. M. Woeppel, D. D. Krahe, E. M. Robbins, A. L. Vazquez and X. T. Cui, Adv. Healthcare Mater., 2024, 13, 2470019 CrossRef.
- J. A. Chikar, J. L. Hendricks, S. M. Richardson-Burns, Y. Raphael, B. E. Pfingst and D. C. Martin, Biomaterials, 2012, 33, 1982–1990 CrossRef CAS PubMed.
- Y. S. Hsiao, E. D. Quiñones, S. C. Yen, J. S. Yu, J. T. Fang, P. L. Chen and R. S. Juang, ACS Appl. Mater. Interfaces, 2023, 15, 21953–21964 CrossRef CAS PubMed.
- P. Sakunpongpitiporn, R. Morarad, W. Naeowong, S. Niamlang and A. Sirivat, RSC Adv., 2024, 14, 1549–1562 RSC.
- E. Sauvage, J. Matta, C. T. Dang, J. Fan, G. Cruzado, F. Cicoira and G. Merle, J. Biomed. Mater. Res., Part A, 2024, 1–10 Search PubMed.
- Y. X. Li, W. Peng, Y. N. Dong, B. R. Fan, W. J. Qian, X. X. Ji, X. Lu, D. L. Gan and P. S. Liu, ACS Appl. Polym. Mater., 2023, 5, 4233–4243 CrossRef CAS.
- K. Sun, S. Zhang, P. Li, Y. Xia, X. Zhang, D. Du, F. H. Isikgor and J. Ouyang, J. Mater. Sci.: Mater. Electron., 2015, 26, 4438–4462 CrossRef CAS.
- N. Terasawa and M. Tanaka, Sens. Actuators, A, 2023, 362, 11460973 CrossRef.
- J. Aerathupalathu Janardhanan, Y. L. Chen, C. T. Liu, H. S. Tseng, P. I. Wu, J. W. She, Y. S. Hsiao and H. H. Yu, Anal. Chem., 2022, 94, 7584–7593 CrossRef CAS PubMed.
- J. Aerathupalathu Janardhanan, A. Valaboju, U. Dhawan, T. H. Mansoure, C.-C. S. Yan, C.-H. Yang, B. Gautam, C.-P. Hsu and H.-h. Yu, Analyst, 2021, 146, 7118–7125 RSC.
- P.-C. Huang, M.-Y. Shen, H.-h. Yu, S.-C. Wei and S.-C. Luo, ACS Appl. Bio Mater., 2018, 1, 160–167 CrossRef CAS.
- C. W. Chang-Jian, E. C. Cho, S. C. Yen, B. C. Ho, K. C. Lee, J. H. Huang and Y. S. Hsiao, Dyes Pigm., 2018, 148, 465–473 CrossRef CAS.
- A. S. Pillai, A. Chandran and S. K. Peethambharan, Appl. Mater. Today, 2021, 23, 100987 CrossRef.
- R. T. Su, S. H. Park, X. Ouyang, S. I. Ahn and M. C. McAlpine, Sci. Adv., 2022, 8, eabl8798 CrossRef CAS PubMed.
- A. Cui, J. L. Yao, J. Xu, R. Wang and L. Y. Hao, Prog. Org. Coat., 2024, 192, 108497 CrossRef CAS.
- T. Cheng, Y.-Z. Zhang, J.-P. Yi, L. Yang, J.-D. Zhang, W.-Y. Lai and W. Huang, J. Mater. Chem. A, 2016, 4, 13754–13763 RSC.
- J. H. Lee, Y. Y. Kim and O. O. Park, J. Mater. Chem. C, 2018, 6, 4106–4113 RSC.
- M. Culebras, Y. Y. Byun, J. Jang, A. Serafin, M. N. Collins, Y. T. Park and C. Cho, Appl. Surface Sci., 2023, 615, 156432 CrossRef CAS.
- J. Kwon, V. Ganapathy, Y. H. Kim, K. D. Song, H. G. Park, Y. Jun, P. J. Yoo and J. H. Park, Nanoscale, 2013, 5, 7838–7843 RSC.
- N. Sanetra, V. Feig, B. Wolfrum, A. Offenhäusser and D. Mayer, Phys. Status Solidi A, 2011, 208, 1284–1289 CrossRef CAS.
- M. R. Abidian, J. M. Corey, D. R. Kipke and D. C. Martin, Small, 2010, 6, 421–429 CrossRef CAS PubMed.
- M. ElMahmoudy, A. M. Charrier, G. G. Malliaras and S. Sanaur, Adv. Mater. Technol., 2018, 3, 1700344 CrossRef.
- D. A. Koutsouras, P. Gkoupidenis, C. Stolz, V. Subramanian, G. G. Malliaras and D. C. Martin, ChemElectroChem, 2017, 4, 2321–2327 CrossRef.
- H.-A. Lin, S.-C. Luo, B. Zhu, C. Chen, Y. Yamashita and H.-h. Yu, Adv. Funct. Mater., 2013, 23, 3212–3219 CrossRef CAS.
- S. S. Guo, Q. F. Meng, J. Qin, Y. Du, L. Wang, P. Eklund and A. le Febvrier, ACS Appl. Mater. Interfaces, 2023, 15(29), 35430–35438 CrossRef CAS PubMed.
- S. C. Raghavan, N. C. Shivaprakash and S. N. Sindhu, Opt. Mater., 2017, 73, 56–63 CrossRef CAS.
- W. Z. Zhang, C. Zhang, J. C. Liu, X. Wang and S. B. Zhu, Sol. Energy Mater. Sol. Cells, 2023, 251, 112146 CrossRef CAS.
- J. L. Niu, S. Chen, W. N. Zhang, W. W. Zhang, K. K. Chai, G. Ye, D. Q. Li, W. Q. Zhou, X. M. Duan and J. K. Xu, Electrochim. Acta, 2018, 283, 488–496 CrossRef CAS.
- F. Pawula, S. Perrot, G. Hadziioannou and G. Fleury, Org. Electron., 2023, 118, 106806 CrossRef CAS.
- Y. H. Lee, J. Oh, S. S. Lee, H. Kim and J. G. Son, ACS Macro Lett., 2017, 6(4), 386–392 CrossRef CAS PubMed.
- K. Chowdhury, S. K. Behura, M. Rahimi and M. H. Gharahcheshmeh, ACS Appl. Energy Mater., 2024, 7(3), 1068–1079 CrossRef CAS.
- S. Deshagani, A. Das, D. Nepak and M. Deepa, ACS Appl. Polym. Mater., 2020, 2(3), 1190–1202 CrossRef CAS.
- R. Garg, G. Balakrishnan, R. B. Rashid, S. A. Gershanok, D. S. Roman, Y. Q. Wang, P. C. Kouassi, J. Rivnay and T. Cohen-Karni, ACS Appl. Nano Mater., 2023, 6(10), 8495–8505 CrossRef CAS.
- J. Tropp, C. P. Collins, X. R. Xie, R. E. Daso, A. S. Mehta, S. P. Patel, M. M. Reddy, S. E. Levin, C. Sun and J. Rivnay, Adv. Mater., 2023, 2306691 Search PubMed.
- S. Shisodia, B. Duponchel, G. Leroy, A. H. Sahraoui, D. P. Singh, C. Poupin, L. Tidahy, R. Cousin, P. Ropa and M. Depriester, Sustainable Energy Fuels, 2022, 6, 3158–3168 RSC.
- X. Y. Li, X. S. Zhao, R. Q. Liu, H. Wang, S. Wang, B. Fan, C. G. Hu and H. B. Wang, J. Mater. Chem. B, 2024, 12, 3092–3102 RSC.
- G. G. Wallace, P. R. Teasdale, G. M. Spinks and L. A. P. Kane-Maguire, Conductive electroactive polymers: intelligent polymer systems, CRC press, 2008 Search PubMed.
- N. A. Zubair, N. A. Rahman, H. N. Lim, R. M. Zawawi and Y. Sulaiman, RSC Adv., 2016, 6, 17720–17727 RSC.
- A. C. Da Silva, C. Amadou-Douah, S. Koiliari, J. Du, R. K. K. Chauhan, T. E. Paterson and I. R. Minev, Macromol. Mater. Eng., 2024, 309(2), 2300263 CrossRef CAS.
- T. H. Mansoure, H. Ayalew, W.-L. Kao, J.-J. Shyue, S.-C. Luo, Y.-C. Cheng and H.-h. Yu, ACS Appl. Energy Mater., 2020, 3(1), 1171–1180 CrossRef CAS.
- C. H. Lin, X. Tang, P. Chen and S. C. Luo, ACS Appl. Bio Mater, 2023, 6, 5695–5707 CrossRef CAS PubMed.
- V. Q. Nguyen, D. Schaming, P. Martin and J. C. Lacroix, ACS Appl. Mater. Interfaces, 2015, 7, 21673–21681 CrossRef CAS PubMed.
- A. A. Ensafi, Electrochem. Biosens., 2019, 1–10 CAS.
- N. Gu and S. Q. Liu, J. Mater. Chem. B, 2020, 8, 3168–3170 RSC.
- C. L. Clark Jr. and C. Lyons, Ann. N. Y. Acad. Sci., 1962, 102, 29 CrossRef PubMed.
- M. Y. Shen, J. F. Chen, C. H. Luo, S. Lee, C. H. Li, Y. L. Yang, Y. H. Tsai, B. C. Ho, L. R. Bao, T. J. Lee, Y. J. Jan, Y. Z. Zhu, S. Cheng, F. Y. Feng, P. Chen, S. Hou, V. Agopian, Y. S. Hsiao, H. R. Tseng, E. M. Posadas and H. H. Yu, Adv. Healthcare Mater., 2018, 7, 1700701 CrossRef PubMed.
- L. Peng, W. Min, R. Chen, L. Zhang, B. Shen, W. Xu and C. Liu, ACS Appl. Mater. Interfaces, 2023, 15(51), 59189–59198 CrossRef CAS PubMed.
- Sweety, S. Paneru and D. Kumar, Mater. Chem. Phys., 2024, 313, 128687 CrossRef CAS.
- R. P. K. C. Kafley, R. A. Sukumaran, S. Kummari, P. K. Vvn and Y. G. Kotagiri, ACS Appl. Nano Mater., 2023, 6, 16915–16926 CrossRef.
- A. L. Soares, B. M. Hryniewicz, A. E. Deller, J. Volpe, L. F. Marchesi, D. E. P. Souto and M. Vidotti, ACS Appl. Nano Mater., 2021, 4, 9945–9956 CrossRef CAS.
- J. Wang, D. Wang and N. Hui, Bioelectrochemistry, 2020, 136, 107595 CrossRef CAS PubMed.
- N. Pandey, M. Mandal, D. Samanta, G. Mukherjee and G. Dutta, Biosens. Bioelectron., 2023, 242, 115733 CrossRef CAS PubMed.
- P. GhavamiNejad, A. GhavamiNejad, H. Zheng, K. Dhingra, M. Samarikhalaj and M. Poudineh, Adv. Healthcare Mater., 2023, 12, 2202362 CrossRef CAS PubMed.
- L. Zhang, L. Wang, S. He, C. Zhu, Z. Gong, Y. Zhang, J. Wang, L. Yu, K. Gao, X. Kang, Y. Song, G. Lu and H.-D. Yu, ACS Appl. Mater. Interfaces, 2023, 15, 3224–3234 CrossRef CAS PubMed.
- P. Murugan, J. Annamalai, R. Atchudan, M. Govindasamy, D. Nallaswamy, D. Ganapathy, A. Reshetilov and A. K. Sundramoorthy, Micromachines, 2022, 13, 304 CrossRef PubMed.
- A. Medhi and D. Mohanta, ECS Adv., 2022, 1, 046504 CrossRef CAS.
- M. M. Dehbali, M. Farahmandpour, S. Hamedi and Z. Kordrostami, Sci. Rep., 2023, 13, 9505 CrossRef PubMed.
- B. Seufert, S. Thomas and A. Takshi, Sensors, 2024, 24, 1283 CrossRef CAS PubMed.
- M. Z. Çetin, N. Guven, R.-M. Apetrei and P. Camurlu, Enzyme Microb. Technol., 2023, 164, 110178 CrossRef PubMed.
- G. Paul, S. Verma, O. Jalil, D. Thakur, C. M. Pandey and D. Kumar, Polym. Adv. Technol., 2021, 32, 1774–1782 Search PubMed.
- J. Prasko, M. Raszka, K. Adamcova, A. Grambal, J. Koprivova, H. Kudrnovska, K. Latalova and J. Vyskocilova, Neuroendocrinol. Lett., 2009, 30, 615–623 Search PubMed.
- R. Hermann, D. Lay, P. Wahl, W. T. Roth and K. Petrowski, Stress, 2019, 22, 664–669 Search PubMed.
- R. R. Wang, M. He, F. Y. Qu, J. Zhang and J. G. Xu, Front. Neurol., 2022, 13, 662385 CrossRef PubMed.
- L. Meng, A. P. F. Turner and W. C. Mak, ACS Appl. Mater. Interfaces, 2021, 13, 54456–54465 CrossRef CAS PubMed.
- L. Meng, A. P. F. Turner and W. C. Mak, Biosens. Bioelectron., 2020, 159, 112181 CrossRef CAS PubMed.
- J. Hao, Z. Zhu, C. Hu and Z. Liu, Anal. Chem., 2022, 94, 4547–4555 CrossRef CAS PubMed.
- A. Bhide, A. Ganguly, T. Parupudi, M. Ramasamy, S. Muthukumar and S. Prasad, ACS Omega, 2021, 6, 6031–6040 CrossRef CAS PubMed.
- Y. P. Wang and Q. Y. Lei, Signal Transduction Targeted Ther., 2018, 3, 30 CrossRef PubMed.
- S. S. Chen, P.-C. Han, W.-K. Kuok, J.-Y. Lu, Y. Gu, T. Ahamad, S. M. Alshehri, H. Ayalew, H.-h. Yu and K. C. W. Wu, Polymers, 2020, 12, 1976 Search PubMed.
- M.-H. Lee, K.-T. Liu, J. L. Thomas, Z.-L. Su, D. O'Hare, T. van Wuellen, J. M. Chamarro, S. Bolognin, S.-C. Luo, J. C. Schwamborn and H.-Y. Lin, ACS Appl. Nano. Mater., 2020, 3, 8027–8036 Search PubMed.
- J. A. Chou, C. L. Chung, P. C. Ho, C. H. Luo, Y. H. Tsai, C. K. Wu, C. W. Kuo, Y. S. Hsiao, H. H. Yu and P. Chen, Front. Chem., 2019, 7, 281 CrossRef CAS PubMed.
- S. C. Luo, J. L. Thomas, H. Z. Guo, W. T. Liao, M. H. Lee and H. Y. Lin, ChemistrySelect, 2017, 2, 7935–7939 CrossRef CAS.
- J. E. An, K. H. Kim, S. J. Park, S. E. Seo, J. Kim, S. Ha, J. Bae and O. S. Kwon, ACS Sens., 2022, 7, 99–108 CrossRef CAS PubMed.
- X. Zhou, X. Zhou, C. Wang and H. Zhou, Chemosphere, 2023, 313, 137489 CrossRef CAS PubMed.
- E. Harding-Smith, D. R. Shaw, M. Shaw, T. J. Dillon and N. Carslaw, Environ. Sci.: Processes Impacts, 2024, 26, 436–450 RSC.
- P. Yáñez-Sedeño, L. Agüí, S. Campuzano and J. M. Pingarrón, Biosensors, 2019, 9, 127 CrossRef PubMed.
- R. Zhang, M. Abdulla, R. Jamal, Y. Ge, W. Zhang, Z. Yu, Y. Yan, Y. Liu and T. Abdiryim, Mater. Lett., 2020, 263, 127206 CrossRef CAS.
- M. O. Farea, H. A. Alhadlaq, Z. M. Alaizeri, A. A. A. Ahmed, M. O. Sallam and M. Ahamed, ACS Omega, 2022, 7, 22492–22499 CrossRef CAS PubMed.
- J. Gupta, S. Juneja and J. Bhattacharya, ACS Omega, 2020, 5, 3172–3180 CrossRef CAS PubMed.
- P. P. Conti, R. S. Andre, L. A. Mercante, L. Fugikawa-Santos and D. S. Correa, Sens. Actuators, B, 2021, 344, 130124 CrossRef CAS.
- L. Jin, K. Yang, L. Chen, R. Yan, L. He, M. Ye, H. Qiao, X. Chu, H. Gao and K. Zhang, Anal. Chem., 2023, 95, 8859–8868 CrossRef CAS PubMed.
- H. S. Dehsari, J. N. Gavgani, A. Hasani, M. Mahyari, E. K. Shalamzari, A. Salehi and F. A. Taromi, RSC Adv., 2015, 5, 79729–79737 RSC.
- L. A. Machhindra and Y.-K. Yen, Chemosensors, 2022, 10, 209 CrossRef CAS.
- L. Wu, L. Lu, L. Zhang, J. Xu, K. Zhang, Y. Wen, X. Duan and F. Yang, Electroanalysis, 2013, 25, 2244–2250 Search PubMed.
- P. Lin, F. Chai, R. Zhang, G. Xu, X. Fan and X. Luo, Microchim. Acta, 2016, 183, 1235–1241 Search PubMed.
- W. Zhang, R. Jamal, R. Zhang, Z. Yu, Y. Yan, Y. Liu, Y. Ge and T. Abdiryim, Phys. Chem. Chem. Phys., 2020, 22, 3592–3603 Search PubMed.
- F. Tian, H. Li, M. Li, C. Li, Y. Lei and B. Yang, Synth. Met., 2017, 226, 148–156 Search PubMed.
- P. Wang, L. Wang, M. Ding, M. Pei and W. Guo, Analyst, 2019, 144, 5866–5874 Search PubMed.
- Jyoti, Deepeka, S. Rana and S. Singhal, Electrochim. Acta, 2023, 471, 143332 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |