Nanoconfinement of ultra-small Bi2Te3 nanocrystals on reduced graphene oxide: a pathway to high-performance sodium-ion battery anodes†
Zhuoying
Cheng
,
Zhuo
Li
,
Yuao
Wang
,
Yiyang
Mao
,
Jun
Yan
*,
Dianxue
Cao
 and
Kai
Zhu
and
Kai
Zhu
 *
*
Key Laboratory of Superlight Materials and Surface Technology, Ministry of Education, College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China. E-mail: kzhu@hrbeu.edu.cn; yanjun198201@vip.163.com
First published on 15th February 2024
Abstract
Bismuth telluride (Bi2Te3) nanomaterials have attracted considerable attention owing to their intriguing physicochemical properties and wide-ranging potential applications arising from their distinctive layered structure and nanoscale size effects. However, synthesizing sub-100 nm ultra-small Bi2Te3 nanocrystals remains a formidable challenge. To date, there has been little investigation on the performance of these ultra-small Bi2Te3 nanocrystals in sodium-ion batteries (SIBs). This study presents a general strategy for synthesizing ultra-small Bi2Te3 nanocrystals on reduced graphene oxide (Bi2Te3/rGO) through a nanoconfinement approach. First-principles calculations and electrochemical kinetic studies confirm that the ultra-small Bi2Te3/rGO composite material can effectively mitigate volumetric expansion, preserve electrode integrity, and enhance electron transfer, Na-ion adsorption, and diffusion capacity. As a result, the Bi2Te3/rGO electrode demonstrates a remarkable initial specific capacity of 521 mA h g−1 at 0.1 A g−1, showcasing outstanding rate behaviour and long-lasting cycle life exceeding 800 cycles at 1 A g−1 while preserving exceptional rate properties. The function of the battery is indicated by ex situ TEM and XPS findings, which propose a conventional dual mechanism involving conversion and alloying. This work paves the way for rapid advancements in Bi2Te3-based SIB anodes while contributing to our understanding of sodium ion storage mechanisms.
1. Introduction
The abundant availability of sodium in nature, its low cost, and the rocking-chair Na-ion storage mechanism have led to significant research interest in sodium-ion batteries (SIBs) as a potential alternative to lithium-ion batteries, as evidenced by the attention they have received.1,2 On the other hand, the Na ion demonstrates reduced reaction kinetics and transport during electrochemical cycling because of its greater ionic radius (around 1.02 Å). It is accompanied by a notable increase in volume, which dramatically affects the electrode's structural stability while charging and discharging.3,4 Consequently, the primary challenge faced by exploring suitable SIB anode materials lies in achieving excellent structural stability while accommodating volume changes induced by Na ion transport and providing rapid Na ion transport capabilities.5,6In recent years, there has been significant emphasis on the development of efficient anode substances to increase the capacity of sodium-ion batteries.7,8 Due to their high theoretical conversion capacity, sulfides, selenides, and metal oxides have gained considerable attention among alternative materials.9,10 Compared with sulfides and oxides, metal tellurides (MTs) exhibit enhanced electrical conductivity and electrochemical activity,9,11 making them promising candidates as anode materials for SIBs. However, further improvements in their rate capability are still necessary to meet practical demands.12,13 Among various MT materials, Bi2Te3 possesses a layered structure similar to graphite and demonstrates adequate theoretical capacity through a combination of conversion and alloying reactions.14,15 Nevertheless, the main obstacle related to Bi2Te3 is its significant alteration in volume while cycling, resulting in the pulverization of the electrode substance and a rapid decline in capacity.16,17 Enhancing the rate capability of MTs while addressing the volume change of Bi2Te3 represents a crucial objective toward advancing SIB anode materials.18
Researchers have developed alternative methods for fabricating Bi2Te3-based nanocomposites to enhance the performance of SIBs that overcome the limitations of conventional electrode preparation methods. One frequently used method includes combining Bi2Te3 with a conductive carbon framework to create nanocomposites, which enhances the stability of the active material's structure and improves its electrical conductivity.19 Several remarkable studies have demonstrated that including nanoscale substances can notably enhance the surface area of contact between the electrode and electrolyte, enlarge ion storage locations, and reduce the length of ion transport routes. These enhancements contribute to the improvement in reversible capacity and kinetic performance.20–22 Moreover, the high electronic conductivity of carbon facilitates accelerated electron transfer, further enhancing the rate capability. For instance, Chong et al. synthesized a composite anode architecture of Bi2Te3 nanosheets enclosed within reduced graphene oxide (rGO) and nitrogen-doped carbon (NC) layers. At a rate of 0.05 A g−1, the composite displayed an impressive initial reversible specific capacity of 322.7 mA h g−1 while also demonstrating outstanding stability throughout multiple cycles and excellent performance at different rates.23 Pang et al. fabricated solvent-reduced Bi2Te3 nanosheets with exceptional rate capability and long-term cycling performance by delivering 364.0 mA h g−1 after 1200 cycles at 5 A g−1.24
This work describes the fabrication of ultra-small Bi2Te3 nanocrystals uniformly attached to reduced graphene oxide (rGO) nanosheets using a facile and versatile nanoconfinement strategy. The pH adjustment of the dispersion of graphene oxide leads to a slight stacking of graphene oxide sheets as a result of reduced electrostatic repulsion. In situ growth of ultra-small Bi2Te3 nanocrystals in these weakly stacked graphene oxide sheets was achieved using confined nano-reactors. By taking advantage of its tiny size, the Bi2Te3/rGO composite material, consisting of just one sheet, is utilized as an anode material for SIBs, exhibiting an impressive maximum capacity of 521 mA h g−1 at 0.1 A g−1 coupled with exceptional rate performance and remarkable cycling stability. The storage mechanism of sodium ions in Bi2Te3/rGO was elucidated through ex situ transmission electron microscopy (TEM) and ex situ X-ray photoelectron spectroscopy (XPS). In addition, successfully assembling a high-performing full cell further displays the potential application of MT anodes for SIBs.
2. Results and discussion
The diagram of synthesizing Bi2Te3 nanocrystals on rGO is depicted in Fig. 1A. First, dilute nitric acid was dropped into a homogeneous dispersion of GO until the pH reaches approximately 4, resulting in a slight accumulation of GO nanosheets. Subsequently, Bi(NO3)3·5H2O was added to the mixture while stirring it in an ice bath to regulate the nucleation and growth speed of the GO dispersion. Through coordination/electrostatic interactions with oxygen-containing functional groups, Bi3+ cations can adsorb on the surface of GO. Then, NH4Cl was added to the dispersion to inhibit the expansion of extremely tiny BiOCl nanocrystals in the interlayer nano region between the loosely arranged GO nanosheets. After subjecting to centrifugal purification and freeze drying, the end product was transformed into a composite aerogel. Utilizing BiOCl/GO nanosheets as precursors and Na2TeO3 as a tellurizing agent, Bi2Te3/GO was obtained through an ion exchange process under hydrothermal conditions. Finally, by subjecting it to a simple annealing treatment at 350 °C, graphene oxide was effectively reduced, leading to the successful synthesis of chemically bonded ultra-small Bi2Te3 on reduced graphene oxide (referred to as Bi2Te3/rGO).The scanning electron microscopy (SEM) images in Fig. 1A and S1† illustrate the even dispersion of Bi2Te3 nanoparticles throughout the rGO surface. The images reveal a close contact between Bi2Te3 nanoparticles and rGO, with loosely arranged GO nanosheets offering numerous locations for the formation of tiny Bi2Te3 nanocrystals and an important interlayer confinement nano-space. For comparison, Bi2Te3 nanoparticles of a larger size (approximately 550 nm on average) were produced by a commonly employed solvothermal method (Fig. S2, ESI†). Weakly stacked GO nanosheets aid in nucleation for ultra-small Bi2Te3 nanocrystals, playing a significant role in interlayer confinement. The microstructure of Bi2Te3–rGO (Fig. 1C–G) was examined using transmission electron microscopy (TEM) analysis, which showed a homogeneous dispersion of tiny Bi2Te3 nanoparticles on the rGO surface with no evidence of clustering. As shown in the high-resolution TEM image (Fig. 1F), a lattice spacing corresponding to the (002) plane of Bi2Te3 at 0.37 nm is observed. Fig. 1G displays the selected area electron diffraction (SAED) pattern that reveals diffraction spots corresponding to the (015), (110), and (125) planes of Bi2Te3. Dark-field TEM imaging and energy-dispersive spectroscopy elemental mapping confirm the presence of elements including Bi, Te, C, and O on both the rGO flakes and ultra-small Bi2Te3 nanoparticles.
Fig. 2A illustrates the XRD pattern of Bi2Te3/rGO, displaying clear peaks at 2θ = 8.6°, 17.4°, 23.6°, 27.6°, 37.8°, 40.4°, 41.1°, and 50.1°, which correspond to the (003), (006), (101), (015), (1010), (0111), (110), and (205) crystallographic planes of Bi2Te3, respectively. These peaks align excellently with the standard card reference JCPDS no. 04-004-7783, confirming the successful synthesis of Bi2Te3/rGO by the ion exchange method. Fig. S3† shows the XRD pattern of BiOCl. Raman spectroscopy, depicted in Fig. 2B, was employed to investigate the carbon state within the composite material. The wavenumbers at around 1589 and 1341 cm−1 are attributed to the sp2-hybridized carbon (g-band) and sp3-hybridized carbon (d-band) in Bi2Te3/rGO.16,25,26 In addition, the rGO content was determined using thermogravimetric analysis (TG). After heating Bi2Te3 to 800 °C in air, it completely transforms into TeO2 and Bi2O3. The TG curve (Fig. 2C) reveals that the rGO content in Bi2Te3/rGO is approximately 3.8 wt%. According to Table S1 in the ESI,† the electrical conductivity of Bi2Te3 nanoparticles is 0.33 × 10−2 S cm−1, while that for Bi2Te3/rGO composites is 1.47 × 10−2 S cm−1, indicating that rGO contributes to the enhancement of electrical conductivity in Bi2Te3/rGO. The N2 adsorption/desorption isotherms of Bi2Te3 and Bi2Te3/rGO (Fig. 2D) display characteristic hysteresis loops,27 indicative of a type-IV mesoporous structure in both composite materials, with the pore sizes of these composites vary from 2 nm to 100 nm. The BET specific surface area of Bi2Te3 was found to be 3.79 m2 g−1, while that of Bi2Te3/rGO significantly increases to approximately 24.21 m2 g−1. This suggests that adding rGO affected the original multichannel structure of Bi2Te3/rGO. The BET findings suggest that Bi2Te3/rGO possesses a significantly increased specific surface area, indicating potential advantages in facilitating electrolyte permeation and providing a greater abundance of active sites for Na ion electrochemical interactions.
The X-ray photoelectron spectroscopy (XPS) technique was employed to investigate the composition and valence states of Bi2Te3/rGO. In the exhaustive scan XPS measurement profile, Bi, Te, C, and O elemental components were observed in the Bi2Te3/rGO sample (Fig. 2E). The C 1s core level spectra exhibited five well-fitted peaks at 288.5, 287.6, 286.2, 285.3, and 284.6 eV, corresponding to the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–Te, C–O, C
O, C–Te, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–C bonds, respectively.9,28Fig. 2G shows the O 1s spectra of Bi2Te3/rGO, where the three fitted peaks at 533.1, 531.3, and 530.2 eV are attributed to the C–O–C, Bi–O–C, and Bi–O bonds, respectively.29,30 It is worth noting that the Bi–O–C chemical bond between rGO and Bi2Te3 facilitates rapid charge transfer while maintaining good morphological and structural stability. The 4f5/2 and Bi 4f7/2 inherent peaks (Fig. 2H) are represented by the two separate peaks at 164.5 eV and 159.5 eV, respectively.28 The binding energy peaks observed at 586.4 eV and 576.1 eV are attributed to the presence of the Te–O bond, indicating the surface oxidation of Te atoms.31 In Fig. 2I, the XPS spectrum of Te 3d shows the presence of two distinct pairs of peaks. The Te 3d3/2 and Te 3d5/2 states linked to the Bi–Te bond in the Bi2Te3 compound can be identified as the peaks at 583.6 eV and 573.2 eV.32
O, and C–C bonds, respectively.9,28Fig. 2G shows the O 1s spectra of Bi2Te3/rGO, where the three fitted peaks at 533.1, 531.3, and 530.2 eV are attributed to the C–O–C, Bi–O–C, and Bi–O bonds, respectively.29,30 It is worth noting that the Bi–O–C chemical bond between rGO and Bi2Te3 facilitates rapid charge transfer while maintaining good morphological and structural stability. The 4f5/2 and Bi 4f7/2 inherent peaks (Fig. 2H) are represented by the two separate peaks at 164.5 eV and 159.5 eV, respectively.28 The binding energy peaks observed at 586.4 eV and 576.1 eV are attributed to the presence of the Te–O bond, indicating the surface oxidation of Te atoms.31 In Fig. 2I, the XPS spectrum of Te 3d shows the presence of two distinct pairs of peaks. The Te 3d3/2 and Te 3d5/2 states linked to the Bi–Te bond in the Bi2Te3 compound can be identified as the peaks at 583.6 eV and 573.2 eV.32
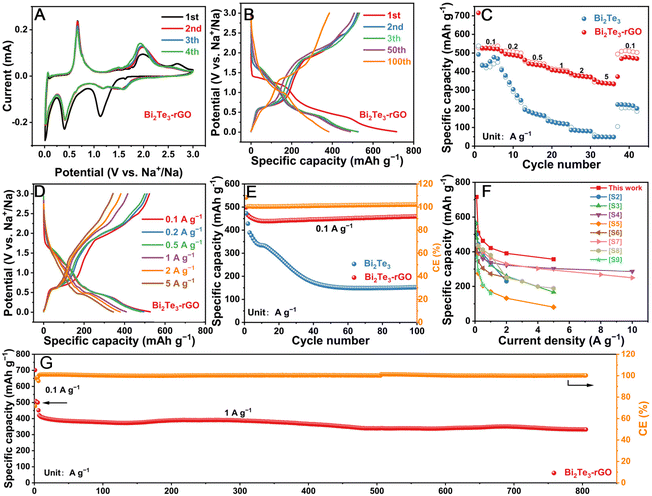
To elucidate the intrinsic advantages and the sodium ion storage mechanism of Bi2Te3, a half-cell system was employed with metal sodium as the counter electrode, using the corresponding electrochemical testing methods. During the first cathodic scan of the cyclic voltammetry(CV) curves, the Bi2Te3 cathodic peaks (Fig. 3A) were detected at 1.1 V, suggesting the development of a solid electrolyte interface (SEI) and the presence of specific unfavorable reactions.33,34 The conversion reaction from Bi2Te3 to Bi (eqn (1)) can be identified as the cause of the cathodic peak appearing at 1.5 V.35,36 The alloy reactions of Bi (eqn (2) and (3)) are represented by the two peaks at 0.4 V and 0.6 V.37 The peak at 0.2 V may be attributed to carbon materials.38 As oxidation occurs, the cliffs at 0.6 V and 0.8 V correspond to the gradual dissociation of the Na3Bi alloy into Bi (eqn (4) and (5)). Furthermore, the oxidation peak at 1.9 V appears as a result of the dissociation reaction of Na2Te (eqn (6)). The CV curves of the Bi2Te3/rGO anode remain highly consistent in the subsequent three cycles, demonstrating the exceptional reversibility of the phase transition throughout the cycling process. Therefore, the entire electrochemical reactions involved in the Bi2Te3/rGO anode for SIBs can be described by the following equations:38
| Bi2Te3 + 6Na+ + 6e− → 3Na2Te + 2Bi | (1) |
| Bi + Na+ + e− → NaBi | (2) |
| NaBi + 2Na+ + 2e− → Na3Bi | (3) |
| Na3Bi → NaBi + 2Na+ + 2e− | (4) |
| NaBi → Bi + Na+ + e− | (5) |
| Na2Te → 2Na+ + Te + 2e− | (6) |
Fig. 3B depicts the galvanostatic charge–discharge (GCD) curve at 0.1 A g−1. Notably, two distinct discharge voltage plateaus are observed at approximately 1.6 V and 0.4 V (vs. Na+/Na), which aligns well with the CV curve. At a current density of 0.1 A g−1, the Bi2Te3/rGO composite material demonstrates a remarkable initial coulombic efficiency (ICE) of 75.2%, with an initial discharge capacity of 719 mA h g−1 and a charge specific capacity of 539 mA h g−1. After undergoing 100 cycles, the discharge capacity remains at 446 mA h g−1 with a cycle efficiency of 91.1%. In contrast, the discharge capacity of the pure Bi2Te3 electrode is close to 450 mA h g−1 at 0.1 A g−1 but significantly decreases to only 50 mA h g−1 at a current density of 5 A g−1. Upon restoring the current density to 0.1 A g−1, the specific capacity only reaches 222 mA h g−1. In sharp contrast, the Bi2Te3/rGO composite material exhibits discharge capacities of 521, 488, 436, 433, and 374 mA h g−1 at 0.1, 0.2, 0.5, 1, and 2 A g−1, respectively. Even at 5 A g−1, it maintains a remarkable specific capacity of 340 mA h g−1. In addition, upon restoring the current to 0.1 A g−1, the Bi2Te3/rGO composite material exhibits a sustained higher specific degree of 476 mA h g−1, demonstrating its superior rate capability compared to pure Bi2Te3 (Fig. 3C). The improved rate efficiency of the Bi2Te3/rGO composite material can be ascribed to the combined impact of the ultra-small Bi2Te3 nanocrystal arrangement and the enhanced chemical bonding between Bi2Te3 and conductive rGO.
Fig. 3D depicts the GCD patterns of Bi2Te3/rGO electrodes at different current densities in the range of 0.1–5 A g−1. The cycling performance was evaluated at a high current density of 1 A g−1. Fig. 3E demonstrates that the specific capacity of the pure Bi2Te3 electrode experiences a sharp decline within the initial 40 cycles, whereas the Bi2Te3/rGO electrode maintains a relatively stable specific capacity of 499 mA h g−1 throughout the cycling process. Notably, the specific capacity and rate performance of the Bi2Te3/rGO composite material significantly surpass those of previously reported bismuth-based anode electrode materials (Fig. 3F, Table S2†). Before engaging in extended cycling at elevated current densities, a preliminary activation test was carried out at a reduced current density of 0.1 A g−1, involving six charge–discharge cycles. During the first 100 cycles, the specific capacity experiences a decrease from 421 to 387 mA h g−1. Remarkably, the system demonstrates exceptional stability with an impressive specific capacity of 345 mA h g−1 achieved after 800 consecutive cycles, resulting in a capacity retention rate of 82% (Fig. 3G). The outstanding performance observed in the anode electrode of Bi2Te3/rGO can be ascribed to its distinctive architectural configuration, wherein minuscule Bi2Te3 nanocrystals establish chemical connections on a three-dimensional interconnected graphene network, facilitating rapid ion and electron transfer across the entire electrode surface.
The capacitance contribution is a crucial parameter for assessing the high rate performance of electrode materials. The dynamic capacitance contribution of the Bi2Te3/rGO electrode was determined by plotting CV profiles at different scan rates (0.2–5 mV s−1), as illustrated in Fig. 4A. The correlation between the peak current (i) and the scan rate (v) in the CV curves conforms to eqn (7), where adjustable parameters a and b are utilized:
| i = avb | (7) |
The slope of log(i) relative to log(v) in the graph represents the value of b. The calculated values for b obtained from anodic peak 1 and cathodic peak 2 are 0.721 and 0.766, respectively (Fig. 4B), implying that the capacitance process contributes predominantly to the capacity. The contributions of ion diffusion and pseudocapacitive diffusion at different scan rates (v) can be derived from eqn (8):
| i(v) = k1v + k2v1/2 | (8) |
To elucidate the enhanced electrochemical capabilities of Bi2Te3/rGO composite materials, electrochemical impedance spectroscopy (EIS) analysis was performed on both the pure Bi2Te3 and Bi2Te3/rGO composite materials, as depicted in Fig. 4E. Each EIS plot comprises a semicircular arc and a sloped region in the high-, medium-, and low-frequency domains.20 The semicircle corresponds to the resistance from solid electrolyte interface (SEI) formation and charge transfer processes between the electrode and the electrolyte.41,42 The sloped region signifies the Warburg diffusion phenomenon associated with sodium ion migration within the electrode, manifesting as a 45° slope angle.43 Consistent with CV and charge–discharge analyses, Fig. 4E reveals that the Bi2Te3/rGO composite electrode exhibits a reduced semicircle diameter compared to its pristine counterpart, indicating diminished interfacial impedance. The inset in Fig. 4E illustrates an equivalent circuit model fitted to the experimental data. Notably, solution resistances (Rs) for pristine and composite electrodes are estimated as 5.14 Ω and 0.88 Ω, respectively. Furthermore, the charge transfer resistance for the Bi2Te3/rGO composite is ∼113.20 Ω, while that for pristine Bi2Te3 nanosheets amounts to 283.00 Ω; this unequivocally demonstrates a significant enhancement in conductivity upon the incorporation of a conductive reduced graphene oxide matrix into the original Bi2Te3 nanosheets.
In addition, Fig. 4F and H illustrate the galvanostatic intermittent titration technique (GITT) plots of Bi2Te3 and Bi2Te3/rGO, respectively, to investigate the diffusion coefficient of the Na ion (DNa+). It can be calculated using eqn (9):44
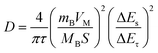 | (9) |
Throughout the discharge and charge processes, the DNa+ values for Bi2Te3/rGO range from 3.9 × 10−12 to 7.38 × 10−10 cm2 s−1, exhibiting a notable increase compared to the measurements of pure Bi2Te3, which vary from 6.84 × 10−16 cm2 s−1 to 1.1 × 10−12 cm2 s−1. Consequently, the diffusion coefficient (DNa+) of Bi2Te3/rGO exhibits a notable enhancement compared to that of pure Bi2Te3. These findings suggest that the two-dimensional structure of Bi2Te3/rGO facilitates Na ion diffusion, thereby improving its rate performance.
Ex situ TEM and ex situ XPS analyses were performed at different sodiation/desodiation stages to acquire a thorough comprehension of the structure evolution and electrochemical mechanism of sodium ion storage in the Bi2Te3/rGO electrode, as illustrated in Fig. 5. Before sodiation under open-circuit voltage, the XPS spectra of Bi 4f exhibited a pair of peaks at 159.1 and 164.6 eV, which corresponded to the Bi3+ states of Bi 4f7/2 and Bi 4f5/2. Notably, an additional peak emerged in the XPS spectrum of Te 3d at 564.1 eV, indicating oxygen adsorption on the Te 3d orbitals.45 Furthermore, compared to the initial position, the peak intensities of Bi3+ 4f7/2, Bi3+ 4f5/2, and Te 3d5/2 were significantly weakened, suggesting the transformation of Bi2Te3/rGO into NaBi and Na2Te, consistent with the results of EDS elemental mapping (Fig. S4†).
Ex situ TEM further demonstrated the morphology evolution of phase transformation during the discharge–charge process with different potential states. At the discharge state (0.01 V), Fig. 5A illustrates that Bi2Te3/rGO undergoes a transition from large grains (approximately 50–100 nm) to uniformly dispersed small grains (around 20 nm). In addition, the existence of lattice fringes measuring 0.332 nm apart in Fig. 5H confirms the formation of a Na3Bi alloy on the (021) plane of Na3Bi with rhombohedral structure (JCPDS no. 97-067-1309), providing further evidence for in situ transformation. The viewpoint is further substantiated by the EDS results (Fig. S3†). Upon being charged back to 1.9 V, the (110) plane of NaBi can be identified from the lattice fringes displayed in Fig. 5I, which have a spacing of 0.338 nm. After charging to 3.0 V, the lattice fringes can be well attributed to Te with a rhombohedral structure (JCPDS no. 97-016-1690) (Fig. 5F and J). Notably, no detectable Bi2Te3 lattice is observed after the discharge/charge process, indicating an irreversible transformation within the Bi2Te3 nanosheet anode.
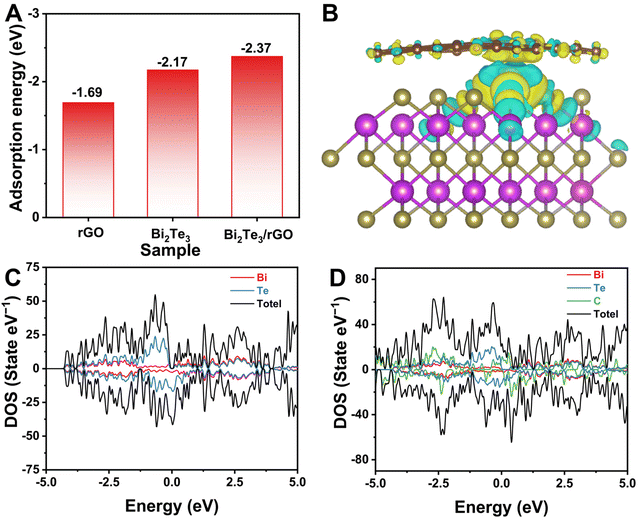
Through the utilization of first-principles calculations using density functional theory (DFT), we have comprehensively elucidated the electronic structure and adsorption energy of the Na ion in the material. Furthermore, we investigated the adsorption capability of the material framework for the Na ion and the calculation model in Fig. S5,† where a 2 × 2 × 1 supercell was constructed to accommodate the adsorption of one Na ion. As depicted in Fig. 6A, the Bi2Te3/rGO interface shows an adsorption energy of the Na ion measuring −2.37 eV, which is comparatively lower than that observed for Bi2Te3 (−2.17 eV). This suggests a pronounced affinity of the Bi2Te3/rGO electrode towards the Na ion, thereby facilitating their adsorption and promoting subsequent electrochemical reactions. Insights into the bonding characteristics of the adsorbed Na atoms were obtained by analyzing the difference in electronic density, which involved subtracting the charge density contributions from Na atoms and bare Bi2Te3 within the combined compound. As illustrated in Fig. 6B, yellow and cyan electron clouds represent electron accumulation and depletion regions within the Bi2Te3/rGO structure. Notably, an evident charge accumulation region around the C atom and an adjacent layer exhibiting electron depletion near the –OH functional group, signifying electron transfer from rGO to Bi2Te3, which enhances the Na ion adsorption capacity. In addition, the total densities of states (DOSs) of all samples are illustrated in Fig. 6C and D. It can be observed that Bi2Te3/rGO exhibits a conductor behaviour near the Fermi energy level, lacking a band gap, which is considerably superior to Bi2Te3, indicating a significant improvement in electronic conductivity due to the rGO confinement effect. Consequently, it can be inferred that preferential adsorption sites for Na ions are more likely to appear around Bi2Te3/rGO rather than on pure Bi2Te3.
The integration of the Bi2Te3/rGO anode into a full cell is possible due to its high capacity and outstanding electrochemical performance, and its evaluation for practical use in SIBs was conducted. We examined the efficiency of the Bi2Te3/rGO full cell by utilizing Na3V2(PO4)3 as a cathode. Fig. S6A† illustrates the operational mechanism of the Bi2Te3/rGO∥Na3V2(PO4)3 full cell. Na+ was extracted from the Na3V2(PO4)3 cathode during the charging process and transferred to the Bi2Te3/rGO anode. During discharging, Na+ escapes from the Bi2Te3/rGO anode and returns to the Na3V2(PO4)3 cathode again. Fig. S6B† illustrates the exceptional cycling stability of the Bi2Te3/rGO∥Na3V2(PO4)3 full cell, which maintains a capacity of 108 mA h g−1 and retains 71% of its capacity after 200 cycles. Moreover, the rate performance of the full cell was evaluated at various current densities (Fig. S6C, ESI†), resulting in discharge capacities of 177, 166, 153, 138, 112, and 100 mA h g−1 at current densities of 0.1, 0.2, 0.5, 1, 2.0, and 5.0 A g−1, correspondingly. Remarkably, upon returning the current density to its original 0.1 A g−1, the reversible capacity rebounded to 160 mA h g−1, indicating outstanding high-rate performance for sodium storage and potential applications in energy devices. Furthermore, the charge–discharge curves at various current densities exhibit weak polarization even at a high current density of 1 A g−1 (Fig. S6D, ESI†), confirming its excellent rate capability.
3. Conclusions
In summary, a universal space-containment strategy was developed to synthesize ultra-small Bi2Te3 nanocrystals on rGO. The procedure entails a viable mechanism by accurately adjusting the stacking characteristics of graphene oxide and the pH level of its aqueous dispersion. A slight stacking of graphene oxide sheets can confine the growth of nanocrystals of Bi2Te3 in the interlayer nanospaces. As a result of the thermal reduction of graphene oxide to rGO, the Bi2Te3/rGO composite material shows a significant capacity for storing Na+. Boasting an impressive reversible capacity of 521 mA h g−1 at 0.1 A g−1, a remarkable rate capacity of 340 mA h g−1 at 5 A g−1, and outstanding cycling stability, it retains 82% of its capacity after 800 cycles. The excellent electrochemical performance is a result of the flexible configuration of the nanoplate structure in two dimensions, which guarantees remarkable structural stability and promotes the swift transfer of sodium ions and electrons. Ex situ TEM and ex situ XPS analyses provide insights into the theoretical basis for the Na+ storage mechanism in Bi2Te3/rGO. Furthermore, the Na ion full cell exhibits good electrochemical performance, combining the Bi2Te3/rGO anode with the Na3V2(PO4)3 cathode. This study introduces an innovative approach for constructing two-dimensional Bi2Te3/rGO anode materials.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Basic Research Support Program for Excellent Young Teachers in Heilongjiang Province and Fundanmental Research Funds for the Central University of China (3072022JC0305). The numerical calculations in this paper have been done on Hefei advanced computing center. Authors thank eceshi (https://www.eceshi.com) for the SEM and EDS mapping tests.References
- G. Dong, L. Li, K. Zhu, J. Yan, G. Wang and D. Cao, Small, 2023, 2208291 CrossRef CAS PubMed.
- M. Di, Z. Song, S. Chen and Y. Bai, Nanoscale, 2023, 15, 19159–19167 RSC.
- R. Hu, Y. Tong, J. Yin, J. Wu, J. Zhao, D. Cao, G. Wang and K. Zhu, Nanoscale Horizons, 2024, 9, 305–316 RSC.
- K. Sada, J. Darga and A. Manthiram, Adv. Energy Mater., 2023, 13, 2302321 CrossRef CAS.
- J. Chen, G. Adit, L. Li, Y. Zhang, D. H. C. Chua and P. S. Lee, Energy Environ. Sci., 2023, 6, e12633 CAS.
- X. Song, X. Li, H. Shan, J. Wang, W. Li, K. Xu, K. Zhang, H. M. K. Sari, L. Lei, W. Xiao, J. Qin, C. Xie and X. Sun, Adv. Funct. Mater., 2023, 2303211 Search PubMed.
- N. LeGe, X.-X. He, Y.-X. Wang, Y. Lei, Y.-X. Yang, J.-T. Xu, M. Liu, X. Wu, W.-H. Lai and S.-L. Chou, Energy Environ. Sci., 2023, 16, 5688–5720 RSC.
- X. Sun, X. Gao, Z. Li, X. Zhang, X. Zhai, Q. Zhang, L. Li, N. Gao, G. He and H. Li, Small Methods, 2023, 2300746 Search PubMed.
- S. Chong, L. Yuan, S. Qiao, M. Ma, T. Li, X. L. Huang, Q. Zhou, Y. Wang and W. Huang, Sci. China Mater., 2023, 66, 2641–2651 CrossRef CAS.
- S. Chong, J. Yang, L. Sun, S. Guo, Y. Liu and H. K. Liu, ACS Nano, 2020, 14, 9807–9818 CrossRef CAS PubMed.
- R. Meija, V. Lazarenko, Y. Rublova, A. Kons, V. Voikiva, J. Andzane, O. Gogotsi, I. Baginskiy, V. Zahorodna, A. Sarakovskis, A. Pludons, A. Sutka, A. Viksna and D. Erts, Sustainable Mater. Technol., 2023, 38, e00768 CrossRef CAS.
- J. H. Moon, H. Seong, G. Kim, Y. Jin, W. Nam, H. Yoo, T. Jung, K. Lee, M. Yang, S. Y. Cho and J. Choi, Appl. Surf. Sci., 2023, 638, 157976 CrossRef CAS.
- Z. Li, M. Han, P. Yu and J. Yu, Adv. Sci., 2023, 2306992 Search PubMed.
- X. Xie, X. Wang, Q. Cai, H. Xie and L. Chen, J. Energy Storage, 2024, 76, 109814 CrossRef.
- R. Tian, H. Zhang, Z. Yuan, Y. Man, J. Sun, J. Bao, M.-S. Wang and X. Zhou, J. Energy Chem., 2024, 89, 250–258 CrossRef CAS.
- X. Sun, B. Zhang, M. Chen, L. Wang, D. Wang, R. Man, S. Iqbal, F. Tian, Y. Qian and L. Xu, Nano Today, 2022, 43, 101408 CrossRef CAS.
- D. Li, J. Hu, C. Wang, L. Guo and J. Zhou, J. Power Sources, 2023, 555, 232387 CrossRef CAS.
- H. T. Zhang, X. G. Luo, C. H. Wang, Y. M. Xiong, S. Y. Li and X. H. Chen, J. Cryst. Growth, 2004, 265, 558–562 CrossRef CAS.
- Y. Zhu, J. Zhao, L. Li, J. Xu, X. Zhao, Y. Mi and J. Jin, Dalton Trans., 2021, 50, 10758–10764 RSC.
- W. Xin, N. Chen, Z. Wei, C. Wang, G. Chen and F. Du, Chem. – Eur. J., 2021, 27, 3745–3752 CrossRef CAS PubMed.
- Z. Dang, W. Meng, D. Zuo, D. Li, L. Jiang and D. Fang, Electrochim. Acta, 2022, 425, 140752 CrossRef CAS.
- H. Yin, D. Han, X. Yu, M. Cao, Z. Hou, C. Li and M.-Q. Zhu, ACS Appl. Energy Mater., 2023, 6, 1155–1175 CrossRef CAS.
- S. Chong, L. Yuan, Q. Zhou, Y. Wang, S. Qiao, T. Li, M. Ma, B. Yuan and Z. Liu, Small, 2023, 19, 2303985 CrossRef CAS PubMed.
- S. Pang, Z. Hu, C. Fan, W. Zhang, Y. Cai, S. Han, J. Liu and J. Liu, Nanoscale, 2022, 14, 1755–1766 RSC.
- J. Cao, J. Zhong, H. Xu, S. Li, H. Deng, T. Wang, L. Fan, X. Wang, L. Wang, J. Zhu, B. Lu and X. Duan, Nano Res., 2022, 15, 2040–2046 CrossRef CAS.
- Q. Peng, S. Zhang, H. Yang, B. Sheng, R. Xu, Q. Wang and Y. Yu, ACS Nano, 2020, 14, 6024–6033 CrossRef CAS PubMed.
- Y. Sun, Q. Wu, K. Zhang, Y. Liu, X. Liang and H. Xiang, Chem. Commun., 2022, 58, 7120–7123 RSC.
- H. Gu, M. Gao, K. Shen, T. Zhang, J. Zhang, X. Zheng, X. Guo, Y. Liu, F. Cao, H. Gu, Q. Kong and S. Xiong, Chin. Chem. Lett., 2023, 109273, DOI:10.1016/j.cclet.2023.109273.
- S. Chong, S. Qiao, L. Yuan, Q. Zhou, T. Li, S. Dong, Y. Wang, M. Ma and W. Huang, Chem. Eng. J., 2023, 461, 141957 CrossRef CAS.
- X. Zhou, A. Wang, X. Zheng, Z. Zhang, J. Song, H. Deng, P. Yan, D. Sun, Y. Yang and Z. Lei, Appl. Surf. Sci., 2024, 648, 159077 CrossRef CAS.
- J. Xu, J. Jiang, H. Tang, Z. Chen, J. Chen, Y. Zhang and C.-S. Lee, Adv. Powder Mater., 2023, 100169, DOI:10.1016/j.apmate.2023.100169.
- C. Guo, S. Yi, R. Si, Y. Wang, H. Liu, F. Yu and J. Li, Chem. Eng. J., 2023, 469, 143845 CrossRef CAS.
- M. Qian, Z. Xu, Z. Wang, B. Wei, H. Wang, S. Hu, L.-M. Liu and L. Guo, Adv. Mater., 2020, 32, 2004835 CrossRef CAS PubMed.
- J. Guo, J. Yang, J. Guan, X. Chen, Y. Zhu, H. Fu, Q. Liu, B. Wei and H. Geng, Chem. Eng. J., 2022, 450, 138007 CrossRef CAS.
- D. Li, J. Zhou, X. Chen and H. Song, ACS Appl. Mater. Interfaces, 2018, 10, 30379–30387 CrossRef CAS PubMed.
- M. A. Al-Tahan, B. Miao, S. Xu, Y. Cao, M. Hou, M. R. Shatat, M. Asad, Y. Luo, A. E. Shrshr and J. Zhang, J. Colloid Interface Sci., 2024, 654, 753–763 CrossRef CAS PubMed.
- Y. Huang, C. Zhu, S. Zhang, X. Hu, K. Zhang, W. Zhou, S. Guo, F. Xu and H. Zeng, Nano Lett., 2019, 19, 1118–1123 CrossRef CAS PubMed.
- X. Liu, Y. Si, K. Li, Y. Xu, Z. Zhao, C. Li, Y. Fu and D. Li, Energy Storage Mater., 2021, 41, 255–263 CrossRef.
- Y.-L. Hou, J.-Z. Chen, B.-H. Zhang, H.-Y. Wang, W.-X. Wen and D.-L. Zhao, Chem. Eng. J., 2024, 479, 147914 CrossRef CAS.
- Y. Xi, X. Wang, H. Wang, M. Wang, G. Wang, J. Peng, N. Hou, X. Huang, Y. Cao, Z. Yang, D. Liu, X. Pu, G. Cao, R. Duan, W. Li, J. Wang, K. Zhang, K. Xu, J. Zhang and X. Li, Adv. Funct. Mater., 2023, 2309701 CrossRef.
- G. Hao, C. Zhang, Z. Chen and Y. Xu, Adv. Funct. Mater., 2022, 32, 2201352 CrossRef CAS.
- Z. Zheng, P. Li, J. Huang, H. Liu, Y. Zao, Z. Hu, L. Zhang, H. Chen, M. S. Wang, D. L. Peng and Q. Zhang, J. Energy Chem., 2020, 41, 126–134 CrossRef.
- L. Zhong, H. Chen, Y. Sheng, Y. Sun, Y. Xiao, B. Cheng and S. Lei, J. Energy Chem., 2023, 90, 294–304 CrossRef.
- Z. Shen, L. Cao, C. D. Rahn, C. Y. Wang and C. Y. Wang, J. Electrochem. Soc., 2013, 160, A1842–A1846 CrossRef CAS.
- Z. Zou, Z. Yu, C. Chen, Q. Wang, K. Zhu, K. Ye, G. Wang, D. Cao and J. Yan, ACS Nano, 2023, 17, 13769–13783 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr06420d |
| This journal is © The Royal Society of Chemistry 2024 |