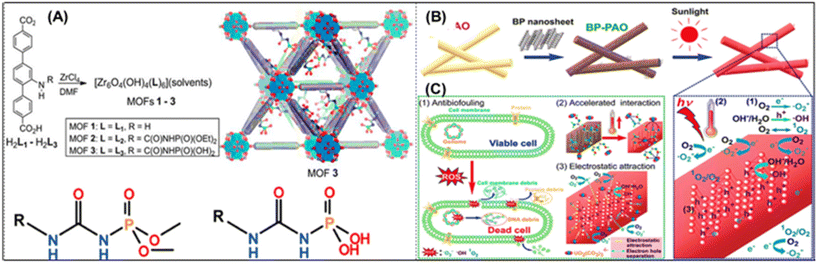
Enhanced uranium extraction from seawater: from the viewpoint of kinetics and thermodynamics
Sania
Shabbir
ab,
Nailiang
Yang
 *ab and
Dan
Wang
*ab and
Dan
Wang
 *ab
*ab
aState Key Laboratory of Biochemical Engineering, Key Laboratory of Biopharmaceutical Preparation and Delivery, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: nlyang@ipe.ac.cn; danwang@ipe.ac.cn
bUniversity of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, P. R. China
First published on 20th January 2024
Abstract
Uranium extraction from seawater (UES) is recognized as one of the seven pivotal chemical separations with the potential to revolutionize global paradigms. The forthcoming decade is anticipated to witness a surge in UES, driven by escalating energy demands. The oceanic reservoirs, possessing uranium quantities approximately 1000-fold higher than terrestrial mines, present a more sustainable and environmentally benign alternative. Empirical evidence from historical research indicates that adsorption emerges as the most efficacious process for uranium recovery from seawater, considering operational feasibility, cost-effectiveness, and selectivity. Over the years, scientific exploration has led to the development of a plethora of adsorbents with superior adsorption capacity. It would be efficient to design materials with a deep understanding of the adsorption from the perspective of kinetics and thermodynamics. Here, we summarize recent advancements in UES technology and the contemporary challenges encountered in this domain. Furthermore, we present our perspectives on the future trajectory of UES and finally offer our insights into this subject.
Detailed research on uranium extraction from seawater from the point of view of kinetics and thermodynamics was summarized. Detailed phenomena of uranium extraction have been discussed along with past and present research trends. The role of a few significant adsorbents including porous, polymeric, biological, and inorganic were summarized in this review. Some factors that affect the process of adsorption in UES are detailed and summarized. For instance, pH, co-existing metal ions, biofouling, and temperature may have significant effects on the adsorption process of uranium. Sea trials from Japan, the USA, and China with different periods along with the adsorption capacities of adsorbents were also summarized.
1 Introduction
With the burgeoning global population humanity is grappling with many challenges, including environmental degradation and energy crises. The 21st century poses a significant conundrum, with traditional fossil fuels such as coal and oil projected to be depleted within the next century.1–3 In response to the global scarcity of non-renewable energy resources, nations are pioneering innovative technologies for a sustainable energy supply.4,5 While the utilization of renewable energy sources as alternatives to fossil fuels has gained traction, considerable constraints persist in their reliability, cost-effectiveness, and environmental impact.6–9 Nuclear fission is emerging as a secure and dependable technology for energy generation, boasting a minimal carbon footprint.10,11 Owing to its efficiency and reduced greenhouse gas emissions, nuclear energy is increasingly being adopted to cater to the global demand and the escalating needs of the growing population.12–14 The recovery of uranium, a primary nuclear element, is paramount for the procurement of uranium resources and the mitigation of pollution. With the mounting demand for uranium, the focus on its recovery has intensified.4,5Terrestrial uranium is the primary global source of uranium fuel, but the global mineable reserves are quite limited.15,16 Seawater is a significant and abundant source for extraction of uranium in a stable complex called Ca2[UO2(CO3)3].17 This proposed resource could potentially address the current energy crisis and ensure future uranium supplies, potentially fulfilling global demand for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years through cost-effective recovery techniques. Ocean mining offers a significant advantage over land mining, with reduced environmental implications including less toxic waste, pollution, and uranium processing. The U(VI) uranyl ion can be effectively removed from aqua-based solutions through various techniques such as membrane separation, chemical precipitation solvent extraction, and adsorption.18–22 Among these, adsorption emerges as a widely adopted method for uranium recovery from seawater, offering fast kinetics, simplicity, cost-effectiveness, minimal secondary pollution, ease of operation, and reusable adsorbents.17,23,24 However, uranium extraction from seawater necessitates efficient chemical separation through uranyl ion equilibrium, balancing conventional techniques such as pre-concentration, crystallization, and co-precipitation due to low concentrations and complex factors. Knowledge of uranium coordination principles assists in devising effective ligands and determining the thermodynamics of adsorption and desorption equilibrium, thereby expediting the process of ligand creation and testing.
000 years through cost-effective recovery techniques. Ocean mining offers a significant advantage over land mining, with reduced environmental implications including less toxic waste, pollution, and uranium processing. The U(VI) uranyl ion can be effectively removed from aqua-based solutions through various techniques such as membrane separation, chemical precipitation solvent extraction, and adsorption.18–22 Among these, adsorption emerges as a widely adopted method for uranium recovery from seawater, offering fast kinetics, simplicity, cost-effectiveness, minimal secondary pollution, ease of operation, and reusable adsorbents.17,23,24 However, uranium extraction from seawater necessitates efficient chemical separation through uranyl ion equilibrium, balancing conventional techniques such as pre-concentration, crystallization, and co-precipitation due to low concentrations and complex factors. Knowledge of uranium coordination principles assists in devising effective ligands and determining the thermodynamics of adsorption and desorption equilibrium, thereby expediting the process of ligand creation and testing.
Direct adsorption technologies are extensively researched for uranium extraction by their functional groups, but conventional methods have significant drawbacks. Uranyl binding restricts uranium uptake and removal, leading to technical difficulties, and the loss of adsorption would take place due to passivation or corrosion caused by marine bacteria and algae in seawater. The interaction between uranium ions and adsorption materials can be reflected through FTIR, suggesting the designing of functional groups on polymeric fiber could be a possible approach to enhance the adsorption capacity. To effectively extract uranium from seawater, a variety of practical adsorbents have been developed.26 It has been proved that amidoxime (AO) groups can effectively extract uranium, but interfering ions in seawater limit their capacity and escalate economic costs for practical applications. Marine materials, despite their limitations, offer eco-friendly, cost-effective, and ocean-resistant alternatives to natural materials. Carbon-based compounds are also commonly used for U extraction from aqueous solutions owing to their exceptional performance in chemical stability, ease of modification, and large surface areas.27–29 Another important issue that needs to be considered is the extraction element ratio of U/V, which is significantly influenced by the type of adsorption materials used.25–27 Further enhancements in materials’ adsorption capacity, selectivity, durability, kinetics, and thermodynamics need to be considered for uranium recovery from natural seawater.
Hollow materials can contribute to thermal aggregation in the process of uranium extraction from seawater by being used as a component in adsorbent designs. By offering more surface area for uranium adsorption, hollow shapes can improve the overall effectiveness of adsorbent materials. Furthermore, the hollow spaces may operate as pathways for enhanced mass movement, making the extraction process more effective. By regulating temperature changes during the extraction process, the controlled thermal characteristics of these materials may also help to optimize the conditions for uranium adsorption from seawater.
However, despite advancements in uranium extraction, only a few economical materials have been developed to rival traditional mining methods, with current cost estimates being $600 per kg per U. Fig. 1 indicates the total cost summary of uranium extraction with different adsorbents at different times. Emerging UES systems are incorporating new adsorption strategies, such as electrochemical and photochemical methods, in addition to traditional surface-based physicochemical adsorption methods. It is possible to promote uranium mass transfer diffusion by disturbing thermodynamic adsorption–desorption equilibrium with an additional electric field. In uranium extraction, electrically or optically driven devices offer more capacity and faster kinetics, potentially leading to a more effective and profitable procedure. This review will provide an understanding of the mechanisms in terms of kinetics and thermodynamics, from the viewpoint of molecules and chemistry, to address the development of materials in UES and perspectives in this field.
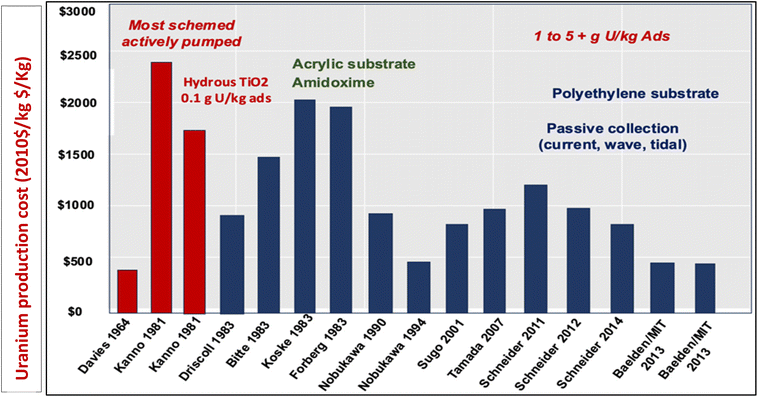 | ||
| Fig. 1 Overview of the cost projection.25 Figure reprinted with permission from ref. 25. Copyright 2015 Elsevier. | ||
2 Uranium in seawater: coordination chemistry
For the development of efficacious adsorbent materials, a molecular-level understanding of uranium's solution coordination chemistry in seawater is crucial. Over the past decade and a half, the coordination chemistry of uranium has garnered considerable attention. The development of easily synthesizable, stable precursors has played a significant role in piquing this interest. These compounds have paved the way for the exploration of uranium with a myriad of potential ligands. Uranium, due to its size and accessibility to various oxidation states, exhibits a unique reactivity and forms intricate coordination complexes that are unattainable with transition metals or lanthanides. The advanced compounds that have been underscored illustrate that the capabilities of uranium are yet to be fully uncovered, necessitating further research to wholly comprehend its potential. From the activation of small molecules to unique magnetic properties, uranium poses a synthetic and spectroscopic conundrum for the upcoming generation of coordination chemists. Research indicates that over 90% of the uranium in seawater is in the stable ionic form [UO2(CO3)3]416− and its derivative, Ca2UO2(CO3)3.30,31 The uranium ion [UO2]2+ presents a linear, centrosymmetric structure with 180 pm-long UO bonds. The uranyl ion is stabilized by its chemically inert double bonds, with minor perturbations.32According to the Hard–Soft-Acid–Base theory, uranium, an actinide, is a potent electron acceptor and can be regarded as a specific type of hard acid. Electron-donating groups augment the capacity of the ligand and uranyl complex, while stability is affected by steric hindrance and weak interactions in the coordination environment. Additional hydrogen bonds could attenuate the connections between the coordination atom and uranium near the equatorial plane while elongating the UO bond length.33 Potential uranyl ligands can be synthesized and screened with the aid of density functional theory (DFT) simulations in tandem with uranium's solution chemistry. This significantly fosters the development of numerous efficient adsorbents, especially for popular organic framework materials with inherent functionalities and structural diversity. Vanadium(V), a potent competitor ion in seawater, also possesses ligand-binding properties that warrant exploration. Research in this domain has yielded a comprehensive understanding of coordination behavior in marine environments, which holds implications for the development of novel, more effective, and highly selective adsorbents for uranium recovery. Uranium (UO22+), which has a linear UO, is a stable molecular species. Its two oxo ligands may be resistant to functionalization and inert to exchange. A growing corpus of research indicates the precise description and understanding of this structure is still necessary.34
3 The mechanism to enhance UES
The primary stage of chemical separation in the procedure for uranium extraction from seawater involves the equilibrium of uranium ion adsorption–desorption at the interfaces between adsorbents and seawater. Achieving optimal chemical separation necessitates the concurrent optimization of both thermodynamic and kinetic parameters. The development and screening of functional ligands can be bolstered by a fundamental understanding of uranium coordination. Studies on adsorption kinetics assist in determining the ideal parameters for batch-size removal operations by examining the rate-controlling step and adsorption mechanism via diverse kinetic models. Among the plethora of kinetic models that exist, the second-order model is considered as the most pertinent and beneficial one. These models encompass Weber and Morris sorption kinetic models, pseudo-first-order models, and pseudo-second-order models.22,35–40 Most studies on the thermodynamics of uranium adsorption indicate that the process is endothermic and spontaneous.41,42 The nature and feasibility of the adsorption process are evaluated by calculating the Gibbs free energy, standard enthalpy, and entropy change. Enthalpy values can be utilized to position sorbents and select the best placement locations by assessing the temperature dependency of uranium extraction in seawater. Different adsorbents exhibit a range of thermodynamic properties, and amidoxime-based adsorbents are identified as the most suitable ones. Structural and thermodynamic investigations can deepen our understanding of uranium coordination chemistry with amidoximes. There is limited research on U(VI) complexation with amidoxime ligands. The thermodynamic behavior results were used to predict the temperature dependence, efficiency, selectivity, and potency of amidoxime ligands for uranium sorption, assessing their potency. Thermodynamic parameters are greatly dependent on the kinetic characteristics of the uranium extraction equilibrium, with ligand accessibility significantly influenced by the conformation of the polymeric adsorbent chain. Currently, effective methods for controlling polymer chain conformation primarily involve random or block copolymerization, which adds hydrophilic copolymer chains. The adsorption capacity of polymers is significantly lower than theoretically calculated, necessitating the use of electrostatic interactions to increase ligand availability. The rapid advancement of computer technology and continuously improving computational chemistry theories and simulation models enable multi-scale research on the connection between polymer adsorbents and uranium extraction. The influence of seawater uranium extraction can be understood from the view of thermodynamics, suggesting that designing adsorbents with high surface areas could enhance adsorption efficiency. The results of the uranium adsorption revealed that the density and specific surface area of the AO groups have a great impact on adsorption capability. The chemistry of the complexes produced in seawater by AO ligands and uranyl is still an open question. A deeper mechanistic understanding of the binding interactions in AO-Uranyl in seawater is needed to create novel, more potent ligands. Uranium adsorption using AO adsorbents is strongly endothermic. Amidoxime-based adsorbents show improved uranium sequestration performance in warm seawater settings due to temperature sensitivity differences between U(VI)- and V(V)-amidoxime ligand interactions.43 The introduction of numerous functional ligands to improve uranium binding has had a significant impact on the evolution of uranium adsorbents. A variety of adsorbent materials, especially those with polymer matrixes, have been developed for UES. Typically, acrylonitrile (AN) monomers are grafted onto polyolefin supports with strong backbones like polyethylene and polypropylene to create AO-based polymers.44 The degree of grafting (DOG) in the radiation-induced graft polymerization (RIGP) process is directly influenced by irradiation parameters and grafting reaction conditions. Adding collaborative features to AO-based adsorbents is another exciting way to increase their ability to bind uranyl ions.45 The development of bifunctional AO-based fibre involved adding EDTA ligands to commercially available PAN fibre and treating the remaining nitrile species with hydroxylamine.46,47 To solve the huge gap between the theoretical DOG and actual use ratio, scientists are being urged to look into the accessibility and kinetic challenges of ligands.The examination of local chemistry and steric factors in well-engineered polymeric adsorbents with AO ligands is of paramount importance due to their attachment to polymer backbones. The regulation of polymer chain conformation can enhance ligand accessibility, yet the synthesis of polymeric adsorbents encounters technological obstacles in modulating chain structure and topology. Further efforts are necessary to develop controlled polymerization techniques that will facilitate the precise fabrication of polymers with structural benefits to surmount kinetic impediments for uranium adsorption. In this case, more work has been done to control the polymerization, thus the structural advantages have been presented to overcome the aforementioned kinetic barriers.
Besides polymer adsorbents, hydrogel-based adsorbents are also being developed, but further research is needed to assess their mechanical strength and salt resistance for field tests. The application of membrane technology in UES is still in its nascent phase, with instances of functionalized membranes being relatively scarce and biofouling posing a significant challenge. Nevertheless, the integration of membrane modules with energy-intensive systems is a necessity. Consequently, a comprehensive techno-economic evaluation is imperative for the membrane-assisted UES system. Prior research has frequently underscored the significance of a high density or concentration of meticulously designed ligands in adsorbents. The suboptimal utilization of functional ligands has emerged as a considerable constraint for the majority of the discussed adsorbents. One of the pivotal technological hurdles is the fabrication of uranium adsorbents via more feasible synthetic methodologies while preserving the efficacy and accessibility of the functional ligands.
The adsorptive prowess of adsorbents tends to diminish rapidly upon cyclic utilization. In real-world marine trials, polymer adsorbents demonstrate superior structural stability and practical application potential, outstripping most novel porous framework adsorbents. The second concern pertains to the adsorbent's resilience during the elution process. The reusability of the adsorbent is impeded by the loss and degradation encountered during the elution process, particularly when employing a potent acid-leaching technique. The elution/regeneration process can be optimized, and the performance decline of the adsorbent can be mitigated by resorting to a non-acidic solution. The evolution of flexible synthetic and processing strategies remains a significant area of interest for molding prospective materials into deployable adsorbents.
The study of novel adsorbent properties requires the establishment of the best-fitting adsorption equilibrium correlation, which is crucial for forecasting adsorption parameters. Mechanistic research is indispensable for a comprehensive exploration of adsorption. Various isotherms, such as Langmuir, Freundlich, Redlich Peterson, Dubinin–Radushkevich, Flory Huggins, Halsey, Sips, and Temkin, are employed, each elucidating distinct aspects of the adsorption process.51,52 Freundlich and Langmuir are the most widely used ones.51,53–55 The applicability of the isotherm model depends on independent parameters and is mathematically simple, with various axes affecting linear analysis accuracy and consistency. Nonlinear statistical functions are deemed more reliable than linear analysis, with a surge in interest in nonlinear optimization modeling over the recent decades due to their simplicity. Langmuir surpasses Freundlich in process prediction and is predominantly harnessed for forecasting adsorption processes, like uranium adsorption, owing to its monolayer surface coverage assumption.
4 Materials for UES
Researchers are developing affordable and efficient adsorbents for seawater uranium extraction. Many suitable adsorbents are used for uranium extraction. Comprehensive details about inorganic adsorbent, polymer adsorbent, biological proteins, MOFs, COFs, and mesoporous adsorbent will be discussed in the following.12,56,574.1 Inorganic material
Inorganic adsorbent is a series of materials which was first adopted for UES. These materials show promising adsorption under optimized conditions. Inorganic materials, such as unbound powders, face some challenges which often compromise the qualities of good adsorption based on lab-scale research. Due to their low cost, high surface area, pore-shape tailoring, and simplicity of synthesis, inorganic adsorbents became the first extractants for uranium adsorption in the 1960s.58 At that time, most inorganic adsorbent research focused on the hydroxides and oxides of metals like magnesium, titanium, and aluminum. The best results among all the investigated adsorbents came from hydrous titania.59,60 Inorganic adsorbents have been the focus of the current study, particularly porous silica as a support or an organo-silica hybrid saturated with organic chelation ligands.61–63 Due to the presence of nanostructured porosity, more information about silica research will be provided. However, it is significant to recognize that silica is unstable under normal circumstances, which may limit its widespread application in the maritime environment.γ-Al2O3 nanosheets were hydrothermally synthesized with supercritical CO2, and the uranium extraction ability was further investigated. A schematic illustration of the proposed mechanism for the formation of γ-Al2O3 nanosheets is shown in Fig. 2A. An isothermal investigation of the material saturation capacity revealed a moderate maximal adsorption of about 10 mg g−1. With an ion exchange mechanism and weak performance in strongly ionic settings like seawater, the conflict between the proton and uranium in solution results in relatively low performance at acidic solution.48 Studies have shown that alkaline and alkali earth cation-containing solutions are better at adsorbing uranium.64 Further studies discovered the use of zerovalent iron nanocomposites that are surface-functionalized with acrylonitrile (AN) and subsequently treated with amidoxime.65 Magnetic adsorbent materials show promising performance in seawater uranium recovery.
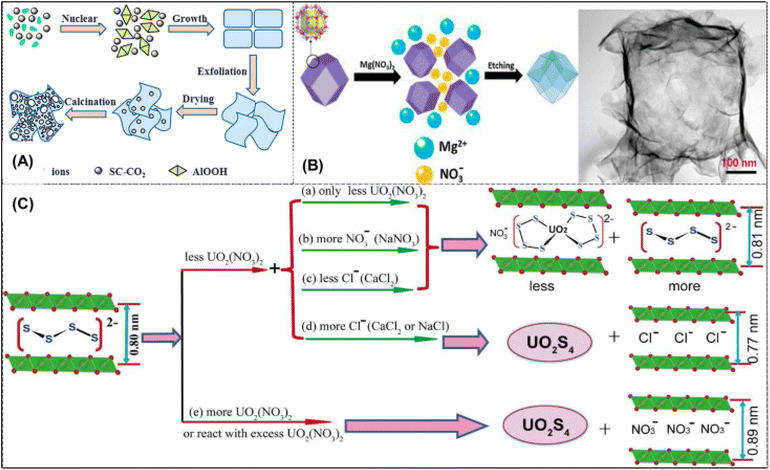 | ||
| Fig. 2 (A) Diagrammatic representation of the suggested process for γ-Al2O3 nano-sheet production.48 Figure reprinted with permission from ref. 48 Copyright 2013 Royal Society of Chemistry. (B) Diagram showing how the ZIF-67 templated Mg–Co LDH was prepared (left). TEM picture of Mg–CO LDH obtained using a cobalt source and a sacrificial ZIF-67 template (right).49 Figure reprinted with permission from ref. 49. Copyright 2017 John Wiley and Sons. (C) [S4]2− binding modalities with UO22+ and Gallery Species arrangements in LDH at varying UO22+ and Anion Concentrations.50 Figure reprinted with permission from ref. 50 Copyright 2015 American Chemical Society. | ||
A very recent study describes the sequestration of uranyl using composite materials made of polysulfide and layered double hydroxide (Fig. 2C). The authors then looked at using these materials to recover uranium from seawater in the region of Tianjin City, China. According to the paper, 78% of the uranium was eliminated in less than 24 hours, indicating quick kinetics and tremendous potential as an adsorbent material. Macro structured Mg–Co layered double hydroxide (LDH) was synthesized through the sacrificial ZIF template, which also functioned as the Co source. The ZIF-67 template's facets started to sprout nanosheets after Mg(NO3)2 was added to it in an aqueous/ethanolic solution. Eventually, these nanosheets generated hollow rhombic dodecahedron structures. When exposed to a brine solution with an ecologically acceptable 3 ppb uranium content, an adsorption of 0.006 mg of uranium per g was obtained.49 Researchers have found that polysulfide/LDH composites work well as uranium extraction adsorbents as well (Fig. 2C). Adsorption effectiveness of Sx-LDH composites for uranyl ions from a variety of aqueous solutions, including seawater, where LDH is Mg/Al layered double hydroxide and polysulfide, was assessed. It was found that the polysulfide/LDH materials, Sx-LDH, show a highly selective UO22+ removal in both aqueous solution and seawater due to the UO22+⋯S2− bonding interactions. These materials have a tremendous potential for uranium capture and outperform other known adsorbents thanks to their uniqueness and low cost for uranium extraction from aqueous media, important to nuclear power.50
The oxygen atoms in uranyl “yl” can also serve as acceptors for hydrogen bonds.66,67 These interactions can be seen in the solid-state molecular structures of uranyl-containing substances.68 Hydrogen bonding interactions have been observed in the pyrrole-based macrocycle of the uranyl Pac-Man solid-state molecular structure despite the absence of a transition metal. In this procedure, two hydrogen atoms from pyrroles mix with an oxygen atom from an endo “yl” molecule. The ligand design ensures that the hydrogen bonding interactions are sustained in solution, according to 1H NMR spectroscopy.69 Computational studies show that there is either no or extremely weak H-bonding between the “yl” oxygens of [UO2(H2O)5]2+ and bulk water. Calculations also show that a weak hydrogen bond exists between the “yl” oxygen in [UO2(H2O)5]2+ and methanol,70–72 but the pentavalent analog of [UO2(H2O)5]+ is expected to form a hydrogen bond since it exhibits a higher negative charge on its “yl” oxygens than uranyl(VI) does.71 New ligands that benefit from the rigid geometry of the uranyl fragment have been made by making use of the capacity of uranyl to receive hydrogen bonds. The ligands form a cavity that encloses the uranyl fragment by having a large number of coordinating groups grouped around a primary hydrogen bond donor. The coordinating groups in these ligands create a cavity that encloses the uranyl fragment by equatorial coordination and hydrogen bonding to the oxo group. Strong complexation of the uranyl ion with three carboxylate-containing arms is demonstrated by the tripodal ligand tris [3-(2-carboxy-4-octadecyl phenoxy) propyl] amine (NPodB). A tertiary amine found in the NPodB ligand establishes a hydrogen bond with the oxygen in the “yl” molecule after being protonated with HCl. Despite the unresolved structural nature of the complex, mass spectrometry suggests a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex developed, and vibrational spectroscopy supports a hydrogen bonding connection. At neutral pH, superb high extraction coefficients can be achieved by these “stereognosis” ligands.68
1 complex developed, and vibrational spectroscopy supports a hydrogen bonding connection. At neutral pH, superb high extraction coefficients can be achieved by these “stereognosis” ligands.68
4.2 Polymeric adsorbent
On account of their robust mechanical characteristics, ability to be mass-produced, and suitability for passive ocean deployment, polymeric adsorbents are potential materials for large-scale seawater field operations. Owing to its strong coordination with uranium, the amidoxime functional group stands out among them as the best option for creating uranium adsorbents. The uranium in seawater may be extracted effectively and precisely by this functional group.17 The large specific surface area, flexibility, and mechanical qualities of amidoxime-based adsorbents make them important components in the recovery of uranium from seawater. These poly(amidoxime) sorbents are long braids with one end attached to the ocean floor and the other end free to float, as shown in Fig. 3A and B. The freestanding braids contribute to enhancing the adsorption of uranium (and other cations) to the poly(amidoxime) braids by interacting with ocean currents. After a specified amount of time, the braids are taken from the water, and the uranium that has been adsorbed is extracted using the appropriate eluant. Poly(amidoxime) sorbents generated up to 3.3 g kg−1 of sorbent after 8 weeks in ocean experiments, demonstrating successful results.73 Advanced polymer-based adsorbents have been used for in-field deployment tests conducted as a result of research programs all around the world, verifying their concept and displaying the highest level of technological competence for suggested seawater uranium adsorbents. Scalability, low material costs, flexibility in graft chain functioning, and a range of support form factors are some advantages of this technology. Understanding of the graft chain molecular structure and uranyl binding environment through direct inspection is still a challenge. Additionally, the effect of salinity and hydrophilicity on the graft chain are still unexplored.44 In simulated seawater with several competing ions, it adsorbs uranium up to 1.6 mg g−1. A braided adsorbent was created by Seko and colleagues, who made lengthy, seaweed-like braids out of amidoxime-functionalized polyethylene fibre and affixed them to the ocean floor with remote-controlled fasteners. The braided-type adsorbent was shown to have a better capacity to adsorb uranium than the stacks of nonwoven fabric because of the enhanced contact between the adsorbent and the seawater.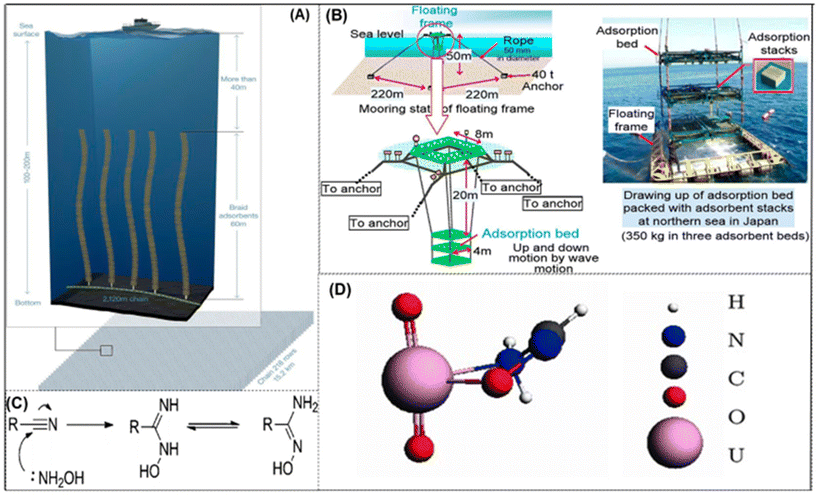 | ||
| Fig. 3 (A) An approach to deploy braided amidoxime-functionalized polymer sorbents in a kelp field simulation is proposed.57 Figure reprinted with permission from ref. 57. Copyright 2017 American Chemical Society. (B) The technique of adsorbent beds was created to remove uranium from seawater. (Left) An example of a stack of adsorbents. (Right) An image captured during open water deployment research of the floating adsorbent frame. Three adsorbent beds, each with 144 stacks of adsorbent, are contained in the frame.76 Figure reprinted with permission from ref. 76. Copyright 2017 Taylor & Francis. (C) Transformation of amidoxime from nitrile.77 Figure reprinted with permission from ref. 77. Copyright 2020 Royal Society of Chemistry. (D) Complexes of uranyl amidoxime,79 figure reprinted with permission from ref. 79. Copyright 2012 American Chemical Society. | ||
The interaction of cyano-containing substances with hydroxylamine is the main source of the amidoxime group (Fig. 3C). It has both a basic (OH) and an acidic site (NH2). The binding of uranium to the ligands was investigated using theoretical simulations and excellent microstructural characterization techniques. Results show uranium is a simple building block in a stable complex, bonding to the electrons in the C–N bond and the lone pair electron in the N–O.74 Amidoxime-based adsorbents have been frequently employed in UES due to the carboxyl group's capacity to improve coordination between the amidoxime group and UO22+ and encourage the dissociation of [UO2(CO3)3]4−.30,75 The highest value ever has been measured for uranium extraction from natural seawater using fabric adsorbents. Most of the amidoxime-based polymeric fibres show good adsorption capacity and formed the uranyl complexes with amidoxime as shown in Fig. 3D. The primary methods for enhancing adsorption capacity involve engineering functional ligands on polymeric backbones and modifying the structures and morphologies of polymers. However, the majority of the polymeric adsorbents had a very low utilization of the functional ligands, which has resulted in subpar enrichment of uranium from seawater.78
When exposed to seawater, only 1% or less of the AO ligands in functionalized polymers were practically utilized. This upsetting reality has prompted people to doubt the industrial potential of adsorbent for large-scale utilization in seas, as well as its design philosophy and processing methods. Researchers have recently been asked to carefully examine the aspects which limit the effectiveness of uranium collection with polymers. The spatial conformation of polymers is a critical problem that has been brought up. The chain conformation of the polymeric adsorbent significantly influences its accessibility, binding capacity, and target diffusion resistance, as it can be adjusted from collapsed to stretched states. Controlled radical polymerization (CRP) techniques are being used to create block copolymers (BCPs) with defined topologies, offering a promising method for altering polymer chain conformation. Through the amazing architectural adaptability of BCPs, there is a lot of opportunity for adjusting polymer chain conformations to enhance particular activities, which creates new opportunities for advancement. Polymer architectures in high-salinity seawater face insufficient ligand utilization and adsorption capacity due to the polyelectrolyte effect, resulting in lower than theoretical values. Lowering uranyl ions’ internal diffusion barriers can increase ligand availability by utilizing electrostatic interactions to control polymer chain conformation. Advancements in computer technology and computational chemistry theories are paving the way for a comprehensive exploration of fundamental scientific questions related to uranium adsorption in aqueous solutions. Inspiring the creation of high-performance polymeric adsorbents for seawater uranium extraction, these fundamental research projects would deepen our grasp of the structure–property relationship.80
4.3 Biological protein
New biological systems have been developed owing to the advances in synthetic biology.81 Bioengineered protein molecules exhibit strong affinity and selectivity for uranyl ions in UES, and synthetic biology research has created functional biomaterials that precisely recognize target molecules.82 When Heide and Wagener first suggested studying biological systems for UES in 1973, they suggested using unicellular green algae as a model system.83Recent advances in protein engineering have revived interest in designing biological systems for selective uranium identification.92,93 It is possible to affordably attain the needed affinity and high selectivity using biological systems that are capable of self-regeneration. Over billions of years, nature has developed methods to differentiate between useful and harmful metal ions with remarkable sensitivity and selectivity. The affinities of these elements can economically mine uranium or other elements from seawater or clean up polluted environments, provided a reliable scaffold is created. Proteins can be visible on the surfaces of living organisms, enabling biological regeneration for recovery and remediation purposes at a reasonable cost. Understanding of the impact of uranyl binding to proteins in potential, native, or synthetic metal-binding sites has made major progress in recent decades.94
The first studied naturally protein-based adsorbent for uranium extraction from natural seawater and nuclear effluent was a low-cost soy protein isolate (SPI) hydrogel.95 The carboxyl or amino groups in the protein were necessary for uranium adsorption. High binding affinities have been discovered for six equatorial oxygen or nitrogen atoms in a coordination arrangement. The SPI hydrogel, with its strong selectivity for uranium, offers low raw material cost. The researchers demonstrated fast kinetics and equilibrium adsorption isotherms for SUP hydrogels (Self-healing Ultra-stretchable and Superabsorbent Polymer Hydrogel, a combination of SodiuAlginate, Polyvinyl alcohol, and Polyacrylamide), offering benefits like accelerated binding, simple separation, quantitative evaluation, and commercial packaging adaptability. A diagrammatic representation of the design of selective and high-affinity uranium nano-traps motivated by the protein biological system is given in Fig. 4A. The study examined the selectivity of SUP for uranyl, metal ions with high affinity, and its binding affinity to seawater-specific metal ions.
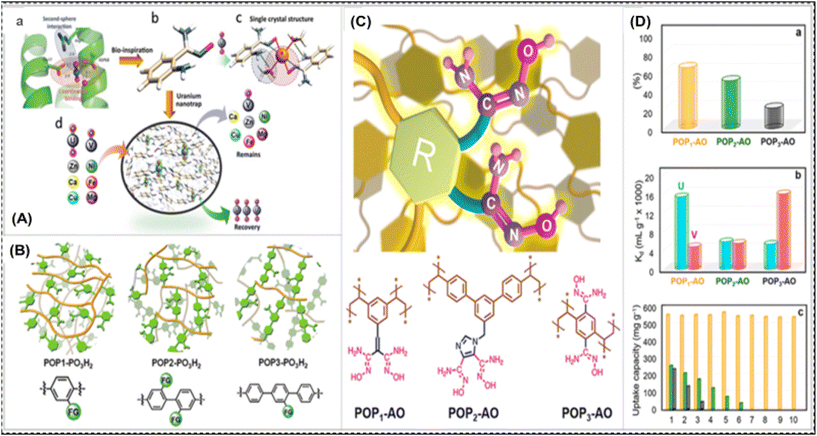 | ||
| Fig. 4 (A) Diagrammatic representation of the design of selective and high-affinity uranium nano-traps, motivated by the protein biological system. (a) SUP protein's uranyl-binding pocket details. (b) The intended configuration of a uranyl-binding moiety was motivated by (a). (c) An overview of the uranyl complex's crystal structure in (b). (d) The creation of uranium nano-traps that is selective and permits uranium enrichment over other metals. (B) A schematic representation of the architectures of POP1-PO3H2, POP2-PO3H2, and POP3-PO3H2, among other phosphoryl-urea functionalized polymers.114 (C) An illustration of di-amidoxime ligands with different R groups acting as particular “hooks” on porous frameworks for uranyl recognition, along with the corresponding structures of POPs functionalized with di-amidoxime. (D) The competitive adsorption and recyclability characteristics of various POPs functionalized with di-amidoxime: (a) the residual uranium adsorption capability of different POPs in the presence of vanadium at identical concentrations; (b) the Kd values of vanadium and uranium for different POPs; (c) the capacity of different POP adsorbents to be recycled during ten consecutive cycles (orange denotes POP1-AO and olive denotes POP2-AO, and black shows POP3-AO) (C and D).115 | ||
Biomacromolecules are responsible for uranium enrichment, and can be used to screen potential ligands by biological systems with high uranium affinity. By achieving a more thermodynamically stable state, these ligands effectively coordinate with uranium. New adsorbents can have exceptional selectivity for uranyl ions in seawater due to their biological ligands. In order to overcome difficulties in the practical application of biocomponent-containing adsorbents in ocean ecosystems, a study served as the inspiration for the development of artificial uranyl nano traps. To establish the principles for regulating ligand spatial arrangements and reducing steric hindrance, the presented theories allow for additional research into the geometry of uranyl coordination complexes.96
Protein-based products face a significant challenge in maintaining the activity of their molecules after immobilization.97 Practical uranyl adsorbents are considered essential due to the high sample volume in ocean uranyl extraction. The secondary structure of proteins is frequently altered by uranyl binding, but proteins can also undergo significant conformational changes, interfere with protein–protein interactions, or even disturb interactions with ligands and DNA.98,99 Some proteins’ binding affinities are considerably increased by phosphorylation, which occurs when phosphate groups are connected directly to the uranyl ion.94 Using this knowledge, model peptides for highly selective and affine uranyl sensors can be logically created.100 Despite the development of uranyl ion-specific model peptides and proteins, new methods and developments in computational biology are still required to investigate the uranyl-binding sites of native proteins in biological systems.100 It is anticipated that additional synthetic peptides and proteins will be created for large-scale uranyl recovery from the ocean and for selective uranyl binding using new biomaterials or hybrid materials. More structural data are needed for uranyl–protein complexes, both in solid state and solution conditions, to fully understand conformational changes caused by uranyl.101,102 In metallomics and proteomics, new approaches utilizing chromatographic and spectroscopic techniques like CE-ICP-MS are required to identify uranyl binding interactions in protein–protein, DNA-binding, and ligand interactions.
Understanding of uranyl ions and other heavy metal ions is necessary for biological remediation because of their extremely detrimental effects on living systems. The growth of chemical biology has significantly benefited metallo proteomics, which effectively utilizes the double-edged sword of uranium.103,104 Research has been conducted to determine if biological materials can be utilized to create effective adsorbents that specifically recognize uranyl ions. Uranyl is synergistically bound by functional groups in proteins, allowing for uranium detection. Biomacromolecule-containing adsorbents face significant challenges in deployment in UES, necessitating further research to demonstrate their viability in this biologically based adsorbent class. Biochemical techniques have created a unique protein with potential for uranyl extraction, offering innovative solutions beyond nanostructured adsorbents. Instead of using conventional polymer fiber or inorganic materials as sorbent materials, the Lai and He groups developed a unique method that uses genetically modified proteins for UES.102 To find protein structures with pockets that may hold the uranyl cation, the Protein Data Bank was first computationally examined. The residue's binding pocket for uranyl coordination in the equatorial plane was modified, with the addition of amino acids. To evaluate the economic viability of biologically based adsorbents compared with existing polymer systems, more study is required.105,106 It is also important to pay more attention to finding non-destructive uranium recovery techniques and evaluating scale-up and deployment options.
4.4 Porous structure
The reported porous structures for UES can be divided into categories such as MOFs, COFs, and Mesoporous adsorbents.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Following “segregated” microwave irradiation, FTIR examination of ultra-microporous carbonaceous COF (CCOF-SCU1) demonstrated a high density of nitrile functionalization.120
3. Following “segregated” microwave irradiation, FTIR examination of ultra-microporous carbonaceous COF (CCOF-SCU1) demonstrated a high density of nitrile functionalization.120
A new COF named BD-TN-AO was recently reported as an adsorbent (Fig. 5B and C) which has shown outstanding kinetic and thermodynamic advantages in uranium adsorption accompanied by a sizeable contact area. The electrostatic interaction between [UO2(CO3)3]4− and the positive surface considerably increased the capacity for uranium extraction. The saturation amount of uranium adsorption was 5.9 mg g−1.
Conjugated carbon–carbon triple bonds are present in recent COFs for uranium adsorption, enabling post-synthetic modification for a variety of functions. Tri-methyl chloride and 2,4-hexadiyne-1,6-diol were microwave-irradiated to produce the COF, also known as TCD. The malo-nitrile post-synthetic treatment gives the COF nitrile functionality, resulting in TCD-CN, which can then interact with hydroxylamine to form amidoxime groups, giving rise to TCD-AO. The study investigated the use of CD and related derivatives in the recovery of uranium from seawater, demonstrating the potential of hydroxyl-functionalized COF TCD-OH.123 TCD achieved 100% adsorption within 20 minutes of contact. For all compounds containing the competing ions indicated above, batch adsorption tests were completed. Uranyl showed notable selectivity, as seen in TCD-OH providing the greatest uptake and selectivity, followed closely by TCD-AO. There was also a sizable uptake and selectivity for TCD-CN and TCD. Although these outcomes are encouraging and its exciting to see positive outcomes in this context. Fig. 6A shows the performance of TCD COF and derivatives in the selective recovery of uranium over 11 other competing ions in an aqueous solution. Carbon-based materials are also commonly used for separation, and it is believed the development of new carbon materials can make surface modification easier, providing electronic pathways for UES. Graphdiyne (GDY), a key research frontier in chemistry and materials science, has experienced rapid development over the past five years, ranking in the Top 10 research areas in the 2020 Research Frontiers report. These materials may offer enhanced conductivity and other properties to optimize the efficiency of the extraction process.124–127 The alkyne-rich and topologically porous structure makes it a good candidate for anchoring the metallic ions. It can be predicted that the development of GDY materials will provide new insight for UES.128–134
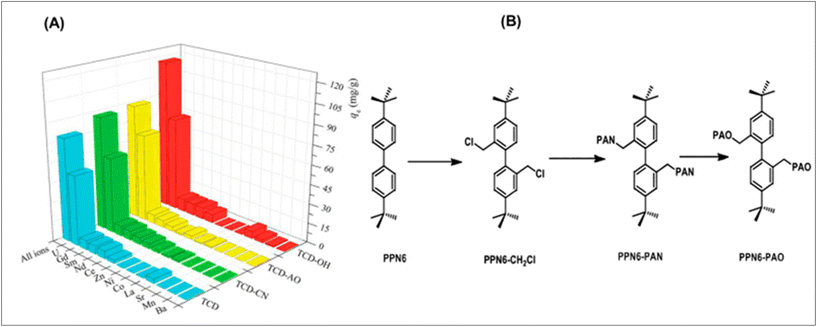 | ||
| Fig. 6 (A) The effectiveness of TCD COF and its derivatives in the aqueous solution for the selective recovery of uranium over 11 other competing ions. The experiment was run for three hours at 25 °C and pH 4.5. At first, each metal was introduced at a concentration of 0.5 mmol L−1.123 (B) Method for generating amidoxime-functionalized POP synthetically (PPN-6-PAO).138 | ||
In contrast to MOFs, the diversity of structures where organic nodes connect linear struts is much more constrained. This is primarily because there are restrictions on the organic node's potential symmetry and the compatibility of the functional groups needed for effective synthesis. The absence of metals in the composition results in a high surface area, low density, and high material stability, allowing for easy post-synthetic chemical functionalization and unique linker design. COFs are gaining attention for catalysis, gas storage, and separation processes, with growing interest in heavy metal extraction, particularly in UES.
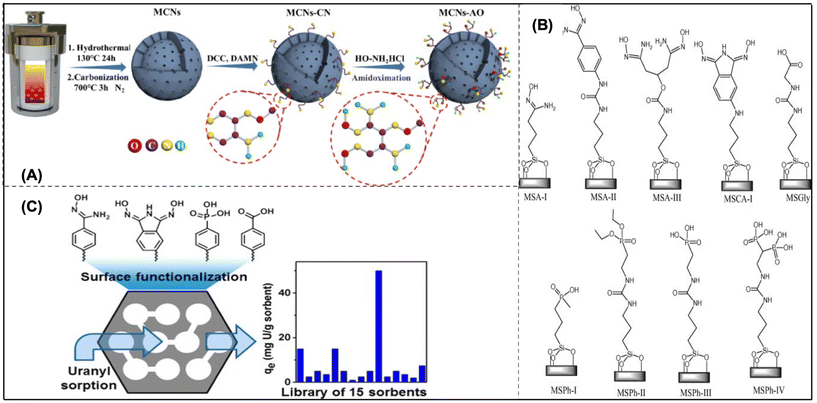 | ||
| Fig. 7 (A) The MCNs-AO preparation scheme.137 (B) Mesoporous silica sorbents with organo functionalization for removing U from both artificial seawater and water.139 (C) Adsorbents made of functionalized mesoporous carbon.140 | ||
Mesoporous silicas (MSs) are also a promising heterogeneous support for uranium extraction from seawater. Since their surfaces are terminated by silanol groups, condensation interactions with organic molecules with tri-alkoxysilane-terminated surfaces can easily modify their surfaces. Because of their huge surface areas and widespread commercial availability, MS materials are a good option for screening testing for newly developed chelating ligands. It is still a challenge to deploy them in areas with seawater due to their instability under an alkaline condition. Direct comparisons are challenging due to a number of factors, including pH ranges, contact periods, phase ratios, grafting levels, and uranyl concentrations used. To solve this problem, Vivero-Escoto et al. created a collection of organo-functionalized MS materials (MSU-H), which is readily available in the marketplace (Fig. 7B). Following comparable grafting and pre-treatment circumstances, the first direct comparison of over nine functionalities was performed. The unfunctionalized MSU-H performed better than all adsorbents in the sorption of deionized water, highlighting the significance of nonspecific binding to the MS support.139 Later on, the scientists also found the background sorption by silanol functionalities cannot be ignored. In this case, scientists modified the MS support with phosphoric acid, and a maximum saturation capacity of 60 mg g−1 was achieved, which eliminated the background adsorption.62
A series of functionalized mesoporous carbon (MC) materials (Fig. 7C) have also proved to be the best adsorbent for UES. After being covalently grafted with amidoxime, carboxyl, and phosphoryl functional groups, these adsorbents were tested for their ability to sorb uranium from aqueous solution.140 Previous studies indicated that ligands produced for grafting had an affinity for U(VI) and were generically classified as phosphoryl, amidoxime, or carboxyl derived. The effects of pH on U(VI) sorption, sorption kinetics, and sorption isotherms were obtained for the phosphoric acid-functionalized MC. Quantitative U(VI) removal from U(VI)-loaded sorbents was achieved by washing with HCl at concentrations higher than 0.01 M. These results indicate that functionalized MC provides a promising platform for the development of novel sorbents for efficient U(VI) extraction. According to the Langmuir model, a thorough examination of sorption by MC-O-PO(OH)2 showed that the highest saturation of the adsorption isotherms was 97 mg g−1 of uranium in acidic water and 67 mg g−1 in artificial seawater. Kinetic analysis revealed rapid U(VI) sorption in seawater simulant, with more gradual sorption in acidic water ultimately yielding a higher sorption. This study demonstrates that mesoporous carbon materials judiciously functionalized with a sorbent ligand have great potential for U(VI) sorption. The development of nanoscale materials has received considerable attention, but how these materials might be successfully applied in industrial-scale activities should be considered in the next step. Given that silica is known to be unstable in alkaline solutions, it is also important to take into account how the material will change over time when exposed to seawater. Last, but not least, it is important to note that early Japanese seawater deployment initiatives depended on a technology that was far less sophisticated but more cost-friendly. The economic efficiency of the adsorbent significantly influenced its conversion to supported polymers, a technological advancement that could significantly alter the process of uranium extraction from seawater.
There are benefits and drawbacks to each type of material. Pre-treatment is needed for carbon-based products for UES.141 Although expensive to produce, MOFs and COFs are promising nanomaterials. Biopolymers are affordable and sustainable, but they are susceptible to the environment. Uranium can be extracted effectively from amidoxime-based materials, but the extraction methods need to be made much simpler and better. Physical and chemical characteristics of different materials are typically various and there are many stages for the adsorption process. There was a significant correlation between the specific surface area and their adsorption capacity.142 Porous structure has an impact on the adsorption capacity as well, where a higher one is produced by a greater porosity.143 With smaller particle sizes, AO-containing adsorbents always present an excellent capacity. Constant efforts are made to improve their morphologies and structures to increase their ability to adsorb uranium in seawater. The use of membrane technology for UES is in its early stages, with a few reports of functionalized membranes with excellent selectivity and affinities for uranium. The membrane modules must be integrated with energy-intensive systems (Table 2).
5 Challenges of working with seawater
The intricacies of seawater pose significant obstacles to efficient uranium extraction. The advanced adsorbents must tackle challenges associated with pH, temperature, coexisting metal ions, and biofouling. To garner the most trustworthy data, the optimal study would either commence in the open ocean or employ periodically replenished ambient seawater. The method of batch interaction with seawater is comparatively more practical, yet it still presents challenges due to the volume necessary for large-scale uranium production and the analytical rigor required for precise quantification. While the low uranium concentration aids in addressing some issues, volumetric and analytical challenges persist. Appropriate pH and ionic strength must be factored in before any significant conclusions can be drawn. Temperature and salinity typically exert direct and indirect influences on the efficacy of adsorbents. Moreover, the impact of biofouling on various marine testing modalities remains elusive.5.1 pH
The efficiency of uranium extraction is influenced by the pH of the solution, as it significantly impacts the adsorption process and the species distribution of compounds.46 The pH's influence on the adsorption process is largely dictated by the adsorbent's functional groups and the metal ion solution chemistry. The quantity of uranium ions present dictates the chemistry of uranyl ions in the solution and its pH effect. Uranium speciation in seawater has been extensively researched, and current knowledge is comprehensive.Early UES studies found that uranium speciation results in the anionic tris carbonate-uranyl complex, UO2(CO3)34−, leading to the development of adsorbents for this complex's extraction.149,150 The typical cations for uranium include UO22+, (UO2)2(OH)22+, (UO2)3(OH)5+, and UO2OH+ in acidic conditions, and the electrostatic interaction between adsorbents and U(VI) would cause binding as shown in Fig. 8A. UO2CO3 or UO2(OH)2 is the main species of U in neutral forms, while UO2(CO3)34− or UO2(OH)3− is the primary species under high pH conditions.151,152 The production of UO2(CO3)34− increases with uranium content, with the polynuclear complex (UO2)2CO3(OH)3− dominating between pH 6.5 and 7.5; from pH 7.5 to 10, UO2(CO3)34− takes its place. This speciation is compared to that observed in naturally occurring saltwater, likely representative of many screening solutions.
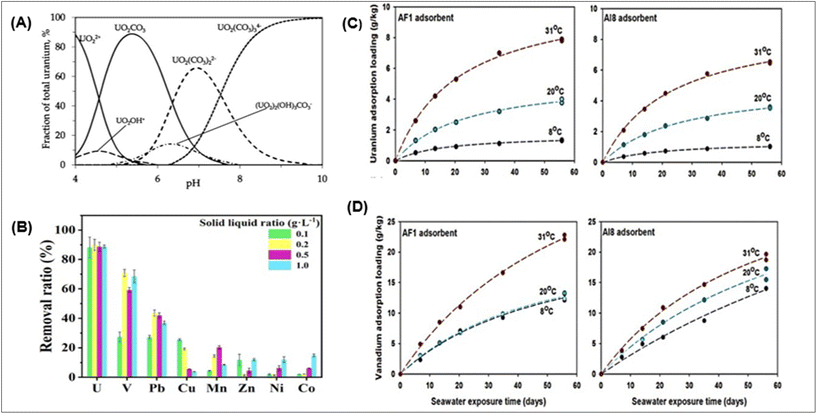 | ||
| Fig. 8 (A) Speciation of uranium.20,41,162,163 (B) The uranium adsorption selectivity of P-HBP-AO.45 (C) Uranium time series measurements; and (D) loadings of vanadium adsorbed at 8, 20, and 318 degrees Celsius using the AF1 and AI8 adsorbents. A one-site ligand saturation model was used to create the fitted lines that were drawn through the data points.75 | ||
5.2 Co-existing ions
The ocean is a high-salinity ecosystem because it contains a variety of metal ions, including Na+, K+, Mg2+, and Ca2+. A chemical knowledge is necessary to bind uranium specifically, escaping from the presence of high concentrations of alkali metals and alkaline earth metal ions in the transition zone.57,153 Uranium, a rare metal ion, is present in seawater at a concentration of around 3.3 μg kg−1, significantly lower than some other coexisting metal ions. It is important to recognize that different adsorbents exhibit varying affinities for the diverse metals present in seawater. In such competitive environments, the binding capacity of uranium adsorbents is considerably reduced. The extraction of uranium by AO adsorbent may be affected by other ions (Table 1).154| Adsorbents | Maximum adsorption capacity | Ref. |
|---|---|---|
| Polysulfide layered double hydroxides | 0.007 | 50 |
| UHMWPE-graft-poly(AO-co-AA) | 0.48 | 84 |
| HW-ACE on AO-functionalized electrode | 0.002 | 85 |
| SUP-hydrogel beads | 0.0092 | 86 |
| AO-OpNpNc fiber | 17.57 | 87 |
| DC-PAO hydrogel | 24.79 | 88 |
| UiO-66-NH2 | 5.52 | 89 |
| M808-4 | 5.83 | 90 |
| 5-AFM membrane adsorbent | 7.46 | 91 |
| BP-PAO fiber | 11.76 | 88 |
| AO functionalized In–Nx–C | 12.7 | 88 |
| Adsorbents | Structural features | Functional merits | Demerits | Application scope | Improvements towards UES |
|---|---|---|---|---|---|
| Inorganic adsorbent | Composed of minerals, and metal or metal oxides etc., a high surface area, rigid | High adsorption capacity, chemical stability, durability, availability of diverse material, regenerability | Limited specificity, biofouling potential, complex synthesis, environmental impact, low adsorption kinetics | Large-scale uranium extraction, integration with UES technologies | Enhanced selectivity, increased adsorption capacity, optimized porosity, tailored surface chemistry, resistance to fouling, stability in harsh conditions |
| Polymeric adsorbents | Composed of organic polymers, surface area can be tailored, flexible | Selective binding, high adsorption capacity, flexibility, and conformational adaptability | Competing ion adsorption, limited temperature range, chemical degradation | Biocompatible, industrial application, flexibility, and conformational adaptability | Selective binding, chemical tunability, cost-effective synthesis, minimize environmental impact |
| Biological proteins | Binding sites, tertiary structure, flexible, specific functional group, selective affinity | Functional diversity, high affinity, selective binding, biocompatibility, structural specificity | Integration complexity, specificity challenges, sale-up challenges, high cost of production | Recycling structural engineering, biofouling resistance, specific optimization | Enhanced selectivity, optimized kinetics, increased hydrophilicity, minimizing the environmental impact |
| Porous adsorbents MOFs, COFs, mesoporous | High surface area, control pore size and distribution, tunability, interconnected pores, low fouling | Scalability, structural stability, chemical functionalization, tailored pore size and distribution, | Temperature sensitivity, biofouling potential, high production cost, complex synthesis | Selective binding, high adsorption capacity, tenability, integration with UES technology | High flexibility of ligand chain, temperature stability, less environmental impact, cost-effective synthesis |
Recent marine experiments show that amidoxime-functionalized polymer adsorbents adsorb metal elements in a relative order of vanadium (14.9%), iron (1.6%), and uranium (1.0%).155 To facilitate discussion, metals that are dissolved in seawater can be broadly categorized into conservative and non-conservative metals, depending on the proportion of original concentration that they contribute to biological, chemical, or geological processes. The overall concentration of uranium in the seawater is relatively uniform because it is a conservative metal; only weak interactions between uranium complexes and suspended particles occur. Non-conservative distribution of aluminium is scavenged-type due to its significant interaction with suspended particles and short marine residence duration. Because both recycling and scavenging techniques have an impact on the distribution of iron and copper, these nonconservative metals show a hybrid distribution. Vanadium(V) has generated a lot of interest, because it is the main competitor for UES in seawater. The concentration of vanadium is lower than uranium (1.9 ppm), but because the vanadium is more tightly coupled to AO-functionalized adsorbents, the capacity of fibre to adsorb uranium decreases.156 Amidoximes are more favourable for complexing with vanadium than their uranium equivalents. Elution of vanadyl ions presents additional challenges.157 Extreme conditions, such as strong acid and high temperature, are required due to the significant interaction between amidoxime groups and vanadium during the elution process, potentially causing irreversible damage to the adsorbents.157 Numerous experimental studies and theoretical research have been conducted to better understand the affinity of amidoxime and vanadium.158,159 There are two primary forms of vanadium in seawater, which are V(IV) and V(V), with V(IV) accounting for 10 to 15% in total.160 The interaction between V(IV) and amidoxime groups is crucial due to the oxidation of V(IV) to V(V) during extraction, causing irreversible damage to adsorbents.157,158,161 When vanadium is bound to the cyclic imide dioxime, the organic group can even substitute the oxygen in vanadyl ions. The resultant non-oxide V(V) complex is more stable than its uranium counterparts, rendering the vanadium-occupied active sites inaccessible to uranyl ions. Eluting vanadium from the adsorbents is challenging even under acidic conditions, given the complex's stability across a broad pH range (Table 3).
| Element | mg kg−1 (ppm) | mol L−1 |
|---|---|---|
| Cl | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.546 |
| Na | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.468 |
| Mg | 1290 | 53 × 10−3 |
| Ca | 413 | 10.3 × 10−3 |
| K | 400 | 10.2 × 10−3 |
| Fe | 0.0034 | 0.5 × 10−9 |
| U | 0.0033 | 14 × 10−9 |
| V | 0.00183 | 36 × 10−9 |
High selectivity and affinity for uranium have been achieved by AO-based fibre P-HBP-AO. The exceptional selectivity and affinity of P-HBP-AO for uranium were confirmed by conducting selective adsorption tests for 12 hours at different solid-to-liquid ratios in an aqueous solution containing several heavy metal ions, as shown in Fig. 8B. The solid-to-liquid ratio in the adsorption test of P-HBP-AO (Poly(2-hydroxyethyl methacrylate)-grafted-poly benzoxazine-ammonium oxalate) fibre refers to the proportion of solid material (fibre) to the liquid medium (absorbing solution) used in the testing process. This is a critical parameter that influences the adsorption characteristics and performance of the fibre. Adjusting this ratio can impact how well the fibre adsorb the liquid and may be crucial in determining the fibre's adsorption capacity and efficiency in practical applications. Compared with most other metal ions, uranium has a far higher capacity for adsorption.45
The fabrication of materials with enhanced selectivity for uranium is advantageous, as it mitigates the influence of coexisting ions on adsorption. To augment the durability of uranium adsorbents, one could consider bolstering structural stability during the preparation phase, through certain procedures such as radiation graft polymerization, hydroxylamine treatment, and hot alkali treatment. Techniques such as ion-imprinted polymers and radiation-induced graft polymerization hold promise for the batch synthesis of high-selectivity materials for uranium extraction, with grafting yield modifiable based on radiation selection and reaction parameters. Recent research endeavours have probed the coordination chemistry of competing metals like iron, copper, lead, and vanadium with glutethimide-dioxide to understand and tackle selectivity issues in adsorbents.41,165,166 Consequently, a principal challenge for UES remains the design of adsorption materials with uranium selectivity at elevated temperatures. Emerging strategies such as ion imprinting technology and protein-engineered techniques have been identified to confer high uranyl ion selectivity to adsorbents.153
5.3 Temperature
Temperature is a crucial factor to consider when locating uranium extraction plants in the ocean. Seawater temperature, which is significantly influenced by location and season, has complex interactions that affect the performance of adsorbents.167 Higher temperatures accelerate both the ligand exchange interactions between amidoxime and uranium groups and the transport of uranyl ions from the bulk solution to the active sites. The efficiency of uranium extraction can be increased by constructing seawater extraction plants in regions with warmer ocean environments. A study highlights the overlooked factors, such as the effect of high temperatures on the resilience of adsorbents, predicting that increasing the solution temperature will enhance the adsorbent's performance.149 Trials at the Tarapur Atomic Power Station underscore the challenge of balancing endothermic reactions and biofouling, with factors like fouling, deployment time, and temperature influencing uranium uptake.168 The outfall canal's uranium uptake increased by nearly 30% due to the increased temperature. Temperature control is an issue that can be easily addressed in lab settings by carefully selecting deployment locations and times. While temperature influences adsorption experiments, its sensitivity is not significant, and minor temperature differences won't notably alter real-world settings. The adsorbent design can be optimized for exothermic uranium binding, allowing deployment below marine phototrophic layers to slow the rate of biofouling.The light-stimulated photocatalytic or photothermal properties of semiconductor materials can elucidate the effects of temperature on uranium adsorption and biofouling. Adsorbents exhibiting high uranium extraction efficiency and anti-biofouling attributes have been synthesized leveraging the photothermal effect. While this may potentially elevate local temperatures, it enhances the interaction between uranium and the adsorbent. In UES, the photocatalytic and photothermal properties of materials can be harnessed to augment adsorption and mitigate biofouling.169,170 Photoactivated sterilization, a potent antibacterial technique, is gaining significant interest in UES due to its ability to activate photoresponsive materials using appropriate wavelengths. By absorbing light energy, photoresponsive materials create radical oxygen species (ROS) and/or hyperthermic conditions, which quickly kill bacteria.171 The ROS can quickly kill bacteria by penetrating bacterial membranes and cell walls, destroying their defence mechanisms, and disrupting cellular respiration and physiological processes.172 Uranium has been extracted from seawater while avoiding biofouling by using a variety of photoresponsive antibacterial compounds, such as photocatalysts, photothermal materials, and photosensitizers. TiO2 and ZnO, common semiconductor materials, were utilized as antibacterial components in the construction of anti-biofouling adsorbents for UES due to their optical response properties. A TiO2-functionalized composite was created by coprecipitating nano-TiO2 particles onto wool fibre prepared with RIGP and functionalized with AO.173 The Wool-AO@TiO2 adsorbent demonstrated potent antibacterial properties while Nano-TiO2 enhanced uranium adsorption and enhanced the antimicrobial properties of the wool fibre.174 UV light irradiation of nano-TiO2/ZnO in water vapor activates ROS, degradation of organic compounds and killing of bacteria, potentially causing microorganism death.175
Research on the enthalpy and entropy of UES under different seawater temperatures provides the endothermic viewpoint in the adsorption process. Thermodynamic data indicate that amidoxime-based adsorbents demonstrate superior uranium adsorption and selectivity in warmer waters. As demonstrated by field studies in Miami, Florida, USA, involving seawater uranium adsorption in warm seawater, high temperatures have a significant impact on seawater uranium mining.75 The first study on the temperature effect was undertaken by Sekiguchi et al., who employed high-density polyethylene fibre modified with amidoxime. The results showed a three times enhancement in uranium adsorption when the temperature increased by 10 °C.167 Overall research indicates that thermodynamics, rather than transport considerations, is principally responsible for the reported rise in uranium extraction rate with temperature.167 Following this approach, many scientists have studied the effect of temperature on uranium adsorption with different adsorbents. Two efficient adsorbents, AF1 and AI8, were recently created by Oak Ridge National Laboratory, who investigate the impact of temperature on the extraction of uranium and vanadium from seawater. These are amidoxime-based adsorbents that are made with radiation-induced graft polymerization (RIGP) technique and hollow-gear-shaped, high surface area poly-ethylene fibre. The grafted co-monomer is the only distinction between these two (AF1: itaconic acid; AI8: vinyl phosphonic acid). Using a flow-through column system and freshly filtered (0.45 mm) seawater from Sequim Bay, Washington, U and V adsorption tests were carried out. The two amidoxime-based polymeric adsorbents showed a significantly reduced temperature response for vanadium and a high positive temperature response for uranium adsorption capacity. Between 8 and 31 °C, the 56-day U adsorption loadings of both adsorbents grew by around six times, but the 56-day V adsorption loadings of AF1 and AI8 increased by only 78 and 37 percent. Fig. 8C represents time series measurements of uranium; and Fig. 8D vanadium adsorption loadings with the AF1 and AI8 adsorbents at different temperature.75 Scientists should find an appropriate way to enhance the localized temperature for sea trials on a large scale.
A possible way to enhance the localized temperature is to combine the UES with photothermal approaches. We have published some research articles for the better understanding of photothermal evaporation phenomena.176–178 For the first time, we have synthesized a hollow multishelled structure (HoMS) using a composite of amorphous Ta2O5 and carbon, which efficiently enhanced the photothermal conversion. Fig. 9 represents the illustration of the very efficient solar-to-vapor production method using amorphous Ta2O5/C HoMS. The high solar evaporation rate in HoMS is due to its precise atomic and composition control, indirect bandgap structure, and unique hollow multishelled structure, which enhances nonradiative relaxation and promotes photothermal conversion. HoMS cavities facilitate mass transport through capillary pumping, while nanopores in the HoMS allow water molecules to evaporate as clusters with reduced energy consumption. The HoMS offers a cost-effective solution for large-scale clean water harvesting, heavy-metal enrichment, and soil remediation, demonstrating potential for large-scale fabrication development.176 Moreover, HoMS, as a special hollow structure, has an adjustable shell composition and an adjustable interlayer environment, thus providing a structural basis for the multi-functionalization of materials, but the synthesis approach is always a big challenge.179 By considering the relationship between the periodic structure in HoMS and chemical reaction, Wang's group provided a new synthetic methodology named the sequential templating approach (STA), rapidly recognized as a universal method180 Later, the unique property of temporal-spatial ordering181 and the physical essence of STA, i.e., the representation of concentration waves, were revealed.182 The unique structure of HoMS has demonstrated the advantages of abundant exposure of efficient surface and optimized mass transport, which can achieve the high-efficiency energy utilization183–186 Herein, introducing suitable photovapor generation materials with UES would enhance the kinetics and thermodynamics for U adsorption simultaneously.
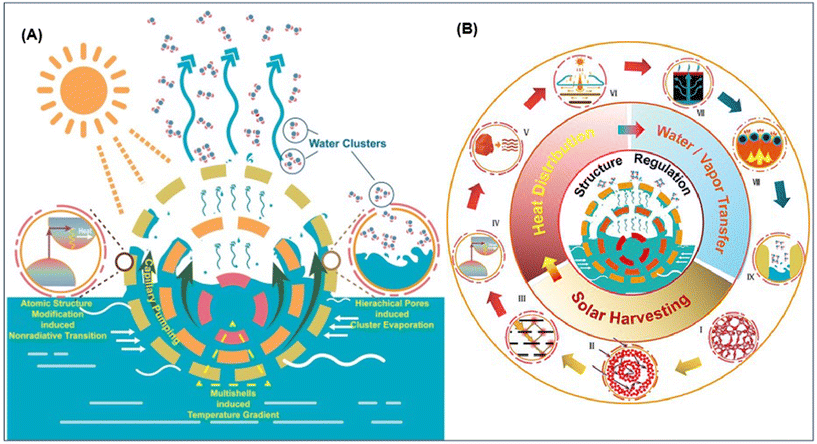 | ||
| Fig. 9 (A) Illustration of the very efficient solar-to-vapor production method using amorphous Ta2O5/C HoMS of amorphous Ta2O5/C HoMS for highly efficient solar-to-vapor production.176 (B) Regulation of the solar vapor generating structure affects each of the subsequent processes in the process, such as heat distribution, water/vapor transfer, and solar harvesting.194 | ||
5.4. Biofouling
Another technical challenge for creating adaptive adsorbents for UES is biofouling. As aforementioned, biofouling inevitably affects the uranium adsorbents placed in the ocean. The UES performance is significantly reduced when marine microorganisms are attached because they prevent uranyl ions from contacting with the adsorbent surfaces. Rigorous procedures are necessary to remove the foulants and biofouling. Feasible ways have been developed in recent years to enhance anti-biofouling performance, such as adding active bacteria-killing antimicrobial components or anti-adhesion coatings to prevent marine organisms from clinging to the adsorbent surfaces. Adsorbents have been successfully produced to be biofouling-proof, and have effectively resisted biofouling for particular microorganisms. Currently, laboratory techniques are used to evaluate antibacterial substances. The relationship between the efficiency of adsorption and anti-biofouling should be further evaluated in the marine environment.Biofouling occurs in four stages, as indicated in Fig. 10A: dense organic film formation, growth of bacteria and diatoms, rough microbial film formation, and large organism attachment inhibiting uranium lift.187 Park et al.188 investigated how biological contamination affected the process of extracting uranium from ocean. As shown in Fig. 10B, the environments of the shallow sea and deep sea were replicated with light and no light irradiation. A significant number of algae cells were seen in the light environment and the uranium adsorption was decreased by 30% after 42 days. Wen et al. synthesized Wool-AO@TiO2, a blend of amidoxime wool fibers and TiO2 nanoparticles, demonstrating strong antibacterial activity against Staphylococcus aureus and Escherichia coli. As shown in Fig. 10C, Guo et al. developed GCZ8A, a biofouling-resistant chitosan-graphene oxide/ZIF, with a maximum uranium absorption capacity of 361.01 mg g−1 and nearly 70% uranium removal in natural seawater (Tables 4–6).
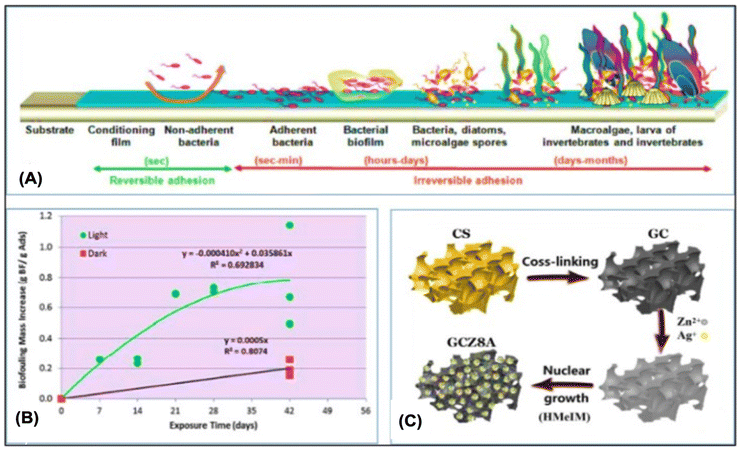 | ||
| Fig. 10 (A) Marine fouling development methods.187 (B) Elevation in the dry mass of the biofouling material with time in both light and dark streams. Biofouling mass is a representation of the biofouling material accumulated during 42 days (g)188 per adsorbent mass (g). (C) Illustrative GCZ8A composites synthesis schematic proposed.56 | ||
| Year | Place | Adsorbent | Time (d) | U-collected | Ref. |
|---|---|---|---|---|---|
| 2011 | South China Sea | AO-based non-woven fabrics | 20 | 0.10 mg g−1 | 189 |
| 2013 | South China Sea | AO-based fibers | 20 | 0.10 mg g−1 | 189 |
| 2015 | Xiamen MSL, PNNL | AO-UHMUPE | 60 | 0.25 mg g−1 | 190 |
| 2018 | East China Sea | Nano fiber-based membranes | 30 | 20 g | 12 |
| 2019 | Dagze Co Salt Lake, Tibet | Polymers | — | 300 g | 12 |
| Year | Place | Adsorbent | Adsorption system |
|---|---|---|---|
| 1991 | The coast of the Pacific Ocean | AO-based fiber | Continuous-flow system |
| 1991 | Imari Bay, Japan | AO-based fiber | Mooring system |
| 1991 | Imari Bay, Japan | AO-based resin | Towing–mooring system |
| 2000 | 6 km offshore at Mutsu Sekine-Hama in Aomori Prefecture, Japan | AO-based fiber | Mooring System |
| 1991–2001 | 7 km offshore from Mustu-Sekine in Aomori prefecture, Japan | AO-based fabric | Stack collection system |
| 2004 | Okinawa, Japan | AO-based fiber | Braid collection system |
| Adsorbents | Absorption system | Saturation capacity (mg g−1) |
|---|---|---|
| 38H | PNNL, flow-through column testing | 4.29 ± 0.24 |
| AI8 | 5.17 ± 0.18 | |
| AF1 | 5.56 ± 0.15 | |
| LCW-2 | 7.05 ± 0.21 | |
| AI8 | PNNL recirculating flume testing | 6.86 ± 0.68 |
| AF1 | 5.93 ± 0.17 | |
| AF8 | 7.04 ± 1.42 | |
| AF1 | WHOI, flow-through column testing | 5.97 ± 0.27 |
| AF1 | WHOI, recirculating flume testing | 9.84 ± 0.48 |
6 Conclusion and outlook
The exploration of seawater uranium extraction as a viable alternative for sustainable nuclear energy expansion commenced with “Project Oyster” in 1950. Innovative methods for function-driven adsorbents have been designed in consideration of enhancing the selectivity, durability, resistance to biofouling, and adsorption capacity. Over the past years, efforts on the physicochemical properties of adsorbents, such as pore size, surface area, surface conformation, and ligand density, have been delivered to enhance the adsorption capabilities. In comparison with conventional physicochemical adsorption techniques, electrochemical and photochemical methodologies have dramatically amplified UES performance. Despite the significant strides made by UES across various strategies, substantial hurdles and challenges persist that impede its progression toward industrialization. This manuscript summarizes recent achievements in UES and underscores the challenges and future research directions for UES systems, emphasizing the necessity for further advancements in fundamental, technical, and engineering facets. Grasping uranium coordination chemistry is pivotal for identifying efficient binding motifs between uranyl and ligands. Uranium extraction necessitates concurrent alterations to the thermodynamic and kinetic factors governing the uranium adsorption equilibrium. Kinetic factors, such as binding site accessibility and mass transfer capacity of uranyl ions, warrant particular attention. The generation of suitable adsorbents for UES, which can be tailored and enhanced, calls for improved synthesis and polymerization procedures. To facilitate a cost-efficiency analysis and a feasible commercialization pathway, more technical efforts are essential to construct standalone prototypes and provide infrastructure for ocean field trials. Nations such as Japan, the United States, and China have implemented offshore platforms and conducted marine field trials pertaining to UES. Research has revealed that uranium extraction in marine contexts is influenced by a multitude of hydrological variables, inclusive of adsorbent properties, seawater temperature, biofouling, water quality, and oceanic depth. Marine engineering is confronted with a plethora of constraints, which need substantial effort to surmount, particularly when navigating the intricate oceanic environment. Prior to undertaking marine engineering, it is imperative to possess a comprehensive understanding of oceanographic conditions, given the significant disparities between results derived from marine field testing and those obtained from seawater modeling. These discrepancies suggest that authentic seawater environments must be utilized to evaluate the efficacy of adsorbents developed within laboratory settings. To optimally select locations and conditions for marine field research, it is critical to assess the impact of seawater temperature and velocity. In addition, the identification of dominant marine species that influence uranium collection is of paramount importance. This knowledge can facilitate the creation of anti-biofouling adsorbents that target specific biological species. The deployment of UES adsorbents and facilities within seawater, particularly those possessing bactericidal activity, may adversely affect regional marine ecosystems. Large-scale ocean field tests for UES would necessitate an analysis of their potential effects.Issues like limited adsorption capacity, selectivity problems, renewability constraints, high production costs, biofouling, and scaling issues are common barriers in uranium adsorbents. Future directions in the development of uranium adsorbents include the use of more computational modeling, nanostructured materials, hybrid materials, biologically inspired designs, multifunctional adsorbents, improved material engineering, and nanostructured materials. Metal–organic frameworks (MOFs) with their high tunability, covalent organic frameworks (COFs) with their structural versatility, nanostructured materials with their increased surface areas, hybrid materials with their combination of different components, and functionalized inorganic adsorbents with their improved selectivity and overall performance are promising types for further practical application. To remove current obstacles and make uranium adsorbents more effective, affordable, and ecologically friendly for large-scale use in uranium extraction from seawater systems, these trends and materials are being continuously explored.144
During sea trials, enhancements have been made to the uranium adsorption capability of AO-based polymeric adsorbents. The substantial gap between laboratory and marine environment implies that the matrix of genuine seawater is complex. Oceanic environmental factors play a significant role in uranium adsorption. Given the dependency of uranium adsorption on oceanic environmental conditions, which can influence the extraction process, the scalability of UES requires a fundamental understanding of environmental elements such as flow rate and ocean temperature. Prolonged exposure of the adsorbents to seawater results in increasingly severe biofouling, which may compromise their capacity and reusability. Since these strategies have not been tested in pilot operations, and any required power is determined by lab-scale evaluations, one needs to link offshore energy sources such as wind and tidal power with UES. Also, coastal nuclear power plants and desalination facilities could significantly reduce the costs.
Currently, the cost of the extracted uranium remains higher than from traditional sources, rendering existing UES unable to economically compete with terrestrial mining. Future research endeavors should be directed towards the consistent enhancement of adsorbent performance, thereby delivering improvements to make a UES system economically viable. The cost of adsorbent production is projected to undergo a gradual decline owing to the decrease in the prices of raw materials and advancements in synthetic techniques. Simultaneously, the expenses associated with mooring and deployment are anticipated to constitute a larger fraction of the overall cost. The cost of offshore deployment and mooring can be effectively mitigated by integrating the sustainable energy source with the UES system, which appears to be a more cost-effective strategy. An efficient, non-destructive elution/regeneration method is imperative to maximize adsorbent reuse, given that adsorbent regeneration is deemed crucial for economic viability.
Transitioning from lab-scale UES to large-scale projects requires considering factors like material design, engineering, interaction with infrastructure, and consideration of variables like the coordination environment of ions, biofouling and corrosion, whereas lab settings favor controlled conditions. Industrial cost-effectiveness, waste management, environmental effects, and regulatory compliance are crucial for large-scale UES, necessitating interdisciplinary cooperation for creating commercially and ecologically sustainable materials and systems. A multimodal strategy is needed to bridge the gap between lab-scale UES and large-scale applications, focusing on maximizing material designs, improving synthesis techniques, and increasing yields. Engineers should focus on large-scale UES solutions, considering reactor design, system integration, and flow rates, while implementing cost-efficiency techniques and prioritizing long-term stability in marine environments for economic feasibility, utilizing materials that can withstand prolonged exposure, resist corrosion, and function across cycles. Implementation of constant monitoring, improvement of adsorbent renewability and reusability, and use of effective regeneration procedures for reduced operating costs is required. Thorough environmental impact assessments are essential for compliance with environmental requirements, and for addressing waste disposal, energy consumption, and ecological damage. Collaborative multidisciplinary research is crucial for comprehensive solutions. Pilot-scale demonstrations and partnerships with business partners validate the practicality and efficiency of UES technologies, bridging the gap between research and industrial application.
This review delves into the contemporary status of UES and proffers recommendations for augmenting its technological robustness and economic feasibility. It necessitates a synergistic collaboration among industrial engineers and scientists across diverse disciplines. Despite formidable challenges, substantial headway has been achieved in commercial viability during the past decade. The recent strides in UES represent merely the tip of the iceberg. Once a significant breakthrough is achieved, it is anticipated to be sustainable and yield considerable positive impacts for the economy and the environment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 92163209, 21931012, 21971244 and 51932001), National Key R&D Program of China (2018YFA0703504, 2021YFB3802600), Beijing Natural Science Foundation (JQ22004).References
- D. Welsby, J. Price, S. Pye and P. Ekins, Nature, 2021, 597, 230–234 CrossRef CAS.
- R. Hanna and D. G. Victor, Nat. Energy, 2021, 6, 568–571 CrossRef.
- K. M. G. Langie, K. Tak, C. Kim, H. W. Lee, K. Park, D. Kim, W. Jung, C. W. Lee, H.-S. Oh, D. K. Lee, J. H. Koh, B. K. Min, D. H. Won and U. Lee, Nat. Commun., 2022, 13, 7482 CrossRef CAS PubMed.
- L. J. Sonter, M. C. Dade, J. E. M. Watson and R. K. Valenta, Nat. Commun., 2020, 11, 4174 CrossRef CAS PubMed.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS.
- R. York, Nat. Clim. Change, 2012, 2, 441–443 CrossRef.
- D. Tong, D. J. Farnham, L. Duan, Q. Zhang, N. S. Lewis, K. Caldeira and S. J. Davis, Nat. Commun., 2021, 12, 6146 CrossRef CAS PubMed.
- A. Q. Al-Shetwi, Sci. Total Environ., 2022, 822, 153645 CrossRef CAS PubMed.
- L. R. Shepherd, Nature, 1974, 249, 717–720 CrossRef CAS.
- A. Bulgac, S. Jin and I. Stetcu, Front. Phys., 2020, 8, 00063 CrossRef.
- Y. Xie, Z. Liu, Y. Geng, H. Li, N. Wang, Y. Song, X. Wang, J. Chen, J. Wang, S. Ma and G. Ye, Chem. Soc. Rev., 2023, 52, 97–162 RSC.
- C. Tsouris, Nat. Energy, 2017, 2, 17022 CrossRef.
- H. Fell, A. Gilbert, J. D. Jenkins and M. Mildenberger, Nat. Energy, 2022, 7, 25–29 CrossRef.
- S. Gabriel, A. Baschwitz, G. Mathonnière, T. Eleouet and F. Fizaine, Ann. Nucl. Energy, 2013, 58, 213–220 CrossRef CAS.
- Y. Pu, T. Qiang, G. Li, X. Ruan and L. Ren, Ecotoxicol. Environ. Saf., 2023, 259, 115053 CrossRef CAS.
- J. Kim, C. Tsouris, R. T. Mayes, Y. Oyola, T. Saito, C. J. Janke, S. Dai, E. Schneider and D. Sachde, Sep. Sci. Technol., 2013, 48, 367–387 CrossRef CAS.
- Z. Wang, H. Hu, L. Huang, F. Lin, S. Liu, T. Wu, N. S. Alharbi, S. O. Rabah, Y. Lu and X. Wang, Chem. Eng. J., 2020, 396, 125272 CrossRef CAS.
- S. Laguel and M. Samar, J. Appl. Water Eng. Res., 2022, 10, 1–16 CrossRef.
- A. Orabi, J. Radiat. Res. Appl. Sci., 2013, 6, 1–10 CAS.
- P. Zaheri and R. Davarkhah, J. Environ. Chem. Eng., 2017, 5, 4064–4068 CrossRef CAS.
- J. Wang and S. Zhuang, Rev. Environ. Sci. Bio/Technol., 2019, 18, 437–452 CrossRef.
- L. Kong, Y. Zhu, M. Wang, Z. Li, Z. Tan, R. Xu, H. Tang, X. Chang, Y. Xiong and D. Chen, J. Hazard. Mater., 2016, 320, 435–441 CrossRef CAS PubMed.
- M. Chaudhary, L. Singh, P. Rekha, V. C. Srivastava and P. Mohanty, Chem. Eng. J., 2019, 378, 122236 CrossRef CAS.
- H. Lindner and E. Schneider, Energy Economics, 2015, 49, 9–22 CrossRef.
- S. Das, Y. Oyola, R. T. Mayes, C. J. Janke, L. J. Kuo, G. Gill, J. R. Wood and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4110–4117 CrossRef CAS.
- H. Guo, H. Wang, N. Zhang, J. Li, J. Liu, A. Alsaedi, T. Hayat, Y. Li and Y. Sun, Chem. Eng. J., 2019, 369, 736–744 CrossRef CAS.
- M. Wang, W. Cheng, T. Wan, B. Hu, Y. Zhu, X. Song and Y. Sun, Chem. Eng. J., 2019, 362, 99–106 CrossRef CAS.
- S. A. Fahad, M. S. Nawab, M. A. Shaida, S. Verma, M. U. Khan, V. Siddiqui, M. Naushad, L. Saleem and I. H. Farooqi, J. Water Process Eng., 2023, 52, 103458 CrossRef.
- C.-Z. Wang, J.-H. Lan, Q.-Y. Wu, Q. Luo, Y.-L. Zhao, X.-K. Wang, Z.-F. Chai and W.-Q. Shi, Inorg. Chem., 2014, 53, 9466–9476 CrossRef CAS PubMed.
- C. J. Leggett, F. Endrizzi and L. Rao, Ind. Eng. Chem. Res., 2016, 55, 4257–4263 CrossRef CAS.
- R. G. Denning, J. Phys. Chem. A, 2007, 111, 4125–4143 CrossRef CAS PubMed.
- S. Fortier and T. W. Hayton, Coord. Chem. Rev., 2010, 254, 197–214 CrossRef CAS.
- R. M. Harris, Z. Zhu, B. A. Tufekci, Deepika, P. Jena, K. A. Peterson and K. H. Bowen, J. Phys. Chem. A, 2023, 127, 7186–7197 CrossRef CAS PubMed.
- H. S. Reynolds, R. Ram, M. I. Pownceby, Y. Yang, M. Chen, J. Tardio, L. Jones and S. K. Bhargava, Trans. Nonferrous Met. Soc. China, 2018, 28, 2135–2142 CrossRef CAS.
- B. Zhang, M. Li, X. Zhang and J. Huang, JOM, 2016, 68, 1990–2001 CrossRef CAS.
- F. Khanramaki, A. S. Shirani, J. Safdari and R. Torkaman, Chem. Eng. Res. Des., 2017, 125, 181–189 CrossRef CAS.
- L. Yang, H. Xiao, Y. Qian, X. Zhao, X.-Y. Kong, P. Liu, W. Xin, L. Fu, L. Jiang and L. Wen, Nat. Sustain., 2022, 5, 71–80 CrossRef.
- Y. Xue, J. Chen, P. Liu, J. Gao, Y. Gui, W. Cheng, F. Ma and Y. Yan, Colloids Surf., A, 2022, 647, 129032 CrossRef CAS.
- O. E. Roshdy, J. Radioanal. Nucl. Chem., 2021, 329, 85–101 CrossRef CAS.
- F. Endrizzi, C. J. Leggett and L. Rao, Ind. Eng. Chem. Res., 2016, 55, 4249–4256 CrossRef CAS.
- N. Wang, X. Zhao, J. Wang, B. Yan, S. Wen, J. Zhang, K. Lin, H. Wang, T. Liu, Z. Liu, C. Ma, J. Li and Y. Yuan, Adv. Sci., 2021, 8, 2102250 CrossRef CAS PubMed.
- A. P. Ladshaw, A. I. Wiechert, S. Das, S. Yiacoumi and C. Tsouris, Materials, 2017, 10(11), 1268 CrossRef PubMed.
- S. Xie, X. Liu, B. Zhang, H. Ma, C. Ling, M. Yu, L. Li and J. Li, J. Mater. Chem. A, 2015, 3, 2552–2558 RSC.
- W.-N. Ren, X.-X. Feng, Y.-L. He, M.-L. Wang, W.-F. Hong, H.-W. Han, J.-T. Hu and G.-Z. Wu, Nucl. Sci. Tech., 2023, 34, 90 CrossRef CAS.
- F. Ma, Y. Gui, P. Liu, Y. Xue and W. Song, Chem. Eng. J., 2020, 390, 124597 CrossRef CAS.
- S. Das, Y. Oyola, R. T. Mayes, C. J. Janke, L. J. Kuo, G. Gill, J. R. Wood and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4103–4109 CrossRef CAS.
- J. Yu, H. Bai, J. Wang, Z. Li, C. Jiao, Q. Liu, M. Zhang and L. Liu, New J. Chem., 2013, 37, 366–372 RSC.
- R. Li, R. Che, Q. Liu, S. Su, Z. Li, H. Zhang, J. Liu, L. Liu and J. Wang, J. Hazard. Mater., 2017, 338, 167–176 CrossRef CAS PubMed.
- S. Ma, L. Huang, L. Ma, Y. Shim, S. M. Islam, P. Wang, L.-D. Zhao, S. Wang, G. Sun, X. Yang and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 3670–3677 CrossRef CAS PubMed.
- S. Kalam, S. A. Abu-Khamsin, M. S. Kamal and S. Patil, ACS Omega, 2021, 6, 32342–32348 CrossRef CAS PubMed.
- J. Wang and X. Guo, Chemosphere, 2020, 258, 127279 CrossRef CAS PubMed.
- H.-K. Chung, W.-H. Kim, J. Park, J. Cho, T.-Y. Jeong and P.-K. Park, J. Ind. Eng. Chem., 2015, 28, 241–246 CrossRef CAS.
- Y. Ma, W. Dou, W. Yang, W. Yang and Q. Pan, J. Radioanal. Nucl. Chem., 2021, 329, 1011–1017 CrossRef CAS.
- A. Yang, Z. Wang and Y. Zhu, Sci. Rep., 2020, 10, 19271 CrossRef CAS PubMed.
- D. Zhang, L. Fang, L. Liu, B. Zhao, B. Hu, S. Yu and X. Wang, Sep. Purif. Technol., 2023, 320, 124204 CrossRef CAS.
- C. W. Abney, R. T. Mayes, T. Saito and S. Dai, Chem. Rev., 2017, 117, 13935–14013 CrossRef CAS PubMed.
- R. V. Davies, J. Kennedy, R. W. McIlroy, R. Spence and K. M. Hill, Nature, 1964, 203, 1110–1115 CrossRef.
- M. Kanno, J. Nucl. Sci. Technol., 1984, 21, 1–9 CrossRef CAS.
- T. Hori, M. Yamawaki and M. Kanno, J. Nucl. Sci. Technol., 1987, 24, 377–384 CrossRef CAS.
- R. K. Singhal, H. Basu, M. K. T. Bassan, M. V. Pimple, V. Manisha, D. K. Avhad, P. Sharma and A. V. R. Reddy, J. Radioanal. Nucl. Chem., 2012, 292, 675–681 CrossRef CAS.
- B. E. Johnson, P. H. Santschi, C.-Y. Chuang, S. Otosaka, R. S. Addleman, M. Douglas, R. D. Rutledge, W. Chouyyok, J. D. Davidson, G. E. Fryxell and J. M. Schwantes, Environ. Sci. Technol., 2012, 46, 11251–11258 CrossRef CAS PubMed.
- Y. Zhao, J. Li, S. Zhang and X. Wang, RSC Adv., 2014, 4, 32710–32717 RSC.
- L. Tan, Q. Liu, X. Jing, J. Liu, D. Song, S. Hu, L. Liu and J. Wang, Chem. Eng. J., 2015, 273, 307–315 CrossRef CAS.
- D. Shao, X. Wang, X. Wang, S. Hu, T. Hayat, A. Alsaedi, J. Li, S. Wang, J. Hu and X. Wang, RSC Adv., 2016, 6, 52076–52081 RSC.
- J.-Y. Kim, A. J. Norquist and D. O'Hare, Dalton Trans., 2003, 2813–2814, 10.1039/B306733P.
- S. V. Krivovichev and P. C. Burns, J. Solid State Chem., 2003, 170, 106–117 CrossRef CAS.
- T. S. Franczyk, K. R. Czerwinski and K. N. Raymond, J. Am. Chem. Soc., 1992, 114, 8138–8146 CrossRef CAS.
- P. L. Arnold, A. J. Blake, C. Wilson and J. B. Love, Inorg. Chem., 2004, 43, 8206–8208 CrossRef CAS PubMed.
- P. Nichols, E. J. Bylaska, G. K. Schenter and W. de Jong, J. Chem. Phys., 2008, 128, 124507 CrossRef PubMed.
- V. Vallet, U. Wahlgren and I. Grenthe, J. Am. Chem. Soc., 2003, 125, 14941–14950 CrossRef CAS PubMed.
- S. Tsushima, Inorg. Chem., 2009, 48, 4856–4862 CrossRef CAS PubMed.
- J. Kim, C. Tsouris, Y. Oyola, C. J. Janke, R. T. Mayes, S. Dai, G. Gill, L.-J. Kuo, J. Wood, K.-Y. Choe, E. Schneider and H. Lindner, Ind. Eng. Chem. Res., 2014, 53, 6076–6083 CrossRef CAS.
- C. W. Abney, R. T. Mayes, M. Piechowicz, Z. Lin, V. S. Bryantsev, G. M. Veith, S. Dai and W. Lin, Energy Environ. Sci., 2016, 9, 448–453 RSC.
- L.-J. Kuo, G. A. Gill, C. Tsouris, L. Rao, H.-B. Pan, C. M. Wai, C. J. Janke, J. E. Strivens, J. R. Wood, N. Schlafer and E. K. D'Alessandro, ChemistrySelect, 2018, 3, 843–848 CrossRef CAS.
- N. Seko, A. Katakai, S. Hasegawa, M. Tamada, N. Kasai, H. Takeda, T. Sugo and K. Saito, Nucl. Technol., 2003, 144, 274–278 CrossRef CAS.
- H.-B. Pan, C. M. Wai, L.-J. Kuo, G. A. Gill, J. S. Wang, R. Joshi and C. J. Janke, Dalton Trans., 2020, 49, 2803–2810 RSC.
- C. Fang-Ting, L. Peng, X. Jie, S. Hu, G. Tao, X. Xiu-Long and W. Xiao-Lin, Chin. Phys. B, 2012, 21, 093102 CrossRef.
- S. Vukovic, L. A. Watson, S. O. Kang, R. Custelcean and B. P. Hay, Inorg. Chem., 2012, 51, 3855–3859 CrossRef CAS PubMed.
- Z. Liu, Y. Lan, J. Jia, Y. Geng, X. Dai, L. Yan, T. Hu, J. Chen, K. Matyjaszewski and G. Ye, Nat. Commun., 2022, 13, 3918 CrossRef CAS PubMed.
- A. S. Ivanov, B. F. Parker, Z. Zhang, B. Aguila, Q. Sun, S. Ma, S. Jansone-Popova, J. Arnold, R. T. Mayes, S. Dai, V. S. Bryantsev, L. Rao and I. Popovs, Nat. Commun., 2019, 10, 819 CrossRef CAS PubMed.
- S. V. Wegner, H. Boyaci, H. Chen, M. P. Jensen and C. He, Angew. Chem., Int. Ed. Engl., 2009, 48, 2339–2341 CrossRef CAS PubMed.
- E. A. Heide, K. Wagener, M. Paschke and M. Wald, Naturwissenschaften, 1973, 60, 431–431 CrossRef CAS.
- J. Hu, H. Ma, Z. Xing, X. Liu, L. Xu, R. Li, C. Lin, M. Wang, J. Li and G. Wu, Ind. Eng. Chem. Res., 2016, 55, 4118–4124 CrossRef CAS.
- C. Liu, P.-C. Hsu, J. Xie, J. Zhao, T. Wu, H. Wang, W. Liu, J. Zhang, S. Chu and Y. Cui, Nat. Energy, 2017, 2, 17007 CrossRef CAS.
- S. Kou, Z. Yang and F. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 2035–2039 CrossRef CAS PubMed.
- X. Xu, L. Xu, J. Ao, Y. Liang, C. Li, Y. Wang, C. Huang, F. Ye, Q. Li, X. Guo, J. Li, H. Wang, S. Ma and H. Ma, J. Mater. Chem. A, 2020, 8, 22032–22044 RSC.
- Y. Li, Y. Zheng, Z. Ahamd, L. Zhu, J. Yang, J. Chen and Z. Zhang, Coord. Chem. Rev., 2023, 491, 215234 CrossRef CAS.
- T. Liu, X. Zhang, H. Wang, M. Chen, Y. Yuan, R. Zhang, Z. Xie, Y. Liu, H. Zhang and N. Wang, Chem. Eng. J., 2021, 412, 128700 CrossRef CAS.
- Z. Zhao, R. Lei, Y. Zhang, T. Cai and B. Han, J. Mol. Liq., 2022, 367, 120514 CrossRef CAS.
- R. Yu, Y. Lu, X. Zhang, W. Chen, X. Chen and L. Li, Desalination, 2022, 539, 115965 CrossRef CAS.
- Y.-W. Lin, Nanomaterials (Basel), 2020, 10, 457 CrossRef CAS PubMed.
- Q. Hu, S. Huang, T. Wei, J. Wang, Z. Huo, T. Zhu, C. Wu and H. Chen, ACS Appl. Polym. Mater., 2022, 4, 2189–2196 CrossRef CAS.
- L. Qi, C. Basset, O. Averseng, E. Quéméneur, A. Hagège and C. Vidaud, Metallomics, 2013, 6, 166–176 CrossRef PubMed.
- M. Cao, Q. Peng, Y. Wang, G. Luo, L. Feng, S. Zhao, Y. Yuan and N. Wang, Int. J. Biol. Macromol., 2023, 242, 124792 CrossRef CAS PubMed.
- C. D. Pemmaraju, R. Copping, D. E. Smiles, D. K. Shuh, N. Grønbech-Jensen, D. Prendergast and A. Canning, ACS Omega, 2017, 2, 1055–1062 CrossRef CAS PubMed.
- F. Shen and L. M. K. Dassama, Chem. Sci., 2023, 14, 8433–8447 RSC.
- C. Basset, O. Averseng, P.-J. Ferron, N. Richaud, A. Hagège, O. Pible and C. Vidaud, Chem. Res. Toxicol., 2013, 26, 645–653 Search PubMed.
- O. Pible, C. Vidaud, S. Plantevin, J.-L. Pellequer and E. Quéméneur, Biochimie, 2010, 19, 2219–2230 CAS.
- A. Garai and P. Delangle, J. Inorg. Biochem., 2020, 203, 110936 CrossRef CAS PubMed.
- O. Carugo, J. Inorg. Biochem., 2018, 189, 1–6 CrossRef CAS PubMed.
- L. Zhou, M. Bosscher, C. Zhang, S. Özçubukçu, L. Zhang, W. Zhang, C. J. Li, J. Liu, M. P. Jensen, L. Lai and C. He, Nat. Chem., 2014, 6, 236–241 CrossRef CAS PubMed.
- T.-N. S. Huynh, D. Bourgeois, C. Basset, C. Vidaud and A. Hagège, Metallomics, 2015, 36, 1374–1382 CAS.
- M. Koohi-Moghadam, H. Wang, Y. Wang, X. Yang, H. Li, J. Wang and H. Sun, Nat. Mach. Intell., 2019, 1, 561–567 CrossRef.
- L. Zhou, M. Bosscher, C. Zhang, S. Ozçubukçu, L. Zhang, W. Zhang, C. J. Li, J. Liu, M. P. Jensen, L. Lai and C. He, Nat. Chem., 2014, 6, 236–241 CrossRef CAS PubMed.
- W. Chouyyok, J. W. Pittman, M. G. Warner, K. M. Nell, D. C. Clubb, G. A. Gill and R. S. Addleman, Dalton Trans., 2016, 45, 11312–11325 RSC.
- H.-C. J. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- X. Guo, H. Yang, Q. Liu, J. Liu, R. Chen, H. Zhang, J. Yu, M. Zhang, R. Li and J. Wang, Chem. Eng. J., 2020, 382, 122850 CrossRef CAS.
- C. W. Abney, K. M. L. Taylor-Pashow, S. R. Russell, Y. Chen, R. Samantaray, J. V. Lockard and W. Lin, Chem. Mater., 2014, 26, 5231–5243 CrossRef CAS.
- F. Carson, V. Pascanu, A. Bermejo Gómez, Y. Zhang, A. E. Platero-Prats, X. Zou and B. Martín-Matute, Chemistry, 2015, 21, 10896–10902 CrossRef CAS PubMed.
- J.-M. Liu, T. Liu, C.-C. Wang, X.-H. Yin and Z.-H. Xiong, J. Mol. Liq., 2017, 242, 531–536 CrossRef CAS.
- Q. Yu, Y. Yuan, J. Wen, X. Zhao, S. Zhao, D. Wang, C. Li, X. Wang and N. Wang, Adv. Sci., 2019, 6, 1900002 CrossRef PubMed.
- Y. Wu, Y. Xie, X. Liu, Y. Li, J. Wang, Z. Chen, H. Yang, B. Hu, C. Shen, Z. Tang, Q. Huang and X. Wang, Coord. Chem. Rev., 2023, 483, 215097 CrossRef CAS.
- Q. Sun, Y. Song, B. Aguila, A. S. Ivanov, V. S. Bryantsev and S. Ma, Adv. Sci., 2021, 8, 2001573 CrossRef CAS PubMed.
- Y. Song, C. Zhu, Q. Sun, B. Aguila, C. W. Abney, L. Wojtas and S. Ma, ACS Cent. Sci., 2021, 7, 1650–1656 CrossRef CAS PubMed.
- H. Fan, J. Gu, H. Meng, A. Knebel and J. Caro, Angew. Chem., Int. Ed. Engl., 2018, 57, 4083–4087 CrossRef CAS PubMed.
- M. Hao, Y. Xie, X. Liu, Z. Chen, H. Yang, G. I. N. Waterhouse, S. Ma and X. Wang, JACS Au, 2023, 3, 239–251 CrossRef CAS PubMed.
- W.-R. Cui, C.-R. Zhang, W. Jiang, F.-F. Li, R.-P. Liang, J. Liu and J.-D. Qiu, Nat. Commun., 2020, 11, 436 CrossRef CAS PubMed.
- M. Hao, Z. Chen, X. Liu, X. Liu, J. Zhang, H. Yang, G. I. N. Waterhouse, X. Wang and S. Ma, CCS Chem., 2022, 4, 2294–2307 CrossRef CAS.
- C. Bai, J. Li, S. Liu, X. Yang, X. Yang, Y. Tian, K. Cao, Y. Huang, L. Ma and S. Li, Microporous Mesoporous Mater., 2014, 197, 148–155 CrossRef CAS.
- M. Carboni, C. W. Abney, S. Liu and W. Lin, Chem. Sci., 2013, 4, 2396–2402 RSC.
- Y. Yuan, B. Niu, Q. Yu, X. Guo, Z. Guo, J. Wen, T. Liu, H. Zhang and N. Wang, Angew. Chem., Int. Ed., 2020, 59, 1220–1227 CrossRef CAS PubMed.
- C. Bai, M. Zhang, B. Li, X. Zhao, S. Zhang, L. Wang, Y. Li, J. Zhang, L. Ma and S. Li, RSC Adv., 2016, 6, 39150–39158 RSC.
- M. Song, R. Cao, X. Chen, C. Wang, X. Xing, W. Li, Y. Li, Y. Liao, W. Zhong, Q. Li and Z. Liu, ACS Nano, 2023, 17, 23359–23373 CrossRef CAS PubMed.
- C. Huang, Y. Li, N. Wang, Y. Xue, Z. Zuo, H. Liu and Y. Li, Chem. Rev., 2018, 118, 7744–7803 CrossRef CAS PubMed.
- Y. Fang, Y. Liu, L. Qi, Y. Xue and Y. Li, Chem. Soc. Rev., 2022, 51, 2681–2709 RSC.
- Y. Gao, Y. Xue, L. Qi, C. Xing, X. Zheng, F. He and Y. Li, Nat. Commun., 2022, 13, 5227 CrossRef CAS PubMed.
- Y. Liu, Y. Gao, F. He, Y. Xue and Y. Li, CCS Chem., 2023, 5, 971–981 CrossRef CAS.
- Y. Gao, Y. Xue, F. He and Y. Li, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2206946119 CrossRef CAS PubMed.
- X. Luan, L. Qi, Z. Zheng, Y. Gao, Y. Xue and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202215968 CrossRef CAS PubMed.
- Y. Fang, Y. Xue, L. Hui, H. Yu and Y. Li, Angew. Chem., Int. Ed. Engl., 2021, 60, 3170–3174 CrossRef CAS PubMed.
- C. Xing, Y. Xue, X. Zheng, Y. Gao, S. Chen and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202310722 CrossRef CAS PubMed.
- Y. Xue, B. Huang, Y. Yi, Y. Guo, Z. Zuo, Y. Li, Z. Jia, H. Liu and Y. Li, Nat. Commun., 2018, 9, 1460 CrossRef PubMed.
- Q. Qi, L. Xu, J. Du, N. Yang and D. Wang, Chem. Res. Chin. Univ., 2021, 37, 1158–1175 CrossRef CAS.
- X. Sun, C. Xu, G. Tian and L. Rao, Dalton Trans., 2013, 42, 14621–14627 RSC.
- B. Li, Q. Sun, Y. Zhang, C. W. Abney, B. Aguila, W. Lin and S. Ma, ACS Appl. Mater. Interfaces, 2017, 9, 12511–12517 CrossRef CAS PubMed.
- Y. Xie, Z. Zhang, Z. Dong, R. Zhou, X. Cao, Y. Liu, B. Hu, H. Yang and X. Wang, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100510 CAS.
- Y. Yue, C. Zhang, Q. Tang, R. T. Mayes, W.-P. Liao, C. Liao, C. Tsouris, J. J. Stankovich, J. Chen, D. K. Hensley, C. W. Abney, D.-E. Jiang, S. Brown and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4125–4129 CrossRef CAS.
- J. L. Vivero-Escoto, M. Carboni, C. W. Abney, K. E. deKrafft and W. Lin, Microporous Mesoporous Mater., 2013, 180, 22–31 CrossRef CAS.
- M. Carboni, C. W. Abney, K. M. L. Taylor-Pashow, J. L. Vivero-Escoto and W. Lin, Ind. Eng. Chem. Res., 2013, 52, 15187–15197 CrossRef CAS.
- Z. Ahmad, Y. Li, J. Yang, N. Geng, Y. Fan, X. Gou, Q. Sun and J. Chen, J. Hazard. Mater., 2022, 425, 127995 CrossRef CAS PubMed.
- W. Chouyyok, C. L. Warner, K. E. Mackie, M. G. Warner, G. A. Gill and R. S. Addleman, Ind. Eng. Chem. Res., 2016, 55, 4195–4207 CrossRef CAS.
- M. J. Comarmond, T. E. Payne, J. J. Harrison, S. Thiruvoth, H. K. Wong, R. D. Aughterson, G. R. Lumpkin, K. Müller and H. Foerstendorf, Environ. Sci. Technol., 2011, 45, 5536–5542 CrossRef CAS PubMed.
- B. Ahmed, Z. Ahmad, A. Khatoon, I. Khan, N. Shaheen, A. A. Malik, Z. Hussain and M. A. Khan, Environ. Sci. Pollut. Res., 2023, 30, 103496–103512 CrossRef CAS PubMed.
- F. Sun and C. He, Chem, 2021, 7, 274–275 CAS.
- K. Yu, H. Pan, Y. Jiang, T. Zhang, H. Zhang, F. Ma, H. Song, Y. Yuan and J. Pan, Desalination, 2023, 566, 116893 CrossRef CAS.
- Y. Yuan, Q. Yu, M. Cao, L. Feng, S. Feng, T. Liu, T. Feng, B. Yan, Z. Guo and N. Wang, Nat. Sustain, 2021, 4, 708–714 CrossRef.
- D. Mei, L. Liu and B. Yan, Coord. Chem. Rev., 2023, 475, 214917 CrossRef CAS.
- G. Tian, S. J. Teat, Z. Zhang and L. Rao, Dalton Trans., 2012, 41, 11579–11586 RSC.
- H. J. Schenk, L. B. Astheimer, E. G. Witte and K. Schwochau, Sep. Sci. Technol., 1982, 17, 1293–1308 CrossRef CAS.
- A. Kausar and H. Bhatti, J. Chem. Soc. Pak., 2013, 35, 1041–1052 Search PubMed.
- D. Panias and A. Krestou, Eur. J. Miner. Process. Environ. Prot., 2004, 4, 113–129 Search PubMed.
- Z. Bai, Q. Liu, H. Zhang, J. Yu, R. Chen, J. Liu, D. Song, R. Li and J. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 18012–18022 CrossRef CAS PubMed.
- A. P. Ladshaw, S. Das, W. P. Liao, S. Yiacoumi, C. J. Janke, R. T. Mayes, S. Dai and C. Tsouris, Ind. Eng. Chem. Res., 2016, 55, 4241–4248 CrossRef CAS.
- G. A. Gill, L.-J. Kuo, C. J. Janke, J. Park, R. T. Jeters, G. T. Bonheyo, H.-B. Pan, C. Wai, T. Khangaonkar, L. Bianucci, J. R. Wood, M. G. Warner, S. Peterson, D. G. Abrecht, R. T. Mayes, C. Tsouris, Y. Oyola, J. E. Strivens, N. J. Schlafer, R. S. Addleman, W. Chouyyok, S. Das, J. Kim, K. Buesseler, C. Breier and E. D'Alessandro, Ind. Eng. Chem. Res., 2016, 55, 4264–4277 CrossRef CAS.
- R. Liu, S. Wen, Y. Sun, B. Yan, J. Wang, L. Chen, S. Peng, C. Ma, X. Cao, C. Ma, G. Duan, S. Shi, Y. Yuan and N. Wang, Chem. Eng. J., 2021, 422, 130060 CrossRef CAS.
- S. P. Kelley, P. S. Barber, P. H. K. Mullins and R. D. Rogers, Chem. Commun., 2014, 50, 12504–12507 RSC.
- B. F. Parker, S. Hohloch, J. R. Pankhurst, Z. Zhang, J. B. Love, J. Arnold and L. Rao, Dalton Trans., 2018, 47, 5695–5702 RSC.
- N. Mehio, J. C. Johnson, S. Dai and V. S. Bryantsev, Phys. Chem. Chem. Phys., 2015, 17, 31715–31726 RSC.
- D. Wang and S. A. Sañudo-Wilhelmy, Mar. Chem., 2009, 117, 52–58 CrossRef CAS.
- N. Mehio, A. S. Ivanov, A. P. Ladshaw, S. Dai and V. S. Bryantsev, Ind. Eng. Chem. Res., 2016, 55, 4231–4240 CrossRef CAS.
- G. I. Park, H. S. Park and S. I. Woo, Sep. Sci. Technol., 1999, 34, 833–854 CrossRef CAS.
- E. S. Ilton, Z. Wang, J.-F. Boily, O. Qafoku, K. M. Rosso and S. C. Smith, Environ. Sci. Technol., 2012, 46, 6604–6611 CrossRef CAS PubMed.
- K. Bruland and M. Lohan, Oceans Mar. Geol., 2003, 6, 23–48 Search PubMed.
- N. Mehio, B. Williamson, Y. Oyola, R. T. Mayes, C. Janke, S. Brown and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4217–4223 CrossRef CAS.
- A. S. Ivanov, C. J. Leggett, B. F. Parker, Z. Zhang, J. Arnold, S. Dai, C. W. Abney, V. S. Bryantsev and L. Rao, Nat. Commun., 2017, 8, 1560 CrossRef PubMed.
- K. Sekiguchi, K. Saito, S. Konishi, S. Furusaki, T. Sugo and H. Nobukawa, Ind. Eng. Chem. Res., 1994, 33, 662–666 CrossRef CAS.
- S. C. Katiyar and S. S. Bajaj, Nucl. Eng. Des., 2006, 236, 881–893 CrossRef CAS.
- M. Chen, T. Liu, X. Zhang, R. Zhang, S. Tang, Y. Yuan, Z. Xie, Y. Liu, H. Wang, K. V. Fedorovich and N. Wang, Adv. Funct. Mater., 2021, 31, 2100106 CrossRef CAS.
- H. Wang, T. Xu, B. Zheng, M. Cao, F. Gao, G. Zhou, C. Ma, J. Dang, W. Yao, K. Wu, T. Liu, Y. Yuan, Q. Fu and N. Wang, J. Hazard. Mater., 2022, 433, 128789 CrossRef CAS PubMed.
- Y. Ren, H. Liu, X. Liu, Y. Zheng, Z. Li, C. Li, K. W. K. Yeung, S. Zhu, Y. Liang, Z. Cui and S. Wu, Cell Rep. Phys. Sci., 2020, 1, 100245 CrossRef.
- T. Wei, Q. Yu and H. Chen, Adv. Healthcare Mater., 2019, 8, 1801381 CrossRef CAS PubMed.
- J. Wen, Q. Li, H. Li, M. Chen, S. Hu and H. Cheng, Ind. Eng. Chem. Res., 2018, 57, 1826–1833 CrossRef CAS.
- H. Ma, F. Zhang, Q. Li, G. Chen, S. Hu and H. Cheng, RSC Adv., 2019, 9, 18406–18414 RSC.
- J.-W. Liou and H.-H. Chang, Arch. Immunol. Ther. Exp., 2012, 60, 267–275 CrossRef CAS PubMed.
- X. Chen, N. Yang, Y. Wang, H. He, J. Wang, J. Wan, H. Jiang, B. Xu, L. Wang, R. Yu, L. Tong, L. Gu, Q. Xiong, C. Chen, S. Zhang and D. Wang, Adv. Mater., 2022, 34, 2107400 CrossRef CAS PubMed.
- X. Chen, P. Li, J. Wang, J. Wan, N. Yang, B. Xu, L. Tong, L. Gu, J. Du, J. Lin, R. Yu and D. Wang, Nano Res., 2022, 15, 4117–4123 CrossRef CAS.
- X. Chen, S. Liu, N. Yang, R. Yu and D. Wang, EcoMat, 2023, 5, e12348 CrossRef.
- J. Wang, J. Wan and D. Wang, Acc. Chem. Res., 2019, 52, 2169–2178 CrossRef CAS PubMed.
- X. Lai, J. Li, B. A. Korgel, Z. Dong, Z. Li, F. Su, J. Du and D. Wang, Angew. Chem., Int. Ed., 2011, 50, 2738–2741 CrossRef CAS PubMed.
- Z. Wang, N. Yang and D. Wang, Chem. Sci., 2020, 11, 5359–5368 RSC.
- P. Cheng, L. Sun, L. Feng, S. Yang, Y. Yang, D. Zheng, Y. Zhao, Y. Sang, R. Zhang, D. Wei, W. Deng and K. Han, Angew. Chem., Int. Ed., 2019, 58, 16087–16091 CrossRef CAS PubMed.
- Y. Wei, N. Yang, K. Huang, J. Wan, F. You, R. Yu, S. Feng and D. Wang, Adv. Mater., 2020, 32, 2002556 CrossRef CAS PubMed.
- X. Zhao, M. Yang, J. Wang and D. Wang, Chem. Res. Chin. Univ., 2023, 39, 630–635 CrossRef CAS.
- J. Zhao, M. Yang, N. Yang, J. Wang and D. Wang, Chem. Res. Chin. Univ., 2020, 36, 313–319 CrossRef CAS.
- W. Han, Y. Wang, J. Wan and D. Wang, Chem. Res. Chin. Univ., 2022, 38, 117–122 CrossRef CAS.
- M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347–4390 CrossRef CAS PubMed.
- J. Park, G. A. Gill, J. E. Strivens, L.-J. Kuo, R. T. Jeters, A. Avila, J. R. Wood, N. J. Schlafer, C. J. Janke, E. A. Miller, M. Thomas, R. S. Addleman and G. T. Bonheyo, Ind. Eng. Chem. Res., 2016, 55, 4328–4338 CrossRef CAS.
- H. Li, J. Wen and X. Wang, Chin. Sci. Bull., 2018, 63, 481–494 CrossRef.
- J. Hu, H. Ma, Z. Xing, X. Liu, L. Xu, R. Li, C. Lin, M. Wang, J. Li and G. Wu, Ind. Eng. Chem. Res., 2016, 55, 4118–4124 CrossRef CAS.
- M. N. Haji, J. A. Drysdale, K. O. Buesseler and A. H. Slocum, Environ. Sci. Technol., 2019, 53, 2229–2237 CrossRef CAS PubMed.
- B. F. Parker, Z. Zhang, L. Rao and J. Arnold, Dalton Trans., 2018, 47, 639–644 RSC.
- J. Guidez and S. Gabriel, EPJ Nucl. Sci. Technol., 2016, 2, 10 CrossRef.
- X. Chen, S. Liu, N. Yang, R. Yu and D. Wang, EcoMat, 2023, 5, e12348 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |