Theoretical investigation of synergistically boosting the anchoring and electrochemical performance of lithiophilic/sulfiphilic transition metal carbides for lithium–sulfur batteries†
Mingyang
Wang
ab,
Jianjun
Mao
c,
Yudong
Pang
a,
Xilin
Zhang
a,
Zongxian
Yang
 *a,
Zhansheng
Lu
*a,
Zhansheng
Lu
 *a and
Shuting
Yang
*bd
*a and
Shuting
Yang
*bd
aSchool of Physics, Henan Normal University and Henan Key Laboratory of Photovoltaic Materials, Xinxiang, Henan 453007, People's Republic of China. E-mail: yzx@htu.edu.cn; zslu@htu.edu.cn
bHenan Battery Research Institute, Xinxiang, Henan 453007, People's Republic of China. E-mail: yangshuting17@163.com
cDepartment of Chemistry, The University of Hong Kong, Pok Fu Lam Road, Hong Kong, People's Republic of China
dSchool of Chemistry and Chemical Engineering Science, Henan Normal University, Xinxiang, Henan 453007, People's Republic of China
First published on 28th November 2023
Abstract
Lithium–sulfur (Li–S) battery is one of the most promising next-generation energy-storage systems with a high energy density and low cost. However, their commercial applications face several challenges, such as the shuttle effect caused by the soluble lithium polysulfide (LiPSs) intermediates and the sluggish sulfur redox reaction. In this article, we systematically investigated the anchoring and electrochemical performance of a series of transition metal carbides (TMCs: TiC, VC, ZrC, NbC, HfC, TaC) as cathode materials for Li–S batteries by theoretical calculations. The lithiophilic/sulfiphilic non-polar (001) surfaces of TMCs can offer moderate binding strength with LiPS intermediates, ensuring good performance of sulfur immobilization. These TMCs can also facilitate lithium diffusion, indicating the good rate performance of Li–S batteries. We also demonstrated that the studied TMCs can be classified into two classes according to their catalytic activity for Li2S decomposition which originated from their different electronic structural features. Furthermore, TiC, ZrC, and HfC exhibited excellent bifunctional electrochemical activity through reducing the Gibbs free energy for sulfur reduction reactions (SRRs) and lowering the barrier for Li2S decomposition which facilitates accelerating electrode kinetics and elevating utilization of sulfur. Our results offer a systematic approach to designing and screening non-polar materials for high-performance Li–S batteries, based on the rational electronic structure and lattice match strategy.
1. Introduction
The increasing demand for energy storage devices in the flourishing sustainable fields of electric vehicles, portable electronic devices, and the large-scale storage of renewable energy has driven the development of high-performance rechargeable batteries. In the last few decades, lithium–sulfur (Li–S) batteries have attracted significant attention and experienced rapid growth because of their extraordinary theoretical energy density, cost-effectiveness and environmentally friendly nature, and are recognized as next-generation energy storage devices.1–4 However, the commercial application of Li–S batteries is still hindered by some challenges, including the insulating nature of sulfur and lithium sulfides which will block the electron transfer, significant volume changes of sulfur materials during charging and discharging at the cathode, and the dissolution of intermediate lithium polysulfides (LiPSs) in the organic electrolyte during the cycling process.5–7 The issues mentioned above will lead to the continual loss of active materials, fast capacity decay, low coulombic efficiency, severe self-discharge, and a poor battery lifespan.8An omni-directional strategy has been considered to address the aforementioned issues of Li–S batteries, e.g. including (1) a blocking layer to block up the LiPSs’ shuttling path;9,10 (2) a conductive matrix to promote electron transport;11,12 (3) anode-protective additives to prevent parasitic reactions between LiPSs and the lithium metal anode;13,14 (4) novel electrolyte configurations to regulate the dissolution and adsorption of LiPSs;15,16 (5) a confinement framework to mitigate the S volume expansion during lithiation and suppress the dissolution of LiPSs;17,18 (6) electrocatalyst materials to improve the decomposition kinetics of Li2S;19,20 (7) cathode anchoring materials to enhance the adsorption ability for LiPSs.21,22
The transformation of long-chain LiPSs to short-chain sulfides contributes to the discharge capacity of Li–S batteries mainly. Therefore, the shuttle effect caused by the dissolution of long-chain LiPSs into the electrolyte has attracted particular attention. To address this problem, the most prevalent solution is to design and search for new cathode anchoring materials to confine the LiPSs and improve sulfur utilization. Carbon materials were selected due to their porous structure, large surface area, and excellent electronic conductivity.23–25 However, the non-polar carbon surface could not offer moderate interactions with polar LiPSs to eliminate the shuttle effect completely. Meanwhile, polar materials, such as oxides and sulfides, show an excellent performance in suppressing the shuttle effect for the stronger polar interactions.11,26,27 Unfortunately, the poor electrical conductivity of these materials leads to the slow conversion of these adsorbed LiPSs. Previous investigations on anchoring LiPSs have made significant progress but cannot restrain the shuttle effect. In addition, catalysts such as sulfides, oxides, and heterostructures were recently incorporated into Li–S batteries to alleviate the shuttle effect by accelerating the conversion kinetics of LiPSs.19,20,28,29 Initially, adsorption and catalysis were only regarded as modulation methods separately. Recently, some research indicated that adsorption and catalysis coexist with each other, and both of them work together to enhance the electrochemical properties of Li–S batteries.19,30
Because of the strongly sulfiphilic surface moieties, carbides always serve as anchoring materials to absorb LiPSs effectively. Transition metal carbides (TMCs) have been of great concern for a long time owing to their remarkable physical properties (e.g. extreme hardness, high melting points, and excellent electrical and thermal conductivity) and intriguing surface chemistry (as potential alternatives to noble-metal catalysts).31,32 Undoubtedly, the unique combination of these properties originates from the electronic structure of TMCs, resulting in strong chemical bonds and interesting catalytic performance. Some previous experimental and theoretical studies verified that TMCs have been used as electrode materials for the oxygen reduction reaction, oxygen evolution reaction, hydrogen evolution reaction, CO oxidation reaction, NO reduction reaction, and water gas shift reaction in the field of fuel cells and related electrocatalytic applications.33–37 TMCs also show the potential in improving the performance of sulfur cathodes, and some studies have shown that TMCs can boost the cycle life and stability of the sulfur cathode, even with high sulfur loading.38–43 On the other hand, transition metal atoms in TMCs exhibit high catalytic activity for the conversion of LiPSs. Recently, there has been growing interest among researchers in utilizing carbide materials to address the shuttle effect and enhance the overall performance of Li–S batteries. Combining the experimental and theoretical methods, Peng et al. concluded that conductive TiC had strong interactions with LiPSs and played a crucial role in the redox kinetics of Li–S batteries.38 Kim et al. showed that tungsten carbides (WC) can not only catalyze the disproportion of polysulfides, but also capture soluble LiPSs.41
Nevertheless, as far as we are concerned, a systematic theoretical understanding of the roles of TMCs in anchoring and conversion of LiPSs is still lacking. Inspired by the exciting experimental discoveries and theoretical predictions, the adsorption ability toward LiPSs, lithium diffusion behavior, the catalyst activation for Li2S decomposition and the electrochemical performance for sulfur reduction reactions on a series of TMC surfaces have been systemically examined by first-principles calculations. The correlation between the electronic structure and the adsorption–catalytic behavior of the TMC surfaces has also been investigated. Our results show that all the non-polar TMC surfaces we investigated possess a moderate interaction with LiPSs, indicative of a remarkable sulfur immobilization, and the binding strength of LiPSs is sensitively dependent on the Li–C and S–TM interaction and the lattice constants of TMCs. In particular, the fast lithium diffusion and low activation energy barriers of Li2S decomposition on the TMC surface benefit the rate performance and energy efficiency of Li–S batteries. Consequently, the different surface behaviors mentioned before are directly dependent on the intrinsic electronic structure characteristics of TMCs. We finally concluded that substituting the metal components in TMCs could regulate their electronic structure properties, and thus influence the adsorption–catalytic reactivity of such materials. Our findings provided the guidance for the use of non-polar TMC surfaces as the possible sulfur host for long cycling stability and promoted the conversion of LiPSs in Li–S batteries.
2. Models and computational methods
Spin-polarized calculations were carried out using the density functional theory (DFT) method as implemented in the Vienna ab initio simulation package (VASP).44 The exchange–correlation functional with generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE) combined with the projector-augmented wave (PAW) method was used to solve the Kohn–Sham equations.45–47 The Kohn–Sham orbital has been expanded using a plane-wave basis set with an energy cutoff of 550 eV. The DFT-D3 method was used to accurately describe the van der Waals (vdWs) interaction between LiPSs and TMCs.48 The Brillouin zone was sampled using a Γ-centered 5 × 5 × 1 k-point mesh with the Monkhorst–Pack49 sampling method during geometry optimization, while a 15 × 15 × 1 mesh was used for electronic structure calculation. The climb-image nudged elastic band (CI-NEB) method50 with five images was used to calculate the energy barriers and the diffusion pathways. More computational details are included in the ESI.†3. Results and discussion
3.1 Geometric and electronic structure of the TMC (001) surface
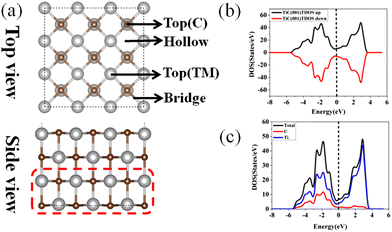
First, the geometric and electronic structures of TiC, VC, ZrC, NbC, HfC, and TaC (the IVB and VB transition metal based carbides) surfaces were investigated systematically. The bulk TMCs have a cubic NaCl structure with a space group of Fm3m, in which each anion (cation) is surrounded by six cations (anions). The basic information of the optimized bulk TMCs is presented in Table S1,† which agrees with the previous results.51–55 The non-polar flat (001) surfaces of the TMCs were selected as the substrate, which has been proven to be the most stable cleavage surface of TMCs among the low-index surfaces theoretically and experimentally.56,57 Simultaneously, the thermal stability of the (001) surface of TMCs was confirmed by the ab initio molecular dynamic (AIMD) simulation in the canonical (NVT) ensemble with the time step set at 1 fs and temperature at 500 K for 10 ps and the detailed information can be seen in Fig. S1.† To investigate the (001) surface of TMCs, the TiC (001) surface was selected as an example due to their similar structures. The optimized TiC (001) surface, along with the possible adsorption sites, is displayed in Fig. 1a. The calculated bond lengths of TM–C, TM–TM and C–C in TMC (001) are shown in Table S1.† The TM–C bond lengths range from 2.084 to 2.356 Å, comparable to the Li–S bond length in LiPSs. The total and projected electronic density of states (DOS) plots are shown in Fig. 1b–c and Fig. S2,† respectively. Spin-polarized calculations were performed for all the systems. The symmetrical characteristics of the DOS plots for the spin-up and spin-down channels shown in Fig. 1b and the upper area of Fig. S2† reveal the nonmagnetic behavior of the TMCs. Moreover, all the TMC surfaces exhibit metallic features with a continuum of energy states and finite DOS at the Fermi energy. As shown in Fig. 1c and the lower area of Fig. S2,† both the TM and C atoms contribute to the finite DOS at the Fermi level. The excellent electronic conductivity of TMCs could address the low conductivity of sulfur and thus improve the electrochemical performance of Li–S batteries.3.2 The adsorption of S8 and LiPSs on the TMC (001) surface
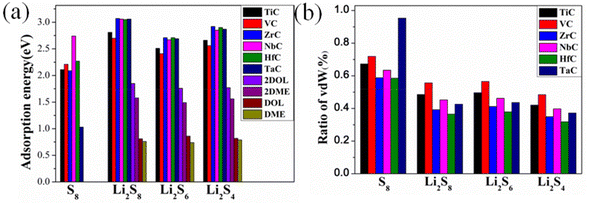
During the discharge/charge process, S8 and Li2S were reduced/oxidized into a series of LiPSs through the incorporation/separation of additional Li. Adsorption energy between the soluble LiPSs with the anchoring material is an important parameter to judge whether the shuttle effect can be suppressed effectively. To reveal the role of TMCs as effective anchoring materials for Li–S batteries, the adsorption behaviors of LiPSs on the TMC (TiC, VC, ZrC, NbC, HfC, TaC) surfaces were investigated systematically. The optimized geometry structures and the bond lengths of S8 and LiPSs are shown in Fig. S3(a) and Table S2,† which are in good accordance with the previous calculations.58 The preferred adsorption configurations of S8 and LiPSs on the (001) facets of the above six TMCs are shown in Fig. 2 and Fig. S4–8,† which were evaluated from different adsorption sites and initial molecular orientations. The adsorption energy (Eads) of LiPSs (or S8) on TMCs was evaluated by eqn (1) (in the ESI†) and shown in Fig. 3a.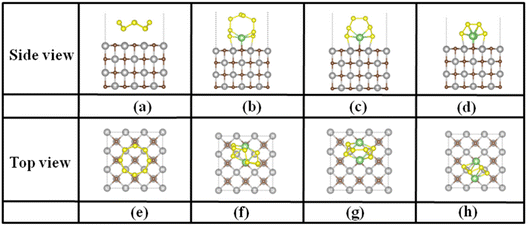 | ||
| Fig. 2 The top and side views of the optimized adsorption structures of (a) and (e) S8, (b) and (f) Li2S8, (c) and (g) Li2S6, (d) and (h) Li2S4, on the TiC (001) surface, respectively. | ||
S8 maintains a parallel adsorption configuration without any obvious deformation but with unconventional van der Waals adsorption (Eads: ∼2.0 eV, adsorption distances: 2.60–2.84 Å) as shown in Fig. 2 and Fig. S4–7.† Four bottom S atoms of S8 were atop TM atoms nonetheless, which will lead to the dissociation of S8 on TaC. The stable adsorption configuration of S8 was observed on the top of C atoms (as shown in Fig. S8a and e†). With regard to the adsorption of LiPSs, similar bonding characteristics with TMCs were obtained. Taking the TiC (001) surface as an example, the LiPSs exhibit a standing-up style with two Li atoms of LiPSs interacting with the surface C atoms to form Li–C bonds, and two S atoms of the LiPSs forming bonds with surface TM atoms, respectively (Fig. 2). Due to the rock–salt structure of the TMCs, LiPS was adsorbed on the TiC (001) substrate with a C2 symmetry. In addition, the Eads values ranging from 2.11 to 2.81 eV were found for S8 and LiPSs on the TiC (001) surface which indicated that TMCs could offer moderate adsorption energies larger than those on graphene and transition metal sulfides (TMSs), similar to those on transition metal nitrides (TMNs) and transition metal oxides (TMOs).59,60 As is known, for LiPSs on TMSs, only the TM or S atoms in TMSs are involved in the interactions. However, on TMCs, both the TM and the C atoms are involved in the interactions with LiPSs, which are similar to those on the TMNs and TMOs, thus endowing stronger binding strength. According to the results, the TMCs can effectively suppress the LiPS shuttling effect in Li–S batteries. The adsorption parameters of LiPSs on different TMCs are summarized in Tables S3–11,† respectively. Furthermore, considering that heavy elements such as Hf and Ta were involved in the studied TMC substrates. The DFT+U correction was used to evaluate the adsorption energies of LiPSs with the HfC, which was taken as an example for testing. We observed a slight change in the adsorption energies of LiPSs, while the trend in adsorption energies is well preserved, as illustrated in Table S12.† Hence, we believe that the chosen PBE/GGA is sufficient for elucidating the adsorption properties of heavy elements involved TMCs to LiPSs.
In order to prevent soluble LiPS dissolution into the electrolyte, stronger interactions between LiPSs and anchoring materials than those of solvent electrolyte molecules are essential. For this purpose, the adsorption of soluble LiPSs on the commonly used electrolyte solvents such as 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) was investigated. To describe the solubility characteristics of LiPSs more accurately, the binding of LiPSs with two DOL or DME molecules was also simulated. The most favorable configurations and adsorption energies of LiPSs on DOLs and DMEs are shown in Fig. S3(b–c),†Fig. 3(a) and Table S13.† The results demonstrated in Fig. 3(a) indicate that the adsorption energies of LiPSs on all TMC substrates are much higher than those of LiPSs on one or two electrolyte molecules (DOL&DME or 2DOL&2DME). Therefore, we could speculate that the shuttle effect could be well alleviated.
The difference in the Eads of LiPSs on the surface of TMCs propels us to explore the mechanism of the anchoring effect. First, we analyzed the geometric features of LiPSs adsorbed on TMCs. As mentioned, both the Li–C and S–TM interactions contribute to the Eads of LiPSs on the TMC substrate. For different LiPSs, as shown in Tables S9–11,† we can see that the Li atoms are found closer to the TMCs than the S atoms, and the shortest distance between Li in LiPSs and the surface decreases with decreasing number of S atoms. For a certain LiPS, taking Li2S8 adsorption as an example, the Li–C bond lengths are about 2.17 Å on TMCs. In comparison, S–TM bond lengths differ significantly from 2.55 to 2.70 Å for different TMCs, indicating that the S–TM interaction caused the difference in the Eads values for different TMC interactions. The results are consistent with the previous research, which indicated that the interaction between sulfur-containing species and the anchoring materials could be dramatically enhanced by introducing TMs.61,62 In summary, the improved interaction of non-polar (001) surfaces was attributed to lithiophilic and sulfiphilic characteristics of TMCs (i.e. the co-formation of S–TM and Li–C interaction).
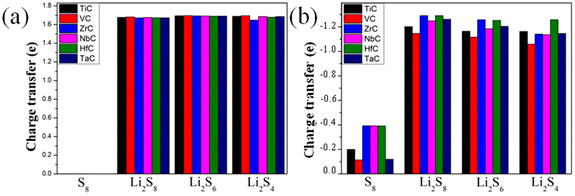
To understand the adsorption mechanism of the TMC surfaces, the contributions of the vdWs and chemical interactions were evaluated by eqn (2) (in the ESI†) and visually illustrated in Fig. 3b. For the adsorption of S8 on all TMCs, vdW interaction accounts for 58.59–94.38%. This phenomenon implies that chemical interaction contributes to the adsorption mechanism (except for the TaC surface). With the lithiation of S8, the chemical interaction increased simultaneously. As a result, the ratio of vdW interaction between LiPSs and TMCs became smaller from of 31.88 to 56.53%. In summary, the anchoring behaviors were exhibited through a combination of vdW and chemical interaction for both S8 and LiPSs, and the chemical interactions increased with deeper lithiation. To obtain further insights into the LiPS binding mechanisms, charge transfer and charge density difference (CDD) analyses were performed for LiPS adsorption on the TMCs. We considered the charge transfer of lithium and sulfur separately because both TM and the nonmetallic(C) atom were involved in the chemical interactions with S and Li in the LiPSs. The Li atoms lost charge and the S atoms obtained charge during the interaction process, and the charge transfer values are displayed in Fig. 4 and Table S14.† All of the LiPS adsorption systems exhibit similar charge transfer values, ranging from 1.67 to 1.69 e for Li atoms and from 1.05 to 1.29 e for S atoms. Comparatively, for the LiPS adsorption on the VC surface, the charge transfer of S is smaller than those on other TMCs, which demonstrates the weaker chemical interactions of LiPSs with VC (as shown in Fig. 3). Plenty of electrons were exchanged between the LiPSs with the TMC hosts, which was consistent with the moderate adsorption energy of the LiPSs. Specifically, this strong interaction is attributed to the cooperation of Li–C and S–TM chemical bonds.
 | ||
| Fig. 4 Charge transfer between the TMCs with Li atoms (a) and S atoms (b) in LiPSs which are in contact with TMCs. | ||
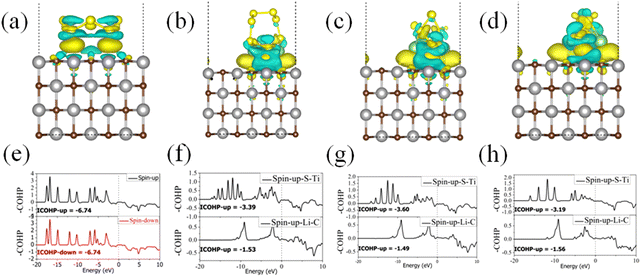
To visualize the charge transfer characteristics, we performed CDD analysis for the adsorption of S8 and LiPSs on TMCs, and the results are presented in Fig. 5a–d and Fig. S9–13,† respectively. For the adsorption of S8, besides the charge transfer that occurred within S8, electron-rich (yellow) regions appeared between S8 and the TMC surfaces. The CDD results further explained the stronger Eads of S8 and the smaller adsorption distance than the conventional vdW adsorption strategy. A more detailed analysis of the crystal orbital Hamilton population (COHP) for the S and TM was conducted, and the results are shown in Fig. 5(e–h). The COHP and the corresponding integration values can efficiently measure the bonding strength of covalent bonds. Quantitative analyses based on COHP confirm the charge transfer analysis and CDD results. For the TiC surface, the spin-up and spin-down bands contribute equally to the four S–Ti bonds with a total integrated COHP (ICOHP) of −6.74. Thus, it can be concluded that there exists chemical covalent bonds between S8 and the TiC (001) surface, resulting from the S–Ti interaction. Combined with charge transfer, CDD and COHP analyses, we can conclude that both vdW interaction and chemical interaction exist between S8 and the TMC surface, except for the TaC surface. The CDD figures illustrate the accumulation of a great amount of charges between the LiPSs and the TMCs. Besides, lots of charges accumulated between the Li and C atoms, as well as between the S and TM atoms, which corroborates that both Li and S participated in a strong chemical interaction. The observed charge depletion and accumulation in the analysis are consistent with the charge transfer values from the Bader charge analysis. The COHP states of S–TM and Li–C have been plotted in Fig. 5(f–h) for the adsorption of Li2S8, Li2S6, Li2S4 on TiC (001) surfaces. The calculated ICOHP values of S–Ti interaction for different LiPS adsorption systems range from −3.60 to −3.19 (only for the spin-up state), which indicates the strong covalent interaction of S–Ti. According to the charge transfer analysis, a plenty of charge was transferred from Li to C, indicating a strong interaction between Li and C atoms. However, the ICOHP of Li–C interactions for different LiPS adsorption systems are relatively large. According to the basic theory of COHP, the COHP mainly measures the covalent bonding interaction. Therefore, the large ICOHP values for the ionic bonds are understandable. Combined with the large charge transfer from Li to C and the large ICOHP values for the Li–C bonds, we can conclude that the Li–C interaction belongs to ionic bonding. Overall, the strong interaction between LiPSs and TMCs mainly originated from the fact that both Li and S in LiPSs were involved in the interaction, resulting in the formation of strong Li–C ionic and S–TM covalent bonds. In contrast to most anchoring materials, the chemical interactions between the LiPSs and the non-polar (001) surface of TMCs are unique. Typically, for most materials, only one kind of atom in the anchoring materials participates in bonding with Li/S atoms in LiPSs. However, for the non-polar (001) surface of TMCs, both Li–C and TM–S contribute to the adsorption effect, resulting in a portion of the chemical interactions contributed by metal–S bonding in addition to Li–C bonding. As a result, the chemical interactions of LiPSs with the (001) surface of TMCs were stronger than some other anchoring materials.
As known, lattice match between the adsorbate and substrate is one of the important factors in adsorption interaction.63 Previous works have proved that the lattice constants of the substrate influence the adsorption of the adsorbate.64 Here, the correlation between the lattice constants and the anchoring ability of TMCs was further explored. As shown in Tables S1 and S9,† there exists a monotonic relationship between the binding energy of Li2S8 and the lattice constants of these TMCs. Importantly, we found that with increasing lattice constants of TMCs, the anchoring ability to LiPSs increased monotonically. However, there is an anomalous result for TaC and NbC, since both the electronegativity of the TM and the lattice constants of TMCs contribute to the adsorption energy, and electronegativity plays an essential role in this case. Although the lattice constant of NbC is a little larger than TaC, the electronegativity of Nb is larger than Ta which implies that it would be more difficult for the S atom to get electrons from the substrate, resulting in a weaker S–Nb bond. In brief, for the NbC and TaC systems, the electronegativity of the TM and lattice constants may have synergistic effects on adsorption energy of LiPSs, which leads to the non-monotonic change of adsorption energy with the lattice constant.
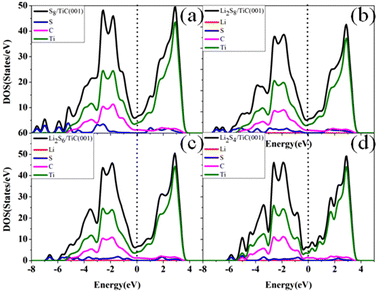
An excellent electronic conductivity of the sulfur hosts could make up the intrinsically insulative nature of sulfur and LiPSs which can further improve the performance of Li–S batteries. Here, the total and projected density of states of S8 and LiPSs adsorbed on the surface of TMCs were calculated to probe the conductive behavior of the systems and the results are shown in Fig. 6 and Fig. S14–18.† The figures demonstrate that although the sulfur-containing species are involved, a considerable amount of electron states exist at the Fermi level in the case of LiPSs adsorbed on TMCs. These results indicate that all the six TMCs can maintain their excellent metallic behavior during discharging and charging processes. Moreover, the metallic behavior of LiPSs adsorbed on TMCs originated from the abundance of electronic states at the Fermi energy level stemming from the d states of TM. Hence all the adsorbed systems possess good electrical conductivity mainly owing to the contribution of electrons from TM atoms, which is essential to expedite electrochemical reactions in Li–S battery systems.
 | ||
| Fig. 6 The total and projected density of states of S8 (a), Li2S8 (b), Li2S6 (c), and Li2S4 (d) on the TiC (001) surface, respectively. | ||
3.3 The diffusion of Li on the TMC (001) surface
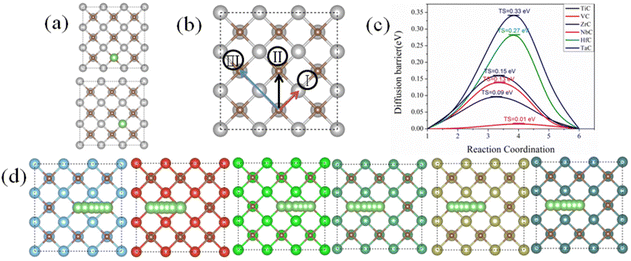
In Li–S batteries, the Li-ion diffusion plays a critical role in transforming of LiPS species and the nucleation/decomposition of Li2S. What is more, the rate capability of the Li–S batteries is also determined mainly by the adsorption and diffusion behavior of Li on the surface of the electrode materials. Therefore, the kinetic performance of TMCs acting as the sulfur hosts was further investigated by calculating the adsorption and diffusion barrier of lithium ions on TMCs. The diffusion barriers and pathways of Li atoms on the surface of TMCs were analyzed using the climbing image nudged elastic band (CI-NEB) method. Herein, the stable adsorption sites, diffusion paths of lithium ion on the (001) facet of TMCs and the minimum energy path (MEP) of Li-ion diffusion are presented in Fig. 7. All the possible adsorption sites for Li ion adsorption were checked carefully, and the detailed test data are listed in Table S15.† The results show that both the top sites of C and TM atoms are the stable adsorption sites, and the C-top is the most stable adsorption site. Therefore, the diffusion paths of Li-ions were designed from one of the C-top sites to another diagonally adjacent C-top site. There are three possible diffusion paths (as shown in Fig. 7b), i.e. Path I with the Li ion crossing the bridge site to the TM-top site, Path II with the Li ion crossing the hollow area constructed by two carbon atoms and TM atoms, and Path III with the Li ion crossing the top of the TM atoms. The calculation results show that for all TMC surfaces Path II has a relatively lower diffusion barrier. The diffusion barriers of Li ions on TiC, VC, ZrC, NbC, HfC and TaC are 0.09, 0.01, 0.33, 0.13, 0.27 and 0.15 eV, respectively, which are all smaller than the diffusion barrier of 0.30 eV on graphene,65 except that on ZrC. Therefore, we conclude that the studied TMCs as host materials could facilitate the diffusion of Li ions, which improves the comprehensive performance of Li–S batteries.3.4 The decomposition of Li2S on the TMC (001) surface
Li2S is the final discharge product in the discharge process of Li–S batteries. However, the low electronic conductivity and high decomposition energy of Li2S lead to a high overpotential and low rate capability. An excellent cathode catalyst that can reduce the decomposition barrier of Li2S could enhance the overall reaction kinetics of the Li–S batteries. Therefore, the kinetic performance of TMCs acting as the sulfur hosts was further investigated by calculating the activation energy barriers of Li2S decomposition on TMCs. Herein, the CI-NEB method was adopted to study the decomposition of Li2S on the surface of TMCs. As shown in Fig. 8 and Fig. S19,† the overall decomposition process of the Li2S molecule on different TMCs was considered as an intact Li2S molecule decomposed into a LiS cluster and a single Li ion. The decomposition barriers of Li2S on TiC, VC, ZrC, NbC, HfC and TaC are 0.57, 1.12, 0.65, 1.13, 0.64, and 1.10 eV, respectively. In contrast to the decomposition barriers of the natural free molecule Li2S (3.39 eV) and that of graphene (1.81 eV),58 all the TMCs have a remarkable catalytic activity in the decomposition of the Li2S molecule. Significantly, TiC, ZrC, and HfC exhibit the most excellent catalytic ability to promote the decomposition of Li2S effectively. According to the results, the decomposition processes of the Li2S molecule on the TMC surface fall into two categories, which are attributed to the different Li2S adsorption structures, as shown in Fig. 8. The adsorption of Li2S on TiC, ZrC, and HfC (IVB transition metals based carbides) surfaces were achieved by the S atom in Li2S bonding with the C atom in the surface. However, the adsorption of Li2S on VC, NbC, and TaC (VB transition metal based carbides) surfaces was achieved by the S atom in Li2S bonding with the transition metal (TM) atom in the surface. The underlying bonding and decomposition mechanism of Li2S on the TMC surface was further investigated via electronic structure analysis. Since Ti, Zr, and Hf are in the same group (e.g. IVB) of the periodic table with the same number of electrons in the outermost partially filled atomic orbital shell, similar adsorption structures were obtained. V, Nb, and Ta are in the VB of the periodic table with the same number of electrons, which have one more valence electron than the transition metals in IVB. For Li2S adsorption, the Li atoms in all systems bond by donating electrons to the nonmetal atom (carbon atom) of the surfaces. However, the S atom in Li2S is bonding by accepting electrons from the surface atom. The V, Nb, and Ta in the VB group have one more valence electron than Ti, Zr, and Hf in the IVB group, which can more easily donate electrons to the S atom to form the S–TM bond. In contrast, on the TiC, ZrC, and HfC surfaces the S atom gets electrons from the C atom to create a strong S–C bond. As shown in Tables S3–8,† after the adsorption of Li2S, the Li–S bonds were elongated. It should be noted that, on the TiC, ZrC, and HfC surfaces, the Li–S bond length increased by 0.33, 0.44, and 0.42 Å, respectively. Nevertheless, the Li–S bond length only increased by 0.11, 0.10 and 0.11 Å on the VC, NbC, and TaC surfaces. Therefore, the strong S–C bond weakens the Li–S bond in the Li2S molecule, which leads to smaller decomposition barriers. The Bader charge analyses were performed for Li2S adsorption on the TMCs. As shown in Table 1, for the adsorption systems of Li2S on TiC, ZrC, and HfC, the substrates transferred more electrons to Li2S than those in the VC, NbC, and TaC adsorption systems, which indicates a more vital chemical interaction between them. To reveal the origin of the above phenomena, the electronic properties of these TMC surfaces were calculated. The calculated total and partial density of states (DOS) plots illustrated in Fig. 9 were used to analyze the unique electronic structures for the non-polar (001) surface of TMCs, accordingly. The total DOS plots of two kinds of surfaces exhibit a significantly distinct peak at the Fermi levels with the TiC group peak featuring hybridization of metal Ti and nonmetal C states. In contrast, the case of the VC group has a clear majority of metal TM states. In addition, Bader charge analysis predicts charge transfer from TM to nonmetal C, and the charges of IVB metal (Ti, Zr, and Hf) in TMCs are slightly higher than those of the VB metal (V, Nb, and Ta) in TMCs due to the difference of electronegativity and electronic structure of the two groups of TM elements. In short, a fundamental difference between the two kinds of surfaces is their highest occupied levels with TiC (001) predominantly of nonmetal (C) nature and VC (001) primarily of metal (TM) nature.66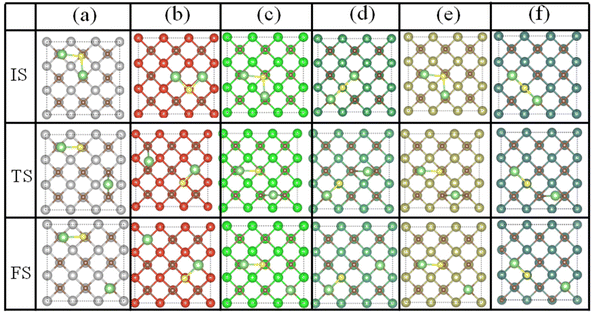 | ||
| Fig. 8 The optimized initial state (IS), transition state (TS), and final state (FS) of Li2S decomposition on the (a) TiC, (b) VC, (c) ZrC, (d) NbC, (e) HfC, and (f) TaC (001) surfaces, respectively. | ||
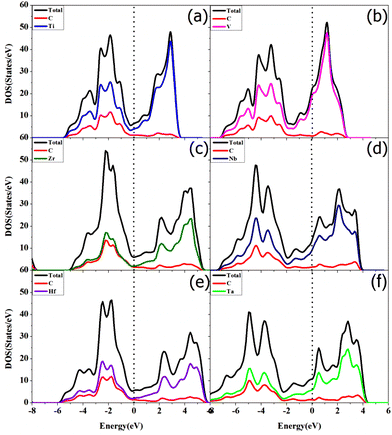 | ||
| Fig. 9 The partial density of states of (a) TiC, (b) VC, (c) ZrC, (d) NbC, (e) HfC, and (f) TaC (001) surfaces, respectively. | ||
| TiC | VC | ZrC | NbC | HfC | TaC | |
|---|---|---|---|---|---|---|
| Charge transfer between Li2S and TMCs (e) | 0.921 | 0.537 | 0.805 | 0.439 | 0.821 | 0.439 |
The intrinsic insulating properties of Li2S will lead to sluggish charge transfer and electrochemical reaction kinetics during the charge/discharge process of Li–S batteries, which requires that the sulfur host should possess good electronic conductivity during the adsorption of Li2S. The electronic conductivity of TMC surfaces before and after adsorption of Li2S species was studied by calculating their total and projected density of states, shown in Fig. 9 and 10. It was found that, in all cases, there was no significant change in the DOS for all the TMCs regardless of Li2S adsorption. Therefore, the electronic conductivity of the TMCs was retained even after Li2S adsorption.
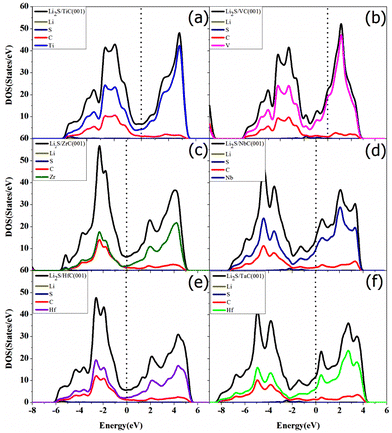 | ||
| Fig. 10 The partial density of states of Li2S on the (a) TiC, (b) VC, (c) ZrC, (d) NbC, (e) HfC, and (f) TaC (001) surfaces, respectively. | ||
3.5 Sulfur reduction reaction (SRR) on the TMC (001) surfaces
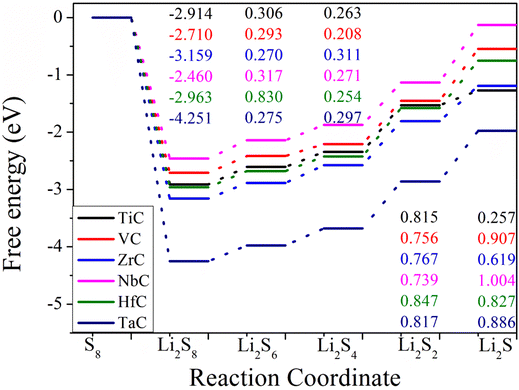
To further understand the electrochemical performance of TMCs, the free energy diagrams of polysulfide conversion were considered. The overall SRR was based on the reversible formation of Li2S from S8 and Li. As shown in Fig. 11, the first steps for all TMC substrates are exothermic with the calculated Gibbs free energies ranging from −2.46 to −4.25 eV, corresponding to the reduction of S8 to Li2S8 spontaneously. The other intermediate reaction steps from Li2S8 to Li2S are endothermic with positive Gibbs free energies. The rate-limiting step in the overall discharge process can be classified into two classes, corresponding to the reduction step of Li2S4 to Li2S2 or Li2S2 to Li2S, respectively. For the IVB group TMCs (e.g. TiC, ZrC, HfC), the rate-limiting step is the reduction step of Li2S4 to Li2S2. However, the rate-limiting step is changed to the reduction step of Li2S2 to Li2S for the VB group TMCs (e.g. VC, NbC, TaC). In brief, we found that the Gibbs free energies of the rate-determining steps on the TMCs are moderate with values of less than 1 eV. The lower Gibbs free energies reveal that the SRR is more thermodynamically favorable on TMCs upon discharging, thereby improving the electrochemical performance of Li–S batteries.4. Conclusions
In summary, based on first-principles calculations, we systematically explored the anchoring and catalytic ability of a series of TMCs for Li–S batteries. It was found that all the non-polar TMC (001) surfaces we investigated had a moderate adsorption ability to LiPSs. The DOS analysis for the adsorption systems of LiPSs on TMCs revealed their metallic properties. Besides, the low Li-ion diffusion barriers are essential to achieve high rate and reaction kinetics. Moreover, the activation barriers of Li2S decomposition on the IVB group TMC (e.g. TiC, ZrC, HfC) surfaces are lower than those on the VB group TMC (e.g. VC, NbC, TaC) surfaces. Furthermore, the observed lower Gibbs free energy for SRR and decomposition energy barriers for catalytic oxidation of Li2S enable accelerated formation and decomposition of Li2S in both discharging and charging processes. To sum up, due to the excellent bifunctionality as anchoring and electrocatalytic ability, together with the intrinsic thermal and thermodynamic stabilities as well as the metallic conductivity of the TMC host materials, IVB group TMCs (e.g. TiC, ZrC, and HfC) are regarded as effective influential sulfur hosts to effectively improve the cycling stability of Li–S batteries. Our work sheds light on a strategy of using the non-polar TMC surface as the anchoring material via multi-element synergy engineering. It provides significant insight into the effective regulation of the electrochemical reactions of Li2S.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 11904084, 22208088 and U2004212), the Henan Center for Outstanding Overseas Scientists (No. GZS202300), and the China Postdoctoral Science Foundation (2021M690933). The simulations are performed on resources provided by the High Performance Computing Center of Henan Normal University.References
- X. Ji and L. F. Nazar, Advances in Li–S batteries, J. Mater. Chem., 2010, 20, 9821–9826 RSC.
- M. Agostini, B. Scrosati and J. Hassoun, An Advanced Lithium–Ion Sulfur Battery for High Energy Storage, Adv. Energy Mater., 2015, 5, 1500481 CrossRef.
- R. Kumar, J. Liu, J.-Y. Hwang and Y.-K. Sun, Recent research trends in Li–S batteries, J. Mater. Chem. A, 2018, 6, 11582–11605 RSC.
- M. R. Kaiser, Z. Han, J. Liang, S.-X. Dou and J. Wang, Lithium sulfide-based cathode for lithium-ion/sulfur battery: recent progress and challenges, Energy Storage Mater., 2019, 19, 1–15 CrossRef.
- B. Liu, R. Fang, D. Xie, W. Zhang, H. Huang, Y. Xia, X. Wang, X. Xia and J. Tu, Revisiting scientific issues for industrial applications of lithium–sulfur batteries, Energy Environ. Mater., 2018, 1, 196–208 CrossRef.
- L. Huang, J. Li, B. Liu, Y. Li, S. Shen, S. Deng, C. Lu, W. Zhang, Y. Xia and G. Pan, Electrode design for lithium–sulfur batteries: problems and solutions, Adv. Funct. Mater., 2020, 30, 1910375 CrossRef CAS.
- Z. Wang, X. Xu, S. Ji, Z. Liu, D. Zhang, J. Shen and J. Liu, Recent progress of flexible sulfur cathode based on carbon host for lithium-sulfur batteries, J. Mater. Sci. Technol., 2020, 55, 56–72 CrossRef CAS.
- K. Park, J. H. Cho, J.-H. Jang, B.-C. Yu, T. Andreah, K. M. Miller, C. J. Ellison and J. B. Goodenough, Trapping lithium polysulfides of a Li–S battery by forming lithium bonds in a polymer matrix, Energy Environ. Sci., 2015, 8, 2389–2395 RSC.
- L. Chen, H. Yu, W. Li, M. Dirican, Y. Liu and X. Zhang, Interlayer design based on carbon materials for lithium–sulfur batteries: a review, J. Mater. Chem. A, 2020, 8, 10709–10735 RSC.
- Z. Wei, Y. Ren, J. Sokolowski, X. Zhu and G. Wu, Mechanistic understanding of the role separators playing in advanced lithium–sulfur batteries, InfoMat, 2020, 2, 483–508 CrossRef CAS.
- T. Zhou, W. Lv, J. Li, G. Zhou, Y. Zhao, S. Fan, B. Liu, B. Li, F. Kang and Q.-H. Yang, Twinborn TiO2–TiN heterostructures enabling smooth trapping–diffusion–conversion of polysulfides towards ultralong life lithium–sulfur batteries, Energy Environ. Sci., 2017, 10, 1694–1703 RSC.
- Z. Gao, Y. Schwab, Y. Zhang, N. Song and X. Li, Ferromagnetic Nanoparticle–Assisted Polysulfide Trapping for Enhanced Lithium–Sulfur Batteries, Adv. Funct. Mater., 2018, 28, 1800563 CrossRef.
- Y. Zhong, Y. Chen, Y. Cheng, Q. Fan, H. Zhao, H. Shao, Y. Lai, Z. Shi, X. Ke and Z. Guo, Li Alginate-Based Artificial SEI Layer for Stable Lithium Metal Anodes, ACS Appl. Mater. Interfaces, 2019, 11, 37726–37731 CrossRef CAS PubMed.
- M. Li, J. Lu, X. Ji, Y. Li, Y. Shao, Z. Chen, C. Zhong and K. Amine, Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes, Nat. Rev. Mater., 2020, 5, 276–294 CrossRef CAS.
- Z. Xu, J. Wang, J. Yang, X. Miao, R. Chen, J. Qian and R. Miao, Enhanced performance of a lithium–sulfur battery using a carbonate–based electrolyte, Angew. Chem., Int. Ed., 2016, 55, 10372–10375 CrossRef CAS PubMed.
- W. Chen, T. Lei, C. Wu, M. Deng, C. Gong, K. Hu, Y. Ma, L. Dai, W. Lv and W. He, Designing safe electrolyte systems for a high–stability lithium–sulfur battery, Adv. Energy Mater., 2018, 8, 1702348 CrossRef.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Porous hollow carbon@ sulfur composites for high–power lithium–sulfur batteries, Angew. Chem., 2011, 123, 6026–6030 CrossRef.
- Q. Pang, D. Kundu and L. F. Nazar, A graphene-like metallic cathode host for long-life and high-loading lithium–sulfur batteries, Mater. Horiz., 2016, 3, 130–136 RSC.
- G. Zhou, H. Tian, Y. Jin, X. Tao, B. Liu, R. Zhang, Z. W. Seh, D. Zhuo, Y. Liu and J. Sun, Catalytic oxidation of Li2S on the surface of metal sulfides for Li–S batteries, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 840–845 CrossRef CAS PubMed.
- R. Xiao, K. Chen, X. Zhang, Z. Yang, G. Hu, Z. Sun, H.-M. Cheng and F. Li, Single-atom catalysts for metal-sulfur batteries: Current progress and future perspectives, J. Energy Chem., 2021, 54, 452–466 CrossRef CAS.
- Q. Fang, M. Fang, X. Liu, P. Yu, J.-C. Ren, S. Li and W. Liu, An asymmetric Ti2CO/WS2 heterostructure as a promising anchoring material for lithium–sulfur batteries, J. Mater. Chem. A, 2020, 8, 13770–13775 RSC.
- T. Li, C. He and W. Zhang, Two-dimensional porous transition metal organic framework materials with strongly anchoring ability as lithium-sulfur cathode, Energy Storage Mater., 2020, 25, 866–875 CrossRef.
- X. Ji, K. T. Lee and L. F. Nazar, A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- J. Guo, Y. Xu and C. Wang, Sulfur-impregnated disordered carbon nanotubes cathode for lithium–sulfur batteries, Nano Lett., 2011, 11, 4288–4294 CrossRef CAS PubMed.
- Y. Xu, Y. Wen, Y. Zhu, K. Gaskell, K. A. Cychosz, B. Eichhorn, K. Xu and C. Wang, Confined sulfur in microporous carbon renders superior cycling stability in Li/S batteries, Adv. Funct. Mater., 2015, 25, 4312–4320 CrossRef CAS.
- X. Liang, C. Hart, Q. Pang, A. Garsuch, T. Weiss and L. F. Nazar, A highly efficient polysulfide mediator for lithium–sulfur batteries, Nat. Commun., 2015, 6, 5682 CrossRef PubMed.
- T. Lei, W. Chen, J. Huang, C. Yan, H. Sun, C. Wang, W. Zhang, Y. Li and J. Xiong, Multi–functional layered WS2 nanosheets for enhancing the performance of lithium–sulfur batteries, Adv. Energy Mater., 2017, 7, 1601843 CrossRef.
- D. Liu, C. Zhang, G. Zhou, W. Lv, G. Ling, L. Zhi and Q.-H. Yang, Catalytic Effects in Lithium–Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect, Adv. Sci., 2018, 5, 1700270 CrossRef PubMed.
- M. Zhang, W. Chen, L. Xue, Y. Jiao, T. Lei, J. Chu, J. Huang, C. Gong, C. Yan, Y. Yan, Y. Hu, X. Wang and J. Xiong, Adsorption-Catalysis Design in the Lithium-Sulfur Battery, Adv. Energy Mater., 2020, 10, 1903008 CrossRef CAS.
- T. Tang and Y. Hou, Chemical Confinement and Utility of Lithium Polysulfides in Lithium Sulfur Batteries, Small Methods, 2020, 4, 1900001 CrossRef CAS.
- W. S. Williams, Cubic Carbides: Solid-state physics explores materials having ionic structure, metallic conductivity, and covalent hardness, Science, 1966, 152, 34–42 CrossRef CAS PubMed.
- S. T. Oyama, Preparation and catalytic properties of transition metal carbides and nitrides, Catal. Today, 1992, 15, 179–200 CrossRef CAS.
- L. K. Ono and B. Roldán-Cuenya, Effect of interparticle interaction on the low temperature oxidation of CO over size-selected Au nanocatalysts supported on ultrathin TiC films, Catal. Lett., 2007, 113, 86–94 CrossRef CAS.
- R. E. Fuentes, H. R. Colón-Mercado and M. J. Martínez-Rodríguez, Pt-Ir/TiC electrocatalysts for PEM fuel cell/electrolyzer process, J. Electrochem. Soc., 2013, 161, F77 CrossRef.
- Y. C. Kimmel, L. Yang, T. G. Kelly, S. A. Rykov and J. G. Chen, Theoretical prediction and experimental verification of low loading of platinum on titanium carbide as low-cost and stable electrocatalysts, J. Catal., 2014, 312, 216–220 CrossRef CAS.
- X. Chu, Z. Fu, S. Li, X. Zhang and Z. Yang, Effects of a TiC substrate on the catalytic activity of Pt for NO reduction, Phys. Chem. Chem. Phys., 2016, 18, 13304–13309 RSC.
- J. A. Rodriguez, P. J. Ramírez and R. A. Gutierrez, Highly active Pt/MoC and Pt/TiC catalysts for the low-temperature water-gas shift reaction: Effects of the carbide metal/carbon ratio on the catalyst performance, Catal. Today, 2017, 289, 47–52 CrossRef CAS.
- H. J. Peng, Z. Ge, C. Xiang, Z. W. Zhang and Z. Qiang, Enhanced Electrochemical Kinetics on Conductive Polar Mediators for Lithium–Sulfur Batteries, Angew. Chem., 2016, 128, 13184–13189 CrossRef.
- T. Zhou, Y. Zhao, G. Zhou, W. Lv, P. Sun, F. Kang, B. Li and Q. H. Yang, An in-plane heterostructure of graphene and titanium carbide for efficient polysulfide confinement, Nano Energy, 2017, 39, 291–296 CrossRef CAS.
- W. Cai, G. Li, K. Zhang, G. Xiao, C. Wang, K. Ye, Z. Chen, Y. Zhu and Y. Qian, Conductive Nanocrystalline Niobium Carbide as High-Efficiency Polysulfides Tamer for Lithium-Sulfur Batteries, Adv. Funct. Mater., 2018, 28, 1704865 CrossRef.
- J. Choi, T.-G. Jeong, B. W. Cho, Y. Jung, S. H. Oh and Y.-T. Kim, Tungsten Carbide as a Highly Efficient Catalyst for Polysulfide Fragmentations in Li–S Batteries, J. Phys. Chem. C, 2018, 122, 7664–7669 CrossRef CAS.
- F. zhou, Z. Li, X. Luo, T. Wu, B. Jiang, L.-L. Lu, H.-B. Yao, M. Antonietti and S.-H. Yu, Low Cost Metal Carbide Nanocrystals as Binding and Electrocatalytic Sites for High Performance Li–S Batteries, Nano Lett., 2018, 18, 1035–1043 CrossRef CAS PubMed.
- Y. Zhang, P. Zhang, B. Li, S. Zhang, K. Liu, R. Hou, X. Zhang, S. R. P. Silva and G. Shao, Vertically aligned graphene nanosheets on multi-yolk/shell structured TiC@C nanofibers for stable Li–S batteries, Energy Storage Mater., 2020, 27, 159–168 CrossRef.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6671–6687 CrossRef CAS PubMed.
- P. Blochl, E. Blöchl and P. Blöchl, Projected augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- H. J. Monkhorst and J. D. Pack, Special points for Brillouin-zone integrations, Phys. Rev. B: Solid State, 1976, 13, 5188 CrossRef.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- J. Mao, S. Li, Y. Zhang, X. Chu and Z. Yang, Density functional study on the mechanism for the highly active palladium monolayer supported on titanium carbide for the oxygen reduction reaction, J. Chem. Phys., 2016, 144, 204703 CrossRef PubMed.
- X. Zhang, Z. Lu and Z. Yang, A first principles study of O2 dissociation on Pt modified ZrC(100) surface, Chem. Phys. Lett., 2016, 649, 141–147 CrossRef CAS.
- S. Wang, X. Zhang, Y. Zhang, J. Mao and Z. Yang, Efficient noble metal nanocatalysts supported on HfC(001) for O2 dissociation, AIP Adv., 2017, 7, 035015 CrossRef.
- D. Kan, X. Zhang, Y. Zhang and Z. Yang, Screening the best catalyst with group 9, 10 and 11 metals monolayer loading on NbC (001) from first-principles study, J. Power Sources, 2018, 378, 691–698 CrossRef CAS.
- Y. Meng, X. Zhang, J. Mao, X. Xu and Z. Yang, The adsorption and dissociation of O2 on Pd and Pt modified TaC (1 0 0) surface: A first principles study, Appl. Surf. Sci., 2018, 439, 845–851 CrossRef CAS.
- S. Zaima, Y. Shibata, H. Adachi, C. Oshima, S. Otani, M. Aono and Y. Ishizawa, Surf. Sci., 1985, 157, 380 CrossRef CAS.
- J. Mao, S. Li, Y. Zhang, X. Chu and Z. Yang, The stability of TiC surfaces in the environment with various carbon chemical potential and surface defects, Appl. Surf. Sci., 2016, 386, 202–209 CrossRef CAS.
- D. Wang, F. Li, R. Lian, J. Xu, D. Kan, Y. Liu, G. Chen, Y. Gogotsi and Y. Wei, 0001-A General Atomic Surface Modification Strategy for Improving Anchoring and Electrocatalysis Behavior of Ti3C2T2 MXene in Lithium–Sulfur Batteries, ACS Nano, 2019, 13, 11078–11086 CrossRef CAS PubMed.
- Q. Zhang, Y. Wang, Z. W. Seh, Z. Fu, R. Zhang and Y. Cui, Understanding the Anchoring Effect of Two-Dimensional Layered Materials for Lithium–Sulfur Batteries, Nano Lett., 2015, 15, 3780–3786 CrossRef CAS PubMed.
- J. Xie, B.-Q. Li, H.-J. Peng, Y.-W. Song, M. Zhao, X. Chen, Q. Zhang and J.-Q. Huang, Implanting Atomic Cobalt within Mesoporous Carbon toward Highly Stable Lithium–Sulfur Batteries, Adv. Mater., 2019, 31, 1903813 CrossRef CAS PubMed.
- M. S. Nahian, R. Jayan and M. M. Islam, Atomic-Scale Insights into Comparative Mechanisms and Kinetics of Na–S and Li–S Batteries, ACS Catal., 2022, 12, 7664–7676 CrossRef CAS.
- N. Li, Y. Zhan, H. Wu, J. Fan and J. Jia, Synergistically boosting the anchoring effect and catalytic activity of MXenes as bifunctional electrocatalysts for sodium–sulfur batteries by single-atom catalyst engineering, Nanoscale, 2023, 15, 2747–2755 RSC.
- L. Wang, W. Hua, X. Wan, Z. Feng, Z. Hu, H. Li, J. Niu, L. Wang, A. Wang and J. Liu, Design rules of a sulfur redox electrocatalyst for lithium–sulfur batteries, Adv. Mater., 2022, 34, 2110279 CrossRef CAS PubMed.
- N. Li, Q. Meng, X. Zhu, Z. Li, J. Ma, C. Huang, J. Song and J. Fan, 0011-Lattice constant-dependent anchoring effect of MXenes for lithium–sulfur (Li–S) batteries: a DFT study, Nanoscale, 2019, 11, 8485–8493 RSC.
- L.-J. Zhou, Z. Hou and L.-M. Wu, First-principles study of lithium adsorption and diffusion on graphene with point defects, J. Phys. Chem. C, 2012, 116, 21780–21787 CrossRef CAS.
- Y. Wang, Z. Fu, X. Zhang and Z. Yang, Understanding the correlation between the electronic structure and catalytic behavior of TiC (001) and TiN (001) surfaces: DFT study, Appl. Surf. Sci., 2019, 494, 57–62 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: A detailed description of models and computational methods; the AIMD simulations at a temperature of 500 K of the TiC(001) surface (Fig. S1); the total and partial density of states of TMC (001) surfaces (Fig. S2); the structures of S8 and LiPSs and the optimized adsorption structures of Li2S8, Li2S6, and Li2S4 with organic molecules (Fig. S3); the optimized adsorption structures of S8 and LiPSs on TMC (001) surfaces (Fig. S4–8); the charge density difference (CDD) of S8 and LiPSs on TMC (001) surfaces (Fig. S9–13); the total and projected density of states of S8 and LiPSs on the TMC (001) surfaces (Fig. S14–18); the minimum energy path of Li2S decomposition on TMC surfaces (Fig. S19); the structure information of TMCs and LiPSs before and after the adsorption interaction (Tables S1–11); the comparison of the adsorption energy for the LiPSs on the HfC(001) surface with or without the DFT+U method (Table S12); the binding energies of Li2S8, Li2S6 and Li2S4 with organic molecules (Table S13); the charge transfer (Δq, in e) of S or Li atoms in the S8 or LiPSs adsorbed on TMCs (Table S14); the detailed test data of Li-ions adsorbed on the (001) surfaces of TMCs (Table S15). See DOI: https://doi.org/10.1039/d3nr04298g |
| This journal is © The Royal Society of Chemistry 2024 |