Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot preparation of pyrazole “turn on” and “turn off” fluorescent sensors for Zn2+ and Cd2+ directly from chalcones via in situ aromatisation†
Alexander
Ciupa

Materials Innovation Factory, University of Liverpool, 51 Oxford Street, Liverpool L7 3NY, UK. E-mail: ciupa@liverpool.ac.uk
First published on 17th July 2024
Abstract
A direct chalcone to pyrazole synthetic route to “turn on” and “turn off” fluorescent sensors for Cd2+ and Zn2+ was developed using CuCl2 as an in situ oxidant. This one-pot approach produced eight novel pyridine based pyrazole fluorescent sensors displaying both “turn on” and “turn off” properties dependent on the substutient on the aryl ring (4-F, 4-Cl, 4-Br, 4-I, 4-CN, 4-NO2, 4-OMe and 3,4-OMe). The results within provide valuable insight for future pyrazole sensor design.
Introduction
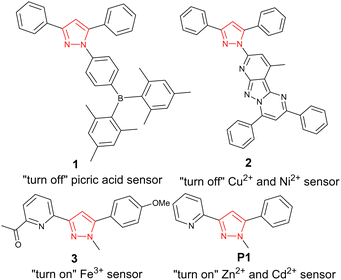
Pyrazole,1 a five-membered heterocycle with two adjacent nitrogen atoms (red in Fig. 1), is a privileged structure2 with a diverse range of biological activities including anti-inflammatory,3 anti-cancer4 and anti-infective5 properties. 3,5 substituted pyrazoles have unique photophysical properties with applications in luminescent dyes6 and fluorescent sensors.7 “Turn off” fluorescent sensors display reductions in fluorescent emission (λem) with analyte, for example 1 with picric acid8 and 2 with Cu2+ and Ni2+ (Fig. 1).9 “Turn on” fluorescent sensors are characterised by increased λem with analyte, 3 demonstrates Fe3+/Fe2+ selectivity10 and P1 displays a “turn on” response with Zn2+ and Cd2+ in MeCN (Fig. 1).11Zinc is involved in a variety of biological functions including gene expression,12 enzyme maintenance13 and neurological functions.14 Cadmium, also a group 12 element sitting below zinc in the periodic table, is a highly toxic pollutant implicated in a variety of cancers.153 and P1 demonstrate structural complexity is not a prerequisite for complex functionality and that simple molecular structures can detect biologically important analytes.10,11
Chalcones,16 also a privileged structure with numerous biological activities,17 are popular precursors for pyrazoles due to the range of inexpensive commercially available starting materials. To date, there are few examples of direct chalcone to pyrazole syntheses, often requiring vigorous heating under acidic conditions18 or use of a catalyst.19 The traditional two step synthesis involves 1,2 addition of hydrazine to the enone10,11,20 forming a pyrazoline21 which is isolated, purified, and undergoes subsequent aromatisation to a pyrazole (blue and red respectively (Scheme 1).
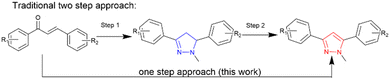 | ||
| Scheme 1 A one step approach to synthesise pyrazole (in red) directly from chalcone avoiding the pyrazoline (in blue) intermediate step. | ||
A range of chemical oxidants have been reported to perform this pyrazoline to pyrazole aromatisation including MnO2,22 FeCl3,23 CoCl224 and CuCl2.25 Previous work10 demonstrated the addition of 8.0 equivalents (eq.) methylhydrazine at room temperature resulted in minor formation of pyrazole (yield 8–18%). Herein we report optimisation of this reaction enabling efficient access to the pyrazole privileged structure directly from chalcone avoiding the requirement to isolate, purify and then aromatise the pyrazoline ring separately. We examined multiple reaction conditions and screened eight different transition metal oxidants. Direct one-pot access to pyrazole sensors facilitated rapid synthesis and screening of eight novel Zn2+ and Cd2+ “turn on” and “turn off” pyrazole sensors.
Results and discussion
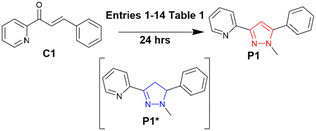
Chalcone C1 is the precursor for P1 and was selected as a model system for reaction screening (Scheme 2).Previous work10 was used as a baseline (entry 1, Table 1) and 1H NMR was used to determine the percentage (%) conversion of C1 to P1 using the ratio of the chemically distinct methyl (CH3) groups in the pyrazoline P1* at approx. 2.90 ppm and the pyrazole methyl (CH3) at approx. 4.30 ppm (shown in blue and red in Fig. 2, see ESI† for full study).
| Entry | Solvent | Temperature (°C) | Eq. H2NNHMe | P1 % conversion |
|---|---|---|---|---|
| 1 | MeOH | 20 | 8.0 | 18 |
| 2 | EtOH | 20 | 8.0 | 10 |
| 3 | iPrOH | 20 | 8.0 | 3 |
| 4 | MeCN | 20 | 8.0 | 11 |
| 5 | THF | 20 | 8.0 | 7 |
| 6 | CH2Cl2 | 20 | 8.0 | 10 |
| 7 | CHCl3 | 20 | 8.0 | 13 |
| 8 | Hexane | 20 | 8.0 | 2 |
| 9 | MeOH | 40 | 8.0 | 30 |
| 10 | MeOH | 60 | 8.0 | 40 |
| 11 | MeOH | 60 | 1.5 | 38 |
| 12 | MeOH | 60 | 2.0 | 40 |
| 13 | MeOH | 60 | 4.0 | 35 |
| 14 | MeOH | 60 | 16 | 32 |
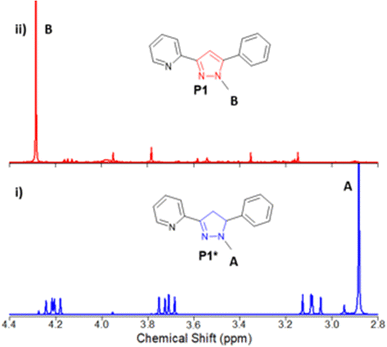 | ||
| Fig. 2 Representative example of 1H NMR study to determine the % conversion of methyl group A in P1* (in blue, entry 18, Table 2, i) to methyl group B in P1 (in red, entry 19, Table 2, ii). | ||
Preliminary conditions produced 18% conversion of C1 to P1 with pyrazoline P1* the residual component (Table 1). Solvent, consisting of the bulk of the reaction mixture, was initially screened using a variety of polar/nonpolar and protic/nonprotic solvents.
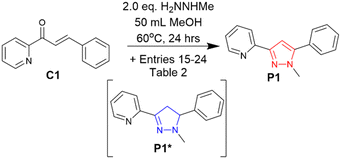
Polar protic solvents MeOH, EtOH and iPrOH (entries 1–3, Table 1) were initially investigated with MeOH providing the best % conversion of 18%. Polar aprotic solvents (MeCN and THF, entry 4 and 5, Table 1) reduced % conversion to 11% and 7% respectively. Chlorinated solvents CH2Cl2 and CHCl3 (entries 6 and 7, Table 1) failed to provide an improvement with 10% and 13% P1 conversion. Interestingly the use of the nonpolar aprotic solvent hexane was highly detrimental to P1 formation with 98% conversion to P1* and only 2% P1 (entry 8, Table 1). Hexane would be an excellent choice for pyrazoline only targeted synthesis. Eight solvents were screened and the preferred solvent for pyrazole formation was MeOH, this was fixed and used for all subsequent reactions. The reaction temperature was varied to 40 °C and then 60 °C with a further improvement on P1% conversion to 30% and 40% respectively (entry 9 and 10, Table 1). The temperature was fixed at 60 °C to provide the best P1 conversion while remaining below both the boiling points of MeOH and methylhydrazine (H2NNHMe). The number of equivalents (eq.) H2NNHMe was varied, surprisingly reducing the eq. of H2NNHMe to 2.0 did not significantly reduce the P1% conversion (entry 12, Table 1). Further increases in eq. H2NNHMe did not yield an improvement therefore according to the principle of atom efficiency and green chemistry26 2.0 eq. H2NNHMe was used for all further reactions.
The initial reaction condition optimisation (Table 1) resulted in a significant increase in P1 pyrazole formation from 18% to 40%, we then investigated if the presence of an oxidant would improve the in situ aromatisation of the pyrazoline to a pyrazole (Scheme 3 and Table 2).
| Entry | Oxidant | Eq. | P1 % conversion |
|---|---|---|---|
| 12 | — | — | 40 |
| 15 | MnO2 | 1.0 | 25 |
| 16 | FeCl3 | 1.0 | 17 |
| 17 | CoCl2 | 1.0 | 12 |
| 18 | NiCl2 | 1.0 | 2 |
| 19 | CuCl2 | 1.0 | 83 |
| 20 | ZnCl2 | 1.0 | 45 |
| 21 | CuSO4 | 1.0 | 64 |
| 22 | Cu(OAc)2 | 1.0 | 78 |
| 23 | CuCl2 | 0.5 | 61 |
| 24 | CuCl2 | 0.25 | 19 |
MnO2 has been reported to be a useful oxidant for pyrazole formation22 however it reduced conversion to 25% (entry 15, Table 2). A similar effect was observed with FeCl3, CoCl2 with the presence of 1.0 eq. NiCl2 highly detrimental to pyrazole formation (entries 16–18, Table 2). 1.0 eq. ZnCl2 produced a slight increase in conversion to 45% (entry 20, Table 2) however the addition of CuCl2 was the most promising with a more than doubling of conversion to 83% (entry 19, Table 2). Encouraged by this result, we investigated two different copper salts, CuSO4 and Cu(OAc)2 which yielded 64% and 78% conversion suggesting the Cu2+ primarily is responsible for the oxidation with CuCl2 the preferred oxidant (entries 21 and 22, Table 2). Reducing the amount of CuCl2 to 0.5 eq. and 0.25 eq. reduced the conversion to 61% and 19% respectively (entries 23 and 24, Table 2) suggesting a full 1.0 eq. is required for maximum conversion. Copper salts are cheap, easily accessible and their ease of handling and low toxicity profile have found widespread use in organic catalysis. Cu2+ mediated aromatisation of cyclohexanone derivatives to phenols,27 Cu2+ catalysed aromatisation of tetrahydrocarbazole to carbazole alkaloids28 and the synthesis of pyrazoles via copper-catalysed relay oxidation have also been reported.29 In summary, we screen eight transition metal as in situ oxidants with Cu2+ based oxidants the most significant with CuCl2 providing the best option for in situ aromatisation. An LC-MS study was conducted to further examine this synthesis under entry 19 conditions (with CuCl2) to monitor the conversion of C1 to P1via the in situP1* intermediate (Fig. 3 and Table 3).
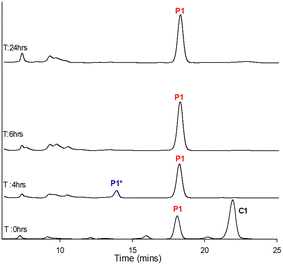 | ||
| Fig. 3 LC-MS study using entry 19 conditions (Table 2). | ||
| Timepoint (h) | P1* (%) | P1 (%) | C1 (%) |
|---|---|---|---|
| 0.0 | — | 27 | 57 |
| 4.0 | 4.0 | 60 | 2.6 |
| 6.0 | 1 | 84 | — |
| 24.0 | — | 83 | — |
After 4.0 hours all chalcone C1 was consumed with P1 the major component with detectable P1*. After 6.0 hours all P1* was aromatised to P1 (Fig. 3 and Table 3). A second LC-MS study was conducted as a negative control using the NiCl2 conditions with contrasting results (Fig. 4 and Table 4).
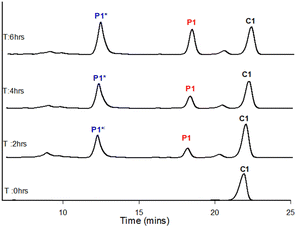 | ||
| Fig. 4 LC-MS study using entry 18 conditions (Table 2). | ||
| Timepoint (h) | P1* (%) | P1 (%) | C1 (%) |
|---|---|---|---|
| 0.0 | — | — | 96.0 |
| 2.0 | 18 | 7.0 | 62.0 |
| 4.0 | 31.0 | 11.0 | 45.0 |
| 6.0 | 36.0 | 14.0 | 39 |
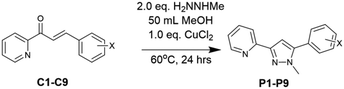
After 2.0 and 4.0 hours there was still significant C1 with P1* and trace amounts of the desired P1 present (Fig. 4 and Table 4). Sampling the mixture at 6.0 hrs showed a minor P1 improvement but with substantial unreacted C1 still remaining, in contrast to the same timepoint with CuCl2. Further sampling at 8.0 and 24.0 hours failed to show improvement (ESI†) in P1 demonstrating the choice of transition metal oxidant was critical to the aromatisation process with CuCl2 the superior choice. With a suitable one-pot method to generate pyrazoles selected we validated it by applying it to chalcones with both electron withdrawing and donating aryl units. Chalcones C1–C9 were prepared via literature methods30 in good to excellent yield (38–92%). The CuCl2 method was applied to P1 with an isolated yield of 57% which is comparable on the overall yield of 58% over two steps previously reported.10 This method was also successfully applied to novel pyrazoles P2–P9 in acceptable yield (38–77%, Scheme 4 and Table 5).
| Entry | X | CuCl2 method yield (%) |
|---|---|---|
| P1 | 4-H | 57 |
| P2 | 4-F | 77 |
| P3 | 4-Cl | 61 |
| P4 | 4-Br | 40 |
| P5 | 4-I | 32 |
| P6 | 4-CN | 57 |
| P7 | 4-NO2 | 47 |
| P8 | 4-OMe | 48 |
| P9 | 3,4-OMe | 38 |
With eight novel potential sensors in hand, we investigated their potential as Zn2+ and Cd2+ fluorescent sensors in MeCN (Fig. 5), this solvent was selected to allow direct comparison with two previous studies on related pyrazoles.10,11 Standard protocols for screening fluorescent sensors in organic solvents were followed throughout.31
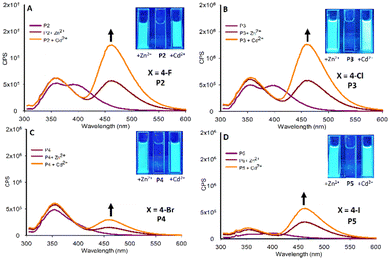 | ||
| Fig. 5 Fluorescence studies of P2–P5 (20 μM, MeCN, λex 295 nm) with 5.0 eq. Zn2+ or Cd2+, insets are pyrazoles at λex 365 nm with the indicated metal, cps is counts per second. | ||
Halogenated pyrazole P2–P5 all display a “turn on” fluorescent response in the presence of both Zn2+ and Cd2+ with a higher λem at 465 nm with Cd2+/Zn2+ (Fig. 5). A similar result was observed with the previously reported sensor11 also displaying λem at 465 nm with Cd2+/Zn2+. The nature of the electronegative halogen influenced “turn on” intensity when X = 4-F P2 (Fig. 5A) and 4-Cl P3 (Fig. 5B) the response is almost identical but as halogen size is increased to 4-Br P4 (Fig. 5C) and 4-I P5 (Fig. 5D) the magnitude of λem is reduced. The halogen series can be summarised as λem intensity at 465 nm: F ≈ Cl > Br < I. These preliminary results suggest the presence of an electronegative group results in a “turn on” response. To test this hypothesis, two additional electronegative pyrazoles were produced pyrazole P6 bearing a strong electronegative cyano (4-CN) group, which is often compared to F, and a highly electronegative nitro (4-NO2 group) pyrazole (Fig. 6A and B).
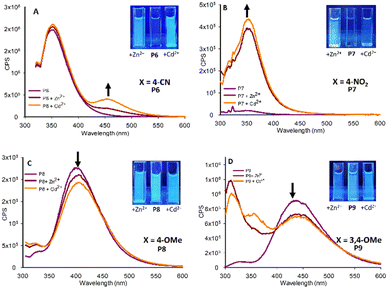 | ||
| Fig. 6 Fluorescence studies of P6–P9 (20 μM, MeCN, λex 295 nm) with 5.0 eq. Zn2+ and Cd2+, insets at λex 365 nm with the indicated metals, cps is counts per second. | ||
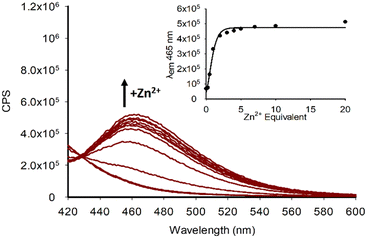
As predicted, pyrazole P6 with a 4-CN group (Fig. 6A) did display a “turn on” response with both Cd2+ and Zn2+ analogous to the halogenated pyrazoles P2–P5 however with reduced intensity at λem 460 nm. Comparing P6 directly with P2, both of which have similar electronegative withdrawing groups, demonstrates that F is the preferred substituent, and that electronegativity alone is not solely responsible for the “turn on” response. This is further confirmed for P7 with a NO2 group displaying “turn on” properties in the presence of Zn2+ and Cd2+ (Fig. 6B) but with λem 350 nm in contrast to λem 460 nm observed in P2–P6 and P8. This indicates the 4-NO2 group is exerting a significant influence on the photophysical properties of this sensor. An interesting observation was the presence of the electron donating 4-OMe group in P8 resulted in a minor “turn off” response with both Zn2+ and Cd2+ (Fig. 6C). A further electron donating pyrazole bearing a 3,4-Dimethyloxyl group P9 was synthesized, this followed the trend of P8 displaying minor “turn off” response in the presence of Zn2+ and Cd2+ (Fig. 6D). In summary this preliminary screen of eight novel pyrazoles suggests the presence of electron withdrawing groups at the 4-position of the aryl ring result in a “turn on” response in the presence of Zn2+ and Cd2+ with the chemistry of the group influencing photophysical properties. Electron donating groups on the aryl ring result in a minor “turn off” response also with Zn2+ and Cd2+. “Turn on” sensors are typically preferred over “turn off” therefore P2 was selected for further investigation with Zn2+ titration experiments confirming an increased λem at 465 nm with Zn2+ reaching a maximum at 5.0 eq. metal with sensor with further increases in Zn2+ resulting in very minor increased fluorescent intensity (Fig. 7).
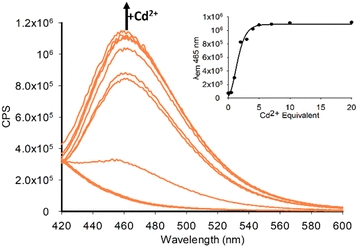
A similar response was observed with Cd2+ however with increased λem at 465 nm at the same concentration suggesting P2 would be more suited as a Cd2+ sensor (Fig. 8).
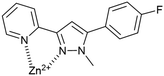
The maximum emission was observed at 5.0 eq. Cd2+ with further increases resulting in minor increase in emission. A limit of detection (LoD) of 1.97 μM for Zn2+ and 0.077 μM for Cd2+ was measured for P2 which is an improvement on the 0.34 μM LoD for P1 with Cd2+ reported previously.11 A proposed binding mechanism for P2 with Zn2+ is shown in Fig. 9 and agrees with the previously reported crystal structure for P1 with Zn2+.11
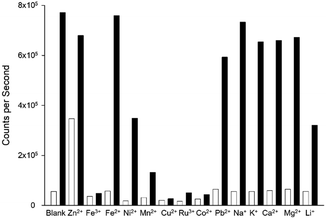
A competition assay was performed to assess P2λem in the presence of a range of competing metals (Fig. 10).
Quenching of the “turn on” response was observed with a range of paramagnetic metals including Ni2+, Mn2+, Cu2+, Ru3+ and Co2+ and this has been observed with similar sensors.10,11 Interestingly the presence of diamagnetic metals Zn2+ and Pb2+ and the group 1 and 2 metals Na+, K+, Ca2+ and Mg2+ did not result in a significant reduction in λem. This suggests P2 could be a useful sensor for the detection of Cd2+ in biological samples containing group 1 and 2 metals once a suitable aqueous based derivative of P2 is developed. The results from the eight novel pyrazoles indicate the 4-position of the aryl ring to be an excellent location for introducing electronegative water solubilising groups to accomplish this. This is the primary focus of the next generation of sensors under development and will be resulted in due course. The one-pot route enables rapid access to novel pyrazoles directly from chalcones greatly accelerating future pyrazole sensors development alongside expediting pyrazole-based molecules with a diverse and wide range of valuable applications.32
Conclusions
A new one pot method to synthesize pyrazoles directly from chalcones was developed using CuCl2 as an in situ oxidant which was validated against a range of electron donating and withdrawing chalcones resulting in eight novel pyrazole based sensors. These sensors were confirmed to display both “turn on” and “turn off” properties dependent on aryl ring substitution. The presence of electronegative groups at the 4-position resulted in a “turn on” sensor with the chemistry of the group influencing the extent of λem wavelength. Electro donating groups displayed a very mild “turn off” response. The 4-position is well suited to a variety of substitution and would be an excellent position for the introduction of water solubilising groups to transition away from a purely organic solvent-based sensor to an aqueous one, this is an ongoing objective and will be reported shortly. This simple modular scaffold would be highly amenable to a multi-sensor approach incorporating multiple chelation sites with valuable applications in real world monitoring. While the focus of this study was efficient access to pyrazoles for the design screening of sensors, this direct one pot approach also enables efficient access to future pyrazoles with applications in anti-cancer, anti-infective and anti-inflammatory screening studies and will be of benefit to the wider chemistry community.32Author contributions
Alexander Ciupa designed, synthesized, characterised, performed all spectroscopy studies and authored the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author acknowledges Steven Robinson for assistance with time-of-flight high resolution mass spectrometry, Glyn Connolly for NMR spectroscopy guidance and Krzysztof Pawlak with fluorescence spectroscopy. This work made use of shared equipment located at the Materials Innovation Factory; created as part of the UK Research Partnership Innovation Fund (Research England) and co-funded by the Sir Henry Royce Institute.References
- Selected examples: (a) S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111(11), 6984 CrossRef CAS; (b) M.-C. Ríos and J. Portilla, Chemistry, 2022, 4, 940 CrossRef; (c) J.-C. Castillo and J. Portilla, Targets Heterocycl. Syst., 2018, 22, 194 CAS.
- R. F. Costa, L. C. Turones, K. V. N. Cavalcante, I. A. Rosa Júnior, C. H. Xavier, L. P. Rosseto, H. B. Napolitano, P. F. S. Da Castro, M. L. F. Neto, G. M. Galvão, R. Menegatti, G. R. Pedrino, E. A. Costa, J. L. R. Martins and J. O. Fajemiroye, Front. Pharmacol., 2021, 12, 666725 CrossRef CAS.
- A. T. Taher, M. T. Mostafa Sarg, N. R. El-Sayed Ali and N. Hilmy Elnagdi, Bioorg. Chem., 2019, 89, 103023 CrossRef CAS.
- B. Insuasty, A. Tigreros, F. Orozco, J. Quiroga, R. Abonía, M. Nogueras, A. Sanchez and J. Cobo, Bioorg. Med. Chem., 2010, 18, 4965 CrossRef CAS PubMed.
- J. V. Faria, P. F. Vegi, A. G. C. Miguita, M. S. Dos Santos, N. Boechat and A. M. R. Bernardino, Bioorg. Med. Chem., 2017, 25, 5891 CrossRef CAS.
- Selected examples: (a) B. Willy and T. J. Mueller, Eur. J. Org. Chem., 2008, 4157 CrossRef CAS; (b) V. Mukundam, S. Sa, A. Kumari, R. Das and K. Venkatasubbaiah, J. Mater. Chem. C, 2019, 7, 12725 RSC; (c) A. C. Murali, P. Pratakshya, P. Patel, P. Nayak, S. Peruncheralathan and K. Venkatasubbaiah, New J. Chem., 2023, 47, 17835 RSC; (d) S. Mukherjee, P. S. Srinivasan and S. Peruncheralathan, Chem. Commun., 2015, 51, 17148 RSC.
- Selected examples: (a) A. Tigreros and J. Portilla, Curr. Chin. Sci., 2021, 1, 197 CrossRef CAS; (b) A. Tigreros and J. Portilla, RSC Adv., 2020, 10, 19693 RSC; (c) M. Verma, A. F. Chaudhry, M. T. Morgan and C. J. Fahmi, Org. Biomol. Chem., 2010, 8, 363 RSC.
- S. Sa, V. Mukundam, A. Kumari, R. Das and K. Venkatasubbaiah, Dalton Trans., 2021, 50, 6204 RSC.
- Y.-Q. Gu, W.-Y. Shen, Y. Mi, Y.-F. Jing, J.-M. Yuan, P. Yu, W.-M. Zhu and F.-L. Hu, RSC Adv., 2019, 9, 35671 RSC.
- A. Ciupa, RSC Adv., 2024, 14, 3519 RSC.
- A. Ciupa, M. F. Mahon, P. A. De Bank and L. Caggiano, Org. Biomol. Chem., 2012, 10, 8753 RSC.
- T. V. O’Halloran, Science, 1993, 261, 715 CrossRef PubMed.
- C. Andreini and I. Bertini, J. Inorg. Biochem., 2012, 111, 150 CrossRef CAS PubMed.
- C. J. Frederickson, Biometals, 2001, 14, 353 CrossRef CAS PubMed.
- G. Genchi, S. M. Sinicropi, G. Lauria, A. Carocci and A. Catalano, Int. J. Environ. Res. Public Health, 2020, 17, 3782 CrossRef CAS.
- P. Singh, A. Anand and V. Kumar, Eur. J. Med. Chem., 2014, 85, 758 CrossRef CAS.
- Selected examples: (a) A. Ciupa, N. J. Griffiths, S. K. Light, P. J. Wood and L. Caggiano, Med. Chem. Commun., 2011, 2, 1011 RSC; (b) M. A. Shalaby, S. A. Rizk and A. M. Fahim, Org. Biomol. Chem., 2023, 21, 5317 RSC; (c) A. Gupta, S. Garg and H. Singh, Anal. Methods, 2020, 12, 5022 RSC; (d) S. Wangngae, T. Pewklang, K. Chansaenpak, P. Ganta, S. Worakaensai, K. Siwawannapong, S. Kluaiphanngam, N. Nantapong, R.-Y. Lai and A. Kamkaew, New J. Chem., 2021, 45, 11566 RSC; (e) A. Ahmad, M. Y. Wani, M. Patel, A. J. F. N. Sobral, A. G. Duse, F. M. Aqlan and A. S. Al-Bogami, Med. Chem. Commun., 2017, 8, 2195 RSC.
- Selected examples: (a) G. Cocconcelli, E. Diodato, A. Caricasole, G. Gaviraghi, E. Genesio, C. Ghiron, L. Magnoni, E. Pecchioli, R. V. Plazzi and G. C. Terstappen, Bioorg. Med. Chem., 2008, 16, 2043 CrossRef CAS; (b) A. Voskiene, V. Mickevicius and G. Mikulskiene, ARKIVOC, 2007, 303 Search PubMed; (c) S. B. Somappa, J. S. Biradar, P. Rajesab, S. Rahber and M. Sundar, Monatsh. Chem., 2015, 146, 2067 CrossRef CAS.
- Selected examples: (a) H. Zhang, Q. Wei, G. Zhu, J. Qu and B. Wang, Tetrahedron Lett., 2016, 57, 2633 CrossRef CAS; (b) S. M. Landge, A. Schmidt, V. Outerbridge and B. Török, Synlett, 2007, 1600 CAS.
- A. Ciupa, P. A. De Bank, M. F. Mahon, P. J. Wood and L. Caggiano, MedChemComm, 2013, 4, 956 RSC.
- B. Varghese, S. N. Al-Busa, F. O. Suliman and S. M. Z. Al-Kindy, RSC Adv., 2017, 7, 46999 RSC.
- I. Bhatnagar and M. V. George, Tetrahedron, 1968, 24, 1293 CrossRef CAS.
- G. S. Ananthnag, A. Adhikari and M. S. Balakrishna, Catal. Commun., 2014, 43, 240 CrossRef CAS.
- J. N. Shah and C. K. Shah, J. Org. Chem., 1978, 43(6), 1266 CrossRef CAS.
- Selected examples: (a) P. D. Lokhande, B. A. Dalvi, V. T. Humne, B. R. Nawghare and A. Kareem, Ind. J. Chem., 2014, 53B, 1091 CAS; V. K. Rao, R. Tiwari, B. S. Chhikara, A. N. Shirazi, K. Parang and A. Kumar, RSC Adv., 2013, 3, 15396 Search PubMed.
- Selected examples: (a) R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC; (b) R. A. Sheldon, Chem. Commun., 2008, 3352 RSC.
- Selected examples: (a) X. Liu, J. Chen and T. Ma, Org. Biomol. Chem., 2018, 16, 8662–8676 RSC; (b) X.-L. Liu, X.-L. Long, Y.-J. Guo, C.-H. Meng, A.-B. Xia and D.-Q. Xu, Asian J. Org. Chem., 2017, 6, 967 CrossRef CAS; (c) H.-C. Tong, R. Reddy and S.-T. Liu, Eur. J. Org. Chem., 2014, 3256 CrossRef CAS.
- B. A. Dalvi and P. D. Lokhande, Tetrahedron Lett., 2018, 59, 2145 CrossRef CAS.
- X. Tang, L. Huang, J. Yang, Y. Xu, W. Wu and H. Jiang, Chem. Commun., 2014, 50, 14793 RSC.
- Selected examples: (a) H. M. Faidallah, M. M. Al-Mohammadi, K. A. Alamry and K. A. Khan, J. Enzyme Inhib. Med. Chem., 2016, 31(S1), 1570163 Search PubMed; (b) J. Majeed and M. Shaharyar, J. Enzyme Inhib. Med. Chem., 2011, 26(6), 819 CrossRef CAS PubMed; (c) Y.-J. Ren, Z.-C. Wang, X. Zhang, H.-Y. Qiu, P.-F. Wang, H.-B. Gong, A.-Q. Jiang and H.-L. Zhu, RSC Adv., 2015, 5, 21445 RSC.
- Selected examples: (a) S. Lohar, S. Pal, M. Mukherjee, A. Maji, N. Demitri and P. Chattopadhyay, RSC Adv., 2017, 7, 25528 RSC; (b) M. Akula, P. Z. El-Khoury, A. Nag and A. Bhattacharya, RSC Adv., 2014, 4, 25605 RSC; (c) Z. Shi, Y. Tu and S. Pu, RSC Adv., 2018, 8, 6727 RSC; (d) Y. Zhang, H. Lui, W. Gao and S. Pu, RSC Adv., 2019, 9, 27476 RSC; (e) X. Wu, Z. Zhang, H. Liu and S. Pu, RSC Adv., 2020, 10, 15547 RSC.
- A. Ansari, A. Abi, M. Asif and S. Shamsuzzaman, New J. Chem., 2017, 41, 16 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj02433h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |