Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymer coated magnesium hydroxide nanoparticles for enhanced wound healing†
Muhammad Khawar
Abbas
ab,
Yasir
Javed
*b,
Naveed Akhtar
Shad
c,
Muhammad
Shahid
d,
Bushra
Akhtar
e,
Effat
Yasin
ab,
Surender Kumar
Sharma
 f and
Nguyen Thi Kim
Thanh
f and
Nguyen Thi Kim
Thanh
 *gh
*gh
aDepartment of Physics, Government College University Faisalabad, Faisalabad, Pakistan
bDepartment of Physics, University of Agriculture, Faisalabad, Pakistan. E-mail: yasir.javed@uaf.edu.pk
cNational Institute of Biotechnology and Genetic Engineering, Jhang Road, Faisalabad, Pakistan
dDepartment of Biochemistry, University of Agriculture, Faisalabad, Pakistan
eDepartment of Pharmacy, University of Agriculture, Faisalabad, Pakistan
fDepartment of Physics, Central University of Punjab, Bathinda, 151401, India
gBiophysics group, Department of Physics and Astronomy, University College London, Gower Street, London WC1E 6BT, UK. E-mail: ntk.thanh@ucl.ac.uk
hUCL Healthcare Biomagnetics and Nanomaterials Laboratories, 21 Albemarle Street, London W1S 4BS, UK
First published on 5th August 2024
Abstract
Wound healing potential is initially evaluated using a chorioallantoic membrane (CAM) assay. Alginate gel containing pristine and polymer-coated magnesium hydroxide nanoparticles (MHNPs) improves the healing response. PEGylated MHNPs have shown a rapid wound-healing process in Gallus gallus domesticus by promoting re-epithelialization as compared to pristine MHNPs. No toxicity or inflammation are observed in the blood, liver, and heart tissues during biochemistry and histopathological examination. Polymer (polyethylene glycol (PEG) and chitosan) functionalized MHNPs are synthesized using a one-step hydrothermal process. The average crystallite size determined from X-ray diffraction analysis of the pristine MHNPs is ∼31.3 nm and upon PEG and chitosan coating it reduces to 23.4 nm and 29.7 nm, respectively. FTIR confirms the presence of functional groups indicating the successful functionalization of the polymers.
1. Introduction
In recent years, pathogens have been treated with metal and metal oxide NPs, which offer broad-spectrum antibacterial properties.1 Metal and metal oxide-based NPs have proven strong antibacterial potential both in preclinical and clinical therapeutic trials.2 The mode of action of NPs while attacking bacterial and cancerous cells is different as compared to medicinal drugs. The exact mechanism adopted by NPs has not yet been elucidated.3 However, it is assumed that they follow the model of introducing oxidative stress, localized discharge of metallic cations, immediate interaction with microbial cell walls, or intracellular disruption of organelles.4,5 The suitable choice of polymer increases the stability, minimizes the aggregation of NPs and improves the inherent properties of NPs.6 Surface modifications of metal oxide NPs with various natural or synthetic polymers are considered a tuning factor for sorption behaviors, redox reactions, changing surface chemistry, and controlling band gaps.7–10 PEG and chitosan are FDA-approved polymers and widely used in many biomedical applications. The conjugation of PEG with NPs is associated with the presence of a chemically active OH group at its linear ends and diverse functional groups at the surface of the NPs.11–14 PEG coating suppresses the immune response by an opsonization process and in this way the retention time or blood circulation time of a drug or NPs in a biological system increases.15 Chitosan is the second most abundant polymer after cellulose.16 The protonation ability of chitosan gives a positive charge on the surface of the NPs17 and can be effective against microbes that modulate the wound healing process.18,19Skin is the largest organ that separates the internal body medium from the external environment. It functions as a first line of defense between harmful pathogens and internal body organs. In this way, it maintains the internal bodily homeostasis. Wounds appear in the case of serious skin disruption; chronic wounds can have negative impacts on health and affect the quality of life.20 Wound healing is a complicated and ongoing process, completed in a series of phases to restore the physical integrity of damaged cells.21,22 In the case of poor hygienic conditions, infectious bacteria like Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) settle down at the wound site and can cause active infection by interfering with the wound healing process. These bacteria discharge various endotoxins, i.e. lipopolysaccharides (E. coli) and TSS-1 (S. aureus),23,24 that can induce fibrin and collagen lysis and slow down the proliferation of fibroblasts and epithelial cells.25 Bacteria consume more oxygen and nutrients at the expense of newly formed tissues, which degrades growth factors. This situation leads to tissue anoxia and thus healing of wounds is further delayed.26 Therefore, applying a strong antibacterial agent on the wound bed which can inhibit the growth of bacteria without damaging the healthy cells is necessary to accelerate the natural healing process of the body.27
Although nanoparticles have shown promising results in wound healing trials, toxicity, in vivo efficacy, scalability, and regularity approval from the FDA are major issues that need to be resolved for advanced research.28 Magnesium hydroxide is bio-safe. The FDA has recommended Mg(OH)2 as a GRAS (generally recognized as safe) ingredient and pH control agent in food and nutritional supplements.29 MHNPs are biocompatible and included in many pharmaceutical products due to their antibacterial action.30,31 The growth inhibition of both G+ and G− bacterial strains is reported by exposing them to MHNPs. The strong antimicrobial and biofilm inhibition actions are associated with the ability of MHNPs to produce a highly localized alkaline environment. Such an environment lowers the production of adenosine triphosphate molecules by consuming local H+, which results in an energy deficit and consequently limits the microbial cellular metabolism.32,33 The small size of NPs improves the cell internalization process and consequently favors antimicrobial action.34 Halbus et al. reported that the bactericidal properties of MHNPs are found in inverse relation to the particle size.5 The MHNPs accelerate the recovery of wounds by preventing bed sores during the healing process.35 Firstly, the process involves the anti-inflammatory action of Mg2+ ions. Secondly, the pro-angiogenic action of MgO in speeding up the healing process is linked with the expression of vascular endothelial growth (VEGF), which triggers angiogenesis, increases the number of blood vessels, and carries more nutrients to the wounded area.36 Thirdly, differentiation and proliferation of endothelial cells are stimulated. It increases the production of IL-12 secreted by neutrophils, which reduces the duration of reepithelization.37 Lastly, the strong antibacterial action of MHNPs towards pyogenic pathogens can increase the chronicity of wounds, making it a good wound-healing agent. Colloidal instability, early biodegradation, and aggregation of MHNPs limit their effectiveness; in the case of PEGylation, the PEG molecule remains over the surfaces of the NPs without entering into the core structure and thus can enhance the shelf life of the NPs,38,39 and in this way, potential biological applications of MHNPs can be optimized.
In the present work, pristine, PEG, and chitosan-functionalized MHNPs are synthesized using a one-step hydrothermal process. The pristine and polymer-coated nano-systems are characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared (FTIR) spectroscopy, photoluminescence12 spectroscopy, ultraviolet-visible spectroscopy (UV-vis spectroscopy) and dynamic light scattering (DLS). The wound healing potential of the pristine and surface-functionalized MHNPs is evaluated using CAM assay and in vivo (G. domesticus). To further understand the process, the antimicrobial response is evaluated on bacterial stains and biofilm inhibition.
2. Experimental section
2.1 Chemicals
The magnesium salt (magnesium nitrate hexahydrate (Mg(NO3)2·6H2O; DAEJUNG, Korea 98%)), PEG-6000 (DAEJUNG, Korea), chitosan (Icon Chemicals, Germany 90%), and NaOH pellets (Icon Chemicals, Germany 98%) were utilized as received.2.2 Synthesis of magnesium hydroxide NPs
MHNPs were synthesized by applying a one-step hydrothermal route. The experimental details were as follows: 0.5 M salt (Mg(NO3)2·6H2O) solution and 1 M base (NaOH) solution were prepared separately in 100 mL distilled water. Then the base solution was added to the salt solution very slowly (drop-wise) under vigorous magnetic stirring. To increase the homogeneity, the final mixture was sonicated for 30 min. Afterwards, the uniform mixture was put into Teflon-lined autoclaves and placed into an oven for 16 h at 180 °C. Then the reaction mixture was centrifuged to obtain the precipitates and washed in triplicate with distilled water to eliminate any by-products. The precipitates were placed in the oven overnight at 80 °C and then the dried precipitates were ground to powder form. Similarly, 0.1 g of PEG and chitosan were added separately to the reaction mixture and stirred for 30 min more before putting the salt solution into the Teflon-lined autoclave for hydrothermal treatment.402.3 Characterization tools
For structure analysis of bare and polymer functionalized MHNPs, XRD (D8 Advance-Bruker) in the 2θ range of 20 to 60° was used. Grain morphology was studied by SEM (NOVA NanoSEM). Surface chemistry was evaluated by FTIR (Agilent Technologies, Cary 630 FTIR). Energy band and optical behavior were determined by UV-vis spectrophotometer and PL spectra. The thermal stability of the pristine, PEGylated, and chitosan functionalized MHNPs was studied using the differential scanning calorimetry-thermogravimetric analysis (DSC-TGA) standard module using SDT Q600 V20.9 Build 20 under N2. Dynamic light scattering (DLS) was performed to determine the hydrodynamic size of the NPs using Zetasizer Ver. 7.11 Serial Number: MAL 1127001 (Malvern Inc). The students’ t-test was applied to analyze the data statistically.2.4 Antibacterial and biofilm inhibition assessment
The Kirby Bauer disk diffusion susceptibility protocol was employed to study the antibacterial activity of the nanomaterials for S. aureus and E. coli bacteria.41 Experiments for both antibacterial action and biofilm inhibition percentage were performed by following the protocols previously reported.422.5 Hemolysis percentage assessment
After written consent and taking ethical permission from university's ethical committee, 10 mL of human blood samples were taken from healthy donors in citrated or heparinized tubes (3.8% sodium citrate or lithium heparin).43 Then a mixture of 3 mL blood cells and 15 mL polystyrene was centrifuged for 5 min. For pH stability, the viscous pellets were washed in triplicate with a 5 mL sterile solution of isotonic phosphate buffer saline (PBS), maintained at 4 °C for 1 h. In this way, the cells were washed and then suspended in chilled and sterile PBS having a final volume of 20 mL. The suspension was kept in ice and diluted with sterile PBS to 7.068 × 108 cells per mL. Three sets of 20 mL solutions of MHNPs, PEG-coated MHNPs, and chitosan-coated MHNPs were formed. Triton X-100 and PBS were the positive and negative controls, respectively. The remaining 180 μL diluted blood cell suspension was kept aseptically in a 2 mL tube. The nanomaterial concentration was kept at 250 μmol L−1. The sample holding tubes were incubated for 30 min at 37 °C with a shaking speed of 80 rpm. Then the tubes were put in wet ice for 5 min and again centrifuged for 5 min. The samples were carefully collected and placed into a 1.5 mL tube where they were diluted with 900 μL of sterile and chilled PBS. Finally, all the tubes were kept in wet ice.44 For validation, three replicates were performed for each nanomaterial sample. Absorbance at wavelength 576 nm was determined by Bausch and Lomb Spectronic 1001 spectrometer using a quartz cuvette. A hemocytometer (Fisher ultra plane, Neubauer ruling) was used to count the cells. The percentage of hemolysis was determined by using eqn (1): | (1) |
2.6 Wound healing properties
 | (2) |
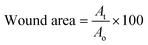 | (3) |
| Group | Avg. weight (g) | Wound healing agent |
|---|---|---|
| Control-1 | 292 ± 5 | Vetericyne plus®, poultry care spray |
| Control-2 | 313 ± 4 | Natural healing |
| A | 295 ± 2 | MHNPs |
| B | 305 ± 2 | PEG-MHNPs |
| C | 307 ± 2 | Chitosan-MHNPs |
3. Results and discussion
3.1 Synthesized material analysis
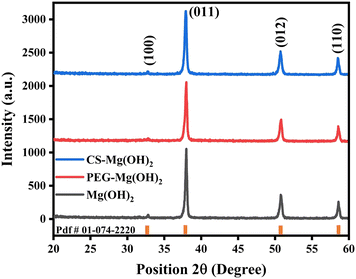
XRD is used as a characterization technique for the structural analysis of the synthesized nanomaterials. XRD patterns of pristine and polymer-functionalized MHNPs are shown in Fig. 1.The formation of single-phase MHNPs is confirmed by XRD with pdf # 01-074-2220 showing the hexagonal structure and belonging to the space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1. Four diffraction peaks are observed at positions 32.8°, 38°, 50.8°, and 58.6° which correspond to the (100), (101), (102), and (110) planes respectively in bare MHNPs. The PEG and chitosan functionalized MHNP patterns have identical behavior; however, the peaks at 38° and 50.8° are minutely shifted towards a lower angle, which may be attributed to the attachment of organic polymer during the reaction, subsequently changing the nanoparticle characteristics. The polymer coating has also shown effects on the structural parameters, which varied as compared to bare MHNPs (Table 2). Debye–Scherrer's relation is used to determine the crystallite size (eqn (4)).
m1. Four diffraction peaks are observed at positions 32.8°, 38°, 50.8°, and 58.6° which correspond to the (100), (101), (102), and (110) planes respectively in bare MHNPs. The PEG and chitosan functionalized MHNP patterns have identical behavior; however, the peaks at 38° and 50.8° are minutely shifted towards a lower angle, which may be attributed to the attachment of organic polymer during the reaction, subsequently changing the nanoparticle characteristics. The polymer coating has also shown effects on the structural parameters, which varied as compared to bare MHNPs (Table 2). Debye–Scherrer's relation is used to determine the crystallite size (eqn (4)).
 | (4) |
| Unit cell parameters | MHNPs | PEG-MHNPs | Chitosan-MHNPs |
|---|---|---|---|
| Crystallite size | 31.3 nm | 23.4 nm | 29.7 nm |
| Lattice cell dimensions | a = 3.149 Å, c = 4.772 Å | a = 3.148 Å, c = 4.771 Å | a = 3.149 Å, c = 4.772 Å |
| Unit cell volume | 4.73 × 10−29 m3 | 4.72 × 10−29 m3 | 4.73 × 10−29 m3 |
| Lattice strain | 1.23 × 10−3 | 1.77 × 10−3 | 1.31 × 10−3 |
The crystallite size of MHNPs is 31.3 nm which is slightly reduced to 29.7 nm in the case of chitosan-coated MHNPs; however, PEG functionalized MHNPs have a much-reduced crystallite size (23.4 nm) and the distinct behavior of crystallite aggregation is observed during the growth process. This variable behavior may be associated with the charge of organic polymers as PEG is a neutral to negatively charged open-branched polymer and chitosan has a positive charge owing to the residual amino groups. This diversity in charge of polymers can certainly dictate crystal growth. Furthermore, these organic moieties can affect the morphology, shape, and size distribution of NPs. The lattice dimensions and cell volume remain almost the same for the three materials while the lattice strain and dislocation densities vary significantly for PEG and chitosan-coated MHNPs.
Texture coefficient (T.C.) is used to determine favorable planes in which the crystalline material shows a preferred tendency to grow. The T.C. is calculated by eqn (5).
 | (5) |
If the T.C. is less than unity (T.C. < 1), the growth direction will be random, and a value more than 1 represents the preferred growth direction of crystals. The plane having the highest value of T.C. indicates the favored direction for crystallite growth.53 The MHNPs have the maximum value of T.C. for the (110) plane, indicating the thermo-dynamical possibility of crystalline growth along this direction. Whereas, when MHNPs are composited with PEG and chitosan, the favorable growth direction changes to (100) (Table 3).
| Position (2θ) | Planes | Texture coefficient | ||
|---|---|---|---|---|
| MHNPs | PEG-MHNPs | Chitosan-MHNPs | ||
| 32.8° | 100 | 0.926 | 1.480 | 1.535 |
| 38° | 011 | 0.927 | 0.810 | 0.840 |
| 50.8° | 012 | 0.874 | 0.879 | 0.780 |
| 58.6° | 110 | 1.262 | 0.829 | 0.842 |
It is believed that during the hydrothermal reaction, water molecules gradually decompose into solvated H+ (hydrogen cations) and OH− (hydroxide anions) under heating and pressure. The increase in temperature plays a vital role in the degree of water dissociation. At room temperature, the magnesium nitrate hexahydrate is not affected too much by the water; however, when the solution is exposed to high temperature and pressure, the magnesium salt is decomposed into Mg2+ cations and reacts with OH−, leading to the formation of Mg(OH)2. The detailed analysis of the mentioned chemical reactions shows that MgOH+ ions, i.e. hydroxo-complexes of Mg2+, contribute to the growth proceeding during the formation of magnesium hydroxide nanocrystals.54
Fig. 2(a) and (b) present the morphology and grain size of the MHNPs, the low and high magnification micrographs of MHNPs show spherical, hexagon, and rectangular-like grains with a mean size of 74 ± 17 nm (Fig. 2c). In the case of PEGylation of MHNPs, the shape of the grains is not much varied as compared to the pristine MHNPs (Fig. 2d and e), whereas grain size is determined as 72 ± 16 nm (Fig. 2f). Fig. 2 shows micrographs of chitosan-coated MHNPs where the mean size is reduced to 55 ± 13 nm (Fig. 2i). The t-test shows that the grain sizes of MHNPs and PEG–MHNPs are not statistically different with p = 0.1007 whereas the gain size of chitosan–MHNPs is statistically different than bare MHNPs (p < 0.0001). The grain size reduction is owing to the surface functionalization of MHNPs, where the polymer has controlled the coalescence of particles, and the composite formation of MHNPs with chitosan has lessened the atomic attachment during the grain growth process.55 XRD results also confirmed the decrease in crystallite size when PEG and chitosan are coated on MHNPs.
 | ||
| Fig. 2 SEM micrographs at different magnifications and corresponding size distribution histograms:55 (a)–(c) MHNPs, (d)–(f) PEG functionalized MHNPs, (g)–(i) chitosan coated MHNPs. | ||
The absorption bands are shown at 289 nm, 285 nm, and 290 nm in the ultraviolet region for MHNPs, PEG-MHNPs, and chitosan-MHNPs, respectively (Fig. 3a). The blue shift in the wavelength from 320 nm to 285 nm in the functionalized MHNPs may be attributed to the attachment of polymer molecules, i.e. PEG and chitosan with MHNP crystals.56 The slight variations and appearance of absorption peaks in the band-gap correspond to the band-to-band transition.57Fig. 3b shows Tauc's plots, which indicate that the band gap of MHNPs is 3 eV and varied with PEG and chitosan-coated NPs as 2.86 eV and 2.94 eV, respectively. Further optical properties investigation by PL shows two emission peaks at 434 nm and 455 nm in all the cases, i.e. bare and polymer-coated MHNPs using an excitation wavelength of 400 nm (Fig. 3c). The emission bands are at the border of the ultraviolet and visible regions and can be attributed to the violet emission. The emission peaks can be attributed to the transition of different Mg2+ and O2− states in the magnesium hydroxide's electronic structure.58 Within the band gap region of the MHNP crystals, the defect states are expected due to the oxygen vacancies.
FTIR spectra are recorded in the range from 650 cm−1 to 4000 cm−1. In the case of pristine MHNPs, the bands at 652 cm−1 and 854 cm−1 wavenumbers are attributed to the metal–oxygen bonding. The band at 1422 cm−1 indicates the symmetrical–asymmetrical vibrations of the carboxylate O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group and 1492 cm−1 shows the stretching modes of –OH. Additionally, two bands at 2938 cm−1 and 3685 cm−1 wave numbers correspond to the out-of-plane bending of C–H and stretching mode of O–H, respectively. It is well established that MgO surfaces when exposed to the atmosphere readily absorb H2O and CO2 molecules, which results in the formation of MHNPs and the emergence of other functional groups in the FTIR spectrum. In PEGylated MHNPs, there are new bands observed at 1105 cm−1, 1342 cm−1, and 1607 cm−1 which belong to C–O–H, C–H, and C
O functional group and 1492 cm−1 shows the stretching modes of –OH. Additionally, two bands at 2938 cm−1 and 3685 cm−1 wave numbers correspond to the out-of-plane bending of C–H and stretching mode of O–H, respectively. It is well established that MgO surfaces when exposed to the atmosphere readily absorb H2O and CO2 molecules, which results in the formation of MHNPs and the emergence of other functional groups in the FTIR spectrum. In PEGylated MHNPs, there are new bands observed at 1105 cm−1, 1342 cm−1, and 1607 cm−1 which belong to C–O–H, C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.59 Whereas in the case of chitosan-coated NPs, C–O–C and C
O bonds, respectively.59 Whereas in the case of chitosan-coated NPs, C–O–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are observed at 1046 cm−1 and 1572 cm−1 wave numbers. The emergence of new bonds confirms the attachment of organic polymers on the surface of the NPs.60Fig. 4b represents the hydrodynamic sizes of the pristine and polymer-coated MHNPs. In the case of polymer coating, i.e. PEG and chitosan, the average grain size increases as compared to pristine MHNPs. Introducing hydrophilic polymers like PEG and chitosan over the NPs tends to increase the material's hydrodynamic size in liquid due to the formation of hydrated layers, which are linked with the long-chain structure of the polymers.61 The charged surface of polymer functionalized NPs induces an electrostatic repulsion that helps to limit the further aggregation of NPs and ensure their suspension stability.62 Therefore, DLS analysis can also confirm the presence of the PEG and chitosan over the MHNPs with the availability of a large number of molecules. The difference in grain size between SEM and hydrodynamic size from DLS can be attributed to the presence of organic molecules i.e. PEG and chitosan on the surface of the MHNPs. The increase in the hydrodynamic size of the bare MHNPs is linked with mild aggregation in the solvent and can be overcome by longer ultrasonication time before DLS measurements.
O bonds are observed at 1046 cm−1 and 1572 cm−1 wave numbers. The emergence of new bonds confirms the attachment of organic polymers on the surface of the NPs.60Fig. 4b represents the hydrodynamic sizes of the pristine and polymer-coated MHNPs. In the case of polymer coating, i.e. PEG and chitosan, the average grain size increases as compared to pristine MHNPs. Introducing hydrophilic polymers like PEG and chitosan over the NPs tends to increase the material's hydrodynamic size in liquid due to the formation of hydrated layers, which are linked with the long-chain structure of the polymers.61 The charged surface of polymer functionalized NPs induces an electrostatic repulsion that helps to limit the further aggregation of NPs and ensure their suspension stability.62 Therefore, DLS analysis can also confirm the presence of the PEG and chitosan over the MHNPs with the availability of a large number of molecules. The difference in grain size between SEM and hydrodynamic size from DLS can be attributed to the presence of organic molecules i.e. PEG and chitosan on the surface of the MHNPs. The increase in the hydrodynamic size of the bare MHNPs is linked with mild aggregation in the solvent and can be overcome by longer ultrasonication time before DLS measurements.
 | ||
| Fig. 4 (a) FTIR, (b) DLS, and (c) TGA profile of bare MHNPs, PEG, and chitosan-coated MHNPs, and (d) first TGA derivative weight loss percentage of MHNPs. | ||
The TGA curves indicate that weight loss for all three nano-systems occurs in different phases (Fig. 4c). Thermal decomposition of the NPs is started soon after the temperature rises; the first weight loss phase is observed below 100 °C, and there is only a 3% weight (inset Fig. 4c) change in the temperature range from 37 to 42 °C, which indicates good water and structural retaining ability that can play a crucial role in the antibacterial activity of the bare and functionalized MHNPs by prolonging the interaction with the cell wall of bacteria.63 For bare MHNPs, the weight loss continues until 300 °C gradually up to 12%. Afterward, 18% weight loss is observed abruptly from 300 °C indicating the removal of absorbed water and decomposition of chemical bonds followed by 8% loss from 600 °C. In the case of PEGylation, there is a gradual weight loss of 20% until 300 °C, and then a further 30% loss is witnessed until 400 °C. In the end, a 12% gradual decrease happened until 750 °C. The chitosan-coated NPs have the least decomposition until 300 °C i.e. 8% followed by the decomposition of the MHNPs until 400 °C (32%). Afterward, there is a gradual decrease of 7–8% until the final temperature, i.e. 1000 °C. The residual masses of Mg(OH)2, PEG - Mg(OH)2, and chitosan—Mg(OH)2 determined from TGA are 58%, 35%, and 46%, respectively. The first derivative weight loss temperatures are also determined for all the materials and it is observed that this temperature is higher in the case of PEG functionalized MHNPs (367 °C) than bare and chitosan conjugated NPs, that is 321 °C and 316 °C, respectively (Fig. 4d). This also indicates the higher stability of PEG-coated MHNPs than bare and functionalized counterparts.
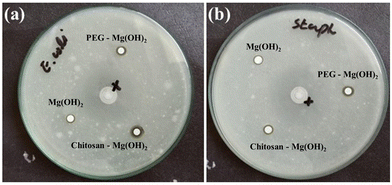
3.2 Antibacterial activity and biofilm inhibition
Before studying the wound healing potential of MHNPs and any potential effects of PEG and chitosan coatings on these properties, it is quite important to evaluate the antibacterial response of all three nano-formulations, as the wounded area is a more exposed and vulnerable site for pathogens to settle down and grow.64 Therefore, the antibacterial action of MHNPs against E. coli and S. aureus bacterial strains was studied. The zones of inhibition of the bacterial strains against the nanomaterials are expressed in Table 4 and shown in Fig. 5, which represents that MHNPs have a good antibacterial response and this potential is enhanced by surface functionalization. It is observed that PEG-modified MHNPs perform significantly better against S. aureus (p = 0.001) whereas E. coli is more affected by chitosan-coated MHNPs (p = 0.002) (Table 4). Uneven bacterial growth is present in both bacterial strains due to the deployment of biofilms in some regions of the Petri plates. The MHNPs with a size less than 70 nm showed better antibacterial action;5 firstly, smaller-size MHNPs offer better cellular internalization where they interact with bacterial DNA and other organelles, and secondly, they adsorbed with the bacterial cell wall and release Mg2+ ions from the hydroxide structure, which damaged the cell wall.65,66 Although the precise bactericidal or bacteriostatic mechanism of MHNPs has not yet been elucidated, generally the antibacterial response of NPs is explained by one of these three suggested models; generation of reactive oxygen species, release of metallic ions, and introduction of nonoxidative stress mechanisms.67 The generation of ROS needs O2 as a precursor but MHNPs have shown an antibacterial response even in anaerobic conditions, indicating that the high concentration of OH−1 ions can directly damage the cell wall due to electrostatic van der Waals interaction.30 The ability of MHNP penetration in the cell membrane and protein leakage of S. aureus is elevated by increasing the concentration of MHNPs. On attaching to the bacterial surface, MHNPs bind themselves with the cell wall, and the penetrated NPs reduce the metabolic activities by inhibiting protein synthesis.4 The bound NPs disintegrate the cell wall structure and cause bacterial death by leakage of cellular contents i.e. leakage of nucleic acid.68 The cell walls of E. coli and S. aureus are composed of a crossed-linked network of peptidoglycan, which is vulnerable to mechanical ruptures, and once this happens, bacteria cannot repair these injuries.69 This cell wall disintegration can be linked with the alkaline nature of MHNPs with a localized pH value of ≈10. This causes the disturbance of bacterial cellular homeostasis.70 The alkaline adaptability of E. coli and S. aureus decreases by increasing the pH value. In the literature, optimum growth of E. coli is recorded from weak acidic conditions to neutral (pH 5.6 to 7.0) at 37 °C71 and E. coli remained resistant up to pH 8 whereas above pH 8, inhibition of E. coli growth increased (55% at pH 10). Likewise, the growth of S. aureus is inhibited 82% at pH ≈ 10 compared to the optimum conditions (pH 6–7) at 30 to 37 °C.| Sample | E. coli (ZOI) mm | S. aureus (ZOI) mm |
|---|---|---|
| Ciprofloxacin (control) | 30 ± 1 | 26 ± 1 |
| MHNPs | 12 ± 1 | 11 ± 1 |
| PEG-MHNPs | 15 ± 2 | 18 ± 1 |
| Chitosan-MHNPs | 21 ± 2 | 14 ± 3 |
The antibacterial action of MHNPs also lies in their unique hygroscopic nature. This property is enhanced by modifying the surfaces of MHNPs with hygroscopic polymers like PEG and chitosan as evidenced by Table 4 where the inhibition zones are increased when MHNPs are coated with PEG and chitosan. Both PEG and chitosan have an excellent ability to retain water.72 The conjugation of these polymers with MHNPs increases the adsorption of water molecules on the surface of the MHNPs and forms a very thin moist meniscus due to capillary action. Thus, the MHNPs remain in contact with the bacteria and damage the bacterial membrane, hence, causing cellular destruction.73,74 PEG and chitosan coatings introduce slightly negative and positive charges respectively on the surface of MHNPs. PEG-coated NPs can develop strong electrostatic interaction with S. aureus as compared to E. coli and that's why PEG-MHNPs have performed well against G+ rather than G− bacteria; conversely, due to the appearance of positive charge, chitosan-coated MHNPs are more effective against E. coli than S. aureus. This electrostatic attraction prolongs the attachment of polymer-coated NPs with bacterial cells and allows invasion from multiple bacterial sites, which reduces the chances to develop resistance against these nanomaterials, consequently causing cell death.
The biofilm inhibition potential of the NPs was also studied for E. coli and S. aureus bacterial strains. Biofilms are three-dimensional, heterogeneous biopolymer colonial structures of planktonic microorganisms organized on an extracellular polymeric matrix or solid surfaces.75 This form of bacteria is stable, stress-resistant, and shows great resilience against unfavorable environmental conditions like temperature, drying out, UV exposure, desiccation, and starvation.76 The formation of a biofilm is due to the development of interconnected complex biochemical aggregation of lipids, proteins, polysaccharides, nucleic acids, and glycol-peptides through maturation, colonization, and adhesion of bacterial cells. In this way, bacterial life organization resembles a multicellular organism and the production of super antigen protein makes it difficult to combat bacteria with traditional antibiotics.77 The results have shown that PEG and chitosan coatings have increased the inhibition potential of MHNPs for both E. coli and S. aureus biofilms and from the two polymers, chitosan-coated MHNPs are more effective against E. coli and S. aureus-based biofilms (p < 0.0001) (Table 5). The biofilm formation can be controlled by limiting the surface colonization of bacteria.78 The biochemical interaction of NPs with biofilms is a step-wise process; (i) introduction of NPs on the biofilm's surface,79 attachment of NPs to the biofilm's surface, and (iii) penetration of NPs into the biofilms, implemented by physicochemical interaction of NPs, extracellular polymeric matrix and environmental conditions. The surface charge induced due to polymer coating,80 initially, develops electrostatic attraction with S. aureus and E. coli and then migrates into the biofilm by diffusion at a different rate. The diffusion rate depends on the size of NPs and the pore size of the biofilm,81 and smaller-sized NPs diffuse faster; moreover, organic molecules from PEG and chitosan disperse more easily into the biofilm matrix.82 This shows that the biofilm inhibition ability of MHNPs is improved by surface coating with PEG and chitosan; however, chitosan coating functions slightly better as compared to PEG. The bacterial growth is correlated with the number of microorganisms in the population and the rate of change with respect to time. The optical density (OD) method determines the portion of the light passing through the bacterial suspension. More scattering of light indicates the presence of more bacteria and vice versa.83 It is observed that chitosan-coated MHNPs have statistically significant loss of optical density (less scattering), indicating inhibition of bacteria more efficiently.
| Samples | Optical density | Inhibition (%) | |||
|---|---|---|---|---|---|
| OD control (PBS) | OD S. aureus | OD E. coli | S. aureus | E. coli | |
| a Significant. b Very significant. c Extremely significant. | |||||
| Positive control (Ciprofloxacin) | 0.207 ± 0.001 | 0.013 ± 0.002 | 0.011 ± 0.002 | 93.7 ± 0.3 | 94.7 ± 0.3 |
| MHNPs | 0.207 ± 0.001 | 0.13 ± 0.02 | 0.12 ± 0.02 | 37.2 ± 1.2 | 42.0 ± 2.5 |
| PEG-MHNPs | 0.207 ± 0.001 | 0.10 ± 0.01 | 0.11 ± 0.01 | 51.7 ± 2.7b | 46.8 ± 1.3a |
| Chitosan-MHNPs | 0.207 ± 0.001 | 0.080 ± 0.01a | 0.070 ± 0.01a | 61.4 ± 1.1c | 66.2 ± 0.5c |
3.3 Hemolytic activity
To evaluate the potential applicability as a therapeutic agent against complex diseases or in medical implants, it is necessary to test their hemocompatibility before recommending their administration in vivo.43 Hemolytic activity is one of the most fundamental tests that explains the interaction of NPs with blood components; it is an obvious finding of the destruction of RBCs measured by spectroscopic analysis, which causes the discharge of hemoglobin in the body fluids. According to the classification made by the American Society for Testing and Materials (ASTM F 756-00, 2000), materials are categorized into three groups: hemolytic (>5% hemolysis), hemolytic (2–5% hemolysis) and non-hemolytic (<2% hemolysis).84 Higher hemolytic activity may cause fatal pathological situations. Therefore, determining the in vitro and in vivo biocompatibility of NPs is a necessary part of preclinical studies. The percentage hemolysis provides the potential of the drug to rupture the cell membrane of erythrocytes85 and, consequently, indicates cytotoxicity of the injected nanomaterials. The hemolytic activity of pristine and surface-coated MHNPs is given in Table 6. It has been noticed that the percentage hemolysis of MHNPs is reduced when combined with organic polymers i.e. from 2.83% to 0.48% and 0.19% for PEG and chitosan, respectively. This indicates higher biocompatibility of polymer surface functionalized MHNPs. Chen et al. showed that the size of the NPs is a major contributor to their uptake efficiency, toxic distribution in the bloodstream, and translocation to different organs as compared to shape, structure, and surface functionalization. This is also consistent with our findings as crystallite size decreases with the introduction of polymers during the synthesis phase of MHNPs.3.4 Wound healing analysis
 | ||
| Fig. 6 Wound healing using CAM assay; (a) C1 = Alginate gel, (b) C2 = No alginate gel, (c) 1K1 = MHNPs, (d) 1K2 = PEG-coated MHNPs, (e) 1K3 = chitosan coated MHNPs. | ||
Keeping the wound bed moist and clean is the most important condition that promotes the healing process, especially in the case of open wounds.86 The hygroscopic nature of MHNPs can be useful in absorbing wound exudates and keeping the wound bed clean, which is usually achieved by ointments. The antibacterial potential of magnesium hydroxide can additionally safeguard the wound from airborne infectious bacteria.87 The swelling behavior of bare and polymer-coated magnesium hydroxide NPs incorporated into the gel is also observed by investigating their ability to absorb water content. It can be observed from Fig. 7 that the swelling behavior of the alginate gel is affected by the addition of NPs. Alginate gel has a higher swelling percentage and this is reduced by the addition of NPs on the gel. From three composite gels, the gel incorporated with the pristine MHNPs has the lowest swelling percentage, whereas, in the case of polymer coatings, i.e. PEG and chitosan, the swelling percentage is increased indicating the role of the polymer over the surface of the MHNPs. Alginate gel containing PEGylated MHNPs swelled relatively more over time (p < 0.0001) than the chitosan-functionalized MHNP modified gel. The swelling saturation in the gels is observed for up to 5 h, and afterward remains the same for 24 h.
 | ||
| Fig. 8 Photographic analysis of wound healing on the 1st, 3rd, 5th, 7th, and 9th day after incision on G. domesticus. | ||
 | ||
| Fig. 9 (a) Wound healing analysis in terms of healing rate (%) on the 3rd, 5th, 7th and 9th day. **Very significant, ***extremely significant, (b) skin remodeling process. | ||
Fig. 9a indicates the percentage of wound healing based on the changes that occurred in the wounds as compared to the state of the wounds on the first day. PEGylated MHNPs and chitosan-coated MHNPs have shown promising potential to close and heal wounds as compared to pristine MHNPs. The wound healing % for bare MHNPs is 41.83 ± 0.17%, 52.44 ± 0.9%, 64.56 ± 1.78%, and 84.77 ± 1.26% for the 3rd, 5th,7th, and 9th day, respectively, whereas, in the case of polymer coating, the healing rate is increased significantly for PEGylated MHNPs, and it is 63.93 ± 1.06%, 70.62 ± 1.7%, 83.49 ± 1.27% and 96.04 ± 0.89% and 64.65 ± 0.91%, 80.03 ± 1.63%, 81.60 ± 0.43% and 89.76 ± 0.2% for chitosan-coated MHNPs. MHNPs are currently used as antacids that can be useful for avoiding bed sores during the healing phase. However, the role of Mg (trace element) in wound healing is still not well defined.89 Pollack has discussed that trace elements like Mg can be useful in the wound-healing process.90 Pastorfide et al. found that the application of MHNPs ointment on the wounds of obstetrical patients is very safe and accelerates wound healing.91 Lange et al. explained that Mg cations can promote wound healing due to their ability to promote β1 integrin-mediated adhesion of dermal fibronectin and migration of keratinocytes to different extracellular matrix proteins.92 Chitosan is a naturally bioactive polymer; the ability of protonation of amino groups with the polysaccharide network of chitosan enabled its solubility in an acidic medium, whereas depolymerization of chitosan into N-acetyl-D-glucosamine gradually triggers the fibroblast proliferation, helps in orderly deposition of collagen and initiates the synthesis of hyaluronic acid at the wound bed. The positively charged chitosan-coated MHNPs might have aggregated with the negatively charged erythrocytes, platelets, and fibrinogen, and this electrostatic interaction facilitates blood hemostasis.21 As we have discussed in the earlier section the coating of chitosan has increased the antibacterial response of MHNPs, and therefore enhanced antibacterial potential might have inhibited the growth of bacteria over the wound bed, prevented inflammation and infection, and also increased the healing process (Fig. 9b). Chen et al. showed that the nontoxic nature of PEG promotes reepithelization when open wounds are treated with PEGylated systems. The appearance of a scar on the skin is very common during the wound healing phase; although the collagen composition of the scar cells is similar to the cells it replaces, there is a difference in the fiber composition of the protein. The scar tissues are affected by the fibrinolysis and fibrinogenesis balance. In normal healthy tissues collagen fibers obtain randomly formed basket weave structures while in fibrosis, fibers cross-link and align themselves in one particular direction.93 As PEG decreases the toxicity and increases the retention time of NPs,39 the wounds treated with PEGylated MHNPs show improved healing and the skin regenerates its normal texture (Fig. 8) and shows that it has the potential to heal the wounds. Less inflammation and negligible scarring are due to the antibacterial response and better tissue adhesion ability of PEGylated MHNPs. Our results suggest that the MHNPs coated with bioactive, nontoxic, and biodegradable polymers like PEG and chitosan can be included in wound healing ointments.
3.5 Toxico-patho-biochemistry examination
To investigate any toxicity induced by the magnesium hydroxide NPs in the case of absorption into the bloodstream, the complete hematological examination and blood serum biochemistry profile of Gallus gallus domesticus were evaluated. The values of red blood cells, white blood cells, hemoglobin, and platelet count are normal in all groups with negligible variations (Table S1, ESI†). Blood and serum biochemistry analysis (Table S2, ESI†) indicates that blood glucose, uric acid, blood urea, serum triglyceride, and serum cholesterol are normal. However, there is a minute increase in the alanine aminotransferase,45 which is also known as serum glutamic-pyruvic transaminase (SGPT), of those chicks that are treated with pristine and polymer-coated MHNPs that indicates a slight variation in the liver functioning which is not considered malfunctioning, high-density lipoprotein (HDL) value, and histopathology shows that the liver is functioning normally. So there are no toxic effects and major variations observed in the blood and serum of chicks.94 All the readings are statistically analyzed by two-way ANOVA followed by Tukey's test and significance is accepted at p < 0.05.Fig. 10 also indicates that there are no residual toxic effects seen on the heart tissues of the chicks. All the cardiac myocytes are normal, inflammation and necrosis are not seen and there is no sign of fatty change and cellular atypia. Similarly, the livers of all the chicks are normal and functioning well, hepatocytes are normal and are surrounded by a central vein and there is no indication of fat deposition or inflammation in the liver tissues. Liver parenchymal changes and high vascularity are not observed, which ruled out the possibility of tumor formation.95 These results have shown that MHNPs in the pristine state and particularly coated with PEG and chitosan induced no intoxication on the organs and can be recommended for use in wound healing recipes for fast recovery.
 | ||
| Fig. 10 Histopathological micrographs of heart and liver tissues of G. domesticus treated with MHNPs. | ||
4. Conclusion
Surface-modified MHNPs, particularly PEG, have accelerated the healing of skin wounds of Gallus gallus domesticus. No toxic effects were observed in blood and serum composition. Histopathological examination of the liver and heart has also confirmed the normal state of these organs. The size reduction due to polymer coating helped the cellular internalization and increased the stability at lower temperatures which enhanced the antibacterial action against bacterial strains. The biofilm inhibition potential of chitosan functionalized magnesium hydroxide NPs was 66.10% and 61.36% against E. coli and S. aureus, respectively. These findings indicate that surface-modified MHNPs, specifically PEGylated NPs, are safe to use in the case of minor dermal injuries/cuts of birds and can be extended to treat mammalian skins.Author contributions
MKA: methodology, formal analysis, writing – original draft, YJ: conceptualization, supervision, resources, writing – review and editing, NAS: resources, formal analysis, writing – review and editing, MS: resources, data curation, BA: validation, writing – review and editing, EY: methodology, data curation, SKS: writing – review and editing, NTKT: funding acquisition, data analysis, writing – critical review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors have no conflict to declare.References
- S. Zhang, et al., Antimicrobial properties of metal nanoparticles and their oxide materials and their applications in oral biology, J. Nanomater., 2022, 2063265 CrossRef CAS.
- F. Aflakian, et al., Nanoparticles-based therapeutics for the management of bacterial infections: a special emphasis on FDA approved products and clinical trials, Eur. J. Pharm. Sci., 2023, 106515 CrossRef CAS PubMed.
- Y. Wang, et al., Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis, Friction, 2023, 11(8), 1371–1394 CrossRef CAS.
- Y. Zhu, et al., Mg(OH)2 nanoparticles enhance the antibacterial activities of macrophages by activating the reactive oxygen species, J. Biomed. Mater. Res., Part A, 2021, 109(11), 2369–2380 CrossRef CAS PubMed.
- A. F. Halbus, T. S. Horozov and V. N. Paunov, Controlling the antimicrobial action of surface modified magnesium hydroxide nanoparticles, Biomimetics, 2019, 4(2), 41 CrossRef CAS PubMed.
- Y. Luo, et al., Size controlled fabrication of enzyme encapsulated amorphous calcium phosphate nanoparticle and its intracellular biosensing application, Colloids Surf., B, 2021, 201, 111638 CrossRef CAS PubMed.
- G. Unnikrishnan, et al., PVA–CS polymeric system conjugated with GO–ZnO–Ag 2 O ternary composite–a multifunctional nanocomposite for wound healing applications, New J. Chem., 2024, 48(19), 8908–8925 RSC.
- H. Liao, et al., An intrinsically self-healing and anti-freezing molecular chains induced polyacrylamide-based hydrogel electrolytes for zinc manganese dioxide batteries, J. Energy Chem., 2024, 89, 565–578 CrossRef CAS.
- J. Yuan, et al., Biomimetic peptide dynamic hydrogel inspired by humanized defensin nanonets as the wound-healing gel coating, Chem. Eng. J., 2023, 470, 144266 CrossRef CAS.
- M. Zhao, et al., Stem Cell-Derived Nanovesicles Embedded in Dual-Layered Hydrogel for Programmed ROS Regulation and Comprehensive Tissue Regeneration in Burn Wound Healing, Adv. Mater., 2024, 2401369 CrossRef CAS PubMed.
- C.-A. Cheng, et al., A responsive mesoporous silica nanoparticle platform for magnetic resonance imaging-guided high-intensity focused ultrasound-stimulated cargo delivery with controllable location, time, and dose, J. Am. Chem. Soc., 2019, 141(44), 17670–17684 CrossRef CAS PubMed.
- H. Huang, et al., One-step fabrication of PEGylated fluorescent nanodiamonds through the thiol-ene click reaction and their potential for biological imaging, Appl. Surf. Sci., 2018, 439, 1143–1151 CrossRef CAS.
- M. Matsumoto, M. Matsusaki and M. J. Mb Akashi, Preparation of biodegradable peptide nanospheres with hetero PEG brush surfaces, Macromol. Biosci., 2014, 14(1), 142–150 CrossRef CAS PubMed.
- B. Yin, et al., Intrapulmonary cellular-level distribution of inhaled nanoparticles with defined functional groups and its correlations with protein corona and inflammatory response, ACS Nano, 2019, 13(12), 14048–14069 CrossRef CAS PubMed.
- L. Shi, et al., Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery, Nanoscale, 2021, 13(24), 10748–10764 RSC.
- C. P. Jiménez-Gómez and J. A. Cecilia, Chitosan: a natural biopolymer with a wide and varied range of applications, Molecules, 2020, 25(17), 3981 CrossRef PubMed.
- S. M. Dadou, et al., Effect of protonation state and N-acetylation of chitosan on its interaction with xanthan gum: a molecular dynamics simulation study, Mar. Drugs, 2017, 15(10), 298 CrossRef PubMed.
- K. Azuma, et al., Chitin, chitosan, and its derivatives for wound healing: old and new materials, J. Funct. Biomater., 2015, 6(1), 104–142 CrossRef CAS PubMed.
- G. Unnikrishnan, et al., Preparation and characterizations of antibacterial and electroactive polymeric composites for wound healing applications, Polym. Compos., 2024, 45(1), 267–285 CrossRef CAS.
- H. Nosrati, et al., Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis, J. Nanobiotechnol., 2021, 19(1), 1–21 CrossRef PubMed.
- S. Ahmed and S. Ikram, Chitosan based scaffolds and their applications in wound healing, Achiev. Life Sci., 2016, 10(1), 27–37 Search PubMed.
- M. Liu, et al., Incorporation of magnesium oxide nanoparticles into electrospun membranes improves pro-angiogenic activity and promotes diabetic wound healing, Biomater. Adv., 2022, 133, 112609 CrossRef CAS PubMed.
- U. Mamat, et al., Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins, Microb. Cell Fact., 2015, 14, 1–15 CrossRef CAS PubMed.
- M. M. Dinges, P. M. Orwin and P. M. Schlievert, Exotoxins of Staphylococcus aureus, Clin. Microbiol. Rev., 2000, 13(1), 16–34 CrossRef CAS PubMed.
- K. Ou, et al., Covalently grafting polycation to bacterial cellulose for antibacterial and anti-cell adhesive wound dressings, Int. J. Biol. Macromol., 2024, 269, 132157 CrossRef CAS PubMed.
- S. Ghildiyal, et al., Wound healing and antimicrobial activity of two classical formulations of Laghupanchamula in rats, J. Ayurveda Integr. Med., 2015, 6(4), 241 CrossRef PubMed.
- J. cQu, et al., Hybrid nanocomposite multinetwork hydrogel containing magnesium hydroxide nanoparticles with enhanced antibacterial activity for wound dressing applications, Polymer, 2022, 251, 124902 CrossRef.
- D. Chen, et al., Key difference between transition state stabilization and ground state destabilization: increasing atomic charge densities before or during enzyme–substrate binding, Chem. Sci., 2022, 13(27), 8193–8202 RSC.
- Y. Liu, et al., Fabrication and research of Mg(OH)2/PCL/PVP nanofiber membranes loaded by antibacterial and biosafe Mg(OH)2 nanoparticles, Polym. Test., 2022, 112, 107635 CrossRef CAS.
- Y. Meng, et al., Antimicrobial activity of nano-magnesium hydroxide against oral bacteria and application in root canal sealer, Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res., 2020, 26, e922920–1 CAS.
- M. Rajkumar, et al., Green synthesis of gelatin-loaded magnesium hydroxide nanocomposite biomaterial using Coleus amboinicus leaf extract for enhanced antibacterial, antioxidant, anticholinergic, and wound healing activities, J. Mater. Res., 2024, 39(4), 548–564 CrossRef CAS.
- J. Tan, et al., Effect of local alkaline microenvironment on the behaviors of bacteria and osteogenic cells, ACS Appl. Mater. Interfaces, 2018, 10(49), 42018–42029 CrossRef CAS PubMed.
- H. Qin, et al., Anti-biofilm properties of magnesium metal via alkaline pH, RSC Adv., 2015, 5(28), 21434–21444 RSC.
- A. Hoshino, et al., Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification, Nano Lett., 2004, 4(11), 2163–2169 CrossRef CAS.
- A. Alimohammad, et al., A study of the effect of magnesium hydroxide on the wound healing process in rats, Med. J. Islamic World Acad. Sci., 2006, 16(4), 165–170 Search PubMed.
- S. Alizadeh, et al., Enhancing Diabetic Wound Healing Through Improved Angiogenesis: The Role of Emulsion–Based Core–Shell Micro/Nanofibrous Scaffold with Sustained CuO Nanoparticle Delivery, Small, 2024, 2309164 CrossRef CAS PubMed.
- R. R. Al-Ajeli, A.-H. M. Al-Hasan and S. S. Al-Mahmood, The Role of Magnesium Oxide Nanoparticles In The Healing Process of Cervical Esophageal Anastomosis in Dogs: An Experimental study, Egypt. J. Vet. Sci., 2022, 53(4), 547–557 Search PubMed.
- S. Shi, et al., High-Fidelity End-Functionalization of Poly (ethylene glycol) Using Stable and Potent Carbamate Linkages, Angew. Chem., Int. Ed., 2020, 59(41), 18172–18178 CrossRef CAS PubMed.
- T. L. Lasseter, et al., Covalently modified silicon and diamond surfaces: resistance to nonspecific protein adsorption and optimization for biosensing, J. Am. Chem. Soc., 2004, 126(33), 10220–10221 CrossRef CAS PubMed.
- D. Jin, et al., Hydrothermal synthesis and characterization of hexagonal Mg (OH) 2 nano-flake as a flame retardant, Mater. Chem. Phys., 2008, 112(3), 962–965 CrossRef CAS.
- J. Hudzicki, Kirby-Bauer disk diffusion susceptibility test protocol. American society for microbiology, 2009. vol. 15, pp. 55–63 Search PubMed.
- M. K. Abbas, et al., Polyethylene glycol dictates the therapeutic response (anticancer and wound healing) of silver oxide nanomaterials, Polym. Adv. Technol., 2023, 34(8), 2606–2619 CrossRef CAS.
- W. Zhou, et al., Synthesis and hemolytic activity of magnesium hydroxide nanoparticles, Adv. Mater. Res., 2014, 971–973, 228–231 Search PubMed.
- M. A. Dobrovolskaia, et al., Method for analysis of nanoparticle hemolytic properties in vitro, Nano Lett., 2008, 8(8), 2180–2187 CrossRef CAS PubMed.
- M. Zahid, et al., Development of hydrogels with the incorporation of Raphanus sativus L. seed extract in sodium alginate for wound-healing application, Gels, 2021, 7(3), 107 CrossRef CAS PubMed.
- I. Liakos, et al., Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents, J. Mater. Chem. B, 2015, 3(8), 1583–1589 RSC.
- A. Krajca and R. Juranova, Anesthesia in poultry, Vet. Med., 1994, 39(1), 23–27 CAS.
- H. Zhao, et al., Function of Chick Subcutaneous Adipose Tissue During the Embryonic and Posthatch Period, Front. Physiol., 2021, 12, 684426 CrossRef PubMed.
- W. Zhang, et al., Preparation of Mg(OH)2 nanosheets and self-assembly of its flower-like nanostructure via precipitation method for heat-resistance application, Integr. Ferroelectr., 2015, 163(1), 148–154 CrossRef CAS.
- P. Wang, et al., Morphology control and growth mechanism of magnesium hydroxide nanoparticles via a simple wet precipitation method, Ceram. Int., 2011, 37(8), 3365–3370 CrossRef CAS.
- R. G. Sibbald, et al., Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers, Adv. Skin Wound Care, 2007, 20(10), 549–558 CrossRef PubMed.
- A. Ullah, et al., Mechanistic insight of dye degradation using TiO2 anchored α-MnO2 nanorods as promising sunlight driven photocatalyst, Mater. Sci. Eng., B, 2021, 271, 115257 CrossRef CAS.
- S. Thanikaikarasan, et al., Characterization of electroplated FeSe thin films, J. Mater. Sci.: Mater. Electron., 2009, 20, 727–734 CrossRef CAS.
- S. Yousefi, et al., Optical properties of MgO and Mg(OH)2 nanostructures synthesized by a chemical precipitation method using impure brine, J. Alloys Compd., 2017, 711, 521–529 CrossRef CAS.
- N. Fleitas-Salazar, et al., Effect of temperature on the synthesis of silver nanoparticles with polyethylene glycol: new insights into the reduction mechanism, J. Nanopart. Res., 2017, 19(3), 113 CrossRef.
- R. De Silva, et al., Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties, Carbohydr. Polym., 2017, 157, 739–747 CrossRef CAS PubMed.
- P. Tyagi and A. Vedeshwar, Grain size dependent optical band gap of CdI2 films, Bull. Mater. Sci., 2001, 24, 297–300 CrossRef CAS.
- S. Yousefi, B. Ghasemi and M. Tajally, PEG-assisted synthesis and formation mechanism of Mg (OH) 2 nanostructures using natural brine, Appl. Phys. A, 2020, 126, 1–11 CrossRef.
- M. H. Zahir, et al., Shape-stabilized phase change materials for solar energy storage: MgO and Mg(OH)2 mixed with polyethylene glycol, Nanomaterials, 2019, 9(12), 1773 CrossRef CAS PubMed.
- M. Barzegar, et al., Green synthesis of magnesium oxide nanoparticles by chitosan polymer and assessment of their photocatalytic activity and cytotoxicity influences, Mater. Chem. Phys., 2023, 301, 127649 CrossRef CAS.
- A. C. Makan, et al., Advanced analysis of polymer emulsions: Particle size and particle size distribution by field-flow fractionation and dynamic light scattering, J. Chromatogr. A, 2016, 1442, 94–106 CrossRef CAS PubMed.
- Z. Rajabimashhadi, et al., Novel Synthesis of Nano Mg (OH) 2 by Means of Hydrothermal Method with Different Surfactants, Nanomaterials, 2023, 13(3), 454 CrossRef CAS PubMed.
- X. Li, Y. Zhao and C. Zhao, Applications of capillary action in drug delivery, iScience, 2021, 24(7), 102810 CrossRef CAS PubMed.
- V. Puca, et al., Microbial species isolated from infected wounds and antimicrobial resistance analysis: Data emerging from a three-years retrospective study, Antibiotics, 2021, 10(10), 1162 CrossRef PubMed.
- N. M. Franklin, et al., Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility, Environ. Sci. Technol., 2007, 41(24), 8484–8490 CrossRef CAS PubMed.
- M. Heinlaan, et al., Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus, Chemosphere, 2008, 71(7), 1308–1316 CrossRef CAS PubMed.
- V. Karunagaran, K. Rajendran and S. Sen, Optimization of biosynthesis of silver oxide nanoparticles and its anticancer activity, Int. J. Nanosci., 2017, 16(05n06), 1750018 CrossRef CAS.
- X. Pan, et al., Investigation of antibacterial activity and related mechanism of a series of nano-Mg(OH)2, ACS Appl. Mater. Interfaces, 2013, 5(3), 1137–1142 CrossRef CAS PubMed.
- J. C. Gumbart, et al., Escherichia coli peptidoglycan structure and mechanics as predicted by atomic-scale simulations, PLoS Comput. Biol., 2014, 10(2), e1003475 CrossRef PubMed.
- A. Truskewycz, et al., Fluorescent magnesium hydroxide nanosheet bandages with tailored properties for biocompatible antimicrobial wound dressings and pH monitoring, ACS Appl. Mater. Interfaces, 2021, 13(24), 27904–27919 CrossRef CAS PubMed.
- P. Philip, et al., Parallel substrate supply and pH stabilization for optimal screening of E. coli with the membrane-based fed-batch shake flask, Microb. Cell Fact., 2018, 17(1), 1–17 CrossRef PubMed.
- M. Nadir, I. Ansyar and P. Khaerani. Effect of various polyethylene glycol concentrations on the growth of seedlings of Indigofera zollingeriana, IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2019 Search PubMed.
- A. Pilarska, et al., Functional polypropylene composites filled with ultra-fine magnesium hydroxide, Open Chem., 2014, 13(1), 000010151520150024 CrossRef.
- H. Li, et al., pH-responsive eco-friendly chitosan–chlorella hydrogel beads for water retention and controlled release of humic acid, Water, 2022, 14(8), 1190 CrossRef CAS.
- S. C. Chew, et al., Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides, mBio, 2014, 5(4), e01536–14 CrossRef PubMed.
- R. M. Donlan, Biofilm formation: a clinically relevant microbiological process, Clin. Infect. Dis., 2001, 33(8), 1387–1392 CrossRef CAS PubMed.
- S. T. Asma, et al., An overview of biofilm formation–combating strategies and mechanisms of action of antibiofilm agents, Life, 2022, 12(8), 1110 CrossRef CAS PubMed.
- L. Shkodenko, I. Kassirov and E. Koshel, Metal oxide nanoparticles against bacterial biofilms: Perspectives and limitations, Microorganisms, 2020, 8(10), 1545 CrossRef CAS PubMed.
- D. Oliinyk, et al., Chorioallantoic membrane assay at the cross-roads of adipose-tissue-derived stem cell research, Cells, 2023, 12(4), 592 CrossRef CAS PubMed.
- E. Fröhlich, The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles, Int. J. Nanomed., 2012, 5577–5591 CrossRef PubMed.
- E. Sahle-Demessie and H. Tadesse, Kinetics and equilibrium adsorption of nano-TiO2 particles on synthetic biofilm, Surf. Sci., 2011, 605(13–14), 1177–1184 CrossRef CAS.
- C. Boyoglu, et al., The intracellular co-localizations of different size of gold nanoparticles, Nanotechnology, 2011, 489–492 CAS.
- K. Stevenson, et al., General calibration of microbial growth in microplate readers, Sci. Rep., 2016, 6(1), 1–7 CrossRef PubMed.
- D. Archana, et al., Chitosan-PVP-nano silver oxide wound dressing: in vitro and in vivo evaluation, Int. J. Biol. Macromol., 2015, 73, 49–57 CrossRef CAS PubMed.
- M. A. Dobrovolskaia and S. E. McNeil, Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines, J. Controlled Release, 2013, 172(2), 456–466 CrossRef CAS PubMed.
- J. P. Junker, et al., Clinical impact upon wound healing and inflammation in moist, wet, and dry environments, Adv. Wound Care, 2013, 2(7), 348–356 CrossRef PubMed.
- X.-Y. Wang, et al., Experimental and first-principle computational exploration on biomass cellulose/magnesium hydroxide composite: Local structure, interfacial interaction and antibacterial property, Int. J. Biol. Macromol., 2021, 191, 584–590 CrossRef CAS PubMed.
- G. C. Gurtner, et al., Wound repair and regeneration, Nature, 2008, 453(7193), 314–321 CrossRef CAS PubMed.
- C. L. Wetteland, et al., Dissociation of magnesium oxide and magnesium hydroxide nanoparticles in physiologically relevant fluids, J. Nanopart. Res., 2018, 20, 1–17 CrossRef CAS.
- S. V. Pollack, Wound healing: a review: III. Nutritional factors affecting wound healing, J. Dermatol. Surg. Oncol., 1979, 5(8), 615–619 CrossRef CAS PubMed.
- G. Pastorfide, et al., Zinc chloride spray-magnesium hydroxide ointment dual topical regimen in the treatment of obstetric and gynecologic incisional wounds, Clin. Ther., 1989, 11(2), 258–263 CAS.
- T. S. Lange, et al., Mg2+ and Ca2+ differentially regulate β1 integrin-mediated adhesion of dermal fibroblasts and keratinocytes to various extracellular matrix proteins, Exp. Cell Res., 1994, 214(1), 381–388 CrossRef CAS PubMed.
- S.-L. Chen, et al., A PEG-based hydrogel for effective wound care management, Cell Transplant., 2018, 27(2), 275–284 Search PubMed.
- C. U. Nwaigwe, et al., Evaluation of the hematological and clinical biochemical markers of stress in broiler chickens, Vet World, 2020, 13(10), 2294–2300 CAS.
- A. Khan, et al., Arsenic Toxicity in Broiler Chicks and its Alleviation with Ascorbic Acid: A Toxico-patho-biochemical Study, Int. J. Agric. Biol., 2013, 15, 1105–1111 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj01909a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |