Sorghum-derived porous carbon for outstanding green supercapacitors†
Fuming
Zhang‡
a,
Hongchao
Lang‡
a,
Jinggao
Wu
b and
Jing
Huang
 *a
*a
aState Key Laboratory of Resource Insects, College of Sericulture, Textile and Biomass Sciences, Westa College, Southwest University, Chongqing 400715, P. R. China. E-mail: hj41012@163.com
bKey Laboratory of Rare Earth Optoelectronic Materials & Devices, College of Chemistry and Materials Engineering, Huaihua University, Huaihua 418000, P. R. China
First published on 22nd November 2023
Abstract
Exploiting high-performance carbonaceous materials from biomass for supercapacitor (SC) electrodes has attracted extensive attention. In this research, hierarchical porous carbon electrodes derived from sorghum have been explored for the construction of symmetrical supercapacitors. A facile carbonization/activation strategy has been employed to synthesize porous carbon with a large specific surface area (∼1271 m2 g−1), an interconnected hierarchical pore structure, and heteroatom doping such as N, O, P, and S. The supercapacitors show a high specific capacitance of ∼483 F g−1 at 1 A g−1, a high energy density of 96.54 W h kg−1 at a power density of 1200 W kg−1 and cycle stability with capacitance retention of 97.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles. This work presents a promising strategy for fabricating biomass-derived porous carbon with high energy density and low loss for commercial application in energy storage systems.
000 charge/discharge cycles. This work presents a promising strategy for fabricating biomass-derived porous carbon with high energy density and low loss for commercial application in energy storage systems.
1. Introduction
With industrial and technological advances over the past few decades, environmental contamination and resource shortages have initiated the expansion of new electrochemical energy storage (EES) devices to address the problems of the energy crisis and carbon neutrality, such as rechargeable batteries, conventional capacitors, and supercapacitors (SCs).1–3 SCs have attracted extensive attention due to their advantages in terms of environmental friendliness, high power density, low cost, ultra-fast charge/discharge speed, and ultralong cycling life.4,5 Currently, SCs are employed in several fields, such as providing peak pulse power, memory backup, capacitive touch sensors, quick charging and an uninterrupted power supply.6,7 According to the mechanism of the energy storage, supercapacitors can generally be divided into electrical double-layer capacitors (EDLCs) and pseudocapacitors (PCs) as well as hybrid supercapacitors.8,9 In principle, EDLCs store energy through the electrostatic adsorption of opposite charges on double-layers at the interface of the electrode/electrolyte.10,11 However, SCs could achieve a high power density (>10 kW kg−1), but still face the deficiencies of limited energy density and a limited operating voltage, which are inadequate for modern commercial electrical equipment.12,13 To overcome the aforementioned limitations, there is a serious need to explore lower-cost and sustainable electrode materials for SCs.14At present, the electrode materials of SCs are categorized as metal oxides, electronically conducting polymers and carbon materials.15,16 Carbon materials, such as activated carbon (AC) and graphene, are the most commonly used platform materials in modern energy storage and conversion.17,18 Compared with activated carbon materials derived from fossil fuels, biomass-derived carbon materials are relatively low cost, easily accessible, and show high rate performance and sustainability, which could make for a reduction in the release of CO2.19–21 Simultaneously, the synthesis of carbon materials by exploring biomass precursors could contribute not only to the environment, but also to economic value.22,23 Additionally, biomass-derived carbon materials are also usually provided with rich in-surface functional groups and easily adjustable pore structures as well as biological structures inherited from the precursor biomass, and have been regarded as promising candidates for supercapacitor electrodes.24,25 Up to now, only a small number of types of biomass, such as bamboo and coconut shells, have been employed as precursors for the fabrication of ACs as electrodes.26 Crab shells are an easily available bio-waste, containing about 20% chitin and 30% protein, both of which are nitrogen-abundant macromolecular polymers. Bamboo possesses excellent flexibility and mechanical durability owing to its well-connected microtexture and multichannel structure, which could be applied to synthesizing carbon with interconnected, multichannel and porous structures.27,28 However, it is still necessary to search for new precursors, which are cheap, accessible and economically valuable.
Sorghum as the fifth grain crop in the world has been widely cultivated in China, with a planting area up to 9.52 million acres and production of up to 2.97 million tons in 2020. Sorghum belongs to the Poaceae and the main component of sorghum grain is carbohydrate, including 75% starch, with multiple proteins such as lysine, arginine, and methionine, and it is rich in vitamins and fat. At presently, sorghum is used for coarse grain and winery. In this context, we have explored a simple pyrolysis method to fabricate cheap but high-performance porous carbon-based electrodes from sorghum and to assemble environmentally benign supercapacitor devices (Scheme 1). Owing to their high specific surface area and heteroatom doping, carbon materials derived from sorghum have shown ultrahigh capacitive properties.
2. Experimental section
2.1 Materials and methods
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 99%), poly(tetrafluoroethylene) (PTFE, Mw: 100.015), and ethanol (
99%), poly(tetrafluoroethylene) (PTFE, Mw: 100.015), and ethanol (![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 99.5%) were purchased from Sigma-Aldrich. Potassium hydroxide (KOH
99.5%) were purchased from Sigma-Aldrich. Potassium hydroxide (KOH ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 85%) and all others reagents were obtained from Adamas-beta®. All chemicals were used without further purification.
85%) and all others reagents were obtained from Adamas-beta®. All chemicals were used without further purification.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and was thoroughly ground in an agate mortar. After grinding, the mixture was further heat treated at 900 °C for 2 hours under the same heating conditions. Finally, the resulting carbon was neutralized, thoroughly dried and named SAC-2. For comparison, different mass ratios of SC/KOH (2
1 and was thoroughly ground in an agate mortar. After grinding, the mixture was further heat treated at 900 °C for 2 hours under the same heating conditions. Finally, the resulting carbon was neutralized, thoroughly dried and named SAC-2. For comparison, different mass ratios of SC/KOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were also investigated under the aforementioned procedure. The products were denoted SAC-1, SAC-3, SAC-4 and SAC-5. Additionally, SAC-6, SAC-7 and SAC-8 were fabricated at 700, 800 and 1000 °C corresponding to a similar procedure to that for SAC-2. During the whole process, all carbonized samples were thoroughly washed with diluted HCl and deionized water, and then dried at 80 °C for 12 h.
2) were also investigated under the aforementioned procedure. The products were denoted SAC-1, SAC-3, SAC-4 and SAC-5. Additionally, SAC-6, SAC-7 and SAC-8 were fabricated at 700, 800 and 1000 °C corresponding to a similar procedure to that for SAC-2. During the whole process, all carbonized samples were thoroughly washed with diluted HCl and deionized water, and then dried at 80 °C for 12 h.
2.2 Characterization
The XRD patterns of all samples were recorded using powder X-ray diffraction (Shimadzu XRD-7000). The surface morphology and structure of the samples were observed using scanning electron microscopy (FESEM, JSM-7800F) and transmission electron microscopy (TEM, JEOL 2100). Nitrogen sorption isotherms were obtained using an Autosorb-1 (Quantachrome Instruments). The specific surface area was calculated with the modified Brunauer–Emmett–Teller (BET) method. The pore size distributions and the pore volume were analyzed from the adsorption branch isotherms by the density functional theory (DFT) method. Moreover, the total pore volume (Vt) was estimated from the amount adsorbed at a relative pressure P/P0 of 0.990. The micropore volume (Vmic) and micropore surface area (Smic) were determined by t-plot theory. Raman spectra were acquired with a Jobin–Yvon HR 800 spectrometer. X-ray photoelectron spectroscopy (XPS) measurements were performed on an Escalab 250xi (Thermo Fisher Scientific, USA). Fourier transform infrared (FT-IR) spectra were recorded on a Thermo Scientific Nicolet iS 50 spectrometer.2.3 Electrochemical measurements
For the two-electrode system, a homogeneous slurry of the electroactive material, polytetrafluoroethylene (PTFE), and acetylene black with a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in ethanol was formed and pasted onto a nickel foam current collector (1 cm × 1 cm), then vacuum dried at 80 °C for 12 h. The loading of the active material for each working electrode was measured as ∼3 mg cm−2. Then, a glass-fiber filter paper (Waterman, GF/B) as a separator and 1 M KOH aqueous solution as the electrolyte were used to assemble a test cell. For the three-electrode system, the working electrode was manufactured via the dispersion of active carbon in a mixture of Nafion and ethanol (1
10 in ethanol was formed and pasted onto a nickel foam current collector (1 cm × 1 cm), then vacuum dried at 80 °C for 12 h. The loading of the active material for each working electrode was measured as ∼3 mg cm−2. Then, a glass-fiber filter paper (Waterman, GF/B) as a separator and 1 M KOH aqueous solution as the electrolyte were used to assemble a test cell. For the three-electrode system, the working electrode was manufactured via the dispersion of active carbon in a mixture of Nafion and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) dropped onto a glassy carbon electrode, accompanied by platinum foil Hg/HgO as the counter and reference electrodes, respectively. Electrochemical characterization was carried out on an electrochemical workstation (Shanghai Chenhua Instrument Co. Ltd, China).
20) dropped onto a glassy carbon electrode, accompanied by platinum foil Hg/HgO as the counter and reference electrodes, respectively. Electrochemical characterization was carried out on an electrochemical workstation (Shanghai Chenhua Instrument Co. Ltd, China).
For a two-electrode system, the gravimetric specific capacitance of a single electrode is calculated with the equation:
| Csp = 2I × Δt/m × ΔV | (1) |
For the three-electrode system, the gravimetric specific capacitance can be calculated with the following equation:
| Csp = I × Δt/m × ΔV | (2) |
The energy density E (W h kg−1) and the power density P (W kg−1) are calculated with the equations:
| E = CspV2/(2 × 3.6) | (3) |
| P = 3600E/Δt | (4) |
Two devices connected in series can power a red light-emitting diode (LED) with a working voltage of 2.5 V.
3. Results and discussion
3.1 Material characterization
The field-emission scanning electron microscope (FESEM) image of SAC-2 is presented in Fig. 1(a), which indicates rough surfaces with honeycomb-like voids and various conchoidal cavities of different sizes. The 3D configuration of SAC-2 confirms the presence of extensive micropores along with substantial mesopores and macropores owing to activation by KOH resulting in the formation of a continuous 3D network of various pores through radically restructuring SC.29 The transmission electron microscope (FETEM) image (Fig. 1(b)) agrees well with the FESEM image. The widespread distribution of micropores is observed, which could contribute to achieving high charge storage capacities.30The X-ray diffraction (XRD) patterns in Fig. 1(c) indicate broad peaks centered at 2θ of ∼22.3° and ∼42.9°, which are attributed to the (002) and (100) planes of graphitic carbon, respectively.31 This phenomenon confirms the amorphous nature and disorder of the structure, which are common pragmatic features for biomass-derived porous carbons owing to the presence of abundant functional groups over the carbon surface.32 Raman spectra of SAC-2 and SC in Fig. 1(d) display a D band (1340 cm−1) and a G band (1580 cm−1), which are ascribed to defects and disorders in the carbon structure and graphitic in-plane stretching from sp2 hybridized carbon, respectively.33 The ratios based on the intensities of the D-to-G bands are 0.928 and 0.636 for SAC-2 and SC, respectively, which demonstrate that the degree of graphitization gradually becomes weaker and the defects conversely strengthen owing to the activation by KOH.34
The N2 adsorption and desorption isotherms are shown in the inset in Fig. 1(e) and the relevant data are listed in Table 1. For SC, typical type IV adsorption–desorption isotherms with H3 hysteresis loops are displayed. SAC-2 exemplifies typical type 1 isotherms with H4 hysteresis loops, which confirms the presence of mesopores.35 Moreover, the adsorption curves for SAC-2 increase sharply at a relative pressure P/P0 between 0.0 and 0.1, which confirms the presence of micropores.36 This phenomenon demonstrates that SAC-2 consists of hierarchical pores, consistent with the pore size distribution at about 3.69 nm in Fig. 1(e). The hierarchical interconnected pore structures could strengthen the properties of carbon electrodes for electrochemical energy storage by providing fast transport channels and enhancing the area reachable by electrolyte ions.37,38 Based on Brunauer–Emmett–Teller (BET) theory, the specific surface areas (SSAs) are ∼1271 and ∼84 m2 g−1 with pore volumes of 1.17 and 0.52 cm3 g−1 and pore diameters of 3.69 and 2.48 nm for SAC-2 and SC, respectively. Obviously, the textural parameters of SAC-2 are higher than those of SC, which is attributed to KOH etching and activation.39 Additionally, KOH concentration and the activation temperature could have an impact on the surface properties and pore texture. As described in Fig. 1(e), the pore ratio obviously depends on the concentration of KOH in the activation process. As the KOH concentration increases, the textural properties also increase (specific surface area: 632.54 vs. 1270.67 m2 g−1; total pore volume: 0.67 vs. 1.17 cm3 g−1; pore diameter: 2.83 vs. 3.69 nm; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 1.5
1 vs. 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; SAC-1 vs. SAC-2) and then gradually decrease in accordance with a further increase in KOH concentration (specific surface area: 1270.67 vs. 1026.35 vs. 975.26 vs. 864.25 m2 g−1; total pore volume: 1.17 vs. 1.04 vs. 0.86 vs. 0.75 cm3 g−1; pore diameter: 3.69 vs. 3.24 vs. 2.93 vs. 2.35 nm; 1.5
1; SAC-1 vs. SAC-2) and then gradually decrease in accordance with a further increase in KOH concentration (specific surface area: 1270.67 vs. 1026.35 vs. 975.26 vs. 864.25 m2 g−1; total pore volume: 1.17 vs. 1.04 vs. 0.86 vs. 0.75 cm3 g−1; pore diameter: 3.69 vs. 3.24 vs. 2.93 vs. 2.35 nm; 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 1
1 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 1
1 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 vs. 1
1.5 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; SAC-2 vs. SAC-3 vs. SAC-4 vs. SAC-5). When the activation temperature increases from 700 to 900 °C, the textural parameters follow a similar tendency (specific surface area: 773.84 vs. 982.43 vs. 1270.67 m2 g−1; total pore volume: 0.62 vs. 0.92 vs. 1.17 cm3 g−1; pore diameter: 2.06 vs. 2.85 vs. 3.69 nm; 700 vs. 800 vs. 900 °C; SAC-2 vs. SAC-6 vs. SAC-7). This phenomenon demonstrates that more volatile components are released from the feedstock with an increase in pyrolysis temperature, which could be beneficial for the formation of pore structures within the carbon materials, resulting in a further increase in textural parameters. But as the activation temperature further increases to 1000 °C, the BET characteristics conversely decrease (specific surface area: 1270.67 vs. 832.67 m2 g−1; total pore volume: 1.17 vs. 0.75 cm3 g−1; pore diameter: 3.69 vs. 2.32 nm; 900 vs. 1000 °C; SAC-2 vs. SAC-8) due to collapse in the pores at high temperature. In general, the individual morphology of SAC-2 with its highly porous structure and large surface area could afford shorter electron channels and rich active sites by forming rich and uniform internal pores, which are favorable to OH− penetration and could significantly enhance electrolyte diffusion, thereby promoting supercapacitive performance.40,41
2; SAC-2 vs. SAC-3 vs. SAC-4 vs. SAC-5). When the activation temperature increases from 700 to 900 °C, the textural parameters follow a similar tendency (specific surface area: 773.84 vs. 982.43 vs. 1270.67 m2 g−1; total pore volume: 0.62 vs. 0.92 vs. 1.17 cm3 g−1; pore diameter: 2.06 vs. 2.85 vs. 3.69 nm; 700 vs. 800 vs. 900 °C; SAC-2 vs. SAC-6 vs. SAC-7). This phenomenon demonstrates that more volatile components are released from the feedstock with an increase in pyrolysis temperature, which could be beneficial for the formation of pore structures within the carbon materials, resulting in a further increase in textural parameters. But as the activation temperature further increases to 1000 °C, the BET characteristics conversely decrease (specific surface area: 1270.67 vs. 832.67 m2 g−1; total pore volume: 1.17 vs. 0.75 cm3 g−1; pore diameter: 3.69 vs. 2.32 nm; 900 vs. 1000 °C; SAC-2 vs. SAC-8) due to collapse in the pores at high temperature. In general, the individual morphology of SAC-2 with its highly porous structure and large surface area could afford shorter electron channels and rich active sites by forming rich and uniform internal pores, which are favorable to OH− penetration and could significantly enhance electrolyte diffusion, thereby promoting supercapacitive performance.40,41
| Sample | S BET | V tot | S mi | S me | S am | V mi | V me | V ma |
|---|---|---|---|---|---|---|---|---|
| a S BET: BET surface area. b V tot: total volume. c S mi: micropore surface area. d S me: mesopore surface area. e S ma: macropore surface area. f V mi: micropore volume. g V me: mesopore volume. h V ma: macropore volume. | ||||||||
| SC | 84.28 | 0.52 | 13.78 | 48.63 | 21.87 | 0.12 | 0.36 | 0.04 |
| SAC-1 | 632.54 | 0.67 | 178.32 | 352.18 | 102.04 | 0.15 | 0.42 | 0.1 |
| SAC-2 | 1270.67 | 1.17 | 287.36 | 642.71 | 340.6 | 0.13 | 0.76 | 0.28 |
| SAC-3 | 1026.35 | 1.04 | 208.62 | 613.54 | 204.19 | 0.11 | 0.64 | 0.29 |
| SAC-4 | 975.26 | 0.86 | 173.25 | 574.32 | 227.69 | 0.07 | 0.48 | 0.31 |
| SAC-5 | 864.25 | 0.75 | 157.42 | 426.18 | 280.65 | 0.12 | 0.37 | 0.26 |
| SAC-6 | 773.84 | 0.62 | 225.36 | 404.16 | 144.32 | 0.08 | 0.36 | 0.18 |
| SAC-7 | 982.43 | 0.92 | 216.42 | 503.41 | 262.6 | 0.18 | 0.53 | 0.21 |
| SAC-8 | 832.67 | 0.75 | 106.24 | 415.27 | 311.16 | 0.06 | 0.46 | 0.23 |
The surface chemical state and elemental composition of SAC-2 have been analyzed by XPS. The survey spectrum in Fig. S1 (ESI†) confirms the surface composition of C, N, O, P and S elements, which demonstrates that the synergetic effect of carbocation with activation could result in self-doping by heteroatoms.42 As shown in Fig. 1(f), the high-resolution C1s spectrum could be divided into five peaks at about 284.8 eV, 285 eV, 286.3 eV, 288.6 eV and 289.1 eV, which could be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, S-sp3C, C–O, C
C, S-sp3C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and π–π*, respectively.43 The N1s spectrum (Fig. 1(g)) is deconvoluted into five peaks at 398.5 eV (pyridinic-N, N6), 399.5 eV (pyrrolic-N, N5), 400.4 eV (quaternary-N, NQ), 401.7 eV (amides/amines or nitrile N, NC) and 404.4 eV (N-oxides, NX).44 Pyridinic-N and pyrrolic-N could enhance the hydrophilicity and carry out a Faraday reaction, which could efficiently promote interfacial reactions. Moreover, the conjugation of the lone-pair electrons of N and graphitic π bonds of carbon would result in distortion of the carbon structure, generating more defects and available active sites, which could enhance the electrochemical performance of supercapacitors.45 The O1s spectrum (Fig. 1(h)) presents two peaks for P
O and π–π*, respectively.43 The N1s spectrum (Fig. 1(g)) is deconvoluted into five peaks at 398.5 eV (pyridinic-N, N6), 399.5 eV (pyrrolic-N, N5), 400.4 eV (quaternary-N, NQ), 401.7 eV (amides/amines or nitrile N, NC) and 404.4 eV (N-oxides, NX).44 Pyridinic-N and pyrrolic-N could enhance the hydrophilicity and carry out a Faraday reaction, which could efficiently promote interfacial reactions. Moreover, the conjugation of the lone-pair electrons of N and graphitic π bonds of carbon would result in distortion of the carbon structure, generating more defects and available active sites, which could enhance the electrochemical performance of supercapacitors.45 The O1s spectrum (Fig. 1(h)) presents two peaks for P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (531.7 eV) and P–O–P/P–O–C (533.2 eV). The extensive oxygen-containing functional groups could elevate the surface wettability of carbon materials, which could contribute to the reaction at the electrode–electrolyte interface.46 As for the deconvoluted P2p spectra in Fig. 1(i), the three peaks correspond to C–P species (131.8 eV, C–P), pyrophosphate/polyphosphate (133.4 eV, PP) and monophosphate/metaphosphate groups (134.4 eV, MP), respectively.47 The S2p spectra in Fig. 1(j) indicate three peaks at 161.8 eV, 164.5 eV and 167.5 eV, which could be assigned to C
O (531.7 eV) and P–O–P/P–O–C (533.2 eV). The extensive oxygen-containing functional groups could elevate the surface wettability of carbon materials, which could contribute to the reaction at the electrode–electrolyte interface.46 As for the deconvoluted P2p spectra in Fig. 1(i), the three peaks correspond to C–P species (131.8 eV, C–P), pyrophosphate/polyphosphate (133.4 eV, PP) and monophosphate/metaphosphate groups (134.4 eV, MP), respectively.47 The S2p spectra in Fig. 1(j) indicate three peaks at 161.8 eV, 164.5 eV and 167.5 eV, which could be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, C–S and oxides of S, respectively.48
S, C–S and oxides of S, respectively.48
The functional groups on the electrode surface were investigated through the Fourier transform infrared (FT-IR) spectra, which present similar vibrational absorptions for SC and SAC-2 (Fig. S2, ESI†). The peak at 617 cm−1 is attributed to aromatic C–H and the C–N vibration (1375 cm−1) is also detected. The peak located at 1620 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching or C
O stretching or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching.49 The absorption peaks in the 3241–3557 cm−1 range refer to the O–H stretching vibration and/or N–H vibration as well as the formation of hydrogen bonds.50 Looking at the spectra of SC and SAC-2, while the peak class remains constant, their wavelengths vary, which could be ascribed to the contribution of functional groups to the reaction.
C stretching.49 The absorption peaks in the 3241–3557 cm−1 range refer to the O–H stretching vibration and/or N–H vibration as well as the formation of hydrogen bonds.50 Looking at the spectra of SC and SAC-2, while the peak class remains constant, their wavelengths vary, which could be ascribed to the contribution of functional groups to the reaction.
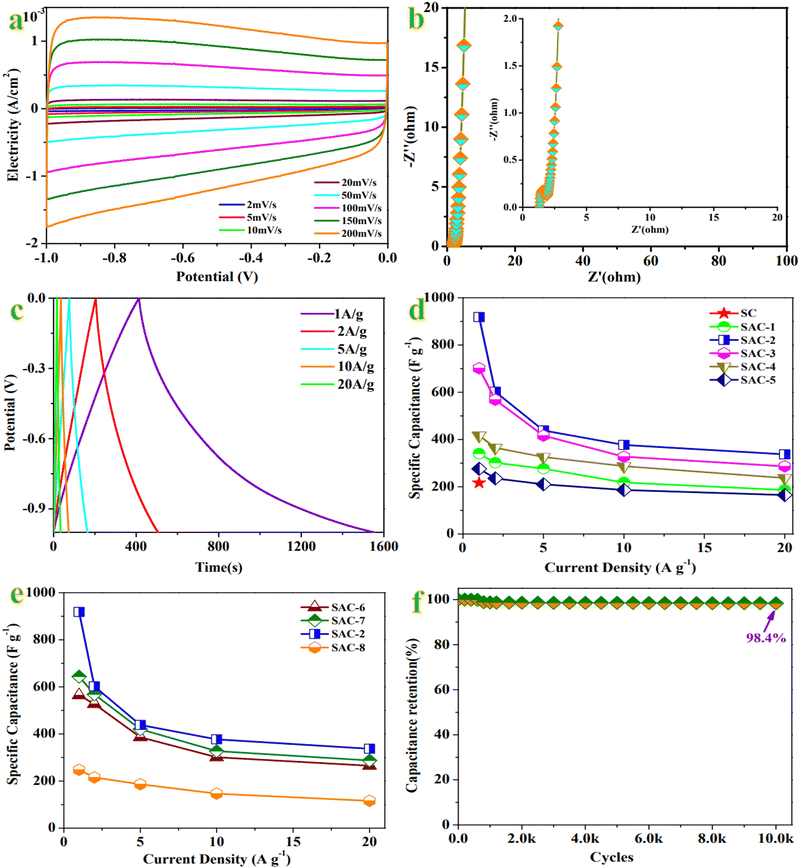
3.2 Electrochemical behavior of the electrode
Thanks to the aforementioned analysis, it could be deduced that SACs are composed of abundant ion transport interfaces and show high conductivity, and so could operate as a feasible electrode material for supercapacitors.51 A three-electrode configuration was explored to investigate the electrochemical behavior of SACs by performing cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) techniques in 2 M KOH as an aqueous electrolyte. The integrated area for the CV of SAC-2 with voltage sweeps from −1 to 0 V at 100 mV s−1 is larger than that of other SAC materials (Fig. S3, ESI†) owing to its hierarchical porous structure and convenient ion transportation channels for the ions K+ and OH−, which demonstrates better electrochemical characteristics of SAC-2 relative to other SAC materials.52 The detailed CV curves for SAC-2 (Fig. 2(a)) still present approximately semi-rectangular shapes with no redox peaks even at scan speeds from 2 mV s−1 to 200 mV s−1, which verifies the prominent rate performance. The slight reversible hump could be attributed to the pseudocapacitive behavior of the doped heteroatoms such as N, O, P and S.53 EIS measurements were performed to investigate the charge transfer kinetics of the electrode material in the electrolyte solution. As described in Fig. 2(b), the Nyquist plots are composed of a semicircle in the high-frequency region and a line in the low-frequency region. The internal resistance refers to the point at which the Nyquist plot and the X-axis intersect, which corresponds to the resistance of the electrolyte, the intrinsic resistance of the substrate, and the resistance at the interface between the electroactive material and the substrate.54 The semicircle in the high-frequency region represents the charge transfer resistance. The slope of the direct line in the low-frequency region denotes the Warburg resistance, representing the ion's resistance to diffusion along the surface of the electrode.55 Based on the Nyquist plots, it could be observed that the SAC-2 electrode has a low internal resistance (∼1.3 Ω) and a small charge transfer resistance as well as a steep line in the low-frequency region, which denotes high electrical conductivity and fast charge transfer kinetics as well as low diffusion resistance.56 Therefore, the powerful conductivity of SAC-2 and its superior ion diffusion together could accelerate reaction kinetics, which ultimately results in superior electrochemical performance57 (Table 2).| Source of biowaste derived AC | Activation method | Surface area of AC (m2 g−1) | Specific capacitance (F g−1) | Electrolyte | Cycle stability |
|---|---|---|---|---|---|
| Coconut shell | Steam | 1532 | 228 at 5 mV s−1 | 6 M KOH | 93% after 3000 cycles |
| Peanut shell | ZnCl2 | 1549 | 340 at 1 A g−1 | 1 M H2SO4 | 95.3% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| Palm kernel shell | KOH | 462.1 | 210 at 0.5 A g−1 | 1 M KOH | 95% after 1000 cycles |
| Watermelon rind | KOH | 2277 | 333.4 at 1 A g−1 | 6 M KOH | 96.8% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| Tea leaves | KOH | 2841 | 330 at 1 A g−1 | 2 M KOH | 92% after 2000 cycles |
| Tea waste buds | KOH | 1610 | 332 at 1 A g−1 | 6 M KOH | 97.8% after 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| Sugarcane bagasse | KOH | 1939.9 | 298 at 1 A g −1 | 1 M H2SO4 | 94.5% after 5000 cycles |
| Withered rose flower | KOH/KNO3 | 1980 | 350 at 1 A g−1 | 6 M KOH | 96.5% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| Jujube fruit | NaOH | 1135 | 460 at 1 A g−1 | 6 M KOH | 92.2% after 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| American poplar fruitwaste | KOH | 942 | 423 at 1 A g −1 | 6 M KOH | 97% after 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| Corncob | KOH | 800 | 390 at 0.5 A g −1 | 1 M H2SO4 | 94% after 5000 cycles |
| Plastic waste (polyethylene terephthalate) | KOH | 2326 | 169 at 0.2 A g −1 | 6 M KOH | 90.6% after 5000 cycles |
| Rice husk | KOH | 3145 | 367 at 5 mV s −1 | 6 M KOH | ≈100% after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Soyabean pods | NaOH | 2612 | 352.6 at 0.5 A g −1 | 1 M Na2SO4 | 94.2% after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Orange peel | KOH | 2160 | 460 at 1 A g−1 | 1 M H2SO4 | 98% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| Mangosteen peels | NaOH | 2623 | 357 at 1 A g−1 | 6 M KOH | 94.5% after 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
GCD tests under various current densities from 1 A g−1 to 20 A g−1 were carried out to investigate the charge and discharge characteristics. GCD curves of SAC-2 (Fig. 2(c)) indicate linear and quasi-symmetric triangular-type behavior with no obvious IR drop, which confirms the effective Coulombic efficiency, and noticeable rate performance.59 The specific capacitances of SAC-2 on the basis of the GCD curves are ∼918, ∼602, ∼438, 377, ∼337 F g−1 in order at 1, 2, 5, 10, 20 A g−1, which reveal the multiplicative performance of SAC-2. Additionally, the specific capacitance of SAC-2 (∼918 F g−1 at 1 A g−1) is superior to that of SC (∼216 F g−1 at 1 A g−1), which could be attributed to the activation by KOH. The specific capacitance of other SACs is compared with SAC-2 at different current densities in Fig. 2(d) and (e). As for the effect of KOH concentration on electrochemical performance (Fig. 2(d)), the specific capacitance first increases along with an increase in KOH concentration (341 vs. 918 F g−1 at 1 A g−1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 1.5
1 vs. 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, SAC-1 vs. SAC-2). And then the specific capacitance conversely decreases when the KOH concentration further increases to 1
1, SAC-1 vs. SAC-2). And then the specific capacitance conversely decreases when the KOH concentration further increases to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (918 vs. 701 vs. 417 vs. 276 F g−1 at 1 A g−1, 1.5
2, (918 vs. 701 vs. 417 vs. 276 F g−1 at 1 A g−1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 1
1 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 1
1 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 vs. 1
1.5 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, SAC-2 vs. SAC-3 vs. SAC-4 vs. SAC-5). With respect to the effect of temperature on the properties (Fig. 2(e)), the specific capacitance increases when the activation temperature increases from 700 to 900 °C (565 vs. 644 vs. 918 F g−1 at 1 A g−1, 700 vs. 800 vs. 900 °C, SAC-6 vs. SAC-7 vs. SAC-2). However, the specific capacitance instead goes down when the activation temperature further increases to 1000 °C (918 vs. 248 F g−1 at 1 A g−1, 900 vs. 1000 °C, SAC-2 vs. SAC-8), due to the collapse of the carbon lattice framework under the conditions of strong reaction with KOH.60 Moreover, the results on the basis of the GCD curves (Table S1, ESI†) are consistent with the trend of the specific capacitance from the CVs.
2, SAC-2 vs. SAC-3 vs. SAC-4 vs. SAC-5). With respect to the effect of temperature on the properties (Fig. 2(e)), the specific capacitance increases when the activation temperature increases from 700 to 900 °C (565 vs. 644 vs. 918 F g−1 at 1 A g−1, 700 vs. 800 vs. 900 °C, SAC-6 vs. SAC-7 vs. SAC-2). However, the specific capacitance instead goes down when the activation temperature further increases to 1000 °C (918 vs. 248 F g−1 at 1 A g−1, 900 vs. 1000 °C, SAC-2 vs. SAC-8), due to the collapse of the carbon lattice framework under the conditions of strong reaction with KOH.60 Moreover, the results on the basis of the GCD curves (Table S1, ESI†) are consistent with the trend of the specific capacitance from the CVs.
The stability of an electrode material is another important parameter for practical applications. Therefore, the durability of SAC-2 was examined by continuous GCD profiles at 10 A g−1, as shown in Fig. 2(f). The SAC-2 electrode preserves 98.4% of its initial capacity after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which reveals outstanding long-term thermal stability, thermal conductivity, and chemical resistance even at high discharge rates.
000 cycles, which reveals outstanding long-term thermal stability, thermal conductivity, and chemical resistance even at high discharge rates.
In general, the superior electrochemical performance of SAC-2 could be ascribed to its rich pore structure. Lei et al have reported that in porous carbons, macropores play the role of electrolyte-buffering reservoirs that improve ion transport into the AC electrode domestic region by minimizing the length of the ion diffusion pathways between the bulk electrolyte and mesopores.61 Mesopores enhance efficient diffusion of electrolyte ions into their own interiors and into micropores through multiple channels. Micropores regulate diffusion through molecular sieve effects and further promote charge storage and capacitance. SAC-2 is composed of hierarchically interconnected microporous–mesoporous structures which provide a large exposed active surface area, which could contribute to shortening the ion diffusion path from the electrolyte to the electrode surface, further leading to high specific capacitance and excellent multiplicative performance at high discharge currents (Fig. 3).62,63 Additionally, the results also demonstrate that a well-balanced micro-to-mesopore ratio of SAC-2 could result in exceptionally high specific capacitance even in a low-concentration (2 M KOH) electrolyte.
3.3 Supercapacitor performance
The CVs. of the SAC//SAC device in Fig. 4(a) varying from 2 to 200 mV s−1 still maintain symmetrical shapes similar to rectangles without obvious deformation even at scan speeds up to 200 mV s−1, which demonstrates fast capacitance behavior with prominent rate performance.64 According to the Nyquist curves for the device in Fig. 4(b), the junction of the plot with the transverse axis in the high-frequency region indicates a low inherent resistance of 1.4 Ω. The straight line with a high slope in the low-frequency region indicates that the ions could be facilely transported from the electrolyte to the electrochemically active sites. Moreover, no obvious semicircle is shown in the high-frequency region, which reveals fast charge transfer at the interface between the electrode and the electrolyte.65,66The charge–discharge curves of the supercapacitor in Fig. 4(c) at a variety of current densities from 1 A g−1 to 20 A g−1 reveal nearly symmetric triangular shapes even at a large current density of 20 A g−1, which demonstrates outstanding reversibility and superior coulombic efficiency.67 Based on the GCD curves, the specific capacitance of the device could be deduced to be up to ∼483, ∼462, ∼434, ∼400 and ∼333 F g−1 as the current density increases in the order 1, 2, 5, 10 and 20 A g−1 in Fig. 4(d), respectively. Even when the current density increases to 20 A g−1, capacitance retention of 68.94% can still be achieved, which presents excellent rate capability. Additionally, the specific capacitance decreases with an increase in current density since electrolyte ions cannot penetrate the interior of the electrode materials at high current densities.68 The Ragone diagram in Fig. 4(e) on the basis of discharge curves at different current densities displays a high energy density of 96.54 W h kg−1 at a power density of 1200 W kg−1, and a comfortable energy density of 66.66 W kg−1 even at a power density as high as 24 kW kg−1, which is comparable or even higher than the values previously reported for porous carbon derived from biomass69–71 and commercial devices (<5 W h kg−1).72 Additionally, the tendency of energy density with the increase in current density could be attributed to the limited pores on the surface being accessed by electrolyte ions at high current density, while the majority of the pores could be utilized at low current density.
The supercapacitor was investigated for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles at 10 A g−1 and the results are shown in Fig. 4(f). Accompanying the increase in charge/discharge time, the capacitance of the device slightly decreases, owing to the destruction of some active sites during continuous charge and discharge processes73 and a capacitance retention of 97.5% after 10
000 charge/discharge cycles at 10 A g−1 and the results are shown in Fig. 4(f). Accompanying the increase in charge/discharge time, the capacitance of the device slightly decreases, owing to the destruction of some active sites during continuous charge and discharge processes73 and a capacitance retention of 97.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 successive cycles can still be achieved. Moreover, the CV curves (inset in Fig. 4(f)) are nearly overlapped, which confirms the prominent recyclability of the device. Two devices connected in series can successfully power a red light-emitting diode (LED) with a working voltage of 2.5 V (Fig. S4, ESI†). The device as a type of EDLC could store charge at the electrode–electrolyte interface by means of electrostatic interaction.74,75 In the process of charging, K+ and OH− in the aqueous solution through electrostatic force could promote ion transport and further form a double electric layer. Simultaneously, the accumulated charge from the electrode could be released during the discharge process.76,77 Above all, the porous carbon derived from sorghum for a supercapacitor provides a valuable reference for the development of environmentally friendly and cost-effective energy storage systems.
000 successive cycles can still be achieved. Moreover, the CV curves (inset in Fig. 4(f)) are nearly overlapped, which confirms the prominent recyclability of the device. Two devices connected in series can successfully power a red light-emitting diode (LED) with a working voltage of 2.5 V (Fig. S4, ESI†). The device as a type of EDLC could store charge at the electrode–electrolyte interface by means of electrostatic interaction.74,75 In the process of charging, K+ and OH− in the aqueous solution through electrostatic force could promote ion transport and further form a double electric layer. Simultaneously, the accumulated charge from the electrode could be released during the discharge process.76,77 Above all, the porous carbon derived from sorghum for a supercapacitor provides a valuable reference for the development of environmentally friendly and cost-effective energy storage systems.
4. Conclusions
In summary, we have successfully engineered porous carbon derived from sorghum via a facile carbonization/activation approach for a supercapacitor. The sorghum-based carbon electrode presents superior specific capacitance of ∼918 F g−1 at 1 A g−1 and excellent rate performance as well as outstanding cycling stability with capacitance retention of 98.4% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Moreover, the assembled supercapacitor also shows a high energy density of 96.54 W h kg−1 at a power density of 1200 W kg−1 and superior cycling performance with the loss of 2.5% capacitance after 10
000 cycles. Moreover, the assembled supercapacitor also shows a high energy density of 96.54 W h kg−1 at a power density of 1200 W kg−1 and superior cycling performance with the loss of 2.5% capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The work reveals the great potential of using sorghum resources for constructing feasible energy storage devices.
000 cycles. The work reveals the great potential of using sorghum resources for constructing feasible energy storage devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge to Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0019) and College Student Innovation and Entrepreneurship Training Program, Southwest University (S202310635215).References
- S. Liu, L. Kang and S. C. Jun, Adv. Mater., 2021, 33, 2004689 CrossRef CAS.
- L. Kang, M. Zhang, J. Zhang, S. Liu, N. Zhang, W. Yao, Y. Ye, C. Luo, Z. Gong, C. Wang, X. Zhou, X. Wu and S. C. Jun, J. Mater. Chem. A, 2020, 8, 24053 RSC.
- S. Liu, Y. Yin, D. Ni, K. S. Hui, M. Ma, S. Park, K. N. Hui, C. Y. Ouyang and S. C. Jun, Energy Storage Mater., 2019, 22, 384 CrossRef.
- S. Liu, L. Kang, J. Zhang, S. C. Jun and Y. Yamauchi, NPG Asia Mater., 2023, 15, 9 CrossRef CAS.
- Z. Guo, X. Han, C. Zhang, S. He, K. Liu, J. Hu, W. Yang, S. Jian, S. Jiang and G. Duan, Chin. Chem. Lett., 2023, 109007 CrossRef.
- C. Gao, J. Huang, Y. Xiao, G. Zhang, C. Dai, Z. Li, Y. Zhao, L. Jiang and L. Qu, Nat. Commun., 2021, 12, 1 CrossRef.
- S. Sahoo, R. P. Kumar, E. Joanni, R. Singh and J.-J. Shim, J. Mater. Chem. A, 2022, 10, 13190 RSC.
- Z. Hao, Z. Meng, X. Li, X. Sun, J. Xu, H. Nan, W. Shi, G. Qi, X. Hu and H. Tian, J. Colloid Interface Sci., 2022, 617, 430 CrossRef CAS PubMed.
- P. Manasa, S. Sambasivam and F. Ran, J. Energy Storage, 2022, 54, 105290 CrossRef.
- W. Zhang, M. Sun, J. Yin, K. Lu, U. Schwingenschlögl, X. Qiu and H. N. Alshareef, Adv. Energy Mater., 2021, 11, 2101928 CrossRef CAS.
- Y. Katsuyama, N. Haba, H. Kobayashi, K. Iwase, A. Kudo, I. Honma and R. B. Kaner, Adv. Funct. Mater., 2022, 32, 2201544 CrossRef CAS.
- W. Deng, W. Liu, H. Zhu, L. Chen, H. Liao and H. Chen, J. Energy Storage, 2023, 72, 108509 CrossRef.
- W. Deng, Y. Xu, X. Zhang, C. Li, Y. Liu, K. Xiang and H. Chen, J. Alloys Compd., 2022, 903, 163824 CrossRef CAS.
- W. N. Deng, Y. H. Li, D. F. Xu, W. Zhou, K. X. Xiang and H. Chen, Rare Met., 2022, 41(10), 3432–3445 CrossRef CAS.
- X. Wen, J. Luo, K. Xiang, W. Zhou, C. Zhang and H. Chen, Chem. Eng. J., 2023, 458, 141381 CrossRef CAS.
- L. Sun, K. Zhuo, Y. Chen, Q. Du, S. Zhang and J. Wang, Adv. Funct. Mater., 2022, 32, 2203611 CrossRef CAS.
- Y. Xu, H. Xue, X. Li, X. Fan, P. Li, T. Zhang, K. Chang, T. Wang and J. He, Nano Res. Energy, 2023, 2, e9120052 CrossRef.
- M. Chen, Q. Cheng, Y. Qian, J. X. He and X. Dong, Electrochim. Acta, 2020, 342, 136046 CrossRef CAS.
- R. Yang, Z. Guo, L. Cai, R. Zhu, Y. Fan, Y. Zhang, P. Han, W. Zhang, X. Zhu, Q. Zhao, Z. Zhu, C. K. Chan and Z. Zeng, Small, 2021, 17, 2103052 CrossRef CAS.
- S. Kandambeth, V. S. Kale, O. Shekhah, H. N. Alshareef and M. Eddaoudi, Adv. Energy Mater., 2022, 12, 2100177 CrossRef CAS.
- A. Zhang, R. Zhao, L. Hu, R. Yang, S. Yao, S. Wang, Z. Yang and Y. M. Yan, Adv. Energy Mater., 2021, 11, 2101412 CrossRef CAS.
- X. Y. Han, J. Li, J. L. Lu, S. Luo, J. Wan, B. X. Li, C. G. Hu and X. L. Cheng, Nano Energy, 2021, 86, 106079 CrossRef CAS.
- X. L. Ye, D. L. Han, G. Y. Jiang, C. J. Cui, Y. Guo, Y. G. Wang, Z. C. Zhang, Z. Weng and Q. H. Yang, Energy Environ. Sci., 2023, 16, 1016 RSC.
- Y. Liu, Y. Jiang, Z. Hu, J. Peng, W. Lai, D. Wu, S. Zuo, J. Zhang, B. Chen, Z. Dai, Y. Yang, Y. Huang, W. Zhang, W. Zhao, W. Zhang, L. Wang and S. Chou, Adv. Funct. Mater., 2020, 31, 2008033 CrossRef.
- J. Zhao, X. Liu, P. Liu, K. Deng, X. Lv, W. Tian, C. Wang, S. Tan and J. Ji, J. Colloid Interface Sci., 2022, 629, 1039 CrossRef.
- S. Luo, L. Xie, F. Han, W. Wei, Y. Huang, H. Zhang, M. Zhu, O. G. Schmidt and L. Wang, Adv. Funct. Mater., 2019, 29, 1901336 CrossRef.
- L. Li, J. S. Nam, M. S. Kim, Y. Wang, S. Jiang, H. Hou and I. D. Kim, Adv. Energy Mater., 2023, 13, 2302139 CrossRef CAS.
- W. Zhou, G. Zeng, H. Jin, S. Jiang, M. Huang, C. Zhang and H. Chen, Molecules, 2023, 28(5), 2147 CrossRef CAS.
- J. Li, Y. W. Wang, W. N. Xu, Y. Wang, B. Zhang, S. Luo, X. Y. Zhou, C. L. Zhang, X. Gu and C. G. Hu, Nano Energy, 2019, 57, 379–387 CrossRef CAS.
- S. Liu, Y. Li, W. Zhang, J. Wang, W. Xu and C. Wang, Dalton Trans., 2023, 52, 10115 RSC.
- T. Kar, S. Godavarthi, S. K. Pasha, K. Deshmukh, L. Martínez-Gómez and M. K. Kesarla, Crit. Rev. Solid State Mater. Sci., 2022, 47, 357 CrossRef CAS.
- X. Wei, Y. Song, L. Song, X. D. Liu, Y. Li, S. Yao, P. Xiao and Y. Zhang, Small, 2021, 17, 2007062 CrossRef CAS.
- C. Wang, Z. Zhou, Q. Tian, X. Cao, Y. Wu, S. Liu and J. Wang, Chem. Eng. J., 2022, 433, 134483 CrossRef CAS.
- X. Zhang, C. Jiang, J. Liang and W. Wu, J. Mater. Chem. A, 2021, 9, 8099 RSC.
- R. Zhang, J. Dong, W. Zhang, L. Ma, Z. Jiang, J. Wang and Y. Huang, Nano Energy, 2022, 91, 106633 CrossRef CAS.
- Q. Zong, C. Liu, H. Yang, Q. Zhang and G. Cao, Nano Today, 2021, 38, 101201 CrossRef CAS.
- H. Huang, J. He, Z. Wang, H. Zhang, L. Jin, N. Chen, Y. Xie, X. Chu, B. Gu and W. Deng, Nano Energy, 2020, 69, 104431 CrossRef CAS.
- J. Tang, T. Mathis, X. Zhong, X. Xiao, H. Wang, M. Anayee, F. Pan, B. Xu and Y. Gogotsi, Adv. Energy Mater., 2021, 11, 2003025 CrossRef CAS.
- W. Zhang, X. Qiu, C. Wang, L. Zhong, F. Fu, J. Zhu, Z. Zhang, Y. Qin, D. Yang and C. C. Xu, Carbon Res., 2022, 1, 1 Search PubMed.
- J. Qian, L. Sun, X. Shi, L. Wu, S. Su, K. Wang and Y. Zhang, Chem. Eng. J., 2022, 429, 132482 CrossRef CAS.
- K. S. Kumar, D. Pandey and J. Thomas, ACS Energy Lett., 2021, 6, 3590 CrossRef CAS.
- Y. Shao, J. H. Fu, Z. Cao, K. Song, R. Sun, Y. Wan, A. Shamim, L. Cavallo, Y. Han, R. B. Kaner and V. C. Tung, ACS Nano, 2020, 14, 7308 CrossRef CAS PubMed.
- F. Qi, Z. Sun, X. Fan, Z. Wang, Y. Shi, G. Hu and F. Li, Adv. Energy Mater., 2021, 11, 2100387 CrossRef CAS.
- Y. Wen, Y. Liu, S. Dang, S. Tian, H. Li, Z. Wang, D. He, Z.-S. Wu, G. Cao and S. Peng, J. Power Sources, 2019, 423, 106 CrossRef CAS.
- Q. Yang, Q. Wang, Y. Long, F. Wang, L. Wu, J. Pan, J. Han, Y. Lei, W. Shi and S. Song, Adv. Energy Mater., 2020, 10, 1903193 CrossRef CAS.
- X. Zhang, X. Yang, G. Sun, S. Yao, Y. Xie, W. Zhang, C. Liu, X. Wang, R. Yang, X. Jin, Z. X. Shen, H. J. Fan and F. Du, Adv. Funct. Mater., 2022, 32, 2204318 CrossRef CAS.
- G. Li, K. Mao, M. Liu, M. Yan, J. Zhao, Y. Zeng, L. Yang, Q. Wu, X. Wang and Z. Hu, Adv. Mater., 2020, 32, 2004632 CrossRef.
- G. Li, Y. Chen, L. Yan, Q. Gong, G. Chen, L. Yang, Q. Wu, X. Wang and Z. Hu, Small, 2022, 18, 2107082 CrossRef CAS.
- Q. Y. Wang, Y. M. Luo, R. Z. Hou, S. Zaman, K. Qi, H. F. Liu, H. S. Park and B. Y. Xia, Adv. Mater., 2019, 31, 1905744 CrossRef CAS.
- Z. Wang, X. Feng, Y. Bai, H. Yang, R. Dong, X. Wang, H. Xu, Q. Wang, H. Li, H. Gao and C. Wu, Adv. Energy Mater., 2021, 11, 2003854 CrossRef CAS.
- D. S. Yu, K. Goh, H. Wang, L. Wei, W. C. Jiang, Q. Zhang, L. M. Dai and Y. Chen, Nat. Nanotechnol., 2020, 15, 811 CrossRef CAS.
- Z. Pan, J. Yang, Q. Zhang, M. Liu, Y. Hu, Z. Kou, N. Liu, X. Yang, X. Ding, H. Chen, J. Li, K. Zhang, Y. Qiu, Q. Li, J. Wang and Y. Zhang, Adv. Energy Mater., 2019, 9, 1802753 CrossRef.
- Y. Guo, X. Hong, Y. Wang, Q. Li, J. Meng, R. Dai, X. Liu, L. He and L. Mai, Adv. Funct. Mater., 2019, 29, 1809004 CrossRef.
- R. Zhou, Y. Fu, K.-A. Chao and C.-H. Cheng, Renewable Energy, 2019, 135, 1445 CrossRef CAS.
- C. Wang, S. Zhai, Z. Yuan, J. Chen, X. Zhang, Q. Huang, Y. Wang, X. Liao, L. Wei and Y. Chen, Electrochim. Acta, 2019, 305, 493 CrossRef CAS.
- A. García-Zaragoza and C. Cerezo-Navarrete, Nanoscale, 2023, 15, 12319 RSC.
- A. Panagiotopoulos, G. Nagaraju, S. Tagliaferri, C. Grotta, P. C. Sherrell, M. Sokolikova, G. Cheng, F. Iacoviello, K. Sharda and C. Mattevi, J. Mater. Chem. A, 2023, 11, 16190 RSC.
- M. A. Yahya, Z. A. Qodah and C. W. Z. Ngah, Renewable Sustainable Energy Rev., 2015, 46, 218 CrossRef CAS.
- M. S. Sokolikova and C. Mattevi, Chem. Soc. Rev., 2020, 49, 3952 RSC.
- Y. Jiang and J. Liu, Energy Environ. Mater., 2019, 2, 30 CrossRef.
- T. S. Mathis, N. Kurra, X. Wang, D. Pinto, P. Simon and Y. Gogotsi, Adv. Energy Mater., 2019, 9, 1902007 CrossRef CAS.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294 RSC.
- S. Fleischmann, Y. Zhang, X. Wang, P. T. Cummings, J. Wu, P. Simon, Y. Gogotsi, V. Presser and V. Augustyn, Nat. Energy, 2022, 7, 222 CrossRef CAS.
- S. Fleischmann, J. B. Mitchell, R. Wang, C. Zhan, D. Jiang, V. Presser and V. Augustyn, Chem. Rev., 2020, 120(14), 6738 CrossRef CAS PubMed.
- S. Vishwanathanab and H. S. S. R. Matte, Chem. Commun., 2023, 59, 9263 RSC.
- K. Han, F. An, F. Yan, H. Chen, Q. Wan, Y. Liu, P. Li and X. Qu, J. Mater. Chem. A, 2021, 9, 15637 RSC.
- G. Cui, Y. Zeng, J. Wu, Y. Guo, X. Gu and X. W. Lou, Adv. Sci., 2022, 9, 2106067 CrossRef CAS.
- X. Chen, T. Shi, K. Zhong, G. Wu and Y. Lu, Chem. Eng. J., 2020, 379, 122240 CrossRef CAS.
- Z. Sun, Y. Zhang, Y. Liu, J. Fu, S. Cheng, P. Cui and E. Xie, J. Power Sources, 2019, 436, 226795 CrossRef CAS.
- H. Shao, Y.-C. Wu, Z. Lin, P.-L. Taberna and P. Simon, Chem. Soc. Rev., 2020, 49, 3005 RSC.
- Y. Zhou, X. Cheng, F. Huang, Z. Sha, Z. Han, J. Chen, W. Yang, Y. Yu, J. Zhang, S. Peng, S. Wu, A. Rider, L. Dai and C. H. Wang, Carbon, 2021, 172, 272 CrossRef CAS.
- H. Tan, Y. Fan, X. Pan, S. Chen, H. Tao, D. Yang, W. Zhang and Z. Li, Ind. Crops Prod., 2023, 191, 115981 CrossRef CAS.
- L. Zhao, Y. Li, M. Yu, Y. Peng and F. Ran, Adv. Sci., 2023, 10, 2300283 CrossRef CAS PubMed.
- G. S. R. Raju, S. Kondrat, N. R. Chodankar, S. K. Hwang, J. H. Lee, T. Long, E. Pavitra, S. J. Patil, K. S. Ranjith, M. V. B. Rao, P. Wu, K. C. Roh, Y. S. Huh and Y. K. Han, J. Mater. Chem. A, 2023, 11, 15540 RSC.
- P. Simon and Y. Gogotsi, Nat. Mater., 2020, 19, 1151 CrossRef CAS PubMed.
- S. Ghosh, S. Barg, S. M. Jeong and K. Ostrikov, Adv. Energy Mater., 2020, 10, 2001239 CrossRef CAS.
- W. Jian, W. Zhang, X. Wei, B. Wu, W. Liang, Y. Wu, J. Yin, K. Lu, Y. Chen, H. N. Alshareef and X. Qiu, Adv. Funct. Mater., 2022, 32, 2209914 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nj04881k |
| ‡ Equal contribution to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |