Carboxymethylcellulose/layered double hydroxide dispersions for topical ocular delivery of non-steroidal anti-inflammatory drugs†
Giuliana
Mosconi
a,
María Lina
Formica
 b,
Santiago D.
Palma
b and
Ricardo
Rojas
b,
Santiago D.
Palma
b and
Ricardo
Rojas
 *a
*a
aInstituto de Investigación en Fisicoquímica de Córdoba (INFIQC), CONICET. Departamento de Fisicoquímica. Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Ciudad Universitaria, Córdoba 5000, Argentina. E-mail: ricardo.rojas@unc.edu.ar
bUnidad de Investigación y Desarrollo en Tecnología Farmacéutica (UNITEFA), CONICET and Departamento de Ciencias Farmacéuticas. Facultad de Ciencias Químicas, Universidad Nacional de Córdoba. Ciudad Universitaria, Córdoba 5000, Argentina
First published on 24th November 2023
Abstract
Drug delivery to ocular tissues is mainly hindered by anatomical and physiological barriers that diminish their bioavailability. In this work, layered double hydroxides (LDHs) intercalated with non-steroidal anti-inflammatory drugs (NSAIDs; ibuprofen, ketoprofen, and ketorolac) were incorporated into carboxymethylcellulose (CMC) dispersions with the aim of adjusting their rheological properties, producing sustained release, and inducing mucoadhesive properties. Pure NSAID-intercalated LDHs (LDH-NSAID) with high drug loading were obtained. The obtained particles showed high aggregation and positive surface charges, which allowed interaction with the negatively charged groups of CMC. Isotonic 1% CMC and 1% LDH-NSAID (w/w) dispersions behaved as viscous, shear-thinning fluids, although a stronger interaction with ketoprofen led to more gel-like behavior. Also, CMC/LDH-NSAID dispersions exhibited a more sustained drug release than that achieved with analogous formulations containing pure drugs, and the modeling of the profile indicated that the release rate was mainly determined by the anion exchange of the intercalated NSAID. Finally, the formulations showed strong mucoadhesion capacity, as they produce significant changes in the zeta potential of mucin dispersions. Further optimization of the particle size and surface properties of the particles is required to modulate the release profile and to enhance the rheological properties of these CMC/LDH-based formulations.
1. Introduction
Ocular drug delivery remains a challenge for pharmaceutical scientists due to the unique structure of the eye, which presents anatomical and physiological barriers that restrict drug permeation. In addition, the drugs to be delivered are generally characterized by low aqueous solubility, which results in low bioavailability.1,2 Among the routes for ocular delivery, the topical one is the most common for treating ocular diseases, as it is the easiest and least invasive. However, topical ocular delivery is still challenging as, in addition to the limitations previously mentioned, rapid clearance processes lead to a low retention time (1–2 minutes) and, consequently, poor drug penetration.3 To achieve and maintain an effective drug concentration, high dosing and frequent administration are usually needed, which lead to a high incidence of side effects and low patient compliance.4,5Thus, uveitis is the most common intraocular inflammatory disease and presents serious therapeutic obstacles. Corticosteroids are usually employed for its treatment, but side effects such as cataract and increased ocular pressure have been associated with their ocular use, which generates a need for the development of alternative therapeutic strategies.6 Thus, non-steroidal anti-inflammatory drugs (NSAIDs), a family of acidic drugs with antipyretic, anti-inflammatory, and pain-relieving effects, have been proposed as substitutes due to their similar anti-inflammatory effects and lower incidence of side effects. However, most NSAIDs exhibit low aqueous solubility and/or bioavailability, resulting in poor effectiveness.4
Hydrogels have been employed to enhance the effectiveness of ocular drug formulations.7 They are hydrophilic networks composed of polymer chains that retain a significant amount of water, resulting in high biocompatibility. Thus, carboxymethylcellulose (CMC) is an inexpensive biopolymer derived from cellulose, containing a high concentration of –OH and COOH groups within its chemical structure. CMC dispersions exhibit (besides excellent biocompatibility and biodegradability) moisturizing and mucoadhesive properties, being commercially used as eye moistener and lubricant formulations.8 However, it presents limitations such as poor mechanical properties and low retention capacity for hydrophilic drugs. These drawbacks can be overcome by incorporating particles, such as silica, carbon nanotubes, and layered double hydroxides (LDHs).9,10
Layered double hydroxides (LDHs) are a class of inorganic solids with a structure based on layers whose positive charge is balanced by anions within the interlayer region.11–13 Their chemical formula can be written as [MII1−xMIIIx(OH)2](Ax/n)·mH2O, where MII and MIII represent divalent and trivalent metal ions (such as Mg2+, Zn2+, Al3+ and Fe3+, commonly used for biomedical applications, due to their low cytotoxicity), while An− denotes the interlayer anion. LDH has been investigated for the oral and topical delivery of NSAIDs, which can be intercalated between the layers and released through anion exchange or by weathering of the hydroxylated layers.14–19 These mechanisms allow for controlled release, depending on the conditions of the surrounding media. LDH also enhances NSAID solubility in acidic environments and provides protection against degradation caused by light and chemical agents. Furthermore, the positively charged LDH particles are expected to interact with the negatively charged cell membranes of the cornea and conjunctiva, prolonging the retention time of NSAIDs, increasing their permeation capacity, and, consequently, enhancing their bioavailability.20 CMC–LDH beads incorporating the antimicrobial drugs amoxicillin, cefalexin and norfloxacin have been developed for oral delivery.9,21,22 These composites have shown controlled drug release, antimicrobial activity, and negligible cytotoxicity. LDHs intercalated with ibuprofen were also incorporated into CMC beads, demonstrating pH-dependent drug release, and enabling controlled release under simulated intestinal conditions.23 However, the properties of CMC dispersions and their applications as topical ocular formulations remain unexplored.
In this study, CMC/LDH dispersions are investigated as eye drop formulations for the delivery of NSAIDs. LDHs intercalated with three different NSAIDs, namely ibuprofen (IB), ketoprofen (KP), and ketorolac (KL), were synthesized, and their structure, chemical composition, and interfacial properties were analyzed. Subsequently, these LDHs were dispersed in CMC, and the physicochemical and rheological properties of the resulting dispersions were evaluated. Finally, the release kinetics from the dispersions were determined and modeled to elucidate the release mechanism and their mucoadhesiveness was studied to evaluate the capacity to achieve enhanced bioavailability.
2. Materials and methods
The following reagents were used without further treatment: MgCl2·6H2O and AlCl3·6H2O (Anedra, Argentina), ketoprofen, ibuprofen and tromethamine ketorolac (Parafarm, Argentina), NaOH and HCl (Cicarelli, Argentina); CMC (Fluka, Germany), NaCl, NaHCO3, CaCl2·2H2O, KCl, NaH2PO3, Na2HPO3 and KBr (Anedra, Argentina). Mucin (type III: partially purified from porcine stomach) was provided by Sigma-Aldrich (Steinheim, Germany). All solutions were prepared with purified water (18 MΩ MilliQ, Millipore System), and the experiments, unless otherwise stated, were performed at room temperature.2.1. Synthesis of LDH-NSAID
NSAID-intercalated LDHs (LDH-NSAID) were obtained by coprecipitation at a constant pH. The effect of hydrothermal treatment under different conditions was evaluated to optimize the NSAID loading (ESI,† Fig. S1 and Table S1).Briefly, a 100 mL aqueous solution (0.30 mol L−1 MgCl2 and 0.10 mol L−1 AlCl3) was added dropwise to 100 mL of a 0.15 mol L−1 solution of ibuprofen (IB), ketoprofen (KP) or ketorolac (KL) under constant stirring and N2 bubbling. The pH was maintained at 9 by controlled addition of a 1 mol L−1 NaOH solution. The obtained solid was aged by hydrothermal treatment at 90 °C for 4 hours and then separated and washed two times with water. The obtained pellets were freeze-dried to obtain the powdered samples LDH-IB, LDH-KP and LDH-KL. A chloride-intercalated Mg–Al LDH (LDH-Cl) was also prepared using the same procedure.
2.2. Characterization of LDH-NSAIDs
Powder X-ray diffraction (PXRD) patterns were recorded with a Phillips X'pert Pro instrument equipped with a Pixcell 1D detector and a CuKα lamp (λ = 1.5408 Å) at 40 kV and 40 mA. The scans were performed in continuous mode (10° min−1) between 5 and 70°. Low angle measurements (2–10°) were performed in step mode (0.05°, 1.2 s) with a Xe detector coupled to a graphite monochromator. FT-IR spectra were recorded with a Thermo Scientific Nicolet iN10 FTIR spectrometer using KBr pellets (1:200 sample:KBr ratio) within a wavenumber range of 4000–400 cm−1, at a 4 cm−1 resolution and 16 scan accumulation. Differential thermal and thermogravimetric analyses (DTA and TGA, respectively) were performed using a SETARAM Setsys Evolution 16/18 instrument, at a 10 °C min−1 temperature slope. The drug content of the LDH-NSAID samples was determined at dispersions of the samples (1 g L−1) in phosphate buffer saline (pH = 7.4), which were equilibrated for 24 hours and then centrifuged. The NSAID concentration in the supernatants was determined by UV–Vis spectrophotometry (Shimadzu UV1601, Japan) at λ = 264 (IB), 261 (KP) and 323 nm (KL) using independent calibration curves in the same media. Scanning electron microscopy (SEM) images were obtained using an FE-SEM Σigma instrument on samples covered with a Cr layer. The Mg/Al ratio was determined by energy dispersive X-ray spectroscopy (EDS) using the same instrument. The hydrodynamic diameter (d) and zeta potential (ζ) were recorded in dispersions of the samples (0.5 g L−1) in water, using a Delsa Nano C instrument (Beckman Coulter, USA).2.3. Preparation and characterization of CMC/LDH-NSAIDs
To characterize the interaction with CMC, 0.5 g L−1 LDH-NSAID dispersions in 0.1 g L−1 CMC and 0.9 g L−1 NaCl solution were prepared. Their d and ζ were determined as previously described and compared to those obtained for LDH-NSAID dispersions in pure water.The CMC dispersions of pure NSAIDs or LDH-NSAIDs were prepared as follows: 0.1 g of CMC, 0.05 g of LDH-NSAIDs, 0.09 g of NaCl and 9.76 g of water were weighted in a beaker and stirred overnight to obtain 1% CMC and 0.5% LDH-NSAID isotonic dispersions (CMC/LDH-NSAID). In the case of the pure drug dispersions (CMC/NSAID), the LDH-NSAID particles were substituted by the equivalent weight of NSAIDs. As a reference, CMC dispersions without drugs nor LDH particles were also prepared.
The rheometric properties of the dispersions (either CMC, CMC/NSAIDs or CMC/LDH-NSAIDs) were investigated using an Anton Paar MCR302 rheometer equipped with a 50 mm plate-plate geometry and a 1.0 mm gap. An amplitude sweep (strain 0.01–100% at a constant angular frequency of 1 Hz) was first performed. Once the linear viscoelastic region (LVR) was determined, a frequency sweep from 0.1 to 100 Hz at a maximum strain into the LVR was recorded. All measurements wereperformed at room temperature.
2.4. Release kinetics
Bicompartimental Franz cells were used to study the drug release kinetics from both CMC/LDH-NSAID and CMC/NSAID dispersions at 37.0 ± 0.5 °C. The receptor compartment was filled with 16 mL of simulated tear buffer (STB) and, in the donor compartment, 1 mL of the corresponding CMC dispersion was added. A semisynthetic acetate cellulose membrane (12 kDa molecular cut-off, Sigma-Aldrich®) was mounted between the donor and the receptor compartments. 1 mL of aliquot was taken from the receptor compartment at predefined times (10, 20, 30 and 45 min, 1, 2, 3, 4, 6 and 24 hours) and replaced by 1 mL of the fresh medium. The concentration of each drug was determined by UV-Vis spectrophotometry as previously described, using calibration curves constructed for each drug in the STB. All experiments were conducted in triplicate and sink conditions were maintained.To determine mechanisms governing NSAID release, the following kinetic models were applied:24
| Zero-order model: %NSAID = %NSAID0 + k0t | (1) |
| Higuchi model: %NSAID = %NSAID0+ kHt1/2 | (2) |
Korsmeyer–Peppas (K–P) model: %NSAID = kPtn; log %NSAID = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kP + n kP + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t t |
2.5. Bioadhesion of LDH-NSAID samples and CMC/LDH-NSAID dispersions
The shifting of the ζ of mucin particles was used to evaluate the mucoadhesive properties of both LDH-NSAID and CMC/LDH-NSAID dispersions.25 Briefly, a mucin dispersion (1 g L−1) in NaCl (0.09%) was contacted with LDH-NSAID (0.5 g L−1) and CMC/NSAID dispersions and NSAID solutions with an equivalent amount of drugs than the previous two. The dispersions were incubated for 1 hour. The ζ of both mucin, NSAID/mucin, LDH-NSAID/mucin and CMC/LDH-NSAID/mucin dispersions were determined.3. Results
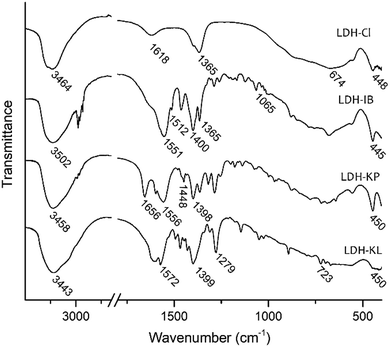
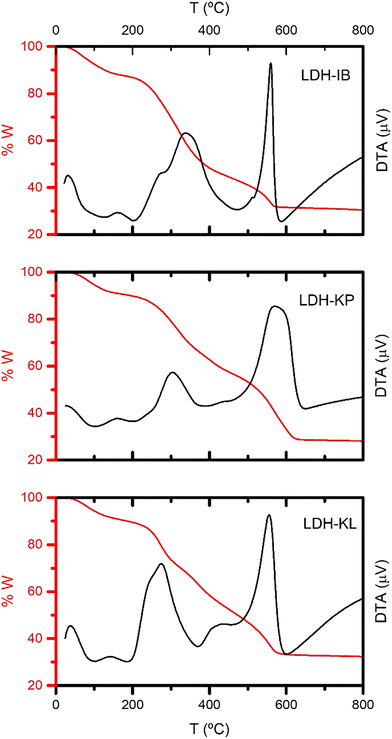
Single, pure phases of Mg-Al LDHs intercalated with the corresponding NSAIDs were obtained by the selected synthesis method, as demonstrated by PXRD patterns (Fig. 1), FT-IR spectra (Fig. 2) and TG-DTA curves (Fig. 3).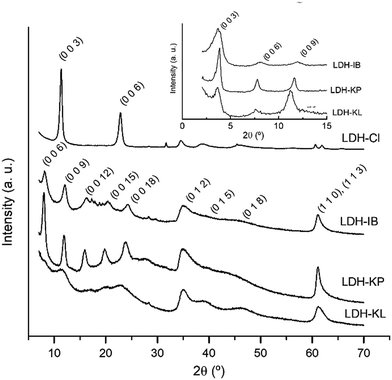 | ||
| Fig. 1 PXRD patterns of LDH-NSAID and LDH-Cl samples. Inset: Low angle measurements of LDH-NSAID samples. | ||
The PXRD patterns of the samples (Fig. 1) were the characteristic of LDH phases. Thus, LDH-Cl showed high-intensity, symmetric peaks at 2θ angles below 30°, which were assigned to (0 0 l) diffraction planes and allowed the determination of an interlayer distance (8.1 Å). Low-intensity, asymmetric peaks obtained at 2θ angles above 30° were assigned to the (0 1 l) and (1 1 l) diffraction planes and a cell parameter of a = 3.04 Å was obtained, similar to that previously obtained for Mg–Al LDHs.26 The intercalation of NSAIDs significantly decreased the 2θ values of (0 0 l) peaks, resulting in the following interlayer distance values: 21.9 Å for LDH-IB, 22.7 Å for LDH-KP, and 23.7 Å for LDH-KL. These values were similar to those previously obtained for LDHs intercalated with ibuprofen27–29 and ketoprofen.16,30,31 The poor intensity and the width of the peaks indicated that the samples had lower crystallinity compared to LDH-Cl.
The FT-IR spectra displayed characteristic bands representing both the LDH structure and the corresponding NSAIDs (Fig. 2). The bands of the LDH structure were similar in all samples as well as in the reference sample LDH-Cl. The band observed between 3443 and 3502 cm−1 was attributed to the O–H stretching mode (νOH) of OH− anions of the layers and water molecules in the interlayer space. Additionally, the broad band at wavenumbers below 1000 cm−1 was assigned to lattice vibrations of the LDH layers.32 Contrarily, the band obtained at 1618 cm−1 for the LDH-Cl sample, due to the bending vibration of the water molecules located at the interlayer, was not observed for the LDH-NSAIDs due to overlapping with the bands of the respective drugs. Spectra of the samples also showed bands corresponding to the intercalated anions. Thus, LDH-Cl only showed a band at 1365 cm−1 corresponding to the intercalation of a significant portion of carbonate anions, intercalated as an impurity.33 This band was absent in the LDH-NSAID samples. The most significant bands of NSAIDs were those corresponding to the symmetric and antisymmetric vibrations of carboxylate anions (νCOO,asym and νCOO,sym). The former was registered at around 1399 cm−1, while the latter was in the range between 1551 and 1572 cm−1. The difference between the wavenumbers of both bands (Δν = νCOO,sym − νCOO,asym) is considered indicative of the type of interaction between the carboxylate groups and the hydroxylated layers.34,35 The values obtained (151, 158 and 173 cm−1 for LDH-IB, LDH-KP and LDH-KL, respectively) indicated that the anions are exclusively attached by electrostatic interactions and hydrogen bonding, without direct coordination binding to the metal ions that constitute the layers. On the other hand, no band at around 1700 cm−1, corresponding to the stretching vibration mode of carboxylic acids, was registered. This absence indicates that the NSAIDs are incorporated almost exclusively in its basic, anionic form. Finally, the FT-IR spectra of the samples showed specific bands of each NSAID: LDH-IB at 1365 cm−1 (νC–C–H), 1512 cm−1 (νC–C,ring) and 1065 cm−1 (νC–C);29 LDH-KP, at 1656 cm−1 (νC=O, ketone) and 1448 cm−1 (δCH2)30,36 and finally LDH-KL, at 1279 cm−1 (δCH2) and 723 cm−1 (δC–C–C).37
The TG/DTA curves of the LDH-NSAID samples (Fig. 3) showed the typical profile of LDH phases intercalated with organic anions. This profile includes three processes: dehydration, dihydroxylation, and loss of the interlayer anion. Dehydration was registered as an endothermal effect near to 100 °C with weight losses up to 15% in all cases. On the other hand, dihydroxylation and interlayer anion oxidation were overlapped in a wide temperature range between 200 and 600 °C in all cases. Two exothermic peaks were recorded in this range, the first was recorded at 340, 300 and 270 °C for LDH-IB, LDH-KP and LDH-KL, respectively, and the second was at near 560 in all cases. Both peaks were assigned to the oxidation of the interlayer anion, and indicated that it was produced in two steps, the former being more dependent on the intercalated drug. The endothermic peak corresponding to the dihydroxylation process, which was clearly observed for the reference sample LDH-Cl (Fig. S2, ESI†) was obscured by the strong exothermic peaks previously exposed, although shoulders were observed at around 380 °C for LDH-KP and LDH-KL. At 800 °C, the overall weight loss was around 70% in all LDH-NSAID samples, which was concordant with a large intercalation of the corresponding NSAIDs.
Once it was confirmed that only a single LDH phase was present, the chemical composition allowed for the determination of the chemical formula of the samples (Table 1). There was good concordance between the experimental and calculated w/w percentages (%Mg, %Al, %AINE, %H2O, and %W800), which confirms the accuracy of the proposed formula. The obtained formulae indicated that the Mg/Al ratio in the samples is lower than that of the starting solution, which was related to the pH selected for the synthesis. Increasing the synthesis pH above 9 is likely to result in an increase in the Mg/Al ratio in the samples. However, it is important to note that higher pH values can also lead to the intercalation of impurities such as carbonate and hydroxyl anions. These impurities would diminish NSAID intercalation and affect the properties of the LDH-NSAID samples. On the other hand, the NSAID/Al ratio (as obtained from the coefficients of the proposed formulae) was quite high in all cases and decreased in the order of LDH-IB > LDH-KP > LDH-KL. The observed order of intercalation of NSAIDs into the LDH layers was attributed to the structural characteristics of the different NSAIDs, which influenced their affinity with the LDH layers. In particular, the specific affinity observed for IB− anions was attributed to lateral interactions between the hydrophobic tails of the drug. The introduction of the polar group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the structure of KP reduced these lateral interactions in the LDH-KP sample. On the other hand, LDH-KL exhibited the lowest NSAID/Al ratio, as KL is a bulky anion that is more challenging to intercalate between the LDH layers, and this study is the first to achieve a such a large intercalation of this drug.
O in the structure of KP reduced these lateral interactions in the LDH-KP sample. On the other hand, LDH-KL exhibited the lowest NSAID/Al ratio, as KL is a bulky anion that is more challenging to intercalate between the LDH layers, and this study is the first to achieve a such a large intercalation of this drug.
| Sample | %Mg | %Al | %AINE | %H2O | %W800 | Formulaa |
|---|---|---|---|---|---|---|
| a Inorganic anions from the synthesis medium are added to compensate the excess charge. | ||||||
| LDH-IB | 13.3 (12.5) | 5.5 (5.2) | 51,5 (45.9) | 12.3 (12.3) | 30.4 (30.6) | Mg0.73Al0.27(OH)2IB0.31Na0.04·0.97H2O |
| LDH-KP | 11.4 (11.8) | 5.3 (5.5) | 47.6 (49.8) | 9.7 (9.5) | 28.1 (29.9) | Mg0.71Al0.29(OH)2KP0.28Cl0.01·0.76H2O |
| LDH-KL | 12.4 (12.6) | 6.4 (6.6) | 44.2 (45.2) | 9.7 (9.6) | 32.3 (33.4) | Mg0.68Al0.32(OH)2KL0.23Cl0.09·0.70H2O |
LDHs presented large d values in the aqueous dispersion that decreased in the order of LDH-IB > LDH-KL > LDH-KP, as determined by DLS (Fig. 4a, green bars). These values are produced by large agglomerates, as demonstrated by the SEM images (Fig. 4c–e), which showed particles with a size of around 150 nm, lamellar aspect, and irregular borders. Their ζ values were positive (Fig. 4b, green bars), as it is typical of LDH nanoparticles,38,39 but lower than that obtained for LDHs intercalated with inorganic anions that are exclusively attached by electrostatic forces such as is the case of chloride-intercalated LDHs.40 The small ζ and large d values indicated that a portion of the NSAID anions were placed at the surface of LDH particles, diminishing the charge and increasing the hydrophobicity of the surface.16,36 Both effects were led to an increase of the particle–particle interactions and, consequently, to the observed LDH-NSAID aggregation. When LDH-NSAIDs are included in CMC dispersions, electrostatic interactions were expected to be produced between the positively charged surface of the aggregates and the negatively charged groups of the polymer. These interactions were explored at a concentration ten times lower than the final dispersions, which allowed the comparison with the results obtained for LDH-NSAID dispersions in pure water (Fig. 4a and b, red columns). All LDH-NSAID samples exhibited negative ζ values in CMC dispersions, although the CMC/LDH-KP dispersion showed a significant diminution in the absolute value. This decrease is consistent with the larger positive ζ potential of LDH-KP, which allowed a stronger interaction with CMC chains. Nevertheless, the interaction between CMC and LDH-NSAID agglomerates did not produce disaggregation. d values larger than that in pure water were obtained, similarly to that obtained for LDH-Cl dispersions in the presence of alginate.41 Both CMC and alginate present a low concentration of negatively charged groups, and they perform as bridges between particles or agglomerates. The d values obtained for CMC/LDH-NSAID dispersions were in the upper limit (10–25 μm) for its use in ophthalmic formulations.42,43 Nevertheless, it can be expected that this agglomerate size can be diminished to more suitable values by repeatedly washing the LDH particles, the direct use of LDH-NSAID dispersions, avoiding the lyophilization step, or the use of stabilizers that increase particle repulsions by electrostatic forces or steric hindrance.
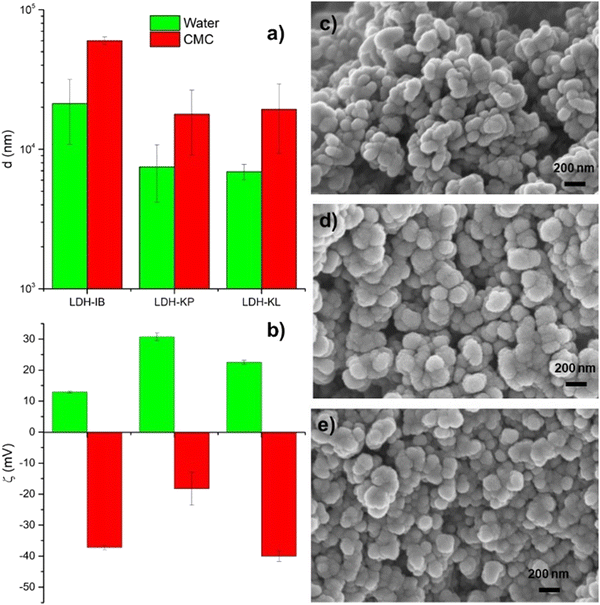 | ||
| Fig. 4 (a) Hydrodynamic diameter and (b) zeta potential of LDH-NSAIDs dispersed in pure water and 0.1% CMC dispersions. SEM images of (c) LDH-IB, (d) LDH-KP and (e) LDH-KL. | ||
Notwithstanding, the dispersions of LDH-NSAID particles obtained in isotonic CMC dispersions (0.5 and 1% w w−1, respectively, 0.9% w w−1 NaCl) were homogeneous and stable. Dispersions containing the pure drug were also obtained in a similar way, but it is important to consider that, while KP and KL were incorporated in their anionic, soluble form, IB was incorporated as particles of their neutral, insoluble form.
Fig. 5 presents the amplitude sweeps of CMC/LDH-NSAID and pure CMC dispersions. Rheology is a critical parameter for ophthalmic formulations, as it determines their physical stability, spreading and ease of applications. Ideally, they present viscoelastic, shear thinning behavior that allows them to flow during administration and blinking while maintain a enough viscosity at rest to prolong their retention. According to the literature, CMC dispersions show different behaviors depending on the polymer concentration.44 It performs as a Newtonian fluid at low concentrations (around 1%) and, generally, viscoelastic properties at high concentrations (around 5%). The amplitude sweeps of CMC, CMC/LDH-IB and CMC/LDH-KL were similar to that previously obtained for CMC dispersions of the same concentration.45 They showed an extended linear viscoelastic (LVE) region where the loss (G′′) and the storage (G′) are constant, and the forces that bind the fluid are not broken. Only at high shear stress, both moduli diminish, which indicates a breakage of the structure. This behavior together with the higher G′′ values compared to G′ was the characteristic of a viscoelastic fluid. In contrast, G′ was larger than G′′ in the LVE region for the CMC/LDH-KP dispersion, which is the characteristic of a gelled/viscoelastic solid. Much larger values of G′ and G′′ were obtained for this dispersion, and the LVE region was shorter. After this region, a gradual decrease of G′ was observed, indicating a steady breakdown of the gel structure.46 A parallel gentle increase of G′′ was observed in this region until a maximum was reached, and then decreased. The first region was associated with the formation of microcracks that evolve to large cracks that break completely the structure of the gel once the maximum has been reached. Then, LDH-KP shows a higher level of structuration, resulting in a gel-like behavior, but the slight variation of both G′ and G′′ after the LVE region suggests that this gel structure is labile. This different behavior of LDH-KP was assigned to the previously exposed stronger interaction with CMC than LDH-IB and LDH-KL.
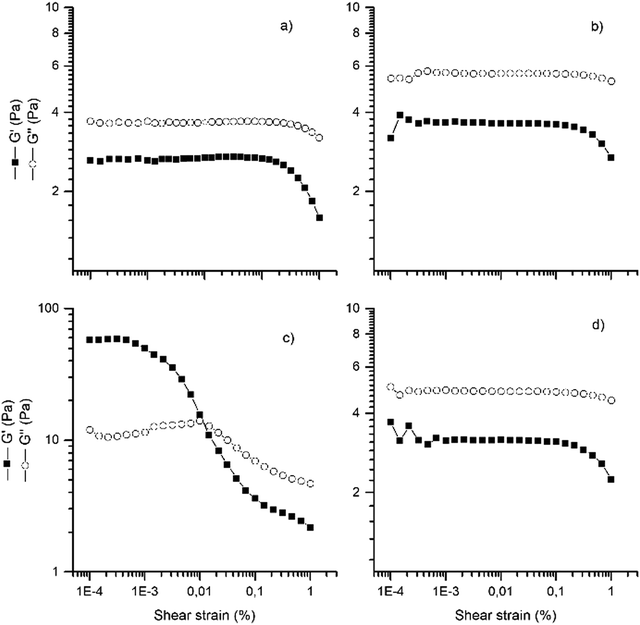 | ||
| Fig. 5 Rheological characterization of CMC dispersions. Amplitude sweeps at room temperature of (a) CMC, (b) CMC/LDH-IB, (c) CMC/LDH-KP (scale x10), and (d) CMC/LDH-KL. | ||
Frequency sweeps were performed at amplitude values within the LVE region, which allowed obtaining apparent viscosity vs frequency curves of the dispersions (ESI,† Fig. S3). The obtained curves indicated that all samples presented shear thinning behavior, in line with that obtained in the amplitude sweeps. Nevertheless, CMC/LDH-KP presented a much lower viscosity as well as a steeper decrease with the frequency. The viscoelastic, shear thinning behavior of the dispersions is expected to ease applications, facilitate its spreading by blinking and prevent a fast draining from the eye surface.
The inclusion of the NSAIDs as a part of the LDH structure in the CMC dispersions produced significant changes in the drug release profiles compared to those containing the pure drug (Fig. 6). Both CMC/LDH-NSAID and CMC/NSAID dispersions showed a sustained release, but the intercalation between the LDH layers led to a release rate decrease in all cases, with a lower cumulative drug release (%NSAID) for CMC/LDH-NSAIDs than for CMC/NSAIDs at any given time. Although the inclusion of NSAIDs in the LDH structure was the main factor that affected the release profile, also differences were found depending on the considered drug. Thus, the cumulative %NSAID at 24 hours was quite larger for CMC/LDH-KL dispersions (75%) than that for CMC/LDH-IB and CMC/LDH-KP (30 and 35%, respectively). This difference in release rates can be attributed to the lower affinity of KL anions for the LDH structure. Besides these general differences, a similar profile was observed at very short times (below 30 minutes) for CMC/LDH-NSAID and CMC/NSAID dispersions. This similar behavior was assigned to the presence of a portion of free NSAIDs in CMC/LDH-NSAID dispersions that were rapidly exhausted. The presence of free NSAIDs was justified by the interaction of carboxylate groups of CMC with the surface of LDH-NSAIDs, displacing the drug from the particle surface.
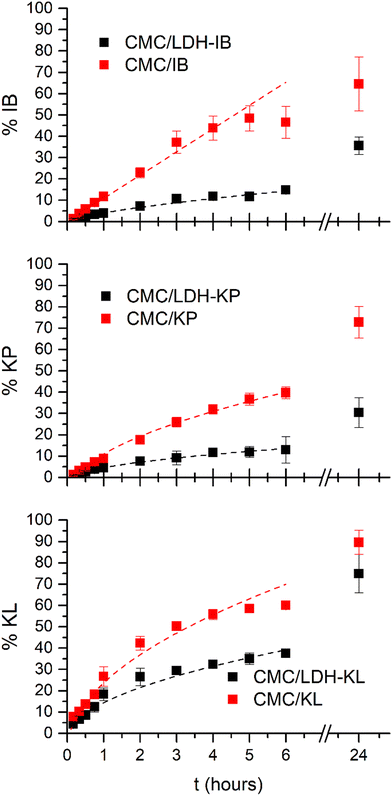 | ||
| Fig. 6 Experimental (squares) and calculated (dashed lines) profiles of cumulative release (%NSAID) from CMC/LDH-NSAID and CMC/NSAID dispersions in contact with the simulated tear fluid (STF). The calculated profiles were obtained using the parameters of the best fits included in Table 2. | ||
Modeling the release profile (Table 2) allowed stablishing the rate determining step of the drug release and gave hints about its mechanism. For CMC/NSAIDs, a distinction can be made between those incorporated to the dispersions as an anion (KP− and KL−) and the one that incorporated in its acidic form (HIB). The former two showed a larger cumulative release at 24 hours than the latter. Also, their best fit was obtained with the Higuchi model, while the K–P model (n = 1.0) produced the best fit for CMC/IB. These results indicated that KP− and KL− anions free and directly diffuse from the CMC/NSAID dispersions to the receptor medium while IB− anions were first dissolved from pure HIB particles, which was the rate determining step of the release process.
| Sample | Zero order | Higuchi | Kornsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|
| %NSAID0 | k 0 (s−1) | R 2 | %NSAID0 | k H (s−1/2) | R 2 | k P (s−n) | n | R 2 | |
| CMC/IB | 1.3 | 10.3 | 0.981 | −13.2 | 27.7 | 0.985 | 10.9 | 1.0 | 0.990 |
| CMC/LDH-IB | 1.5 | 2.4 | 0.940 | −2.07 | 6.7 | 0.976 | 4.1 | 0.7 | 0.992 |
| CMC/KP | 2.1 | 6.9 | 0.981 | −9.0 | 20.0 | 0.992 | 6.2 | 1.0 | 0.976 |
| CMC/LDH-KP | 1.9 | 2.1 | 0.940 | −1.6 | 6.2 | 0.991 | 3.3 | 0.8 | 0.981 |
| CMC/KL | 12.1 | 9.6 | 0.894 | −8.2 | 31.9 | 0.979 | 22.2 | 0.7 | 0.965 |
| CMC/LDH-KL | 8.4 | 5.6 | 0.891 | −2.7 | 17.1 | 0.992 | 13.5 | 0.6 | 0.984 |
For CMC/LDH-NSAID dispersions, the lower release rate indicated that the inclusion of the drug in the structure of LDHs controlled its release profile. The best fits were obtained with the Higuchi model in the case of LDH-KP and LDH-KL. Drug release from LDH particles is produced, at neutral mediums such as STF, by an ion exchange mechanism,47 whose rate is determined by anion diffusion (particularly chloride, carbonate and phosphate anions) between the layers. This process was quite slower than diffusion through loosely bonded hydrogels such as CMC, which explains that the lower release rate for CMC/LDH-NSAIDs compared to that for CMC/NSAIDs forms dispersions., In the case of CMC/LDH-IB, the K–P model presented the best fit for LDH-IB and the n value (=0.7) indicated that both dissolution and diffusion shared the control of the release. The lower solubility of this drug and stronger interactions with the LDH structure produces a slower detachment from the LDH-IB particles. This solvation process at the surface of LDH-IB particles determined, together with diffusion, the release rate.
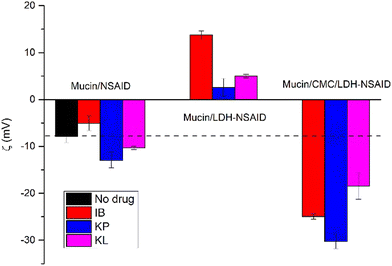
Finally, the mucoadhesion capacity of the dispersions was evaluated by mucin experiments (Fig. 7). Both the cornea and conjunctiva are covered by a mucous layer mainly composed of mucin, a glycoprotein of a large molecular weight and negative charging under physiological conditions. In our experiments, mucin-only dispersions exhibited a ζ value of −7 ± 1 mV, similar to that found in the bibliography.48 This value is only slightly changed upon interactions with different NSAIDs, indicating that the drugs present a weak interaction with mucin. In contrast, a significant change was produced in the presence of both LDH-NSAID particles and CMC/LDH-NSAIDs, indicating a strong interaction between both systems and mucin and therefore, a good mucoadhesion capacity. Compared to free mucin, ζ values increased, as was expected due to the interaction with the positively charged LDH-NSAID particles. Nevertheless, an additional interaction was produced due to hydrophobic interactions, which allows the inversion of ζ values. Concordantly, the most positive value was obtained for IB, the most hydrophobic NSAIDs. On the other hand, the presence of CMC/LDH-NSAID dispersions diminished ζ values which is consistent with the ζ value of CMC/LDH-NSAIDs and the mucoadhesion capacity of CMC, which is produced by a specific interaction of moderate intensity including hydrogen bonding and dehydration that allows overcoming the repulsive electrostatic interactions between CMC and mucin.49 Thus, CMC/LDH-NSAIDs present a significant mucoadhesion capacity that is favorable for a longer residence time and drug permeation of the NSAIDs included in these dispersions. This mucoadhesion, together with the sustained release produced from this dispersions, and their viscous behavior make formulation of NSAIDs as CMC/LDH dispersions a simple and promising strategy to enhance the effectiveness of these drugs and allow their application as substitutes of corticosteroids in the treatment of conditions such as uveitis.
4. Conclusions
LDHs have been successfully intercalated with three NSAIDs: ibuprofen, ketoprofen, and ketorolac. The synthesis method employed resulted in obtaining pure phases with high loading of the respective drugs. Although the method led to aggregation of nanoparticles caused by the presence of the drugs on the particle surface, their positive ζ allowed their interactions with anionic groups of CMC. Stable dispersions of LDH-NSAID and carboxymethylcellulose (CMC) were produced as a result. The obtained dispersions were viscous and exhibited shear thinning behavior, except for CMC/LDH-KP, which displayed a gel-like consistency due to stronger interactions between the inorganic particles and the polymer chains. CMC/LDH-NSAID dispersions showed a sustained drug release, significantly slower than that of dispersions containing pure drugs. The main release mechanism was anion exchange between the intercalated drug and anions present in the release media. This process was slower than the direct diffusion of NSAIDs from CMC/NSAID dispersions. Finally, both LDH-NSAID and CMC/LDH-NSAID presented mucoadhesive properties. The former presents both electrostatic and hydrophobic interactions with mucin while the latter is produced by specific interactions of the polymer. Based on these findings, CMC/LDH dispersions show promising properties as drug delivery systems for topical ocular administration. Thus, simple and low-cost chemical technology could be an alternative for optimizing ophthalmic formulations. Further research and development efforts are required to maximize the disaggregation of the particles, to optimize their rheological properties and to modulate their release rate.Conflicts of interest
There are no conflicts to declare.Acknowledgements
GM thanks CONICET for his postdoctoral grant. MLF, SDP and RR are members of CONICET's scientific career. The authors wish to acknowledge the assistance of the CONICET and UNC, Argentina, both of which provided facilities for this work. The economic support by CONICET, ANPCyT (PICT2020-1781) SeCyT-UNC and MinCyT-CBA is gratefully acknowledged. The rheological determinations were performed at CEPROCOR-MinCyT-CBA, with the aid of Dr Karina Bierbrauer. The SEM images were obtained at the Laboratorio de Microscopía Electrónica y Análisis por Rayos X (LAMARX).References
- C. Torres-Luna, X. Fan, R. Domszy, N. Hu, N. S. Wang and A. Yang, Eur. J. Pharm. Sci., 2020, 154, 105503 CrossRef CAS.
- C. R. Lynch, P. P. D. Kondiah, Y. E. Choonara, L. C. du Toit, N. Ally and V. Pillay, Front. Bioeng. Biotechnol., 2020, 8, 1–18 Search PubMed.
- S. K. Motwani, S. Chopra, S. Talegaonkar, K. Kohli, F. J. Ahmad and R. K. Khar, Eur. J. Pharm. Biopharm., 2008, 68, 513–525 CAS.
- M. Ahuja, A. S. Dhake, S. K. Sharma and D. K. Majumdar, AAPS J., 2008, 10, 229–241 CrossRef CAS PubMed.
- S. Kirchhof, A. M. Goepferich and F. P. Brandl, Eur. J. Pharm. Biopharm., 2015, 95, 227–238 CrossRef CAS PubMed.
- V. M. B. Fiorelli, P. Bhat and C. Stephen Foster, Ocul. Immunol. Inflammation, 2010, 18, 116–120 CrossRef.
- A. M. Ribeiro, A. Figueiras and F. Veiga, J. Pharm. Pharm. Sci., 2015, 18, 683–695 CAS.
- B. Gupta, V. Mishra, S. Gharat, M. Momin and A. Omri, Pharmaceuticals, 2021, 14, 1021 CrossRef.
- S. Barkhordari and M. Yadollahi, Appl. Clay Sci., 2016, 121–122, 77–85 CrossRef CAS.
- F. Cao, Y. Wang, Q. Ping and Z. Liao, Int. J. Pharm., 2011, 404, 250–256 CrossRef CAS.
- R. Rojas, G. Mosconi, J. Pablo and G. A. Gil, Appl. Clay Sci., 2022, 224, 106514 CrossRef CAS.
- C. Taviot-Guého, V. Prévot, C. Forano, G. Renaudin, C. Mousty and F. Leroux, Adv. Funct. Mater., 2017, 28, 1703868 CrossRef.
- V. R. L. Constantino, M. P. Figueiredo, V. R. Magri, D. Eulálio, V. R. R. Cunha, A. C. S. Alcântara and G. F. Perotti, Pharmaceutics, 2023, 15, 413 CrossRef CAS PubMed.
- V. Rives, M. del Arco and C. Martín, Appl. Clay Sci., 2014, 88–89, 239–269 CrossRef CAS.
- V. Rives, M. Del Arco and C. Martín, J. Controlled Release, 2013, 169, 28–39 CrossRef CAS.
- R. Rojas, A. F. Jimenez-Kairuz, R. H. Manzo and C. E. Giacomelli, Colloids Surf., A, 2014, 463, 37–43 CrossRef CAS.
- U. Costantino, V. Ambrogi, M. Nocchetti and L. Perioli, Microporous Mesoporous Mater., 2008, 107, 149–160 CrossRef CAS.
- L. Perioli, V. Ambrogi, L. di Nauta, M. Nocchetti and C. Rossi, Appl. Clay Sci., 2011, 51, 407–413 CrossRef CAS.
- C. Pagano, L. Latterini, A. Di Michele, F. Luzi, D. Puglia, M. Ricci, C. A. V. Iborra and L. Perioli, Pharmaceutics, 2020, 12, 1–15 CrossRef PubMed.
- R. Rojas, D. Aristizabal Bedoya, C. Vasti and C. E. Giacomelli, in Layered Double Hydroxides (LDHs), ed. I. T. Sherman, Nova Press, New York, 2015, pp. 101–120 Search PubMed.
- S. Behzadi Nia, M. Pooresmaeil and H. Namazi, Int. J. Biol. Macromol., 2020, 155, 1401–1409 CrossRef CAS PubMed.
- N. B. Allou, A. Yadav, M. Pal and R. L. Goswamee, Carbohydr. Polym., 2018, 186, 282–289 CrossRef CAS.
- S. Barkhordari, M. Yadollahi and H. Namazi, J. Polym. Res., 2014, 21, 454 CrossRef.
- P. Costa and J. M. S. Lobo, Eur. J. Pharm. Sci., 2001, 13, 123–133 CrossRef CAS.
- H. A. Abd El-Rehim, A. E. Swilem, A. Klingner, E. S. A. Hegazy and A. A. Hamed, Biomacromolecules, 2013, 14, 688–698 CrossRef CAS.
- R. Botan and S. de Bona Sartor, Layered Double Hydroxide Polymer Nanocomposites, 2020, pp. 205–229 Search PubMed.
- R. Rojas, M. C. Palena, A. F. Jimenez-Kairuz, R. H. Manzo and C. E. Giacomelli, Appl. Clay Sci., 2012, 62–63, 15–20 CrossRef CAS.
- E. E. Gaskell, T. Ha and A. R. Hamilton, Ther. Delivery, 2018, 9, 653–666 CrossRef CAS PubMed.
- C. V. Luengo, M. C. Crescitelli, N. A. Lopez and M. J. Avena, J. Pharm. Sci., 2021, 110, 1779–1787 CrossRef CAS.
- M. S. San Román, M. J. Holgado, B. Salinas and V. Rives, Appl. Clay Sci., 2012, 55, 158–163 CrossRef.
- M. Park, H. Kim, D. Park, J. Yang and J. Choy, Bull. Korean Chem. Soc., 2012, 33, 1827–1828 CrossRef CAS.
- J. T. Kloprogge and R. L. Frost, in Layered Double Hydroxides: Present and Future, ed. V. Rives, Nova Science Publishers, New York, 2001, pp. 139–192 Search PubMed.
- C. R. Gordijo, C. a S. Barbosa, A. M. Da Costa Ferreira, V. R. L. Constantino and D. de Oliveira Silva, J. Pharm. Sci., 2005, 94, 1135–1148 CrossRef CAS.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley and Sons, New York, 5th edn, 1997 Search PubMed.
- F. Wypych, G. G. C. Arízaga and J. E. F. da Costa Gardolinski, J. Colloid Interface Sci., 2005, 283, 130–138 CrossRef CAS PubMed.
- R. Rojas, Y. G. Linck, S. L. Cuffini, G. A. Monti and C. E. Giacomelli, Appl. Clay Sci., 2015, 109–110, 119–126 CrossRef CAS.
- Z. P. Xu, Z. Gu, X. Cheng, F. Rasoul, A. K. Whittaker and G. Q. M. Lu, J. Nanopart. Res., 2011, 13, 1253–1264 CrossRef CAS.
- Z. P. Xu, Y. Jin, S. Liu, Z. P. Hao and G. Q. M. Lu, J. Colloid Interface Sci., 2008, 326, 522–529 CrossRef CAS.
- M. Pavlovic, R. Huber, M. Adok-Sipiczki, C. Nardin and I. Szilagyi, Soft Matter, 2016, 12, 4024–4033 RSC.
- C. Vasti, V. Pfaffen, E. Ambroggio, M. R. Galiano, R. Rojas and C. E. Giacomelli, Appl. Clay Sci., 2017, 137, 151–159 CrossRef CAS.
- C. Vasti, A. Borgiallo, C. E. Giacomelli and R. Rojas, Colloids Surf., A, 2017, 533, 316–322 CrossRef CAS.
- M. Uddin, A. Mamun, M. Kabir, J. Setu, S. Zaman, Y. Begum and M. Amran, J. Adv. Med. Pharm. Sci., 2017, 14, 1–17 Search PubMed.
- 789-Particulate matter in ophtalmic solutions, United States Pharmacopeial Convention (USP, 41st ed.).
- A. Benchabane and K. Bekkour, Colloid Polym. Sci., 2008, 286, 1173–1180 CrossRef CAS.
- A. Potanin, Colloids Surf., A, 2019, 562, 54–60 CrossRef CAS.
- G. Schramm, A practical approach to Rheology and Rheometry, Thermo Electron, Karlsruhe, 2nd edn, 2004 Search PubMed.
- Y. Salguero, L. Valenti, R. Rojas and M. C. García, Mater. Sci. Eng., C, 2020, 111, 110859 CrossRef CAS.
- I. Sousa, A. Mendes, R. F. Pereira and P. J. Bártolo, Mater. Lett., 2014, 134, 263–267 CrossRef CAS.
- Q. D. Pham, S. Nöjd, M. Edman, K. Lindell, D. Topgaard and M. Wahlgren, Int. J. Pharm., 2021, 610, 121245 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: DRX patterns of samples obtained with different synthesis methods. Mg/Al ratio, NSAID contents of this samples, TG curves of LDH-Cl sample and dynamic viscosity (η) vs. shear rate (γ) curves for CMC and CMC/LDH-NSAID dispersions. See DOI: https://doi.org/10.1039/d3nj04434c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |