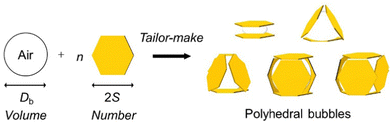
Shape design of aqueous bubbles stabilized with millimeter-sized polymer plates†
Yuri
Sakurai‡
a,
Rina
Kakiuchi‡
a,
Masaki
Hayashi
a,
Tomoyasu
Hirai
 bc,
Yoshinobu
Nakamura
bc and
Syuji
Fujii
bc,
Yoshinobu
Nakamura
bc and
Syuji
Fujii
 *bc
*bc
aDivision of Applied Chemistry, Environmental and Biomedical Engineering, Graduate School of Engineering, Osaka Institute of Technology, 5-16-1, Omiya, Asahi-ku, Osaka 535-8585, Japan
bDepartment of Applied Chemistry, Faculty of Engineering, Osaka Institute of Technology, 5-16-1 Omiya, Asahi-ku, Osaka 535-8585, Japan. E-mail: syuji.fujii@oit.ac.jp
cNanomaterials Microdevices Research Center, Osaka Institute of Technology, 5-16-1, Omiya, Asahi-ku, Osaka 535-8585, Japan
First published on 27th November 2023
Abstract
Bubbles with polyhedral morphologies were fabricated using polymer plates as a stabilizer. Precise control of the shape and size of the bubbles could be performed by tuning the size ratio of the air bubble and plate, controlling the number of plates adsorbed to the bubble, and coalescence of multiple bubbles.
Solid particles with proper hydrophilic–hydrophobic balance are well known to adsorb to air–water interfaces to stabilize bubbles in aqueous media.1–8 The particle-stabilized bubbles are called “armored bubbles” and show excellent stability (>15 years)9 due to irreversible adsorption of the particles at the interface due to high adsorption energy. Thanks to their high stability, the bubbles have found applications as a platform toward porous materials,10 microreactors11 and carriers for gaseous materials.12 Until now, inorganic particles (e.g. silica13,14 and alumina15), organic particles (e.g. synthetic polymer particles,16–21 semi-synthetic polymer particles22 and fatty acid particles23,24), and natural particles5 (e.g. protein,25 algae,26 bacterial spores and vegetative cells27 and aquatic hyphomycete spores28) with (near)spherical, rod-shaped and atypical morphologies have been widely utilized as a bubble stabilizer. The particle-stabilized bubbles have been produced by methods similar to those utilized to produce bubbles stabilized by molecular surfactants. Production methods include mixing of gas, liquid and solid particles in a mechanical manner (shaking and blender agitation), and injection of pre-/in situ-formed bubbles into a liquid dispersion of particles (foam column and microfluidic).7,29 In many cases, the size of the particulate stabilizer is smaller than those of the bubbles, and near-spherical and spherocylindrical bubbles are generally produced.7,9,30,31 Shape design of the bubbles is a fascinating research topic, which should be developed in order to expand the diversity and opportunities of the bubbles as a functional system.
Recently, we studied aqueous bubbles stabilized with (sub)millimeter-sized polymer plates and confirmed that polyhedral bubbles with various shapes could be produced when the sizes of the plate and air bubble were comparable.32 In our previous study, the bubbles were produced simply by handshaking of air, water and plates in one sample bottle, and they were polydispersed in shape and size, which could not be well controlled. Additionally, it was difficult to optimize the size ratios of the plate and air bubble, and therefore the shapes of the polyhedral bubbles were not well defined. In this communication, we introduce a tailor-made method to fabricate polyhedral bubbles, whose shape and size can be precisely controlled, in a building-up manner utilizing millimeter-sized hexagonal polymer plates monodispersed in shape and size as a stabilizer (Fig. 1).
The size ratio (Db/2S) between the air bubble (diameter, Db) and the plates (diagonal length, 2S) has been proven to play an important role in predicting the shape and size of the resulting bubbles.32 In the case of near-spherical bubbles, Db is larger than 2S. Polyhedral bubbles can be fabricated when the sizes of Db and 2S are comparable. In this study, hexagonal poly(ethylene terephthalate) (PET) plates with 2S of 2 mm and the thickness (T) of 38 μm were utilized to stabilize the bubbles (Fig. S1, ESI†). For the plates to adsorb to the air–water interface, the plates should show hydrophobic surface nature. To this end, the PET plates were surface modified using trichloro(1H,1H,2H,2H-heptadecafluorodecyl)silane, as previously reported (ESI†).33–35 The contact angles of the water droplet on the PET before and after surface modification in air were 73 ± 1° and 111 ± 2°, and those of the air bubble in water were 118 ± 2° and 84 ± 1°, respectively, indicating successful hydrophobization.
The building-up method was applied to control the shape and size of the bubbles precisely. The advantage of this method is that the shape and size of the bubbles can be manually controlled on demand. To begin with, the volumes of bubbles (Vb) required to fabricate bubbles with sandwich, triangular, tetrahedral, pentahedral, and hexahedral shapes using the PET plate were calculated based on eqn (1).
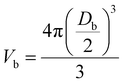 | (1) |
Next, we worked on precise control of the bubble shape by adsorbing 2–6 plates to the air bubbles with the calculated volumes (Fig. S2, ESI†). Here, the plates are rigid enough not to bend. First, bare air bubbles (ca. 1.5–10.0 μL) were adsorbed on the top of the PET board (width: ca. 50 mm, length: ca. 80 mm, thickness: ca. 5 mm, surface modified in the same manner with the PET plates), submerged horizontally in a deionized water pool. Next, the PET plates floating on the planar air–water surface were brought into the bulk water phase one by one using a pipette tip, and the target numbers of plates were adsorbed to the air bubbles on the PET board. Here, the air bridged the plate and the pipette tip, when the plate was brought into the water phase, and the plates could be easily manipulated in the water phase. The PET plates spontaneously adsorbed to the surface of the air bubbles on the PET board, resulting in the formation of polyhedral bubbles. It took approximately 2 min to fabricate the polyhedral bubbles.
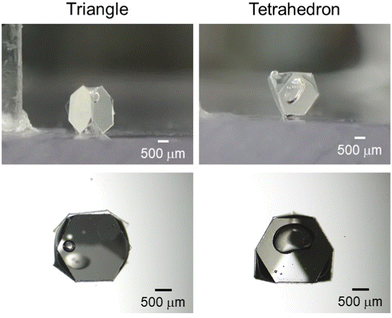
A digital camera and a stereomicroscope were used to observe the bubbles from the side and top directions, respectively (Fig. S3, ESI†). The results showed that the bubbles with sandwich, triangular, tetrahedral, pentahedral and hexahedral shapes were prepared using air bubbles with volumes of ca. 1.5 μL, 2.5 μL, 5.5 μL, 7.0 μL and 10.0 μL, with two, three, four, five and six plates. To our best knowledge, these are the lowest numbers of particles that have ever been used to stabilize air bubbles. Recently, these kinds of polyhedral morphologies have been also reported in particle-stabilized emulsion36,37 and liquid marble33–35 systems. Bare satellite air bubbles with a number-average Heywood diameter of 380 ± 290 μm were observed to adsorb on the surface of the plate in contact with the aqueous phase. These were the air bubbles that were bridging the pipette tip and plate in the water during the preparation, which eventually disappeared (within 24 h) due to Ostwald ripening38 (Fig. S4, ESI†). (The bare satellite bubbles dissolved into water and absorbed to the polyhedral bubbles and the bulk air phase.)
The position of the PET plates at the air–water interface was clarified by scanning electron microscopy observation of the PET plate, which was brought from the bulk water phase to the planar air–water interface, followed by ethyl-2-cyanoacrylate (ECA) vapor treatment (ESI†).39 Anionic polymerization of ECA occurred when the ECA vapor came into contact with water, which acted as a catalyst, resulting in the formation of a poly(ethyl-2-cyanoacrylate) (PECA) film. By ECA treatment, the bare air–water surface, where no plate was adsorbed, was covered with PECA film and the PET plate was trapped at the air–water interface. It was clarified that the plate adsorbed at the air–water interface, contacting one large face and a partial side face with the air (Fig. S5, ESI†). This position was different from that observed in the liquid marble system,34 where the PET plate adsorbed contacting only one large face to the interface. This difference should come from the difference in the adsorption direction: the PET plate adsorbed from the water phase to the air–water interface in the bubble system and from the air phase to the air–water interface in the liquid marble system. The adsorption energy (ΔG) can be calculated using eqn (2) (ESI†):32
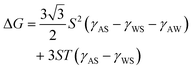 | (2) |
The bubbles were stable and could keep their three dimensional structures at least for 5 days. (Note that the volume of the air bubble gradually decreased due to Ostwald ripening.38 The air bubble dissolved into water and absorbed to the bulk air phase. This decrease of volume could be easily observed in the hexahedral bubble system, see Fig. S3 and S4, ESI.†) Interestingly, dots appeared on the surface of the PET plates adsorbed at the air–water interface <24 h after preparation in all bubble systems (Fig. S3 bottom row and Fig. S4, ESI†). In the case of the sandwich-shaped bubble system, the size and number density of the dots were 127 ± 122 μm and 17 mm−2. To clarify the cause of this phenomenon, the PET film, whose surface was hydrophobically modified by the same method with the PET plates, was floated on a planar air–water interface in a plastic Petri dish, sealed with a lid, and allowed to stand for 24 h to prepare similar conditions as the plates adsorbed on the bubble surface, and the changes occurring on the film surface were observed. As a result, similar dots appeared on the film surface in contact with the air phase (Fig. S6, ESI†). A drop of ECA was placed on the film with dots and allowed to stand for 6 h in a closed system, resulting in the formation of a PECA film with the same shape as the dots (Fig. S6, ESI†). This result confirmed that the dots are water droplets existing in the air phase. These water droplets should be formed by condensation of water vapor on the surface of the plate stabilizing the bubbles.43–46
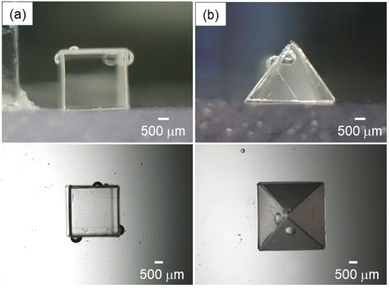
From the above study, it was clear that bubbles with controlled shape can be manually fabricated. However, in the triangular bubble system, the plate and gas were not in complete contact on one side of the plate adsorbing to the bubble, and both air-plate and water-plate interfaces were observed. This structure suggests that a bubble volume of 2.5 μL is insufficient for the creation of well-defined triangular bubbles. Additionally, in the tetrahedral bubble system, there was a gap between the plates, suggesting that a bubble volume of 5.5 μL is too large. Based on these results, it is clear that the bubble volumes determined from the handshaking method were not optimal volumes for precise control of bubble shape. This is because it is difficult to produce bubbles with optimal volume by the handshaking method. Therefore, in order to determine the optimal size ratio and precisely control the bubble shape, the number of plates adsorbed on the bubbles was fixed and the volumes of the bubbles were precisely controlled. As a result, we succeeded in producing bubbles with well-defined triangular and tetrahedral shapes by setting the bubble volumes to 3.5 μL and 4.5 μL, respectively (Fig. 2). It was also possible to fabricate hexahedral and pyramidal shaped bubbles using square and triangle plates (Fig. 3). It was confirmed that the sizes of the bubbles could be controlled by changing the size of the plates: hexahedral and pyramidal shaped bubbles with side lengths between 2 mm and 4 mm (Fig. S7 and S8, ESI†).
The shape and size of the bubbles could also be controlled by coalescence (jointing) of multiple bubbles via application of external mechanical stress (Fig. 4). The jointing of two hexahedral bubbles using a metal wire resulted in the formation of a cuboid-shaped bubble. When jointing, two PET plates existing between two bubbles (jointing point) flipped up to the bubble surface (ESI,† Movie S1). The bubble could keep its cuboid shape, which is thermodynamically unstable, at least for 48 h thanks to the high adsorption energy of the plates at the air–water interface and interfacial jamming effect.34,47,48 It is noteworthy that the cuboid bubbles could be only realized by the tailor-made method developed in this study, but not by the previous hand-shaking method.32 The method developed in this study is not at the stage of mass production. Mass production of shape-controlled polyhedral bubbles might be achieved using Shirasu porous glass.49 Automation of the production of polyhedral bubbles in a precise manner could be possible using microfluidics, an auto-dispenser and an automated manipulator.50
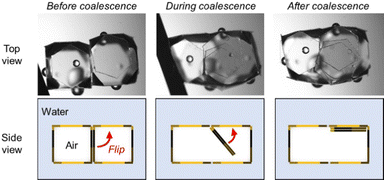 | ||
| Fig. 4 Stereo micrographs and schematic representation of the jointing process of two hexahedral bubbles to form a cuboid bubble. | ||
In conclusion, we demonstrated that shape-designed particle-stabilized polyhedral bubbles can be fabricated by adsorbing plates to the air bubble in a building-up manner. The shape and size of the bubbles could be precisely controlled by tuning the volume of the air bubble and number of plates adsorbed to the bubble. Coalescence of multiple polyhedral bubbles by application of external mechanical stress could also work to design the shape and size of the bubbles. The bubbles studied in this study should contribute to the development of the research fields involving interface chemistry, materials chemistry, physics, chemical engineering and soft matter in an interdisciplinary manner.
Author contributions
Yuri Sakurai: conceptualization, methodology, investigation. Rina Kakiuchi: methodology, investigation. Masaki Hayashi: methodology, investigation. Tomoyasu Hirai: methodology. Yoshinobu Nakamura: methodology. Syuji Fujii: conceptualization, methodology, investigation, writing – original draft, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Panac Co. Ltd for the kind donation of the PET films. This work was supported by JSPS-DAAD Bilateral Joint Research Project (Grant Numbers JPJSBP120203509 and 57521644), a Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant Number JP20H02803 and JP16H04207) and Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (JSPS KAKENHI Grant Number JP15H00767)”.Notes and references
- W. Ramsden and F. Gotch, Proc. R. Soc. London, 1904, 72, 156–164 CrossRef.
- B. S. Murray, Curr. Opin. Colloid Interface Sci., 2007, 12, 232–241 CrossRef CAS.
- T. N. Hunter, R. J. Pugh, G. V. Franks and G. J. Jameson, Adv. Colloid Interface Sci., 2008, 137, 57–81 CrossRef CAS PubMed.
- T. Horozov, Curr. Opin. Colloid Interface Sci., 2008, 13, 134–140 CrossRef CAS.
- S. Lam, K. P. Velikov and O. D. Velev, Curr. Opin. Colloid Interface Sci., 2014, 19, 490–500 CrossRef CAS.
- A. L. Fameau, A. Carl, A. Saint-Jalmes and R. von Klitzing, ChemPhysChem, 2015, 16, 66–75 CrossRef CAS PubMed.
- S. Fujii and Y. Nakamura, Langmuir, 2017, 33, 7365–7379 CrossRef CAS.
- A.-L. Fameau and S. Fujii, Curr. Opin. Colloid Interface Sci., 2020, 50, 101380 CrossRef CAS.
- S. Fujii, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2006, 128, 7882–7886 CrossRef CAS PubMed.
- A. R. Studart, U. T. Gonzenbach, I. Akartuna, E. Tervoort and L. J. Gauckler, J. Mater. Chem., 2007, 17, 3283 RSC.
- D. Dedovets, Q. Li, L. Leclercq, V. Nardello-Rataj, J. Leng, S. Zhao and M. Pera-Titus, Angew. Chem., Int. Ed., 2022, 61, e202107537 CrossRef CAS.
- M. Ito, H. Mayama, Y. Asaumi, Y. Nakamura and S. Fujii, Langmuir, 2020, 36, 7021–7031 CrossRef CAS PubMed.
- E. Dickinson, R. Ettelaie, T. Kostakis and B. S. Murray, Langmuir, 2004, 20, 8517–8525 CrossRef CAS.
- B. P. Binks and T. S. Horozov, Angew. Chem., Int. Ed., 2005, 44, 3722–3725 CrossRef CAS.
- U. T. Gonzenbach, A. R. Studart, E. Tervoort and L. J. Gauckler, Angew. Chem., Int. Ed., 2006, 45, 3526–3530 CrossRef CAS PubMed.
- R. G. Alargova, D. S. Warhadpande, V. N. Paunov and O. D. Velev, Langmuir, 2004, 20, 10371–10374 CrossRef CAS PubMed.
- D. Dupin, J. R. Howse, S. P. Armes and D. P. Randall, J. Mater. Chem., 2008, 18, 545–552 RSC.
- S. Fujii, M. Mochizuki, K. Aono, S. Hamasaki, R. Murakami and Y. Nakamura, Langmuir, 2011, 27, 12902–12909 CrossRef CAS.
- V. Poulichet, A. Huerre and V. Garbin, Soft Matter, 2016, 13, 125–133 RSC.
- S. Nakayama, S. Hamasaki, K. Ueno, M. Mochizuki, S. Yusa, Y. Nakamura and S. Fujii, Soft Matter, 2016, 12, 4794–4804 RSC.
- Y. Nishizawa, T. Watanabe, T. Noguchi, M. Takizawa, C. Song, K. Murata, H. Minato and D. Suzuki, Chem. Commun., 2022, 58, 12927–12930 RSC.
- H. A. Wege, S. Kim, V. N. Paunov, Q. Zhong and O. D. Velev, Langmuir, 2008, 24, 9245–9253 CrossRef CAS.
- A. L. Fameau, A. Saint-Jalmes, F. Cousin, B. Houinsou Houssou, B. Novales, L. Navailles, J. Emile, F. Nallet, C. Gaillard, F. Boue and J. P. Douliez, Angew. Chem., Int. Ed., 2011, 50, 8264–8269 CrossRef CAS PubMed.
- A.-L. Fameau, S. Lam and O. D. Velev, Chem. Sci., 2013, 4, 3874 RSC.
- E. S. Basheva, P. A. Kralchevsky, N. C. Christov, K. D. Danov, S. D. Stoyanov, T. B. Blijdenstein, H. J. Kim, E. G. Pelan and A. Lips, Langmuir, 2011, 27, 2382–2392 CrossRef CAS.
- W. R. Bare, N. B. Jones and E. J. Middlebrooks, J. Water Pollut. Control Fed., 1975, 153–169 CAS.
- W. Boyles and R. Lincoln, Appl. Microbiol., 1958, 6, 327–334 CrossRef CAS PubMed.
- S. H. Iqbal and J. Webster, Trans. Br. Mycol. Soc., 1973, 60, 37-IN32 Search PubMed.
- P. Stevenson, Foam Engineering: Fundamentals and Applications, Wiley, Chichester Hoboken, 2012 Search PubMed.
- A. B. Subramaniam, M. Abkarian, L. Mahadevan and H. A. Stone, Nature, 2005, 438, 930 CrossRef PubMed.
- A. B. Subramaniam, M. Abkarian, L. Mahadevan and H. A. Stone, Langmuir, 2006, 22, 10204–10208 CrossRef CAS.
- Y. Sakurai, R. Kakiuchi, T. Hirai, Y. Nakamura and S. Fujii, Langmuir, 2023, 39, 3800–3809 CrossRef CAS PubMed.
- F. Geyer, Y. Asaumi, D. Vollmer, H. J. Butt, Y. Nakamura and S. Fujii, Adv. Funct. Mater., 2019, 29, 1808826 CrossRef.
- J. Fujiwara, F. Geyer, H. J. Butt, T. Hirai, Y. Nakamura and S. Fujii, Adv. Mater. Interfaces, 2020, 7, 2001573 CrossRef CAS.
- J. Fujiwara, A. Yokoyama, M. Seike, N. Vogel, M. Rey, K. Oyama, T. Hirai, Y. Nakamura and S. Fujii, Mater. Adv., 2021, 2, 4604–4609 RSC.
- R. Koike, Y. Iwashita and Y. Kimura, Langmuir, 2018, 34, 12394–12400 CrossRef CAS PubMed.
- Y. Iwashita, Curr. Opin. Colloid Interface Sci., 2020, 49, 94–106 CrossRef CAS.
- W. Ostwald, Z. Phys. Chem., 1897, 22U, 289–330 CrossRef.
- N. Vogel, J. Ally, K. Bley, M. Kappl, K. Landfester and C. K. Weiss, Nanoscale, 2014, 6, 6879–6885 RSC.
- K. D. Danov and P. A. Kralchevsky, Colloid J., 2012, 74, 172–185 CrossRef CAS.
- R. H. Dettre and R. E. Johnson, Jr., J. Phys. Chem., 1965, 69, 1507–1515 CrossRef CAS.
- H. Yan, K. Kurogi, H. Mayama and K. Tsujii, Angew. Chem., Int. Ed., 2005, 44, 3453–3456 CrossRef CAS.
- E. Schmidt, W. Schurig and W. Sellschopp, Tech. Mech. Thermodyn., 1930, 1, 53–63 Search PubMed.
- J. W. Rose and L. R. Glicksman, Int. J. Heat Mass Transfer, 1973, 16, 411–425 CrossRef CAS.
- H. Tsuchiya, K. Manabe, T. Gaudelet, T. Moriya, K. Suwabe, M. Tenjimbayashi, K.-H. Kyong, F. Gillot and S. Shiratori, New J. Chem., 2017, 41, 982–991 RSC.
- B. El Fil, G. Kini and S. Garimella, Int. J. Heat Mass Transfer, 2020, 160, 120172 CrossRef CAS.
- H. M. Jaeger, Soft Matter, 2015, 11, 12–27 RSC.
- M. Cui, T. Emrick and T. P. Russell, Science, 2013, 342, 460–463 CrossRef CAS PubMed.
- M. Kukizaki, Colloids Surf., A, 2009, 340, 20–32 CrossRef CAS.
- P. Garstecki, I. Gitlin, W. DiLuzio, G. M. Whitesides, E. Kumacheva and H. A. Stone, Appl. Phys. Lett., 2004, 85, 2649–2651 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nj04411d |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2024 |