Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High-yield and chirality-selective isolation of single-walled carbon nanotubes using conjugated polymers and small molecular chaperones†
D.
Just
 *a,
A.
Dzienia
*a,
A.
Dzienia
 a,
K. Z.
Milowska
a,
K. Z.
Milowska
 bc,
A.
Mielańczyk
bc,
A.
Mielańczyk
 a and
D.
Janas
a and
D.
Janas
 *a
*a
aDepartment of Chemistry, Silesian University of Technology, B. Krzywoustego 4, 44-100, Gliwice, Poland. E-mail: dominik.just@polsl.pl; dawid.janas@polsl.pl
bCIC nanoGUNE, Donostia-San Sebastián 20018, Spain
cIkerbasque, Basque Foundation for Science, Bilbao 48013, Spain
First published on 22nd November 2023
Abstract
Single-walled carbon nanotubes (SWCNTs) have potential for a wide range of applications in diverse fields, but the heterogeneous properties of the synthesized mixtures of SWCNT types hinder the realization of these aspirations. Recent developments in extractive purification methods of polychiral SWCNT mixtures have somewhat gradually alleviated this problem, but either the yield or purity of the obtained fractions remains unsatisfactory. In this work, we showed the possibility of simultaneously achieving both the aforementioned goals, commonly considered mutually exclusive, via the enhancement of the capabilities of the conjugated polymer extraction (CPE) technique. We found that combining small molecular species, which alone are unwanted in the system, with a selective poly(9,9′-dioctylfluorenyl-2,7-diyl-alt-6,6′-(2,2′-bipyridine)) polymer increased the concentration of the harvested SWCNTs by an order of magnitude while maintaining near-monochiral purity of the materials. The conducted modeling revealed that the presence of these additives facilitated the folding of conjugated polymers around (6,5) SWCNTs, leading to a substantial increase in the concentration and quality of the SWCNT suspension. The obtained results lay the foundation for the widescale implementation of the CPE of usually scarcely available chirality-defined SWCNTs owing to the molecular chaperones expediting the folding of the conjugated polymers.
New conceptsSingle-walled carbon nanotubes (SWCNTs) have groundbreaking potential for application in diverse fields, but their polydispersity has hampered the realization of this goal for the past three decades. Unfortunately, the purification of SWCNTs, like any other nanomaterial, is either selective or efficient, so only negligible amounts of SWCNTs of defined characteristics can be harvested nowadays. In this work, we report on how to resolve this stalemate using enhanced conjugated polymer extraction in an organic medium. The introduction of relatively low molecular weight compounds into the extraction system increases the concentration of the obtained monochiral SWCNTs by an order of magnitude without negatively affecting the purity of the fractions. A comprehensive analysis of the system, supported by modeling, reveals that the newly added compounds act as polymer chaperones and promote appropriate folding of polymers around SWCNTs, thereby boosting both the selectivity and the yield of differentiation. In light of the widespread use of polymers, the discovery that their conformation can be tuned this way has multidisciplinary importance, laying the foundation for developing novel processing techniques for a broad spectrum of materials. |
1. Introduction
Conjugated polymer extraction (CPE) is a valuable method to obtain monochiral single-walled carbon nanotubes (SWCNTs), which are indispensable for the development of disruptive applications based on them. Extensive studies have revealed several factors that affect the selectivity of CPE of SWCNTs. An appropriate combination of polymer identity and characteristics,1–9 the composition of raw SWCNTs,2,10–12 and the extraction conditions13–21 have exuded several protocols that enabled the enrichment of the obtained fractions with specific chiralities.1,2,6,7,16,19,22–24 Nonetheless, despite a decade of research in this area, only (6,5), (7,3) and (7,5) SWCNTs have been separated with monochiral purity.1,2,22 Consequently, the vast majority of studies employing pure SWCNTs are based on the isolation of the above-mentioned SWCNTs from the SG65i raw material, i.e. (6,5) by poly(9,9′-dioctylfluorenyl-2,7-diyl-alt-6,6′-(2,2′-bipyridine)) – (PFO-BPy6,6′) or (7,5) by poly(9,9′-dioctylfluorenyl-2,7-diyl) – (PFO). Unfortunately, while the selectivity for these chiralities is impressive, the isolation efficiency is typically mediocre, reducing the utility of the elaborated purification protocols. However, because the methods to improve the extraction efficiency via optimization of the system's composition or process parameters have not been sufficiently successful regarding chirality-selective isolation,13–21 it is imperative to explore alternative routes to tackle this challenge.Polythiophenes have piqued the curiosity of the optoelectronic researchers for years due to their favorable light emission properties.25–27 In the context of SWCNTs, it has been reported that highly ordered polythiophene–SWCNT nanohybrids can be successfully employed in solar cells.1,18,28–31 The results showed that hybrids based on regioregular polythiophenes, such as regioregular-poly(3-hexyltiophene) – (rr-P3HT), and SWCNTs create highly crystalline domains by π–π stacking. Energy transfer from rr-P3HT to SWCNTs has been observed, which is extremely effective even in areas where crystalline domains on the tubes agglomerated. Additionally, polythiophene–SWCNT hybrids have been shown to be useful for the development of thin-film transistors.32–36 The application of regioregular poly(3-dodecyltiophene) – (rr-P3DDT) gave rise to the formation of a supramolecular structure, well-connected with SWCNTs, which further enhanced the performance. Furthermore, since thiophene structures have an optimal bandgap for their effective use in nanocarbon-based devices, it is clear that the formation of hybrids of chirality-pure SWCNTs and thiophene-based copolymers can generate promising materials for photonics and electronics.
Unfortunately, because of the exceptional affinity of polythiophenes to SWCNTs, they can extract a wide range of SWCNTs, so the highly concentrated dispersions they produce by CPE are inherently polychiral. The investigations of the process of SWCNT extraction with poly(9,9-dioctylfluorene) (PFO), poly(9,9′-dioctylfluorenyl-2,7-diyl-alt-bithiophene) – (F8T2), poly(9,9′-dioctylfluorenyl-2,7-diyl-alt-benzothiadiazole) – (F8BT), and poly(3-hexylthiophene-2,5-diyl) – (P3HT) revealed that the binding strength of the polymers to the SWCNTs can be arranged in the order of PFO < F8T2–F8BT < P3HT, which correlates inversely with the selectivity of the mentioned polymers.1,18,28–31 The binding of PFO to SWCNTs is weak, mediated mostly through π–π stacking, while polymers containing heteroatoms, such as polythiophenes, are attracted to the SWCNT surface due to the lone electron pairs interacting with the sp2 lattice. Bonding through lone electron pairs occurs irrespective of the SWCNT type on which the polymer molecules adsorb; hence, as the separation yield increases, it inversely influences the selectivity. In another report, double sonication tests were conducted using P3HT and F8BT, which confirmed that the order in which the polymers were added was important.1,18,28–31 The described results confirmed the hypothesis of substantially different binding energies of the polymers to SWCNTs. However, this raises the question of whether it is possible to exploit the high affinity of polythiophenes to SWCNTs and overcome the discussed limitation of CPE for SWCNT extraction without compromising the process selectivity.
Liu et al. reported that polymer blends where one component acts as a SWCNT extractor and the other as an enhancer gave positive outcomes.37 The tests were performed with P3DDT as the enhancer and poly(9-(1-octylnoyl)-9H-carbazole-2,7-diyl) (PCO) as the extractor of semiconducting SWCNTs (s-SWCNTs). It was found that P3DDT had a stronger binding force to s-SWCNTs than PCO, and when introduced into a PCO/SWCNT dispersion, it replaced PCO on the surface. Consequently, the displaced PCO solubilized more s-SWCNTs, which increased the concentration of these species in the suspension. Moreover, Hou et al. reported that the extraction yield of F8BT could be improved by sonicating the obtained supernatant in the presence of poly(9,9-dihexyl-2,7-fluorene-alt-3,6-(9-phenyl)carbazole) – (PDFP). The results showed that the simultaneous use of PDFP and F8BT allows for the extraction of a broad spectrum of s-SWCNTs with notably increased concentrations. The authors noted that PDFP acted as a second layer coating on F8BT-wrapped s-SWCNTs. This enhanced the stability of the SWCNT suspension by creating a thicker and more uniform coating around the SWCNTs. Consequently, the extracted SWCNTs were less prone to precipitation during centrifugation employed after homogenization. Despite these useful strategies to increase the yield of semiconducting SWCNTs, they did not exhibit chiral selectivity, leading only to a suspension of a broad spectrum of semiconducting SWCNTs.
In the presented work, we showed significant enhancement of monochiral SWCNT extraction via simultaneous combination of a chiral-selective conjugated polymer, acting as an extractor, with a non-selective low-molecular weight or short-chain additive based on a thiophene backbone as an extraction enhancer. The combination of PFO-BPy6,6′ as a selective extractor and a palette of non-selective species substantially increased the separation efficiency while maintaining the process's high selectivity. A detailed evaluation of the structure–activity relationship revealed a noticeable increase in the isolation efficiency due to the fact that the additives acted as chaperones for the utilized conjugated polymers, facilitating their deposition on the surface of SWCNTs. Our discovery allows the popular CPE process to be more feasible for large-scale applications, paving the way for its commercial implementation in the extraction of highly desirable near-monochiral SWCNTs.
2. Results and discussion
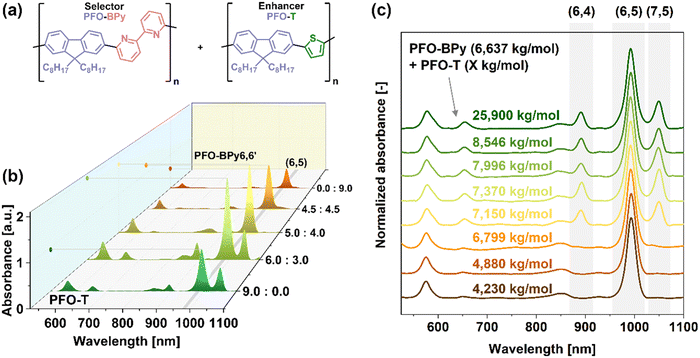
2.1. PFO-T and PFO polymer blends with PFO-BPy6,6′
Dispersions using various alkyl-grafted polythiophene derivatives have previously been reported on materials such as CoMoCAT or HiPco.2,8,38 Unfortunately, these polymers are not chiral selective4,21 in contrast to the aforementioned polyfluorene formulations,22 hence, they cannot be employed for SWCNT enrichment. In addition, we report that an alternating copolymer formed using both units, e.g., poly(9,9′-dioctylfluorenyl-2,7-diyl-alt-2,5-(3-dodecyltiophene)) (PFO-3DDT) is non-selective to a large extent (Fig. S5 and S6, ESI†).37 This outcome suggests that “molecular matching” of PFO is lost upon the incorporation of a thiophene subunit. The inclusion of such moiety can provide the polymer's backbone with additional flexibility (due to the shorter persistence length) and is the source of strong interactions with SWCNTs through lone electron pairs located on the heteroatoms. This can lead to the deposition on virtually every type of SWCNT present in the raw material. When a variety of structural analogs of such polymers were tested, we observed that a structurally similar thiophene-containing copolymer, i.e., poly(9,9′-dioctylfluorenyl-2,7-diyl-alt-2,5-tiophene) – (PFO-T), which is a commonly used PFO-3DDT without an alkyl chain on the thiophene ring, exhibited some degree of selectivity when combined with raw SWCNT materials of either small (![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) ∼ 0.80 nm) or medium diameter (
∼ 0.80 nm) or medium diameter (![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif) ∼ 1.00 nm). The data showed the affinity for (6,5) SWCNTs while processing (6,5)-enriched CoMoCAT and HiPco SWCNTs (Fig. S5 and S6, ESI†). Nonetheless, the extracted species still had a polychiral nature, presumably due to similar features to those of PFO-3DDT. Concomitantly, it should be noted that PFO-BPy6,6′, a polymer commonly employed in CPE to isolate (6,5) SWCNTs, also operates in this range (Fig. S5 and S6, ESI†). Although it enabled near exclusive separation of (6,5) SWCNTs from CoMoCAT SWCNTs containing only several SWCNT types (Fig. S5, ESI†), the fractions obtained from more heterogeneous HiPco SWCNTs usually contained many SWCNT types, in particular those of greater than 25° chiral angles (Fig. S6, ESI†). Therefore, we considered whether the combination of two polymers (PFO-BPy6,6′ and PFO-T) would exhibit synergistic tendencies as their SWCNT affinity scopes overlap (Fig. 1).
∼ 1.00 nm). The data showed the affinity for (6,5) SWCNTs while processing (6,5)-enriched CoMoCAT and HiPco SWCNTs (Fig. S5 and S6, ESI†). Nonetheless, the extracted species still had a polychiral nature, presumably due to similar features to those of PFO-3DDT. Concomitantly, it should be noted that PFO-BPy6,6′, a polymer commonly employed in CPE to isolate (6,5) SWCNTs, also operates in this range (Fig. S5 and S6, ESI†). Although it enabled near exclusive separation of (6,5) SWCNTs from CoMoCAT SWCNTs containing only several SWCNT types (Fig. S5, ESI†), the fractions obtained from more heterogeneous HiPco SWCNTs usually contained many SWCNT types, in particular those of greater than 25° chiral angles (Fig. S6, ESI†). Therefore, we considered whether the combination of two polymers (PFO-BPy6,6′ and PFO-T) would exhibit synergistic tendencies as their SWCNT affinity scopes overlap (Fig. 1).
As expected, using only PFO-T and (6,5)-enriched CoMoCAT yielded a polydisperse mixture of SWCNTs, in which (6,5) chirality was the dominant fraction. In contrast, the utilization of PFO-BPy6,6′, which has known affinity to this SWCNT chirality, produced a near monochiral supernatant with an absorbance (A) of A(6,5)@S11 of 0.576. To our surprise, when PFO-T was used in conjunction with PFO-BPy6,6′, it drastically enhanced its extraction capabilities. Equivalent amounts of PFO-T and PFO-BPy6,6 (4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 mg mg−1) doubled the concentration of (6,5) SWCNTs in the suspension, giving the absorbance of A(6,5)@S11 = 1.24, while maintaining selectivity. Moreover, a slight excess of PFO-T (5.0
4.5 mg mg−1) doubled the concentration of (6,5) SWCNTs in the suspension, giving the absorbance of A(6,5)@S11 = 1.24, while maintaining selectivity. Moreover, a slight excess of PFO-T (5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 mg mg−1) further increased the amount of extracted (6,5) SWCNTs, reaching as high absorbance values as A(6,5)@S11 = 1.79. This tripled the original concentration, without any apparent loss of purity. Unfortunately, a further increase of PFO-T amount (6.0
4.0 mg mg−1) further increased the amount of extracted (6,5) SWCNTs, reaching as high absorbance values as A(6,5)@S11 = 1.79. This tripled the original concentration, without any apparent loss of purity. Unfortunately, a further increase of PFO-T amount (6.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 mg mg−1) disrupted the system's selectivity, resulting in a polychiral mixture resembling the parent material isolated previously by PFO-T (Fig. S5, ESI†).
3.0 mg mg−1) disrupted the system's selectivity, resulting in a polychiral mixture resembling the parent material isolated previously by PFO-T (Fig. S5, ESI†).
To examine the possible reason for the enhanced (6,5) concentration, we synthesized several batches of PFO-T with varying molecular characteristics (Table S2, ESI†). As shown in Fig. 1c, the observed enhancement occurred only for short PFO-T chains, e.g., weight average molecular weights (Mw) of 4230, 4880 kg mol−1, and 6799 kg mol−1 (corresponding to ca. 8–14 dioctylfluorene-thiophene repeatable units). When the Mw of the PFO-T enhancer exceeded that of the PFO-BPy6,6′ selector (Mw = 7150 kg mol−1 (PFO-T) vs. Mw = 6640 kg mol−1 (PFO-BPy6,6′)), the selectivity reduced and statistical distribution of SWCNTs in the supernatant was noted. Based on these observations, it could be inferred that to reach satisfactory selectivity the engaged flexible PFO-T chains should not influence the alignment of selective PFO-BPy6,6′ on the SWCNT surface, which has a more rigid structure.39 Therefore, to enable the adjustment of PFO-T conformation to occupy SWCNT areas not covered by PFO-BPy6,6′, the length of the polymer chains should be relatively short. As previously reported, short conjugated polymers exhibit poor ability to suspend SWCNTs.15 Even though this is considered a disadvantage, making certain batches of conjugated polymers of limited use for CPE of SWCNTs, herein, in our mixed-extractor system, this disadvantage was highly beneficial. Therefore, we capitalized on short PFO-T chains possessing higher binding strength to SWCNTs than PFO-BPy6,6′ that cannot readily suspend a range of SWCNTs. Thus, under these conditions, attachment could only occur to those SWCNTs already wrapped and solubilized by the selective polymer, promoting their suspension in organic media.
To gain greater insight into the process (and verify the above-mentioned hypotheses), we also evaluated combinations of two polymers with well-proven selectivity toward (6,5) and (7,5)-chiralities, i.e., PFO-BPy6,6′ and PFO. It should be noted that compared with PFO-T, PFO is more rigid, which enables near-monochiral isolation of (7,5) species. However, regardless of the polymer ratios of the PFO:PFO-BPy6,6′ blends depicted in Fig. S7 (ESI†), the desired outcome was not obtained. The two polymers interacted antagonistically, weakening each other's selectivity, owing to their strong preference for different SWCNT types. Nonetheless, the experiments provided important information about the mechanics of the conjugated polymer extraction using more than one polymer extractor at a time.
The observed competitive adsorption, previously reported for bile salt surfactants contending for access to the SWCNT surface,40 occurred for our polymer/SWCNT systems suspended in organic media. This behavior was confirmed via two-step sonication. The first step involved the preparation of a PFO-based suspension, rich in (7,5) SWCNTs. The second step was the addition of a certain amount of PFO-BPy6,6′ polymer, followed by agitation. The spectrum of the generated suspension (Fig. S8, ESI†) resembled a fraction obtained using the same amounts of polymer in a single sonication approach (Fig. S7, ESI†), thus validating that a dynamic exchange of conjugated polymers occurred on the SWCNT surface (since the polymers were not long enough to cause irreversible SWCNT wrapping15,37).
Regarding selectivity, since a larger excess of the PFO polymer was required to reveal the presence of (7,5) SWCNTs in the suspended material, it proved that PFO-BPy6,6′ had a higher binding strength to SWCNTs than PFO. It appeared that unpaired electrons in PFO-BPy6,6′ (this time on the nitrogen atoms, not sulfur, as in the case of PFO-T) again provided stronger interactions with SWCNTs than only π-bonds present in the system based on the latter conjugated polymer. Therefore, at this point, it was concluded that the facilitator of the extraction should possess an affinity toward the same SWCNT types as the main extractor polymer, and to enhance the process, its length should not be excessive to prevent interference with wrapping of the said polymer on SWCNTs. Additionally, it should contain heteroatoms to promote high extraction efficiency. To verify whether PFO-T promotes SWCNT purification due to the presence of sulfur, another non-selective polymer (PFO-BPy5,5′) was synthesized and evaluated. Structurally, the polymer was similar to PFO-BPy6,6′ (Fig. 2a); however, the arrangement of the polymer chain was considerably different. As shown in the results, PFO-BPy5,5′ independently extracted a broad spectrum of different SWCNT species (Fig. S9, ESI†), probably due to its rather linear conformation, compared to the helical PFO-BPy6,6′39 one. This greatly facilitated adsorption onto different SWCNT species. Again, when combined with PFO-BPy6,6′, a dramatic increase in SWCNT concentration was noted without affecting the spectral purity. Thus, the presence of a sulfur atom in the polymer was not necessary to enhance the yield of the separation. Analogously for PFO-T addition, we found that (a) the ratio between PFO-BPy6,6′ and PFO-BPy5,5′ was important, and (b) the molecular weight of the second polymer should not be excessive (Fig. S10, ESI†). The highest yield was obtained for 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 mg mg−1 combination using short chain PFO-BPy5,5′ as the extraction promoter. The copolymer with a lower range of molecular weights favored the selective extraction of SWCNTs. In addition, the heterocyclic subunit of the PFO-BPy5,5′ molecule was planarly larger than the heterocycle in PFO-T, which explained why the optimum molecular weight of PFO-BPy5,5′ was smaller than that of PFO-T.
4.5 mg mg−1 combination using short chain PFO-BPy5,5′ as the extraction promoter. The copolymer with a lower range of molecular weights favored the selective extraction of SWCNTs. In addition, the heterocyclic subunit of the PFO-BPy5,5′ molecule was planarly larger than the heterocycle in PFO-T, which explained why the optimum molecular weight of PFO-BPy5,5′ was smaller than that of PFO-T.
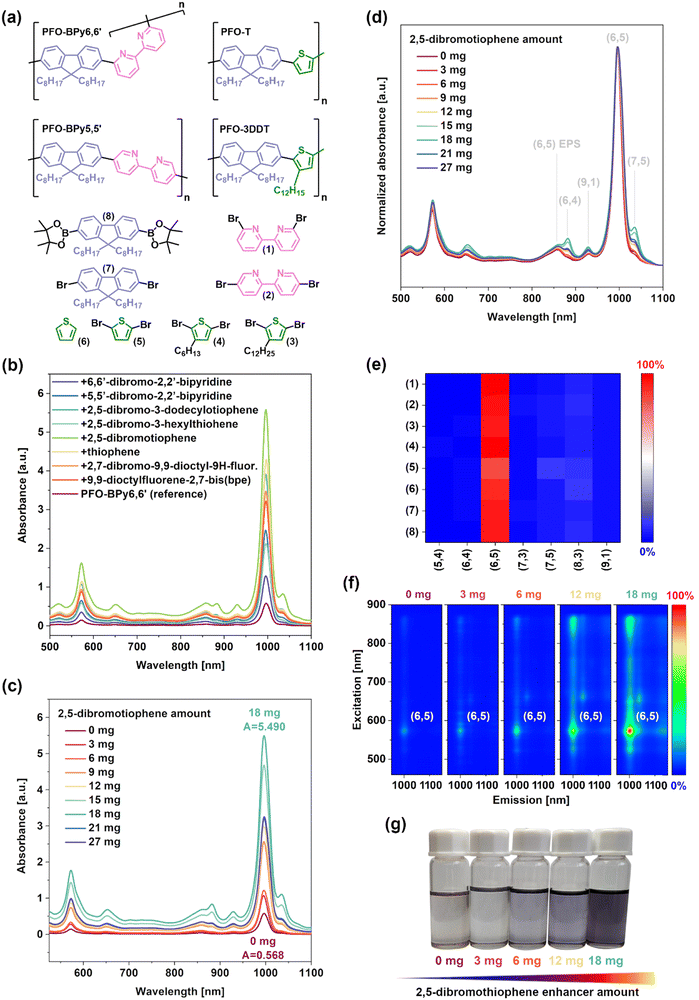
2.2. Small molecular enhancers
Even though the synthetic pathway used in this study was highly simplified, the preparation of the short-chain conjugated polymers via Suzuki polycondensation was a time-consuming and challenging to control process since a stepwise increase of oligomer size was achieved using a sequence of chemical reactions.22 Unfortunately, there is no reasonable alternative to obviate this problem. This motivated us to explore whether other commercially available enhancers with low molecular weight compounds could be used instead of PFO-T or PFO-BPy5,5′ oligomers. Since the building blocks of PFO-BPy6,6′, PFO-BPy5,5′, PFO-T, and PFO-3DDT (Fig. 2a) were responsible for their interactions with the SWCNT surface, we assumed that these deconvoluted molecular fragments may be useful for enhancing the CPE of SWCNTs. Therefore, we evaluated whether monomers used for the synthesis of the studied polymers, or their derivatives, could display an enhanced performance (Fig. 2).The experiments were conducted using 9,9-dioctylfluorene-2,7-diboronic acid bis(pinacol)ester, 2,7-dibromo-9,9-dioctylfluorene, 6,6′-dibromo-2,2′-bipyridine, 5,5′-dibromo-2,2′-bipyridine, thiophene, 2,5-dibromotiophene, 2,5-dibromo-3-hexyltiophene, and 2,5-dibromo-3-dodecylthiophene (Fig. 2a). By themselves, they were unable to suspend SWCNTs. However, upon combination of these polymer precursors with a selective PFO-BPy6,6′ polymer, a dramatic increase in SWCNT concentration was noted (Fig. 2b). While the absorbance of the reference SWCNT suspension rich in (6,5) SWCNTs was only A = 0.569, after the introduction of the indicated compounds, the yield dramatically increased. Each compound, regardless of the fluorene, thiophene or bipyridyl subunit, exhibited a capability to enhance yields while exhibiting little to no influence on extraction selectivity. The best results were obtained for 2,5-dibromothiophene, which had an order of magnitude higher SWCNT concentration (A = 5.567) with a negligible impact on purity (Fig. 2b and Fig. S11, ESI†). Nevertheless, thiophene and 2,5-dibromo-3-dodecylthiophene themselves also achieved noteworthy SWCNT concentrations. Enhancers based on the fluorene subunit showed similar activity regardless of the presence of bromine or boronic ester functional groups, and bipyridines were the weakest.
It was also found that the overall SWCNT concentration (Fig. 2c) and the purity of the suspension (Fig. 2d) were highly dependent on the 2,5-dibromothiophene content. The highest extraction yield was obtained for 18 mg of the enhancer per 1.5 mg of SWCNTs and 6 mg of PFO-BPy6,6′. Thus, the most favorable results were obtained for a weight ratio of the enhancer to polymer of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This value may appear high, but it should be noted that such molecular enhancers must be very easy to remove by washing via simple filtration without the need to engage tedious dialytic processes commonly used for SWCNT purification.23,41,42 Another aspect for examination was the performance per mole of individual enhancers to rule out the key role of this parameter, given the significant differences in the structures within the examined series (Table S3, ESI†). At this point, 2,5-dibromothiophene offered the best performance (Fig. S12, ESI†). In terms of application perspectives, the use of this molecule is very cost-effective to enhance the concentration of highly-valuable near-monochiral (6,5) SWCNTs, with a net price on the order of just $100 per kg. Interestingly, regardless of the enhancer type introduced, the purity of the obtained suspensions was notable as the content of (6,5) SWCNTs approached unity (Fig. 2e). Finally, it should be highlighted that the concentration increase of the desired type of SWCNTs upon addition of enhancers (in this case, 2,5-dibromothiophene) was visible to the naked eye (Fig. 2f).
1. This value may appear high, but it should be noted that such molecular enhancers must be very easy to remove by washing via simple filtration without the need to engage tedious dialytic processes commonly used for SWCNT purification.23,41,42 Another aspect for examination was the performance per mole of individual enhancers to rule out the key role of this parameter, given the significant differences in the structures within the examined series (Table S3, ESI†). At this point, 2,5-dibromothiophene offered the best performance (Fig. S12, ESI†). In terms of application perspectives, the use of this molecule is very cost-effective to enhance the concentration of highly-valuable near-monochiral (6,5) SWCNTs, with a net price on the order of just $100 per kg. Interestingly, regardless of the enhancer type introduced, the purity of the obtained suspensions was notable as the content of (6,5) SWCNTs approached unity (Fig. 2e). Finally, it should be highlighted that the concentration increase of the desired type of SWCNTs upon addition of enhancers (in this case, 2,5-dibromothiophene) was visible to the naked eye (Fig. 2f).
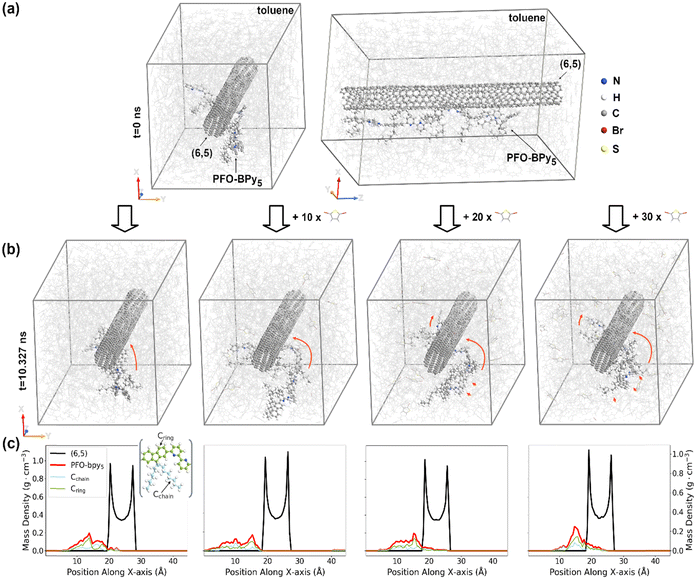
2.3. Elucidation of the mechanism of the enhanced extraction
As mentioned in the introduction, it is possible to modulate the characteristics of SWCNT suspension using chemical compounds both in organic and aqueous solutions.43–45 For instance, reduction–oxidation processes could affect the arrangement of surfactants on the surface of SWCNTs in water, causing their complete reorganization. Regarding organic media, redox dopants were used in the sorting of SWCNTs using CPE, wherein the aromatic structures, such as polyfluorenes, were employed.46 It was noted that the controlled oxidation of SWCNTs could be used to enhance the selectivity of the polymers in the extraction of semiconducting SWCNTs. Since such an effect was not observed for the enrichment of supernatants with SWCNTs of particular chirality, we utilized molecular dynamic and time-stamped force-bias Monte Carlo modeling to understand the impact of 2,5-dibromothiophene on the interactions between (6,5) SWCNT, PFO-BPy6,6′ polymer, and solvent (toluene) molecules.The MD/MC simulation results presented in Fig. 3 clearly indicate that the interactions between SWCNT and PFO-BPy6,6′ polymer could be enhanced via the addition of 2,5-dibromothiophene molecules to the solution. After the same amount of simulated time, the polymer wrapping around SWCNTs advanced the most in the simulation, containing an additional 30 molecules of 2,5-dibromothiophene (enhancer:polymer weight ratio = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), as shown in Fig. 3b.
3), as shown in Fig. 3b.
The addition of 30 molecules of 2,5-dibromothiophene to the polymer-SWCNT system brought closer first the polymer backbone, which started wrapping around SWCNTs, and then the polymer side chains (Fig. S13, ESI†). Consequently, in the presence of 30 2,5-dibromothiophene molecules, both polymer backbone and polymer side chains were positioned much closer to the SWCNTs than in the absence of 2,5-dibromothiophene molecules (cf. first and forth mass density profiles from the left shown in Fig. 3c). For a smaller concentration of 2,5-dibromothiophene (10 and 20 molecules in the simulation box corresponding to the 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and 16
9 and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 enhancer
9 enhancer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polymer weight ratios) changes were observed in the interaction between the nanotube and polymer after 10.327 ns of MD/MC simulations and resembled earlier stages of MD/MC simulation of system with higher concentration of 2,5-dibromothiophene. This suggested that the strength of the nanotube–polymer interaction and, thus, the speed of polymer wrapping around the SWCNTs could be increased by increasing the concentration of 2,5-dibromothiophene in the system. Upon closer inspection of the interactions between all components in the system (Fig. S13, ESI†), we observed that a high concentration of 2,5-dibromothiophene (30 molecules) affected the solvent–nanotube interactions and reduced the probability of finding solvent molecules in the direct vicinity of nanotube lateral surface.
polymer weight ratios) changes were observed in the interaction between the nanotube and polymer after 10.327 ns of MD/MC simulations and resembled earlier stages of MD/MC simulation of system with higher concentration of 2,5-dibromothiophene. This suggested that the strength of the nanotube–polymer interaction and, thus, the speed of polymer wrapping around the SWCNTs could be increased by increasing the concentration of 2,5-dibromothiophene in the system. Upon closer inspection of the interactions between all components in the system (Fig. S13, ESI†), we observed that a high concentration of 2,5-dibromothiophene (30 molecules) affected the solvent–nanotube interactions and reduced the probability of finding solvent molecules in the direct vicinity of nanotube lateral surface.
Additional ab-initio computation was conducted to examine what causes the discovered coordinated organization of PFO-BPy6,6′ on the surface of SWCNTs in the presence of 2,5-dibromothiophene, which was found to be the most efficient molecular extraction enhancer. Spin polarized density functional theory calculations using hybrid exchange–correlation functional (B3LYP) of HOMO and LUMO level of this compound (EHOMO = −5.882 eV, ELUMO = −0.663 eV) revealed that it could promote energy transfer to PFO-BPy6,6′, which, in turn, could transfer it to (6,5) SWCNTs (Fig. 4). It was previously reported that charge transfer could occur between the polymer and SWCNTs wrapped with a polymer dispersant.29–31,42,46 Herein, we showed that in such a novel three-component extraction system, a molecular chaperone exhibits favorable electronic interactions with the polymer. Consequently, it may give rise to beneficial polymer conformation reconfiguration, enabling the polymer to wrap the desired SWCNT species more efficiently.
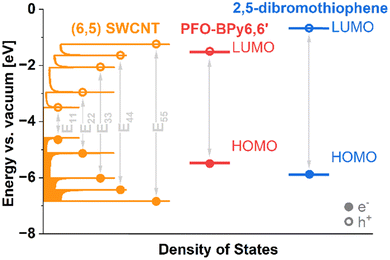 | ||
| Fig. 4 Energy levels of (6,5) SWCNT, 2,5-dibromothiophene and PFO-BPy6,6′, which form two type-I heterojunctions. The density of (6,5) SWCNT states (shown up to E55 transition) was taken from ref. 47 and aligned with the Fermi level calculated in this work (EF = −4.041 eV). HOMO and LUMO levels of 2,5-dibromothiohene and PFO-BPy6,6′ were also calculated in this study (cf. ESI† file). | ||
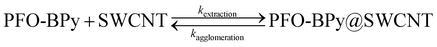
Quickly and properly folded polymer molecules in the presence of these molecular chaperones are much less likely to unwrap from SWCNTs with which they exhibit molecular matching, making the desired PFO-BPy6,6′@(6,5) SWCNT complexes more stable. Creation of these nanohybrids (typically promoted by sonication or shear mixing) is a reversible process, and the obtained results strongly suggested that the addition of these extraction enhancers promotes the kinetics of the forward process (kextraction), counteracting the opposite one (kagglomeration) leading to material sedimentation.
It was previously reported that physical interactions48–50 may stabilize certain polymer conformations, which may explain the observed enhancement of both yield and purity. These findings are in agreement with our modeling results, revealing that the inclusion of 2,5-dibromothiophene affected the rotation of the polymer chains in space. Interestingly, the discovered phenomenon was valid when purifying complex SWCNT mixtures such as HiPco. Despite the presence of multitude of SWCNT chiralities in the raw material, upon addition of the 2,5-dibromothiophene enhancer, the PFO-BPy6,6′ extractor experienced a significant increase in its affinity to (6,5) SWCNTs (Fig. S14, ESI†). While (6,5) chirality was a minority SWCNT type in the starting material, upon the processing, it became the most abundant fraction due to enhanced wrapping of such SWCNTs with PFO-BPy6,6′ caused by the additive.
3. Conclusions
In conclusion, we showed a new type of system for the purification of SWCNT mixtures, which in the unprocessed state has known limited application potential. The implementation of the prepared conjugated polymers produced high-quality (6,5) SWCNT dispersions, but, as in other reports, the yield of the process was unsatisfactory. We discovered that the incorporation of additives in the form of chemical compounds or oligomers into the extraction system improved the concentration of the extracted SWCNT species by an order of magnitude.A systematic empirical approach combined with modeling revealed that the aforementioned enhancers acted as polymer chaperones, refining the process of wrapping the desired SWCNT types with the polymers. This effect was explained by the uncovered charge transfer between the PFO-BPy6,6′ selective polymer and 2,5-dibromothiophene extraction promoter, which, among a spectrum of tested compounds, provided the most considerable improvement of the extraction yield.
Our findings constitute an important milestone toward SWCNT implementation in real-life applications, by making these promising materials much more available. The high cost of purifying SWCNTs is a major impediment hindering their deployment in various application fields, so the proposed technique that greatly increased the concentration of the harvested species is an attractive solution to this pressing problem.
4. Experimental
All the information about the materials and methods is provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the National Science Centre, Poland (under the SONATA program, Grant agreement UMO-2020/39/D/ST5/00285) for supporting the research and the Polish National Agency for Academic Exchange for enabling scientific discussions in a project funded within the Strategic Partnerships program (BPI/PST/2021/1/00039) that helped us interpret a selection of the phenomena described above. Dominik Just would also like to acknowledge the support of the Silesian University of Technology (04/020/BKM23/1079).References
- A. Nish, J. Y. Hwang, J. Doig and R. J. Nicholas, Highly Selective Dispersion of Single-Walled Carbon Nanotubes Using Aromatic Polymers, Nat. Nanotechnol., 2007, 2(10), 640–646, DOI:10.1038/nnano.2007.290.
- H. Ozawa, N. Ide, T. Fujigaya, Y. Niidome and N. Nakashima, One-Pot Separation of Highly Enriched (6,5)-Single-Walled Carbon Nanotubes Using a Fluorene-Based Copolymer, Chem. Lett., 2011, 40(3), 239–241, DOI:10.1246/cl.2011.239.
- H. Ozawa, T. Fujigaya, Y. Niidome, N. Hotta, M. Fujiki and N. Nakashima, Rational Concept to Recognize/Extract Single-Walled Carbon Nanotubes with a Specific Chirality, J. Am. Chem. Soc., 2011, 133(8), 2651–2657, DOI:10.1021/ja109399f.
- H. W. Lee, Y. Yoon, S. Park, J. H. Oh, S. Hong, L. S. Liyanage, H. Wang, S. Morishita, N. Patil, Y. J. Park, J. J. Park, A. Spakowitz, G. Galli, F. Gygi, P. H. S. Wong, J. B. H. Tok, J. M. Kim and Z. Bao, Selective Dispersion of High Purity Semiconducting Single-Walled Carbon Nanotubes with Regioregular Poly(3-Alkylthiophene)s, Nat. Commun., 2011, 2, 541, DOI:10.1038/ncomms1545.
- J. Gao, M. A. Loi, E. J. F. De Carvalho and M. C. Dos Santos, Selective Wrapping and Supramolecular Structures of Polyfluorene-Carbon Nanotube Hybrids, ACS Nano, 2011, 5(5), 3993–3999, DOI:10.1021/nn200564n.
- M. Tange, T. Okazaki and S. Iijima, Influence of Structure-Selective Fluorene-Based Polymer Wrapping on Optical Transitions of Single-Wall Carbon Nanotubes, Nanoscale, 2014, 6(1), 248–254, 10.1039/c3nr03812b.
- M. Tange, T. Okazaki and S. Iijima, Selective Extraction of Large-Diameter Single-Wall Carbon Nanotubes with Specific Chiral Indices by Poly(9,9-Dioctylfluorene-Alt-Benzothiadiazole, J. Am. Chem. Soc., 2011, 133(31), 11908–11911, DOI:10.1021/ja204698d.
- M. Tange, T. Okazaki and S. Iijima, Selective Extraction of Semiconducting Single-Wall Carbon Nanotubes by Poly(9,9-Dioctylfluorene-Alt-Pyridine) for 1.5 Mm Emission, ACS Appl. Mater. Interfaces, 2012, 4(12), 6458–6462, DOI:10.1021/am302327j.
- H. Ozawa, N. Ide, T. Fujigaya, Y. Niidome and N. Nakashima, Supramolecular Hybrid of Metal Nanoparticles and Semiconducting Single-Walled Carbon Nanotubes Wrapped by a Fluorene-Carbazole Copolymer, Chem. – Eur. J., 2011, 17(48), 13438–13444, DOI:10.1002/chem.201101669.
- K. S. Mistry, B. A. Larsen and J. L. Blackburn, High-Yield Dispersions of Large-Diameter Semiconducting Single-Walled Carbon Nanotubes with Tunable Narrow Chirality Distributions, ACS Nano, 2013, 7(3), 2231–2239, DOI:10.1021/nn305336x.
- G. J. Brady, Y. Joo, S. Singha Roy, P. Gopalan and M. S. Arnold, High Performance Transistors via Aligned Polyfluorene-Sorted Carbon Nanotubes, Appl. Phys. Lett., 2014, 104, 083107, DOI:10.1063/1.4866577.
- J. Wang and T. Lei, Separation of Semiconducting Carbon Nanotubes Using Conjugated Polymer Wrapping, Polymers, 2020, 1, 1–29, DOI:10.3390/polym12071548.
- N. A. Rice, A. V. Subrahmanyam, S. E. Laengert and A. Adronov, The Effect of Molecular Weight on the Separation of Semiconducting Single-Walled Carbon Nanotubes Using Poly(2,7-Carbazole)s, J. Polym. Sci. A Polym. Chem., 2015, 53(21), 2510–2516, DOI:10.1002/pola.27715.
- P. Imin, F. Cheng and A. Adronov, The Effect of Molecular Weight on the Supramolecular Interaction between a Conjugated Polymer and Single-Walled Carbon Nanotubes, Polym. Chem., 2011, 2(6), 1404–1408, 10.1039/c1py00023c.
- N. Berton, F. Lemasson, F. Hennrich, M. M. Kappes and M. Mayor, Influence of Molecular Weight on Selective Oligomer-Assisted Dispersion of Single-Walled Carbon Nanotubes and Subsequent Polymer Exchange, Chem. Commun., 2012, 48(19), 2516–2518, 10.1039/c2cc17508h.
- F. Jakubka, S. P. Schießl, S. Martin, J. M. Englert, F. Hauke, A. Hirsch and J. Zaumseil, Effect of Polymer Molecular Weight and Solution Parameters on Selective Dispersion of Single-Walled Carbon Nanotubes, ACS Macro Lett., 2012, 1(7), 815–819, DOI:10.1021/mz300147g.
- H. Zhu, L. Hong, H. Tanaka, X. Ma and C. Yang, Facile Solvent Mixing Strategy for Extracting Highly Enriched (6,5) Single-Walled Carbon Nanotubes in Improved Yield, Bull. Chem. Soc. Jpn., 2021, 94(4), 1166–1171, DOI:10.1246/bcsj.20200370.
- J. Y. Hwang, A. Nish, J. Doig, S. Douven, C. W. Chen, L. C. Chen and R. J. Nicholas, Polymer Structure and Solvent Effects on the Selective Dispersion of Single-Walled Carbon Nanotubes, J. Am. Chem. Soc., 2008, 130(11), 3543–3553, DOI:10.1021/ja0777640.
- R. Si, L. Wei, H. Wang, D. Su, S. H. Mushrif and Y. Chen, Extraction of (9,8) Single-Walled Carbon Nanotubes by Fluorene-Based Polymers, Chem. – Asian J., 2014, 9(3), 868–877, DOI:10.1002/asia.201301350.
- N. Stürzl, F. Hennrich, S. Lebedkin and M. M. Kappes, Near Monochiral Single-Walled Carbon Nanotube Dispersions in Organic Solvents, J. Phys. Chem. C, 2009, 113(33), 14628–14632, DOI:10.1021/jp902788y.
- A. Dzienia, D. Just and D. Janas, Solvatochromism in SWCNTs Suspended by Conjugated Polymers in Organic Solvents, Nanoscale, 2023, 15(21), 9510–9524, 10.1039/d3nr00392b.
- A. Dzienia, D. Just, P. Taborowska, A. Mielanczyk, K. Z. Milowska, S. Yorozuya, S. Naka, T. Shiraki and D. Janas, Mixed-Solvent Engineering as a Way around the Trade-Off between Yield and Purity of (7,3) Single-Walled Carbon Nanotubes Obtained Using Conjugated Polymer Extraction, Small, 2023, 19, 2304211, DOI:10.1002/smll.202304211.
- J. Ouyang, H. Shin, P. Finnie, J. Ding, C. Guo, Z. Li, Y. Chen, L. Wei, A. J. Wu, S. Moisa, F. Lapointe and P. R. L. Malenfant, Impact of Conjugated Polymer Characteristics on the Enrichment of Single-Chirality Single-Walled Carbon Nanotubes, ACS Appl. Polym. Mater., 2022, 4(8), 6239–6254, DOI:10.1021/acsapm.2c01022.
- Y. Li, M. Zheng, J. Yao, W. Gong, Y. Li, J. Tang, S. Feng, R. Han, Q. Sui, S. Qiu, L. Kang, H. Jin, D. Sun and Q. Li, High-Purity Monochiral Carbon Nanotubes with a 1.2 Nm Diameter for High-Performance Field-Effect Transistors, Adv. Funct. Mater., 2022, 32(1), 2107119, DOI:10.1002/adfm.202107119.
- N. N. Linh, T. T. T. Duong, N. Hien and V. Q. Trung, Synthesis of Polythiophene Containing Heterocycle on the Side Chain: A Review, Vietnam J. Chem., 2020, 1, 1–9, DOI:10.1002/vjch.201900184.
- K. Pal, A. Si, R. Stephen and S. Thomas, Conductive Polymer Nanocomposites for Organic Light-Emitting Diodes (OLEDs), Handbook of Polymer and Ceramic Nanotechnology, 2021 Search PubMed.
- I. F. Perepichka, D. F. Perepichka, H. Meng and F. Wudl, Light-Emitting Polythiophenes, Adv. Mater., 2005, 17, 2281–2305, DOI:10.1002/adma.200500461.
- S. D. Stranks, A. M. R. Baker, J. A. Alexander-Webber, B. Dirks and R. J. Nicholas, Production of High-Purity Single-Chirality Carbon Nanotube Hybrids by Selective Polymer Exchange, Small, 2013, 9(13), 2245–2249, DOI:10.1002/smll.201202434.
- T. Schuettfort, H. J. Snaith, A. Nish and R. J. Nicholas, Synthesis and Spectroscopic Characterization of Solution Processable Highly Ordered Polythiophene-Carbon Nanotube Nanohybrid Structures, Nanotechnology, 2010, 21(2), 025201, DOI:10.1088/0957-4484/21/2/025201.
- S. N. Habisreutinger, T. Leijtens, G. E. Eperon, S. D. Stranks, R. J. Nicholas and H. J. Snaith, Enhanced Hole Extraction in Perovskite Solar Cells through Carbon Nanotubes, J. Phys. Chem. Lett., 2014, 5(23), 4207–4212, DOI:10.1021/jz5021795.
- S. D. Stranks, S. N. Habisreutinger, B. Dirks and R. J. Nicholas, Novel Carbon Nanotube-Conjugated Polymer Nanohybrids Produced By Multiple Polymer Processing, Adv. Mater., 2013, 25, 4365–4371, DOI:10.1002/adma.201205250.
- H. Wang, B. Hsieh, G. Jiménez-Osés, P. Liu, C. J. Tassone, Y. Diao, T. Lei, K. N. Houk and Z. Bao, Solvent Effects on Polymer Sorting of Carbon Nanotubes with Applications in Printed Electronics, Small, 2015, 11(1), 126–133, DOI:10.1002/smll.201401890.
- H. Wang, G. I. Koleilat, P. Liu, G. Jiménez-Osés, Y. C. Lai, M. Vosgueritchian, Y. Fang, S. Park, K. N. Houk and Z. Bao, High-Yield Sorting of Small-Diameter Carbon Nanotubes for Solar Cells and Transistors, ACS Nano, 2014, 8(3), 2609–2617, DOI:10.1021/nn406256y.
- H. Wang, Y. Li, G. Jiménez-Osés, P. Liu, Y. Fang, J. Zhang, Y. C. Lai, S. Park, L. Chen, K. N. Houk and Z. Bao, N-Type Conjugated Polymer-Enabled Selective Dispersion of Semiconducting Carbon Nanotubes for Flexible CMOS-like Circuits, Adv. Funct. Mater., 2015, 25(12), 1837–1844, DOI:10.1002/adfm.201404126.
- A. Chortos, G. I. Koleilat, R. Pfattner, D. Kong, P. Lin, R. Nur, T. Lei, H. Wang, N. Liu, Y. C. Lai, M. G. Kim, J. W. Chung, S. Lee and Z. Bao, Mechanically Durable and Highly Stretchable Transistors Employing Carbon Nanotube Semiconductor and Electrodes, Adv. Mater., 2016, 28(22), 4441–4448, DOI:10.1002/adma.201501828.
- H. Wang, P. Wei, Y. Li, J. Han, H. R. Lee, B. D. Naab, N. Liu, C. Wang, E. Adijanto, B. C. K. Tee, S. Morishita, Q. Li, Y. Gao, Y. Cui and Z. Bao, Tuning the Threshold Voltage of Carbon Nanotube Transistors by N-Type Molecular Doping for Robust and Flexible Complementary Circuits, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(13), 4776–4781, DOI:10.1073/pnas.1320045111.
- D. Liu, P. Li, X. Yu, J. Gu, J. Han, S. Zhang, H. Li, H. Jin, S. Qiu, Q. Li and J. Zhang, A Mixed-Extractor Strategy for Efficient Sorting of Semiconducting Single-Walled Carbon Nanotubes, Adv. Mater., 2017, 29(8), 1603565, DOI:10.1002/adma.201603565.
- F. Chen, B. Wang, Y. Chen and L. J. Li, Toward the Extraction of Single Species of Single-Walled Carbon Nanotubes Using Fluorene-Based Polymers, Nano Lett., 2007, 7(10), 3013–3017, DOI:10.1021/nl071349o.
- Z. Li, W. Chen, J. Liu and D. Jiang, Can Linear Conjugated Polymers Form Stable Helical Structures on the Carbon Nanotubes, ACS Appl. Mater. Interfaces, 2022, 14(43), 49189–49198, DOI:10.1021/acsami.2c14771.
- J. A. Fagan, Aqueous Two-Polymer Phase Extraction of Single-Wall Carbon Nanotubes Using Surfactants, Nanoscale Adv., 2019, 3307–3324, 10.1039/c9na00280d.
- B. Mirka, N. A. Rice, P. Williams, M. N. Tousignant, N. T. Boileau, W. J. Bodnaryk, D. Fong, A. Adronov and B. H. Lessard, Excess Polymer in Single-Walled Carbon Nanotube Thin-Film Transistors: Its Removal Prior to Fabrication Is Unnecessary, ACS Nano, 2021, 15(5), 8252–8266, DOI:10.1021/acsnano.0c08584.
- S. Schneider, J. Lefebvre, N. J. Diercks, F. J. Berger, F. Lapointe, J. Schleicher, P. R. Malenfant and J. Zaumseil, Phenanthroline Additives for Enhanced Semiconducting Carbon Nanotube Dispersion Stability and Transistor Performance, ACS Appl. Nano Mater., 2020, 3(12), 12314–12324, DOI:10.1021/acsanm.0c02813.
- A. Hirano, T. Tanaka, Y. Urabe and H. Kataura, PH- and Solute-Dependent Adsorption of Single-Wall Carbon Nanotubes onto Hydrogels: Mechanistic Insights into the Metal/Semiconductor Separation, ACS Nano, 2013, 7(11), 10285–10295, DOI:10.1021/nn4046776.
- S.-Y. Ju, M. Utz and F. Papadimitrakopoulos, Enrichment Mechanism of Semiconducting Single-Walled Carbon Nanotubes by Surfactant Amines, J. Am. Chem. Soc., 2009, 131(19), 6775–6784, DOI:10.1021/ja809054c.
- J. Wang, T. Dat Nguyen, Q. Cao, Y. Wang, M. Y. C. Tan and M. B. Chan-Park, Selective Surface Charge Sign Reversal on Metallic Carbon Nanotubes for Facile Ultrahigh Purity Nanotube Sorting, ACS Nano, 2016, 10(3), 3222–3232, DOI:10.1021/acsnano.5b05795.
- Z. Li, J. Ding, J. Lefebvre and P. R. L. Malenfant, Dopant-Modulated Conjugated Polymer Enrichment of Semiconducting SWCNTs, ACS Omega, 2018, 3(3), 3413–3419, DOI:10.1021/acsomega.8b00383.
- http://www.photon.t.u-tokyo.ac.jp/~maruyama/kataura/1D_DOS.html, (Accessed 2023-09-27).
- S. Shao and L. Wang, Through-space Charge Transfer Polymers for Solution-processed Organic Light-emitting Diodes, Aggregate, 2020, 1(1), 45–56 CrossRef.
- P. Sozzani, A. Comotti, S. Bracco and R. Simonutti, Cooperation of Multiple CH⋯π Interactions to Stabilize Polymers in Aromatic Nanochannels as Indicated by 2D Solid State NMR, Chem. Commun., 2004, 768–769, 10.1039/b316855g.
- H. Huang, L. Yang, A. Facchetti and T. J. Marks, Organic and Polymeric Semiconductors Enhanced by Noncovalent Conformational Locks, Chem. Rev., 2017, 117(15), 10291–10318, DOI:10.1021/acs.chemrev.7b00084.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01687k |
| This journal is © The Royal Society of Chemistry 2024 |