Open Access Article
Open Access ArticleAnti-Schistosomal activity and ADMET properties of 1,2,5-oxadiazinane-containing compound synthesized by visible-light photoredox catalysis†
Kennosuke
Itoh
 *ab,
Hiroki
Nakahara
*ab,
Hiroki
Nakahara
 a,
Atsushi
Takashino
a,
Aya
Hara
a,
Akiho
Katsuno
a,
Yuriko
Abe
a,
Takaaki
Mizuguchi
a,
Atsushi
Takashino
a,
Aya
Hara
a,
Akiho
Katsuno
a,
Yuriko
Abe
a,
Takaaki
Mizuguchi
 ab,
Fumika
Karaki
ab,
Fumika
Karaki
 ab,
Shigeto
Hirayama
ab,
Kenichiro
Nagai
ab,
Shigeto
Hirayama
ab,
Kenichiro
Nagai
 b,
Reiko
Seki
b,
Noriko
Sato
b,
Kazuki
Okuyama
c,
Masashi
Hashimoto
c,
Ken
Tokunaga
b,
Reiko
Seki
b,
Noriko
Sato
b,
Kazuki
Okuyama
c,
Masashi
Hashimoto
c,
Ken
Tokunaga
 d,
Hitoshi
Ishida
d,
Hitoshi
Ishida
 e,
Fusako
Mikami
f,
Kofi Dadzie
Kwofie
f,
Hayato
Kawada
f,
Bangzhong
Lin
g,
Kazuto
Nunomura
g,
Toshio
Kanai
g,
Takeshi
Hatta
f,
Naotoshi
Tsuji
f,
Junichi
Haruta
g and
Hideaki
Fujii
e,
Fusako
Mikami
f,
Kofi Dadzie
Kwofie
f,
Hayato
Kawada
f,
Bangzhong
Lin
g,
Kazuto
Nunomura
g,
Toshio
Kanai
g,
Takeshi
Hatta
f,
Naotoshi
Tsuji
f,
Junichi
Haruta
g and
Hideaki
Fujii
 ab
ab
aLaboratory of Medicinal Chemistry, School of Pharmacy, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo 108-8641, Japan. E-mail: itok@pharm.kitasato-u.ac.jp
bMedicinal Research Laboratories, School of Pharmacy, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo 108-8641, Japan
cDepartment of Material Science, Graduate School of Science, Josai University, 1-1 Keyakidai, Sakado, Saitama 350-0295, Japan
dDivision of Liberal Arts, Center for Promotion of Higher Education, Kogakuin University, 2665-1 Nakano-machi, Hachioji, Tokyo 192-0015, Japan
eGraduate School of Science and Engineering, Department of Chemistry, Materials and Bioengineering, Kansai University, 3-3-35 Yamate-cho, Suita, Osaka 564-8680, Japan
fDepartment of Parasitology and Tropical Medicine, Kitasato University School of Medicine, 1-15-1 Kitazato, Minami-ku, Sagamihara, Kanagawa 252-0374, Japan
gDrug Innovation Center Lead Exploration Unit, Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadagaoka, Suita, Osaka 565-0871, Japan
First published on 26th September 2024
Abstract
The incorporation of saturated nitrogen-containing heterocycle 1,2,5-oxadiazinane into small molecules represents a compelling avenue in drug discovery due to its unexplored behavior within biological systems and incomplete protocols for synthesis. In this study, we present 1,2,5-oxadiazinane, an innovative heterocyclic bioisostere of piperizin-2-one and novel chemotype of the anti-schistosomal drug praziquantel (PZQ), which has been the only clinical drug available for three decades. PZQ is associated with significant drawbacks, including poor solubility, a bitter taste, and low metabolic stability. Therefore, the discovery of a new class of anti-schistosomal agents is imperative. To address this challenge, we introduce a pioneering method for the synthesis of 1,2,5-oxadiazinane derivatives through the cycloaddition of nitrones with N,N,N′,N′-tetraalkyldiaminomethane in the presence of an IrIII complex photosensitizer. This transformative reaction offers a streamlined route to various kinds of 1,2,5-oxadiazinanes that is characterized by mild reaction conditions and broad substrate scope. Mechanistic investigations suggest that the photoredox pathway underlies the [3 + 3] photocycloaddition process. Thus, based on bioisosteric replacement, we identified a remarkable molecule as a new chemotype of a potent anti-schistosomal compound that not only exhibits superior solubility, but also retains the potent biological activity inherent to PZQ.
Introduction
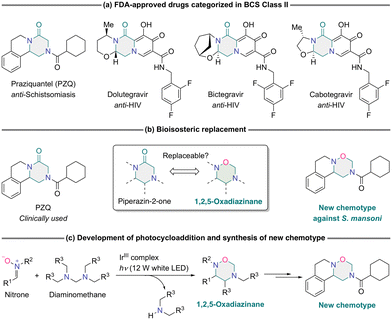
As vital scaffolds for designing and developing novel drugs, saturated nitrogen-containing heterocycles have immense potential for the field of drug development.1 Their sp3 hybridized atoms, providing three-dimensional shapes in drug candidates and facilitating multiple interactions with biological systems, are key to the effectiveness of these molecules.2,3 Moreover, they can significantly improve the pharmacokinetic/pharmacodynamic (PK/PD) properties of drug candidates, thereby increasing their clinical success rates.4 Given these promising attributes, the proposition of novel synthetic methodologies for saturated nitrogen-containing heterocycles is not just fascinating, but a compelling avenue of investigation.5–12Our research has been focused on the prospect of integrating a saturated nitrogen-containing heterocycle, 1,2,5-oxadiazinane, instead of the incumbent moiety piperazin-2-one, which is present in FDA-approved drugs. Notable examples include the anti-schistosomal agent praziquantel (PZQ),13–15 along with the antiretroviral drugs dolutegravir, bictegravir, and cabotegravir,16 which are renowned for their efficacy against human immunodeficiency virus (HIV) (Scheme 1a). This approach, which can be advocated as a bioisosteric replacement strategy, revolutionizes drug design by ingeniously replacing an atom or functional group with another. This method not only improves the physicochemical and ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties of molecules, but maintains their biological efficacy.17,18 The pharmaceutical agents referenced above belong to biopharmaceutics classification system (BCS) class II, which is characterized by high permeability and low solubility.19 Consequently, their gastrointestinal dissociation rates are sluggish, leading to limited bioavailability. In such instances, bioisosteric replacement has proven effective in enhancing the intrinsic properties of these drugs.
Of particular interest is PZQ, which has been exclusively employed as a therapeutic agent for schistosomiasis—one of the neglected tropical diseases—for over three decades, with no other agent surpassing it to this day.20 However, despite its long-standing clinical use, PZQ is plagued by unresolved issues, including low solubility,21,22 poor metabolic stability,23 and a bitter taste.24 In 2012, the Global Health Innovative Technology Fund (GHIT), along with Merck & Co., Inc., the Bill and Melinda Gates Foundation, and the European & Developing Countries Clinical Trials Partnership (EDCTP), established the Pediatric Praziquantel Consortium to address this challenge.25 Through their dedicated efforts, they successfully developed arPraziquantel (L-praziquantel), a chiral switch for PZQ, as well as orodispersible tablets. In 2023, they received a positive scientific opinion on arPraziquantel from the European Medicines Agency (EMA).26,27 Very recently, in 2024, Sprague, Marchant, and colleagues merely discovered a new chemotype of anti-schistosomal agent.28 Regarding the low solubility of PZQ in aqueous media, the carbonyl group of the piperazin-2-one ring does not appear to increase the solubility of PZQ, as the molecule contains rings that lack polarity or are weakly polarized. However, crystallographic analyses indicate that the carbonyl oxygen of piperazin-2-one plays a pivotal role in mediating intermolecular hydrogen bonding, thereby contributing to the overall aggregation and reduced solubility.29 Although replacing the piperazine ring with the piperazin-2-one ring of PZQ presents a viable strategy for disrupting these hydrogen-bonding interactions, it unfortunately results in a complete loss of biological activity in the resulting piperazine derivative.20b,30 This underscores the critical importance of the carbonyl oxygen within the piperazin-2-one ring of PZQ for exerting its anti-schistosomal effects. Consequently, the prospect of bioisosteric replacement within PZQ by substituting the piperazin-2-one ring with a 1,2,5-oxadiazinane ring presents a significant challenge. However, it remains feasible due to the non-classical isosteric relationship between divalent ether oxygen and the carbonyl group.31 The embedded oxygen atom can compensate for the role of the carbonyl oxygen, maintaining essential interactions with the Schistosoma target molecule while ameliorating the solubility provided by the weakened intermolecular hydrogen bonding attributed to the carbonyl oxygen of the piperazin-2-one ring in PZQ.
In this study, we present a pioneering approach to bioisosteric replacement wherein the piperazin-2-one ring is replaced with the 1,2,5-oxadiazinane ring to yield a new chemotype of potent anti-schistosomal molecule, an unprecedented achievement (Scheme 1b). To build a 1,2,5-oxadiazinane ring, we developed efficient cycloaddition reactions between a diverse array of nitrones and N,N,N′,N′-tetraalkyldiaminomethanes in the presence of iridium(III) complex photosensitizer (IrIII complex) (Scheme 1c). This methodology facilitated the synthesis of a wide range of 1,2,5-oxadiazinane derivatives. In contrast, thermal reactions32 and an ultraviolet-induced process33 are challenging. Furthermore, we elucidated the plausible mechanism underlying the photocycloaddition reaction and demonstrated the scalability of our approach by performing gram-scale synthesis of a 1,2,5-oxadiazinane derivative utilizing a custom-designed photochemical flow reactor. The present integrated study not only expands the chemical diversity accessible through bioisosteric replacement, but offers practical insights into the synthesis and mechanistic understanding of 1,2,5-oxadiazinane derivatives.
Results and discussion
Reaction optimization
Initially, we screened IrIII complexes I–XI to evaluate their abilities to promote the cycloaddition reactions of nitrone 1a (50 mM) with N,N,N′,N′-tetramethyldiaminomethane (2a) (500 mM) under high-intensity light irradiation (12 W white LEDs), leading to the formation of 1,2,5-oxadiazinane 3aa (Table 1). Notably, in the absence of an IrIII complex, no reaction was observed, and 1a was recovered (entry 1). IrIII complexes I and II yielded only trace amounts of 3aa (entries 2 and 3), whereas IrIII complexes III, IV, V, and VI exhibited limited promotion of the cycloaddition reactions, resulting in low yields of 3aa (entries 4–7) and the formation of dimethylamine as a byproduct. IrIII complex VII facilitated the desired reaction to afford a moderate yield of 3aa (entry 8), whereas IrIII complex VIII emerged as the optimal mediator, resulting in a good yield of 3aa (entry 9). Reducing the amount of VIII slowed the reaction, which was complete in 94 hours, yielding 3aa in a comparable yield to entry 9 (entry 10). Decreasing the concentration of 2a (100 mM) led to a decrease in the yield of 3aa (entry 11). Compared with VIII, which features a CF3 group at position 5, the newly prepared IrIII complex IX, with a CF3 group at position 4 of the pyridine ring in the ppy ligand, decelerates the desired reaction, resulting in a lower yield of 3aa (entry 12). Conversely, ruthenium(II) (RuII) complexes X and XI were not suitable as photochemical mediators (entries 13 and 14). Considering the redox potential of the excited states [E* (IrIII*/IrII) and E* (RuII*/RuI)] and ground states [E1/2 (IrIII/IrII) and E1/2 (RuII/RuI)] of the IrIII complexes and RuII complexes,34,35 the ideal complex should function as a potent oxidizing reagent for 2a and a powerful one-electron-reducing reagent for the reaction intermediate. Ultimately, IrIII complex VIII emerged as the optimal photosensitizer, distinguished by its remarkable oxidizing ability [E* (IrIII*/IrII) = +1.41 V] and one-electron-reducing ability [E1/2 (IrIII/IrII) = −1.26 V] (Fig. S3, Tables S2 and S3†). The presence of fluorine groups in the ppy ligands of IrIII complexes VI–IX likely reduces the phosphorescence radiative rate constant and spin-orbit coupling strength of the photoexcited species.36 The above-mentioned photophysical phenomena would lead to an increased triplet lifetime of the IrIII complexes, ultimately improve the reaction efficiency. Additionally, the screening results suggest that IrIII complexes play a crucial role in facilitating a reductive quenching process that promotes photocycloaddition.37 Furthermore, 1a was recovered in the absence of 2a, indicating the absence of photodecomposition and the stability of 1a in the presence of the photoexcited IrIII complex. This accentuates the indispensable role of 2a in inducing the desired reaction. Employing CH2Cl2 as the solvent yielded the best results compared to other solvents (MeCN: 8 h, 65% yield; MeOH: 25 h, 29%).| Entry | [M]b | Yieldc (%) | E*d (Mn*/Mn−1) | E 1/2 (Mn/Mn−1) |
|---|---|---|---|---|
| a Experiments were conducted using nitrone 1a and N,N,N′,N′-tetramethyldiaminomethane (2a) in deaerated CH2Cl2 employing an IrIII complex and irradiated with a 1-m LED strip (λ = 400–780 nm, 12 W, 60 white LEDs per m). b M = Ir or Ru. c Isolated yield. d Electrical potentials are provided in ref. 34, 35, and the ESI,† with units in volts. e 0.5 mM of VIII was used, and the reaction time was 94 h. f 100 mM of 2a was used. g The reaction time was 24 h. | ||||
| 1c | None | 0 | None | None |
| 2 | I | Trace | +1.22 | −0.88 |
| 3 | II | Trace | +1.65 | −0.79 |
| 4 | III | 21 | +0.81 | −1.31 |
| 5 | IV | 19 | +0.66 | −1.51 |
| 6 | V | 17 | +1.03 | −1.44 |
| 7 | VI | 46 | +0.97 | −1.23 |
| 8 | VII | 67 | +1.21 | −1.37 |
| 9 | VIII | 86 | +1.41 | −1.26 |
| 10e | VIII | 82 | +1.41 | −1.26 |
| 11f | VIII | 46 | +1.41 | −1.26 |
| 12 | IX | 65 | +1.37 | −1.26 |
| 13g | X | 0 | +0.77 | −1.33 |
| 14g | XI | 0 | +1.45 | −0.80 |
Scope
Having established the optimized reaction conditions, our investigation focused on exploring the scope of nitrones 1b–1u and N,N,N′,N′-tetraalkyldiaminomethanes 2b–2d (Scheme 2). Reactions involving 1b–1l, featuring aryl, heteroaryl, and cyclohexyl groups as R1, and 2a gave 3ba–3la, with low to moderate yield. Notably, N-tert-butyl nitrone 1m and π-extended derivatives 1n–1s, which are susceptible to decomposition under UV irradiation,33 underwent successful cyclization to afford 3ma–3sa in this visible-light-driven reaction. Reactions employing cyclic nitrones 1t and 1u proceeded smoothly, yielding tricyclic 3ta and tetracyclic 3ua products. N,N,N′,N′-tetraalkyldiaminomethanes 2b–2e in reactions with 1a, 1t, and 1u yielded 3ab, 3tb, 3ub, 3uc, 3ud, and 3ue in low to reasonable yields. We successfully conducted single-crystal X-ray diffraction analysis of 3ab and determined the relative stereochemistry of 3ue as trans in NMR.Mechanistic study
To elucidate the reaction mechanism of the formal [3 + 3] photocyclization, we conducted quenching experiments on IrIII complex VIII. The emission of VIII was attenuated upon addition of 1a and 2a, yielding Stern–Volmer plots (Scheme 3a) and uncovering Stern–Volmer constants (KSV) of 1.01 × 104 M−1 for 1a and 2.86 × 103 M−1 for 2a. Notably, the molar concentration of 2a (500 mM) was 10-times higher than that of 1a (50 mM), resulting in a quenching efficiency ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 for KSV [1a]
2.8 for KSV [1a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KSV [2a]. This finding suggests that the quenching biases 2a rather than 1a. Deaerated CH2Cl2 was essential, indicating that the reaction requires the triplet excited state of VIII. Subsequently, the quantum yield for the formation of 3aa (Φ3aa) was determined by dividing the rate of 3aa formation (1.05 × 10−8 mol s−1) by the rate of Fe(II) production (4.63 × 10−8 Einsteins s−1) during the photolysis of K3[Fe(C2O4)3],38,39 resulting in a value of 0.23 (Scheme 3b). As the quantum yield was less than 1.00, we excluded the non-photochemical radical chain mechanism. We updated the data for the photophysical properties of VIII due to an unclear E1/2 (IrIII/IrII) value in our previous study.40 Therefore, we further scrutinized the cyclic voltammetry conditions in analyzing VIII and determined the electrochemical potentials for the ground state of IrIII complex VIII [Ec (IrIII/IrII) = −1.26 V versus Fc/Fc+ in CH3CN; Ea (IrIII/IrIV) = +1.30 V versus Fc/Fc+ in CH3CN]. The 0–0 transition frequency of VIII was reported previously to be 2.67 V.40 Thus, the excited state potential of IrIII complex VIII* can be calculated as E* (Ir*III/IrII) = +1.41 V versus Fc/Fc+ in CH3CN and E* (Ir*III/IrIV) = −1.37 V versus Fc/Fc+ in CH3CN. The potential of 2a [Epa (2a/2a˙+) = +0.52 V versus Fc/Fc+ in CH3CN] was reported previously.40 We observed that the electrochemical potential of 1a ranged from −1.60 V to +1.70 V versus Fc/Fc+ in CH3CN, consistent with the reported potentials of (Z)-N-(phenylmethylene)methanamine N-oxide.41 The triplet energies of VIII and 1a are within a narrow range, noted at 2.67 eV (ref. 40) and 2.0 eV,42 respectively. This suggests the feasibility of energy transfer between the photoexcited VIII and the ground state 1a, and potentially leads to the decomposition of 1a under suitable conditions. However, in the absence of 2a, no decomposition of 1a occurred in the presence of VIII under visible-light irradiation (λ = 400–780 nm), indicating that energy transfer did not occur. Considering the electron transfer between 1a and the Ir species,43a,b it is conceivable that this process is reversible in our investigation, consistent with the findings of those who developed and elucidated the [3 + 3] dipolar cycloaddition of nitrones with aryl cyclopropanes via visible-light acridinium-based organophotoredox catalysis.43b
KSV [2a]. This finding suggests that the quenching biases 2a rather than 1a. Deaerated CH2Cl2 was essential, indicating that the reaction requires the triplet excited state of VIII. Subsequently, the quantum yield for the formation of 3aa (Φ3aa) was determined by dividing the rate of 3aa formation (1.05 × 10−8 mol s−1) by the rate of Fe(II) production (4.63 × 10−8 Einsteins s−1) during the photolysis of K3[Fe(C2O4)3],38,39 resulting in a value of 0.23 (Scheme 3b). As the quantum yield was less than 1.00, we excluded the non-photochemical radical chain mechanism. We updated the data for the photophysical properties of VIII due to an unclear E1/2 (IrIII/IrII) value in our previous study.40 Therefore, we further scrutinized the cyclic voltammetry conditions in analyzing VIII and determined the electrochemical potentials for the ground state of IrIII complex VIII [Ec (IrIII/IrII) = −1.26 V versus Fc/Fc+ in CH3CN; Ea (IrIII/IrIV) = +1.30 V versus Fc/Fc+ in CH3CN]. The 0–0 transition frequency of VIII was reported previously to be 2.67 V.40 Thus, the excited state potential of IrIII complex VIII* can be calculated as E* (Ir*III/IrII) = +1.41 V versus Fc/Fc+ in CH3CN and E* (Ir*III/IrIV) = −1.37 V versus Fc/Fc+ in CH3CN. The potential of 2a [Epa (2a/2a˙+) = +0.52 V versus Fc/Fc+ in CH3CN] was reported previously.40 We observed that the electrochemical potential of 1a ranged from −1.60 V to +1.70 V versus Fc/Fc+ in CH3CN, consistent with the reported potentials of (Z)-N-(phenylmethylene)methanamine N-oxide.41 The triplet energies of VIII and 1a are within a narrow range, noted at 2.67 eV (ref. 40) and 2.0 eV,42 respectively. This suggests the feasibility of energy transfer between the photoexcited VIII and the ground state 1a, and potentially leads to the decomposition of 1a under suitable conditions. However, in the absence of 2a, no decomposition of 1a occurred in the presence of VIII under visible-light irradiation (λ = 400–780 nm), indicating that energy transfer did not occur. Considering the electron transfer between 1a and the Ir species,43a,b it is conceivable that this process is reversible in our investigation, consistent with the findings of those who developed and elucidated the [3 + 3] dipolar cycloaddition of nitrones with aryl cyclopropanes via visible-light acridinium-based organophotoredox catalysis.43b
Based on these findings, we propose a plausible reaction mechanism in Scheme 3c. The ground state IrIII complex (IrIII) absorbs light to generate the photoexcited IrIII complex (Ir*III), which oxidizes 2via SET, followed by proton transfer to generate the α-aminoalkyl radical A and the ground state IrII complex (IrII). α-Aminoalkyl radical A is generated in a regioselective manner based on a stereoelectronic rule.44,45 Nitrone 1 traps A to form the resonance-stabilized radical B. Subsequently, B receives an electron from the ground state IrIII and a proton, yielding N-benzylhydroxylamine C and regenerating the ground state IrIII. N-Benzylhydroxylamine C then cyclizes, accompanied by proton transfer to one of the tertiary amine nitrogens, releasing the secondary amine D.
Flow synthesis and derivatization
To achieve multi-gram-scale synthesis of a target molecule in drug discovery, we developed the photochemical flow reactor and applied it to the reaction of 1t (11.6 mmol) with 2b (Scheme 4). The reaction proceeded smoothly, yielding 1.88 g (6.70 mmol) of 3tb in a single photochemical flow operation (Fig. S2†). Subsequently, we employed 518 mg (1.85 mmol) of 3tb for acylative debenzylation in the presence of cyclohexanecarboxylic anhydride, Pd(OH)2/C, under a hydrogen atmosphere, thereby completing the synthesis of 4.Biological activity
Our ongoing investigation focused on evaluating and comparing the anti-schistosomal activities of 4 and PZQ against Schistosoma mansoni (Table 2; see ESI† for videos of the worm movement). Remarkably, 4 exhibited comparable activity and a rapid onset of action against adult worms. Marchant and colleagues have conducted meticulous research to elucidate the interaction of PZQ with the transient receptor potential ion channel of the melastatin subfamily of S. mansoni, Sm.TRPMPZQ.46 According to their recent findings, the piperazin-2-one carbonyl of PZQ forms a crucial hydrogen bond with Sm.TRPMPZQ, contributing to binding energy.46b Therefore, the remarkable biological activity of 4 is particularly surprising, as it lacks this essential functional group.| Compoundb | Motility and survival of the wormsc,d,e | |||||
|---|---|---|---|---|---|---|
| Adult (♂) | Adult (♀) | |||||
| a Mice were inoculated with approximately 250 cercariae and underwent a 6-week incubation period. Subsequently, the mice were euthanized to harvest the adult schistosomes from the mesenteric veins using tweezers or by perfusion. All procedures were performed in compliance with the ethical guidelines of the Kitasato University Animal Experiment Ethics Committee (approval no. 2022-074, 2023-123). b The molar concentration of compounds was 10 μM. c The motility and survival of the worms were evaluated based on a 0 to 4 viability scale: 0 indicated full motility with worms adherent to the well bottom; 1 indicated partial detachment with weak movement; 2 represented full detachment with weak movement; 3 was characterized by no pairing and varied movement intensity, or no movement but still alive; 4 denoted deceased worms. A treatment was deemed lethal if no worm movement was observed for 1 minute. Data are presented as the mean of three independent experiments. d The health status of the adult worms, including their motility and mortality, was assessed using an inverted microscope. e Post-treatment, the parasites were monitored daily for 5 days (120 hours). f DMSO served as the vehicle control. | ||||||
| 1 h | 24 h | 120 h | 1 h | 24 h | 120 h | |
| PZQ | 3.7 | 3.7 | 3.3 | 3.7 | 3.3 | 3.3 |
| 4 | 3.0 | 3.0 | 3.0 | 2.0 | 2.0 | 2.3 |
| Nonef | 0 | 0 | 0 | 0 | 0 | 0 |
ADMET property
Next, we comprehensively assessed the melting point, solubility, membrane permeability, stability under acidic pH, and metabolic stability of 4 to evaluate its potential as a lead compound in the discovery of a new chemotype demonstrating an anti-schistosomal effect (Table 3). Comparing the melting points of PZQ13 and 4, we noted that 4 exhibited a significantly lower melting point, indicating weaker molecular packing.3a,d,g,47,48 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D value of PZQ could not be estimated due to its limited solubility in phosphate-buffered saline (PBS) at pH 7.4. In contrast, 4, a new chemotype, demonstrated sufficient solubility in PBS, yielding a favorable value of 4.1, which is suitable for oral administration.49 Consequently, 4 is expected to have enhanced solubility in PBS at pH 7.4. Conversely, due to its significantly low solubility, PZQ was not detected in high-performance liquid chromatography with ultraviolet detection.13,21 To elucidate the notable solubility of 4, we assessed its molecular polarity by calculating the dipole moments of both PZQ and 4 (Fig. S6†).50 These computational studies were performed using the B3LYP hybrid functional and a 6-31G** basis set within Gaussian16.51 The solvent effects of water were considered using the polarizable continuum model with the integral equation formalism variant (IEFPCM). The computations yielded dipole moment values of 1.97 debye for PZQ and 3.96 debye for 4. The dipole moment value of 4 was significantly larger than that of PZQ, corroborating its substantially greater solubility in polar solvents compared to PZQ. In permeability studies employing the Caco-2 cell assay, 4 demonstrated adequate permeability through human epithelial cells.22a,52,53 Evaluation of its stability under acidic pH revealed resilience despite the presence of the N,O-acetal structure. We then investigated the metabolic stability of both PZQ and 4 in liver microsomes. Although PZQ underwent faster metabolism than expected,23a4 exhibited even greater attenuation than PZQ, in contrast to our initial expectations.
D value of PZQ could not be estimated due to its limited solubility in phosphate-buffered saline (PBS) at pH 7.4. In contrast, 4, a new chemotype, demonstrated sufficient solubility in PBS, yielding a favorable value of 4.1, which is suitable for oral administration.49 Consequently, 4 is expected to have enhanced solubility in PBS at pH 7.4. Conversely, due to its significantly low solubility, PZQ was not detected in high-performance liquid chromatography with ultraviolet detection.13,21 To elucidate the notable solubility of 4, we assessed its molecular polarity by calculating the dipole moments of both PZQ and 4 (Fig. S6†).50 These computational studies were performed using the B3LYP hybrid functional and a 6-31G** basis set within Gaussian16.51 The solvent effects of water were considered using the polarizable continuum model with the integral equation formalism variant (IEFPCM). The computations yielded dipole moment values of 1.97 debye for PZQ and 3.96 debye for 4. The dipole moment value of 4 was significantly larger than that of PZQ, corroborating its substantially greater solubility in polar solvents compared to PZQ. In permeability studies employing the Caco-2 cell assay, 4 demonstrated adequate permeability through human epithelial cells.22a,52,53 Evaluation of its stability under acidic pH revealed resilience despite the presence of the N,O-acetal structure. We then investigated the metabolic stability of both PZQ and 4 in liver microsomes. Although PZQ underwent faster metabolism than expected,23a4 exhibited even greater attenuation than PZQ, in contrast to our initial expectations.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, melting point, solubility, membrane permeability, stability under acidic pH, and metabolic stability of 4versus PZQ
D, melting point, solubility, membrane permeability, stability under acidic pH, and metabolic stability of 4versus PZQ
| Comp. | M.p. (°C) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Da Da |
Sol.a | Caco-2 cell permeabilityb | Remaining ratio (%) in acidic and neutral mediac | Remaining ratio (%) in liver microsomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Papp | Class | pH 1.2 | pH 7.4 | Human | Mouse | ||||||||
| 1 h | 5 h | 1 h | 5 h | 10 min | 60 min | 10 min | 60 min | ||||||
| a PBS (pH 7.4) was used as the buffer. b Employing a well-established method demonstrated in ref. 53 that measured the rate of flux of compounds across polarized Caco-2 cell monolayers (Papp: × 10−6 cm s). c A disintegration testing instrument and conditions were utilized for the evaluation. d Reported value in ref. 13. e N.E. stands for “not estimated”. f N.D. stands for “not detected”. g Insoluble in water. | |||||||||||||
| PZQ | 136–138d | N.E.e | N.D.f,g | 37.3 | High | 93.1 | 95.0 | 103.2 | 100.1 | 87.5 | 49.8 | 11.5 | 0.1 |
| 4 | 106–107 | 4.1 | ≧95 | 30.5 | High | 95.1 | 94.9 | 95.2 | 93.3 | 47.3 | 5.9 | 2.7 | 0.2 |
We also performed a cell viability assay in HeLa cells and found no detrimental effects when comparing 4 and PZQ (Fig. 1a). Furthermore, we performed a cardiac safety assay using human induced pluripotent stem cell (iPSC)-derived cardiomyocytes, iCell® Cardiomyocytes2,54 to compare the cardiotoxic effects of 4 with those of PZQ. PZQ and 4 were added to cardiomyocytes, and the resulting calcium ion influx was monitored using a fluorescent indicator. This process generated calcium (Ca2+) oscillation profiles (Fig. 1b). The Ca2+ flux signal in each well provided a view of the beating profiles of the cardiac cells over 20 seconds for both untreated cells and cells treated with PZQ or 4. The peak height (amplitude) represents the maximum change in calcium flux during a cardiac cycle; a higher peak amplitude suggests stronger contraction of the cardiac cells, whereas a lower peak amplitude indicates weaker contraction. The peak width represents the duration of the calcium transient. A broader peak indicates longer duration of calcium presence, suggesting prolonged contraction or slower relaxation of the cardiomyocytes. Conversely, a narrower peak indicates a shorter contraction time or quicker relaxation. These metrics provide valuable insights into the timing of the cardiac cycle and are crucial for evaluating the potential of compounds to prolong the QT-interval, which is associated with the onset of arrhythmias. We evaluated the impact of E-4031, a known human ether-a-go-go-related gene (hERG) channel blocker, as a positive control, which induced significant cardiac toxicity (Fig. S8†).55,56 Upon addition of PZQ at various concentrations (0.2 μM, 2 μM, 20 μM), no changes in the pulse waveforms or beat intervals were observed within the 60 minutes before and after addition. In contrast, the addition of 20 μM of 4 did not alter the pulse waveforms, but resulted in shortened beat intervals for 60 minutes. At 2 μM of 4, the pulse waveforms remained unchanged, but a slight shortening of beat intervals was observed for up to 30 minutes post-addition, eventually reverting to the pre-addition state within 60 minutes (Fig. S9†). The addition of 0.2 μM of 4 did not affect the pulse waveforms and beat intervals for 60 minutes. Notably, there were no signs of QT-interval prolongation, early after-depolarization, or cardiomyocyte cell cycle arrest. Thus, we concluded that both compounds exhibit potential liabilities to cardiovascular safety.
Conclusions
Our investigation into replacing the piperazin-2-one ring of PZQ with a 1,2,5-oxadiazinane ring successfully identified a novel chemotype with highly potent anti-schistosomal activity. This derivative demonstrates significant efficacy against S. mansoni while largely preserving the potent biological activity of the parent compound. Notable attributes include excellent solubility, resilience to acid exposure, and potential liabilities to cardiovascular safety and cell viability. These results advocate for the 1,2,5-oxadiazinane moiety as a bioisostere of piperazin-2-one. Our ongoing efforts are focused on enhancing the metabolic stability of this molecule and conducting further structure–activity relationship studies to surpass the efficacy of PZQ. Our exploration of bioisosteric replacement and the discovery of this anti-schistosomal molecule were facilitated by our pivotal synthetic methodology involving the formal [3 + 3] photocycloaddition reaction between nitrones and N,N,N′,N′-tetraalkyldiaminomethanes, yielding a diverse array of 1,2,5-oxadiazinane structures. Utilizing a photochemical flow reactor, we achieved multi-gram-scale synthesis of an intermediate for the target molecule, which is crucial for drug discovery. The proposed mechanism for the photocycloaddition is convincing and opens new avenues for synthesizing novel saturated nitrogen-containing heterocycles that are potentially useful in bioisosteric replacement.Data availability
The data supporting this study's findings are available in the ESI† of this article. Videos for the worm movement assay are also included.Author contributions
K. I. conceived the research, executed the experiments, analyzed the data, wrote the manuscript, and supervised the project; H. N. synthesized the anti-schistosomal molecule, optimized the photocycloaddition, and developed the photoflow synthesis; A. K. optimized the photoflow synthesis; A. T. and Y. A. optimized the photocycloaddition; A. H. carried out the kinetic experiments to determine the quantum yield and Stern–Volmer constants; T. M., F. K., and S. H. provided valuable advice to advance the research; K. N. and R. S. conducted SCXRD, HRMS, and FAB-MS analyses; N. S. performed NMR experiments; K. O. and M. H. conducted emission experiments at 77 K and elemental analysis; K. T. executed the computational study; H. I. synthesized RuII complexes and provided helpful advice for photochemistry experiments; F. M., K. D. K., H. K., T. H., and N. T. performed and evaluated the worm movement assays and provided valuable information on advanced Schistosomiasis research; B. L., K. N., T. K., and J. H. not only performed and evaluated ADMET assays and provided guidance on drug discovery in the pharmaceutical industry; H. F. provided fruitful advice on the medicinal chemistry research in academia and pharmaceutical industry and supervised the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under grant number JP24ama121054.Notes and references
- C. M. Marson, Adv. Heterocycl. Chem., 2017, 121, 13–33 CrossRef.
- H. Chen, O. Engkvist and T. Kogej, in The Practice of Medicinal Chemistry, ed. C.G. Wermuth, D. Aldous, P. Raboisson and D. Rogan, Academic Press, London, 4th edn, 2015, pp. 384–385 Search PubMed.
- (a) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef PubMed; (b) G. Wuitschik, E. M. Carreira, B. Wagner, H. Fischer, I. Parrilla, F. Schuler, M. Rogers-Evans and K. Müller, J. Med. Chem., 2010, 53, 3227–3246 CrossRef PubMed; (c) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529–2591 CrossRef PubMed; (d) F. Lovering, Med. Chem. Commun., 2013, 4, 515–519 RSC; (e) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef PubMed; (f) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef PubMed; (g) W. Wei, S. Cherukupalli, L. Jing and X. Liu, Drug Discovery Today, 2020, 25, 1839–1845 CrossRef PubMed; (h) M. A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 1839–1845 CrossRef PubMed; (i) N. A. Meanwell and O. Loiseleur, J. Agric. Food Chem., 2022, 70, 10942–10971 CrossRef PubMed; (j) N. A. Meanwell and O. Loiseleur, J. Agric. Food Chem., 2022, 25, 1839–1845 Search PubMed.
- (a) O. Méndez-Lucio and J. L. Medina-Franco, Drug Discovery Today, 2017, 22, 120–126 CrossRef PubMed; (b) W. Wei, S. Cherukupalli, L. Jing, X. Liu and P. Zhan, Drug Discovery Today, 2020, 22, 120–126 Search PubMed; (c) J. Shearer, J. L. Castro, A. D. G. Lawson, M. MacCoss and R. D. Taylor, J. Med. Chem., 2022, 65, 8699–8712 CrossRef PubMed.
- (a) G. Wuitschik, M. Rogers-Evans, A. Buckl, M. Bernasconi, M. Märki, T. Godel, H. Fischer, B. Wagner, I. Parrilla, F. Schuler, J. Schneider, A. Alker, W. B. Schweizer, K. Müller and E. M. Carreira, Angew. Chem., Int. Ed., 2008, 47, 4512–4515 CrossRef PubMed; (b) J. A. Burkhard, B. Wagner, H. Fischer, F. Schuler, K. Müller and E. M. Carreira, Angew. Chem., Int. Ed., 2010, 49, 3524–3527 CrossRef PubMed; (c) J. A. Burkhard, G. Wuitschik, M. Rogers-Evans, K. Müller and E. M. Carreira, Angew. Chem., Int. Ed., 2010, 49, 9052–9067 CrossRef PubMed; (d) J. A. Burkhard, C. Guérot, H. Knust, M. Rogers-Evans and E. M. Carreira, Org. Lett., 2010, 12, 1944–1947 CrossRef PubMed; (e) C. Guérot, B. H. Tchitchanov, H. Knust and E. M. Carreira, Org. Lett., 2011, 13, 780–783 CrossRef PubMed.
- (a) A. P. Mityuk, A. V. Denisenko, O. P. Dacenko, O. O. Grygorenko, P. K. Mykhailiuk, D. M. Volochnyuk, O. V. Shishkin and A. A. Tolmachev, Synthesis, 2010, 493–497 Search PubMed; (b) A. V. Denisenko, A. P. Mityuk, O. O. Grygorenko, D. M. Volochnyuk, O. V. Shishkin, A. A. Tolmachev and P. K. Mykhailiuk, Org. Lett., 2010, 12, 4372–4375 CrossRef PubMed; (c) A. A. Kirichok, I. Shton, M. Kliachyna, I. Pishel and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2017, 56, 8865–8869 CrossRef PubMed; (d) B. A. Chalyk, M. V. Butko, O. O. Yanshyna, K. S. Gavrilenko, T. V. Druzhenko and P. K. Mykhailiuk, Chem. – Eur. J., 2017, 23, 16782–16786 CrossRef PubMed; (e) V. V. Levterov, O. Michurin, P. O. Borysko, S. Zozulya, I. V. Sadkova, A. A. Tolmachev and P. K. Mykhailiuk, J. Org. Chem., 2018, 83, 14350–14361 CrossRef PubMed; (f) A. A. Kirichok, I. O. Shton, I. M. Pishel, S. A. Zozulya, P. O. Borysko, V. Kubyshkin, O. A. Zaporozhets, A. A. Tolmachev and P. K. Mykhailiuk, Chem. – Eur. J., 2018, 24, 5444–5449 CrossRef PubMed; (g) D. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2023, 62, e202304246 CrossRef CAS PubMed; (h) A. A. Kirichok, H. Tkachuk, Y. Kozyriev, O. Shablykin, O. Datsenko, D. Granat, T. Yegorova, Y. P. Bas, V. Semirenko, I. Pishel, V. Kubyshkin, D. Lesyk, O. Klymenko-Ulianov and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2023, 62, e202311583 CrossRef CAS PubMed; (i) A. Denisenko, P. Garbuz, N. M. Voloshchuk, G. Al-Maali, P. Borysko and P. K. Mykhailiuk, Nat. Chem., 2023, 15, 1155–1163 CrossRef CAS PubMed; (j) V. V. Levterov, Y. Panasiuk, K. Sahun, O. Stashkevych, V. Badlo, O. Shablykin, I. Sadkova, L. Bortnichuk, O. Klymenko-Ulianov, Y. Holota, L. Lachmann, P. Borysko, K. Horbatok, I. Bodenchuk, Y. Bas, D. Dudenko and P. K. Mykhailiuk, Nat. Commun., 2023, 14, 5608 CrossRef CAS PubMed.
- (a) C.-V. T. Vo, G. Mikutis and J. W. Bode, Angew. Chem., Int. Ed., 2013, 52, 1705–1708 CrossRef PubMed; (b) C.-V. T. Vo, M. U. Luescher and J. W. Bode, Nat. Chem., 2014, 6, 310–314 CrossRef PubMed; (c) W.-Y. Siau and J. W. Bode, J. Am. Chem. Soc., 2014, 136, 17726–17729 CrossRef PubMed; (d) C.-V. T. Vo and J. W. Bode, J. Org. Chem., 2014, 79, 2809–2815 CrossRef PubMed; (e) M. U. Luescher, K. Geoghegan, P. L. Nichols and J. W. Bode, Aldrichimica Acta, 2015, 48, 43–48 Search PubMed; (f) M. U. Luescher and J. W. Bode, Org. Lett., 2016, 18, 2652–2655 Search PubMed; (g) Y. Wang and J. W. Bode, J. Am. Chem. Soc., 2019, 141, 9739–9745 CrossRef PubMed; (h) M. K. Škopić, F. Losch, A. E. McMillan, N. Willeke, M. Malenica, L. Bering, J. Bode and A. Brunschweiger, Org. Lett., 2022, 24, 1383–1387 CrossRef PubMed; (i) J. Götz, M. K. Jackl, C. Jindakun, A. N. Marziale, J. André, D. J. Gosling, C. Springer, M. Palmieri, M. Reck, A. Luneau, C. E. Brocklehurst and J. W. Bode, Sci. Adv., 2023, 9, eadj2314 CrossRef PubMed.
- (a) M. Yar, E. M. McGarrigle and V. K. Aggarwal, Angew. Chem., Int. Ed., 2008, 47, 3784–3786 CrossRef PubMed; (b) M. G. Unthank, B. Tavassoli and V. K. Aggarwal, Org. Lett., 2008, 10, 1501–1504 CrossRef PubMed; (c) M. Yar, E. M. McGarrigle and V. K. Aggarwal, Org. Lett., 2009, 11, 257–260 CrossRef PubMed; (d) S. P. Frintz, T. H. West, E. M. McGarrigle and V. K. Aggarwal, Org. Lett., 2012, 14, 6370–6373 CrossRef PubMed; (e) S. P. Frintz, J. V. Matlock, E. M. McGarrigle and V. K. Aggarwal, Chem. – Eur. J., 2013, 19, 10827–10831 CrossRef PubMed; (f) J. A. Matlock, T. D. Svejstrup, P. Songara, S. Overington, E. M. McGarrigle and V. K. Aggarwal, Org. Lett., 2015, 17, 5044–5047 CrossRef CAS PubMed; (g) A. Fawcett, A. Murtaza, C. H. U. Gregson and V. K. Aggarwal, J. Am. Chem. Soc., 2019, 141, 4573–4578 CrossRef CAS PubMed; (h) J. L. Tyler, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2021, 60, 11824–11829 CrossRef CAS PubMed; (i) J. L. Tyler, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2022, 61, e202114235 CrossRef CAS PubMed.
- (a) K. E. Gettys, Z. Ye and M. Dai, Synthesis, 2017, 49, 2589–2604 CrossRef CAS; (b) Z. Ye, S. Adhikari, Y. Xia and M. Dai, Nat. Commun., 2018, 9, 721 CrossRef PubMed.
- A. V. Chernykh, A. N. Tkachenko, I. O. Feskov, C. G. Daniliuc, N. A. Tolmachova, D. M. Volochnyuk and D. S. Radchenko, Synthesis, 2016, 27, 1824–1827 CAS.
- Z. Dobi, T. Holczbauer and T. Soós, Eur. J. Org. Chem., 2017, 1391–1395 CrossRef.
- A. J. Boddy, D. P. Affron, C. J. Cordier, E. L. Rivers, A. C. Spivey and J. A. Bull, Angew. Chem., Int. Ed., 2019, 58, 1458–1462 CrossRef PubMed.
- H. I. El-Subbagh and A. A. Al-Badr, in Analytical Profiles of Drug Substances and Excipients, ed. H. G. Brittain, Academic Press, San Diego, 1998, vol. 25, pp. 463–500 Search PubMed; The Merck Index Online. “Praziquantel”, can be found under https://merckindex.rsc.org/monographs/m9107 (accessed 26 April 2024).
- C. R. Caffrey, N. El-Sakkary, P. Mäder, R. Krieg, K. Becker, M. Schlitzer, D. H. Drewry, J. L. Vennerstrom and C. G. Grevelding, in Neglected Tropical Dieaes: Drug Discovery and Development, ed. D. C. Swinney, M. P. Pollastri, R. Mannhold, H. B. Buschmann and J. Holenz, Wiley-VCH Verlag GmbH & Co. KGaA., 2019, vol. 77, pp. 187–299 Search PubMed.
- (a) P. Andrews, H. Thomas, R. Pohlke and J. Seubert, Med. Res. Rev., 1983, 3, 147–200 CrossRef PubMed; (b) A. G. P. Ross, P. B. Bartley, A. C. Sleigh, G. R. Olds, Y. Li, G. M. Williams and D. P. McManus, N. Engl. J. Med., 2002, 346, 1212–1220 CrossRef PubMed; (c) D. P. McManus, D. W. Dunne, M. Sacko, J. Utzinger, B. J. Vennervald and X. N. Zhou, Nat. Rev. Dis., 2018, 4, 13 Search PubMed; (d) C. M. Thomas and D. J. Timison, Curr. Top. Med. Chem., 2018, 18, 1575–1584 CrossRef PubMed; (e) S.-K. Park and J. S. Marchant, Trends Parasitol., 2020, 36, 182–194 CrossRef CAS PubMed; (f) N. C. Lo, F. S. M. Bezerra, D. G. Colley, F. M. Fleming, M. Homeida, N. Kabatereine, F. M. Kabole, C. H. King, M. A. Mafe, N. Midzi, F. Mutapi, J. R. Mwanga, R. M. R. Ramzy, F. Satrija, J. R. Stothard, M. S. Traoré, J. P. Webster, J. Utzinger, X.-N. Zhou, A. Danso-Appiah, P. Eusebi, E. S. Loker, C. O. Obonyo, R. Quansah, S. Liang, M. Vaillant, M. H. Murad, P. Hagan and A. Garba, Lancet Infect. Dis., 2022, 22, e327–e335 CrossRef PubMed.
- A. Wiesner, M. Skrońska, G. Gawlik, M. Marcinkowska, P. Zagrodzki and P. Paśko, Aids Behav., 2023, 27, 1441–1468 CrossRef PubMed.
- N. Brown, in Bioisosteres in Medicinal Chemistry, ed. N. Brown, Wiley-VCH, Weinheim, 1st edn, 2012, pp. 3–29 Search PubMed; D. A. Smith and D. S. Millan, in Bioisosteres in Medicinal Chemistry, ed. N. Brown, Wiley-VCH, Weinheim, 1st edn, 2012, pp. 31–51 Search PubMed; P. Ciapetti and B. Giethlen, in The Practice of Medicinal Chemistry, ed. C. G. Wermuth, D. Aldous, P. Raboisson and D. Rogan, Academic Press, London, 4th edn, 2015, pp. 181–220 Search PubMed.
- (a) G. A. Patani and E. J. LaVoie, Chem. Rev., 1996, 96, 3147–3176 CrossRef PubMed; (b) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529–2591 CrossRef PubMed; (c) N. Brown, Mol. Inf., 2014, 33, 458–462 CrossRef PubMed; (d) N. A. Meanwell, J. Agric. Food Chem., 2023, 71, 18087–18122 CrossRef PubMed.
- M. Lindenberg, S. Kopp and J. B. Dressmana, Eur. J. Pharm. Biopharm., 2004, 58, 265–278 CrossRef PubMed.
- (a) F. Ronketti, A. V. Ramana, X. C. Ming, L. Pica-Mattoccia, D. Ciolic and M. H. Todd, Bioorg. Med. Chem. Lett., 2007, 17, 4154–4157 CrossRef CAS PubMed; (b) W.-L. Wang, L.-J. Song, X. Chen, X.-R. Yin, W.-H. Fan, G.-P. Wang, C.-X. Yu and B. Feng, Molecules, 2013, 18, 9163–9178 CrossRef CAS; (c) M. Patra, K. Ingram, A. Leonidova, V. Pierroz, S. Ferrari, M. N. Robertson, M. H. Todd, J. Keiser and G. Gasser, J. Med. Chem., 2013, 56, 9192–9198 CrossRef CAS PubMed; (d) Z.-X. Wang, J.-L. Chen and C. Qiao, Chem. Biol. Drug Des., 2013, 82, 216–225 CrossRef CAS PubMed; (e) Y. Zheng, L. Dong, C. Hu, B. Zhao, C. Yang, C. Xia and D. Sun, Bioorg. Med. Chem. Lett., 2014, 24, 2481–2484 CrossRef PubMed.
- I. D'Abbrunzo, G. Procida and B. Perissutti, Pharmaceutics, 2024, 16, 27–38 CrossRef PubMed.
- (a) G.-E. Dinora, R. Julio, C. Nelly, Y.-M. Lilian and H. J. Cook, Int. J. Pharm., 2005, 295, 93–99 CrossRef PubMed; (b) N. El-Lakkany, S. H. S. El-Din and L. Heikal, Eur. J. Drug Metab. Pharmacokinet., 2012, 37, 289–299 CrossRef PubMed; (c) A. D. A. Silva, M. A. Sarcinelli, B. F. C. Patricio, M. H. C. Chaves, L. M. Lima, P. M. Parreiras, P. F. Pinto, L. D. Prado and H. V. A. Rocha, J. Drug Deliv. Sci. Technol., 2023, 81, 104260 CrossRef.
- (a) N. Castro, R. Medina, J. Sotelo and H. Jung, Antimicrob. Agents Chemother., 2000, 44, 2903–2904 CrossRef PubMed; (b) P. Olliaro, P. Delgado-Romero and J. Keiser, J. Antimicrob. Chemother., 2014, 69, 863–870 CrossRef PubMed; (c) N. Abla, J. Keiser, M. Vargas, N. Reimers, H. Haas and T. Spangenberg, PLoS Negl. Trop. Dis., 2017, 11, e0005942 CrossRef PubMed.
- T. Meyer, H. Sekljic, S. Fuchs, H. Bothe, D. Schollmeyer and C. Miculka, PLoS Negl. Trop. Dis., 2009, 3, e357 CrossRef PubMed.
- “The Pediatric Praziquantel Consortium”, can be found under https://www.pediatricpraziquantelconsortium.org/, 2024 (accessed 01 May 2024).
- “European Medicines Agency (EMA) adopts a positive scientific opinion on arPraziquantel”, can be found under https://www.who.int/news/item/07-02-2024-european-medicines-agency-(ema)-adopts-a-positive-scientific-opinion-on-arpraziquantel, 2024 (accessed 01 May 2024).
- E. K. N'Goran, M. R. Odiere, R. A. Aka, M. Ouattara, N. A. D. Aka, B. Ogutu, F. Rawago, W. M. Bagchus, M. Bödding, E. Kourany-Lefoll, A. Tappert, X. Yin, D. Bezuidenhout, H. Badenhorst, E. Huber, B. Dälken and O. H.-A. Saflo, Lancet Infect. Dis., 2023, 23, 867–876 CrossRef PubMed.
- D. J. Sprague, S.-K. Park, S. Gramberg, L. Bauer, C. M. Rohr, E. G. Chulkov, E. Smith, L. Scampavia, T. P. Spicer, S. Haeberlein and J. S. Marchant, Nat. Struct. Mol. Biol., 2024, 31, 1386 CrossRef CAS PubMed.
- (a) J. C. Espinosa-Lara, D. Guzman-Villanueva, J. I. Arenas-García, D. Herrera-Ruiz, J. Rivera-Islas, P. Romań-Bravo, H. Morales-Rojas and H. Höpfl, Cryst. Growth Des., 2013, 13, 169–185 CrossRef; (b) A. Borrego-Sánchez, C. Viseras, C. Aguzzi and C. I. Sainz-Díaz, Eur. J. Pharm. Sci., 2016, 92, 266–275 CrossRef PubMed.
- V. B. R. Silva, B. R. K. L. Campos, J. F. Oliveira, J.-L. Decout and M. C. A. Lima, Bioorg. Med. Chem., 2017, 25, 3259–3277 CrossRef PubMed.
- P. Ciapetti and B. Giethlen, in The Practice of Medicinal Chemistry, ed. C. G. Wermuth, D. Aldous, P. Raboisson and D. Rogan, Academic Press, London, 4th edn, 2015, p. 187, (Table 8.5) Search PubMed.
- (a) F. G. Riddell and E. S. Turner, Tetrahedron, 1979, 35, 1311–1314 CrossRef; (b) K. Bell, M. P. Coogan, M. B. Gravestock, D. W. Knight and S. R. Thornton, Tetrahedron Lett., 1997, 38, 8545–8552 CrossRef; (c) K. K. Afanaseva, M. M. Efremova, S. V. Kuznetsova, A. V. Ivanov, G. L. Starova and A. P. Molchanov, Tetrahedron, 2018, 74, 5665–5673 CrossRef.
- K. Itoh, A. Takashino, A. Ohtsuka, M. Kobe, S. Sawamura, R. Kato, S. Hirayama, F. Karaki, T. Mizuguchi, N. Sato, K. Tokunaga, Y. Toda, H. Suga, H. Ishida and H. Fujii, ChemPhotoChem, 2020, 4, 388–392 CrossRef.
- J. D. Wraver III and R. Overfield, in Springer Handbook of Inorganic Photochemistry, ed. D. W. Bahnemann and A. O. T. Patrocinio, Springer Nature Switzerland AG, 2022, pp. 1481–1508 Search PubMed.
- (a) K. Teegardin, J. I. Day, J. Chan and J. Weaver, Org. Process Res. Dev., 2016, 20, 1156–1163 CrossRef; (b) J. Zheng, X. Dong and T. P. Yoon, Org. Lett., 2020, 22, 6520–6525 CrossRef PubMed; (c) M. A. Bryden and E. Zysman-Colman, Chem. Soc. Rev., 2021, 50, 7587–7680 RSC; (d) J. Tang, L. Ren, J. Li, Y. Wang, D. Hu, X. Tong and C. Xia, Org. Lett., 2022, 24, 3582–3587 CrossRef PubMed; (e) N. Holmberg-Douglas and D. A. Nicewicz, Chem. Rev., 2022, 122, 1925–2016 CrossRef PubMed.
- (a) X. Li, B. Minaev, H. Ågren and H. Tian, Eur. J. Inorg. Chem., 2011, 2517–2524 CrossRef; (b) Y. Chen, C. Liu and L. Wang, Tetrahedron, 2019, 75, 130686 CrossRef.
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef PubMed; (b) K. Nakajima, Y. Miyake and Y. Nishibayashi, Acc. Chem. Res., 2016, 49, 1946–1956 CrossRef PubMed; (c) D. Staveness, I. Bosque and C. R. J. Stephenson, Acc. Chem. Res., 2016, 49, 2295–2306 CrossRef PubMed; (d) P. R. D. Murray, J. H. Cox, N. D. Chiappini, C. B. Roos, E. A. McLoughlin, B. G. Hejna, S. T. Nguyen, H. H. Ripberger, J. M. Ganley, E. Tsui, N. Y. Shin, B. Koronkiewicz, G. Qiu and R. R. Knowles, Chem. Rev., 2022, 122, 2017–2291 CrossRef PubMed.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, in Handbook of Photochemistry, CRC Press, Boca Raton, 3rd edn, 2006, pp. 601–616 Search PubMed.
- K. Itoh, S. Nagao, K. Tokunaga, S. Hirayama, F. Karaki, T. Mizuguchi, K. Nagai, N. Sato, M. Suzuki, M. Hashimoto and H. Fujii, Chem. – Eur. J., 2021, 27, 5171–5178 CrossRef PubMed.
- K. Itoh, N. Ishii, A. Takashino, A. Hara, S. Kon, T. Mizuguchi, F. Karaki, S. Hirayama, Y. Shibagaki, K. Nagai, N. Sato, K. Tokunaga, M. Suzuki, M. Hashimoto and H. Fujii, J. Photochem. Photobiol., A, 2022, 434, 114239 CrossRef.
- P. H. R. Oliveira, É. A. Tordato, J. A. C. Vélez, P. S. Carneiro and M. W. Paixaõ, J. Org. Chem., 2023, 88, 6407–6419 CrossRef PubMed.
- D. R. Cyr, T. Mathew, K. Ashok, P. K. Das and M. V. George, J. Photochem. Photobiol., A, 1991, 60, 161–174 CrossRef.
- (a) G. Haun, A. N. Paneque, D. W. Almond, B. E. Austin and G. Moura-Letts, Org. Lett., 2019, 21, 1388–1392 CrossRef PubMed; (b) Y. Xu, H.-X. Gao, C. Pan, Y. Shi, C. Zhang, G. Huang and C. Feng, Angew. Chem., Int. Ed., 2023, 62, e202310671 CrossRef PubMed.
- F. D. Lewis, Acc. Chem. Res., 1986, 19, 401–405 CrossRef.
- F. D. Lewis and T.-I. Ho, J. Am. Chem. Soc., 1980, 102, 1751–1752 CrossRef.
- (a) S.-K. Park, G. S. Gunaratne, E. G. Chulkov, F. Moehring, P. McCusker, P. I. Dosa, J. D. Chan, C. L. Stucky and J. S. Marchant, J. Biol. Chem., 2019, 294, 18873–18880 CrossRef PubMed; (b) S.-K. Park, L. Friedrich, N. A. Yahya, C. M. Rohr, E. G. Chulkov, D. Maillard, F. Rippmann, T. Spangenberg and J. S. Marchant, Sci. Transl. Med., 2021, 13, eabj5832 CrossRef CAS PubMed; (c) W. Le Clec'h, F. D. Chevalier, A. C. A. Mattos, A. Strickland, R. Diaz, M. McDew-White, C. M. Rohr, S. Kinung'hi, F. Allan, B. L. Webster, J. P. Webster, A. M. Emery, D. Rollinson, A. G. Djirmay, K. M. Al Mashikhi, S. Al Yafae, M. A. Idris, H. Moné, G. Mouahid, P. LoVerde, J. S. Marchant and T. J. C. Anderson, Sci. Transl. Med., 2021, 13, eabj9114 CrossRef PubMed; (d) C. M. Rohr, D. J. Spraguea, S.-K. Parka, N. J. Malcolma and J. S. Marchant, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2217732120 CrossRef PubMed; (e) D. Sprague, M. Kaethner, S.-K. Park, C. M. Rohr, J. L. Harris, D. Maillard, T. Spangenberg, B. Lundström-Stadelmann and J. S. Marchant, ACS Med. Chem. Lett., 2023, 14, 1537–1543 CrossRef PubMed; (f) S.-K. Park, D. J. Sprague, C. M. Rohr, E. G. Chulkov, I. Petrow, S. Kumar and J. S. Marchant, J. Biol. Chem., 2024, 300, 105528 CrossRef PubMed.
- M. Ishikawa and Y. Hashimoto, J. Med. Chem., 2011, 54, 1539–1554 CrossRef PubMed.
- M. A. Walker, Expert Opin. Drug Discovery, 2014, 9, 1421–1433 CrossRef PubMed.
- (a) C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeny, Adv. Drug Delivery Rev., 1997, 23, 3–25 CrossRef CAS; (b) M. C. Wenlock, R. P. Austin, P. Barton, A. M. Davis and P. D. A. Leeson, J. Med. Chem., 2003, 46, 1250–1256 CrossRef CAS PubMed; (c) M. J. Waring, Expert Opin. Drug Discovery, 2010, 5, 235–248 CrossRef CAS PubMed.
- F. Pereira and J. Aires-de-Sousa, J. Cheminf., 2018, 10, 43 Search PubMed.
- Gaussian 16, Revision C.01, ed. M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- G. Kigen and G. Edwards, BMC Pharmacol. Toxicol., 2017, 18, 20 CrossRef PubMed.
- I. Hubatsch, E. G. E. Ragnarsson and P. Artursson, Nat. Protoc., 2007, 2, 2111–2119 CrossRef PubMed.
- K. Raniga, A. Nasir, N. T. N. Vo, R. Vaidyanathan, S. Dickerson, S. Hilcove, D. Mosqueira, G. R. Mirams, P. Clements, R. Hicks, A. Pointon, W. Stebbeds, J. Francis and C. Denning, Cell Stem Cell, 2024, 31, 292–311 CrossRef PubMed.
- J. Tamargo, R. Caballero, R. Gómez, C. Valenzuela and E. Delpón, Cardiovasc. Res., 2004, 62, 9–33 CrossRef PubMed.
- M. C. Sanguinetti and N. K. Jurkiewicz, J. Gen. Physiol., 1990, 96, 195–215 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The data that support the findings of this study are available in the ESI of this article. Videos for the worm movement assay are also included. CCDC 2224678. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4md00599f |
| This journal is © The Royal Society of Chemistry 2024 |