Open Access Article
Open Access ArticleRational design of NT-PSMA heterobivalent probes for prostate cancer theranostics†
Santo
Previti
 *ab,
Sacha
Bodin
*ab,
Sacha
Bodin
 cd,
Emmanuelle
Rémond
cd,
Emmanuelle
Rémond
 a,
Delphine
Vimont
c,
Elif
Hindié
cde,
Clément
Morgat
cd and
Florine
Cavelier
a,
Delphine
Vimont
c,
Elif
Hindié
cde,
Clément
Morgat
cd and
Florine
Cavelier
 *a
*a
aPôle Chime Balard, IBMM, UMR 5247 CNRS, Université Montpellier, ENSCM, F-34000 Montpellier, France. E-mail: florine.cavelier@umontpellier.fr; Tel: +33 448792134
bDepartment of Chemical, Biological, Pharmaceutical, and Environmental Sciences, University of Messina, Viale Stagno d'Alcontres 31, 98166 Messina, Italy. E-mail: spreviti@unime.it; Tel: +39 090 676 5669
cCNRS, EPHE, INCIA UMR 5287, University of Bordeaux, F-33400 Talence, France
dDepartment of Nuclear Medicine, CHU Bordeaux, F-33000 Bordeaux, France
eInstitut Universitaire de France, F-75000 Paris, France
First published on 16th September 2024
Abstract
Targeting the prostate-specific membrane antigen (PSMA) with radiopharmaceuticals for imaging and/or therapy has demonstrated significant advancement in the management of prostate cancer patients. However, PSMA targeting remains unsuccessful in prostate cancers with low expression of PSMA, which account for 15% of cases. The neurotensin receptor-1 (NTS1) has been highlighted as a suitable oncotarget for imaging and therapy of PSMA-negative prostate cancer lesions. Therefore, heterobivalent probes targeting both PSMA and NTS1 could improve the prostate cancer management. Herein, we report the development of a branched hybrid probe (JMV 7489) designed to target PSMA and/or NTS1 bearing relevant pharmacophores and DOTA as the chelating agent. The new ligand was synthesized with a hybrid approach, which includes both syntheses in batch and in the solid phase. Saturation binding experiments were next performed on HT-29 and PC3-PIP cells to derive Kd and Bmax values. On the PC3-PIP cells, [68Ga]Ga-JMV 7489 displayed good affinity towards PSMA (Kd = 53 ± 17 nM; Bmax = 1393 ± 29 fmol/106 cells) in the same range as the corresponding reference monomer. A lower affinity value towards NTS1 was depicted (Kd = 157 ± 71 nM; Bmax = 241 ± 42 fmol/106 cells on PC3-PIP cells; Kd = 246 ± 1 nM; Bmax = 151 ± 44 fmol/106 cells on HT-29 cells) and, surprisingly, it was also the case for the corresponding monomer [68Ga]Ga-JMV 7089. These results indicate that the DOTA macrocycle and the linker are critical elements to design heterobivalent probes targeting PSMA and NTS1 with high affinity towards NTS1.
1. Introduction
Prostate cancer is the fifth cause of cancer-related death in men all over the world (375![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases in 2020), with a 5-year survival rate of 31% at the late stage.1,2 Recently, radiolabelled prostate-specific membrane antigen (PSMA) inhibitors have made a spectacular entry in clinical practice both for imaging (with [68Ga]Ga-PSMA-11) and therapy (with [177Lu]Lu-PSMA-617), as shown in Chart 1.3,4 However, PSMA targeting remains unsuccessful in prostate cancers with low expression of PSMA, which account for 15% of cases. To bypass these challenges, the development of hybrid probes addressing two distinct targets expressed by tumoral prostate tissues was found to be a valid approach.5–7 In this context, the neurotensin (NT) system was found to be involved in the growth of prostate cancer cells.8,9 NT is a trideca-neuropeptide that is implicated in the modulation of several physiological pathways, such as the dopaminergic system, pain transmission, and vasodilatation.10 After the release from endocrine N cells, NT acts by binding with NTS1 and NTS2, both belonging to the G-protein coupled receptor family, and NTS3, a sortilin-like receptor.10 NTS1 was found to be overexpressed in androgen-independent human prostate cancer cells, and their growth is positively influenced by subnanomolar concentrations of NT.8,11 In the presence of SR-48692 (Chart 1), a selective NTS1 antagonist, the NT-induced growth of prostate cancer cells is stopped.12 In human samples, NTS1 has been highlighted as a valid oncotarget for imaging and therapy of PSMA-negative prostate cancer lesions.7–9 Several radiolabelled probes have been developed to target NTS1 based on two strategies: non-peptide based probes and NT[8-13] based-probes (Chart 1). Nonpeptide-based radiotracers are intrinsically stable and have been derived from the NTS1 antagonist SR142948A. For diagnostic and therapeutic procedures (theranostics), DOTA chelate was added to the SR142948A structure yielding 3BP-227 (ref. 13), which has been applied in a small series of patients with limited performances due to slow pharmacokinetics with persistent uptake in the blood.14 Recent pre-clinical optimizations of SR142948A hold promise for NTS1-targeting.15,16 Considering the peptide-based probes, they are highly sensitive to in vivo metabolism and several strategies were carried out to increase the stability against endopeptidases, and therefore enhance tumour uptake. The most advanced radiopharmaceutical in terms of human use is DOTA-NT-20.3 radiolabelled with 68Ga, but with low tumour uptake in humans.17 Our consortium published the preclinical results of [68Ga]Ga-JMV 6659 with high tumour uptake on xenografted mice, standing as a potential competitor to [177Lu]Lu-3BP-227 and [68Ga]Ga-DOTA-NT-20.3, pending human application.18
000 cases in 2020), with a 5-year survival rate of 31% at the late stage.1,2 Recently, radiolabelled prostate-specific membrane antigen (PSMA) inhibitors have made a spectacular entry in clinical practice both for imaging (with [68Ga]Ga-PSMA-11) and therapy (with [177Lu]Lu-PSMA-617), as shown in Chart 1.3,4 However, PSMA targeting remains unsuccessful in prostate cancers with low expression of PSMA, which account for 15% of cases. To bypass these challenges, the development of hybrid probes addressing two distinct targets expressed by tumoral prostate tissues was found to be a valid approach.5–7 In this context, the neurotensin (NT) system was found to be involved in the growth of prostate cancer cells.8,9 NT is a trideca-neuropeptide that is implicated in the modulation of several physiological pathways, such as the dopaminergic system, pain transmission, and vasodilatation.10 After the release from endocrine N cells, NT acts by binding with NTS1 and NTS2, both belonging to the G-protein coupled receptor family, and NTS3, a sortilin-like receptor.10 NTS1 was found to be overexpressed in androgen-independent human prostate cancer cells, and their growth is positively influenced by subnanomolar concentrations of NT.8,11 In the presence of SR-48692 (Chart 1), a selective NTS1 antagonist, the NT-induced growth of prostate cancer cells is stopped.12 In human samples, NTS1 has been highlighted as a valid oncotarget for imaging and therapy of PSMA-negative prostate cancer lesions.7–9 Several radiolabelled probes have been developed to target NTS1 based on two strategies: non-peptide based probes and NT[8-13] based-probes (Chart 1). Nonpeptide-based radiotracers are intrinsically stable and have been derived from the NTS1 antagonist SR142948A. For diagnostic and therapeutic procedures (theranostics), DOTA chelate was added to the SR142948A structure yielding 3BP-227 (ref. 13), which has been applied in a small series of patients with limited performances due to slow pharmacokinetics with persistent uptake in the blood.14 Recent pre-clinical optimizations of SR142948A hold promise for NTS1-targeting.15,16 Considering the peptide-based probes, they are highly sensitive to in vivo metabolism and several strategies were carried out to increase the stability against endopeptidases, and therefore enhance tumour uptake. The most advanced radiopharmaceutical in terms of human use is DOTA-NT-20.3 radiolabelled with 68Ga, but with low tumour uptake in humans.17 Our consortium published the preclinical results of [68Ga]Ga-JMV 6659 with high tumour uptake on xenografted mice, standing as a potential competitor to [177Lu]Lu-3BP-227 and [68Ga]Ga-DOTA-NT-20.3, pending human application.18
 | ||
| Chart 1 Structures of [177Lu]Lu-PSMA-617, [68Ga]Ga-PSMA-11, SR-48692, SR142948A, and probes targeting NTS1. The structure of DOTA was solely reported in [177Lu]Lu-PSMA-617. | ||
Overall, the development of hybrid probes targeting both PSMA and NTS1 could improve the management of prostate cancer.7 Starting from all these considerations, herein we report a design study for the synthesis of a novel branched PSMA/NTS1-targeting heterobivalent probe (JMV 7489), which was then radiolabelled with 68Ga and tested in vitro to derive important features for the Structure–Activity Relationship (SAR) study. The in vivo evaluation was not the primary objective of this work. The design of the new hybrid was based on the two pharmacophores of PSMA and NT (Chart 2). With regard to PSMA, the binding region Glu-urea-Lys (also known as EUK), which fits well into the Zn2+ active-sites and S1 and S1′ of PSMA, was maintained. Several linkers between the PSMA binding region and chelating agents have been reported in the literature.19,20 Among these studies, Zha et al. reported in 2018 the evaluation of the radioligand 1 (Chart 2), which contains the O-(carboxymethyl)-Tyr-Phe moiety as a linker.21 The incorporation of this linker was found to be productive in terms of binding affinity towards PSMA, with affinity values in the low nanomolar range. For this reason, the linker of compound 1 was selected to be incorporated in JMV 7489. Regarding the NT arm, we decided to incorporate the minimum sequence for biological activity, namely the C-terminal portion (NT[8-13]), in which the Arg8–Arg9 fragment was replaced with Lys8–Lys9 (JMV 438, Chart 2).22 This substitution is widely known in the literature, since the introduction of Lys instead of Arg is more feasible in terms of synthesis, resulting in analogues with comparable biological properties of NT[8-13].22,23 Two residues of β-Ala were incorporated to the N-terminus as linkers, and a residue of Glu, useful to merge the two pharmacophores, was inserted. The corresponding NT monomer (JMV 7089) was also synthesized for the SAR study. Data available in the literature on the radioligand 1 targeting PSMA is used as a reference.21
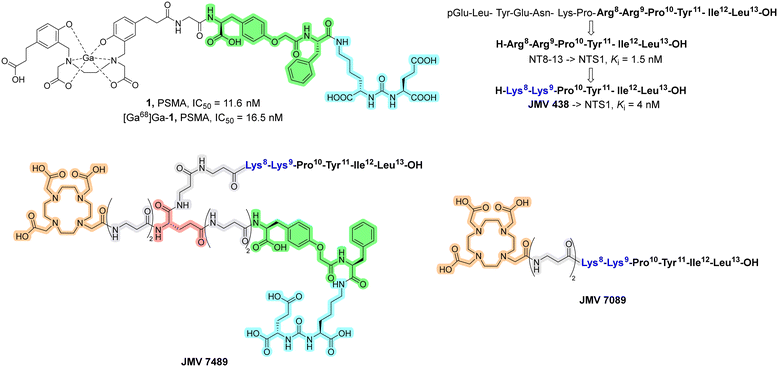 | ||
| Chart 2 Structure and biological activity of compound 1.21 NT and its modified analogues with biological activity towards NTS1.22 Structure of the branched heterobivalent probes JMV 7489 and JMV 7089: DOTA, β-Ala linkers, Glu, O-(carboxymethyl)-Tyr-Phe fragments, and PSMA binding regions are reported in orange, gray, red, green, and light blue, respectively. The NT arm is reported as the amino acid sequence. | ||
2. Results and discussion
Synthetic procedures
After the design of JMV 7489, several synthetic procedures have been evaluated. The possibility to synthesize the hybrid probe by solid-phase peptide synthesis (SPPS) was the priority. Synthesis of both ligands as monomers using SPPS is widely reported in the literature.18,24–29 As both pharmacophores are C-terminal, the Glu and Leu residues of PSMA and NT pharmacophores, respectively, could serve as the first amino acid to be loaded on the resin. At the same time, the presence of two C-terminal portions allowed the use of SPPS for only one pharmacophore. Moreover, JMV 7489 is a branched heterobivalent probe carrying non-peptide bonds. Therefore, the synthesis in batch was also evaluated. Starting from all these points, JMV 7489 was synthesized with a hybrid synthetic approach, which includes both SPPS and synthesis in batch. In particular, the O-(carboxymethyl)-Tyr fragment of compound 1 was synthesized in solution starting from H-Tyr-OtBu 2, whose amino group was coupled with Fmoc-β-Ala-OH in order to incorporate the first N-Fmoc-protected β-Ala residue (Scheme 1). The resulting intermediate 3 was treated with methyl bromoacetate in the presence of K2CO3, and the methyl ester of intermediate 4 was then hydrolysed to the corresponding carboxylic acid 5 under mild conditions, using CaCl2 with NaOH to preserve the Fmoc-protecting group. In parallel, the NT arm was synthesized both in batch and by SPPS (Scheme 1). Considering the need to have the C-terminus protected with acid labile protecting groups, the fragment H-Try(tBu)-Ile-Leu-OtBu 9 was synthesized in solution following the Fmoc-chemistry. The remaining NT arm carrying acid-labile protecting groups and the Glu residue with the γ-COOH as methyl ester, Fmoc-Glu(OMe)-β-Ala-β-Ala-Lys(Boc)-Lys(Boc)-Pro-OH 10 was synthesized by SPPS using the 2-chloro trityl chloride (2-CTC) resin. The cleavage conditions, namely DCM/trifluoroethanol (TFE)/acetic acid (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), allowed us to obtain the intermediate 10, preserving the acid-labile protecting groups. Because of the presence of Pro at the C-terminus of fragment 10, the coupling reaction between the acid 10 and amine 9 provided the nonapeptide 11 without racemization. After purification, the γ-OMe ester of Glu was hydrolysed as described above for intermediate 5, and the resulting carboxylic acid 12 was obtained. The PSMA binding motif was synthesized starting from commercially available Fmoc-Glu(tBu)-OH (Scheme 2), which was loaded on the previously activated 2-CTC resin (loading: 0.77 mmol g−1). Subsequently, the amino group was deprotected with 20% piperidine in DMF, dried, and moved in a round-bottom flask containing the isocyanate intermediate 13, which was obtained by treatment of H-Lys(Fmoc)-OtBu with triphosgene in the presence of triethylamine (TEA). After that, the resin was moved in an SPPS syringe, treated with 20% piperidine in DMF, and Fmoc-Phe-OH was coupled. After the Fmoc-removal, fragment 5 was coupled, followed by a further incorporation of Fmoc-β-Ala-OH.
1), allowed us to obtain the intermediate 10, preserving the acid-labile protecting groups. Because of the presence of Pro at the C-terminus of fragment 10, the coupling reaction between the acid 10 and amine 9 provided the nonapeptide 11 without racemization. After purification, the γ-OMe ester of Glu was hydrolysed as described above for intermediate 5, and the resulting carboxylic acid 12 was obtained. The PSMA binding motif was synthesized starting from commercially available Fmoc-Glu(tBu)-OH (Scheme 2), which was loaded on the previously activated 2-CTC resin (loading: 0.77 mmol g−1). Subsequently, the amino group was deprotected with 20% piperidine in DMF, dried, and moved in a round-bottom flask containing the isocyanate intermediate 13, which was obtained by treatment of H-Lys(Fmoc)-OtBu with triphosgene in the presence of triethylamine (TEA). After that, the resin was moved in an SPPS syringe, treated with 20% piperidine in DMF, and Fmoc-Phe-OH was coupled. After the Fmoc-removal, fragment 5 was coupled, followed by a further incorporation of Fmoc-β-Ala-OH.
Thus, the NT arm 12 was coupled: although this coupling was performed between two large peptide chains, the desired product turned out to be the main peak (around 65%) in LC-MS analysis. The introduction of fragments 5 and 12 can be considered as the crucial step of the synthetic pathway. After that, two additional residues of Fmoc-β-Ala-OH and DOTA(tBu)3-OH were incorporated. Lastly, the peptide was cleaved from the resin using a mixture of TFA/TIS/H2O (95/2.5/2.5) for 7 h and purified by preparative HPLC.
In vitro characterization
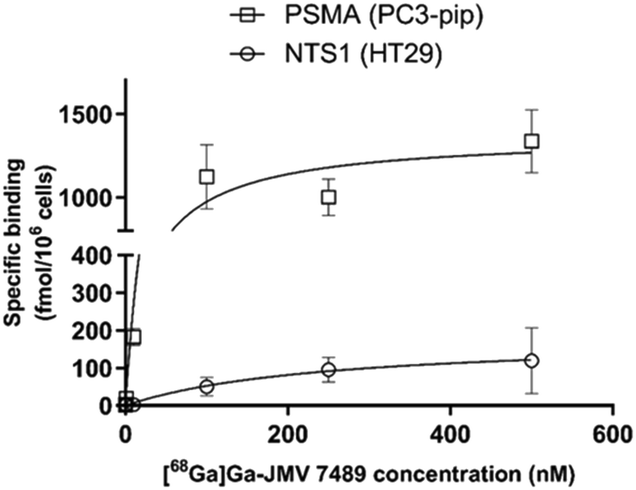
JMV 7489 was next radiolabelled with the positron-emitter 68Ga (mean activity at end-of-synthesis of 340 ± 136 MBq) with good decay-corrected yield of 62.8 ± 20.5%. The apparent molar activity was 16.6 ± 6.6 GBq μmol−1. The hydrophilicity was −3.93 ± 0.42 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4), similar to other peptide-based radiopharmaceuticals.18 Saturation binding experiments were then performed on HT-29 (NTS1+/PSMA−) and PC3-PIP cells (NTS1+/PSMA+) to derive Kd and Bmax values (Fig. 1). Both HT-29 and PC3-PIP cells were obtained from the University of Bordeaux (FR). On the PC3-PIP cells, [68Ga]Ga-JMV 7489 displayed good affinity towards PSMA (Kd = 53 ± 17 nM; Bmax = 1393 ± 29 fmol/106 cells) and lower affinity towards NTS1 (Kd = 157 ± 71 nM; Bmax = 241 ± 42 fmol/106 cells). Similar values were obtained regarding NTS1-binding on HT-29 cells (Kd = 246 ± 1 nM; Bmax = 151 ± 44 fmol/106 cells). Full displacement of the probe was observed in the saturation binding studies when using 10 μM of NT or 10 μM 2-PMPA to compete with the binding, suggesting full-specificity of the bindings at NTS1 and PSMA. Compared with the PSMA monomer (compound 1), the affinity value of [68Ga]Ga-JMV 7489 towards PSMA is in the same range, suggesting limited impact of the NT moiety in the PSMA binding of [68Ga]Ga-JMV 7489.
D7.4), similar to other peptide-based radiopharmaceuticals.18 Saturation binding experiments were then performed on HT-29 (NTS1+/PSMA−) and PC3-PIP cells (NTS1+/PSMA+) to derive Kd and Bmax values (Fig. 1). Both HT-29 and PC3-PIP cells were obtained from the University of Bordeaux (FR). On the PC3-PIP cells, [68Ga]Ga-JMV 7489 displayed good affinity towards PSMA (Kd = 53 ± 17 nM; Bmax = 1393 ± 29 fmol/106 cells) and lower affinity towards NTS1 (Kd = 157 ± 71 nM; Bmax = 241 ± 42 fmol/106 cells). Similar values were obtained regarding NTS1-binding on HT-29 cells (Kd = 246 ± 1 nM; Bmax = 151 ± 44 fmol/106 cells). Full displacement of the probe was observed in the saturation binding studies when using 10 μM of NT or 10 μM 2-PMPA to compete with the binding, suggesting full-specificity of the bindings at NTS1 and PSMA. Compared with the PSMA monomer (compound 1), the affinity value of [68Ga]Ga-JMV 7489 towards PSMA is in the same range, suggesting limited impact of the NT moiety in the PSMA binding of [68Ga]Ga-JMV 7489.
This value is also 3-fold greater than for other NT-PSMA hybrids5 and is similar to PSMA monomers used in the clinic.30 Regarding the NT pharmacophore, the affinity value was however decreased compared to our previous series of monomers.18 To better understand this finding, the corresponding NT monomer (JMV 7089, i.e. DOTA-β-Ala-β-Ala-JMV438) was synthesized, radiolabelled with 68Ga, and investigated on HT-29 cells. All details of the synthesis, radiolabelling, and quality control of JMV 7089 and [68Ga]Ga-JMV 7089 were reported in the ESI.† Surprisingly, the affinity value of [68Ga]Ga-JMV 7089 was also decreased (Kd = 154.7 ± 13.2 nM; Bmax = 144 ± 52 fmol/106 cells) compared to JMV438 (ref. 22) (39-fold decrease) meaning that the linker and the DOTA macrocycle are mainly responsible for the loss of affinity. Therefore, the integration of the NT-pharmacophore into the DOTA-β-Ala-β-Ala-NT-PSMA heterobivalent probe was quite well tolerated at NTS1 (1.6-fold decrease in affinity only compared with the monomer).
Overall, the DOTA macrocycle and the linker appear as critical structures to design NT-PSMA branched hybrid probes with high affinity towards NTS1. Table 1 below summarized the affinity values of the various compounds used for the design of [68Ga]Ga-JMV 7489.
3. Materials and methods
Chemical synthesis, characterization, and biological evaluation methods are fully described in the ESI.†4. Conclusions
In this original article, the synthesis and biological investigation of a radiolabelled, branched PSMA/NTS1 heterobivalent probe is reported. The new dual targeting agent was synthesized with a hybrid synthetic pathway, which includes both synthesis in batch and the solid phase. This approach resulted to be feasible and the designed hybrid was obtained in good yields. The synthetic method herein described lends itself well to the development of branched heterobivalent probes harbouring any type of substituent and pseudo-peptide bonds. The biological evaluation of the radiolabelled compound revealed that the binding towards PSMA is preserved and showed good affinity. Regarding NTS1 binding, we observed that the DOTA macrocycle and the linker are critical moieties that interfere the most with the receptor binding.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
SP: writing – original draft, investigation, and methodology; SB: investigation and methodology; ER: resources, writing – review & editing; EH: writing – review & editing; CM: conceptualization, funding acquisition, supervision, writing – review & editing; FC: conceptualization, funding acquisition, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Institut National du Cancer (INCa PLBIO 2017, THERACAN project) and was achieved in the framework of the NewMOON network from the University of Bordeaux.Notes and references
- P. Rawla, World J. Oncol., 2019, 10, 63–89 CrossRef CAS PubMed.
- Prostate Cancer: Statistics, https://www.cancer.net/cancer-types/prostate-cancer/statistics, (accessed June 15 2024).
- M. J. Roberts, T. Maurer, M. Perera, M. Eiber, T. A. Hope, P. Ost, S. Siva, M. S. Hofman, D. G. Murphy, L. Emmett and W. P. Fendler, Nat. Rev. Urol., 2023, 20, 23–47 CrossRef PubMed.
- O. Sartor, J. de Bono, K. N. Chi, K. Fizazi, K. Herrmann, K. Rahbar, S. T. Tagawa, L. T. Nordquist, N. Vaishampayan, G. El-Haddad, C. H. Park, T. M. Beer, A. Armour, W. J. Perez-Contreras, M. DeSilvio, E. Kpamegan, G. Gericke, R. A. Messmann, M. J. Morris and B. J. Krause, N. Engl. J. Med., 2021, 385, 1091–1103 CrossRef CAS PubMed.
- X. Ma, M. Wang, H. Wang, T. Zhang, Z. Wu, M. V. Sutton, V. V. Popik, G. Jiang and Z. Li, Bioconjugate Chem., 2019, 30, 1314–1322 CrossRef CAS PubMed.
- B. Mitran, Z. Varasteh, A. Abouzayed, S. S. Rinne, E. Puuvuori, M. De Rosa, M. Larhed, V. Tolmachev, A. Orlova and U. Rosenstrom, Cancers, 2019, 11, 1371 CrossRef CAS PubMed.
- R. Schollhammer, M. L. Quintyn Ranty, H. de Clermont Gallerande, F. Cavelier, I. E. Valverde, D. Vimont, E. Hindie and C. Morgat, Cancers, 2023, 15, 2345 CrossRef CAS PubMed.
- C. Morgat, A. Chastel, V. Molinie, R. Schollhammer, G. Macgrogan, V. Velasco, B. Malavaud, P. Fernandez and E. Hindie, Int. J. Mol. Sci., 2019, 20, 1721 CrossRef CAS PubMed.
- T. He, M. Wang, H. Wang, H. Tan, Y. Tang, E. Smith, Z. Wu, W. Liao, S. Hu and Z. Li, Eur. J. Nucl. Med. Mol. Imaging, 2019, 46, 2199–2207 CrossRef CAS PubMed.
- P. Sarret and F. Cavelier, Reference Module in Neuroscience and Biobehavioral Psychology, 2018, pp. 1–17 Search PubMed.
- G. P. Amorino, P. D. Deeble and S. J. Parsons, Oncogene, 2007, 26, 745–756 CrossRef CAS PubMed.
- L. Seethalakshmi, S. P. Mitra, P. R. Dobner, M. Menon and R. E. Carraway, Prostate, 1997, 31, 183–192 CrossRef CAS PubMed.
- J. Schulz, M. Rohracker, M. Stiebler, J. Goldschmidt, O. S. Grosser, F. Osterkamp, A. Pethe, U. Reineke, C. Smerling and H. Amthauer, J. Nucl. Med., 2016, 57, 1120–1123 CrossRef CAS PubMed.
- R. P. Baum, A. Singh, C. Schuchardt, H. R. Kulkarni, I. Klette, S. Wiessalla, F. Osterkamp, U. Reineke and C. Smerling, J. Nucl. Med., 2018, 59, 809–814 CrossRef CAS PubMed.
- G. O. Fonseca Cabrera, X. Ma, W. Lin, T. Zhang, W. Zhao, L. Pan, X. Li, T. E. Barnhart, E. Aluicio-Sarduy, H. Deng, X. Wu, K. P. Rakesh, Z. Li, J. W. Engle and Z. Wu, J. Nucl. Med., 2024, 65, 1250–1256 CrossRef PubMed.
- T. Zhang, X. Ma, M. Xu, J. Cai, J. Cai, Y. Cao, Z. Zhang, X. Ji, J. He, G. O. F. Cabrera, X. Wu, W. Zhao, Z. Wu, J. Xie and Z. Li, Eur. J. Nucl. Med. Mol. Imaging, 2024, 51, 3322–3333 CrossRef CAS PubMed.
- M. Hodolic, W. Y. Wu, Z. Zhao, F. Yu, I. Virgolini and F. Wang, Eur. J. Nucl. Med. Mol. Imaging, 2021, 48, 1229–1234 CrossRef CAS PubMed.
- R. Fanelli, A. Chastel, S. Previti, E. Hindie, D. Vimont, P. Zanotti-Fregonara, P. Fernandez, P. Garrigue, F. Lamare, R. Schollhammer, L. Balasse, B. Guillet, E. Rémond, C. Morgat and F. Cavelier, Bioconjugate Chem., 2020, 31, 2339–2349 CrossRef CAS PubMed.
- A. Abouzayed, K. Seitova, F. Lundmark, V. Bodenko, M. Oroujeni, V. Tolmachev, U. Rosenstrom and A. Orlova, Front. Oncol., 2023, 13, 1221103 CrossRef CAS PubMed.
- G. Capasso, A. Stefanucci and A. Tolomeo, Eur. J. Med. Chem., 2024, 263, 115966 CrossRef CAS PubMed.
- Z. Zha, K. Ploessl, S. R. Choi, Z. Wu, L. Zhu and H. F. Kung, Nucl. Med. Biol., 2018, 59, 36–47 CrossRef CAS PubMed.
- S. Previti, M. Vivancos, E. Rémond, S. Beaulieu, J. M. Longpre, S. Ballet, P. Sarret and F. Cavelier, Front. Chem., 2020, 8, 406 CrossRef CAS PubMed.
- S. Previti, M. Desgagne, D. Tourwe, F. Cavelier, P. Sarret and S. Ballet, J. Pept. Sci., 2023, 29, e3471 CrossRef CAS PubMed.
- Y. Jia, W. Shi, Z. Zhou, N. K. Wagh, W. Fan, S. K. Brusnahan and J. C. Garrison, Nucl. Med. Biol., 2015, 42, 816–823 CrossRef CAS PubMed.
- L. Schindler, J. Moosbauer, D. Schmidt, T. Spruss, L. Gratz, S. Ludeke, F. Hofheinz, S. Meister, B. Echtenacher, G. Bernhardt, J. Pietzsch, D. Hellwig and M. Keller, Cancers, 2022, 14, 4922 CrossRef CAS PubMed.
- J. Carlos Dos Santos, B. Beijer, U. Bauder-Wust, M. Schafer, K. Leotta, M. Eder, M. Benesova, C. Kleist, F. Giesel, C. Kratochwil, K. Kopka, U. Haberkorn and W. Mier, J. Nucl. Med., 2020, 61, 70–79 CrossRef PubMed.
- M. Mosayebnia, S. Rezaeianpour, P. Rikhtechi, Z. Hajimahdi, D. Beiki, F. Kobarfard, O. Sabzevari, M. Amini, K. Abdi and S. Shahhosseini, Iran. J. Pharm. Res., 2018, 17, 917–926 CAS.
- M. Mosayebnia, Z. Hajimahdi, D. Beiki, M. Rezaeianpour, M. Hajiramezanali, P. Geramifar, O. Sabzevari, M. Amini, D. Hatamabadi and S. Shahhosseini, Bioorg. Chem., 2020, 99, 103743 CrossRef CAS PubMed.
- S. Bodin, S. Previti, E. Jestin, D. Vimont, I. Ait-Arsa, F. Lamare, E. Rémond, E. Hindie, F. Cavelier and C. Morgat, ACS Omega, 2023, 8, 6994–7004 CrossRef CAS PubMed.
- E. Gourni, C. Canovas, V. Goncalves, F. Denat, P. T. Meyer and H. R. Maecke, PLoS One, 2015, 10, e0145755 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00491d |
| This journal is © The Royal Society of Chemistry 2024 |