Open Access Article
Open Access ArticleEffect of CO2 release behavior on the crosslinking degree of alginate hydrogels prepared with CaCO3 and carbonated water†
Ryota
Teshima
 *a,
Shigehito
Osawa‡
*a,
Shigehito
Osawa‡
 bc,
Kaoru
Hirose
bc,
Kaoru
Hirose
 d,
Yayoi
Kawano§
d,
Yayoi
Kawano§
 d,
Akihiko
Kikuchi
d,
Akihiko
Kikuchi
 e,
Takehisa
Hanawa
e,
Takehisa
Hanawa
 d and
Hidenori
Otsuka
d and
Hidenori
Otsuka
 abc
abc
aDepartment of Chemistry, Graduate School of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku, Tokyo 162-8601, Japan. E-mail: 1322595@ed.tus.ac.jp
bDepartment of Applied Chemistry, Faculty of Science, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku, Tokyo 162-8601, Japan
cWater Frontier Research Center (WaTUS), Research Institute for Science and Technology, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku, Tokyo 162-8601, Japan
dDepartment of Pharmacy, Faculty of Pharmaceutical Sciences, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan
eDepartment of Materials Science and Technology, Faculty of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika, Tokyo 125-8585, Japan
First published on 5th June 2024
Abstract
Carbonated water facilitates the gelation of alginate and CaCO3 solutions. The rapid release of CO2 from the hydrogels after gelation resulted in a faster pH increase, lower amount of CaCO3 dissolution, and lower crosslinking degree. The CO2 release behavior, i.e., “post-gelation condition,” affects the degree of crosslinking, an essential parameter for determining hydrogel properties.
Hydrogels—soft materials containing water molecules in a three-dimensional network structure of crosslinked polymers—are widely applied in life sciences.1–3 In particular, hydrogels composed of biopolymers such as polysaccharides4,5 and proteins6,7 have attracted attention owing to their safety, biocompatibility, and ability to form supramolecular assemblies. These biopolymers can be physically crosslinked by changing the solution environment, such as the pH.8,9 Hydrogels based on physical crosslinking have been widely studied for biomedical applications. However, when the pH of the entire solution is reduced by adding acidic agents, the acidic agents often remain in the hydrogel and cause acidification, which can harm living organisms.10
Recently, a new gelation system introducing carbon dioxide (CO2) gas into biopolymer solutions has attracted attention.11–18 In this gelation method, CO2 functions as an acidic agent that reduces the system's pH and is released into the atmosphere after gelation, preventing acidification of the hydrogel. Floren et al. successfully gelled silk fibroin without acidification by introducing high-pressure CO2.15 In commonly used methods, polysaccharides such as alginate or pectin are crosslinked with Ca2+via physical interactions with the carboxy groups of the polymer using CaCO3 as a Ca2+ source;11–13 Gurikov et al. demonstrated high-pressure CO2 into a mixture of alginate and CaCO3 to reduce the pH of the solution and produce Ca2+ from CaCO3 for forming an alginate hydrogel.11 Hydrogels prepared with high-pressure CO2 have a controlled porous structure composed of CO2 that can be applied as an effective cell scaffold16,17 because it aids in nutrient penetration and cell migration. In addition, this gelation method can be hybridized with other biopolymers, such as lignin16 and carrageenan,18 to achieve enhanced functionality. The effects of CO2 introduction and release conditions on hydrogel properties have been studied to broaden the hydrogel application scope but are not fully understood. In particular, the effect of the CO2 release behavior after gelation, that is, the post-gelation conditions, is unclear because of the preconceived notion that the hydrogel properties are controlled by pre-gelation conditions, such as polymer and crosslinker concentrations. Martins et al. examined the effect of the CO2 depressurization rate on the hydrogel structure, revealing that faster depressurization corresponded to a higher degree of CO2 gas expansion and complicated the comparison of hydrogel physicochemical properties, such as the crosslinking degree.17 In addition, the effects of the depressurization rate on the mechanical structure of hydrogels remain debatable,16,17 as pressure changes complicate the physicochemical comparison of the hydrogel structures. An understanding of the effects of CO2 release conditions on hydrogel properties, especially the crosslinking degree, would greatly expand the hydrogel application scope from the materials field to the medical field, because the crosslinking degree affects both the physicochemical (e.g., mechanical strength and swelling ratio) and biological (e.g., cell migration) properties of hydrogels.
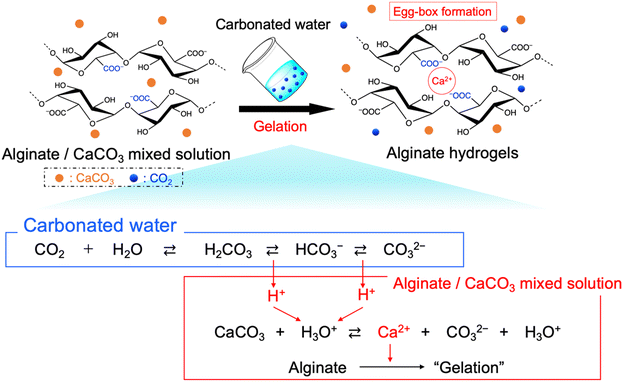
In this study, we prepared the alginate hydrogels (Alg-gel) with CaCO3 and carbonated water and investigated the effect of CO2 release from the hydrogels on the degree of crosslinking. The gelation mechanism of the alginate with CaCO3 and carbonated water is shown in Fig. 1. In previously reported gelation methods using high-pressure CO2,11–18 precise control of the CO2 pressure was essential for controlling the physicochemical properties of hydrogels, but this makes comparison of hydrogel properties difficult. In contrast, our method enabled facile hydrogel synthesis under ambient conditions and, hence, the comparison of the physicochemical properties of the hydrogels without the effect of CO2 gas expansion. As previously reported,19–21 Alg-gels were prepared with carbonated water. The relationship between the concentration of calcium-binding units in alginate (concentration of carboxy groups in guluronic acid) and CaCO3 is shown in Table S1 (ESI†). When carbonated water with the pH of 3.64 ± 0.06 (n = 3, mean ± standard deviation)20 was added to the alginate/CaCO3 mixed solution, an Alg-gel was obtained within 4 min (Fig. S1a, ESI†). A scanning electron microscopy (SEM) image of the cross-section of the Alg-gel showed a porous structure, indicating that the alginate was crosslinked by Ca2+ (Fig. S1b, ESI†). Furthermore, the phase-contrast image of the Alg-gel revealed the CaCO3 crystals in the hydrogel (Fig. S1c, ESI†). To control the release of CO2 from the Alg-gel and CaCO3 dissolution, we adjusted the contact area between the Alg-gel surface and the atmosphere. Specifically, we prepared two types of Alg-gels with different air contact areas (Fig. 2). First, Alg-gel sheet was prepared and incubated in Petri dish such that only the top surface of the gel was in contact with the atmosphere. The Alg-gel sheet was cut into 17.5-mm disks for evaluation. Second, Alg-gel sheet was prepared in the same manner, hollowed out into a 17.5-mm disk 10 min after preparation, and incubated on a wire mesh so that the entire surface of the gel was in contact with the atmosphere.
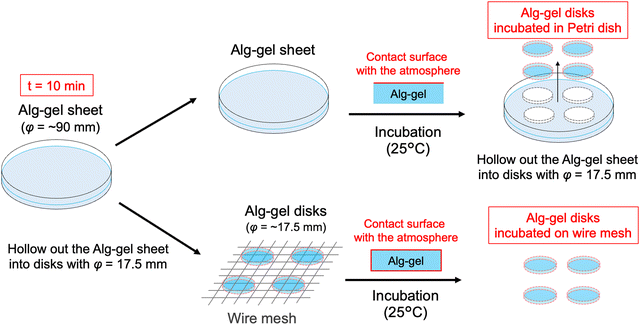 | ||
| Fig. 2 Schematic of Alg-gel disks incubated in a Petri dish and on a wire mesh. t = 0 refers to the time as carbonated water was added. | ||
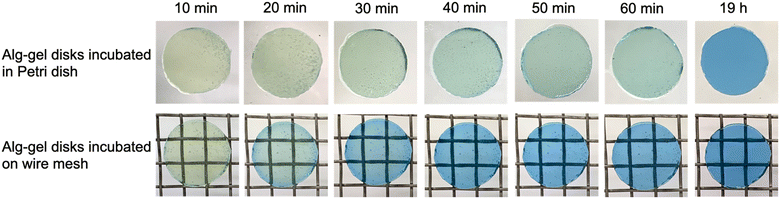
To visually capture the release behavior of CO2 from the Alg-gel, the Alg-gel was stained with bromothymol blue (BTB) as a pH indicator, which appeared yellow, green, and blue under acidic, neutral, and alkaline conditions, respectively (Fig. 3). The Alg-gel disk incubated in the Petri dish changed slightly to neutral over 60 min, indicating that CO2 was released slowly into the atmosphere. However, the Alg-gel disk incubated on the wire mesh became alkaline at t = 20 min, and the entire gel disk became alkaline at t = 40 min. These results indicated that the CO2 in the Alg-gel disk incubated on the wire mesh was released quickly into the atmosphere because all the surfaces of the gel were in contact with the atmosphere. Thereafter, the Alg-gel disk incubated in the Petri dish turned completely blue. Interestingly, the color change of the Alg-gel disk incubated on the wire mesh began around the gel disk. Details regarding the behavior of CO2 release from each surface would be interesting to investigate in the future.
To quantitatively evaluate the pH change behavior shown in Fig. 3, the surface pH values of the Alg-gels in the Petri dish and on the wire mesh were measured over 300 min (Fig. 4). The difference in surface pH between the Alg-gel surface exposed to the atmosphere—denoted as the front surface—and that in contact with the bottom of the dish—denoted as the back surface—was examined (Fig. S2, ESI†). Unfortunately, the pH of the side surface of the Alg-gel disk could not be measured, because the side surface area was too small. The surface pH values of the Alg-gel disks incubated in a Petri dish are shown in Fig. 4a. The pH of the front surface increased faster than that of the back surface. The release of CO2 from the Alg-gel disk incubated in the Petri dish delayed the pH change on the back surfaces. Notably, no pH gradient appeared in the mixture of CaCO3 and carbonated water without alginate, implying that carbonate ions and CO2 exhibited different behaviors in the hydrogels.20 At t = 300 min, the pH values of the front and back surfaces were almost equal (8.5), indicating that all the CO2 in the Alg-gel disk was released. In contrast, different release behavior of CO2 was observed for the Alg-gel disks incubated on wire mesh (Fig. 4b). At t = 10 min, the pH of the Alg-gel disks was higher on the front surface than on the back surface because the gel disks were incubated in Petri dishes. However, at t = 30 min, the pH values of the front and back surfaces were identical. Thus, the Alg-gel disks incubated on the wire mesh immediately exhibited no delay in pH change because the front and back surfaces of the gel disk were in contact with the atmosphere. At t = 90 min, the pH values of the front and back surfaces converged to 8.5, as indicated by the BTB color change shown in Fig. 3. In addition, the pH of the front surface of the Alg-gel disks incubated on the wire mesh increased faster than those of the disks incubated in the Petri dish. This may be due to the release of CO2 from the entire surface of Alg-gel disks incubated on the wire mesh, as shown in Fig. 3. These results suggest that the Alg-gel disks on the wire mesh released CO2 faster into the atmosphere than those in the Petri dish.
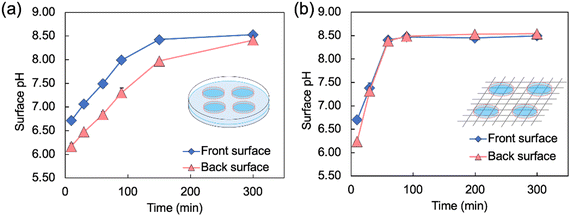 | ||
| Fig. 4 Changes in the surface pH of Alg-gel disks incubated (a) in a Petri dish* and (b) on a wire mesh over time (t = 0 refers to the time when carbonated water was added). The thickness of the Alg-gel disks was 3.46 ± 0.26 mm. Values are expressed as the mean ± SE obtained from n = 3–4. * This result has been reproduced from previous our study.19 | ||
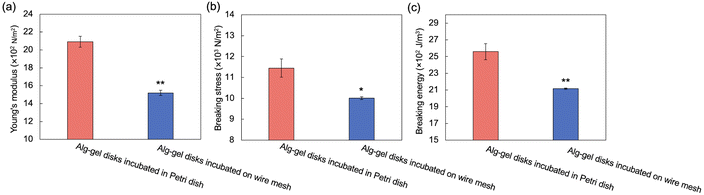
The incubation method of the Alg-gel disks influences the release behavior of CO2 from the gel disks, which motivated us to compare the degrees of crosslinking. The rapid release of CO2 from the hydrogels after gelation may result in lower amount of CaCO3 dissolution, and lower crosslinking degree. As shown in Fig. 3 and 4, for the Alg-gel disks incubated on the wire mesh, CO2 release was complete after only 90 min. Therefore, the disks were incubated on the wire mesh for 90 min to achieve complete CO2 release and then transferred to the Petri dish to prevent moisture loss. The procedure used to prepare the Alg-gel disks for mechanical evaluations is shown in Fig. 5. As shown in Fig. S3 (ESI†), the thicknesses of the disks incubated in the Petri dish and on the wire mesh were almost the same. After adjusting the conditions, Young's moduli of the Alg-gel disks were measured via a compression test (Fig. 6a). The Young's modulus was calculated from the slope of 3% compressive strain, and the Young's modulus of Alg-gel disks incubated in the Petri dish were significantly higher than those of Alg-gel disks incubated on the wire mesh. Furthermore, we investigated the mechanical properties of the Alg-gel disks incubated in a Petri dish and wire mesh. The Alg-gel disks were compressed to 87% strain, and the breaking stress and energy were calculated. All the stress–strain curves are shown in Fig. S4 (ESI†). The breaking stress of the Alg-gel disks incubated in the Petri dish was significantly higher than that of the disks incubated on the wire mesh (Fig. 6b). Additionally, the breaking energy of the disks incubated in the Petri dish was significantly higher than that of the disks incubated on the wire mesh (Fig. 6c). These results indicate that the degree of crosslinking differed between the Alg-gel disks incubated in the Petri dish and on the wire mesh and that the behavior of CO2 release from the Alg-gel affected the degree of crosslinking. Additionally, the Alg-gel disk incubated on the wire mesh had a higher swelling ratio than that incubated in the Petri dish (Fig. 7). These results indicate that the degree of crosslinking of the Alg-gel disks incubated on the wire mesh was lower than that of the Alg-gel disks incubated in the Petri dish. Therefore, the rapid release of CO2 from the hydrogels after gelation resulted in faster pH increase, lower amount of CaCO3 dissolution, and lower crosslinking degree.
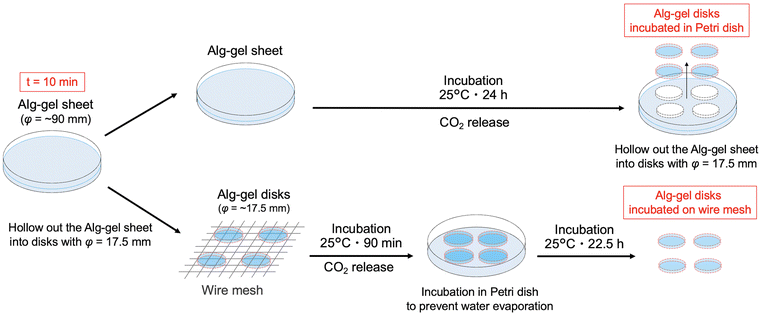 | ||
| Fig. 5 Schematic preparation of the Alg-gel disks incubated in the Petri dish and on the wire mesh for mechanical evaluations. t = 0 refers to the time as carbonated water was added. | ||
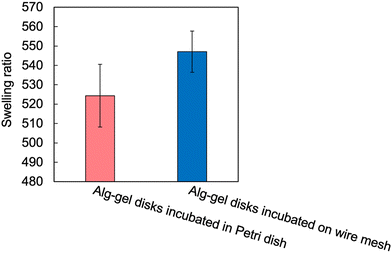 | ||
| Fig. 7 Swelling ratio of Alg-gel disks in a Petri dish and on a wire mesh. Values are expressed as the mean ± SE obtained from n = 5–6. | ||
In general, the degree of hydrogel crosslinking is an important parameter governing physicochemical properties, such as the swelling ratio and mechanical strength, and biological properties, such as the migration of encapsulated cells. The degree of crosslinking of hydrogels tends to be controlled by “pre-gelation parameters” such as the polymer and crosslinker concentrations. However, the crosslinking degree of the hydrogels prepared with CO2 as the acidic agent and CaCO3 as the crosslinking agent source was affected by post-gelation conditions, namely the contact area between the hydrogel and the atmosphere after gelation. The rapid release of CO2 from the hydrogels after gelation lowered the system's pH and reduced the crosslinking degree. In future studies, the polymer network structure and potential applications of these hydrogels as functional materials should be examined in detail. Especially, the molecular weight of alginate may affect these properties and applications. In addition, elucidating the mass balance and kinetics of the gelation reaction may contribute to the control of gelation reactions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Kimica Corp. (Tokyo, Japan) for providing alginates. This work was supported by Grants-in-Aid for Research Activities from the Masason Foundation [grant numbers: GD14469, GD9675, and GD2825] and Grants-in-Aid for Extracurricular Activities for Students at Tokyo University of Science Parents Associations (Kouyoukai) [grant number: 2019-15].References
- X. Liu, S. Zhou, B. Cai, Y. Wang, D. Deng and X. Wang, Biomater. Sci., 2022, 10, 3480 RSC.
- S. H. Jeong, M. Kim, T. Y. Kim, H. Kim, J. H. Ju and S. K. Hahn, ACS Appl. Bio Mater., 2020, 3, 5040 CrossRef CAS PubMed.
- H. Mamada, A. Kemmochi, T. Tamura, Y. Shimizu, Y. Owada, Y. Ozawa, K. Hisakura, T. Oda, N. Ohkohchi, Y. Kawano and T. Hanawa, Polym. Adv. Technol., 2022, 33, 125 CrossRef CAS.
- C. J. Maxwell, A. M. Soltisz, W. W. Rich, A. Choi, M. A. Reilly and K. E. Swindle-Reilly, J. Biomed. Mater. Res. A, 2022, 110, 1621 CrossRef CAS PubMed.
- F. Marin, M. C. Tanzi and P. Petrini, Int. J. Biol. Macromol., 2012, 51, 681 CrossRef PubMed.
- Ł. Mazurek, M. Szudzik, M. Rybka and M. Konop, Biomolecules, 2022, 12, 1852 CrossRef PubMed.
- S. Indrakumar, A. Joshi, T. K. Dash, V. Mishra, B. Tandon and K. Chatterjee, Int. J. Biol. Macromol., 2023, 233, 123569 CrossRef CAS PubMed.
- A. E. Terry, D. P. Knight, D. Porter and F. Vollrath, Biomacromolecules, 2004, 5, 768 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Prog. Polym., 2012, 37, 106 CrossRef CAS PubMed.
- H. R. Moreira, F. Munarin, R. Gentilini, L. Visai, P. L. Granja, M. C. Tanzi and P. Petrini, Carbohydr. Polym., 2014, 103, 339 CrossRef CAS PubMed.
- P. Gurikov, S. P. Raman, D. Weinrich, M. Fricke and I. Smirnova, RSC Adv., 2015, 5, 7812 RSC.
- I. Preibisch, L. M. Ränger, P. Gurikov and I. Smirnova, Gels, 2020, 6, 28 CrossRef CAS PubMed.
- P. Gurikov and I. Smirnova, Gels, 2018, 4, 14 CrossRef PubMed.
- S. Napavichayanun, W. Bonani, Y. Yang, A. Motta and P. Aramwit, Adv. Wound Care, 2019, 8, 452 CrossRef PubMed.
- M. L. Floren, S. Spilimbergo, A. Motta and C. Migliaresi, Biomacromolecules, 2012, 13, 2060 CrossRef CAS PubMed.
- S. Quraishi, M. Martins, A. A. Barros, P. Gurikov, S. P. Raman, I. Smirnova, A. R. C. Duarte and R. L. Reis, J. Supercrit. Fluids, 2015, 105, 1 CrossRef CAS.
- M. Martins, A. A. Barros, S. Quraishi, P. Gurikov, S. P. Raman, I. Smirnova, A. R. C. Duarte and R. L. Reis, J. Supercrit. Fluids, 2015, 106, 152 CrossRef CAS.
- M. Alnaief, B. Mohammad, M. Aljarrah and R. M. Obaidat, Jordan J. Phys., 2019, 12, 79 Search PubMed.
- R. Teshima, S. Osawa, M. Yoshikawa, Y. Kawano, H. Otsuka and T. Hanawa, Int. J. Biol. Macromol., 2024, 254, 127928 CrossRef CAS PubMed.
- R. Teshima, S. Osawa, Y. Kawano, T. Hanawa, A. Kikuchi and H. Otsuka, ACS Omega, 2023, 8, 7800 CrossRef CAS PubMed.
- R. Teshima, Y. Kawano, T. Hanawa and A. Kikuchi, Polym. Adv. Technol., 2020, 31, 3032 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, molar concentration of alginate and CaCO3, SEM and phase-contrast image of Alg-gel, definition of Alg-gel surfaces, thickness changes of Alg-gel and stress–strain curves of Alg-gel. See DOI: https://doi.org/10.1039/d4ma00257a |
| ‡ Current affiliation: Department of Biomedical Engineering, Faculty of Life Sciences, Toyo University, 48-1 Oka, Asaka, Saitama 351-8510, Japan. |
| § Current affiliation: Department of Clinical Pharmacy, Graduate School of Pharmaceutical Sciences, Nagoya City University, 3-1 Tanabe-dori, Mizuho, Nagoya 467-8603, Japan. |
| This journal is © The Royal Society of Chemistry 2024 |