Open Access Article
Open Access ArticleA tailored polyoxometalate-derived RuW/g-C3N4-based electrocatalyst for enhanced hydrogen evolution reaction†
Soyeb
Pathan
 *ab,
Menon
Ankitha
*ab,
Menon
Ankitha
 c,
Ajith Arjun
Mohan
c,
Ajith Arjun
Mohan
 d,
Neermunda
Shabana
c,
Yongfeng
Tong
e and
P. Abdul
Rasheed
d,
Neermunda
Shabana
c,
Yongfeng
Tong
e and
P. Abdul
Rasheed
 *cd
*cd
aResearch and Development Cell (RDC), Parul Institute of Applied Sciences, Parul University, Waghodia, Vadodara, Gujarat 391760, India. E-mail: khan_9751@yahoo.com
bDepartment of Chemistry, Parul Institute of Applied Sciences, Parul University, Waghodia, Vadodara, Gujarat 391760, India
cDepartment of Chemistry, Indian Institute of Technology Palakkad, Palakkad, Kerala 678 557, India
dDepartment of Biological Sciences and Engineering, Indian Institute of Technology Palakkad, Palakkad, Kerala 678 557, India. E-mail: abdulrasheed@iitpkd.ac.in
eQatar Environment and Energy Research Institute, Hamad Bin Khalifa University, P.O. box 5825, Doha, Qatar
First published on 21st November 2023
Abstract
Graphitic carbon nitride (g-C3N4) and its composites for the hydrogen evolution reaction (HER) have gained immense attention owing to their facile synthesis technique and favorable catalytic performance. Herein we present a composite prepared using a g-C3N4 and H3PW12O40 polyoxometalate (POM) electrocatalyst synthesized by a novel approach for HER application. The electrocatalyst is synthesized by the complexation of a tailored POM, i.e. Ru-substituted polyoxometalate (PW11Ru), with melamine followed by calcination under an inert atmosphere. The proposed monomer complexation strategy utilizing well-defined modulated PW11Ru as a metal precursor provided a unique approach for the preparation of a highly dispersed matrix to form a RuW/g-C3N4 composite. The HER activity of the obtained RuW/g-C3N4 composite is evaluated and compared with individual components of composites by performing several control experiments. RuW/g-C3N4 exhibited improved electrochemical activity towards the HER with an overpotential of approximately 266 mV at 10 mA cm−2 with a Tafel slope of 63 mV dec−1. This new approach using tailored-transition–metal substituted POMs and melamine as the monomer precursors will be expected to contribute to the development of well-defined cluster-embedded 2D-carbon materials with enhanced electrochemical activities.
Introduction
Hydrogen evolution via electrocatalytic water splitting is regarded as one of the foremost encouraging routes to generate energy using environmentally friendly routes.1–3 Two-dimensional (2D) materials have attracted the curiosity of the research community due to their rich edge states, exceptional conductivity, unique structural properties, subsequent contribution to electron/mass transfer, and relatively low cost in energy applications.4 Furthermore, the structural morphology and tunable chemical compositions of these materials can be used for targeted function modulations. Various 2D nanostructures including MXenes, graphitic carbon nitride (g-C3N4), graphene, and transition-metal dichalcogenides have been evaluated for their electrocatalytic properties with favorable efficiency in the electrolysis of water.5–12 On comparing with metal clusters or bulk materials, atomically dispersed single-atom or single-atom alloy catalysts are believed to be superior to homogeneous and heterogeneous catalysts.13 Modifying the metal sites at the atomic level bestows the catalysts with utmost atomic utilization, exceptional electronic properties, improved metal-support interaction, and positive synergy between the active catalytic sites and carbon substrates.14–16 Despite these advantages, many carbon-based electrocatalytic systems exhibited disadvantages, such as loss of catalytically active sites due to carbon surface destruction under high electrical potentials,17–19 aggregation of isolated metals caused due to higher mobility of individual metal atoms under harsh reaction conditions, as well as complications associated with the identification of the origin of the catalytic activity and structure–activity relationship.20,21In this context, polyoxometalates (POMs), which are atomically well-defined polynuclear metal oxide anions extend unparalleled opportunities to synthesize atomically dispersed active metal centers.22–24 Recently, various well-defined composites of POMs have been reported as efficient electrocatalysts25–28 as well as precursors for the hydrogen evolution reaction (HER).29–32 The studies revealed that the presence of POMs assisted in inducing activity in an otherwise inactive counterpart via a synergistic effect. POMs incorporated into a 2D-carbon matrix also exhibited enhanced activity in energy applications as compared to the parent materials.33–36 The high specific surface energy of isolated atoms in the carbon matrix caused easy migration of individual atoms and resulted in agglomerated large clusters. Thus, it would be interesting to attain “further dispersion” of the isolated metal center via the “coordination islands” concept.37,38 This protocol is expected to minimize or prevent agglomeration of isolated atoms by the incorporation of a metal in lacuna of the POMs to form transition-metal substituted polyoxometalates (TMSPOMs) followed by incorporation to C3N4 sites.
Considering these advantages, well-defined bimetallic clusters embedded in carbon catalysts have been developed (Scheme 1(a)) for electrocatalytic applications. Hydrothermal synthesis of a carbon embedded W–Mo heterodimer catalyst, which was derived from TMSPOMs, was synthesized by Zhang and co-workers.39 The synthesized electrocatalyst exhibited Pt-like HER activity with high stability, showing higher activity than analogous Mo–Mo and W–W homodimer catalysts. Wagberg et al. reported electrocatalysts based on carbon-sub-nanometer FeCoW clusters controlled by different TMSPOMs as precursors.40 Furthermore, multi-step synthesis of single-atom Al and O co-doped Mo2N quantum dots on conductive N-doped graphene from the well-defined Anderson-typed POM anion clusters ((NH4)3[AlMo6O24H6]) for hydrogen production in alkaline media has also been reported.41 It is clear that most of the results comprised: (1) the use of a pre-synthesized 2D-carbon matrix for incorporation of either parent POMs or TMSPOMs and (2) hydrothermal synthesis. Furthermore, synthesis using TMSPOMs as precursors for these groups of materials remains unexplored. Thus, there is a need for the simplified synthesis of tailored carbon-materials with the advantage of TMSPOMs derived from well-defined clusters.
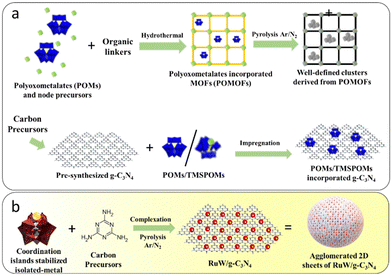 | ||
| Scheme 1 (a) Various protocols for the synthesis of well-defined nanostructures embedded in a carbon matrix and (b) a monomer complexation approach. | ||
Hence, in view of the scarce but encouraging literature reports on the synthesis and electrocatalytic activities of these materials, the possibility of combining well-defined molecular model systems paves the way to designing new atomically dispersed nanostructures embedded in g-C3N4 electrocatalysts. Here, g-C3N4 incorporated with well-defined RuW clusters through melamine complexation and its atomic-scale modulation of TMSPOM is reported. This is done by synthesizing Ru-substituted phosphotungstate and subsequent calcination under an inert atmosphere (Scheme 1(b)). As far as we are aware, the specified technique has only been used in two publications. The formation of single atoms (arrays) within g-C3N4 frameworks by the simple heat treatment of a complex between commercially available H4SiW12O40 and melamine has been reported by Antonietti's group.42 Recently our group reported g-C3N4 incorporated well-defined clusters of RuW derived from Ru-substituted phosphotungstate.43 Ru was selected for atomic-scale modification of phosphotungstic acid due to its remarkable electrocatalytic activities and relatively low cost compared to Pt (33% less).44–47 Significantly, the RuW/g-C3N4 composite exhibited enhanced electrochemical activity towards the HER in comparison with individual components. The composite showed an overpotential of around 266 mV at 10 mA cm−2 with a Tafel slope of 63 mV dec−1.
Experimental
Materials
12-Tungstophosphoric acid, ruthenium trichloride, melamine, sodium dihydrogen phosphate, disodium hydrogen phosphate, sulphuric acid, potassium hexacyanoferrate, acetic acid, sodium bicarbonate, and potassium chloride were purchased from Sigma Aldrich, India.Synthesis of Ru-substituted phosphotungstate (PW11Ru)
Synthesis of PW11Ru was carried out using a reported procedure with slight modification.48 Briefly, H3PW12O40·nH2O (PW12; 1 mM) was dissolved in 15 mL of water containing 0.6 mL acetic acid and the pH was adjusted to 5.0 using a saturated sodium bicarbonate solution. RuCl3 (1 mM) was dissolved in the minimum amount of hot water and added to the PW12 solution. The final pH of the solution was adjusted to 5.0. The solution was refluxed with stirring for 1 h and the product was filtered. The filtrate was refrigerated overnight. The product was isolated by filtration followed by washing with acetone to obtain brownish-black PW11Ru.Synthesis of RuW/g-C3N4
RuW/g-C3N4 was synthesized with slight modification in the reported procedure.43 0.3 g of PW11Ru was dissolved in the minimum quantity of water and 0.5 g of melamine was added to it and dispersed thoroughly by stirring at 50 °C for 2 h. The final product was washed by centrifugation (5000 rpm; 5 min) with water and dried at 80 °C to obtain a complex of PW11Ru and melamine (PW11Ru–Mel). The obtained PW11Ru–Mel was calcined in a tube furnace at 650 °C (with a ramp rate of 2 °C min−1) for 5 h under an inert atmosphere to obtain RuW/g-C3N4.Electrochemical characterization
All electrochemical experiments were performed using a CHI6005 workstation. Initially, the glassy carbon electrode (GCE) was cleaned and 6 μL of the catalyst dispersion (1 mg mL−1) is drop cast on it followed by drying at ambient temperature for 3 h. The cyclic voltammogram (CV) was measured for different electrodes in 0.1 M KCl with 5 mM [Fe(CN)6]3−/4− at a scan rate of 100 mV s−1. Graphite felt was used as the counter electrode and Ag/AgCl was used as the reference electrode. The polarization curves were measured by performing linear sweep voltammetry (LSV) in 0.5 M H2SO4 between 0.1 and −0.7 V and the reference electrode was calibrated against a reversible hydrogen electrode (RHE). The electrochemical active surface area (ECSA) was evaluated by running CV at different scan rates and the durability was evaluated by a continuous 500 CV cycles.Results and discussion
Material characterization
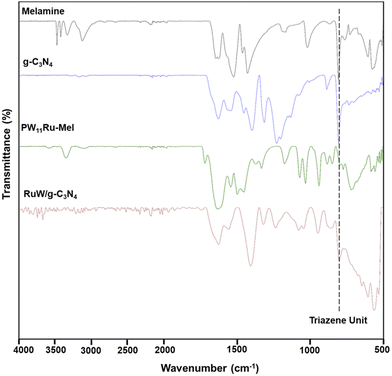
The Fourier transform infrared (FTIR) spectra (Bruker Alpha II) of pristine melamine, g-C3N4, PW11Ru–Mel, and RuW/g-C3N4 are presented in Fig. 1. Compared to melamine, the synthesized g-C3N4 appears different.49 FTIR of g-C3N4 shows multiple bands in the region 3077–3267![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1, 1631
cm−1, 1631![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1, and 1204–1565
cm−1, and 1204–1565![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−1, which could be attributed to N–H stretching mode, C
cm−1, which could be attributed to N–H stretching mode, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N, respectively. The PW11Ru–Mel exhibited characteristic bands related to PW11Ru at 1076 and 1036 cm−1 – υas (P–Oa), 943 cm−1 – υas (W–Ob), 887 cm−1 – υas (W–Oc–W), and 853 cm−1 – υas (W–Od–W).50 The typical bands of melamine appeared with no appreciable shift in wavenumber, suggesting the complexation without any changes happening to the structure of the POM. After calcination under an Argon atmosphere, the synthesized RuW/g-C3N4 composites showed the disappearance of several bands of Individual components. The decrease in the number of bands of melamine and PW11Ru may be due to the disintegration of the Keggin unit and the subsequent formation of Ru and W-nanoclusters in the g-C3N4 matrix. The FT-IR of RuW/g-C3N4 showed a band at 945 cm−1 with an additional band at 1233 cm−1 that may be due to the W–O and W–C stretching, respectively, indicating the presence of tungsten-oxide and tungsten-carbide phase in RuW/g-C3N4. Another band at 802 cm−1 corresponding to triazine aromatic repeating units is observed, which confirms the presence of the g-C3N4 matrix in the RuW/g-C3N4.51
N and C–N, respectively. The PW11Ru–Mel exhibited characteristic bands related to PW11Ru at 1076 and 1036 cm−1 – υas (P–Oa), 943 cm−1 – υas (W–Ob), 887 cm−1 – υas (W–Oc–W), and 853 cm−1 – υas (W–Od–W).50 The typical bands of melamine appeared with no appreciable shift in wavenumber, suggesting the complexation without any changes happening to the structure of the POM. After calcination under an Argon atmosphere, the synthesized RuW/g-C3N4 composites showed the disappearance of several bands of Individual components. The decrease in the number of bands of melamine and PW11Ru may be due to the disintegration of the Keggin unit and the subsequent formation of Ru and W-nanoclusters in the g-C3N4 matrix. The FT-IR of RuW/g-C3N4 showed a band at 945 cm−1 with an additional band at 1233 cm−1 that may be due to the W–O and W–C stretching, respectively, indicating the presence of tungsten-oxide and tungsten-carbide phase in RuW/g-C3N4. Another band at 802 cm−1 corresponding to triazine aromatic repeating units is observed, which confirms the presence of the g-C3N4 matrix in the RuW/g-C3N4.51
Scanning electron microscopy was carried out using a Carl Zeiss Gemini SEM 300. The image of the synthesized g-C3N4 (Fig. 2(a)) shows stacked 2D sheets with non-uniform pores. The SEM of PW11Ru–Mel (Fig. 2(b)) displays the typical bulk morphology without any structural characteristics. Interestingly, the composite (RuW/g-C3N4) formed by the calcination of melamine in the presence of PW11Ru, i.e. calcination of compound (PW11Ru–Mel) synthesized using the monomer complexation approach, exhibits a “poly dispersed spherical” morphology (Fig. 2(c)) as compared to its counterparts. However, evaluation of the SEM images of the composite (Fig. 2(d)) revealed the presence of folded 2D sheets of RuW/g-C3N4. Thus, it is predicted that due to high wt% of PW11Ru, the RuW-clusters incorporated 2D-sheets agglomerated together to form an overall non-uniform spherical morphology.
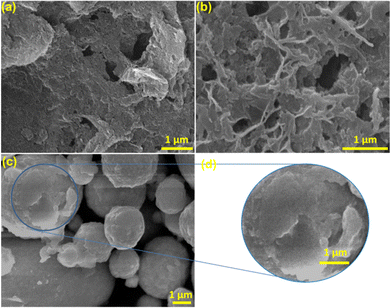 | ||
| Fig. 2 SEM images of (a) g-C3N4, (b) PW11Ru-Mel, and (c) RuW/g-C3N4; (d) magnified image of RuW/g-C3N4. | ||
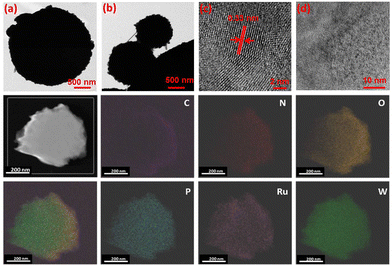
The spherical morphology for the bulk RuW/g-C3N4 was further confirmed by transmission electron microscopy (TEM: Tecnai G2, F30) and it is given in Fig. 3(a)–(d), which shows the polydispersed spherical microparticles. Besides, the periphery of dense microstructures shows spicules. For finer extrapolation of the nanostructure of the composites, the material was subject to ultrasonication for an extended period of 30–40 min before TEM imaging.
The interplanar distance was measured for the composites (Fig. 3(c)) and found to be 0.34 nm corresponding to the (002) plane of g-C3N4.52 At higher magnification (Fig. 3(d)), well-dispersed nano-clusters (<2 nm) of RuW(-oxides) throughout the g-C3N4 matrix were observed. This was evident by the elemental mapping of the composite material (Fig. 3), which confirmed the uniform dispersion of elements throughout the g-C3N4 matrix. Moreover, no evidence could be observed for the formation of large metal(s) clusters even in the presence of higher W loading. Thus, the described method of complexation is a promising method for the synthesis of well-dispersed metal-centers.
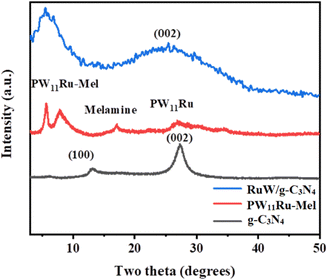
The XRD diffractogram of g-C3N4, PW11Ru–Mel, and RuW/g-C3N4 is presented in Fig. 4. For g-C3N4, the typical peaks at 12.8° and 27.4° correspond to (100) and (002) planes was observed. These peaks can be ascribed to the layered structure of the tri-s-triazine units53 and the interlayer stacking of the aromatic system.53,54 The presence of a Keggin unit in PW11Ru–Mel was confirmed by the sharp peaks in the region 6° and 9° as well as broad peaks in the 25–30° region.43
The broadness of the peak suggests the loss of crystallinity of the Keggin structure after the complexation between PW11Ru and the melamine monomer. On calcination of PW11Ru–Mel, the formed RuW/g-C3N4 was expected to exhibit a decrease in the number of peaks due to the partial breakdown of the Keggin unit to the corresponding oxide. Thus, RuW/g-C3N4 showed a single broad peak in the region 4°–10°. Moreover, the presence of a broad peak with maxima at around 26° confirmed the presence of the g-C3N4 matrix in the RuW/g-C3N4. Furthermore, no additional peaks of PW11Ru are observed in the region 20–35° and this could be due to the decomposition of the Keggin unit as well as higher dispersion of the corresponding oxides in the g-C3N4 matrix.
XPS measurement was conducted on a Kratos Axis Ultra platform with an Alkα X-ray source of 1486.6 eV and a total energy resolution of around 0.1 eV. The measurement is conducted under a UHV of 10–10 mbar at room temperature. A hemisphere analyser with a take-off angle of 90° to the surface is utilized for signal acquisition. The high-resolution (HR) spectra are measured with a step size of 0.1 eV and a pass energy of 10 eV and the wide scan is with a step size of 1 eV and a pass energy of 100 eV. All binding energy positions are calibrated to the C 1s at 284.8 eV (C–C). The XPS survey spectrum of RuW/g-C3N4 is shown in Fig. S1 (ESI†) and it indicates the presence of C, N, O, Ru, and W, which is an indication of the presence of g-C3N4 and PW11Ru in the composite.55,56
Fig. 5 shows the high-resolution (HR) spectra of C 1s, Ru 3d, W 4f and O 1s with deconvolution GL(30) after a proper Shirley background subtraction. Fig. 5(a) shows the C 1s, in which a proper fitting gives 4 related peaks corresponding to the C–C at 284.8 eV, C–O at 286.5 eV, and two N related signals at 287.0 eV (C–N) and 288.5 eV (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), respectively.57–59Fig. 5(b) presents the Ru 3d with the Ru 3d5/2 located at 280.9 eV and 282.3 eV for Ru4+ (solid-red-line) and Ru5+ (dash-blue-line), respectively.60–62 The related Ru 3d3/2 are given at 284.9 eV (to 280.9 eV) and 286.4 eV (to 282.3 eV) with a spin-orbital splitting of 4.2 eV (for simplicity the C 1s component was hidden). Fig. 5(c) exhibits the W6+ valence state with the W 4f7/2 located at 35.6 eV, which corresponds to the W–O bond.63,64 A blue doublet with W 4f7/2 at 32.9 eV and a green doublet at 33.9 eV can be assigned to the W4+ and W5+ states, which can probably be assigned to the W–C bond.29,64 A loss feature at the higher BE side of 41.4 eV is also observed, which is a signature of the W6+ states.65–69 This again confirms the presence of WO3 in the composites. The corresponding O 1s signal is given in Fig. 5(d), where the different oxidation components, including the W–O/Ru–O at 530.4 eV, the metal hydroxide (M–OH) at 531.8 eV, the carbon related C–O at 533.1 eV and the carbonate (O–C
N), respectively.57–59Fig. 5(b) presents the Ru 3d with the Ru 3d5/2 located at 280.9 eV and 282.3 eV for Ru4+ (solid-red-line) and Ru5+ (dash-blue-line), respectively.60–62 The related Ru 3d3/2 are given at 284.9 eV (to 280.9 eV) and 286.4 eV (to 282.3 eV) with a spin-orbital splitting of 4.2 eV (for simplicity the C 1s component was hidden). Fig. 5(c) exhibits the W6+ valence state with the W 4f7/2 located at 35.6 eV, which corresponds to the W–O bond.63,64 A blue doublet with W 4f7/2 at 32.9 eV and a green doublet at 33.9 eV can be assigned to the W4+ and W5+ states, which can probably be assigned to the W–C bond.29,64 A loss feature at the higher BE side of 41.4 eV is also observed, which is a signature of the W6+ states.65–69 This again confirms the presence of WO3 in the composites. The corresponding O 1s signal is given in Fig. 5(d), where the different oxidation components, including the W–O/Ru–O at 530.4 eV, the metal hydroxide (M–OH) at 531.8 eV, the carbon related C–O at 533.1 eV and the carbonate (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 534.2 eV, can be distinguished.70 The O 1s indicates the possible RuxW(OH)y species in the composites.
O) at 534.2 eV, can be distinguished.70 The O 1s indicates the possible RuxW(OH)y species in the composites.
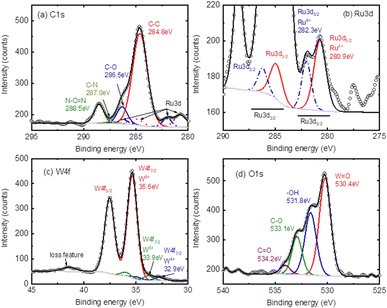 | ||
| Fig. 5 The core level spectra of (a) C 1s, (b) Ru 3d, (c) O 1s and (d) W 4f of the RuW/g-C3N4 composite. | ||
Inductively coupled plasma (ICP) analysis was carried out to evaluate the concentration of Ru and W in the RuW/g-C3N4 composite and it was found that the percentage of Ru is only 3.7 and the concentration of W is around 59.5% (Table S1, ESI†). This shows that the presence of Ru in the final composite is much less. In addition, we have made Ru/g-C3N4 and W/g-C3N4 composites with the ratio of 3.7 and 59.5% of Ru and W respectively by simply mixing g-C3N4 with salts of Ru and W to compare the HER performances.
Electrochemical analysis
The RuW/g-C3N4 modified GCE was evaluated and compared with the RuW and g-C3N4 modified GCE for its electrochemical performance. Fig. S2 (ESI†) shows the CV of different modified electrodes in which the peak currents of the GCE increased considerably by 30–40 μA for both anodic and cathodic peaks after modification with PW11Ru and g-C3N4. The peak current is further enhanced by 20–30 μA for the RuW/g-C3N4 modified GCE. The large surface area and promising electrical conductivity of the composite could accelerate the electron transfer rate and enhance the HER performance.The HER activities of the RuW/g-C3N4 samples were studied in 0.5 M H2SO4 solution with a specific amount of sample loaded on a GCE and compared with a bare GCE and Pt/C. As shown in Fig. 6(a), the GCE showed very weak electrocatalytic activity while the Pt/C showed the best HER activity. The onset overpotential of Pt/C is approximately 7 mV, and the overpotential is 69 mV (at 10 mA cm−2). The overpotential of PW11Ru was 446 mV while the corresponding value of g-C3N4 is 427 mV. However, the overpotential of the RuW/g-C3N4 composite was 266 mV, which is around 40% lower than the HER overpotential of g-C3N4 and around 37% lower than the HER overpotential of PW11Ru. These results showed that the HER performance of the RuW/g-C3N4 composite is significantly better compared to its individual components. In addition, at the same overpotential of 266 mV, the current density of RuW/g-C3N4 is 10 mA cm−2, which is higher than that of PW11Ru (2.7 mA cm−2) and g-C3N4 (3.69 mA cm−2) (Fig. S3, ESI†).
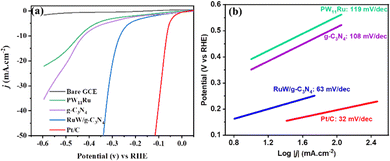 | ||
| Fig. 6 (a) HER polarization curve and (b) the corresponding Tafel plots of PW11Ru, g-C3N4, and RuW/g-C3N4 composites in comparison with Pt/C. | ||
The Tafel slopes of PW11Ru, g-C3N4, and the RuW/g-C3N4 composite are given in Fig. 6(b) by fitting the linear portion of the polarization curves. The Tafel slope for Pt/C (32 mV dec−1) is lower than all samples for commercial Pt/C. The Tafel slopes for PW11Ru and g-C3N4 are 119 mV dec−1 and 108 mV dec−1, respectively. The Tafel slope for the composite is 63 mV dec−1, which is much lower than that of pure g-C3N4 and PW11Ru and it confirms that the incorporation of PW11Ru on g-C3N4 can significantly enhance the electrocatalyst performance. Here, the enhanced HER performance of RuW/g-C3N4 arises due to the Ru metal centers embedded on the g-C3N4 backbone, which can enhance the HER performance. A comparison table showing the HER performance of POM/g-C3N4-based catalysts is presented in Table 1. The HER performance of the RuW/g-C3N4 composite is comparable with the reported Ru-based POM materials, with a Tafel slope of 63 mV dec−1 even with a lower content of Ru (only 3.7%).
| Material | Overpotential@10 mA cm−2 (mV) | (Tafel slope) (mV dec−1) | Ref. |
|---|---|---|---|
| Co-WS2/P-WO2.9 | 146 and 120 (acidic and alkaline) | 86 and 74 (acidic and alkaline) | 75 |
| {Cu2(3-bptzp)3(H2O)4[SiW12O40]}H2O (Keggin-type) | 59.4 (alkaline media) | 74 (alkaline media) | 76 |
| Co2P/WC@NC/CNTs | 171 and 198, (0.5 M H2SO4, 1.0 M KOH), | 65 and 42 (acidic and alkaline) | 77 |
| [PW11MO39]5-@Ru-rGO | −0.41 and −1.65 (vs. Ag/AgCl) (acidic and alkaline) | 36.56, 107.95 (acidic and alkaline) | 78 |
| POM derived Co/WN | 143 (both acidic and alkaline media) | 90 and 118 (acidic and alkaline) | 79 |
| Ru-MoO2@PC | 230 (alkaline) | 86.6 (alkaline) | 80 |
| Ru doped WO3 | 124 (acid) | 28, 17.6, and 83 (acidic, alkaline, and neutral) | 81 |
| 2D [Co2(TIB)2(PMo12O40)]·Cl·4H2O | 137 mV (acidic) | 59 (acidic) | 82 |
| CoW12/clicked g-C3N4 | 230 | 67 | 83 |
| g-C3N4-boron nitride and chitosan | 520 (acidic) | 150 (acidic) | 84 |
| RuW/g-C3N4 | 266 (acidic) | 63 (acidic) | This work |
As control experiments, the HER performances of the Ru/g-C3N4 and W/g-C3N4 composites were compared with RuW/g-C3N4 and the results are given in Fig. S4 (ESI†). As shown in Fig. S4 (ESI†), the HER activity of these composites is much less compared to RuW/g-C3N4. It shows that there is appropriate synergy between the precursor and g-C3N4 matrix during the monomer complexation approach, while it is not observed in Ru/g-C3N4 and W/g-C3N4 composites. The control experiments confirm the improved activity as compared to their counterparts, i.e. Ru/g-C3N4, suggesting synergy between Ru and W in RuW/g-C3N4 catalyzed HER. Thus, Ru and W both are predicted as active sites and the electronic interactions between these two metals can catalyze the hydrogen evolution.71 In addition, the presence of WO3 can transfer free electrons to Ru, which results in an interfacial electric field and this electron-rich region on the Ru surface can enhance the binding capability with protons.72,73 Moreover, WO3 in an acidic environment can form a hydrogen tungsten bronze compound (HyWO3) through H insert/insert out, which results in hydrogen transfer from the active site to the WO3 substrate.73,74
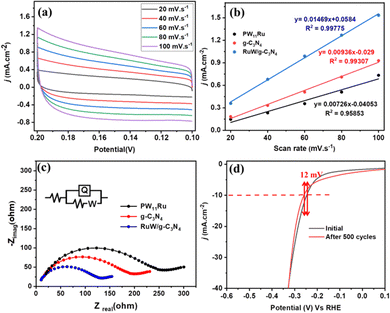
To evaluate the interactions between PW11Ru and the g-C3N4 matrix quantitatively, charge-transfer resistance (Rct) and electrochemical active surface area (ECSA) were utilized. Fig. 7(a) shows the CVs of RuW/g-C3N4 at different scan rates and it shows that the current density increases with scan rate. The slope of the current density at −0.15 V and scan rate (Fig. 7(b)) provides double-layer capacitance (Cdl), which can be used as proportional to ECSA. The Cdl of RuW/g-C3N4 was calculated as 14.7 mF cm−2, which was 1.56 times that of g-C3N4 (9.36 mF cm−2), and 2 times that of PW11Ru (7.26 mF cm−2). This increase in Cdl value confirms the enhanced electrical conductivity of the composite due to the incorporation of metal centers into the g-C3N4 matrix.
The Rct values were measured from electrochemical impedance measurements. Fig. 7(c) depicts the typical Nyquist plots of PW11Ru, g-C3N4, and the RuW/g-C3N4 composite, and the Rct value was assessed (inset of Fig. 7(c)). The Rct values are lower for the RuW/g-C3N4 (145 Ω) composite in comparison with PW11Ru (258 Ω) and g-C3N4 (190 Ω) and this corresponds to enhanced electron transfer kinetics of the composite, which is corroborated by the CV data. In addition, the stability of the catalysts is also evaluated, which is an important parameter when considering practical applications. The polarization curve showed a negligible difference of 12 mV after 500 scan cycles in the onset potential (Fig. 7(d)). This negligible difference in the overpotential confirms the long-term stability of the catalyst even in the absence of a binder molecule.
The present work aimed to simplify the preparation of bimetallic-supported C3N4 from tailored POM. Due to the known remarkable electrocatalytic activities of Ru, we have decided to use Ru for the substitution of one of the W from phosphotungstate. We believe that a simplified approach will be useful for developing similar bimetallic catalytic systems with different transition metals. This protocol has numerous advantages, such as straight forward synthetic route, the privilege of module metallic compositions as POMs can be designed at the molecular level, low-cost reagents, non-hydrothermal conditions, and no use of organic templates (in the case of MOF-derived nanoclusters).
Conclusion
In summary, the present synthesis protocols demonstrated the development of well-defined RuW-clusters embedded in a 2D-C3N4 matrix via straightforward monomer complexation. Furthermore, FT-IR, SEM and TEM showed retention of 2D-C3N4 in the composites. The presence of individual elements as well as composition was confirmed by TEM-mapping and XPS analysis. TEM mapping showed uniform dispersion of Ru and W throughout the matrix, without agglomeration of metallic clusters. XPS analysis also revealed the presence of two types of W (based on oxidation states), associated with W–O and W–C bonds in addition to the Ru–O bond. The HER catalytic activity of the RuW/g-C3N4 composite was evaluated and compared with PW11RuO40, g-C3N4, Ru/g-C3N4, W/g-C3N4 and Pt/C. The RuW/g-C3N4 composite exhibited improved electrochemical activity towards the HER with an overpotential of approximately 266 mV at 10 mA cm−2 with a Tafel slope of 63 mV dec−1. The novel approach using tailored-TMSPOMs and melamine as the monomer precursors is expected to contribute to the development of other base-metal-derived well-defined cluster-embedded 2D-carbon materials with enhanced electrochemical activities.Author contributions
Conceptualization-SP, PAR; formal analysis-MA, AMA, NS; investigation-SP, PAR, YT; methodology-SP; supervision-SP, PAR; validation-MA, AMA, PAR; writing – original draft-MA, NS, AMA, YT; writing – review & editing-SP, PAR.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SP acknowledges Parul University for financial support (IMR: CR4D/IMSL/053 dated 11/02/2022). PAR acknowledges the Ramalingaswami re-entry fellowship (BT/RLF/Re-entry/75/2020) from the Department of Biotechnology (DBT), Govt. of India. The authors are thankful to SAIF, IIT Bombay and CIF, IIT Palakkad for characterization facilities. We acknowledge the Nanotechnology Research Centre (NRC), SRIMST for providing the XPS analysis facility.Notes and references
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS.
- Q. Xue, X.-Y. Bai, Y. Zhao, Y.-N. Li, T.-J. Wang, H.-Y. Sun, F.-M. Li, P. Chen, P. Jin, S.-B. Yin and Y. Chen, J. Energy Chem., 2022, 65, 94–102 CrossRef CAS.
- Q. Zhu, Y. Qu, D. Liu, K. W. Ng and H. Pan, ACS Appl. Nano Mater., 2020, 3, 6270–6296 CrossRef CAS.
- S. Intikhab, V. Natu, J. Li, Y. Li, Q. Tao, J. Rosen, M. W. Barsoum and J. Snyder, J. Catal., 2019, 371, 325–332 CrossRef CAS.
- R. Meshkian, M. Dahlqvist, J. Lu, B. Wickman, J. Halim, J. Thörnberg, Q. Tao, S. Li, S. Intikhab, J. Snyder, M. W. Barsoum, M. Yildizhan, J. Palisaitis, L. Hultman, P. O. Å. Persson and J. Rosen, Adv. Mater., 2018, 30, 1706409 CrossRef PubMed.
- Y. Qu, Y. Ke, Y. Shao, W. Chen, C. T. Kwok, X. Shi and H. Pan, J. Phys. Chem. C, 2018, 122, 25331–25338 CrossRef CAS.
- J. Wen, J. Xie, X. Chen and X. Li, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS.
- K. Geethalakshmi, T. Y. Ng and R. Crespo-Otero, J. Mater. Chem. C, 2016, 4, 8429–8438 RSC.
- R. J. Toh, Z. Sofer, J. Luxa, D. Sedmidubský and M. Pumera, Chem. Commun., 2017, 53, 3054–3057 RSC.
- H. Liang, H. Shi, D. Zhang, F. Ming, R. Wang, J. Zhuo and Z. Wang, Chem. Mater., 2016, 28, 5587–5591 CrossRef CAS.
- H. Pan, Sci. Rep., 2014, 4, 5348 CrossRef CAS PubMed.
- X.-F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Acc. Chem. Res., 2013, 46, 1740–1748 CrossRef CAS PubMed.
- S. Ji, Y. Chen, X. Wang, Z. Zhang, D. Wang and Y. Li, Chem. Rev., 2020, 120, 11900–11955 CrossRef CAS PubMed.
- A. Wang, J. Li and T. Zhang, Nat. Rev. Chem., 2018, 2, 65–81 CrossRef CAS.
- G. Yilmaz, S. B. Peh, D. Zhao and G. W. Ho, Adv. Sci., 2019, 6, 1901129 CrossRef CAS PubMed.
- D. Zhao, Z. Zhuang, X. Cao, C. Zhang, Q. Peng, C. Chen and Y. Li, Chem. Soc. Rev., 2020, 49, 2215–2264 RSC.
- X. X. Wang, M. T. Swihart and G. Wu, Nat. Catal., 2019, 2, 578–589 CrossRef CAS.
- F. M. Oliveira, I. Danylo, V. Mazánek, M. Veselý, R. Gusmão and Z. Sofer, Mater. Adv., 2022, 3, 4348–4358 RSC.
- M. J. Hülsey, J. Zhang and N. Yan, Adv. Mater., 2018, 30, 1802304 CrossRef.
- R. Qin, P. Liu, G. Fu and N. Zheng, Small Methods, 2018, 2, 1700286 CrossRef.
- M. J. Hülsey, V. Fung, X. Hou, J. Wu and N. Yan, Angew. Chem., Int. Ed., 2022, 61, e202208237 CrossRef.
- M. J. Hülsey, G. Sun, P. Sautet and N. Yan, Angew. Chem., Int. Ed., 2021, 60, 4764–4773 CrossRef.
- D. Zang and H. Wang, Polyoxometalates, 2022, 1, 9140006 CrossRef.
- C. Singh, A. Haldar, O. Basu and S. K. Das, Inorg. Chem., 2021, 60, 10302–10314 CrossRef CAS.
- D. Jana, H. K. Kolli, S. Sabnam and S. K. Das, Chem. Commun., 2021, 57, 9910–9913 RSC.
- D. M. Fernandes, M. P. Araújo, A. Haider, A. S. Mougharbel, A. J. S. Fernandes, U. Kortz and C. Freire, ChemElectroChem, 2018, 5, 273–283 CrossRef CAS.
- R. Liu, G. Zhang, H. Cao, S. Zhang, Y. Xie, A. Haider, U. Kortz, B. Chen, N. S. Dalal, Y. Zhao, L. Zhi, C.-X. Wu, L.-K. Yan, Z. Su and B. Keita, Energy Environ. Sci., 2016, 9, 1012–1023 RSC.
- X.-L. Wang, Y.-J. Tang, W. Huang, C.-H. Liu, L.-Z. Dong, S.-L. Li and Y.-Q. Lan, ChemSusChem, 2017, 10, 2402–2407 CrossRef CAS PubMed.
- Y. Hou, H. Pang, L. Zhang, B. Li, J. Xin, K. Li, H. Ma, X. Wang and L. Tan, J. Power Sources, 2020, 446, 227319 CrossRef CAS.
- Z. Zeb, Y. Huang, L. Chen, W. Zhou, M. Liao, Y. Jiang, H. Li, L. Wang, L. Wang, H. Wang, T. Wei, D. Zang, Z. Fan and Y. Wei, Coord. Chem. Rev., 2023, 482, 215058 CrossRef CAS.
- Z.-Y. Tian, X.-Q. Han, J. Du, Z.-B. Li, Y.-Y. Ma and Z.-G. Han, ACS Appl. Mater. Interfaces, 2023, 15, 11853–11865 CrossRef CAS.
- J. Miao, Z. Lang, X. Zhang, W. Kong, O. Peng, Y. Yang, S. Wang, J. Cheng, T. He, A. Amini, Q. Wu, Z. Zheng, Z. Tang and C. Cheng, Adv. Funct. Mater., 2019, 29, 1805893 CrossRef.
- X.-L. Zhai, J. Liu, L.-Y. Hu, J.-C. Bao and Y.-Q. Lan, Chem. – Eur. J., 2018, 24, 15930–15936 CrossRef CAS PubMed.
- R. Gong, D. Mitoraj, D. Gao, M. Mundszinger, D. Sorsche, U. Kaiser, C. Streb, R. Beranek and S. Rau, Adv. Sustainable Systems, 2022, 6, 2100473 CrossRef CAS.
- Y. Gao, Z. Lang, F. Yu, H. Tan, G. Yan, Y. Wang, Y. Ma and Y. Li, ChemSusChem, 2018, 11, 1082–1091 CrossRef CAS PubMed.
- R. Liu and C. Streb, Adv. Energy Mater., 2021, 11, 2101120 CrossRef CAS.
- A. Kondinski and T. N. Parac-Vogt, Front. Chem., 2018, 6, 346 CrossRef PubMed.
- Y. Yang, Y. Qian, H. Li, Z. Zhang, Y. Mu, D. Do, B. Zhou, J. Dong, W. Yan, Y. Qin, L. Fang, R. Feng, J. Zhou, P. Zhang, J. Dong, G. Yu, Y. Liu, X. Zhang and X. Fan, Sci. Adv., 2020, 6, eaba6586 CrossRef CAS PubMed.
- X.-B. Han, X.-Y. Tang, Y. Lin, E. Gracia-Espino, S.-G. Liu, H.-W. Liang, G.-Z. Hu, X.-J. Zhao, H.-G. Liao, Y.-Z. Tan, T. Wagberg, S.-Y. Xie and L.-S. Zheng, J. Am. Chem. Soc., 2019, 141, 232–239 CrossRef CAS.
- Y. Huang, W. Zhou, W. Kong, L. Chen, X. Lu, H. Cai, Y. Yuan, L. Zhao, Y. Jiang, H. Li, L. Wang, L. Wang, H. Wang, J. Zhang, J. Gu and Z. Fan, Adv. Sci., 2022, 9, 2204949 CrossRef CAS PubMed.
- B. Kumru, D. Cruz, T. Heil and M. Antonietti, Chem. Mater., 2020, 32, 9435–9443 CrossRef CAS.
- N. Shabana, A. M. Arjun, K. Rajendran, S. Pathan and P. A. Rasheed, Anal. Methods, 2023, 15, 587–595 RSC.
- R. Ye, Y. Liu, Z. Peng, T. Wang, A. S. Jalilov, B. I. Yakobson, S.-H. Wei and J. M. Tour, ACS Appl. Mater. Interfaces, 2017, 9, 3785–3791 CrossRef CAS PubMed.
- D. H. Kweon, M. S. Okyay, S.-J. Kim, J.-P. Jeon, H.-J. Noh, N. Park, J. Mahmood and J.-B. Baek, Nat. Commun., 2020, 11, 1278 CrossRef CAS PubMed.
- J. Mahmood, F. Li, S.-M. Jung, M. S. Okyay, I. Ahmad, S.-J. Kim, N. Park, H. Y. Jeong and J.-B. Baek, Nat. Nanotechnol., 2017, 12, 441–446 CrossRef CAS PubMed.
- Y. Ding, K.-W. Cao, J.-W. He, F.-M. Li, H. Huang, P. Chen and Y. Chen, Chin. J. Catal., 2022, 43, 1535–1543 CrossRef CAS.
- P. Shringarpure and A. Patel, Inorg. Chim. Acta, 2009, 362, 3796–3800 CrossRef CAS.
- N. E. Mircescu, M. Oltean, V. Chiş and N. Leopold, Vib. Spectrosc., 2012, 62, 165–171 CrossRef CAS.
- M. S. S. Balula, I. C. M. S. Santos, J. A. F. Gamelas, A. M. V. Cavaleiro, N. Binsted and W. Schlindwein, Eur. J. Inorg. Chem., 2007, 1027–1038 CrossRef CAS.
- M. Bledowski, L. Wang, A. Ramakrishnan, O. V. Khavryuchenko, V. D. Khavryuchenko, P. C. Ricci, J. Strunk, T. Cremer, C. Kolbeck and R. Beranek, Phys. Chem. Chem. Phys., 2011, 13, 21511–21519 RSC.
- Y. Li, X. Liu, L. Tan, Z. Cui, X. Yang, Y. Zheng, K. W. K. Yeung, P. K. Chu and S. Wu, Adv. Funct. Mater., 2018, 28, 1800299 CrossRef.
- R. Malik, V. K. Tomer, N. Joshi, T. Dankwort, L. Lin and L. Kienle, ACS Appl. Mater. Interfaces, 2018, 10, 34087–34097 CrossRef CAS PubMed.
- R. Malik, N. Joshi and V. K. Tomer, Coord. Chem. Rev., 2022, 466, 214611 CrossRef CAS.
- X. Li, J. Zhang, L. Shen, Y. Ma, W. Lei, Q. Cui and G. Zou, Appl. Phys. A, 2009, 94, 387–392 CrossRef CAS.
- M. Tong, J. Yang, Q. Jin, X. Zhang, J. Gao and G. Li, J. Mater. Sci., 2019, 54, 10656–10669 CrossRef CAS.
- H. Xu, T. Zhang, Y. Gu, X. Yan, N. Lu, H. Liu, Z. Xu, Y. Xing, Y. Song, Z. Zhang and M. Yang, Microchim. Acta, 2020, 187, 163 CrossRef CAS.
- S. Gong, Z. Jiang, S. Zhu, J. Fan, Q. Xu and Y. Min, J. Nanopart. Res., 2018, 20, 310 CrossRef.
- J.-S. Li, Y.-J. Tang, C.-H. Liu, S.-L. Li, R.-H. Li, L.-Z. Dong, Z.-H. Dai, J.-C. Bao and Y.-Q. Lan, J. Mater. Chem. A, 2016, 4, 1202–1207 RSC.
- K. Wang, Q. Chen, Y. Hu, W. Wei, S. Wang, Q. Shen and P. Qu, Small, 2018, 14, 1802132 CrossRef PubMed.
- Y. Feng, P. Huang, Z. Zhou, X. Ding, L. Liu, X. Liu and J. Kang, Nanoscale Res. Lett., 2019, 14, 86 CrossRef.
- J. Wojciechowska, E. Gitzhofer, J. Grams, A. M. Ruppert and N. Keller, Materials, 2018, 11, 2329 CrossRef PubMed.
- H. Wang, C. Wang, Y. Yang, M. Zhao and Y. Wang, Catal. Sci. Technol., 2017, 7, 405–417 RSC.
- L. Wang, B. Wu, W. Li, S. Wang, Z. Li, M. Li, D. Pan and M. Wu, Adv. Biosystems, 2018, 2, 1700191 CrossRef.
- W. Feng, Y. Ding, Y. Liu and R. Lu, Mater. Chem. Phys., 2006, 98, 347–352 CrossRef CAS.
- S.-M. Wang, L. Liu, W.-L. Chen, E.-B. Wang and Z.-M. Su, Dalton Trans., 2013, 42, 2691–2695 RSC.
- D. Xu, W.-L. Chen, J.-S. Li, X.-J. Sang, Y. Lu, Z.-M. Su and E.-B. Wang, J. Mater. Chem. A., 2015, 3, 10174–10178 RSC.
- Y. G. Shen and Y. W. Mai, J. Mater. Res., 2000, 15, 2437–2445 CrossRef CAS.
- Y. Zhao, W. Hu, Y. Xia, E. Smith, Y. Zhu, C. Dunnill and D. H. Gregory, J. Mater. Chem., 2007, 17, 4436–4440 RSC.
- B. Sivaranjini, R. Mangaiyarkarasi, V. Ganesh and S. Umadevi, Sci. Rep., 2018, 8, 8891 CrossRef CAS PubMed.
- U. Joshi, S. Malkhandi, Y. Ren, T. L. Tan, S. Y. Chiam and B. S. Yeo, ACS Appl. Mater. Interfaces, 2018, 10, 6354–6360 CrossRef CAS.
- L. Peng, L. Su, X. Yu, R. Wang, X. Cui, H. Tian, S. Cao, B. Y. Xia and J. Shi, Appl. Catal., B, 2022, 308, 121229 CrossRef CAS.
- E. V. Miu, J. R. McKone and G. Mpourmpakis, J. Am. Chem. Soc., 2022, 144, 6420–6433 CrossRef CAS PubMed.
- J. Park, S. Lee, H. E. Kim, A. Cho, S. Kim, Y. Ye, J. W. Han, H. Lee, J. H. Jang and J. Lee, Angew. Chem., Int. Ed., 2019, 58, 16038–16042 CrossRef CAS.
- Y. Wang, S. Yun, J. Shi, Y. Zhang, J. Dang, C. Dang, Z. Liu, Y. Deng and T. Yang, J. Colloid Interface Sci., 2022, 625, 800–816 CrossRef CAS.
- X.-L. Wang, Y. Tian, Z.-H. Chang and H. Lin, ACS Sustainable Chem. Eng., 2020, 8, 15696–15702 CrossRef CAS.
- C. Yue, N. Liu, Y. Li, Y. Liu, F. Sun, W. Bao, Y. Tuo, Y. Pan, P. Jiang, Y. Zhou and Y. Lu, J. Colloid Interface Sci., 2023, 645, 276–286 CrossRef CAS.
- A. A. Ensafi, E. Heydari-Soureshjani and B. Rezaei, Int. J. Hydrogen Energy, 2017, 42, 5026–5034 CrossRef CAS.
- L. Men, T. Shi, J. Li, X. Li, B. Sun, Q. Pan and Z. Su, Int. J. Hydrogen Energy, 2022, 47, 27452–27459 CrossRef CAS.
- J.-Y. Han, S.-H. Cai, J.-Y. Zhu, S. Yang and J.-S. Li, Chem. Commun., 2022, 58, 100–103 RSC.
- H. Liu, G. Tan, M. Li, Z. Zhang, M. Getaye Sendeku, Y. Li, Y. Kuang and X. Sun, Chem. Eng. J., 2023, 458, 141414 CrossRef CAS.
- L. Wang, A. Wang, Z.-Z. Xue, J.-X. Hu, S.-D. Han and G.-M. Wang, Inorg. Chem., 2022, 61, 18311–18317 CrossRef CAS PubMed.
- S. Shahsavarifar, M. Masteri-Farahani and M. R. Ganjali, Langmuir, 2022, 38, 12124–12131 CrossRef CAS PubMed.
- P. S. Kumar and P. Prakash, J. Environ. Chem. Eng., 2023, 11, 109045 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XPS survey spectra, cyclic voltammogram, current density data, ICP analysis and HER polarization curve. See DOI: https://doi.org/10.1039/d3ma01010d |
| This journal is © The Royal Society of Chemistry 2024 |