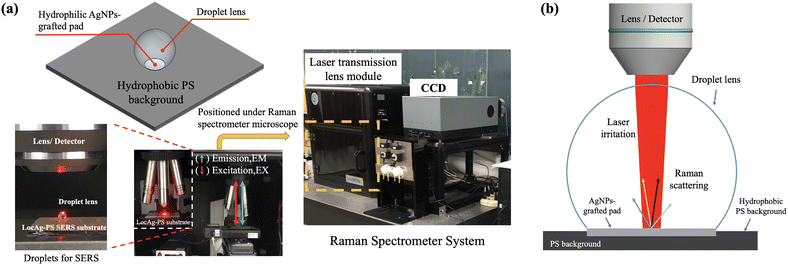
Utilization of microdroplets as optical lenses for surface-enhanced Raman spectroscopy (SERS) enhancement on localized silver nanoparticle-decorated porous silicon substrates†
Chia-Wen
Tsao
 *ab and
Zi-Yi
Yang
a
*ab and
Zi-Yi
Yang
a
aDepartment of Mechanical Engineering, National Central University, No. 300, Zhongda Rd., Zhongli District, Taoyuan City 320, Taiwan. E-mail: cwtsao@ncu.edu.tw
bDepartment of Medical Research, Cathay General Hospital, No. 280, Renai Rd. Sec. 4, Taipei City, 106, Taiwan
First published on 18th September 2024
Abstract
Surface-enhanced Raman spectroscopy (SERS) is a widely used analytical technique known for its high sensitivity and broad applicability. Despite its potential, SERS faces challenges related to detection sensitivity and reproducibility. This study proposes an innovative method to enhance SERS performance by employing water microdroplets as optical lenses on localized silver nanoparticle-decorated porous silicon (LocAg-PS) substrates. The hydrophobic nature of the LocAg-PS substrate not only ensures precise positioning of the microdroplet lenses on the silver nanoparticle grafted pad (AgNP pad) but also forms a plano-convex-like microdroplet lens for the focusing of the excitation laser and the collection of scattered light. Experimental results demonstrate that using microdroplet lenses enhances the SERS signal intensity and reproducibility, providing a rapid and cost-effective solution for advanced SERS analysis.
1. Introduction
Surface-enhanced Raman spectroscopy (SERS) has emerged as a highly sensitive analytical tool with a wide range of scientific and industrial applications.1,2 SERS is also a versatile analytical platform that can effectively integrate with microfluidics,3,4 mass spectrometry,5,6 artificial intelligence,7 or machine learning8 technologies, creating a fully functional lab on a chip microdevice. Originating from the fundamental principle of Raman scattering, SERS amplifies Raman scattering through the interaction of laser light with specially engineered metallic nanostructures through electromagnetic and chemical enhancement. This enhancement allows molecules to be detected and analyzed even in extremely low concentrations by amplifying their signals to detectable levels.9 SERS also provides a detailed view of chemical structures, offering “molecular fingerprints” of substances. It allows for the identification and quantification of molecules, even at extremely low concentrations down to the zeptomole level.10 The ability of SERS to provide highly sensitive and detailed information has made it indispensable in many scientific domains, which has found broad applications in various fields, including environmental pollutant monitoring,11 fungi, bacteria, and virus detection,12 food quality and safety analysis,13 medical diagnosis,14 cancer detection and imaging,15etc.While SERS holds significant potential as a highly sensitive tool for various applications, it is hindered by various challenges.16–18 One of the issues is the detection sensitivity. SERS sensitivity largely depends on hot spots, which are regions with extremely high electromagnetic field enhancement. These hot spots are often localized in specific areas of the SERS substrate and must be precisely identified for effective detection. Under controlled conditions, single-molecule level detection can be achieved.19 Another predominant challenge lies in the effective positioning of the molecule of interest in the hot spot region for maximal SERS enhancement. Their random and irregular occurrence often results in a lack of uniformity and control, leading to signal enhancement inconsistencies across different SERS substrate regions. This heterogeneity significantly impacts the reproducibility of SERS signals, a crucial requirement for any reliable analytical technique. These issues have motivated the ongoing search to develop a robust, reliable, and universally applicable SERS substrate. Various methodologies have been proposed to construct SERS hot spots for improved performance.20 For instance, Li et al. demonstrated a cost-effective effect of generating hot spots by flame aerosol self-assembly for the solid SERS substrate.21 Deng et al. developed hierarchical superhydrophobic MF/Ag nanocomposite array substrates by combining colloidal lithography with various interfacial chemical reactions.22 Several recent review papers also elaborate on the current status of advanced SERS substrate techniques.23,24
SERS enhancement has recently benefited from significant advancements in integrating optical lenses. Various studies have previously explored the potential of using microlenses in SERS. For example, Milenko's group25,26 melted optical fiber ends to form a spherical-shaped lens. Research has demonstrated that the fiber lenses can effectively provide desirable light focusing for property collection. Vlatko Gašparić attached silica microspheres to a silicon substrate and found distinct Raman enhancement (hotspot area) compared to a plain silicon substrate.27 Tran et al. externally modified a Raman microscope to incorporate a solid immersion lens to perform Raman experiments for polymer analysis.28 In addition to silica microspheres, researchers have also used cured polydimethylsiloxane (PDMS),29 UV-curable adhesive,30 or SU-8 photoresist31 on SERS substrates as a microlens for signal collection. In particular, an innovative study by Jin et al. uses a 3D nanoplasmonic well for light focusing, which is formed by curing the liquid PDMS and metal ion microdroplet complex. Because the spherical shape of PDMS is well integrated with metallic nanoparticles, a strong Raman signal was collected at the center location by the optical focusing effects.32 Another noteworthy investigation by Kim et al. highlighted that the solid-state lens can be used to enhance signals, and aqueous microlenses can also be used to capture gaseous molecules. They also showed that the electromagnetic wave had been focused by the lens for SERS analysis.33 Similarly, in the realm of neuroscience, the integration of optical techniques such as high-spatiotemporal-resolution photoacoustic imaging with voltage-sensitive dyes has demonstrated improvements in visualizing and analyzing complex biological signals.34 These advancements underscore the potential of optical enhancements in overcoming challenges related to signal detection and SERS resolution. Despite the promising results, the integration of microlenses in SERS faces challenges. Issues such as high costs, fabrication complexity, and extended analysis times hinder practical implementation. This highlights the need for a simpler, more cost-effective, and versatile microlens solution.
In our previous report,35 we demonstrated a simple and low-cost contact deposition process to generate localized nanoparticle-decorated porous silicon (LocAg-PS) for SERS analysis due to the hydrophobic porous silicon background. The sample can be auto-aligned and dried to the confined silver nanoparticle-grafted region for concentrating the sample. This paper introduces an innovative approach utilizing water droplets as microlenses for enhanced SERS analysis. Leveraging the surface hydrophobicity of the LocAg-PS substrate, the water microdroplet lenses are precisely positioned atop the LocAg-PS pads. The use of the microdroplet lens is aimed at overcoming the hot spot and sensitivity limitations while building upon the demonstrated benefits of microlenses for SERS analysis.
2. Experiment
2.1 Materials and reagents
A 6 inch silicon test grade wafer, with a resistivity of 1–10 ohm cm and a 3 nm thick gold E-beam plating was purchased from Advanced Furnace Systems Corp. (Tainan, Taiwan). Hydrofluoric acid (HF. 49% concentration), hydrogen peroxide (H2O2, 31% concentration), acetone (100% concentration), and isopropanol (99.8% concentration) were obtained from BASF Corp (Taipei, Taiwan). Rhodamine 6G (R6G) was sourced from Alfa Aesar (MA, USA). Ethanol (99.5% concentration), methanol (99.9% concentration), and HPLC-grade water were obtained from J.T. Baker Chemical Company (Phillipsburg, NJ, USA). Silver nitrate (99.99%, powder form) was purchased from Advanced Furnace Systems Corp. (Tainan, Taiwan). Melamine was purchased from Sigma-Aldrich Co. LLC (Saint Louis, MO, USA).The silver nanoparticle deposition solutions were prepared by first adding 96.18, 128.24, and 160.3 mg of silver nitrate to 8 mL of MS-grade water to achieve concentrations of 12, 16, and 20 mM. Then the solution was mixed with AgNO3(aq) with H2O2 and HF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.225, volume ratio).
1.225, volume ratio).
2.2 Porous silicon fabrication
The porous silicon (PS) surface was fabricated by the metal-assisted chemical etching (MACE) process.36 The 3 nm thick Au-coated silicon substrate was cut into 1.5 × 1.5 cm2 and immersed in a HF/H2O2/ethanol solution (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, volume ratio) for etching for 20 minutes. After etching, the PS substrate was cleaned with methanol, and a nitrogen gun was blown to dry the PS substrate.
5, volume ratio) for etching for 20 minutes. After etching, the PS substrate was cleaned with methanol, and a nitrogen gun was blown to dry the PS substrate.
2.3 Contact angle measurement
The hydrophobicity of the LocAg-PS surface was obtained using an OCA 15EC contact angle measuring system from DataPhysics Instruments GmbH, Germany. To measure the water contact angle (CA), we pipet 1–2 μL of water onto the LocAg-PS surface. Then, we capture the water droplet image to calculate the CA using the built-in software for contact angle measurement (SCA software for OCA).2.4 Field emission scanning electron microscopy
Field emission scanning electron microscopy (FE-SEM) was conducted by using a Hitachi FESEM (HITACHI S-4300, Krefeld, Germany) at the Precious Instrument Utilization Center at National Central University, Taiwan. To obtain the FE-SEM image, the LocAg-PS samples were cut into 0.5 × 0.5 cm2 sizes and affixed to the SEM holder to take top FE-SEM images.2.5 Enhancement factor
The enhancement factor (EF) was estimated based on the 612 cm−1 characterized peak for R6G and 704 cm−1 characterized peak for melamine in the following equation37 | (1) |
3. Results and discussion
3.1 Localized silver nanoparticle-decorated porous silicon surface (LocAg-PS) fabricated by the contact depiction method
Nobel nanoparticles are required to deposit on the nanostructured surface for SERS analysis. In previous work,35 the deposition solution was directly deposited onto the PS surface. The deposition volume and weightiness constrain the LocAg-PS pad size. A minimum pad radius of 513.0 μm was reported by using a 1 μL deposition volume. To further minimize the pad size and generate high contact angle droplets for our proposed focusing lens for advanced SERS analysis, we proposed a modified contact deposition method in this paper. As shown in Fig. 1, the PS substrate fabricated by MACE was fixed on a Z-axis stage and positioned to a 1 μL silver nanoparticle deposition solution droplet (Ag-Drop) attached to the pipette tip. Once the droplet contracts with the PS surface, move the Z-axis stage to a height (H) to press the droplet for ∼10 seconds and gradually position it down to the original position to avoid over-pressing it. Stay for 2 minutes to allow for sufficient electroless deposition of the silver nanoparticles (Ag-NPs) onto the PS surface and finally remove the droplet (pipette) to complete the procedure. After the droplet contact deposition, the substrate was cleaned with methanol and N2, and the Ag-NP pads were blown dry to create the Ag-NP pads on the LocAg-PS substrate.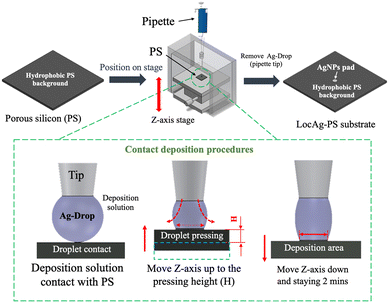 | ||
| Fig. 1 Schematic illustration of the LocAg-PS fabrication procedure by the contact deposition method. | ||
The Ag-NP pad size on the LocAg-PS substrate fabricated by the contact deposition approach is highly correlated to the Z-axis stage positioning height (H). To validate the height correlation, we tested the contact deposition heights at 15 mm, 20 mm, and 25 mm. Based on the SEM measurements in Fig. 2, the deposit areas had radii of 204.5, 212.0, and 388.9 μm, respectively. A lower H resulted in smaller Ag-NP pads, with a 15 mm deposition height producing the smallest pad size. In our initial investigation, we also tested a deposition height of 10 mm. While it created a LocAg-PS pad size of less than 200 μm, its repeatability was poor because the Ag-Drop often stuck to the pipette tip instead of the PS silicon surface. Thus, we chose 15 mm as the optimal deposition height for subsequent experiments. Compared to our previously reported method, this 15 mm contact deposition approach successfully reduced the Ag-NP pad size by 41.9% using the same droplet deposition volume of 1 μL.
 | ||
| Fig. 2 The SEM images of the silver nanoparticle-decorated LocAg-PS surface with 15, 20, and 25 mm contract-pressure height (H). The Ag-Drop concentration is 20 mM. | ||
In addition to the deposition height, the concentration of Ag-Drop in the contact deposition process primarily influences the density of Ag-NP grafting, consequently affecting the SERS performance. Fig. 3a–c show the SEM imaging of LocAg-PS surfaces with varying Ag-Drop concentrations of 12 mM, 16 mM, and 20 mM, respectively. With increased concentrations of silver nitrate in Ag-Drop, a denser distribution of nanoparticles closer to the center of the AgNP pad was observed, resulting in an enhanced SERS signal. As depicted in Fig. 3d, all of the SERS analyses exhibited characteristic peaks of the R6G sample at 612, 774, 1189, and 1310 cm−1, representing (C–C–C) ring deformation vibration, (C–H) bending vibration, C–H and N–H bending vibration, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration, respectively. The peaks at 1364 and 1509 cm−1 corresponded to C–C bonding.38 Among the tested conditions, the 20 mM concentration exhibited the highest SERS signal intensity. The enhancement factor of the LocAg-PS substrate was measured at 3.86 × 105 under the 15 mm deposition height and 20 mM Ag-Drop deposition condition. Therefore, this setup was selected for subsequent experiments.
C stretching vibration, respectively. The peaks at 1364 and 1509 cm−1 corresponded to C–C bonding.38 Among the tested conditions, the 20 mM concentration exhibited the highest SERS signal intensity. The enhancement factor of the LocAg-PS substrate was measured at 3.86 × 105 under the 15 mm deposition height and 20 mM Ag-Drop deposition condition. Therefore, this setup was selected for subsequent experiments.
Fig. 4 summarizes the sizes of Ag-NP pads created using Ag-Drop volumes of 1, 2, 5, and 10 μL at varying concentrations. The data suggests that the primary factor influencing the pad radius is the Ag-Drop volume rather than the concentration. Specifically, increasing the Ag-Drop volume resulted in larger Ag-NP pad sizes, measuring 238.5 ± 11.7 μm for 1 μL, 312.1 ± 5.1 μm for 2 μL, 377.3 ± 27.9 μm for 5 μL, and 1037.4 ± 112.9 μm for 10 μL at 20 mM Ag-Drop concentration.
 | ||
| Fig. 4 Correlation of the Ag-NP pad size on the LocAg-PS substrate with various Ag-Drop volumes. The contract-pressing height was fixed at 15 mm, and error bars were averaged from four measurements. The raw data is available in ESI† Table S1. | ||
3.2 Microdroplet lens for SERS enhancement
The LocAg-PS surface, characterized by its hydrophilic Ag-NP pad on a hydrophobic PS background, effectively confines deposited sample droplets due to surface tension. As depicted in Fig. 5a, the confinement of the water droplet leads to the creation of a plano-convex-like microdroplet lens. For SERS analysis, the microdroplet lens was assembled on the LocAg-PS substrate and positioned under a Raman spectrometer microscope equipped with a 633 nm laser source. The laser light was directly irradiated on the water droplet on the LocAg-PS substrate. The Raman scattered light was reflected through the water droplet into the optical microscope and transmitted into the CCD to collect the Raman spectrum.The microdroplet will spread out of the hydrophilic AgNP-grafted pad (SERS effective area) to the hydrophobic PS background due to the force balance between surface tension and gravity. It is highly correlated to the AgNP-grafted pad size and the microdroplet volume. Besides, the curvature of the microdroplet lens fundamentally influences the focus of laser irradiation and Raman signals, which can be determined by the water contact angle of the microdroplet lens. Fig. 6a presents the evaluation of water contact angles (Y-axis) on various Ag-NP pad sizes, which were fabricated using 1, 2, 5, and 10 μL Ag-Drop conditions (as indicated in the labels). The measurements were taken with water droplets ranging from 1 to 10 μL deposited on the LocAg-PS surface (X-axis). For all Ag-NP pad sizes, as the droplet volume increases, the contact angle proportionally rises until it reaches the maximum capacity of the pads. Beyond this threshold, excessive sample volume causes the droplet to spread out the effective SERS sensing region of the Ag-NP pad, primarily due to the force balance between surface tension and gravity effects. The maximum sample capacity varies depending on the Ag-NP pad size around 1.5–2.5 μL.
 | ||
| Fig. 6 (a) Correlation between contact angle and droplet volume with different Ag-Drop deposition volumes for 20 mM concentrations. The contact angle measurement images are available in ESI† Fig. S1. (b) Optical simulations for 0°, 30°, 130°, and 150° contact angle microdroplet lenses. The original simulation result data is available in ESI† Fig. S2. | ||
For optimal SERS enhancement by the microdroplet lens approach, a higher sample contact angle is advantageous as it facilitates a better focusing effect, while a smaller sample volume is preferable for less sample consumption without spreading out of the Ag-NP pad; therefore, 1.0 μL microdroplet lens volume is preferred. We simulate the focusing effect under different water contact angles for 0° (no lens), 30° (10 μL Ag-Drop fabricated Ag-NP pad), 130° (5 μL Ag-Drop fabricated Ag-NP pad) to 150° (1 & 2 μL Ag-Drop fabricated Ag-NP pad) that circulated in Fig. 6a. Simulation software (PhyDemo) was used to simulate the focusing effect of the microdroplet lens on the LocAg-PS SERS substrate. The Ag-pad size is set up as 235 μm in radius (15 mm contract-pressing height as previously discussed), and the laser size is assumed 100 μm in diameter. As presented in Fig. 6b, it shows the focused path of the excitation (EX) laser beam irradiating through the microdroplet lens, concentrating light onto the AgNP hotspots on the LocAg-PS SERS substrate to create a strong electromagnetic field, and therefore expecting an enhanced SERS spectrum emission (EM) from the microdroplet lens. As displayed in Fig. 6b, by increasing the droplet contact angle, better laser irradiation and Raman signal focusing effects can be obtained. Therefore, our subsequent SERS analyses will focus on employing a 1.0 μL microdroplet lens volume on the 235 μm Ag-NP pad on the LocAg-PS SERS substrate.
The simulations assume that the laser irradiates at the center of the microdroplet lens. However, during the actual SERS measurements, the position of the laser beam needs to be manually pre-adjusted to the microdroplet lens. Consequently, the laser beam is more likely to irradiate away from the center of the droplet and rarely gets perfectly aligned with the droplet's center. Fig. 7a I–IV list the potential laser irradiation locations on the microdroplet lens, excluding the scenario where the laser irradiates outside the droplet. Since the AgNP grafted pad serves as the SERS active region, for laser irradiation inside the AgNP pad (cases II and III in Fig. 7b), Raman signals are generated, and the laser light is more focused on the Ag-grafted region with the presence of the microdroplet lens, as previously discussed. More importantly, when the laser irradiates outside the effective AgNP pad, such as in the cases of I and IV in Fig. 7b, no SERS signals can be generated without the droplet (displayed by the red-dashed box in Fig. 7b). However, with the presence of the microdroplet lens, the laser was refracted toward the Ag-grafted pad and generated SERS signals for analysis. These findings demonstrate that the microdroplet lens not only enhances the focusing effect for a stronger signal but also auto-guides the laser light toward the AgNP pad, enabling more effective SERS analysis. This feature allows users to easily locate hotspots for optimized SERS signals.
 | ||
| Fig. 7 Schematic illustration of (a) laser irradiation locations on the microdroplet lens, and (b) laser irradiation scenarios: cases I and IV: with a microdroplet lens, the laser is guided toward the AgNP-grafted pad. Cases II and III: laser focusing effect from the microdroplet lens. The red solid line shows the irradiation light path with a microdroplet, while the red dashed line indicates the irradiation light path when there is no microdroplet. The green solid line shows the refraction light path. The original simulation result data are available in ESI† Fig. S3. | ||
3.3 SERS analysis by the microdroplet lens
SERS analysis was performed to evaluate the LocAg-PS performance, and R6G (concentration: 10−5 M to 10−9 M) was selected as the standard sample for SERS analysis. For standard SERS analysis, one of the simplest and most effective methods is directly drying sample droplets onto the LocAg-PS pad. This approach capitalizes on the hydrophobic nature of the porous silicon background and the hydrophilic properties of the LocAg-PS surface. As a result, the droplet sample naturally aligns and concentrates on the LocAg-PS pad for SERS analysis (Fig. 8a).Given that our target was to minimize the LocAg-PS pad size to around 238 μm in radius, we anticipated that the sample was well distributed across the surface, and no significant inhomogeneity, such as the coffee ring effect, was observed (Fig. S4†). To further enhance the SERS analysis by the optical lens, after sample drying on the LocAg-PS pad, we introduced an additional 1 μL water droplet deposited onto the LocAg-PS surface as an optical focusing lens (Fig. 8b). However, using this sample dry and then adding the microdroplet lens approach, it took approximately ∼20 minutes for a 1 μL sample droplet to completely dry on the LocAg-PS surface at room temperature (∼20 °C). An alternative approach is proposed: directly applying the sample-containing droplet as a lens onto the LocAg-PS substrate without the sample drying step. This enables rapid analysis without the need for the sample drying step, streamlining SERS for rapid analysis (Fig. 8c).
Although the microdroplet lens approach provides a rapid and straightforward method for SERS analysis, using laser light in the liquid-phase microdroplet may lead to photothermal consideration, which induces heat and expedites droplet evaporation. Therefore, we evaluated the droplet evaporation by measuring the change in water contact angle, as shown in Fig. 9a. Under ambient conditions, a 1 μL droplet typically requires approximately 20 minutes to evaporate. Conversely, when it is subjected to laser irradiation, droplet evaporation accelerates, with complete drying occurring in approximately 15 minutes. Although laser irradiation accelerates droplet evaporation by about 25% (from 15 to 20 minutes), the microdroplet lens approach remains effective due to its simplicity in locating hotspots for SERS analysis. In actual SERS analysis, the laser spot irradiated on the microdroplet lens can be visually observed through the CCD camera. As illustrated in Fig. 9b, when the laser was irradiated away from the center of the microdroplet lens, a scattering halo and optical fringes around the droplet were observed under laser irradiation by the CCD camera image. The laser spot exhibited an oval shape instead of a round shape. Based on this observation, we adjusted the microdroplet lens towards the droplet's center, and the laser spot became circular. This adjustment based on the CCD image allows us to rapidly search for and locate the optimal hot spot position for SERS analysis.
 | ||
| Fig. 9 (a) Water microdroplet (1 μL) evaporation under laser irradiation and room environment. The Raman spectrometer laser energy (633 nm wavelength, 2.12 mW laser with 75% lens filtration). The raw data is available in ESI† Table S2. (b) Schematic illustration of laser positioning based on observation. | ||
The entire procedure, from sample/droplet lens deposition to SERS analysis, takes approximately 3 minutes, with only 1–2 minutes required for SERS data collection while the laser is on during this period (Movie S1†). With this operation time, as presented in Fig. 9b, the microdroplet lens maintains a high contact angle over 140°, ensuring efficient optical effects for SERS enhancement.
Fig. 10a shows the SERS spectrum on the LocAg-PS substrate with 10−5–10−9 M R6G sample concentrations and compares between the water lens, sample lens, and complete drying (as control) conditions. Results proved that both the water droplet lens (red line) and sample droplet lens (green line) can not only effectively detect the R6G sample showing all of the characteristic peaks, but also present higher signal intensity compared with the standard complete drying approach (control, black line). To further demonstrate the applicability of the microdroplet lens approach to real sample analysis, we selected melamine as our sample. Melamine is a compound commonly found in various consumer products, including plastics, adhesives, countertops, dishware, and food products. Due to its widespread use, detecting melamine in food products has become a critical concern, especially after several high-profile contamination incidents.39,40Fig. 10b demonstrates the efficacy of using a microdroplet lens for SERS in detecting melamine. The characteristic peaks of melamine at 704, 873, 983, 1120, 1256, and 1396 cm−1 were successfully observed.41,42
 | ||
| Fig. 10 Raman spectrum of completely dry, water droplet lens and sample droplet lens methods with (a) R6G sample and (b) melamine sample with concentration from 10−5–10−9 M. (c) and (d) Summarization of SERS signal intensities for R6G and melamine, respectively. The signal intensity values were obtained from the 612 cm−1 characteristic peak for R6G and 704 cm−1 characteristic peak for melamine. The full procedure of SERS analysis by the microdroplet lens (sample) is available in the ESI† Movie S1. | ||
Similar to the standard R6G sample, both the water lens (red line) and the sample lens (green line) exhibited stronger Raman signals compared to the drying condition (black line). The enhancement provided by the microdroplet lens is particularly notable at lower concentrations. For the lowest concentration of 10−9 M, no Raman signal was detected under the drying condition, indicating that standard SERS without the microdroplet lens is insufficient for such low analyte concentrations. In contrast, the water and sample lens conditions enabled the detection of melamine. Fig. 10c and d summarize the signal intensities of R6G at the characteristic peak of 612 cm−1 and melamine at the characteristic peak of 704 cm−1. The results show that the signal intensities are 2 to 5 times higher for both the water and sample droplet lenses than for the complete drying condition. This enhancement can be attributed to the microdroplet lens's ability to direct and focus the laser onto hotspots, increasing the electromagnetic field's intensity. As a result, there is an increased signal-to-noise ratio, thereby improving the sensitivity of SERS analysis. The sample droplet lens method generates a stronger signal than the water droplet lens method. We hypothesize that this enhanced signal is due to the greater spatial freedom offered by the sample droplet lens. This increased freedom allows the sample molecules to diffuse more freely across the silver nanoparticle surface, resulting in a more efficient SERS analysis (Fig. S5†). As a consequence, the lowest 10−9 M sample concentration exhibits a stronger enhancement factor of 5.21 × 108 (water lens) and 1.39 × 109 (sample lens) for R6G and 2.93 × 108 (water lens) and 6.58 × 108 (sample lens), for melamine. These results illustrate the significant signal enhancement facilitated by the microdroplet lens method.
Conclusions
This study presents a novel approach to enhancing SERS by utilizing microdroplet lenses on LocAg-PS substrates. A modified contact deposition method enables precise control over AgNP pad sizes, achieving dimensions as small as 238.5 μm in radius. The hydrophilic AgNP pads on the hydrophobic PS background enable the creation of a plano-convex-like microdroplet that acts as an optical lens, concentrating laser energy and Raman signals to enhance SERS analysis.We demonstrated that the unique hydrophobicity of the LocAg-PS substrate can not only enhance laser focus and signal collection but can also self-guide the laser toward the AgNP pad center for a more reliable SERS analysis. In addition to using water microdroplets as lenses, we also directly employed sample droplets as optical lenses for enhanced SERS analysis. Notably, the sample droplet lens approach streamlines SERS analysis by eliminating the need for sample drying, significantly reducing analysis time.
Compared to other microdroplet-based SERS techniques, our method offers simplicity, cost-effectiveness, and good performance without the need for complex fabrication processes or specialized equipment. Our approach leverages the natural hydrophobicity of the LocAg-PS substrate to precisely position water microdroplets as optical lenses, avoiding the need for additional complex fabrication steps. To demonstrate the practical utility and sensitivity of this approach for real-world applications, melamine was used as the analyte. Our SERS analyses demonstrated that water and sample droplet lenses provided 2 to 5 times higher SERS signal intensity than the drying method on LocAg-PS substrates. These findings highlight the potential of microdroplet lenses as a cost-effective and rapid solution for advancing SERS applications across various fields.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Dr. Chia-Wen Tsao is responsible for supervision, project administration, methodology, funding acquisition, conceptualization, and writing, reviewing, and editing. Mr. Zi-Yi Yang is responsible for SERS substrate fabrication, optical path simulation, Raman data acquisition and analysis, and drafting and editing the initial figures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from National Science and Technology Council, Taiwan (grand no. MOST 111-2221-E-008-065-MY3) and the cooperation between National Central University and Cathay General Hospital. The authors also gratefully acknowledge Mr. You-Shan Zheng for his initial trials of the microdroplet lens. The use of Raman spectroscope equipment belonging to the Precision Instrument Center of National Central University was also greatly appreciated. Additionally, we acknowledge using an artificial intelligence (AI) tool (ChatGPT 4.0) for refining specific graphical elements, including the laser, droplet, and porous silicon substrate image in table of contents entry of this manuscript.References
- J. Langer, D. Jimenez de Aberasturi, J. Aizpurua, R. A. Alvarez-Puebla, B. Auguié, J. J. Baumberg, G. C. Bazan, S. E. J. Bell, A. Boisen, A. G. Brolo, J. Choo, D. Cialla-May, V. Deckert, L. Fabris, K. Faulds, F. J. García de Abajo, R. Goodacre, D. Graham, A. J. Haes, C. L. Haynes, C. Huck, T. Itoh, M. Käll, J. Kneipp, N. A. Kotov, H. Kuang, E. C. Le Ru, H. K. Lee, J. F. Li, X. Y. Ling, S. A. Maier, T. Mayerhöfer, M. Moskovits, K. Murakoshi, J. M. Nam, S. Nie, Y. Ozaki, I. Pastoriza-Santos, J. Perez-Juste, J. Popp, A. Pucci, S. Reich, B. Ren, G. C. Schatz, T. Shegai, S. Schlücker, L. L. Tay, K. G. Thomas, Z. Q. Tian, R. P. Van Duyne, T. Vo-Dinh, Y. Wang, K. A. Willets, C. Xu, H. Xu, Y. Xu, Y. S. Yamamoto, B. Zhao and L. M. Liz-Marzán, ACS Nano, 2020, 14, 28–117 CrossRef CAS PubMed.
- X. X. Han, R. S. Rodriguez, C. L. Haynes, Y. Ozaki and B. Zhao, Nat. Rev. Methods Primers, 2022, 1, 87 CrossRef.
- R. Panneerselvam, H. Sadat, E.-M. Höhn, A. Das, H. Noothalapati and D. Belder, Lab Chip, 2022, 22, 665–682 RSC.
- J. Wang, Y.-C. Kao, Q. Zhou, A. Wuethrich, M. S. Stark, H. Schaider, H. P. Soyer, L. L. Lin and M. Trau, Adv. Funct. Mater., 2022, 32, 2010296 CrossRef CAS.
- D. S. Burr, W. L. Fatigante, J. A. Lartey, W. Jang, A. R. Stelmack, N. W. McClurg, J. M. Standard, J. R. Wieland, J.-H. Kim, C. C. Mulligan and J. D. Driskell, Anal. Chem., 2020, 92, 6676–6683 CrossRef CAS PubMed.
- L. Veliz, T. T. Cooper, I. Grenier-Pleau, S. A. Abraham, J. Gomes, S. H. Pasternak, B. Dauber, L. M. Postovit, G. A. Lajoie and F. Lagugné-Labarthet, ACS Sens., 2024, 9, 272–282 CrossRef CAS PubMed.
- X. Bi, L. Lin, Z. Chen and J. Ye, Small Methods, 2024, 8, 2301243 CrossRef PubMed.
- Q. Hu, C. Sellers, J. S.-I. Kwon and H.-J. Wu, Digit. Chem. Eng., 2022, 3, 100020 CrossRef PubMed.
- R. R. Jones, D. C. Hooper, L. Zhang, D. Wolverson and V. K. Valev, Nanoscale Res. Lett., 2019, 14, 231 CrossRef PubMed.
- D. Xu, F. Teng, Z. Wang and N. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 21548–21553 CrossRef CAS PubMed.
- T. T. X. Ong, E. W. Blanch and O. A. H. Jones, Sci. Total Environ., 2020, 720, 137601 CrossRef CAS PubMed.
- J. Xia, W. Li, M. Sun and H. Wang, Nanomaterials, 2022, 12, 3572 CrossRef CAS PubMed.
- L. Jiang, M. M. Hassan, S. Ali, H. Li, R. Sheng and Q. Chen, Trends Food Sci. Technol., 2021, 112, 225–240 CrossRef CAS.
- V. Moisoiu, S. D. Iancu, A. Stefancu, T. Moisoiu, B. Pardini, M. P. Dragomir, N. Crisan, L. Avram, D. Crisan, I. Andras, D. Fodor, L. F. Leopold, C. Socaciu, Z. Bálint, C. Tomuleasa, F. Elec and N. Leopold, Colloids Surf., B, 2021, 208, 112064 CrossRef CAS PubMed.
- M. Vendrell, K. K. Maiti, K. Dhaliwal and Y.-T. Chang, Trends Biotechnol., 2013, 31, 249–257 CrossRef CAS PubMed.
- C. Zong, M. Xu, L.-J. Xu, T. Wei, X. Ma, X.-S. Zheng, R. Hu and B. Ren, Chem. Rev., 2018, 118, 4946–4980 CrossRef CAS PubMed.
- C. Deriu, S. Thakur, O. Tammaro and L. Fabris, Nanoscale Adv., 2023, 5, 2132–2166 RSC.
- A. Bernat, M. Samiwala, J. Albo, X. Jiang and Q. Rao, J. Agric. Food Chem., 2019, 67, 12341–12347 CrossRef CAS PubMed.
- L. M. Almehmadi, S. M. Curley, N. A. Tokranova, S. A. Tenenbaum and I. K. Lednev, Sci. Rep., 2019, 9, 12356 CrossRef PubMed.
- A. Shiohara, Y. Wang and L. M. Liz-Marzán, J. Photochem. Photobiol., C, 2014, 21, 2–25 CrossRef CAS.
- H. Li, P. Merkl, J. Sommertune, T. Thersleff and G. A. Sotiriou, Adv. Sci., 2022, 9, 2201133 CrossRef CAS PubMed.
- Z. Deng, P. Wen, N. Wang and B. Peng, Sens. Actuators, B, 2019, 288, 20–26 CrossRef CAS.
- K. Ge, Y. Hu and G. Li, Biosensors, 2022, 12, 941 CrossRef CAS PubMed.
- C. Lin, Y. Li, Y. Peng, S. Zhao, M. Xu, L. Zhang, Z. Huang, J. Shi and Y. Yang, J. Nanobiotechnol., 2023, 21, 149 CrossRef PubMed.
- K. Milenko, S. S. Fuglerud, S. B. Kjeldby, R. Ellingsen, A. Aksnes and D. R. Hjelme, Opt. Lett., 2018, 43, 6029–6032 CrossRef CAS PubMed.
- K. Milenko, S. S. Fuglerud, A. Aksnes, R. Ellingsen and D. R. Hjelme, J. Lightwave Technol., 2020, 38, 2081–2085 CAS.
- V. Gašparić, S. Taccheo, H. Gebavi, D. Ristić and M. Ivanda, J. Raman Spectrosc., 2020, 51, 165–175 CrossRef.
- W. Tran, L. G. Tisinger, L. E. Lavalle and A. J. Sommer, Appl. Spectrosc., 2015, 69, 230–238 CrossRef CAS PubMed.
- F. Yang, P. Wen, W. Jia, G. Li, C. Yang, B. Li, D. Li and L. Chen, Analyst, 2021, 146, 6132–6138 RSC.
- C. S. Woodhead, J. Roberts, Y. J. Noori, Y. Cao, R. Bernardo-Gavito, P. Tovee, A. Kozikov, K. Novoselov and R. J. Young, 2D Mater., 2017, 4, 015032 CrossRef.
- F. Yang, P. Wen, G. Li, Z. Zhang, C. Ge and L. Chen, Biomed. Opt. Express, 2021, 12, 4795–4806 CrossRef PubMed.
- C. M. Jin, J. B. Joo and I. Choi, Anal. Chem., 2018, 90, 5023–5031 CrossRef CAS PubMed.
- Y.-T. Kim, D. Kim, S. Park, A. Zhexembekova, M. Byeon, T. E. Hong, J. Lee and C. Y. Lee, Adv. Opt. Mater., 2021, 9, 2101209 CrossRef CAS.
- W. Pang, B. Zhu, H. Li, Y. Zhou, C. M. Woo, X. Huang, T. Zhong, H. Lo, L. Wang, P. Lai and L. Nie, Laser Photonics Rev., 2024, 2400165 CrossRef.
- C.-W. Tsao, Y.-S. Zheng, Y.-S. Sun and Y.-C. Cheng, Analyst, 2021, 146, 7645–7652 RSC.
- X. Li and P. W. Bohn, Appl. Phys. Lett., 2000, 77, 2572–2574 CrossRef CAS.
- E. C. Le Ru, E. Blackie, M. Meyer and P. G. Etchegoin, J. Phys. Chem. C, 2007, 111, 13794–13803 CrossRef CAS.
- Y. Q. Wang, S. Ma, Q. Q. Yang and X. J. Li, Appl. Surf. Sci., 2012, 258, 5881–5885 CrossRef CAS.
- G. Qiao, T. Guo and K. K. Klein, Appetite, 2010, 55, 190–195 CrossRef CAS PubMed.
- C. M. Gossner, J. Schlundt, P. Ben Embarek, S. Hird, D. Lo-Fo-Wong, J. J. Beltran, K. N. Teoh and A. Tritscher, Environ. Health Perspect., 2009, 117, 1803–1808 CrossRef CAS PubMed.
- A. Hussain, D.-W. Sun and H. Pu, Food Addit. Contam.: Part A, 2019, 36, 851–862 CrossRef CAS PubMed.
- X. Du, H. Chu, Y. Huang and Y. Zhao, Appl. Spectrosc., 2010, 64, 781–785 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lc00550c |
| This journal is © The Royal Society of Chemistry 2024 |