Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electric field temporal interference stimulation of neurons in vitro†
Annika
Ahtiainen
 *a,
Lilly
Leydolph
*a,
Lilly
Leydolph
 b,
Jarno M. A.
Tanskanen
b,
Jarno M. A.
Tanskanen
 a,
Alexander
Hunold
bc,
Jens
Haueisen
a,
Alexander
Hunold
bc,
Jens
Haueisen
 bd and
Jari A. K.
Hyttinen
bd and
Jari A. K.
Hyttinen
 a
a
aFaculty of Medicine and Health Technology, Tampere University, 33520, Tampere, Finland. E-mail: annika.ahtiainen@tuni.fi
bInstitute of Biomedical Engineering and Informatics, Technische Universität Ilmenau, 98693, Ilmenau, Germany
cneuroConn GmbH, 98693, Ilmenau, Germany
dDepartment of Neurology, Jena University Hospital, 07747 Jena, Germany
First published on 12th July 2024
Abstract
Electrical stimulation (ES) techniques, such as deep brain and transcranial electrical stimulation, have shown promise in alleviating the symptoms of depression and other neurological disorders in vivo. A new noninvasive ES method called temporal interference stimulation (TIS), possesses great potential as it can be used to steer the stimulation and possibly selectively modulate different brain regions. To study TIS in a controlled environment, we successfully established an in vitro ‘TIS on a chip’ setup using rat cortical neurons on microelectrode arrays (MEAs) in combination with a current stimulator. We validated the developed TIS system and demonstrated the spatial steerability of the stimulation by direct electric field measurements in the chip setup. We stimulated cultures of rat cortical neurons at 28 days in vitro (DIV) by two-channel stimulation delivering 1) TIS at 653 Hz and 643 Hz, resulting in a 10 Hz frequency envelope, 2) low-frequency stimulation (LFS) at 10 Hz and 3) high-frequency stimulation (HFS) at 653 Hz. Unstimulated cultures were used as control/sham. We observed the differences in the electric field strengths during TIS, HFS, and LFS. Moreover, HFS and LFS had the smallest effects on neuronal activity. Instead, TIS elicited neuronal electrophysiological responses, especially 24 hours after stimulation. Our ‘TIS on a chip’ approach eludicates the applicability of TIS as a method to modulate neuronal electrophysiological activity. The TIS on a chip approach provides spatially steerable stimuli while mitigating the effects of high stimulus fields near the stimulation electrodes. Thus, the approach opens new avenues for stimulation on a chip applications, allowing the study of neuronal responses to gain insights into the potential clinical applications of TIS in treating various brain disorders.
1 Introduction
A promising new technique known as temporal interference stimulation (TIS) has been proposed to open up new possibilities in the treatment of neurological and psychiatric conditions. TIS is a non-invasive electrical stimulation (ES) method that promises targeting of neuronal structures at specific depths.1 Traditional non-invasive brain stimulation methods, such as transcranial magnetic stimulation (TMS) and transcranial electrical current stimulation (tES), have lacked selectivity and effectiveness to therapeutically address target structures at the necessary depth. Hence, the primary option has been invasive deep brain stimulation (DBS), despite its associated risks.2–4 TIS, however, possesses considerable promise, as it can be steered to affect specific deep regions without modulating the superficial layers of the brain.1,5 TIS has been demonstrated to have the ability to target deep brain structures and modulate brain functions in vivo.6 TIS has been reported to cause minimal side effects and high tolerability.7–10 Previously, TIS at theta or gamma frequency envelopes has been used to modulate brain activity in the hippocampus,6,8,11 striatum,8,12 and motor cortex,7,9 demonstrating a targeted stimulation with minimal exposure to overlying brain areas.6,12 Specifically, theta-frequency TIS has been shown to modulate hippocampal activity, enhance memory performance,6,11 and alter functional connectivity after TIS.6 When targeted to the striatum, TIS has been shown to improve motor performance, and modulate the activity in the targeted structures.12 Another TIS study showed increased neuromodulatory influence between the striatum and motor cortex.13 In contrast, higher frequency TIS has been shown to facilitate motor learning, while gamma-frequency TIS enhanced motor cortex excitability compared to sham.7 However, there have been no previous studies of TIS on a chip for in vitro neuronal systems; thus, our approach potentially offers new ways for studying the effects of TIS and provides methods for neuronal stimulation in vitro, especially for lab-on-a-chip approaches.TIS is realized using two high-frequency sinusoidal currents with different frequencies (i.e., f2 > f1 > 600 Hz) that, when interfering in the volume conductor, form an interference signal with a low-frequency “envelope” Δf (Δf = f2 − f1), usually between 1 and 100 Hz. As the high-frequency fields are above the frequency range defined by the cell membrane time constant, cells – and neurons – do not react individually to the high frequencies. However, the low-frequency interference field of the resulting beat frequency Δf has the potential to influence neuronal transmembrane potential14–16 through the nonlinearity of the ion channels charging the membrane capacitance.17–20 Therefore, TIS has recently drawn attention from researchers as it could offer new ways of modulating the neuronal systems and treating neurological conditions such as Parkinson's disease and chronic pain.21–27 So far, TIS has been demonstrated using computational studies23,25,28–31 and in vivo applications with rodents1,32 and human subjects.6,7,33 However, before our work, TIS had not been tested with in vitro cell cultures, where the effects of electrical field interference (EFI) on neuronal cells on chip and their interactions could be assessed more in detail.
In this study, the aim was to establish and assess a new in vitro ‘TIS on a chip’ platform for neuronal cell cultures. The stimulation setup consists of an EFI current stimulator, electrodes for the stimulation, and a microelectrode array (MEA) system, capable of recording and monitoring the resulting stimulus fields and neuronal electrical activity in real time.34 We validated the developed setup by utilizing different ES paradigms to compare the effects of various stimulation modalities on previously characterized neuronal cultures35,36in vitro. We investigated how neurons respond to TIS and its low-frequency (LFS) and high-frequency (HFS) counterparts, both in short term and over an extended period. We observed that TIS highly affected the electrophysiological activity of neurons, especially 24 hours after stimulation. Furthermore, we measured the electric field strengths of the stimuli and demonstrated the spatial steerability of TIS in our setup. We showed and validated the usability of our in vitro TIS setup, which innovatively integrates electrical current stimulation and electrophysiology recording setup on a chip. Our system provides a new way to assess the modulatory effects of TIS on neuronal electrophysiology, and therefore, to study the potential of TIS in cellular models of therapeutic applications.
2 Experimental section
2.1 Cell culture
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 neurons. In the same way, neurons were seeded on coverslips and μwells for further characterization, but the cell counts were ½ or ¼ of that was used for MEAs, respectively. Neuron cultures were maintained in neurobasal plus medium with 2% B-12 Plus supplement, 1% P/S, and 1% GlutaMAX supplement (all purchased from Thermo Fisher Scientific). Cells were fed by replacing at least half of the medium three times a week, and always after recording electrical activity. Cell growth and morphology were constantly monitored using a Nikon Eclipse Ts2 (Nikon Corporation, Japan) microscope.
000 neurons. In the same way, neurons were seeded on coverslips and μwells for further characterization, but the cell counts were ½ or ¼ of that was used for MEAs, respectively. Neuron cultures were maintained in neurobasal plus medium with 2% B-12 Plus supplement, 1% P/S, and 1% GlutaMAX supplement (all purchased from Thermo Fisher Scientific). Cells were fed by replacing at least half of the medium three times a week, and always after recording electrical activity. Cell growth and morphology were constantly monitored using a Nikon Eclipse Ts2 (Nikon Corporation, Japan) microscope.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), microtubule associated protein 2 (MAP2; PA1-10005, chicken, 1
1000), microtubule associated protein 2 (MAP2; PA1-10005, chicken, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 or 1
1000 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000), postsynaptic density protein 95 (PSD-95; MA1-045, mouse, 1
2000), postsynaptic density protein 95 (PSD-95; MA1-045, mouse, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), and synaptophysin (SYN; MA5-14532, rabbit, 1
200), and synaptophysin (SYN; MA5-14532, rabbit, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). The secondary antibodies used were goat anti-mouse 488 (A32723; 1
50). The secondary antibodies used were goat anti-mouse 488 (A32723; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), goat anti-Rabbit 555 (A-21428; 1
500), goat anti-Rabbit 555 (A-21428; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), and goat anti-chicken 647 (A32933; 1
500), and goat anti-chicken 647 (A32933; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500). All the antibodies were purchased from Thermo Fisher Scientific (Waltham, MA, USA), except for AB5804, which was purchased from Sigma-Aldrich (St. Louis, MO, USA), and all were diluted in 5% (v/v) goat serum (Sigma-Aldrich) in Dulbecco's phosphate-buffered saline (DPBS). Samples were stained with DAPI (4′,6-diamidino-2-phenylindole; D1306, Thermo Fisher Scientific) prior to mounting (10–15 min at RT). Samples were mounted with ProLong™ Gold Antifade Mountant (P10144, Thermo Fisher Scientific; 24 hours in the dark at RT). The coverslips were stored at +4 °C until imaging with an Olympus IX51 fluorescence microscope with an Olympus DP30BW camera (Olympus Corporation, Hamburg, Germany). Fiji (ImageJ, National Institute of Health, USA) software was used to process the images.
500). All the antibodies were purchased from Thermo Fisher Scientific (Waltham, MA, USA), except for AB5804, which was purchased from Sigma-Aldrich (St. Louis, MO, USA), and all were diluted in 5% (v/v) goat serum (Sigma-Aldrich) in Dulbecco's phosphate-buffered saline (DPBS). Samples were stained with DAPI (4′,6-diamidino-2-phenylindole; D1306, Thermo Fisher Scientific) prior to mounting (10–15 min at RT). Samples were mounted with ProLong™ Gold Antifade Mountant (P10144, Thermo Fisher Scientific; 24 hours in the dark at RT). The coverslips were stored at +4 °C until imaging with an Olympus IX51 fluorescence microscope with an Olympus DP30BW camera (Olympus Corporation, Hamburg, Germany). Fiji (ImageJ, National Institute of Health, USA) software was used to process the images.
2.2 Stimulation setup and system validation
MEA wells were connected to the DC-STIMULATOR MC (neuroConn GmbH, Germany), which provided the stimulation currents to the culture medium via platinum electrodes. An illustration of the stimulation and recording setup is shown in Fig. 1A. MEA signals from neurons were recorded prior to, during, and after stimulation with the MEA2100 system (Multi Channel Systems MCS GmbH), which enabled real-time monitoring of the effects of stimulus on neurons. Stimuli were applied via four platinum rod electrodes (1 mm × 0.5 mm × 12 mm) through an in-house 3D-printed MEA cap composed of Biomed Amber Resin (Formlabs GmbH, Germany). The cap was printed with a stereolithography 3D printer Form 3B+ (Formlabs GmbH, Germany) and designed using Solidworks 2021 (Dassault Systèmes SolidWorks Corp., France). Various cap designs were tested and validated prior to the final design to establish the desired electric fields. The cap consisted of four elevated holes for inserting the platinum rod electrodes that were submerged in the cell medium. Additionally, the cap had two air holes for a ventilation tube (0.3 mbar; 5% CO2 + 19% O2 + 75% N2). Holes for the platinum rods (1.2 × 0.8 mm) were inserted at a 25° angle to obtain appropriate TIS conductance.The generated currents for TIS and high frequencies were initially confirmed with measurements using an oscilloscope (MP720665 from Multicomp Pro, Premier Farnell Ltd, USA) (ESI† Fig. S1A and B), as well as thereafter with the MEA2100 system, which was also used during the experiments to record electrical activity of neurons.
The stimulation and recording setups were used to stimulate rat cortical neurons on MEAs (Fig. 1B). We used four different stimulation groups – temporal interference stimulation (TIS), high-frequency stimulation (HFS), low-frequency stimulation (LFS), and control/sham for neurons (n = 16 MEAs; 4 MEAs per group). TIS was realized with two slightly different high-frequency currents (f1 and f2) that produced a low beat frequency (Δf), which fell into the range to which neurons could respond (Fig. 1C). To compare the effects of TIS, HFS and LFS with frequencies corresponding to that of TIS, HFS and LFS were also used to stimulate neurons (Fig. 1D; see Section 2.3).
2.3 Stimulation and MEA recordings
The electrical stimulus (TIS/HFS/LFS) was applied for five minutes at 28 days in vitro (DIV). The final stimulation parameters were chosen to be 450 μA delivered from both stimulation sites, and a 5 minute stimulation time. These parameter values were selected because they induced relatively high stimulation field amplitudes without being seemingly harmful to the neurons based on a pilot experiment run prior to the full-scale experiment reported herein. Neuronal electrical activity was recorded before stimulation/sham, immediately after, one hour after, 24 hours after, and one week after stimulation/sham (Fig. 1E). MEAs were always allowed to settle in the preamplifier for five minutes before the first recording. For the stimulation, MEAs were not moved from the recording site until the first stimulation was conducted immediately after recording the baseline electrical activity. ES (or sham) was applied for five minutes. For those MEAs that did not receive any stimulation, the stimulation was simulated by keeping the MEAs outside the incubator in the MEA headstage for the same amount of time as those MEAs that were stimulated (TIS/HFS/LFS). After the ES (or sham), at least half of the cell medium was replaced, and MEAs were put back in the incubator. Data were collected and analyzed by comparing the stimulation effects to the percentual change in the control cultures at each time point in question (see Section 2.4.3 for more details). The purpose of using LFS (at fL = Δf = 10 Hz) and HFS (at f1 = 653 Hz) was to compare these stimulation paradigms alone to enable evaluation of the effects of interference stimulation with respect to control cultures without stimulation. Hence, the stimulation parameters, such as the applied stimulation currents, were kept equal for all stimulation modalities (TIS/HFS/LFS). The stimulation frequencies were selected according to the hardware limitations so that the f1 and f2 were the highest possible prime numbers below 700 Hz (hardware bandwidth limit) with a 10 Hz difference. Detailed stimulation paradigms are available in Table 1.| TIS | LFS | HFS | Control | |
|---|---|---|---|---|
| Current (μA) | 450 | 450 | 450 | — |
| Frequency (Hz) | Channel 1: 653 | Channel 1: 10 | Channel 1: 653 | — |
| Channel 2: 643 | Channel 2: 10 | Channel 2: 653 | ||
| Ventilation during stimulation recordings | + | + | + | + |
| Days in vitro (DIV) for stimulations | 28 | 28 | 28 | 28 |
| n (MEAs) | 4 | 4 | 4 | 4 |
2.4 Data analysis
Raw electrophysiological data were further analyzed with Matlab 2022a (MathWorks, Inc., Natick, MA, USA) using a previously published, widely used tool for spike sorting,37 also implemented in.35 Briefly, a second-order bandpass (300–3000 Hz) elliptic filter with a threshold of ±5σ (σ = median (|x|/0.6745), x = bandpass filtered signal) and 1.5 second detector dead time after each spike was used for sorting. From the sorted, filtered signals, we analyzed the timestamps of neuronal spikes and other parameters of the electrical activity, such as spike rate, burst rate, burst duration, spikes in bursts, burst spike ratio (BSR%), and interspike interval (ISI) in bursts (details in Table 3). These values were extracted from the data using a tool developed previously in our group.38,39 Signal traces and impact electrode data (ESI† Fig. S9) were extracted from the analyzed data and plotted with Matlab 2022a.
To observe stimulation electric field strengths, bipolar signals were calculated from the unfiltered MEA data recorded during the different stimulation modalities. Bipolar signals were calculated by subtracting the signals from two adjacent microelectrodes for all microelectrodes (cf., Fig. 2D for the MEA layout) in both horizontal and vertical directions, resulting in two sets of bipolar signals (later denoted as Ex [mV mm−1] and Ey [mV mm−1], respectively). The bipolar signals were converted to field strengths, given the microelectrode distance of 200 μm. The sets of the EFI field strengths for all the used stimulation modalities and the microelectrodes are illustrated in Fig. 3 and S2.†
Power spectral density (PSD) analysis was conducted for the same MEA data used in the stimulation electric field strength analysis. Briefly, PSD and spectrogram analysis were performed on raw unfiltered signals and analyzed for one electrode signal (electrode 44) that was the same for all stimulation modalities. Welch's power spectral density estimates40 were calculated for the unfiltered and filtered signals. For filtering, an IIR notch filter at a) 653, 643, and 50 Hz (TIS), b) 653 and 50 Hz (HFS), c) 10 and 50 Hz (LFS), and 50 Hz (control/sham) was applied and plotted with Matlab 2022a. The PSD and spectrogram results for all the used stimulation modalities are illustrated in Fig. 4 and S3–S6.†
3 Results and discussion
3.1 Electrical stimulation concept and validation on microelectrode arrays
We established an in vitro stimulation and recording setup by combining a current stimulator with a MEA system that can record neuronal electrical activity. The stimulus was conducted via platinum rod electrodes to cells located on the MEA electrode area. Fig. 2A–C shows the design of the 3D-printed cap through which the platinum electrodes were inserted. The directions from the platinum electrodes to the MEA area (with the neurons) is illustrated in Fig. 2D. Fig. 2E illustrates the schematics of the TIS setup with two electrode pairs fed with currents of 450 μA.Prior to stimulation of cells, the stimulation system that comprised of the stimulator and the MEA recording system was verified without any cell cultures. Using only the cell culture medium, all stimulations (TIS, HFS, and LFS) were observed in the MEA2100 recordings (Fig. 2F). Fig. 2G shows 100 ms close-ups of each of the stimulations. The LFS had the lowest frequency at 10 Hz, the HFS had high-frequency characteristics at 653 Hz, and the TIS had 10 Hz envelopes, along with the high-frequency signals within the envelopes, as expected.
Next, we evaluated and validated the system with cell cultures; Fig. 3A shows the entire MEA electrode area of one representative TI-stimulated neuronal culture. Depending on the electrode in question, the TIS amplitudes were substantially higher, especially compared to control and LF-stimulated cultures (Table 2). The recorded TIS amplitude was the lowest around the reference electrode (electrode number 15), which was also internally grounded in the system. The amplitudes were the highest further away from the reference electrode.
| Minimum values; mean ± SD | Maximum values; mean ± SD | |||||
|---|---|---|---|---|---|---|
| Voltage (mV) | E x (mV mm−1) | E y (mV mm−1) | Voltage (mV) | E x (mV mm−1) | E y (mV mm−1) | |
| Ctrl | −0.4; −0.1 ± 0.1 | −2.0; −0.9 ± 0.5 | −2.1; −0.9 ± 0.5 | 0.4; 0.1 ± 0.1 | 2.2; 0.9 ± 0.5 | 2.1; 0.9 ± 0.5 |
| TIS | −2.7; −2.0 ± 0.5 | −5.9; −1.8 ± 1.2 | −4.1; −1.4 ± 0.7 | 2.7; 2.0 ± 0.5 | 5.7; 1.8 ± 1.2 | 4.5; 1.4 ± 0.8 |
| HFS | −1.2; −0.7 ± 0.2 | −3.1; −1.1 ± 0.8 | −3.3; −1.1 ± 0.7 | 1.0; 0.7 ± 0.2 | 4.7; 1.0 ± 0.8 | 2.8; 1.0 ± 0.7 |
| LFS | −0.9; −0.3 ± 0.1 | −2.2; −1.0 ± 0.5 | −2.9; −1.0 ± 0.5 | 0.6; 0.3 ± 0.1 | 3.7; 1.2 ± 0.6 | 3.6; 1.1 ± 0.6 |
As the field potentials in MEA are recorded against a common reference electrode (electrode number 15), we also calculated the local electric field strengths in both horizontal and vertical directions (Ex [mV mm−1] and Ey [mV mm−1], respectively) for control, TIS, HFS, and LFS-stimulated cultures on MEA. The calculated field strengths during TIS are shown in Fig. 3B and the other stimulation modalities (HFS, LFS, control/sham) can be found in ESI† Fig. S2. For the TIS-stimulated MEA, the electric field strengths also varied within the electrode area, being the highest in the middle of the electrode area in both vertical and horizontal directions and lowest at the edges of the electrode area (Fig. 3B). Specifically, amongst the 59 electrodes and different field directions considered, the local interference electric field strengths were highest between the electrode pairs in the middle of the electrode array in both horizontal and vertical directions – a result fitting nicely with the theoretical consideration of the EFI. For the other stimulation modalities, the local electric field strengths were lower compared to TIS (Fig. 3C, D and Table 2). TIS promises selective targeting of different spatial areas of neural populations, while at the same time minimizing off-target effects.1,42–44 Notably, our findings indicate a spatial selectivity of the introduced TIS system, thus potentially facilitating targeting specific stimulation areas. Contrarily, with conventional stimulation methods, the local electric field strengths are the highest in proximity to the stimulation electrodes.45,46 Our results highlight the potential of TIS to produce high local electrical interference fields further away from the stimulus electrodes. Hence, our results indicate that TIS can open new prospects for spatially targeted ES applications not only in vivo but also in vitro.
We also validated the setup by characterizing TIS signal power content over frequency (power spectral density [PSD]). Notably, the stimulation frequencies of f1 = 653 Hz and f2 = 643 Hz were clearly visible in the unfiltered signal that was recorded during stimulation (Fig. 4A and B). Also, the spectrogram analysis that reveals the spectrum of frequencies over time demonstrated clear bands for the stimulation frequencies (f1 and f2) during the entire stimulation (Fig. 4C and D). The corresponding results for the other stimulation modalities (HFS, LFS, and control/sham) are available in ESI† Fig. S3–S6.
3.2 Neuronal cell cultures
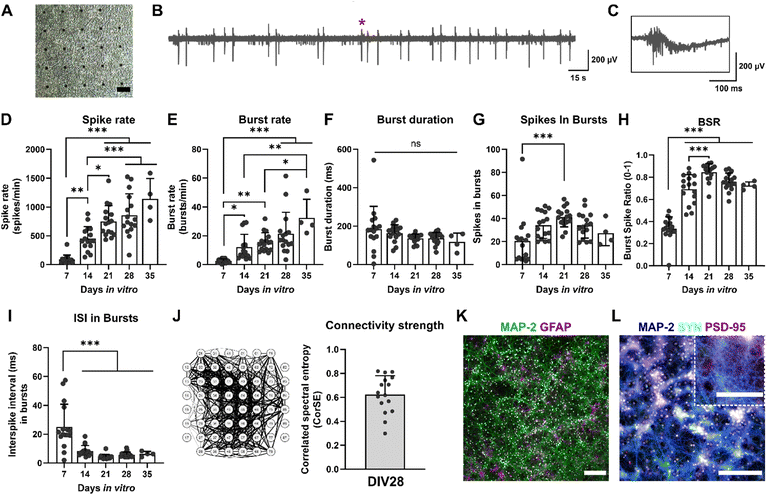
Primary rat cortex neurons were used to assess the impact of TIS on neurons. Neuronal cultures were followed for 35 days on MEAs (representative images of the cultures at DIV28 in Fig. 5A). Fig. 5B and C shows the representative signal traces of neurons at DIV28.The electrical activity of the cultures was evaluated weekly, starting with DIV7. The assessed electrophysiological features and their descriptions are listed in Table 3. All statistical tests and details of the maturation of the cultures can be found in ESI† Table S1. The spike rates and burst rates rose steadily until DIV35 for all the cultures, as expected (Fig. 5D and E). The burst duration shortened toward DIV35 but remained statistically unchanged (Fig. 5F). Also, the number of spikes in bursts reduced toward DIV35 and was significantly higher at DIV21 than DIV7 (Fig. 5G). The ratio of spikes involved in bursts (BSR) was lower at DIV7 but rose rapidly already from DIV14 (Fig. 5H). The interspike interval (ISI) in bursts was the highest at DIV7 and decreased significantly for DIV14, 21, 28, and 35 (Fig. 5I). The number of active electrodes was still 59 at DIV35, except for one MEA (ESI† Fig. S1C). Furthermore, the connectivity of the neuronal cultures was evaluated with correlation spectral entropy (CorSE) analysis. In general, the cultures had a high neuronal network connectivity across the MEA area (0.62 ± 0.16 out of 1; Fig. 5J).
| Electrophysiological features | Description | Unit/scale |
|---|---|---|
| Number of active electrodes | The number of active electrodes in MEA. An electrode is considered active if it has ≥10 spikes per min (otherwise excluded from the analysis) | 0–60 |
| Spike rate | Number of spikes per minute (spike detection threshold ±5σ) | 1 min−1 |
| Burst rate | Number of bursts per minute. Burst consists of ≥3 spikes | 1 min−1 |
| Burst duration | The average duration of a burst | ms |
| Spikes in bursts | The average number of spikes in bursts | ≥0 |
| Burst spike ratio (BSR%) | Percentage of spikes involved in bursts | 0–1 (1 = 100%) |
| Interspike interval (ISI) in bursts | Average time between sequential spikes in bursts | ms |
| Correlated spectral entropy (CorSE) | Evaluates MEA signal synchronicity by calculating the correlations of time-varying spectral entropies between the MEA channels (connection threshold ≥0.75) | 0–1 |
The viability of the cultures was analyzed at DIV28. The cultures had very few dead cells (viability 83 ± 10%, ESI† Fig. S1D). Furthermore, at DIV29, cultures were stained with immunocytochemistry (ICC), and the cultures expressed their typical neuronal and astrocytic proteins, as expected (Fig. 5K). Moreover, pre- and postsynaptic proteins, indicated by synaptophysin (SYN) and postsynaptic density protein-95 (PSD-95), were present, further implying functional connections between the neurites in the cultures (Fig. 5L). The electrophysiological properties and other characteristics of the cultures, such as cell composition and morphology, resembled those in our previous studies using the same cells.35,36 The neurons exhibited a relatively high electrophysiological activity, similarly to what we have previously observed.36 Hence, the neuronal cultures exhibited their typical electrophysiological and morphological features; the functional networks exhibited robust spiking and bursting activities, as also observed previously by others.47,48
3.3 Electrical stimulation of neurons in vitro
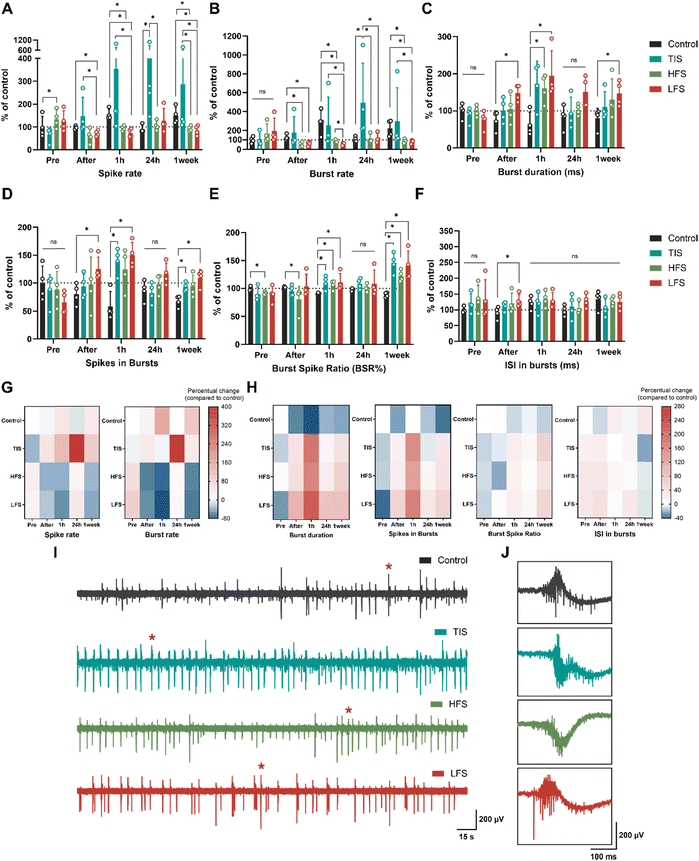
At DIV28, neurons were subjected to ES or sham for five minutes (n = 4 MEAs/group, as described in Fig. 1). All statistical tests with details can be found in ESI† Tables S2–S4. Prior to electrical stimulation, all the cultures exhibited very similar activity except for the HFS cultures, which had slightly elevated spike rates (+34%, p = 0.0286; Mann–Whitney U test) and smaller BSRs than control (−8%, p = 0.0286) (Fig. 6A and E). Moreover, neurons were electrically active also during TIS, which was revealed after filtering the stimulation frequencies from the measured neuronal signals (ESI† Fig. S4). However, the spiking activity was lower during stimulation compared to baseline activity measured before the stimulation.Directly after the five-minute stimulation, LFS cultures experienced decreased spike rates (−26%), burst rates (−52%), elevated burst duration (+105%), number of spikes in bursts (+61%), and ISI (+41%) compared to control cultures (Fig. 6A–D and F; p = 0.0286 for all). As expected, HFS did not affect the neurons as much as the LFS, but the HFS cultures had decreased burst rates (−59%) and BSR (−20%) compared to control cultures right after the stimulation (Fig. 6B and E; p = 0.0286). Furthermore, one hour after stimulation, LFS and HFS showed decreased spiking (−51% and −37%, respectively), and bursting (−80% and −70%, respectively), increased burst duration (+227 for LFS and 171% for HFS) and BSRs (+22% for LFS and +17% for HFS), and an increased number of spikes in bursts (+178%) for the LFS (p = 0.0286 for all). Moreover, TIS cultures had an elevated number of spikes in bursts (+159%) and BSR (+22%) one hour after the stimulation compared to the control cultures (p = 0.0286), and the differences were emphasized the next day.
Twenty-four hours after the stimulation, TIS cultures had significantly increased spike rates and burst rates by +397% and +389%, respectively (Fig. 6A, B and G; p = 0.0286 for both). There were no differences in electrical activity for the LFS and HFS cultures after 24 hours compared to control cultures, but the differences in spike rates and burst rates were significant compared to TI-stimulated cultures (p = 0.0286). This result is also in line with a previous in vivo study that reported hippocampal TIS to improve associative memory 24 hours after stimulation, but not earlier, after 90 minutes (Δf = 5 Hz).11 Burst durations, spikes in bursts, BSRs, and ISI in bursts remained unchanged for all the stimulation conditions 24 hours after the stimulation (Fig. 6C–F and H).
Cultures were also assessed one week after the stimulation, and LFS and HFS cultures still showed decreased spike (−40% and −34%, respectively) and burst rates (−61% and −57%, respectively) compared to control and TI-stimulated cultures (p = 0.0286 for all). Also, burst duration was higher (+86%) for the LFS, as were the number of spikes in bursts and BSR (+60% and 58%, respectively, p = 0.0286). Furthermore, TI-stimulated cultures showed elevated numbers of spikes in bursts and increased BSRs one week after the stimulation compared to control cultures (+36% and +64%, p = 0.0286). The ISI in bursts remained unchanged for all one week after the stimulation (Fig. 6F).
Taken together, TI-stimulation was the only form of ES applied that elicited the neuronal electrophysiological activity as seen in increased spiking and bursting activity after the stimulation (Table 4). The temporally interfering electrical fields increased the neuronal electrical activity, and the trend was already visible one hour after the stimulation, but the effect was emphasized 24 hours after the stimulation. On the contrary, the “conventional” LFS suppressed neuronal electrical activity instead of eliciting it. This suppressing effect was evident immediately after stimulation and lasted up to a week. The HFS had less impact on the neuronal cultures, but seemed to impede electrical activity, especially one hour and one week after the applied stimulus. The percentual changes compared to the changes in the control cultures are depicted in Fig. 6G for the spike rates and burst rates and in Fig. 6H for the rest of the parameters. All stimulation results are summarized in Table 4.
| Control | TIS | HFS | LFS | |||||
|---|---|---|---|---|---|---|---|---|
| Time point | % | % | p | % | p | % | p | |
| Spike rate | After | −4 | 53 | ns | −37 | ns | −26 | * |
| +1 h | 50 | 136 | ns | −37 | * | −51 | * | |
| +24 h | −12 | 397 | * | 20 | ns | 45 | ns | |
| +1 week | 41 | 102 | ns | −34 | * | −40 | * | |
| Burst rate | After | 25 | 41 | ns | −59 | * | −52 | * |
| +1 h | 170 | −5 | ns | −70 | * | −80 | * | |
| +24 h | 1 | 389 | * | 12 | ns | 17 | ns | |
| +1 week | 111 | 42 | ns | −57 | * | −61 | * | |
| Burst duration | After | −28 | 40 | ns | 45 | ns | 105 | * |
| +1 h | −40 | 192 | ns | 171 | * | 227 | * | |
| +24 h | −18 | 15 | ns | 26 | ns | 84 | ns | |
| +1 week | −21 | 41 | ns | 66 | ns | 86 | * | |
| Spikes in bursts | After | −22 | 21 | ns | 26 | ns | 61 | * |
| +1 h | −46 | 159 | * | 131 | ns | 178 | * | |
| +24 h | −14 | −5 | ns | 14 | ns | 35 | ns | |
| +1 week | −32 | 36 | * | 38 | ns | 60 | * | |
| BSR% | After | 1 | −5 | ns | −20 | * | 3 | ns |
| +1 h | −9 | 22 | * | 17 | * | 22 | * | |
| +24 h | −3 | 11 | ns | 3 | ns | 11 | ns | |
| +1 week | −11 | 64 | * | 38 | * | 58 | * | |
| ISI in bursts | After | −9 | 23 | ns | 32 | ns | 41 | * |
| +1 h | 22 | 0 | ns | 10 | ns | 5 | ns | |
| +24 h | −2 | 13 | ns | 8 | ns | 31 | ns | |
| +1 week | 33 | −21 | ns | −5 | ns | −6 | ns | |
The representative signal traces 24 hours after the stimulation for all the culture conditions are presented in Fig. 6I and J. Moreover, the functional connectivity changes 24 hours after the stimulation, when the effects were the most prominent, were evaluated using CorSE analysis. ESI† Fig. S7 represents the CorSE figure of a representative MEA of each group 24 hours after the stimulation and the results of the analysis. The connectivity results indicated that none of the stimulation methods affected the connectivity strengths of the cultures despite the changes in neuronal spiking and bursting, especially due to the TIS. Representative raster plots for each stimulation modality are presented in ESI† Fig. S8.
The reasons for the observed impact in neuronal cultures are multifaceted – starting with the parameters of the stimulation, including the applied stimulation current and duration. Most brain stimulation studies, including the original study by Grossmann et al.,1 use a 20 minute stimulation time, as it has been shown to induce direct effects and aftereffects in electroencephalograms (EEGs).49,50 Moreover, in recent in vivo studies, longer stimulation times, ranging from ten minutes to over half an hour, have been applied.6,9,12 However, these long stimulation durations were not feasible in our study setup, as our purpose was to demonstrate the TIS in vitro setup and the effects of short stimulation. Whereas a number of TIS studies have reported clear effects in certain applications, e.g., Piao et al.9 found no significant effects. Our setup will open future research possibilities to assess TIS in a controlled in vitro environment. Using our in vitro tools, future studies can now assess different cell targets, stimulation envelopes, and carrier frequencies that have been applied in vivo.6,8–12 Due to present hardware limitations, we were not able to replicate the Grossman setup1 with respect to the carrier frequency. It would be helpful to systematically investigate the influence of different carrier frequencies. However, our results suggest that the frequency limitation of our stimulator was not significant for our conclusions and demonstrate the applicability of TIS at the utilized carrier frequencies. Our in vitro ‘TIS on a chip’ setup would open new avenues to explore the mechanisms of various stimulus parameters, including stimulus frequency combinations.
As hypothesized in the TIS theory, HFS at 653 Hz did not evoke increased electrophysiological responses in neurons, nor did the LFS at 10 Hz. Instead, HFS and LFS seemed to suppress neuronal spiking and bursting activity, and this suppressive effect seemed to persist even one week after stimulation. This suggests that LFS and HFS may have affected neurons, for example, by changing their excitability through long-term depression or potentiation and alterations in synaptic plasticity.51 Furthermore, previous research suggests that the effects of HFS and LFS on neuronal activity are highly dependent on the specific stimulus site and parameters but that they could suppress epileptiform activity.52–55 Moreover, emerging evidence suggests that high-frequency conduction blocks might block or inhibit the propagation of action potentials along axons, thereby contributing to the observed effects.23 However, it is noteworthy to acknowledge that, in general, the ES parameters used, aspects of the experimental setup such as invasiveness, and consequently the results usually vary highly across studies, both in vitro and in vivo.
We also evaluated whether the different stimulation modalities affected neurons differently depending on their location on the microelectrode area. The spike rates for the individual electrodes were analyzed in a similar manner to Section 3.3. The spatial analysis revealed consistent results – the electrophysiological spiking activity of neurons increased 24 hours after TIS (ESI† Fig. S9A). However, there were no significant spatial differences observed between the middle (n = 16 electrodes) and edge (n = 43 electrodes) electrodes in any (TIS/HFS/LFS/control) of the stimulated cultures (ESI† Fig. S9B). This observed network-wide TIS impact is influenced by dynamic interactions, synaptic strengths, functional connectivity, and action potential propagation in the cortical network, all of which contribute to the activation of neurons across the whole culture area.56–58 Moreover, as shown in Section 3.3, the effects of TIS are long-lasting, suggesting sustained modulation of neuronal activity over time and across the cell neuronal culture. Therefore, further investigation and a larger microelectrode surface area may be needed to elucidate the precise spatiotemporal effects of TI-stimulation in vitro.
4 Conclusions
Temporal interference stimulation (TIS) utilizes slightly differing frequencies that superimpose spatially and temporally to create a low-frequency beating amplitude electric field. In this study, we introduced an experimental in vitro setup that enabled us to explore the effects of TIS on neuronal electrophysiology at the cellular and network levels. We successfully established, verified, and validated the ‘TIS on a chip’ setup for non-invasive stimulation of neurons with and without cell cultures. Using direct MEA electrode voltage measurements, we demonstrated the capability of TIS to provide spatially targetable stimulation fields in vitro. Moreover, employing neuronal cultures on MEAs, we observed pronounced effects of TIS on these cultures, resulting in a prominent electrophysiological response. TIS on a chip approach holds promise for assessing the interactions between neural cells and in vitro controlled EFI stimulation for brain modulation purposes. TIS offers better spatial control over the stimulation while minimizing unwanted effects on non-targeted brain regions, thus making it an attractive subject of study for future stimulation methods both in vitro and in vivo. Our study provides insight into the modulatory effects of different stimulation systems and their influence on neuronal electrophysiology. Our in vitro ‘TIS on a chip’ application enables spatiotemporal control over the modulation of stimulation fields, thereby providing a demonstration of using TIS on a chip to gain a deeper understanding of the mechanism and applicability of TIS in vivo.Data availability
ESI† is available for this paper. Datasets are available on request: the raw data supporting the conclusions of this article will be made available by the authors upon a reasonable request. Correspondence and requests for materials should be addressed to E-mail: annika.ahtiainen@tuni.fi. All original MATLAB codes used in the paper are publicly available.Author contributions
Conceptualization: J. A. K. H., J. H., A. H., A. A.; data curation: A. A., L. L.; formal analysis: A. A.; funding acquisition: J. A. K. H., J. H.; investigation: A. A., L. L.; methodology: A. A., L. L.; project administration: J. A. K. H., J. H., J. M. A. T.; resources: J. A. K. H., J. M. A. T., J. H., A. H.; software: A. A., J. M. A. T.; supervision: J. A. K. H, J. H.; validation: A. H., J. A. K. H., J. H., J. M. A. T., A. A., L. L.; visualization: A. A.; writing – original draft: A. A.; writing – review & editing: J. A. K. H., J. M. A. T., J. H., A. H., A. A.Conflicts of interest
A. H. is partially employed with neuroConn GmbH. Otherwise, the authors declare no conflict of interest.Acknowledgements
The authors acknowledge the doctoral school of Faculty of Medicine and Health Technology, Tampere University, for supporting the work of A. A. and the Tampere Imaging Facility for their services. The authors acknowledge the Academy of Finland – funded Centre of Excellence in Body-On-Chip Research (grant number 353178) for supporting laboratory equipment. Otherwise, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.Notes and references
- N. Grossman, D. Bono, N. Dedic, S. B. Kodandaramaiah, A. Rudenko, H.-J. Suk, A. M. Cassara, E. Neufeld, N. Kuster, L.-H. Tsai, A. Pascual-Leone and E. S. Boyden, Cell, 2017, 169, 1029–1041.e16 CrossRef CAS PubMed.
- J. K. Krauss, N. Lipsman, T. Aziz, A. Boutet, P. Brown, J. W. Chang, B. Davidson, W. M. Grill, M. I. Hariz, A. Horn, M. Schulder, A. Mammis, P. A. Tass, J. Volkmann and A. M. Lozano, Nat. Rev. Neurol., 2021, 17, 75–87 CrossRef.
- A. M. Lozano, N. Lipsman, H. Bergman, P. Brown, S. Chabardes, J. W. Chang, K. Matthews, C. C. McIntyre, T. E. Schlaepfer, M. Schulder, Y. Temel, J. Volkmann and J. K. Krauss, Nat. Rev. Neurol., 2019, 15, 148–160 CrossRef PubMed.
- A. Videnovic and L. V. Metman, Mov. Disord., 2008, 23, 343–349 CrossRef.
- J. Dmochowski and M. Bikson, Cell, 2017, 169, 977–978 CrossRef CAS.
- I. R. Violante, K. Alania, A. M. Cassarà, E. Neufeld, E. Acerbo, R. Carron, A. Williamson, D. L. Kurtin, E. Rhodes, A. Hampshire, N. Kuster, E. S. Boyden, A. Pascual-Leone and N. Grossman, Nat. Neurosci., 2023, 26(11), 1994–2004 CrossRef CAS.
- R. Ma, X. Xia, W. Zhang, Z. Lu, Q. Wu, J. Cui, H. Song, C. Fan, X. Chen, R. Zha, J. Wei, G.-J. Ji, X. Wang, B. Qiu and X. Zhang, Front. Neurosci., 2022, 15, 800436 CrossRef.
- P. Vassiliadis, E. Stiennon, F. Windel, M. J. Wessel, E. Beanato and F. C. Hummel, J. Neural Eng., 2024, 21, 024001 CrossRef.
- Y. Piao, R. Ma, Y. Weng, C. Fan, X. Xia, W. Zhang, G. Q. Zeng, Y. Wang, Z. Lu, J. Cui, X. Wang, L. Gao, B. Qiu and X. Zhang, Brain Sci., 2022, 12, 1194 CrossRef PubMed.
- Y. Zhang, Z. Zhou, J. Zhou, Z. Qian, J. Lü, L. Li and Y. Liu, Front. Hum. Neurosci., 2022, 16, 918470 CrossRef PubMed.
- T. Popa, E. Beanato, M. J. Wessel, P. Menoud, F. Windel, P. Vassiliadis, I. R. Violante, K. Alania, P. Dzialecka, N. Grossman, E. Neufeld and F. C. Hummel, 2023, DOI:10.1101/2023.10.11.554933.
- M. J. Wessel, E. Beanato, T. Popa, F. Windel, P. Vassiliadis, P. Menoud, V. Beliaeva, I. R. Violante, H. Abderrahmane, P. Dzialecka, C.-H. Park, P. Maceira-Elvira, T. Morishita, A. M. Cassara, M. Steiner, N. Grossman, E. Neufeld and F. C. Hummel, Nat. Neurosci., 2023, 26(11), 2005–2016 CrossRef CAS PubMed.
- P. Vassiliadis, E. Beanato, T. Popa, F. Windel, T. Morishita, E. Neufeld, J. Duque, G. Derosiere, M. J. Wessel and F. C. Hummel, Nat. Hum. Behav., 2024 DOI:10.1038/s41562-024-01901-z.
- M. Bikson, M. Inoue, H. Akiyama, J. K. Deans, J. E. Fox, H. Miyakawa and J. G. R. Jefferys, J. Physiol., 2004, 557, 175–190 CrossRef CAS.
- J. K. Deans, A. D. Powell and J. G. R. Jefferys, J. Physiol., 2007, 583, 555–565 CrossRef CAS.
- F. Karimi, A. Attarpour, R. Amirfattahi and A. Z. Nezhad, Phys. Med. Biol., 2019, 64, 235010 CrossRef.
- S. Ghosh, A. K. Bera and S. Das, J. Theor. Biol., 1999, 200, 299–305 CrossRef CAS PubMed.
- L. Mosgaard, K. Zecchi, T. Heimburg and R. Budvytyte, Membranes, 2015, 5, 495–512 CrossRef CAS PubMed.
- L. D. Mosgaard, K. A. Zecchi and T. Heimburg, Soft Matter, 2015, 11, 7899–7910 RSC.
- T. Heimburg, Biophys. J., 2012, 103, 918–929 CrossRef CAS.
- N. Grossman, M. S. Okun and E. S. Boyden, JAMA Neurol., 2018, 75, 1307 CrossRef.
- W. Guo, Y. He, W. Zhang, Y. Sun, J. Wang, S. Liu and D. Ming, Front. Neurosci., 2023, 17, 1092539 CrossRef PubMed.
- E. Mirzakhalili, B. Barra, M. Capogrosso and S. F. Lempka, Cell Syst., 2020, 11, 557–572.e5 CrossRef CAS PubMed.
- A. Opitz and W. J. Tyler, Nat. Biomed. Eng., 2017, 1, 632–633 CrossRef.
- S. Rampersad, B. Roig-Solvas, M. Yarossi, P. P. Kulkarni, E. Santarnecchi, A. D. Dorval and D. H. Brooks, NeuroImage, 2019, 202, 116124 CrossRef PubMed.
- X. Song, X. Zhao, X. Li, S. Liu and D. Ming, J. Neural Eng., 2021, 18, 036003 CrossRef.
- Z. Zhu and L. Yin, Front. Hum. Neurosci., 2023, 17, 1266753 CrossRef.
- S. Bahn, C. Lee and B. Kang, Hum. Brain Mapp., 2023, 44, 1829–1845 CrossRef PubMed.
- S. Lee, J. Park, D. S. Choi, C. Lee and C.-H. Im, Comput. Biol. Med., 2022, 143, 105337 CrossRef PubMed.
- S. Lee, J. Park, D. S. Choi, S. Lim, Y. Kwak, D. P. Jang, D. H. Kim, H. B. Ji, Y. B. Choy and C.-H. Im, J. Neural Eng., 2022, 19, 056003 CrossRef.
- S. Lee, C. Lee, J. Park and C.-H. Im, Sci. Rep., 2020, 10, 11730 CrossRef CAS.
- V. G. Carmona-Barrón, I. S. F. Del Campo, J. M. Delgado-García, A. J. De La Fuente, I. P. Lopez and M. A. Merchán, Front. Neuroanat., 2023, 17, 1128193 CrossRef.
- Z. Zhu, Y. Xiong, Y. Chen, Y. Jiang, Z. Qian, J. Lu, Y. Liu and J. Zhuang, Neural Plast., 2022, 2022, 1–7 CrossRef.
- C. Thomas, P. Springer, G. Loeb, Y. Berwaldnetter and L. Okun, Exp. Cell Res., 1972, 74, 61–66 CrossRef PubMed.
- A. Ahtiainen, B. Genocchi, J. M. A. Tanskanen, M. T. Barros, J. A. K. Hyttinen and K. Lenk, Int. J. Mol. Sci., 2021, 22, 12770 CrossRef CAS.
- A. Ahtiainen, I. Annala, M. Rosenholm, S. Kohtala, J. Hyttinen, J. M. A. Tanskanen and T. Rantamäki, Neuropharmacology, 2023, 229, 109481 CrossRef CAS.
- F. J. Chaure, H. G. Rey and R. Q. Quiroga, J. Neurophysiol., 2018, 120, 1859–1871 CrossRef CAS.
- F. E. Kapucu, J. M. A. Tanskanen, J. E. Mikkonen, L. Ylä-Outinen, S. Narkilahti and J. A. K. Hyttinen, Front. Comput. Neurosci., 2012, 6, 38 Search PubMed.
- I. A. Välkki, K. Lenk, J. E. Mikkonen, F. E. Kapucu and J. A. K. Hyttinen, Front. Comput. Neurosci., 2017, 11, 40 CrossRef.
- P. Welch, IEEE Trans. Audio Electroacoust., 1967, 15, 70–73 CrossRef.
- F. E. Kapucu, I. Välkki, J. E. Mikkonen, C. Leone, K. Lenk, J. M. A. Tanskanen and J. A. K. Hyttinen, Front. Comput. Neurosci., 2016, 10, 112 CrossRef PubMed.
- Z. Esmaeilpour, G. Kronberg, D. Reato, L. C. Parra and M. Bikson, Brain Stimul., 2021, 14, 55–65 CrossRef.
- X. Su, J. Guo, M. Zhou, J. Chen, L. Li, Y. Chen, X. Sui, H. Li and X. Chai, IEEE Trans. Neural Syst. Rehabil. Eng., 2021, 29, 418–428 Search PubMed.
- E. Acerbo, A. Jegou, C. Luff, P. Dzialecka, B. Botzanowski, F. Missey, I. Ngom, S. Lagarde, F. Bartolomei, A. Cassara, E. Neufeld, V. Jirsa, R. Carron, N. Grossman and A. Williamson, Front. Neurosci., 2022, 16, 945221 CrossRef PubMed.
- Y. Huang, A. A. Liu, B. Lafon, D. Friedman, M. Dayan, X. Wang, M. Bikson, W. K. Doyle, O. Devinsky and L. C. Parra, eLife, 2017, 6, e18834 CrossRef.
- C. Ineichen, N. R. Shepherd and O. Sürücü, Front. Hum. Neurosci., 2018, 12, 468 CrossRef.
- D. A. Wagenaar, J. Pine and S. M. Potter, BMC Neurosci., 2006, 7, 11 CrossRef.
- T. Hyvärinen, A. Hyysalo, F. E. Kapucu, L. Aarnos, A. Vinogradov, S. J. Eglen, L. Ylä-Outinen and S. Narkilahti, Sci. Rep., 2019, 9, 17125 CrossRef PubMed.
- F. H. Kasten, J. Dowsett and C. S. Herrmann, Front. Hum. Neurosci., 2016, 10, 245 Search PubMed.
- T. Neuling, S. Rach and C. S. Herrmann, Front. Hum. Neurosci., 2013, 7, 161 Search PubMed.
- B. C. Albensi, D. R. Oliver, J. Toupin and G. Odero, Exp. Neurol., 2007, 204, 1–13 CrossRef.
- S. Ahn, S. Jo, S. B. Jun, H. W. Lee and S. Lee, Front. Comput. Neurosci., 2017, 11, 39 CrossRef.
- S.-N. Lim, C.-Y. Lee, S.-T. Lee, P.-H. Tu, B.-L. Chang, C.-H. Lee, M.-Y. Cheng, C.-W. Chang, W.-E. J. Tseng, H.-Y. Hsieh, H.-I. Chiang and T. Wu, Neuromodulation, 2016, 19, 365–372 CrossRef PubMed.
- E. Paschen, C. Elgueta, K. Heining, D. M. Vieira, P. Kleis, C. Orcinha, U. Häussler, M. Bartos, U. Egert, P. Janz and C. A. Haas, eLife, 2020, 9, e54518 CrossRef CAS PubMed.
- Y. Schiller and Y. Bankirer, J. Neurophysiol., 2007, 97, 1887–1902 CrossRef PubMed.
- W. Singer, Neuron, 1999, 24, 49–65 CrossRef CAS PubMed.
- T. P. Vogels and L. F. Abbott, J. Neurosci., 2005, 25, 10786–10795 CrossRef CAS.
- M. Diesmann, M.-O. Gewaltig and A. Aertsen, Nature, 1999, 402, 529–533 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lc00224e |
| This journal is © The Royal Society of Chemistry 2024 |