 Open Access Article
Open Access ArticleDepolymerization of PET with ethanol by homogeneous iron catalysts applied for exclusive chemical recycling of cloth waste†
Nor Wahida Binti
Awang
ab,
Muhammad Aidel Bin Ratno
Hadiyono
b,
Mohamed Mehawed
Abdellatif
a and
Kotohiro
Nomura
 *a
*a
aDepartment of Chemistry, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo 192-0397, Japan. E-mail: ktnomura@tmu.ac.jp
bFaculty of Applied Sciences, Universiti Teknologi MARA Sarawak Branch, 94300 Kota Samarahan, Sarawak, Malaysia
First published on 6th August 2024
Abstract
Acid-, base-free depolymerization of poly(ethylene terephthalate) (PET) with ethanol catalyzed by FeCl3, FeBr3 (1.0–5.0 mol%) gave diethyl terephthalate (DET) and ethylene glycol (EG) exclusively (98–99%, 160–180 °C), and FeCl3 showed better catalytic performance in terms of activity. The FeCl3 catalyst enabled exclusive, selective depolymerization of PET from textile waste to afford DET (and recovered cotton waste), strongly suggesting the possibility of chemical recycling of cloth waste by the transesterification in this catalysis.
Keywords: Depolymerization; PET; Chemical recycling; Textile waste management; Homogeneous catalyst.
1 Introduction
Chemical recycling, chemical conversion of used plastics to raw materials (monomers), has been recognized as an important technology for addressing concerns about plastic waste,1–4 although the percentage is still low in the world (e.g. 0.1% in Europe5 and ca. 3% in Japan).6 Conversion of plastic waste to value-added chemicals, called upcycling, has also been considered as an important technology in terms not only of circular economy, but also of development of chemical processes from new alternative resources to fossil oil. Polyesters, exemplified as poly(ethylene terephthalate) (PET), are widely used commodity thermoplastics, and PET has been reused as transparent bottles partly by so-called mechanical recycling through a process of collection, sorting, cleaning, melting and reprocessing. However, due to inferior quality of PET reused resin compared to the fresh material derived from petroleum, there has been a strong demand to increase the percentage of called “closed-loop recycling”, and an importance of chemical recycling, conversion to the same quality as fresh resin, has been pronounced recently.7–17Although many studies have been reported to date concerning depolymerization of polyesters including PET,12–44 most methods require harsh (high temperature, pressure) conditions in the presence of excess base/acid and/or inorganic salts. For example, the method of recovery and purification of dimethyl terephthalate (DMT, known as methanolysis) or bis(2-hydroxyethyl) terephthalate (BHET, known as glycolysis) requires excess inorganic/organic bases, acids, and additives (inorganic salts, ionic liquid etc.). The process also requires separation of the target compound(s) with by-products and subsequent purification (and also wastewater treatment etc.); these cause inferior quality of the recycled materials and a concern that the recycled products are more expensive in the present process. Moreover, in some methods, the effective alcohol used is limited to methanol and use of other alcohols leads to a decrease in efficiency.
In more detail, in the depolymerization with ethylene glycol (EG), called glycolysis, PET was treated in the presence of Zn(OAc)2 catalyst combined with a base such as Na2CO3,24 1,3-dimethylurea (at ca. 190 °C, called Lewis acid–base synergetic catalysis),30 or a mixture of p-toluene sulfonic acid (or methane sulfonic acid) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD, 5 mol%, 180 °C),31,32 or the reactions were conducted in the presence of transition metal-containing ionic liquids (such as [bmin]2[CoCl4], [bmin][FeCl4], [bmin]2[ZnCl4] etc.; bmin = 1-butyl-3-methylimidazolium) at 170 °C.29 Moreover, in the depolymerization of PET with methanol, called methanolysis, harsh conditions at high temperature (e.g. 280–310 °C) and high pressure (ca. 4 MPa) are generally required.12–17 Copresence of inorganic base (K2CO3etc.) was effective to reduce the harsh conditions.12–17 The depolymerization with n-butanol was conducted in the presence of ZnCl2 and [HO3S-(CH2)3-NEt3]Cl at 205 °C.28
More recently, approaches for conducting the methods (for chemical recycling and upcycling) under mild conditions (at 25 °C) by using excess K2CO3 (or KOCH3, or TBD) in methanol–CH2Cl2,38 or dimethyl carbonate in the presence of excess Li(OMe)39,40 have also been suggested. The depolymerization of PET using FeCl3·6H2O combined with sulfonic acid conducted at 100 °C was also reported.34 These methods, however, required excess base38–40 and/or the reactions did not proceed exclusively.34,38 Development of efficient “acid-, base-free” catalysts for chemical recycling of polyesters has thus been an attractive and important subject. Efforts should contribute to providing a simplified purification and separation process for obtaining recycled resins in a rather efficient manner (more suitable for achievement of “closed-loop chemical recycling”).
We recently demonstrated that the development of efficient transesterification catalysts can solve the problem, because this type of degradation and repolymerization (polycondensation and condensation polymerization) is a transesterification. Our laboratory thus focused on using Cp′TiCl3 [Cp′ = C5H5 (Cp), C5Me5 (Cp*)], efficient transesterification of aliphatic fatty acid esters (FAEs),45 and calcium oxide (CaO), efficient transesterification of triglycerides (plant oils)46 and FAEs,47 to depolymerize aliphatic polyesters,45,47 and demonstrated that these catalysts enabled the depolymerization not only of poly(ethylene adipate) (PEA) and poly(butylene adipate) (PBA),45,47 but also of PET and poly(butylene terephthalate) (PBT)42,43 with various alcohols (Scheme 1). Moreover, La(acac)3 (acac = acetylacetonato) also depolymerized PET as well as other polyesters by treating with methanol.41,48 CaO catalyst has been widely used for transesterification of triglycerides (plant oil),49–51 for which catalyst pretreatment (calcination at high temperature like 300 °C)47 would be necessary. Therefore, development using simple, commercially available catalysts has been an attractive target.
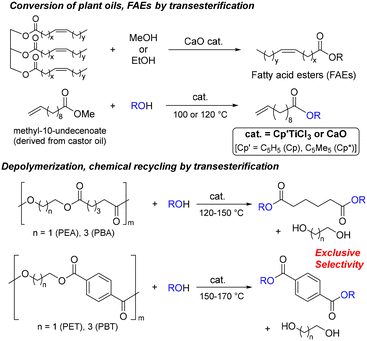 | ||
| Scheme 1 Acid-, base-free catalytic transesterification of methyl-1-undecenoate (fatty acid ester, FAE) and polyesters with alcohol.42,43,45–47 Reprinted with permissions from ref. 42 (Copyright 2023 by the authors; licensee MDPI) and ref. 43 (Copyright 2024 Springer Nature). | ||
Importantly, these depolymerizations of polyesters with alcohol especially using both titanium and calcium catalysts gave raw materials (monomers) exclusively (>99% conversion, >99% selectivity), and the finding enabled us to demonstrate one-pot closed-loop chemical recycling (one pot depolymerization–repolymerization) demonstrated by PBA with ethanol (Scheme 2).42 Moreover, aminolysis of polyesters to afford amides could be achieved by using Cp′TiCl3 catalyst.52
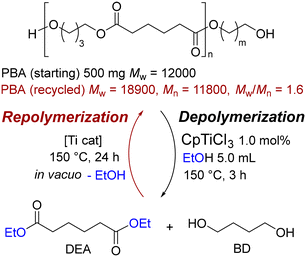 | ||
| Scheme 2 Closed-loop chemical recycling (one-pot depolymerization and re-condensation polymerization) of PBA by one-pot depolymerization/re-polycondensation.42 Reprinted with permission ref. 42. Copyright 2023 by the authors; licensee MDPI. | ||
We previously communicated that FeCl3 and FeBr3 (readily available commercially) were effective catalysts for transesterification of FAE (methyl-1-undecenoate).45 In this paper, we thus report that FeCl3 and FeBr3 are also effective as catalysts for depolymerization of PET with alcohol. Moreover, we applied this homogeneous catalysis to the selective depolymerization of polyesters from a plastic mixture (polyester and polyethylene) and a mixture of PET and cotton (often employed in our daily life as clothes) in order for this method to be applied in a chemical recycling process (obtaining raw materials) from practical wastes. We demonstrate that the method enabled quantitative chemical recycling of cloth waste, which has been considered as an important subject for the achievement of a circular economy.53–56
2 Results and discussion
2.1 Depolymerization of PET with ethanol catalyzed by FeCl3, FeBr3
Depolymerizations of PET with ethanol were conducted in a sealed glass reaction tube containing a PET sheet (by cutting a PET drink bottle, as reported previously),42,52 ethanol (5.0 mL, anhydrous) and a prescribed amount of FeCl3 or FeBr3 (anhydrous), and the reaction mixture was stirred magnetically at 180 °C (or 160 °C etc.; Scheme 3). According to the method in our previous report,42 the conversions of PET were estimated by 13C NMR spectra and the yields of DET were analyzed by GC [vs. internal standard (IS, mesitylene) using a calibration curve]. As described below, the reaction mixtures (carried out under the conditions in Table 1) after careful removal of the volatile (ethanol) were completely soluble in CDCl3 (without any insoluble precipitates), and, as reported previously,42 no residual resonances ascribed to carbonyl groups except those of DET were seen after dissolving the reaction mixture in CDCl3. Detailed procedures are described in the Experimental section and selected NMR spectra and GC charts are shown in the ESI.† Selected results are summarized in Table 1. | ||
| Scheme 3 Acid-, base-free catalytic transesterification (depolymerization) of PET with ethanol catalyzed by FeCl3, FeBr3. | ||
| Run | Catalystb (mol%) | Temp. (°C) | Time (h) | Conv.c (%) | Yieldd (%) |
|---|---|---|---|---|---|
| a Conditions: 500 mg poly(ethylene terephthalate) (PET) (prepared by cutting a PET bottle), and 5.0 mL ethanol. b Based on monomer unit in PET. c Estimated by 13C NMR spectra. d GC yield of DET vs. internal standard (mesitylene). | |||||
| 1 | FeCl3 (5.0) | 180 | 18 | >99 | >99 |
| 2 | FeCl3 (5.0) | 180 | 12 | >99 | 98 |
| 3 | FeCl3 (5.0) | 160 | 18 | >99 | 97 |
| 4 | FeCl3 (5.0) | 160 | 18 | >99 | 98 |
| 5 | FeCl3 (5.0) | 160 | 24 | >99 | 98 |
| 6 | FeCl3 (5.0) | 120 | 18 | >99 | 46 |
| 7 | FeCl3 (3.0) | 180 | 18 | >99 | 97 |
| 8 | FeCl3 (3.0) | 180 | 24 | >99 | >99 |
| 9 | FeCl3 (3.0) | 180 | 30 | >99 | >99 |
| 10 | FeCl3 (1.0) | 180 | 18 | >99 | 21 |
| 11 | FeCl3 (1.0) | 180 | 30 | >99 | 97 |
| 12 | FeCl3 (1.0) | 180 | 48 | >99 | >99 |
| 13 | FeBr3 (5.0) | 180 | 18 | >99 | >99 |
| 14 | FeBr3 (5.0) | 160 | 18 | >99 | 87 |
| 15 | FeBr3 (5.0) | 120 | 18 | >99 | 18 |
| 16 | FeBr3 (3.0) | 180 | 18 | >99 | >99 |
| 17 | FeBr3 (3.0) | 180 | 30 | >99 | >99 |
| 18 | FeBr3 (1.0) | 180 | 18 | >99 | 34 |
| 19 | FeBr3 (1.0) | 180 | 30 | >99 | 97 |
| 20 | FeBr3 (1.0) | 180 | 48 | >99 | >99 |
It was revealed that, as expected from the results of the transesterification of methyl-10-undecenoate with alcohol,39 depolymerizations of PET with ethanol in the presence of FeCl3 (5.0 mol%) proceeded at 180 °C to afford DET quantitatively (runs 1 and 2). The recovered yields consisting of EG and DET after careful removal of ethanol were very close to those calculated (Table S1†). The exclusive formation of DET and EG was also confirmed by 1H NMR and 13C NMR spectra (Fig. 1; additional NMR spectra are shown in ESI†). The reactions conducted in the presence of 3.0 mol% of FeCl3 at 180 °C (runs 7–9) or conducted at 160 °C (5.0 mol% FeCl3, runs 3–5) also gave DET and EG exclusively, and the results are reproducible (runs 3 and 4). DET was obtained as a sole product after removal of volatiles (including EG partially), as shown in Fig. S18† (time course monitored by 13C NMR spectra).
 | ||
| Fig. 1 (a) 1H NMR and (b) 13C NMR spectra (in CDCl3 at 25 °C) of the reaction mixture (at 180 °C for 18 h, run 1) after removal of ethanol. IS (internal standard) = mesitylene. | ||
In contrast, the reaction conducted with low FeCl3 loading (1.0 mol%, runs 10–12) revealed that DET yield (by GC) was low after 18 h, and the yield increased with time and eventually reached >99% after 48 h [DET yield 21% (18 h), 97% (30 h), >99% (48 h), 180 °C], as observed in the depolymerization of PET with ethanol using Cp'TiCl3 (ref. 36) and CaO (ref. 37) catalysts. The activity of FeCl3 thus was rather low compared to that of Cp'TiCl3 under the same conditions [DET yield 92% (CpTiCl3), 94% (Cp*TiCl3, Ti 1.0 mol% at 150 °C after 24 h)].42
The reaction catalyzed by FeCl3 conducted at 120 °C led to a decrease in the yield of DET (run 6). In both cases (runs 6 and 10), only one resonance ascribed to the carbonyl group in DET was observed in the region around 155–170 ppm (ascribed to carbonyl carbon), whereas the reaction mixtures after removal of volatiles were completely soluble in CDCl3 [Fig. S17 (run 6) and S19† (run 10)]. Several resonances (probably ascribed to the oligomer) were seen in the spectra with observation of several additional peaks (with their retention time longer than those of DET) in the GC chromatogram. These results thus suggest that, as observed in the depolymerization of PET with ethanol catalyzed by Cp'TiCl3 (ref. 36) and CaO (ref. 37) catalysts, PET was first depolymerized to afford an oligomer mixture and eventually converted to DET over the time course of the reaction. One probable explanation we may consider is the formation of ethyl(hydroxyethyl) terephthalate (EHTP), as an intermediate in this depolymerization event, as demonstrated in the reaction of PET with ethanol catalyzed by CaO.37
Fig. 2 shows selected photographs of the reaction mixture in the depolymerization of PET catalyzed by FeCl3 under various conditions. As shown in Fig. 2a, the reaction mixture with low FeCl3 loading at 180 °C first became cloudy pale yellow after 18 h (Fig. 2a left) and became a clear solution after 30 h (Fig. 2a right) with quantitative formation of DET (confirmed by GC and NMR spectra). Similar colour changes are observed in Fig. 2b, and the reaction mixture with incomplete conversion to DET was a cloudy pale yellow, the colour eventually changing to a clear solution with complete conversion to DET. These observations could also suggest the above assumption that PET was first converted to an oligomer mixture and then converted to DET eventually.
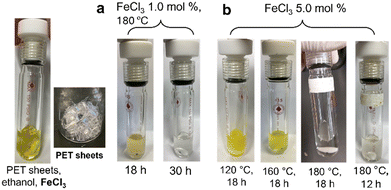 | ||
| Fig. 2 Reaction mixture of PET depolymerization with ethanol in the presence of FeCl3. Reaction mixture with (a) FeCl3 (1.0 mol%) at 180 °C (runs 10, 11) and (b) FeCl3 (5.0 mol%) (runs 1–3, 6). | ||
Similarly, depolymerization of PET with ethanol proceeded in the presence of FeBr3, and the reactions conducted at 180 °C (with FeBr3 at 3.0 and 5.0 mol%) gave DET in quantitative yields (runs 13, 16, 17), whereas the DET yield in the reaction with low FeBr3 loading (1.0 mol%) was initially low (34%, run 18) and reached a quantitative value after 48 h (run 20). However, the conversions at 160 °C (run 14) and 120 °C (run 15) were low compared to those in the presence of FeCl3 (runs 3, 4, 6). It is thus concluded that FeCl3 is a suitable catalyst in PET depolymerization. The colour in the reaction mixture initially showed red and changed from cloudy pale red to a clear solution with quantitative formation of DET (Fig. S38†).
2.2 Selective depolymerization of PET with ethanol from a mixture with polyethylene and cloth waste
It should be noted that depolymerizations of textile waste samples (yellow, white, black, shown in Fig. 3) with ethanol catalyzed by FeCl3 afforded DET quantitatively. As shown in Fig. 3a as well as in Table 2 (runs 21 and 22), the yellow textile sample consisting of a mixture of PET (65%) and cotton (35%) was treated with ethanol in the presence of FeCl3 (5.0 mol% to PET) to afford DET (>99% yield, very small amount of dyes remaining) along with recovery of cotton quantitatively (>99% recovery yield); the complete conversion to DET could be achieved in 16 h (run 22). Similarly, reactions of the white textile sample (Fig. 3b, 100% PET) and the black sample (Fig. 3c, 100% PET) with ethanol also afforded PET (runs 23–25, Table 2), and the results were reproducible (runs 23, 24). Moreover, as shown in Fig. S20 and S21,† no other resonances ascribed to DET (and EG remaining) were observed in the NMR spectra after removal of volatiles from the reaction mixture. These results thus clearly indicate that the present method can be applied to chemical recycling of cloth waste to afford raw materials exclusively without any accompanying by-products.| Run | Textile sampleb (composition) | Time (h) | PET conv.c (%) | DET yieldd (%) | Cottone (wt% (mg)) |
|---|---|---|---|---|---|
| a Conditions: textile pieces 200 mg, ethanol 5.0 mL, FeCl3 5.0 mol% for PET (calculated on the basis of monomer unit in PET only). b Samples shown in Fig. 3. c Estimated by 13C NMR spectra. d GC yield vs. internal standard (mesitylene). e Based on weight recovered as solid after filtration and drying in vacuo. | |||||
| 21 | Yellow (PET 65%, cotton 35%) | 12 | >99 | 96 | >99 (69 mg) |
| 22 | Yellow (PET 65%, cotton 35%) | 16 | >99 | >99 | >99 (70 mg) |
| 23 | White (PET 100%) | 18 | >99 | >99 | — |
| 24 | White (PET 100%) | 18 | >99 | 98 | — |
| 25 | Black (PET 100%) | 18 | >99 | >99 | — |
Moreover, as summarized in Table 3 as well as Fig. 4, the method can be applied to the selective depolymerization of PET from a mixture of PET and cotton or polyethylene (PE). Importantly, DET was recovered from the mixture in quantitative yields (runs 26 and 27) along with quantitative recovery of cotton or PE. These results also indicate that the present method can be applied to separation of polyesters from PE and cotton waste by the exclusive depolymerization.
| Run | Sampleb (composition) | PET conv.c (%) | DET yieldd (%) | Cottone (wt% (mg)) |
|---|---|---|---|---|
| a Conditions: 500 mg poly(ethylene terephthalate) (PET) prepared by cutting a drink bottle, 200 mg of PE or cotton textile, and 5.0 mL ethanol. b Samples shown in Fig. 4. c Estimated by 13C NMR spectra. d GC yield vs. internal standard (mesitylene). e Based on weight recovered as solid after filtration and dried in vacuo. | ||||
| 26 | PET (500 mg) + cotton (200 mg) | >99 | 98 | >99 (200 mg) |
| 27 | PET (500 mg) + PE (200 mg) | >99 | >99 | >99 (198 mg) |
3 Conclusions
We have demonstrated that FeCl3 (FeBr3) catalyzed acid-, base-free depolymerization (transesterification) of PET with ethanol to afford diethyl terephthalate (DET) and ethylene glycol (EG) exclusively (98–99% yield, 1.0–5.0 mol% Fe, 160–180 °C); FeCl3 showed better catalyst performance in terms of activity. The reaction gave by-products in negligible amounts that facilitates the purification. Use of FeCl3 should be emphasized in terms of wide availability (cheap, used in industry) without pretreatment (as required for CaO catalyst, calcination at 300 °C etc.),37,40 whereas the observed catalytic activity by FeCl3 was rather low compared to that of Cp'TiCl3.35 The method can be applied to selective depolymerization of PET from textile (cloth) waste to afford DET with exclusive recovery of cotton waste. The method can also be applied to selective depolymerization of polyesters from a mixture of plastic waste (consisting of polyolefins etc.). We believe that the method could provide a possibility of a clean chemical recycling process in the presence of a commercially available catalyst (FeCl3). We are currently exploring the possibility of conducting the reactions under mild conditions without compromising the exclusive selectivity via catalyst development.4 Experimental
All depolymerization experiments were carried out under a nitrogen atmosphere in a drybox. PET sheets were prepared by cutting a PET drink bottle. Anhydrous-grade ethanol (>99.5%, Kanto Chemical Co., Inc.) was used as received. Anhydrous-grade FeCl3 (97.0%) and FeBr3 (98.0%) were purchased from Aldrich Chemical Co. and were used as received in the drybox under nitrogen atmosphere. Textile samples were received from companies (by donation for research purposes).All 1H and 13C NMR measurements were performed at 25 °C with a Bruker AV500 spectrometer (500.13 MHz and 125.77 MHz, respectively) using CDCl3 as a solvent. SiMe4 was used as a reference at 0.00 ppm (chemical shifts were reported as ppm). The GC chromatograms were recorded using a Shimadzu gas chromatograph (GC-2014, Shimadzu Corp., Tokyo, Japan) equipped with a flame ionization detector (FID) using nitrogen as a carrier gas. Conditions were as follows (DB-1MS column, 30 m × 0.250 mm × 0.25 μm): column temperature, 80 °C (4 min) followed by increasing up to 320 °C (20 °C min−1) [injection at 300 °C, flow (column) at 1.71 mL min−1].
4.1 General procedure for the depolymerization of PET through transesterification
In a drybox under nitrogen atmosphere, the prescribed amount of FeCl3 or FeBr3 (anhydrous grade), 500 mg of PET sheets (or 200 mg of textile), and 5.0 mL of ethanol (anhydrous grade) were placed under a nitrogen atmosphere into an oven-dried 15.0 mL scale pressure reaction tube with a screw cap. The reaction mixture was stirred for a prescribed time and temperature using an alumina bath. After completion of the reaction, the mixture was cooled to room temperature and washed with CHCl3 (ca. 3 mL). The volatiles (CHCl3, ethanol, etc.) were removed in vacuo. The internal standard was added to the resultant residue then the yield was estimated using GC (quantitative analysis using a calibration curve vs. internal standard). The resultant reaction mixtures were analyzed by using 1H (500.13 MHz) and 13C{1H} (125.77 MHz) NMR spectra in CDCl3 at 25 °C. Selective transesterifications of PET from the mixture with polyethylene or cotton were conducted under similar conditions, except that 500 mg of PET sheets and 200 mg of cotton or polyethylene were used.Data availability
Data are contained within the article and the ESI† including (i) additional results for depolymerization of PET with ethanol catalyzed by FeCl3 and FeBr3, (ii) GC chromatograms of the resultant mixtures for depolymerization of PET through transesterification with ethanol using FeCl3 and FeBr3, (iii) selected 13C NMR spectra of the resultant depolymerization mixtures, (iv) selected 1H NMR spectra of the resultant depolymerization mixtures, (v) photos for selected experimental trials.Author contributions
KN designed the project (conceptualization, catalyst selection, methodology, supervision) including funding acquisition. NWBA and MABRH conducted reactions, analysis and MMA helped in conducting experiments, analysis (GC, NMR etc.) and data analysis including drawing. KN wrote the manuscript, and NWBA and MMA helped in the preparation. All authors have discussed the results and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was partly supported by JST-CREST (grant number JPMJCR21L5), and the authors express their thanks to Ms. Yuriko Ohki and Mr. Asahi Tanaka (Tokyo Metropolitan University) for some technical support. MABRH thanks Universiti Teknologi MARA Sarawak Branch and Faculty of Science, Tokyo Metropolitan University for financial support. KN expresses his thanks to Prof. Wenjuan Zhang (Beijing Institute of Fashion Technology) for sending the test samples of cloth waste for conducting these experiments.References
- G. W. Coates and Y. D. Getzler, Chemical recycling to monomer for an ideal, circular polymer economy, Nat. Rev. Mater., 2020, 5, 501–516 CrossRef CAS.
- D. I. Collias, M. I. James and J. M. Layman, Circular Economy of Polymers: Topics in Recycling Technologies, American Chemical Society, Washington, DC, 2021 Search PubMed.
- J. C. Worch and A. P. Dove, 100th anniversary of macromolecular science viewpoint: Toward catalytic chemical recycling of waste (and future) plastics, ACS Macro Lett., 2020, 9, 1494–1506 CrossRef CAS PubMed.
- M. Chu, Y. Liu, X. Lou, Q. Zhang and J. Chen, Rational design of chemical catalysis for plastic recycling, ACS Catal., 2022, 12, 4659–4679 CrossRef CAS.
- Plastic Waste Management Institute, An Introduction to Plastic Recycling in Japan, https://www.pwmi.or.jp/ei/plastic_recycling_2022.pdf, (accessed May 2024) Search PubMed.
- Plastics Europe, The Circular Economy for Plastics – A European Analysis 2024, https://plasticseurope.org/knowledge-hub/the-circular-economy-for-plastics-a-european-analysis-2024/, (accessed May 2024) Search PubMed.
- X. Zhang, M. Fevre, G. O. Jones and R. M. Waymouth, Catalysis as an enabling science for sustainable polymers, Chem. Rev., 2018, 118, 839–885 CrossRef CAS PubMed.
- S. Westhues, J. Idel and J. Klankermayer, Molecular catalyst systems as key enablers for tailored polyesters and polycarbonate recycling concepts, Sci. Adv., 2018, 4, eaat9669 CrossRef CAS.
- A. Basterretxea, C. Jehanno, D. Mecerreyes and H. Sardon, Dual organocatalysts based on ionic mixtures of acids and bases: A step toward high temperature polymerizations, ACS Macro Lett., 2019, 8, 1055–1062 CrossRef CAS PubMed.
- J. Payne and M. D. Jones, The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities, ChemSusChem, 2021, 14, 4041–4070 CrossRef CAS.
- M. Häußler, M. Eck, D. Rothauer and S. Mecking, Closed-loop recycling of polyethylene-like materials, Nature, 2021, 590, 423–427 CrossRef PubMed.
- R. D. Allen and M. I. James, in Circular Economy of Polymers: Topics in Recycling Technologies, ed. D. I. Collias, M. I. James and J. M. Layman, ACS Symposium Series, American Chemical Society, Washington, DC, 2021, pp. 61–80 Search PubMed.
- D. Paszun and T. Spychaj, Chemical recycling of poly(ethylene terephthalate), Ind. Eng. Chem. Res., 1997, 36, 1373–1383 CrossRef CAS.
- D. Damayanti and H. S. Wu, Strategic possibility routes of recycled PET, Polymers, 2021, 13, 1475 CrossRef PubMed.
- A. McNeeley and Y. A. Liu, Assessment of PET depolymerization processes for circular economy. 1. Thermodynamics, chemistry, purification, and process design, Ind. Eng. Chem. Res., 2024, 63, 3355–3399 CrossRef CAS.
- C. Jehanno, M. M. Pérez-Madrigal, J. Demarteau, H. Sardon and A. P. Dove, Organocatalysis for depolymerization, Polym. Chem., 2019, 10, 172–186 RSC.
- M. D. de Dios Caputto, R. Navarro, J. L. Valentín and Á. Marcos-Fernández, Chemical upcycling of poly(ethylene terephthalate) waste: Moving to a circular model, J. Polym. Sci., 2022, 60, 3269–3283 CrossRef CAS.
- L. Dupont and V. Gupta, Degradative transesterification of terephthalate polyesters to obtain DOTP plasticizer for flexible PVC, J. Vinyl Technol., 1993, 15, 100–104 CrossRef CAS.
- J. W. Chen and L. W. Chen, The glycolysis of poly(ethylene terephthalate), J. Appl. Polym. Sci., 1999, 73, 35–40 CrossRef CAS.
- C. H. Chen, C. Y. Chen, Y. W. Lo, C. F. Mao and W. T. Liao, Studies of glycolysis of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. I. Influences of glycolysis conditions, J. Appl. Polym. Sci., 2001, 80, 943–948 CrossRef CAS.
- S. H. Mansour and N. E. Ikladious, Depolymerization of poly(ethylene terephthalate) wastes using 1,4-butanediol and triethylene glycol, Polym. Test., 2002, 21, 497–505 CrossRef CAS.
- H. Kurokawa, M. Ohshima, K. Sugiyama and H. Miura, Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol, Polym. Degrad. Stab., 2003, 79, 529–533 CrossRef CAS.
- K. Troev, G. Grancharov, R. Tsevi and I. Gitsov, A novel catalyst for the glycolysis of poly(ethylene terephthalate), J. Appl. Polym. Sci., 2003, 90, 1148–1152 CrossRef CAS.
- R. López-Fonseca, I. Duque-Ingunza, B. De Rivas, S. Arnaiz and J. I. Gutierrez-Ortiz, Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts, Polym. Degrad. Stab., 2010, 95, 1022–1028 CrossRef.
- K. Fukushima, O. Coulembier, J. M. Lecuyer, H. A. Almegren, A. M. Alabdulrahman, F. D. Alsewailem, M. A. Mcneil, P. Dubois, R. M. Waymouth and H. W. Horn, Organocatalytic depolymerization of poly(ethylene terephthalate), J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1273–1281 CrossRef CAS.
- K. Fukushima, D. J. Coady, G. O. Jones, H. A. Almegren, A. M. Alabdulrahman, F. D. Alsewailem, H. W. Horn, J. E. Rice and J. L. Hedrick, Unexpected efficiency of cyclic amidine catalysts in depolymerizing poly(ethylene terephthalate), J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1606–1611 CrossRef CAS.
- M. Imran, W. A. Al-Masry, A. Mahmood, A. Hassan, S. Haider and S. M. Ramay, Manganese-, cobalt-, and zinc-based mixed-oxide spinels as novel catalysts for the chemical recycling of poly(ethylene terephthalate) via glycolysis, Polym. Degrad. Stab., 2013, 98, 904–915 CrossRef CAS.
- S. Liu, Z. Wang, L. Li, S. Yu, C. Xie and F. Liu, Butanol alcoholysis reaction of polyethylene terephthalate using acidic ionic liquid as catalyst, J. Appl. Polym. Sci., 2013, 130, 1840–1844 CrossRef CAS.
- Q. Wang, Y. Geng, X. Lu and S. Zhang, First-row transition metal-containing ionic liquids as highly active catalysts for the glycolysis of poly(ethylene terephthalate) (PET), ACS Sustainable Chem. Eng., 2015, 3, 340–348 CrossRef CAS.
- B. Liu, W. Fu, X. Lu, Q. Zhou and S. Zhang, Lewis acid–base synergistic catalysis for polyethylene terephthalate degradation by 1,3-dimethylurea/Zn(OAc)2 deep eutectic solvent, ACS Sustainable Chem. Eng., 2019, 7, 3292–3300 CrossRef CAS.
- C. Jehanno, J. Demarteau, D. Mantione, M. C. Arno, F. Ruipérez, J. L. Hedrick, A. P. Dove and H. Sardon, Selective chemical upcycling of mixed plastics guided by a thermally stable organocatalyst, Angew. Chem., Int. Ed., 2021, 60, 6710–6717 CrossRef CAS PubMed.
- S. Kaiho, A. A. R. Hmayed, K. R. Delle Chiaie, J. C. Worch and A. P. Dove, Designing thermally stable organocatalysts for poly(ethylene terephthalate) synthesis: Toward a one-pot, closed-loop chemical recycling system for PET, Macromolecules, 2022, 55, 10628–10639 CrossRef CAS.
- K. R. Delle Chiaie, F. R. McMahon, E. J. Williams, M. J. Price and A. P. Dove, Dual-catalytic depolymerization of polyethylene terephthalate (PET), Polym. Chem., 2020, 11, 1450–1453 RSC.
- M. Rollo, F. Raffi, E. Rossi, M. Tiecco, E. Martinell and G. Ciancaleoni, Depolymerization of polyethylene terephthalate (PET) under mild conditions by Lewis/Brønsted acidic deep eutectic solvents, Chem. Eng. J., 2023, 456, 141092 CrossRef CAS.
- M. D. de Dios Caputto, R. Navarro, J. L. Valentín and A. Marcos-Fernandez, Tuning of molecular weight and chemical composition of polyols obtained from poly(ethylene terephthalate) waste recycling through the application of organocatalysts in an upcycling route, J. Cleaner Prod., 2024, 454, 142253 CrossRef CAS.
- J.-T. Du, Q. Sun, X.-F. Zeng, D. Wang, J.-X. Wang and J.-F. Chen, ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate, Chem. Eng. Sci., 2020, 220, 115642 CrossRef CAS.
- S. Shirazimoghaddam, I. Amin, J. A. Faria Albanese and N. R. Shiju, Chemical recycling of used PET by glycolysis using niobia-based catalysts, ACS Eng. Au, 2023, 3, 37–44 CrossRef CAS PubMed.
- D. D. Pham and J. Cho, Low-energy catalytic methanolysis of poly(ethyleneterephthalate), Green Chem., 2021, 23, 511–525 RSC.
- S. Tanaka, J. Sato and Y. Nakajima, Capturing ethylene glycol with dimethyl carbonate towards depolymerisation of polyethylene terephthalate at ambient temperature, Green Chem., 2021, 23, 9412–9416 RSC.
- S. Tanaka, M. Koga, T. Kuragano, A. Ogawa, H. Ogiwara, K. Sato and Y. Nakajima, Depolymerization of polyester fibers with dimethyl carbonate-aided methanolysis, ACS Mater. Au, 2024, 4, 335–345 CrossRef CAS.
- R. Abe, N. Komine, K. Nomura and M. Hirano, La(iii)-Catalysed degradation of polyesters to monomers via transesterifications, Chem. Commun., 2022, 58, 8141–8144 RSC.
- Y. Ohki, Y. Ogiwara and K. Nomura, Depolymerization of polyesters by transesterification with ethanol using (cyclopentadienyl)titanium trichlorides, Catalysts, 2023, 13, 421 CrossRef CAS.
- P. Unruean, P. Padungros, K. Nomura and B. Kitiyanan, Efficient chemical depolymerization of polyethylene terephthalate via transesterification with ethanol using CaO catalyst, J. Mater. Cycles Waste Manage., 2024, 26, 731–740 CrossRef CAS.
- Y. Peng, J. Yang, C. Deng, J. Deng, L. Shen and Y. Fu, Acetolysis of waste polyethylene terephthalate for upcycling and life-cycle assessment study, Nat. Commun., 2023, 14, 3249 CrossRef CAS PubMed.
- K. Nomura, T. Aoki, Y. Ohki, S. Kikkawa and S. Yamazoe, Transesterification of methyl-10-undecenoate and poly(ethylene adipate) catalyzed by (cyclopentadienyl)titanium trichlorides as model chemical conversions of plant oils and acid-, base-free chemical recycling of aliphatic polyesters, ACS Sustainable Chem. Eng., 2022, 10, 12504–12509 CrossRef CAS.
- P. Unruean, K. Nomura and B. Kitiyanan, High conversion of CaO-catalyzed transesterification of vegetable oils with ethanol, J. Oleo Sci., 2022, 71, 1051–1062 CrossRef CAS PubMed.
- S. Sudhakaran, S. H. Siddiki, B. Kitiyanan and K. Nomura, CaO catalyzed transesterification of ethyl 10-undecenoate as a model reaction for efficient conversion of plant oils and their application to depolymerization of aliphatic polyesters, ACS Sustainable Chem. Eng., 2022, 10, 12864–12872 CrossRef CAS.
- N. Kobayashi, N. Komine, K. Nomura, H. Hirano and M. Hirano, La(III)-catalyzed depolymerization of poly(l-lactic acid) yielding chiral lactates, Bull. Chem. Soc. Jpn., 2023, 96, 1324–1330 CrossRef.
- M. Kouzu and J. Hidaka, Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review, Fuel, 2012, 93, 1–12 CrossRef CAS.
- A. Esipovich, S. Danov, A. Belousov and A. Rogozhin, Improving methods of CaO transesterification activity, J. Mol. Catal. A: Chem., 2014, 395, 225–233 CrossRef CAS.
- D. M. Marinković, M. V. Stanković, A. V. Veličković, J. M. Avramović, M. R. Miladinović, O. O. Stamenković, V. B. Veljković and D. M. Jovanović, Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives, Renewable Sustainable Energy Rev., 2016, 56, 1387–1408 CrossRef.
- Y. Ogiwara and K. Nomura, Chemical upcycling of PET into a morpholine amide as a versatile synthetic building block, ACS Org. Inorg. Au, 2023, 3, 377–383 CrossRef CAS PubMed.
- BBC, https://www.bbc.com/future/article/20200710-why-clothes-are-so-hard-to-recycle, (accessed May 2024).
- S. Singhal, S. Agarwal and N. Singhal, Chemical recycling of waste clothes: A smarter approach to sustainable development, Environ. Sci. Pollut. Res., 2023, 30, 54448–54469 CrossRef CAS.
- Scientific American Magazine, https://www.scientificamerican.com/article/chemists-are-figuring-out-how-to-recycle-our-clothes, (accessed June 30 2024).
- Chemistry World, https://www.chemistryworld.com/features/recycling-clothing-the-chemical-way/4010988.article, (accessed June 30 2024).
Footnote |
| † Electronic supplementary information (ESI) available: (i) Additional results for depolymerization of PET with ethanol by FeCl3 and FeBr3, (ii) GC chromatograms of the resultant mixtures for depolymerization of PET through transesterification with ethanol using FeCl3 and FeBr3, (iii) selected 13C NMR spectra of the resultant depolymerization mixtures, (iv) selected 1H NMR spectra of the resultant depolymerization mixtures, (v) photos for selected experimental trials. See DOI: https://doi.org/10.1039/d4im00081a |
| This journal is © Institute of Process Engineering of CAS 2025 |


