Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controllable tertiary amine-promoted photoactivation metal-free carbonylation of aryl sulfonium salts to aryl carboxylic acid derivatives†
Jiajun
Zhang
ab,
Le-Cheng
Wang
ab,
Yuanrui
Wang
a and
Xiao-Feng
Wu
 *ab
*ab
aDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, Liaoning, China. E-mail: xwu2020@dicp.ac.cn
bLeibniz-Institut für Katalyse e.V., 18059 Rostock, Germany
First published on 22nd October 2024
Abstract
Conventional transition metal-catalyzed carbonylative reactions are a powerful tool for the direct incorporation of electrophilic reagents, CO, and nucleophilic reagents into high-value-added products. Although these metal-catalyzed carbonylation strategies can efficiently synthesize carbonylated compounds, metal-free systems remain an attractive direction in carbonylative reactions. Inspired by the achievements in metal-free radical carbonylation, herein we describe a photochemical method for the carbonylation of aryl sulfonium salts using photoexcitation of electron donor–acceptor (EDA) complexes. This strategy is metal-free and widely applicable, enabling ready access to a wide range of aryl carboxylic acid derivatives in a simplified manner. Notably, by choosing different amines, the reaction intermediates can be captured and then quenched stepwise. It has the potential to be a direct green alternative to conventional carbonylation methods for the synthesis of aryl carboxylic acid derivatives.
Introduction
The carbonyl group can be used as a pharmacophore or linking group and therefore plays a prominent role in drug design and development. Among the carboxylic acid derivatives, esters have been used to solve metabolic problems due to their susceptibility to cleavage in organisms, most commonly to form prodrugs.1,2 The carbonyl and amino groups in amides, which act as a HBA (hydrogen bond acceptor) and a HBD (hydrogen bond donor), respectively,3,4 can form various hydrogen bonding interactions with target proteins and improve the binding of drugs to target proteins (Fig. 1a).5,6 Therefore, the development of convenient and efficient methods for synthesizing carbonyl-containing compounds has been a long-standing concern. In organic synthesis, one of the most commonly used methods for synthesizing esters and amides is direct coupling of electrophilic acyl chlorides with nucleophilic reagents.7–9 However, the synthesis of acid chlorides typically requires high-energy and corrosive reagents such as thionyl chloride,10 oxalyl chloride,11 or POCl3, all of which are undesired in organic synthesis and cause difficulties when used in large quantities. These characteristics make the development of more efficient acylation schemes a priority.Another method is the transition metal-catalyzed carbonylation, which allows the formation of a wide variety of amides, esters and related products directly from available carbon monoxide and widely sourced aromatic (pseudo)halides (Fig. 1b, (1)). Since the first report on the palladium-catalyzed direct synthesis of carbonyl compounds from aryl halides by Heck and co-workers in 1974,12 significant progress has been made in the preparation of aryl carboxylic acid derivatives through palladium-catalyzed carbonylation of aromatic (pseudo)halides.13–25 Various inexpensive metals, such as Ni,26,27 Mo,28,29 Co,30,31 Cu,32,33etc., have been gradually developed and used in the carbonylation of aryl halides to synthesize arylamides and esters. Noteworthily, Arndtsen and co-workers (Fig. 1b, (2)) synthesized acid chlorides via palladium-catalyzed carbonylation using carbon monoxide as a C1 source and converted them along with nucleophilic reagents into aryl carboxylic acid derivatives.34,35 A few years later, their group successfully reported the synthesis and conversion of aroyl DMAP salts (Fig. 1b, (3)) into aromatic carboxylic acid derivatives.36–38
Radical carbonylation chemistry allows obtaining carbonylation products in a metal-free manner.39–45 Such a reaction would be an attractive supplement to transition metal-catalyzed strategies. Lei and co-workers (Fig. 1b, (4)) reported the first metal-free carbonylation reaction of aryl halides in 2012.46 This reaction confirmed that aryl halides can generate aryl radicals under the mediation of potassium tert-butoxide and 1,10-phenanthroline, which supported the formation of a series of aryl tert-butyl esters. Subsequently, metal-free carbonylation of aryl radicals continues to receive attention and is thriving. Shortly thereafter, the groups of Wangelin47 and Xiao48 reported that aryl diazonium salts, which are synthesized from aryl amines, allowed for the production of a series of aryl esters under conditions where fluorescein or eosin Y was used as a photosensitizer (Fig. 1b, (6)). A few years later, Polyzos49 described an iridium-catalyzed visible light mediated aromatic radical amidation (Fig. 1b, (5)). However, exploring new methods in aryl radical carbonylation is still crucial, not only because of the availability of starting materials but also because simple methods and broader substrates need to be considered.
The generation of aryl radicals through EDA complexes has become a green and sustainable method for aryl C–H bond functionalization.50–52 In the last few years, Ritter's group53–56 and other groups57–59 have described a wide range of aromatic hydrocarbon functionalization reactions via site-selective C–H pre-thianthrenation of arenes. Furthermore, aryl sulfonium salts, as a common electron acceptor, support the generation of EDA complexes using N-reagents as electron donors.60–62 Inspired by these works, we report herein a tertiary amine-promoted aryl radical carbonylation of aryl sulfonium salts (Fig. 1c). This protocol, in combination with aryl C–H thianthrenation, can provide an efficient method for the preparation of esters and amides via site-selective C–H carbonylation of arenes under metal-free conditions.
Results and discussion
We employed an aryl thianthrenium salt (1a) and benzyl alcohol as model substrates, and performed an extensive screening for metal-free carbonylation under blue LED irradiation (Kessil 40 W, 456 nm). After a broad assessment of the reaction parameters, we found that an optimum isolation yield of 92% was obtained for 1a (0.4 mmol), 2a (benzyl alcohol, 0.2 mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 0.6 mmol), acetonitrile (MeCN) as a solvent, and 50 bar CO (Table 1, entry 1). A suitable electron donor (tertiary amine) is critical to the success of the reaction, and there was a significant decrease in the yield when other tertiary amines were used as electron donors (Table 1, entries 2–10). The yield was significantly lower when the reaction was carried out in 1,2-dichloroethane (DCE) and toluene compared to acetonitrile (MeCN) (Table 1, entries 11 and 12). When the pressure of carbon monoxide was lowered (Table 1, entry 13), the yield was significantly reduced, which may be related to the decarbonylative reaction of the formed acyl radical intermediate. The target product 3 could not be detected when the electron donor or light was omitted (Table 1, entries 14 and 15). Then we tried to reduce the equivalent of 1a and DBU, and the yield decreased with the decrease of 1a and DBU.| Entry | Variation from standard conditions | Yieldb |
|---|---|---|
| a 1a (0.4 mmol), 2a (0.2 mmol), G (0.6 mmol), CO (50 bar), MeCN (2 mL), 40 W blue LEDs, 24 h. b Yields were determined by GC-FID analysis using n-dodecane as the internal standard. c Isolated yield. A, triethylamine; B, 4-methylmorpholine; C, N-isopropyl-N-methyl-tert-butylamine; D, quinuclidine; E, N,N,N′,N‘-tetramethylethylenediamine; F, 1,4-diazabicyclo[2.2.2]octane; G, 1,8-diazabicyclo[5.4.0]undec-7-ene; H, N,N,N′,N′′,N′′-pentamethyldiethylenetriamine; I, N,N-dimethylaniline; J, 4-dimethylaminopyridine (DMAP). | ||
| 1 | None | 93% (92%)c |
| 2 | A instead of G | Trace |
| 3 | B instead of G | Trace |
| 4 | C instead of G | Trace |
| 5 | D instead of G | 13% |
| 6 | E instead of G | Trace |
| 7 | F instead of G | 12% |
| 8 | H instead of G | Trace |
| 9 | I instead of G | Trace |
| 10 | J instead of G | 28% |
| 11 | Toluene instead of MeCN | 35% |
| 12 | DCE instead of MeCN | 21% |
| 13 | 10 bar CO | <5% |
| 14 | w/o DBU | ND |
| 15 | w/o blue LEDs, 80 °C | ND |
| 16 | 1a (0.2 mmol), 2a (0.3 mmol) | 48% |
| 17 | G (1.0 equiv.) | 52% |
| 18 | G (2.5 equiv.) | 79% |
Next, we explored the compatibility of the photoactivation carbonylation method with various aryl thianthrenium salts and nucleophilic reagents as pathways to obtain different aryl carboxylic acid derivatives (Fig. 2). Various electron-donating groups of aryl thianthrenium salts provided high yields of the target compounds under standard conditions, where 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) acted as an electron donor (3–6 and 8). The reaction was also compatible with functional groups such as acyl, cyano, and heterocyclic groups, providing moderate yields (12–14). Moreover, various phenols, alcohols, and benzyl alcohols with various substituents were tested under our optimal conditions, affording excellent yields of the desired products. When electron-withdrawing, electron-donating, o-substituted, and benzyl alcohols were used, the corresponding target products were obtained in moderate to excellent yields (15–20). The target products were obtained in good yields under standard conditions for substrates containing halogen substituents (22–24). It is worth noting that a two-site nucleophilic reagent like 4-(hydroxymethyl)phenol (25) could afford the corresponding products in only trace amounts. From the reaction mixture, a mixture of mono- and double-carbonylation products could be detected together with some compounds from 4-(hydroxymethyl)phenol oxidation. When the carbonylation reaction was carried out using the optimal conditions, phenols containing different substituents were transformed in moderate to excellent reaction yields for the corresponding target products (26–33). Unfortunately, other types of nucleophilic reagents such as alkyl alcohols, aryl amines, and alkyl amines were not compatible with the system. The compatibility of nucleophilic reagents remains a potential challenge in this photochemical carbonylation reaction.
To overcome this limitation, the exploration of new photochemical methods was carried out. Inspired by the work of Arndtsen, we envisioned whether acyl radicals could be directly coupled with the N-radicals present in this system to form relatively stable acyl amine salts, and then nucleophilic reagents could be added to the reaction system to achieve compatibility with a wider range of substrates. We used a variety of N-reagents to initiate the reaction. Surprisingly, in the one-pot, two-step approach, the previously incompatible cyclohexanol of the reaction system could give the corresponding ester when DMAP was used as an electron donor. Subsequently, after screening a series of relevant conditions, we finally used aryl thianthrenium salt 1a (0.6 mmol), DMAP (1.2 mmol), CO (50 bar), and MeCN (2 mL) to react under blue LED irradiation for 36 hours. Then, cyclohexanol (0.2 mmol) and NEtiPr2 (0.2 mmol) were added to the mixture and reacted at room temperature for 16 hours as the optimal conditions, obtaining the corresponding ester with a separation yield of 92% (Table 2, entry 1). Other tertiary amines could not afford the esterification product 47 through this one-pot two-step method (Table 2, entries 2–10). This may be due to the fact that the corresponding acyl amine salts generated from other tertiary amines are not sufficiently stable. When the pressure of CO was lowered, the reaction yield decreased to 41% (Table 2, entry 13). The reaction could not occur under conditions where DMAP or blue light irradiation was not present (Table 2, entries 14 and 15). This indicates that DMAP and blue light irradiation are critical. When the blue light irradiation time was reduced to 24 hours, the reaction yield decreased to 82% (Table 2, entry 16).
| Entry | Variation from standard conditions | Yieldb |
|---|---|---|
| a 1a (0.6 mmol), electron donor (1.2 mmol), MeCN (2 mL), CO (50 bar), 40 W blue LEDs, 36 h. Then, cyclohexanol (0.2 mmol) and NEtiPr2 (0.2 mmol) were added to this reaction system at r.t. for 16 h. b Yields were determined by GC-FID analysis using n-dodecane as the internal standard. c Isolated yield. | ||
| 1 | None | 93% (92%)c |
| 2 | A instead of J | ND |
| 3 | B instead of J | ND |
| 4 | C instead of J | ND |
| 5 | D instead of J | ND |
| 6 | E instead of J | ND |
| 7 | F instead of J | ND |
| 8 | G instead of J | ND |
| 9 | H instead of G | ND |
| 10 | I instead of G | ND |
| 11 | DMF instead of MeCN | Trace |
| 12 | Toluene instead of MeCN | 39% |
| 13 | 10 bar CO | 41% |
| 14 | w/o DMAP | NR |
| 15 | w/o blue LEDs, 80 °C | NR |
| 16 | Blue LEDs, 24 h | 82% |
Subsequently, under the optimal conditions of a DMAP-mediated one-pot two-step process for photoactivation, we expanded the substrate range. Various aryl thianthrenium salts could be used in the DMAP-mediated carbonylative reaction to produce the corresponding products in more appreciable yields (34–39). The electron pairs of heteroatoms in heterocycle-containing nucleophilic reagents can coordinate with metal catalysts, thereby decreasing and limiting the catalytic activity of metal catalysts.63 Notably, heterocyclic benzyl alcohols were compatible with this metal-free carbonylative reaction system affording excellent yields (40, 42, and 43). Importantly, reagents with two nucleophilic sites that are incompatible with DBU-mediated carbonylation reactions (45 and 46) could be transformed in this new system. Alkyl alcohols and N-nucleophilic reagents were also compatible with this protocol. Anilines and primary and secondary alkyl amines were successfully converted under the reaction conditions. Amines substituted with electron-withdrawing substituents such as trifluoromethyl, trifluoromethoxy, and cyano were compatible and afforded the target compounds in 51–91% yields (53, 55, 56, and 61). Meanwhile, various electron-donating substituents such as methoxy, thiomethyl and phenoxy substituted amines were compatible and the target compounds were obtained in 64–86% yields (54, 59, and 60). For halogen-substituted anilines, the target products were obtained in good yields under the standard conditions (57 and 58). The range of various heterocyclic amines was then examined under the same reaction conditions (62, 63, 65 and 66). Different alkyl amines could be obtained in good yields in this case.
As shown in Fig. 3, cyclic amines of different ring sizes, including cyclopentyl (68, 74%), cyclohexyl (69, 90%), and even alkyl amines with larger tensile rings, such as 3-oxetanyl (71, 76%) and cyclopropyl (70, 71%), delivered the desired carbonylation products in good yields. We demonstrated the synthetic value of this strategy using natural products, bioactive molecules, and late-stage modifications of complex multiple aryl complexes. Encouragingly, bioactive alcohols and phenols such as vitamin E (73), estrone (74), DL-menthol (75), dehydroabietic amines (76), amino acid derivatives (77), and ezetimibe (78) were successfully converted into the corresponding carbonylated products. It is noteworthy that compounds with multiple substitutions on the aryl groups could be site-selectively generated to the corresponding thianthrenes, both of which could react with nucleophilic reagents to form the corresponding esters (36, 39, 79 and 80), which are difficult to achieve in other similar methods. These positive results show the potential for this strategy to be employed in the late-stage modification of complex molecules.
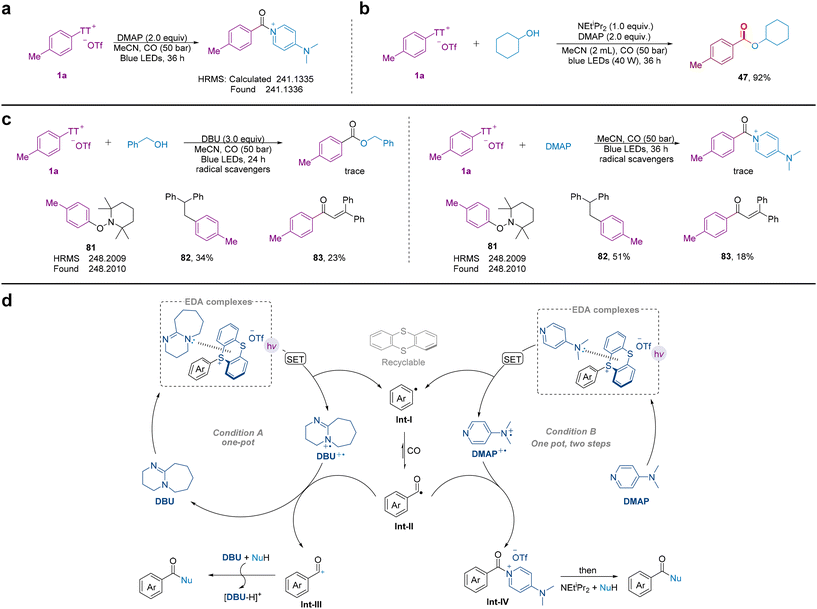
The formation of EDA complexes with aryl sulfonium salts has been demonstrated in the literature.60–62 It can be observed that when DBU is added to the solution of aryl sulfonium salt (1a) in MeCN, there is no significant change in colour. However, when the mixture is irradiated with blue light at 456 nm for 20 minutes, it clearly turns into yellow (Fig. 4a). In the UV absorption spectrum, the absorption curve of the mixture of 1a and DBU shows a red shift, which becomes more pronounced after blue light irradiation. In the system of 1a and DMAP, 400 nm blue light irradiation is required for a significant red shift in the absorption curve (Fig. 4b). This may be caused by the formation of different EDA complexes.
In the absence of a nucleophilic reagent and NEtiPr2, we also conducted reactions to identify the key intermediates involved in the method (Fig. 5a). To our delight, we could detect the corresponding intermediates by HRMS. At the same time, we attempted a one-step reaction and modified the reaction conditions. It was found that the one-step method is also compatible with the reaction using alkyl alcohol as a nucleophile, and the separation yield could reach up to 92% (Fig. 5b). The addition of radical scavengers to reaction system A (Fig. 5c, left) and reaction system B (Fig. 5c, right), respectively, completely inhibited the formation of the desired product under other standard conditions. Additionally, the aryl radical capture product 81 was detected by HRMS, and when the radical capture reagent was 1,1-diphenylethylene, both aryl and acyl radicals could be detected. These results suggest that aryl radicals and acyl radicals are most likely involved in the process.
Based on the above experimental results and reported literature, we proposed a mechanism for the generation of aryl radicals by the formation of EDA complexes from aryl thianthrenium salts and tertiary amines for this methodology (Fig. 5d). Initially, DBU or DMAP is combined with aryl sulfonium salts to produce the corresponding EDA complex, which produces the aryl radical, DBU˙+ or DMAP˙+, and the thianthrene. The thianthrene can be recovered and reused for aryl activation. Subsequently, the aryl radical captures CO, forming an acyl radical. Under condition A, the acyl radical is oxidized by DBU˙+ to an acyl cation, and finally the nucleophilic reagent reacts with the acyl cation in the presence of a base to produce the corresponding acyl compound. Under condition B, the acyl radical is combined with DMAP˙+ to form the corresponding aroyl-DMAP salt, which acts as an active species allowing the reaction with more abundant N-, O-nucleophilic reagents to obtain the corresponding acyl compounds.
Conclusions
In summary, we have developed a universal strategy for the three-component construction of aryl carboxylic acid derivatives in a metal-free manner. In combination with site-selective thianthrenation of aromatic hydrocarbons, esterification and amidation of arenes were achieved and late modification of complex molecules could be accomplished.64 The multifunctionality of the protocol is proven by the compatibility with phenols, alkyl alcohols, anilines and alkyl amines containing various functional groups, which allows for the generation of synthetically valuable and pharmaceutically relevant aryl carboxylic acid derivatives. We expect this work to further promote the development of metal-free radical carbonylation chemistry.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Key R&D Program of China (2023YFA1507500) and the Chinese Academy of Sciences Dalian Institute of Chemical Physics (DICP).References
- K. Beaumont, R. Webster, L. Gardner and K. Dack, Curr. Drug Metab., 2003, 4, 461–485 CrossRef CAS PubMed.
- D. H. Jornada, G. F. D. S. Fernandes, D. E. Chiba, T. R. F. D. Melo, J. L. D. Santos and M. C. Chung, Molecules, 2015, 21, 42 CrossRef PubMed.
- A. Johansson, P. Kollman, S. Rothenberg and J. McKelvey, J. Am. Chem. Soc., 1974, 96, 3794–3800 CrossRef CAS.
- H. Zhao, S. Tang, X. Xu and L. Du, Int. J. Mol. Sci., 2017, 18, 4 CrossRef PubMed.
- S. Kumari, A. V. Carmona, A. K. Tiwari and P. C. Trippier, J. Med. Chem., 2020, 63, 12290–12358 CrossRef CAS PubMed.
- T. Wieland and M. Bodanszky, The World of Peptides: A Brief History of Peptide Chemistry, Springer, 1991 Search PubMed.
- B. Lu, W.-J. Xiao and J.-R. Chen, Molecules, 2022, 27, 517 CrossRef CAS PubMed.
- M. T. Sabatini, L. T. Boulton, H. F. Sneddon and T. D. Sheppard, Nat. Catal., 2019, 2, 10–17 CrossRef CAS.
- J. R. Dunetz, J. Magano and G. A. Weisenburger, Org. Process Res. Dev., 2016, 20, 140–177 CrossRef CAS.
- A. Leggio, E. L. Belsito, G. D. Luca, M. L. D. Gioia, V. Leotta, E. Romio, C. Sicilianoa and A. Liguori, RSC Adv., 2016, 6, 34468–34475 RSC.
- L. A. Carpino, B. J. Cohen, K. E. Stephens Jr., S. Y. Sadat-Aalaee, J. H. Tien and D. C. Langridge, J. Org. Chem., 1986, 51, 3732–3734 CrossRef CAS.
- A. Schoenberg, I. Bartoletti and R. F. Heck, J. Org. Chem., 1974, 39, 3318–3326 CrossRef CAS.
- A. Brennführer, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 4114–4133 CrossRef PubMed.
- C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC.
- W. Mägerlein, A. F. Indolese and M. Beller, Angew. Chem., Int. Ed., 2001, 40, 2856–2859 CrossRef.
- J. R. M. Rachel, H. Munday and S. L. Buchwald, J. Am. Chem. Soc., 2008, 130, 2754–2755 CrossRef PubMed.
- Y. Hu, J. Liu, Z. Lü, X. Luo, H. Zhang, Y. Lan and A. Lei, J. Am. Chem. Soc., 2010, 132, 3153–3158 CrossRef CAS PubMed.
- W. Magerlein, M. Beller and A. F. Indolese, J. Mol. Catal. A: Chem., 2000, 156, 213–221 CrossRef CAS.
- Y. A. Kolekar and B. M. Bhanage, J. Org. Chem., 2021, 86, 14028–14035 CrossRef CAS PubMed.
- T. Xu and H. Alper, J. Am. Chem. Soc., 2014, 136, 16970–16973 CrossRef CAS PubMed.
- J. Y. Wang, A. E. Strom and J. F. Hartwig, J. Am. Chem. Soc., 2018, 140, 7979–7993 CrossRef CAS PubMed.
- J. McNulty, J. J. Nair and A. Robertson, Org. Lett., 2007, 9, 4575–4578 CrossRef CAS PubMed.
- S. N. Gockel and K. L. Hull, Org. Lett., 2015, 17, 3236–3239 CrossRef CAS PubMed.
- J.-B. Peng, D. Li, H.-Q. Geng and X.-F. Wu, Org. Lett., 2019, 21, 4878–4881 CrossRef CAS PubMed.
- Y. L. Gerardo, M. Torres and B. A. Arndtsen, Science, 2020, 368, 319–323 Search PubMed.
- N. Shen, C. W. Cheung and J.-A. Ma, Chem. Commun., 2019, 55, 13709–13712 RSC.
- C. W. Cheung, M. L. Ploeger and X. Hu, Chem. Sci., 2018, 9, 655–659 RSC.
- W. Ren and M. Yamane, J. Org. Chem., 2010, 75, 8410–8415 CrossRef CAS PubMed.
- D. L. B. Roberts, L. Alcaraz, T. Luker and M. J. Stocks, Org. Lett., 2010, 12, 4280–4283 CrossRef PubMed.
- M.-Y. She, D.-W. Xiao, B. Yin, Z. Yang, P. Liu, J.-L. Li and Z. Shi, Tetrahedron, 2013, 69, 7264–7268 CrossRef CAS.
- T. Imamoto, T. Kusumoto and M. Yokoyama, Bull. Chem. Soc. Jpn., 1982, 55, 643–644 CrossRef CAS.
- J. Liu, R. Zhang, S. Wang, W. Sun and C. Xia, Org. Lett., 2009, 11, 1321–1324 CrossRef CAS PubMed.
- P. K. Prasad and A. Sudalai, Adv. Synth. Catal., 2014, 356, 2231–2238 CrossRef CAS.
- J. S. Quesnel and B. A. Arndtsen, J. Am. Chem. Soc., 2013, 135, 16841–16844 CrossRef CAS PubMed.
- J. S. Quesnel, L. V. Kayser, A. Fabrikant and B. A. Arndtsen, Chem. – Eur. J., 2015, 21, 9550–9555 CrossRef CAS PubMed.
- J. S. Quesnel, A. Fabrikant and B. A. Arndtsen, Chem. Sci., 2016, 7, 295–300 RSC.
- P.-L. Lagueux-Tremblay, A. Fabrikant and B. A. Arndtsen, ACS Catal., 2018, 8, 5350–5354 CrossRef CAS.
- P.-L. Lagueux-Tremblay, C. Augereau, P. Nair, K. M. Tam and B. A. Arndtsen, ACS Catal., 2022, 12, 13394–13399 CrossRef CAS.
- T. Kawamoto, T. Fukuyama, B. Picard and I. Ryu, Chem. Commun., 2022, 58, 7608–7617 RSC.
- B. Lu, Z. Zhang, M. Jiang, D. Liang, Z. W. He, F. S. Bao, W. J. Xiao and J. R. Chen, Angew. Chem., Int. Ed., 2023, 62, e202309460 CrossRef CAS PubMed.
- B. Lu, M. Xu, X. Qi, M. Jiang, W.-J. Xiao and J.-R. Chen, J. Am. Chem. Soc., 2022, 144, 14923–14935 CrossRef CAS PubMed.
- N. Micic and A. Polyzos, Org. Lett., 2018, 20, 4663–4666 CrossRef CAS PubMed.
- A. Cartier, E. Levernier, V. Corcé, T. Fukuyama, A. L. Dhimane, C. Ollivier, I. Ryu and L. Fensterbank, Angew. Chem., Int. Ed., 2019, 58, 1789–1793 CrossRef CAS PubMed.
- L. Lu, D. Cheng, Y. Zhan, R. Shi, C.-W. Chiang and A. Lei, Chem. Commun., 2017, 53, 6852–6855 RSC.
- W.-W. Ding, Z.-Y. He, M. Sayed, Y. Zhou, Z.-Y. Han and L.-Z. Gong, Nat. Synth., 2024, 3, 507–516 CrossRef.
- H. Zhang, R. Shi, A. Ding, L. Lu, B. Chen and A. Lei, Angew. Chem., Int. Ed., 2012, 51, 12542–12545 CrossRef CAS PubMed.
- M. Majek and A. J. von Wangelin, Angew. Chem., Int. Ed., 2014, 54, 2270–2274 CrossRef PubMed.
- W. Guo, L. Q. Lu, Y. Wang, Y. N. Wang, J. R. Chen and W. J. Xiao, Angew. Chem., Int. Ed., 2014, 54, 2265–2269 CrossRef PubMed.
- J. A. Forni, N. Micic, T. U. Connell, G. Weragoda and A. Polyzos, Angew. Chem., Int. Ed., 2020, 59, 18646–18654 CrossRef CAS PubMed.
- B. Liu, C.-H. Lim and G. M. Miyake, J. Am. Chem. Soc., 2017, 139, 13616–13619 CrossRef CAS PubMed.
- A. Kamal, V. Srivastava and S. Singh, New J. Chem., 2023, 47, 14605–14609 RSC.
- M. J. Cabrera-Afonso, A. Granados and G. A. Molander, Angew. Chem., Int. Ed., 2022, 61, e202202706 CrossRef CAS PubMed.
- F. Berger, M. B. Plutschack, J. Riegger, W. Yu, S. Speicher, M. Ho, N. Frank and T. Ritter, Nature, 2019, 567, 223–228 CrossRef CAS PubMed.
- B. Lansbergen, P. Granatino and T. Ritter, J. Am. Chem. Soc., 2021, 143, 7909–7914 CrossRef CAS PubMed.
- R. Sang, S. E. Korkis, W. Su, F. Ye, P. S. Engl, F. Berger and T. Ritter, Angew. Chem., Int. Ed., 2019, 58, 16161–16166 CrossRef CAS PubMed.
- P. S. Engl, A. P. Häring, F. Berger, G. Berger, A. Pérez-Bitrián and T. Ritter, J. Am. Chem. Soc., 2019, 141, 13346–13351 CrossRef CAS PubMed.
- R. A. Roberts, B. E. Metze, A. Nilova and D. R. Stuart, J. Am. Chem. Soc., 2023, 145, 3306–3311 CrossRef CAS PubMed.
- H. Meng, M.-S. Liu and W. Shu, Chem. Sci., 2022, 13, 13690–13707 RSC.
- X.-Y. Chen, Y.-N. Li, Y. Wu, J. Bai, Y. Guo and P. Wang, J. Am. Chem. Soc., 2023, 145, 10431–10440 CrossRef CAS PubMed.
- L. van Dalsen, R. E. Brown, J. A. Rossi-Ashton and D. J. Procter, Angew. Chem., Int. Ed., 2023, 62, e202303104 CrossRef CAS PubMed.
- A. Dewanji, L. van Dalsen, J. A. Rossi-Ashton, E. Gasson, G. E. M. Crisenza and D. J. Procter, Nat. Chem., 2022, 15, 43–52 CrossRef PubMed.
- K. Sun, A. Shi, Y. Liu, X. Chen, P. Xiang, X. Wang, L. Qu and B. Yu, Chem. Sci., 2022, 13, 5659–5666 RSC.
- H.-J. Ai, Y. Yuan and X.-F. Wu, Chem. Sci., 2022, 13, 2481–2486 RSC.
- (a) J. Zhang and X.-F. Wu, Org. Lett., 2023, 25, 2162–2166 CrossRef CAS PubMed; (b) J. Zhang, L.-C. Wang, Z.-P. Bao and X.-F. Wu, Chem. Sci., 2023, 14, 7637–7641 RSC; (c) J. Zhang, L.-C. Wang, Y. Wang, B.-H. Teng and X.-F. Wu, J. Catal., 2024, 432, 115454 CrossRef CAS; (d) Y. Guo, L. Wei, H. Jiang and C. Qi, Asian J. Org. Chem., 2024, 13, e202400142 CrossRef CAS; (e) Y.-H. Zhao, X.-W. Gu and X.-F. Wu, Org. Lett., 2024, 26, 6507–6511 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General comments, general procedure, analytic data, and NMR spectra. See DOI: https://doi.org/10.1039/d4gc04502e |
| This journal is © The Royal Society of Chemistry 2024 |