Metal-free catalytic nucleophilic substitution of primary alcohols with secondary phosphine oxides†
Xiantao
Ma
 *a,
Xiaoyu
Yan
a,
Jing
Yu
a,
Jiarui
Guo
a,
Jiakun
Bian
a,
Ran
Yan
a,
Qing
Xu
*a,
Xiaoyu
Yan
a,
Jing
Yu
a,
Jiarui
Guo
a,
Jiakun
Bian
a,
Ran
Yan
a,
Qing
Xu
 *bc and
Li-Biao
Han
*bc and
Li-Biao
Han
 *bd
*bd
aCollege of Chemistry and Chemical Engineering, Green Catalysis & Synthesis Key Laboratory of Xinyang City, Xinyang Normal University, Xinyang, Henan 464000, China. E-mail: xiantaoma@126.com
bZhejiang Yangfan New Materials Company, Ltd., Shangyu, Zhejiang 312369, China
cCollege of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang 325035, China. E-mail: qing-xu@wzu.edu.cn
dCollege of Chemistry & Chemical Engineering, Shaoxing University, Shaoxing, Zhejiang 312000, China. E-mail: hlb@shoufuchem.com
First published on 27th November 2024
Abstract
Due to their inert nature and low reactivities, the dehydrative version of the MA reaction has not been achieved under metal-free conditions. Here, we develop a direct and efficient metal-free nucleophilic substitution reaction of primary alcohols with secondary phosphine oxides using trimethyliodosilane (TMSI) as the catalyst, providing a simple and green method for synthesis of useful tertiary phosphine oxides. This method is very effective as it can be extended to various primary alcohols, including benzylic and allylic, and even the more inert primary aliphatic alcohols. Many products can be obtained with high purity by simple washing and/or recrystallization under column chromatography-free conditions. This method can also be scaled up easily and applied in one-pot step-wise transformations to synthesize useful chemicals. Mechanism studies suggested that the reaction can be considered as a dehydrative version of the Michaelis–Arbuzov reaction and that both alcohol dehydroxylation and dehydrogenation processes may be involved in the reaction.
Organophosphoryl compounds R1R2P(O)R3 bearing a P(O) structure are not only important intermediates in organic and pharmaceutical synthesis1,2 but also effective catalysts in organic synthesis.3 For example, tertiary phosphine oxides can be used for the preparation of various phosphine ligands,4 or directly used as organocatalysts in various reactions such as catalytic Mitsunobu reactions.3
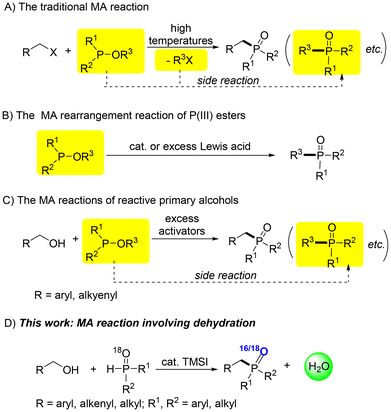
Numerous methods have been developed, such as the Michaelis–Arbuzov (MA) reaction,5 TM-catalyzed cross-coupling reactions of P(O)–H compounds with alkyl halides,6 carboxylic acids and even hydrazones7 and TM-catalyzed addition of P(O)–H compounds to various unsaturated bonds.8 Among them, the famous MA reactions of P(III) esters with alkyl halides, which were developed more than one hundred years ago, remain the most important method for the large-scale preparation (Scheme 1A).5 Despite the advantages of MA reactions, there are still some drawbacks, such as the use of toxic alkyl halides and the inevitable side reactions caused by the low-boiling byproduct alkyl halides under harsh reaction conditions. Then, Lewis acid catalyzed/mediated MA rearrangements of P(III) esters (Scheme 1B)9 and more recently MA reactions of primary alcohols (limited to reactive benzyl and allylic alcohols) instead of alkyl halides were also developed as greener alternatives (Scheme 1C)10 of the classic MA reactions. However, the use of unstable and commercially unavailable P(III) esters, stoichiometric amounts of activators, the side reactions of trivalent phosphorus esters, and the limited substrate scope narrowed the wide application of these methods. Therefore, avoiding the usage of toxic, unstable and reactive alkyl halides and P(III) esters may lead to a more practical and greener version of the MA method.
We have also worked on phosphorus chemistry11 and activation and applications of alcohols.12 We noticed that the dehydration of alcohols with P(O)–H compounds may be an important advancement of the MA reaction; however, metal (M)-free direct dehydrative nucleophilic substitution of the more inert and much less reactive primary alcohols with P(O)–H compounds has yet to be explored.13,14 Herein, we report an efficient TMSI-catalyzed dehydrative substitution reaction of inert primary alcohols with secondary phosphine oxides, producing useful tertiary phosphine oxides (Scheme 1D). Mechanism studies suggested that the reaction can be considered as a dehydrative version of the MA reaction and that both alcohol dehydroxylation and dehydrogenation processes may be involved in the reaction.
Without catalysts, no reaction of benzyl alcohol (1a) and diphenylphosphine oxide (2a) occurred when the neat mixture was heated at 80 °C (Table 1, run 1). To our delight, when HCl and trimethylsilyl chloride (TMSCl) were used as the catalyst, the formation of a trace amount of the target 3aa could be observed (runs 2 and 3). Further screening of haloid acids (HX) and trimethylsilyl halides (TMSX) showed that TMSX is better than the corresponding HX (runs 4–7), with TMSI being the most effective catalyst that afforded 90% yield of 3aa (run 7). In contrast, tetrabutylammonium iodide (TBAI), a good catalyst for our previous alcohol-based MA reaction,10e was not effective at all in this reaction (run 8). Therefore, the most effective TMSI was still chosen as the catalyst for the reaction. Catalyst loading and reaction temperature were further screened, but no better results could be obtained (runs 9–13). Moreover, the common organic solvents were investigated, but the target 3aa was obtained only in lower yields (runs 14–20). Finally, a careful study on highly concentrated solution was performed (runs 21–25), and the target 3aa could be obtained in a yield of 77% (run 22), which may be an alternative to the solvent-free method. Moreover, during this work, we found that the reaction mixture turned from the liquid state at the beginning to the solid state at the end of the reaction.15 We then attempted a simple way to purify the product. Thus, after several trials, in small scale reactions, 3aa could be isolated by simply washing the reaction mixture with a mixed solvent of ethyl acetate/petroleum ether (v/v 1/1) and/or by recrystallizing with a mixed solvent of MeOH/water (v/v 1/1) under column chromatography-free conditions.14 This simple purification method makes the reaction more practical and easier to conduct, hence being greener by saving chemicals and shortening the product purification process.
| Run | Cat. (mol%) | Solvent | 3aa%b |
|---|---|---|---|
| a Unless otherwise noted, the mixture of 1a (0.60 mmol), 2a (0.50 mmol), a catalyst and a solvent (0.5 mL) sealed under N2 in a 10 mL Schlenk tube was heated at 80 °C for 12 h and then analyzed by TLC/GC-MS. b Isolated yields based on 2a. c The yield was obtained simply by washing the reaction mixture with a mixed solvent of ethyl acetate/petroleum ether (v/v 1/1) (out of parentheses) or by recrystallizing the product with a mixed solvent of MeOH/water (v/v 1/1) (in parentheses). d 40 °C. e 60 °C. f 100 °C. g Solvent (0.05 mL). h Solvent (0.1 mL). i Solvent (0.2 mL). | |||
| 1 | None | None | 0 |
| 2 | HCl (10) | None | Trace |
| 3 | TMSCl (10) | None | Trace |
| 4 | HBr (10) | None | 50 |
| 5 | TMSBr (10) | None | 75 |
| 6 | HI (10) | None | 80 |
| 7 | TMSI (10) | None | 90 (84)c |
| 8 | TBAI (10) | None | 0 |
| 9 | TMSI (5) | None | 74 |
| 10 | TMSI (30) | None | 85 |
| 11d | TMSI (10) | None | 4 |
| 12e | TMSI (10) | None | 36 |
| 13f | TMSI (10) | None | 90 |
| 14 | TMSI (10) | Toluene | 52 |
| 15 | TMSI (10) | Dioxane | 32 |
| 16 | TMSI (10) | DCE | 3 |
| 17 | TMSI (10) | EtOAc | 21 |
| 18 | TMSI (10) | DMF | 0 |
| 19 | TMSI (10) | THF | 29 |
| 20 | TMSI (10) | CHCl3 | 7 |
| 21g | TMSI (10) | Toluene | 77 |
| 22h | TMSI (10) | Toluene | 77 |
| 23i | TMSI (10) | toluene | 62 |
| 24h | TMSI (10) | Xylene | 77 |
| 25h | TMSI (10) | Dioxane | 70 |
With the optimized conditions in hand (Table 1, run 7), we explored the scope of this metal-free reaction of primary alcohols and phosphine oxides. As shown in Table 2, both electron-rich and -deficient group-substituted benzylic alcohols including those bearing methoxy and halogen groups and 2-naphthylmethanol all reacted efficiently with 2a to afford good to high yields of the desired products (runs 1–8). A heteroaryl methanol, 2-thienyl methanol, also afforded the target product in a high yield of 92% (run 9). This method could also be extended to ferrocene methanol, which afforded the target product in 88% yield (run 10). For allylic alcohols such as cinnamyl alcohol, 4-F-cinnamyl alcohol, and 3,3-dimethyl allyl alcohol, all specifically afforded linear products in high yields without the formation of potential branched regiomers (runs 11–13), revealing the high regioselectivity of the reaction. However, the reaction of a primary propargylic alcohol could not yield the target product at present (run 14). For less reactive primary aliphatic alcohols, the reactions were more sluggish under standard conditions, and moderate yields of the products could be obtained under modified conditions (runs 15–18). Steric more bulky secondary and tertiary alcohols were also investigated, but no target products were obtained at present (runs 19 and 20), which indicates that this reaction is a complementary method to the known reactions of alcohols and P(O)H compounds that mainly work well with other types of alcohols (Scheme 1A).13 Moreover, this method could also be extended to diols, e.g., the reaction of 1,4-benzenedimethanol and 2a proceeded smoothly to afford 84% yield of the target product (run 21).
| a Unless otherwise noted, see run 7 of Table 1 for details. Isolated yields based on 2. b The yields in parentheses were obtained by recrystallizing the products with a mixed solvent of MeOH/water (v/v 1/1). c Products purified by normal column chromatography. d 100 mol% TMSI, 120 °C. e 0.5 mL MeOH was added. f 20 mol% TMSI. g 100 °C. |
|---|

|
Other phosphine oxides were also investigated. Similarly, both electron-rich and -deficient diarylphosphine oxides reacted efficiently with 1a to afford good to high yields of the desired products (runs 22–25). The reactions of steric more bulky phenyl t-butyl phosphine oxide, dicyclohexyl phosphine oxide, and diisopropyl phosphine oxide were not efficient under the standard conditions. At a higher temperature, the reactions afforded moderate yields of the target products (runs 26–28).
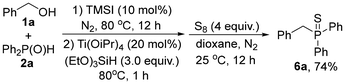
The reaction can be easily scaled up on a gram scale. For example, a 10 mmol reaction of 1a and 2a readily reacted to afford 3aa in a good yield of 82% under neat conditions (eqn (1), run 1) and in a yield of 84% under highly concentrated solution conditions, respectively (eqn (1), run 2). Moreover, this reaction can also be well utilized for step-wise one-pot preparation of phosphine ligands, e.g., after the reaction of 1a and 2a is completed, addition of Ti(Oi-Pr)4 and (EtO)3SiH can readily reduce intermediate 3aa to tertiary phosphine 4a in a high yield (eqn (2)).16 Similarly, step-wise one-pot reactions of the in situ generated 3aa with BH3·HCl or elemental sulfur could readily afford the valuable products 5a or 6a in 76% and 74% yields, respectively (eqn (3) and (4)).17 All these examples showed the practicality and great potential of this new method in synthetic applications.
 | (1) |
 | (2) |
 | (3) |
 | (4) |
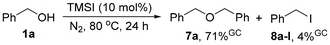
The mechanism of the reaction was then investigated.15 First, the generation of dibenzyl ether (7a) and benzyl iodide (8a-I) was observed by GC-MS in a blank reaction of benzyl alcohol (1a) catalyzed by TMSI (eqn (5)). Next, no reaction of dibenzyl ether (7a) and diphenylphosphine oxide (2a) was observed under the standard conditions (eqn (6)), suggesting that ethers should not be the active intermediate in the reaction. In contrast, the reaction of benzyl bromide (8a-Br) and diphenylphosphine oxide (2a) successfully afforded 3aa in 75% yield, which could be further enhanced to 80% by adding 10 mol% TBAI (eqn (7)). This result is consistent with those observed in the catalyst screening stage using HX as the catalyst (Table 1), as the textbook reaction of alcohols and HX can generate the corresponding halides. The above results indicate that benzyl halides should be the key intermediates in the reaction, especially in the formation of P(III) ester benzyl diphenylphosphinite (9a) through a nucleophilic substitution reaction with the tautomer of2a,9a,e because the basic reactions of alkyl halides with P(O)–H compounds have been known18 and because a TMSI-catalyzed MA rearrangement of 9a could also afford a high yield of 3aa under the standard conditions (eqn (8)). Besides, though ether 7a was generated as the major product from 1a with only catalytic TMSI (eqn (5)), it could be easily converted into the corresponding halide 8a-I with a stoichiometric amount of TMSI (eqn (9)), revealing that the initial formation of unwanted ethers does not affect the reaction outcome at all.
 | (5) |
 | (6) |
 | (7) |
 | (8) |
 | (9) |
On the other hand, in a short time reaction of 8a-Br and 2a, in addition to product 3aa, another compound with a very close mass value (M, 293.1056) to the mass value of 3aa (M + H, 293.1091), which is most possibly the cation of phosphonium salt 10a, was also observed by HRMS analysis of the reaction mixture (eqn (10)), suggesting that the reaction of in situ generated halides 8a and 2a may also form phosphonium salts 10. Therefore, another reaction path via the formation of phosphonium salts 10 as another key intermediate cannot be excluded completely.
 | (10) |
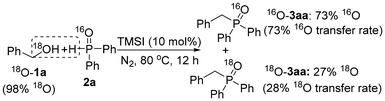
Isotope labelling reactions were further investigated to get a deeper insight into the reaction mechanism.15 First, the reaction of 1a and 18O-2a (86 atom% 18O as prepared and determined) afforded mainly 18O-3aa (72 atom% 18O, ca. 84% 18O transfer rate based on 18O-2a) under the standard conditions (eqn (11)); conversely, the reaction of 18O-1a (98 atom% 18O as prepared and determined) with normal 2a afforded mainly normal 16O-3aa (73 atom% 16O, ca. 73% 16O transfer rate based on 16O-2a) under the same conditions (eqn (12)). These results suggested that (1) the reaction mainly proceeded with the retention of the P–O bond, and (2) the alcohol's OH group mainly worked as the leaving group, which is well consistent with the supposal that the reaction starts with a TMSI-promoted transformation of alcohols 1 to iodides 8 as the key intermediate, followed by C–P bond formation of 8 and 2.
 | (11) |
 | (12) |
However, the formation of 27 atom% 18O-3aa (ca. 28% 18O transfer rate based on 18O-1a) in the latter reaction (eqn (12)) implied that another mechanism may also work in the reaction. As the TMSI-catalyzed MA rearrangement of 9a afforded a high yield of 3aa (eqn (8)), the only explanation for this result is that 18O-9a with good 18O maintenance from 18O-1a was generated and worked as a key intermediate in this process. Therefore, as shown in path a of Scheme 2, before being completely converted into the corresponding iodides 8 by TMSI and similar to the esterification reactions of alcohols and carboxylic acids, a certain amount of 18O-1a may possibly undergo a direct dehydration reaction with 2a′ (the tautomer of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9a,e) to yield the P(III) ester intermediate 18O-9a with the maintenance of 18O of 1a in the molecule,19 followed by a MA rearrangement of 18O-9a to finally afford 18O-3aa. However, as only 27 atom% 18O-3aa was obtained even when using 98 atom% 18O-1a (eqn (12)), which corresponds to only a 28% 18O transfer rate, this process (Scheme 2, path a) should be a minor pathway in the reaction mechanism.
9a,e) to yield the P(III) ester intermediate 18O-9a with the maintenance of 18O of 1a in the molecule,19 followed by a MA rearrangement of 18O-9a to finally afford 18O-3aa. However, as only 27 atom% 18O-3aa was obtained even when using 98 atom% 18O-1a (eqn (12)), which corresponds to only a 28% 18O transfer rate, this process (Scheme 2, path a) should be a minor pathway in the reaction mechanism.
Therefore, most alcohols 1 should be gradually transferred to iodides 8 in the presence of TMSI (Scheme 2, path b). During the process, the byproducts of TMSI, such as TMS2O and TMSOH, may be recovered and converted back into TMSI by HI,20 furnishing the catalytic cycle and generating water as the only byproduct of the whole reaction. Meanwhile, upon transformation into its tautomer 2′, phosphine oxides 2 may react with iodides 8 to yield P(III) ester intermediate 9via nucleophilic substitution reactions (path b1). Then, TMSI promoted the MA rearrangement of 9 to afford final product 3. However, the reaction of 2 and 8 to form phosphonium salt 10, followed by elimination of HI to afford 3 is also a possible way that cannot be excluded completely at present (path b2), as 10a was observed in the reaction of 2a and 8a-Br (eqn (10)).
In conclusion, a direct and M-free dehydrative nucleophilic substitution reaction of more inert primary alcohols and secondary phosphine oxides was developed, which leads to a simple and green method for synthesis of useful tertiary phosphine oxides. This method can be extended to a variety of primary alcohols, can be easily scaled up, and can be applied in one-pot synthesis of valuable phosphine ligands, phosphine–boron compounds, and tertiary phosphine sulfides. This reaction may be a good complementary method to the known dehydrative nucleophilic substitution reactions of secondary or tertiary alcohols with P(O)–H compounds in the synthesis of important phosphoryl compounds and can be considered as a dehydrative version of the Michaelis–Arbuzov reaction, which has not been reported yet. Mechanism studies suggested that both alcohol dehydroxylation and dehydrogenation processes may be involved in the reaction. Further extension of the method for developing new methods for synthesis of other organophosphorus compounds is underway.
Data availability
The authors confirm that the data supporting the findings of this study are available in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (22101243 and 21672163), the Young Backbone Teachers Programs in Universities of Henan Province (2023GGJS097), the Scientific and Technological Innovation Talents in Universities of Henan Province (25HASTIT003), the Nanhu Scholars Program for Young Scholars of XYNU, Postgraduate research and innovation Fund of XYNU (2024KYJJ007) Postgraduate research and innovation Fund and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2022R01021) for financial support.References
- For reviews, see: (a) D. Hérault, D. H. Nguyen, D. Nuel and G. Buono, Reduction of secondary and tertiary phosphine oxides to phosphines, Chem. Soc. Rev., 2015, 44, 2508 RSC; (b) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, A comprehensive overview of directing groups applied in metal-catalysed C-H functionalisation chemistry, Chem. Soc. Rev., 2018, 47, 6603 RSC.
- (a) P. Finkbeiner, J. P. Hehn and C. Gnamm, Phosphine Oxides from a Medicinal Chemist's Perspective: Physicochemical and in Vitro Parameters Relevant for Drug Discovery, J. Med. Chem., 2020, 63, 7081 CrossRef CAS PubMed; (b) M. Voráčová, M. Zore, J. Yli-Kauhaluoma and P. Kiuru, Harvesting phosphorus-containing moieties for their antibacterial effects, Bioorg. Med. Chem., 2023, 96, 117512 CrossRef PubMed; (c) X. Mao, J. Chen, Y. Yao, D. Liu, H. Wang and Y. Chen, Progress in phosphorylation of natural products, Mol. Biol. Rep., 2024, 51, 697 CrossRef CAS PubMed; (d) Y. Volkova, A. Scherbakov, Y. Dzichenka, A. Komkov, F. Bogdanov, D. Salnikova, A. Dmitrenok, A. Sachanka, D. Sorokin and I. Zavarzin, Design and synthesis of phosphoryl-substituted steroidal pyridazines (Pho-STPYRs) as potent estrogen receptor alpha inhibitors: targeted treatment of hormone-dependent breast cancer cells, RSC Med. Chem., 2024, 15, 2380 RSC.
- For a review, see: S. Kotani and M. Nakajima, Recent advances in asymmetric phosphine oxide catalysis, Tetrahedron Lett., 2020, 61, 151421 CrossRef CAS . For selected recent examples, see: (a) R. H. Beddoe, K. G. Andrews, V. Magné, J. D. Cuthbertson, J. Saska, A. L. Shannon-Little, S. E. Shanahan, H. F. Sneddon and R. M. Denton, Redox-neutral organocatalytic Mitsunobu reactions, Science, 2019, 365, 910 CrossRef CAS PubMed; (b) Y. Zou, J. J. Wong and K. N. Houk, Computational exploration of a redox-neutral organocatalytic Mitsunobu reaction, J. Am. Chem. Soc., 2020, 142, 16403 CrossRef CAS PubMed; (c) L. Zhou, S. Perulli, M. M. Mastandrea, P. Llanes, J. Lai and M. A. Pericàs, Development of a robust immobilized organocatalyst for the redox-neutral mitsunobu reaction, Green Chem., 2021, 23, 8859 RSC; (d) D. Song, C. Zhang, Y. Cheng, L. Chen, J. Lin, C. Zheng, T. Liu, Y. Ding, F. Ling and W. Zhong, Development of a more efficient catalyst for the redox-neutral organocatalytic mitsunobu reaction by DFT-guided catalyst design, Green Synth. Catal., 2024, 5, 290, DOI:10.1016/j.gresc.2023.11.002.
- For reviews, see: (a) A. L. Clevenger, R. M. Stolley, J. Aderibigbe and J. Louie, Trends in the Usage of Bidentate Phosphines as Ligands in Nickel Catalysis, Chem. Rev., 2020, 120, 6124 CrossRef CAS PubMed; (b) T. Imamoto, Searching for Practically Useful P-Chirogenic Phosphine Ligands, Chem. Rec., 2016, 16, 2659 CrossRef PubMed.
- (a) B. A. Arbuzov, Michaelis-Arbusow-und perkow-reaktionen, Pure Appl. Chem., 1964, 9, 307 CrossRef CAS; (b) A. K. Bhattacharya and G. Thyagarajan, Michaelis-arbuzov rearrangement, Chem. Rev., 1981, 81, 415 CrossRef CAS; (c) A. Jasiak, G. Mielniczak, K. Owsianik, M. Koprowski, D. Krasowska and J. Drabowicz, Solvent-Free Michaelis–Arbuzov Rearrangement under Flow Conditions, J. Org. Chem., 2019, 84, 2619 CrossRef CAS PubMed.
- G. Lavén, M. Kalek, M. Jezowskaa and J. Stawinski, Preparation of benzylphosphonates via a palladium(0)-catalyzed cross-coupling of H-phosphonatediesters with benzyl halides. Synthetic and mechanistic studies, New J. Chem., 2010, 34, 967 RSC.
- (a) S. P. Bew, R. A. Brimage, D. L. Hughes, L. Legentil, S. V. Sharma and M. A. Wilson, Expedient synthesis of substituted (diphenylphosphinoylmethyl) benzenes, J. Org. Chem., 2007, 72, 2655 CrossRef CAS PubMed; (b) W. Miao, Y. Gao, X. Li, Y. Gao, G. Tang and Y. Zhao, Copper-Catalyzed Synthesis of Alkylphosphonates from H-Phosphonates and N-Tosylhydrazones, Adv. Synth. Catal., 2012, 354, 2659 CrossRef CAS; (c) J. Yang, T. Chen and L.-B. Han, C–P bond-forming reactions via C–O/P–H cross-coupling catalyzed by nickel, J. Am. Chem. Soc., 2015, 137, 1782 CrossRef CAS PubMed; (d) Y. Makida, K. Usui, S. Ueno and R. Kuwano, Palladium-catalyzed benzylic substitution of benzyl carbonates with phosphorus nucleophiles, Chem. Lett., 2017, 46, 1814 CrossRef CAS; (e) A. Hosseinian, F. A. H. Nasab, S. Ahmadi, Z. Rahmani and E. Vessally, Decarboxylative cross-coupling reactions for P(O)-C bond formation, RSC Adv., 2018, 8, 26383 RSC; (f) L.-X. Liu, D. Zhou, J.-Y. Dong, Y.-B. Zhou, S.-F. Yin and L.-B. Han, Transition-Metal-Free C-P Bond Formation via Decarboxylative Phosphorylation of Cinnamic Acids with P(O)H Compounds, J. Org. Chem., 2018, 83, 4190 CrossRef CAS PubMed; (g) J.-S. Zhang, T. Q. Chen, Y. B. Zhou, S.-F. Yin and L.-B. Han, Catalytic sp3C-N Bond Cleavage: Ni-Mediated Phosphorylation of Alkylnitriles, Org. Lett., 2018, 20, 6746 CrossRef CAS PubMed.
- For reviews, see: (a) J. Yang, J. Xiao, Y. Zhou, T. Chen, S. Yin and L.-B. Han, Recent Advances in the Synthesis of Organophosphorus Compounds via Cross Coupling between Readily Available Materials and P-H Compounds, Chin. J. Org. Chem., 2017, 37, 1055 CrossRef CAS; (b) Y. Gao, G. Tang and Y. Zhao, Recent Advances of Phosphorus-Centered Radical Promoted Difunctionalization of Unsaturated Carbon-Carbon Bonds, Chin. J. Org. Chem., 2018, 38, 62 CrossRef CAS; (c) Q. Xu and L.-B. Han, Metal-catalyzed additions of H-P(O) bonds to carbon-carbon unsaturated bonds, J. Organomet. Chem., 2011, 696, 130 CrossRef CAS.
- (a) P.-Y. Renard, P. Vayron, E. Leclerc, A. Valleix and C. Mioskowski, Lewis Acid Catalyzed Room-Temperature Michaelis–Arbuzov Rearrangement, Angew. Chem., Int. Ed., 2003, 42, 2389 CrossRef CAS PubMed; (b) P.-Y. Renard, P. Vayron and C. Mioskowski, Trimethylsilyl Halide-Promoted Michaelis-Arbuzov Rearrangement, Org. Lett., 2003, 5, 1661 CrossRef CAS PubMed; (c) G. G. Rajeshwaran, M. Nandakumar, R. Sureshbabu and A. K. Mohanakrishnan, Lewis Acid-Mediated Michaelis-Arbuzov Reaction at Room Temperature: A Facile Preparation of Arylmethyl/Heteroarylmethyl Phosphonates, Org. Lett., 2011, 13, 1270 CrossRef CAS PubMed; (d) W. Dabkowski, A. Ozarek, S. Olejniczak, M. Cypryk, J. Chojnowski and J. Michalski, Studies on the Efficient Generation of PhosphorusCarbon Bonds via a Rearrangement of PIII Esters Catalysed by Trimethylhalosilanes, Chem. – Eur. J., 2009, 15, 1747 CrossRef CAS PubMed; (e) C. Li, Q. Wang, J.-Q. Zhang, J. Ye, J. Xie, Q. Xu and L.-B. Han, Water determines the products: an unexpected Brønsted acid-catalyzed PO–R cleavage of P(III) esters selectively producing P(O)–H and P(O)–R compounds, Green Chem., 2019, 21, 2916 RSC.
- (a) R. J. Barney, R. M. Richardson and D. F. Wiemer, Direct Conversion of Benzylic and Allylic Alcohols to Phosphonates, J. Org. Chem., 2011, 76, 2875 CrossRef CAS PubMed; (b) R. M. Richardson and D. F. Wiemer, Direct Conversion of Benzylic and Allylic Alcohols to Diethyl Phosphonates, Org. Synth., 2012, 90, 145 Search PubMed; (c) G. G. Rajeshwaran, M. Nandakumar, R. Sureshbabu and A. K. Mohanakrishnan, Lewis Acid-Mediated Michaelis–Arbuzov Reaction at Room Temperature: A Facile Preparation of Arylmethyl/Heteroarylmethyl Phosphonates, Org. Lett., 2011, 13, 1270 CrossRef CAS PubMed; (d) N. Iranpoor, H. Firouzabadi, K. R. Moghadam and E. Etemadi-Davan, Triphenylphosphine/2,3-Dichloro-5,6dicyanobenzoquinone (PPh3/DDQ) System for Conversion of Alcohols and Thiols into Trialkyl Phosphonates, Asian J. Org. Chem., 2015, 4, 1289 CrossRef CAS; (e) X. T. Ma, Q. Xu, H. Li, C. Su, L. Yu, X. Zhang, H. Cao and L.-B. Han, Alcohol-based Michaelis–Arbuzov reaction: an efficient and environmentally-benign method for C-P(O) bond formation, Green Chem., 2018, 20, 3408 RSC.
- (a) Q. Xu and L.-B. Han, Palladium-Catalyzed Asymmetric Hydrophosphorylation of Norbornenes, Org. Lett., 2006, 8, 2099 CrossRef CAS PubMed; (b) Q. Xu, C.-Q. Zhao and L.-B. Han, Stereospecific Nucleophilic Substitution of Optically Pure H-Phosphinates: A General Way for the Preparation of Chiral P-Stereogenic Phosphine Oxides, J. Am. Chem. Soc., 2008, 130, 12648 CrossRef CAS PubMed; (c) Q. Xu and L.-B. Han, Metal-catalyzed additions of H-P(O) bonds to carbon-carbon unsaturated bonds, J. Organomet. Chem., 2011, 696, 130 CrossRef CAS; (d) Q. Xu, R. Shen, Y. Ono, R. Nagahata, S. Shimada, M. Goto and L.-B. Han, A new oxapalladacycle generated via ortho C-H activation of phenylphosphinic acid: an efficient catalyst for Markovnikov-type additions of E-H bonds to alkynes, Chem. Commun., 2011, 47, 2333 RSC; (e) Q. Xu, Y.-B. Zhou, C.-Q. Zhao, S.-F. Yin and L.-B. Han, Transition Metal-Catalyzed Efficient and Green Transformations of P(O)-H Compounds to Functional Organophosphorus Compounds, Mini-Rev. Med. Chem., 2013, 13, 824 CrossRef CAS PubMed; (f) Q. Li, T. Chen, Q. Xu and L.-B. Han, Rhodium- and Iridium-Catalyzed Asymmetric Addition of Optically Pure P-Chiral H-Phosphinates to Aldehydes Leading to Optically Active α-Hydroxyphosphinates, Chem. – Eur. J., 2016, 22, 6213 CrossRef CAS PubMed; (g) X. Ma, Q. Xu, H. Li, C. Su, L. Yu, X. Zhang, H. Cao and L.-B. Han, Alcohol-based Michaelis–Arbuzov reaction: an efficient and environmentally-benign method for C–P(O) bond formation, Green Chem., 2018, 20, 3408 RSC.
- (a) Q. Xu, J. Chen, H. Tian, X. Yuan, S. Li, C. Zhou and J. Liu, Catalyst-Free Dehydrative α-Alkylation of Ketones with Alcohols: Green and Selective Autocatalyzed Synthesis of Alcohols and Ketones, Angew. Chem., Int. Ed., 2014, 53, 225 CrossRef CAS PubMed; (b) Q. Xu, H. Xie, P. Chen, L. Yu, J. Chen and X. Hu, Organohalide-catalyzed dehydrative O-alkylation between alcohols: a facile etherification method for aliphatic ether synthesis, Green Chem., 2015, 17, 2774 RSC; (c) Q. Xu, H. Xie, E.-L. Zhang, X. Ma, J. Chen, X.-C. Yu and H. Li, Selective catalytic Hofmann N-alkylation of poor nucleophilic amines and amides with catalytic amounts of alkyl halides, Green Chem., 2016, 18, 3940 RSC; (d) X. Ma, L. Yu, C. Su, Y. Yang, H. Li and Q. Xu, Efficient Generation of C–S Bonds via a By-Product-Promoted Selective Coupling of Alcohols, Organic Halides, and Thiourea, Adv. Synth. Catal., 2017, 359, 1649 CrossRef CAS; (e) Y. Yang, Z. Ye, X. Zhang, Y. Zhou, X. Ma, H. Cao, H. Li, L. Yu and Q. Xu, Efficient Dehydrative Alkylation of Thiols with Alcohols Catalyzed by Alkyl Halides, Org. Biomol. Chem., 2017, 15, 9638 RSC; (f) X. Ma, J. Yu, R. Yan, M. Yan and Q. Xu, Promoting effect of crystal water leading to catalyst-free synthesis of heteroaryl thioether from heteroaryl chloride, sodium thiosulfate pentahydrate, and alcohol, J. Org. Chem., 2019, 84, 11294 CrossRef CAS PubMed; (g) X. Ma, Y. Zhu, J. Yu, R. Yan, X. Xie, L. Huang, Q. Wang, X. P. Chang and Q. Xu, Water oxidation by Brønsted acid-catalyzed in situ generated thiol cation: dual function of the acid catalyst leading to transition metal-free substitution and addition reactions of S–S bonds, Org. Chem. Front., 2022, 9, 3204 RSC.
- The Dehydrative nucleophilic substitution reactions of alcohols with P(O)–H compounds have emerged as one of the most attractive methods for C–P(O) bond formation; however, the methods are still limited to more reactive secondary or tertiary π-activated benzylic, allylic, and propargylic alcohols. For a review, see: (a) L. Chen, Y.-X. Zou, X.-Y. Liu and X.-J. Gou, Dehydrative Cross-Coupling and Related Reactions between Alcohols (C-OH) and P(O)-H Compounds for C-P Bonds Formation, Adv. Synth. Catal., 2019, 361, 3490 CrossRef CAS. For recent examples, see: (b) Y. Nishibayashi, I. Wakiji and M. Hidai, Novel propargylic substitution reactions catalyzed by thiolate-bridged diruthenium complexes via allenylidene intermediates, J. Am. Chem. Soc., 2000, 122, 11019 CrossRef CAS; (c) Y.-F. Qiu, S.-P. Chen, J.-H. Cao, M. Li, Z.-J. Quan, X.-C. Wang and Y.-M. Liang, Iron(II)-Catalyzed Bisphosphorylation Cascade Cycloisomerization of γ-Hydroxyl Ynones and Diphenylphosphine Oxides: Synthesis of Highly Substituted Bisphosphorylated Dihydrofuran Derivatives, Org. Lett., 2022, 24, 2264 CrossRef CAS PubMed; (d) B. Xiong, C. Shi, Y. Ren, W. Xu, Y. Liu, L. Zhu, F. Cao, K.-W. Tang and S.-F. Yin, Zn-Catalyzed Dehydroxylative Phosphorylation of Allylic Alcohols with P(III)-Nucleophiles, J. Org. Chem., 2024, 89, 3033 CrossRef CAS PubMed; (e) H. F. Zhuang, P. Wan, C. X. Miao, Y. Yang, S. Y. Liang and F. Han, Heteropolyacid-Catalyzed Phosphorylation of Secondary Aromatic Alcohols with H-Phosphine Oxides in DMC: A Simple Protocol for C-P Bond Formation, J. Org. Chem., 2024, 89, 2397 CrossRef CAS PubMed.
- Montchamp and Chen independently reported Pd-catalyzed, t-BuONa-mediated or Fe-catalyzed reactions of some primary alcohols with hypophosphorous acid or secondary phosphine oxides, but these reactions preceded with the formation of alkene and aldehyde/P(III) ester intermediates and thus do not belong to direct nucleophilic substitution reactions and MA reactions: (a) L. Coudray and J.-L. Montchamp, Green, Palladium-Catalyzed Synthesis of Benzylic H-Phosphinates from Hypophosphorous Acid and Benzylic Alcohols, Eur. J. Org. Chem., 2008, 410 Search PubMed; (b) L. Chen, Y. Zhu, T. Chen, L. Liu, J.-S. Zhang and L.-B. Han, Direct C-OH/P(O)-H dehydration coupling forming phosphine oxides, Org. Biomol. Chem., 2018, 16, 5090 RSC; (c) L. Gan, C. Ye, T. Pi, L. Wang, C. Li, L. Liu, T. Huang, T. Chen and L.-B. Han, Ligand-Free Iron-Catalyzed Construction of C-P Bonds via Phosphorylation of Alcohols: Synthesis of Phosphine Oxides and Phosphine Compounds, J. Org. Chem., 2024, 89, 7047 CrossRef CAS PubMed.
- See the ESI for details.†.
- Due to its sensitive nature toward air, the generation of 10a was confirmed by its conversion into the phosphonium salt [PPh2(CH2Ph)2]B r.
- R. F. Cheng and C.-J. Li, Csp3–PIII Bond Formation via Cross-Coupling of Umpolung Carbonyls with Phosphine Halides Catalyzed by Nickel, Angew. Chem., Int. Ed., 2023, 62, e202301730 CrossRef CAS PubMed.
- (a) E. N. Tsvetkov, N. A. Bondarenko, I. G. Malakhova and M. I. Kabachnik, A simple synthesis and some synthetic applications of substituted phosphide and phosphinite anions, Synthesis, 1986, 198 CrossRef CAS; (b) M. Sasaki, J. Collin and B. Henri, Reactions of P-Cl compounds in presence of SmI2, Tetrahedron Lett., 1991, 32, 2493 CrossRef CAS; (c) R. K. Haynes, R. N. Freeman, C. R. Mitchell and S. C. Vonwiller, Preparation of Enantiomerically Pure Tertiary Phosphine Oxides from, and Assay of Enantiomeric Purity with, (Rp)- and (Sp)-tert-Butylphenylphosphinothioic Acids, J. Org. Chem., 1994, 59, 2919 CrossRef CAS; (d) R. J. Cohen, D. L. Fox, J. F. Eubank and R. N. Salvatore, Mild and efficient Cs2CO3-promoted synthesis of phosphonates, Tetrahedron Lett., 2003, 44, 8617 CrossRef CAS.
- Though it may close to the esterification reactions of carboxylic acids, how this dehydration step occurs is not clear yet and may need deeper mechanistic studies.
- B. O. Pray, L. H. Sommer, G. M. Goldberg, G. T. Kerr, P. A. Di Giorgio and F. C. Whitmore, Trimethylhalosilane Preparations, J. Am. Chem. Soc., 1948, 70, 433 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc04409f |
| This journal is © The Royal Society of Chemistry 2025 |