Open Access Article
Open Access ArticleCatalytic three-component carboamination of unactivated alkenes with primary sulfonamides†
Ying
Zhang
 ,
Kai-Dian
Li
,
Kai-Dian
Li
 ,
Chang-Quan
Zhou
,
Chang-Quan
Zhou
 ,
Zhi-Xi
Xing
and
Huan-Ming
Huang
,
Zhi-Xi
Xing
and
Huan-Ming
Huang
 *
*
School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China. E-mail: huanghm@shanghaitech.edu.cn
First published on 26th August 2024
Abstract
Catalytic multicomponent reactions offer an efficient synthetic approach for constructing multiple chemical bonds in a single step. However, the carboamination of unactivated alkenes, involving nitrogen radical species directly from N–H precursors, is infrequent due to the high bond energy and challenges in step matching. Herein we demonstrate a catalytic radical three-component reaction using simple primary sulfonamides, which provides a novel method for constructing a library of complex architectures through carbon–nitrogen and carbon–carbon bond formation. The newly developed method demonstrates a high degree of tolerance towards various functional groups, proving to be highly efficient in the late-stage modification of complex drug molecules and natural products under very mild conditions.
Introduction
The ability to design sustainable and atom-economical synthetic transformations always creates a revolution in organic synthesis.1 Catalytic multicomponent reactions (MCRs) are very powerful synthetic methods in the construction of carbon–carbon and carbon–heteroatom bonds to generate complex architectures in a single reaction vessel.2 Radical cross-couplings have emerged as valuable methods for forming C(sp3)–C(sp3) and C(sp3)–X bonds under mild conditions, driven by advancements in transition-metal catalysis,3 photoredox chemistry4 and electrosynthesis.5 Recently, nitrogen-centered radicals (NCRs) have proven to be versatile reactive intermediates, successfully employed in multicomponent reactions (MCRs) for the generation of nitrogen-containing motifs. This achievement has been realized through suitable excited photocatalysts6 or electrochemical conditions.5c Nevertheless, pre-functionalization of nitrogen-based precursors is consistently required, often involving multiple steps.6,7Remarkably, Knowles,8 Rovis9 and others6 developed elegant examples regarding remote C–H functionalization triggered by nitrogen centered radical species generated from the N–H bonds directly (Scheme 1A). Intramolecular and intermolecular hydroamination of unactivated alkenes represent another powerful synthetic transformation for constructing nitrogen-containing motifs.10 For example, Knowles and co-workers achieved the intermolecular anti-Markovnikov hydroamination of unactivated alkenes with sulfonamide through a proton coupled electron transfer (PCET)11 approach (Scheme 1B, up).12 Doyle and her team utilized a catalytic amount of phosphine and photocatalysis to achieve hydroamination of unactivated alkenes with primary sulfonamides via α-scission from phosphoranyl radicals (Scheme 1B, down).13a Additionally, Knowles,10c Molander,13b Moeller,13c Qin,13d Xu,13e Nevado,13f Ohmiya,13g and others6 also achieved intramolecular cascade reaction triggered nitrogen centered radical intermediates directly generated from N–H bonds. However, these valuable transformations primarily focus on remote C–H functionalization, intramolecular cyclization and hydroamination (Scheme 1A and B). Although several noteworthy efforts have been made, including those of Scheidt14a and Xia & Yang,14b the direct generation of nitrogen-centered radicals (NCRs) from N–H bonds, followed by their participation in catalytic multicomponent reactions (MCRs), is still rare due to the high bond dissociation free energy (BDFE) associated with N–H bonds.12,13a Furthermore, the control of radical intermediates and the sequential achievement of fundamental radical addition/coupling steps in multicomponent reactions (MCRs) pose significant challenges.
Herein, we envisioned that the N–H bond of primary sulfonamides (BDFE ∼ 105 kcal mol−1, E1/2 = +2.6 V versus SCE in MeCN)12,13a could be converted to the corresponding nitrogen centered radical, and then sequential radical addition steps could be controlled by polar effects (Scheme 1C).15 The catalytic three-component carboamination reactions between primary sulfonamides and olefins offer a sustainable method for synthesizing secondary sulfonamide derivatives. These reactions achieve a remarkable 100% atom utilization efficiency, making them environmentally friendly. This approach clearly showcases its green credentials by minimizing the E-factor and maximizing atom economy, thereby demonstrating the environmental benefits of the current method.16 These derivatives are versatile motifs found in bioactive molecules and drugs.17
Results and discussion
Optimization
With these considerations in mind, we started to investigated this three-component carboamination reaction by employing primary sulfonamide 1a, unactivated olefin 2a and methyl acrylate 3a under visible light conditions (Table 1). Unfortunately, the desired product 4 could not be formed under either condition A (entry 1)12a or condition B (entry 2).13a Additionally, the coupling product 4 also could not be generated with 4 mol% Ir–F and 2 equivalents of K2CO3 with mixture solvent (PCF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tBuOH = 1
tBuOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.2 M, entry 3) as previously applied in the synthesis of cyclic amines by our group.18 Pleasingly, the coupling product 4 could be formed in 17% yield with K3PO4 as a base instead of K2CO3 (entry 4). X-ray crystallographic analysis confirmed the structure of product 4.18 Interestingly, the yield of 4 increased dramatically to 67% when a catalytic amount of K3PO4 was used (entry 5). With a catalytic amount of iridium based photoredox catalyst (Ir–F) and K3PO4, the desired coupling product 4 could be obtained in 84% isolated yield (entry 6) using PhCF3 as reaction solvent. Then, different solvents (entries 7–10) were also investigated, and we did not observe any better results. Despite efforts to decrease the loading of the photoredox catalyst or alter the amounts of coupling partners or change the reaction time or use different photocatalysts or different bases, the yield of product 4 did not increase (entries 11–19). Finally, we demonstrated that the base, photocatalyst, and visible light were necessary to achieve this three-component coupling reaction successfully (entry 20).
1, 0.2 M, entry 3) as previously applied in the synthesis of cyclic amines by our group.18 Pleasingly, the coupling product 4 could be formed in 17% yield with K3PO4 as a base instead of K2CO3 (entry 4). X-ray crystallographic analysis confirmed the structure of product 4.18 Interestingly, the yield of 4 increased dramatically to 67% when a catalytic amount of K3PO4 was used (entry 5). With a catalytic amount of iridium based photoredox catalyst (Ir–F) and K3PO4, the desired coupling product 4 could be obtained in 84% isolated yield (entry 6) using PhCF3 as reaction solvent. Then, different solvents (entries 7–10) were also investigated, and we did not observe any better results. Despite efforts to decrease the loading of the photoredox catalyst or alter the amounts of coupling partners or change the reaction time or use different photocatalysts or different bases, the yield of product 4 did not increase (entries 11–19). Finally, we demonstrated that the base, photocatalyst, and visible light were necessary to achieve this three-component coupling reaction successfully (entry 20).
| Entry | Deviation from standard conditions | Yieldb % | Entry | Deviation from standard conditions | Yieldb % |
|---|---|---|---|---|---|
| a Optimization of the reaction conditions: 1a (0.2 mmol, 1.0 equiv.), 2a (0.4 mmol, 2.0 equiv.), 3a (0.4 mmol, 2.0 equiv.), Ir–F (4 mol%), and K3PO4 (40 mol%) in PhCF3 (1.0 mL, 0.2 M) at room temperature under irradiation with 30 W blue LEDs with a cooling fan for 24 hours. b Yields determined by 1H NMR with nitromethane as an internal standard. c Yields of the isolated product. | |||||
| 1 | Condition A | 0 | 10 | MeCN as solvent | 65 |
| 2 | Condition B | 0 | 11 | 2 mol% Ir–F was used | 78 |
| 3 |
Ir–F (4 mol%), K2CO3 (2 equiv.) as base and PhCF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tBuOH = 1 tBuOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.2 M) as solvent 1 (0.2 M) as solvent |
0 | 12 |
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1.5 3a = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used 1 was used |
85 (82)c |
| 13 | 12 h | 69 | |||
| 4 |
Ir–F (4 mol%), K3CO4 (2 equiv.) as base and PhCF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tBuOH = 1 tBuOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.2 M) as solvent 1 (0.2 M) as solvent |
17 | 14 | 48 h | 80 |
| 5 |
Ir–F (4 mol%), K3CO4 (40 mol%) as base and PhCF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tBuOH = 1 tBuOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.2 M) as solvent 1 (0.2 M) as solvent |
67 | 15 | 4-CzIPN as photocatalyst | <5 |
| 16 | Ru(bpy)3(PF6)2 as photocatalyst | <5 | |||
| 6 | None | 88 (84)c | 17 | Na3PO4/Cs2CO3/[2.4.6]-collidine/K2HPO4 as base | 6/80/29/36 |
| 7 | tBuOH as solvent | 63 | 18 | PhCF3 (0.4 M) | 83 |
| 8 | PhCI as solvent | 65 | 19 | PhCF3 (0.1 M) | 84 |
| 9 | DCM as solvent | 73 | 20 | No base/photocatalyst/light/50 °C | 0/0/0/0 |

|
|||||
Reaction scope
Have established the reaction conditions, we started to investigate the primary sulfonamide scope of catalytic radical three-component reactions (Scheme 2). The aromatic ring with various substituents, such as alkyl, fluoride, chloride, bromide, trifluoromethyl, and methoxyl could be tolerated with good to excellent yields (4–14). Additionally, thiophene (15), amine (16), cyclopropyl (17), methyl (18) and the anti-inflammatory drug celecoxib (19) were also compatible under the current reaction conditions, and the desired products 15–19 could be produced in 64%–84% isolated yields. Unfortunately, secondary sulfonamide was not compatible in our catalytic system. Furthermore, different unactivated olefins were also screened, mono-substituted olefins containing ketone (20), silicon (21), halide (22 and 23), and alkyl (24) functional groups were all tolerated with moderate to excellent yields. We then examined a variety of functional groups in the 1,2-disubstituted olefin component including ether (25), diverse protecting groups (26 and 28), ester (27), phenyl (29), phthalimide (30), diethyl (31), and carbocycles (32–34). The further results of inner olefins (35 and 36) also showed a good performance in our system.Gratifyingly, the standard reaction conditions tolerate a wide range of Michael acceptors containing different functional groups, such as alkyne (37), alkyl (38 and 43), nitrile (39), bromide (40), chloride (41), sulfoxide (42), and ketone (44), with moderate to high yields (Scheme 3). Furthermore, we also applied this atom-economical method in the late-stage modification of natural products. When 7-ketolithocholic methyl ester (45), carvone (46), cedrol (47), fenofibric acid (48), androsterone (49) and gemfibrozil (50) derived olefins were employed, the three-component products 45–50 could be obtained in 42% to 81% isolated yields, respectively. Unfortunately, when an α,β-unsaturated Michael acceptor was employed as a coupling partner, the desired three-component coupling product could not be formed.
 | ||
| Scheme 3 Michael acceptor scope and late-stage modification of catalytic radical three-component reactions. | ||
Mechanistic study
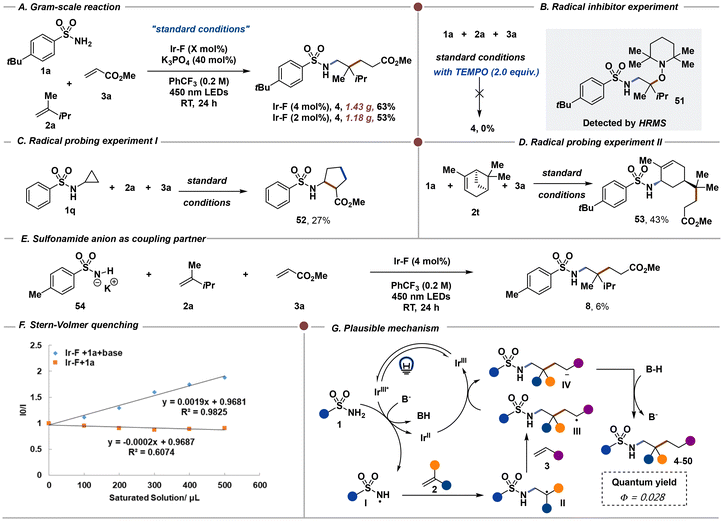
We also scaled up this three-component reaction, and the desired product 4 could be obtained with 63% isolated yield. When the photocatalyst was reduced to 2 mol%, the yield of 4 was still achievable at a 53% isolated yield (Scheme 4A). With two equivalents of TEMPO, the coupled product 4 could not be produced and the TEMPO-adduct 51 could be detected by HRMS (Scheme 4B). Meanwhile, when the sulfonamide 1q containing a three membered ring was employed under the standard conditions, the cyclized product 52 was obtained in 27% isolated yield, indicating that the nitrogen centered radical was formed which resulted in the ring opening and cyclization process (Scheme 4C).19 Additionally, the unactivated alkene 2t was also employed under the standard conditions, and the ring opening product 53 could be produced in 43% isolated yield, which also demonstrated that radical intermediates were involved in this three-component reaction (Scheme 4D). Using cyclic voltammetry, we found that 4-methylbenzenesulfonamide 1e undergoes oxidation at +2.21 V vs. SCE in MeCN. Therefore, direct electron transfer between this sulfonamide 1e and the excited state of Ir–F (Ered1/2[*Ir(III)/Ir(II)] = +1.21 V vs. SCE in MeCN)20 is unlikely. Furthermore, the pKa of benzenesulfonamide in MeCN is approximately 27,12a making it unlikely to be deprotonated by K3PO4. We also found that the oxidation of the corresponding potassium sulfonamide 54 occurs at approximately +1.28 V vs. SCE in MeCN using cyclic voltammetry, which did not match the oxidation potential of Ir–F. Furthermore, we performed the three-component reaction between 54, 2a and 3a with 4 mol% Ir–F (Scheme 4E), and the desired coupling product 8 was obtained in only 6% yield. This result indicates that the oxidation of the sulfonamide anion to form nitrogen radical species is unlikely. Additionally, using Cs2CO3, a weaker base (pKa = 10.3) also provides the desired product 4 with similar yield (Table 1, entry 17). A Stern–Volmer quenching experiment was also performed and the excited photocatalyst Ir–F could only be quenched with a mixture of sulfonamide 1a and base (Scheme 4F). Based on the current mechanistic results and recent hydroamination of alkenes by Knowles and co-workers,12a we propose the following mechanism shown in Scheme 4G. The nitrogen-centered radical I is likely generated through the proton-coupled electron-transfer (PCET) pathway. After radical addition between I and unactivated alkene 2, the newly formed radical intermediate II could further react with the electron-deficient alkene 3 to produce the radical intermediate III which could be further reduced to the anion intermediate IV. After the protonation step, the final coupled products 4–50 were produced efficiently. The quantum yield of this three-component reaction was measured (∅ = 0.028) which demonstrated that chain reaction is unlikely to have occurred under the current reaction conditions.Conclusions
In summary, we have demonstrated a catalytic radical three-component reaction employing primary sulfonamides as nitrogen radical precursors. This mild and atom-economical method successfully coupled the primary sulfonamide, unactivated olefin and Michael acceptor with only a catalytic amount of photocatalyst and a simple inorganic base. This coupling reaction could tolerate a variety of functional groups and natural product modification. We hope that this newly developed method will be quickly adopted by the synthetic community and inspire further development regarding nitrogen centered radical triggered multicomponent reactions.Data availability
The data supporting this article have been included as part of the ESI.† CCDC 2335224 (4) contains the supplementary crystallographic data for this paper.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (22201179) and the startup funding from ShanghaiTech University. We sincerely thank Prof. Chao-Dan Pu, Prof. Jiajun Yan, Zhuo Zhao, Shu-Ya Wen and Zixiao Wang for help with the mechanistic study, CV measurements and X-ray analysis.References
- (a) B. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed; (b) P. A. Wender and B. L. Miller, Nature, 2009, 460, 197–201 CrossRef CAS PubMed.
- (a) B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323–8359 CrossRef CAS PubMed; (b) B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef PubMed; (c) B. Ganem, Acc. Chem. Res., 2009, 42, 463–472 CrossRef CAS PubMed.
- For selected recent reviews, see: (a) J. Choi and G. C. Fu, Science, 2017, 356, eaaf7230 CrossRef PubMed; (b) M. Yan, J. C. Lo, J. T. Edwards and P. S. Baran, J. Am. Chem. Soc., 2016, 138, 12692–12714 CrossRef CAS PubMed; (c) A. Studer and D. P. Curran, Nat. Chem., 2014, 6, 765–773 CrossRef CAS PubMed; (d) Q.-S. Gu, Z.-L. Li and X.-Y. Liu, Acc. Chem. Res., 2020, 53, 170–181 CrossRef CAS PubMed; (e) F. Wang, P. Chen and G. Liu, Acc. Chem. Res., 2018, 51, 2036–2046 CrossRef CAS.
- For selected recent reviews, see: (a) A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V. Oakley, N. L. Reed, H. A. Sakai, C. P. Seath and D. W. C. MacMillan, Chem. Rev., 2022, 122, 1485–1542 CrossRef CAS PubMed; (b) X.-Y. Yu, J.-R. Chen and W.-J. Xiao, Chem. Rev., 2021, 121, 506–561 CrossRef CAS PubMed; (c) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS; (d) D. Staveness, I. Bosque and C. R. J. Stephenson, Acc. Chem. Res., 2016, 49, 2295–2306 CrossRef CAS PubMed; (e) H.-M. Huang, P. Bellotti and F. Glorius, Acc. Chem. Res., 2022, 55, 1135–1147 CrossRef CAS PubMed; (f) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (g) F.-D. Lu, G.-F. He, L.-Q. Lu and W.-J. Xiao, Green Chem., 2021, 23, 5379–5393 RSC.
- For selected recent reviews, see: (a) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed; (b) H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Chem. Rev., 2019, 119, 6769–6787 CrossRef CAS; (c) P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 3339–3350 CrossRef CAS PubMed; (d) K.-J. Jiao, Y.-K. Xing, Q.-L. Yang, H. Qiu and T.-S. Mei, Acc. Chem. Res., 2020, 53, 300–310 CrossRef CAS PubMed; (e) B. A. Frontana-Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099–2119 RSC.
- For selected recent reviews, see: (a) X.-Y. Yu, Q.-Q. Zhao, J. Chen, W.-J. Xiao and J.-R. Chen, Acc. Chem. Res., 2020, 53, 1066–1083 CrossRef CAS; (b) K. Kwon, R. T. Simons, M. Nandakumar and J. L. Roizen, Chem. Rev., 2022, 122, 2353–2428 CrossRef CAS; (c) C. Pratley, S. Fenner and J. A. Murphy, Chem. Rev., 2022, 122, 8181–8260 CrossRef CAS; (d) J. M. Ganley, P. R. D. Murray and R. R. Knowles, ACS Catal., 2020, 10, 11712–11738 CrossRef CAS; (e) H. Jiang and A. Studer, CCS Chem., 2019, 1, 38–49 CrossRef CAS; (f) J. Davies, S. P. Morcillo, J. J. Douglas and D. Leonori, Chem. – Eur. J., 2018, 24, 12154–12163 CrossRef CAS PubMed; (g) M. D. Kärkäs, ACS Catal., 2017, 7, 4999–5022 CrossRef; (h) T. Xiong and Q. Zhang, Chem. Soc. Rev., 2016, 45, 3069–3087 RSC; (i) S. Z. Zard, Chem. Soc. Rev., 2008, 37, 1603–1618 RSC; (j) H. Jiang and A. Studer, Chem. Soc. Rev., 2020, 49, 1790–1811 RSC; (k) C. Song, X. Shen, F. Yu, Y. He and S. Yu, Chin. J. Org. Chem., 2020, 40, 3748–3759 CrossRef CAS.
- For selected recent examples, see: (a) H. Zhang, W. Pu, T. Xiong, Y. Li, X. Zhou, K. Sun, Q. Liu and Q. Zhang, Angew. Chem., Int. Ed., 2013, 52, 2529–2533 CrossRef CAS; (b) D. Wang, F. Wang, P. Chen, Z. Lin and G. Liu, Angew. Chem., Int. Ed., 2017, 56, 2054–2058 CrossRef CAS; (c) H. Xiao, H. Shen, L. Zhu and C. Li, J. Am. Chem. Soc., 2019, 141, 11440–11445 CrossRef CAS PubMed; (d) Y. Zhang, H. Liu, L. Tang, H. J. Tang, L. Wang, C. Zhu and C. Feng, J. Am. Chem. Soc., 2018, 140, 10695–10699 CrossRef CAS PubMed; (e) H. Jiang, G. Seidler and A. Studer, Angew. Chem., Int. Ed., 2019, 58, 16528–16532 CrossRef CAS PubMed; (f) Y. Moon, B. Park, I. Kim, G. Kang, S. Shin, D. Kang, M.-H. Baik and S. Hong, Nat. Commun., 2019, 10, 4117 CrossRef PubMed; (g) B. Yang, X. Ren, X. Shen, T. Li and Z. Lu, Chin. J. Chem., 2018, 36, 1017–1023 CrossRef CAS.
- G. J. Choi, Q. Zhu, D. C. Miller, C. J. Gu and R. R. Knowles, Nature, 2016, 539, 268–271 CrossRef CAS PubMed.
- (a) J. C. K. Chu and T. Rovis, Nature, 2016, 539, 272–275 CrossRef PubMed; (b) D.-F. Chen, J. C. K. Chu and T. Rovis, J. Am. Chem. Soc., 2017, 139, 14897–14900 CrossRef CAS; (c) S. M. Thullen, S. M. Treacy and T. Rovis, J. Am. Chem. Soc., 2019, 141, 14062–14067 CrossRef CAS.
- For selected recent reviews and excellent examples, see: (a) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed; (b) L. Huang, M. Arndt, K. Gooßen, H. Heydt and L. J. Gooßen, Chem. Rev., 2015, 115, 2596–2697 CrossRef CAS PubMed; (c) C. B. Roos, J. Demaerel, D. E. Graff and R. R. Knowles, J. Am. Chem. Soc., 2020, 142, 5974–5979 CrossRef CAS PubMed; (d) D. C. Miller, J. M. Ganley, A. J. Musacchio, T. C. Sherwood, W. R. Ewing and R. R. Knowles, J. Am. Chem. Soc., 2019, 141, 16590–16594 CrossRef CAS PubMed; (e) A. J. Musacchio, B. C. Lainhart, X. Zhang, S. G. Naguib, T. C. Sherwood and R. R. Knowles, Science, 2017, 355, 727–730 CrossRef CAS PubMed; (f) S. W. Lardy and V. A. Schmidt, J. Am. Chem. Soc., 2018, 140, 12318–12322 CrossRef CAS PubMed; (g) H. Jiang and A. Studer, Chem. – Eur. J., 2019, 25, 7105–7109 CrossRef CAS; (h) S.-M. Jia, Y.-H. Huang, Z.-L. Wang, F.-X. Fan, B.-H. Fan, H.-X. Sun, H. Wang and F. Wang, J. Am. Chem. Soc., 2022, 144, 16316–16324 CrossRef CAS PubMed.
- (a) D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016–4093 CrossRef CAS PubMed; (b) P. R. D. Murray, J. H. Cox, N. D. Chiappini, C. B. Roos, E. A. McLoughlin, B. G. Hejna, S. T. Nguyen, H. H. Ripberger, J. M. Ganley, E. Tsui, N. Y. Shin, B. Koronkiewicz, G. Qiu and R. R. Knowles, Chem. Rev., 2022, 122, 2017–2291 CrossRef CAS PubMed.
- (a) Q. Zhu, D. E. Graff and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 741–747 CrossRef CAS; (b) B. G. Hejna, J. M. Ganley, H. Shao, H. Tian, J. D. Ellefsen, N. J. Fastuca, K. N. Houk, S. J. Miller and R. R. Knowles, J. Am. Chem. Soc., 2023, 145, 16118–16129 CrossRef CAS PubMed.
- For selected recent elegant examples, see: (a) A. J. Chinn, K. Sedillo and A. G. Doyle, J. Am. Chem. Soc., 2021, 143, 18331–18338 CrossRef CAS PubMed; (b) S. Zheng, Á. Gutiérrez-Bonet and G. A. Molander, Chem, 2019, 5, 339–352 CrossRef CAS; (c) H.-C. Xu and K. D. Moeller, J. Am. Chem. Soc., 2010, 132, 2839–2844 CrossRef CAS PubMed; (d) X. Wang, D. Xia, W. Qin, R. Zhou, X. Zhou, Q. Zhou, W. Liu, X. Dai, H. Wang, S. Wang, L. Tan, D. Zhang, H. Song, X. Y. Liu and Y. Qin, Chem, 2017, 2, 803–816 CrossRef CAS; (e) L. Zhu, P. Xiong, Z.-Y. Mao, Y.-H. Wang, X. Yan, X. Lu and H.-C. Xu, Angew. Chem., Int. Ed., 2016, 55, 2226–2229 CrossRef CAS PubMed; (f) W. Shu, A. Genoux, Z. Li and C. Nevado, Angew. Chem., Int. Ed., 2017, 56, 10521–10524 CrossRef CAS PubMed; (g) Y. Sato, Y. Miyamoto, T. Matsui, Y. Sumida and H. Ohmiya, Chem Catal., 2023, 3, 100736 CrossRef CAS.
- (a) N. Tanaka, J. L. Zhu, O. L. Valencia, C. R. Schull and K. A. Scheidt, J. Am. Chem. Soc., 2023, 145, 24486–24492 CAS; (b) N. Ma, L. Guo, D. Qi, F. Gao, C. Yang and W. J. Xia, Org. Lett., 2021, 23, 6278–6282 CrossRef CAS PubMed.
- (a) B. P. Roberts, Chem. Soc. Rev., 1999, 28, 25–35 RSC; (b) A. Ruffoni, R. C. Mykura, M. Bietti and D. Leonori, Nat. Synth., 2022, 1, 682–695 CrossRef.
- P. Jessop, Green Chem., 2020, 22, 13–15 RSC.
- (a) S. Apaydın and M. Török, Bioorg. Med. Chem. Lett., 2019, 29, 2042–2050 CrossRef PubMed; (b) C. Zhao, K. P. Rakesh, L. Ravidar, W.-Y. Fang and H.-L. Qin, Eur. J. Med. Chem., 2019, 162, 679–734 CrossRef CAS PubMed.
- Y. Zhang, S.-S. Chen, K.-D. Li and H.-M. Huang, Angew. Chem., Int. Ed., 2024, 63, e202401671 CrossRef CAS PubMed.
- D. H. White, A. Noble, K. I. Booker-Milburn and V. K. Aggarwal, Org. Lett., 2021, 23, 3038–3042 CrossRef CAS PubMed.
- M. S. Lowry, J. I. Goldsmith, J. D. Slinker, R. Rohl, R. A. Pascal, G. G. Malliaras and S. Bernhard, Chem. Mater., 2005, 17, 5712–5719 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2335224. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4gc02879a |
| This journal is © The Royal Society of Chemistry 2024 |