Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
What to do with polyurethane waste? The environmental potential of chemically recycling polyurethane rigid foam†
Martin
Pillich
 ,
Johannes
Schilling
,
Johannes
Schilling
 ,
Luca
Bosetti
,
Luca
Bosetti
 and
André
Bardow
and
André
Bardow
 *
*
Energy and Process Systems Engineering, ETH Zurich, 8092 Zurich, Switzerland. E-mail: abardow@ethz.ch
First published on 18th September 2024
Abstract
Chemical recycling of plastics has gathered momentum to manage plastic waste and replace fossil-based feedstocks. However, chemical recycling of complex polymers, such as polyurethane (PU) rigid foam, can yield a variety of intermediates. But which intermediates reduce environmental impacts the most when produced from PU is currently unclear. In this work, we assess the potential of chemical recycling of PU rigid foam waste to reduce environmental impacts compared to state-of-the-art treatment options, such as incineration and landfilling. For this purpose, we extend the environmental potential methodology to account for any possible recycling product. We then calculate the environmental potential for six ideal closed-loop recycling options and one experimentally demonstrated recycling option based on a patent. All analyzed chemical recycling options for PU rigid foam to various intermediates are shown to offer a substantial environmental potential to reduce multiple environmental impacts. The best performing option recovers both polyol and isocyanate and can decrease climate change impacts by 3.8–5.6 kgCO2eq. per kg PU treated. However, PU rigid foam recycling to low-value intermediates, such as benzene, does not seem promising due to burden shifting actually increasing half of the analyzed environmental impacts. We further determine the minimal conversion rates required to reduce environmental impacts by chemical recycling of PU rigid foam. Our environmental potential analysis assists the decision-making process for product prioritization in recycling and identifies (side-)products whose recovery is worth investigating from an environmental perspective.
1 Introduction
Current plastic production pathways demand large amounts of fossil resources and emit substantial amounts of greenhouse gas emissions.1,2 The fossil demands and greenhouse gas emissions of the plastics industry already exceed what our planet can endure, making current plastic production environmentally unsustainable.3 The predicted increase in plastic demand will further challenge the sustainability of the plastics industry. Beyond the environmental impacts of plastic production, a significant share of the produced plastics ends up as mismanaged plastic waste. This mismanaged plastic waste poses risks to human health and the environment.4–7 Even when managed properly in landfills and incinerators, plastic waste still causes considerable environmental impacts.7–9 Viewed holistically, the current plastic life cycle, from cradle to grave, is unsuitable for a sustainable future.A key option to improve the sustainability of the plastic life cycle is recycling.3 Plastic recycling tackles challenges in both production and waste management by offering:
(a) an alternative plastic production pathway based on a new carbon source that thus decreases the demand for fossil resources, and
(b) a novel way to treat plastic waste with potentially lower environmental impacts than current waste treatment options.
Following this rationale, EU legislation has begun to mandate a circular economy.10,11 Plastic recycling aligns with this legislation as it ensures a circular economy by design.
Plastic recycling has been researched extensively for several large-volume polymers.12–19 Generally, two approaches are considered: mechanical recycling and chemical recycling. The ISO 15270 norm20 defines mechanical recycling as the “processing of plastics waste into secondary raw material or products without significantly changing the chemical structure of the material”. Typical mechanical recycling technologies revolve around the extrusion of plastic waste to new plastic granulates or the utilization of solvents to purify the plastic waste into new plastic raw material. In contrast, chemical recycling encompasses technologies that substantially change the chemical structure of the material. These technologies employ, e.g., depolymerization, pyrolysis, and gasification.21 The output of these technologies, e.g., monomers, typically serves as a feedstock to the value chains of the chemical industry. A more detailed explanation and clear delineation are provided by Ragaert et al.21 While the current plastic recycling industry predominantly relies on mechanical recycling,1 in recent years, the industry has been increasingly interested in chemical recycling. Chemical recycling enables the recycling of plastics unsuitable for mechanical recycling while also potentially avoiding the accumulation of impurities, a typical disadvantage of mechanical recycling. For some plastic wastes, the first commercial chemical recycling plants are either under construction or are close to commissioning.22–24
For certain large-volume polymers, such as polyethylene terephthalate (PET) or high-density polyethylene (HDPE), multiple recycling options have been developed. In contrast, polyurethanes are a polymer class with less mature recycling options thus far.25–27 Polyurethanes (PU) represent the 6th largest polymer by production volume and consist of an isocyanate and a polyol.28 PU is predominantly found in soft foams (36%), rigid foams (32%), as well as coatings and adhesives (20%).28 For soft foams, some recycling options have been investigated,29 and a small number of projects are attempting commercialization, e.g., PUReSmart.30,31 For rigid foams, several research projects32,33 are currently exploring recycling options beyond lab scale, but, to the best of the authors’ knowledge, no commercial plants are currently in operation. Due to this lack of commercial recycling options, the majority of PU waste is presently landfilled or incinerated.34 Recycling PU rigid foam could thus close an important gap to a sustainable plastics future.
However, both mechanical and chemical recycling of PU rigid foams are challenging tasks. PU rigid foams are thermosets that cannot be remelted and reformed into new products. This characteristic leads to an inherent deterioration of properties when mechanically recycling rigid foams, e.g., through shredding. With this inherent deterioration of properties, rigid foams can only be mechanically recycled a limited number of times, and thus, the mechanical recycling of PU rigid foam is unsuitable as a long-term alternative to the current plastic life cycle.35 In contrast, PU rigid foam could potentially be recycled an unlimited number of times via chemical recycling. In particular, PU has varying formulations, applications, characteristics, and diverse precursors with a wide range of distinct chemical bonds.36 These chemical bonds can be selectively targeted by a multitude of chemical recycling reactions, which yield a wide range of possible recycling products from PU.37 This diversity leads to numerous chemical recycling options for PU,35,38–40 which complicates the identification of a preferred option to focus development efforts on.
Promising recycling options are so-called closed-loop chemical recycling options, which recycle PU to its direct precursors.41 Currently, PU's direct precursors are produced in elaborate chemical processes based on fossil resources. Closed-loop chemical recycling of PU bypasses these elaborate chemical processes and could thus reduce environmental impacts.
Nonetheless, plastic recycling does not necessarily reduce environmental impacts compared to the current treatment options.41 Hence, research resources could be wasted on developing recycling technologies that do not provide any environmental benefits endangering a fast and efficient transition to a sustainable plastic industry. Therefore, PU recycling research should focus on recycling options that have the potential to reduce environmental impacts. For this purpose, an analysis is required to determine if and to what extent recycling options for PU waste are environmentally beneficial compared to state-of-the-art waste treatment.
In this work, we analyze the environmental potential that chemical recycling of PU rigid foam offers compared to current waste treatment options. The environmental potential is a consistent, LCA-based method proposed by Meys et al.,41 which robustly quantifies the maximum possible reduction of environmental impacts when implementing a future chemical recycling option (see Section 2). This method enables a technology-independent analysis of pathways at an early stage of process development. So far, the environmental potential analyzed by Meys et al.41 has not been applied to PU and therefore is limited to recycling to direct precursors, i.e., isocyanates and polyols in the case of polyurethanes. We extend this method to consider any possible recycling product while maintaining its low data requirement and technology independence. We apply the environmental potential to recycling products throughout the value chain of PU-rigid-foam production and compare the recycling options to 3 current waste treatment options (see Section 3). This calculation allows us to assess the viability of chemical recycling options for PU rigid foam in reducing environmental impacts.
2 The environmental potential of recycling polyurethane rigid foam
As introduced by Meys et al.,41 the environmental potential of future chemical recycling options is calculated using a comparative LCA between the treatment routes of a currently available conventional waste treatment option (WT) and an idealized novel chemical recycling option (CR). The idealized novel chemical recycling option represents a best-case scenario with the lowest possible environmental impact (see Section 2.1). Formally, the environmental potential EPot is calculated using the following equation: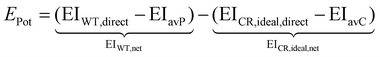 | (1) |
A negative environmental potential of a recycling option indicates that reducing the environmental impact with this recycling option is not possible, i.e., the minimal environmental impact EICR, ideal, net of the ideal recycling option is already greater than the environmental impact of the current waste treatment (EIWT, net). Conversely, if the environmental potential of a recycling option is positive, there is the possibility to decrease the environmental impact by utilizing the recycling option (EICR, ideal, net < EIWT, net). Furthermore, the value of the positive environmental potential of a recycling option provides an upper limit for the environmental impact of any additional process steps that go beyond the idealized chemical recycling (e.g., additional energy requirements).
In this section, we describe the calculation of the environmental potential for various chemical recycling options of PU rigid foam. First, we explain how to derive the minimal environmental impact of a recycling option (Section 2.1). Then, we introduce the 7 recycling options for PU rigid foam considered in this work (Section 2.2). Finally, we describe the system boundaries and assumptions for calculating the environmental potential of the PU-rigid-foam recycling options (Section 2.3).
2.1 Calculating the minimal environmental impact of a recycling option
Chemical recycling is based on an underlying chemical reaction:| aPlastic Waste → yProducts + zResiduals | (2) |
This underlying chemical reaction describes the amount of plastic waste a turned into y amount of products and z amount of residuals. The minimal environmental impact of a chemical recycling option is determined based on an ideal chemical recycling process, which facilitates the underlying chemical reaction by assuming:
1. a stoichiometric reaction with 100% conversion,
2. the required heat input is only the reaction enthalpy,
3. other process aspects, such as separation, preheating, cooling, etc., are neglected, and
4. all products are pure.
Any actual chemical recycling process will have a higher environmental impact than the ideal one (cf. discussion in Section 4.1). The calculation of the minimal environmental impact is possible at an early stage of development as it only requires knowledge of the types and amounts of products and residuals from plastic waste. Furthermore, no knowledge or assumptions about the technology facilitating the chemical recycling option are needed, making the results of the analysis technology-independent.
2.2 Considered recycling products of polyurethane rigid foams
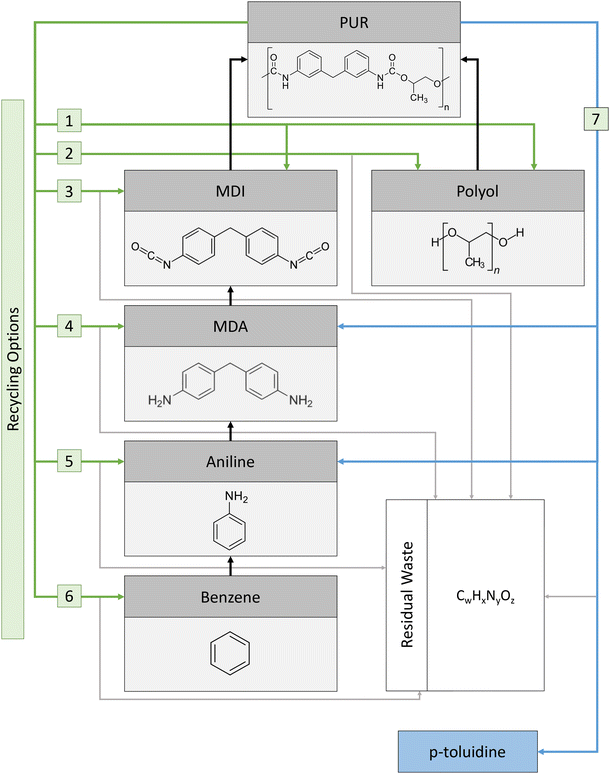
The presented method is applicable to any chemical recycling product. This work focuses on closed-loop recycling options for PU rigid foam due to the promising aspects mentioned in Section 1. For closed-loop chemical recycling of PU rigid foam, we consider the precursors of the PU-rigid-foam value chain as possible products, as shown in Fig. 1. The two main precursors of PU rigid foams are isocyanate and polyols.42 Isocyanate and polyols usually constitute around 90 weight% of the final PU-rigid-foam product, with the remainder being additives.34 We assume methylene diphenyl diisocyanate (MDI) as the isocyanate precursor in our analysis (see left black arrow leading to PU at the top of Fig. 1), which is a common isocyanate in the production of PU rigid foam.34 Generally, the recovery of solely the direct precursors of PU rigid foam, i.e., MDI + polyol, from chemical recycling is not guaranteed, as considered in previous work by Meys et al.41 Thus, we additionally consider the main precursors used in the value chain of MDI production as possible recycling products, namely methylenedianiline (MDA), MDA precursor aniline, and aniline precursor benzene (grey boxes to the left of PU in Fig. 1). For the sake of simplicity, any additional reactants required for producing these precursors (e.g., phosgene to produce MDI from MDA) are not considered, as they do not represent the main mass flows in an assumed stoichiometric reaction.Common polyols used for PU rigid foams are short-chain polyether polyols.34 A typical short-chain polyether polyol is polypropylene glycol with an average molecular weight of 400 g mol−1, also called PPG-400. We assume PPG-400 as the only polyol precursor in our analysis (see the right black arrow leading to PU at the top of Fig. 1). The precursor of PPG-400, propylene oxide, is not considered a recycling product, as the recycling of polyols from PU has been studied extensively and is considered feasible.36,43
Since additives constitute only a small mass of the PU rigid foam and are often unknown,44 we exclude possible additives from our analysis for the sake of simplicity. In summary, based on these 5 precursors (i.e., polyol, MDI, MDA, aniline, and benzene), we consider 6 possible recycling reactions for closed-loop recycling, namely PU-rigid-foam recycling to:
(1) both PU-rigid-foam precursors: MDI + polyol,
(2) only polyol,
(3) only MDI,
(4) only the MDI precursor MDA,
(5) only the MDA precursor aniline, and
(6) only the aniline precursor benzene.
Generally, chemically recycling PU rigid foam can produce product mixtures that contain not solely the precursors of PU-rigid-foam production. Such precursors are then categorized under open-loop chemical recycling. We analyze an exemplary product mixture containing non-precursors as a 7th option to show the applicability of the presented method to any possible recycling mixture. The analyzed exemplary mixture consists of the products aniline, MDA, and p-toluidine, the non-precursor product, taken from a patent by Covestro,45 a major PU-rigid-foam producer. The composition of the patent mixture represents experimental data from pyrolysis experiments, a chemical recycling technology currently under investigation as an option for a large variety of plastics.46
While the product composition is specific for the experimental setup described by the patent, other experimental setups for PU rigid foam recycling yield the same main precursors (i.e., aniline, MDA, and polyol).36,47–51 By analyzing the patent mixture (7th option) which also contains a non-precursor (toluidine), we showcase the method's adaptability to any experimental setup and demonstrate the method's potential to directly support experimental research.
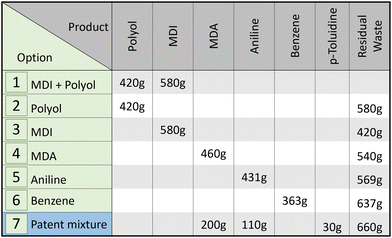
We assume that the recovered product mass of each closed-loop recycling option is equal to 100% of the mass needed for production (see Fig. 2), in line with the assumed ideal chemical recycling with 100% conversion (cf. Section 2.1). Since PU can have a wide variety of formulations and hence no definitive precursor ratio is required for PU-rigid-foam production, we assume that 1 kg PU rigid foam consists of 580 g MDI and 420 g polyol (see Fig. 2, option 1, – for details of the calculation, see Section 1 of the ESI†). This composition matches the range of available PU rigid foams and is based on a patent by Covestro.45 The neglect of any additives is in line with the ideal recycling assumption underlying the environmental potential. The mass needed for the production of precursors is based on reaction stoichiometry and the molecular structure, e.g., 2 mol of aniline is required to stoichiometrically produce 1 mol of MDA. Detailed calculations can be found in the ESI, Section 2.† For the patent mixture (option 7), product mass flows are taken from the aforementioned patent.45 For all recycling options, we assume the mass not considered as a product to be residual waste, whose amount and composition are obtained from an elemental mass balance (see Section 3 of the ESI† for details). Consequently, as a product is located further back in the value chain, the mass of the product decreases and the mass of residual waste increases.
2.3 System boundaries and assumptions
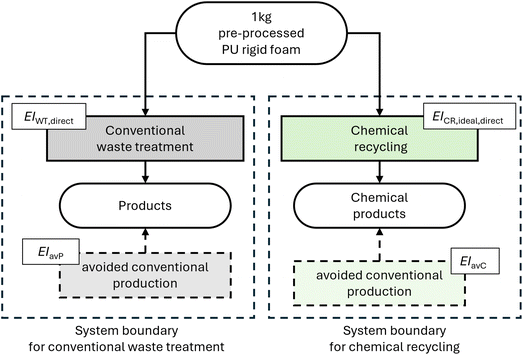
The environmental potential calculation considers the whole life cycle of PU from cradle to grave for both compared routes, i.e., the chemical recycling route and the benchmark waste treatment route. Due to the comparative nature of the LCA performed in this study (see equation (1)), the environmental potential is not affected by identical activities in both routes, and therefore identical activities are excluded from our analysis. Namely, we omit the modeling of the PU-rigid-foam production, the use phase of the rigid foams, and any collection, sorting, and preprocessing activities before the end-of-life stage. We assume the recycling processes to take the exact same input as any other waste treatment option, due to the modeling of an ideal chemical recycling, i.e., a best-case. Hence, no additional sorting or treatment for the chemical recycling is required. Further, we assume the location of both routes to be the same and thus exclude transport in our analysis. We define the treatment of 1 kg of pre-processed PU rigid foam waste at the treatment plant as our functional unit for both routes. Fig. 3 illustrates the system boundaries of the two compared systems.All required LCA data is taken from ecoinvent 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 unless stated otherwise in this section. We assume Europe as the location of all processes; hence, all data used represents the European average. See ESI, Section 4,† for a detailed list of the used datasets. We use the Environmental Footprint 3.0
52 unless stated otherwise in this section. We assume Europe as the location of all processes; hence, all data used represents the European average. See ESI, Section 4,† for a detailed list of the used datasets. We use the Environmental Footprint 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 as our impact assessment method and quantify all 16 major impact categories to analyze potential burden shifting, i.e., a decrease in some environmental impacts accompanied by an increase in other environmental impacts.
53 as our impact assessment method and quantify all 16 major impact categories to analyze potential burden shifting, i.e., a decrease in some environmental impacts accompanied by an increase in other environmental impacts.
An overview of the assumed treatment options is shown in Table 1. For the conventional route, the environmental impact EIWT,direct is calculated for 3 currently available conventional waste treatment options:
1. incineration of PU rigid foam waste in municipal solid waste incinerators with energy recovery (WtE),
2. landfilling of PU rigid foam waste in a sanitary landfill (landfill), and
3. incineration of PU rigid foam waste in cement kilns replacing lignite (cement kiln).
| Conventional waste treatment EIWT, direct | Avoided conventional production EIavP | Chemical recycling EICR, ideal, direct | Avoided conventional production EIavC |
|---|---|---|---|
| Waste incineration with energy recovery (WtE) | District heat and electricity grid mix | Ideal chemical recycling | Chemical products from 7 recycling options |
| Landfill | None | ||
| Cement kiln | Lignite |
The environmental impact of the waste incineration EIWT, direct is calculated based on primary data from Meys et al.41 and Doka et al.54
The environmental impact of the avoided products EIavP is calculated based on the following assumptions:
1. for municipal solid waste incinerators with energy recovery, we assume the recovered energy to replace the electricity grid mix and district heating,
2. for landfilling, no avoided products are assumed, and
3. for waste incineration in cement kilns, we consider the avoided product to be lignite, as incinerating PU rigid foam will avoid the incineration of lignite in the cement kiln.
For the chemical recycling route, the minimal net environmental impact EICR,ideal,net is calculated for the 7 recycling options described in Section 2.2. The minimal environmental impact EICR,ideal,direct includes the emissions from the heat supply and residual waste treatment. Heat supply to all recycling options is assumed to be from natural gas boilers. Residual waste of each recycling option is assumed to be treated in municipal solid waste incinerators with energy recovery as modeled in the conventional incineration routes.
The avoided environmental impact of the conventional production EIavC for all considered recycling options is calculated with data from CarbonMinds,55 which is consistent with ecoinvent 3.8.52
3 Results: the environmental potential of PU-rigid-foam recycling
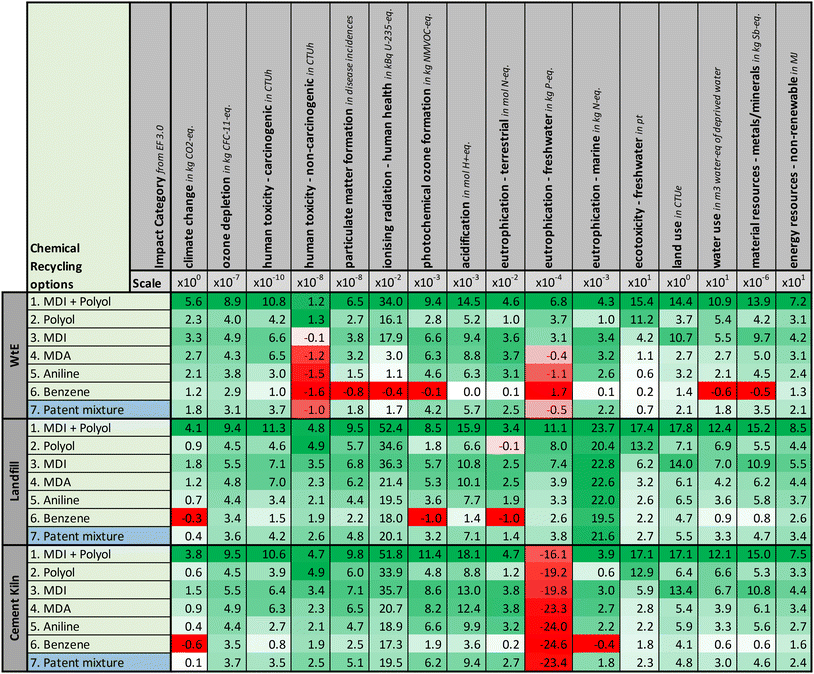
We calculate the environmental potential for the 7 recycling options, each compared to the 3 conventional treatment options (see Section 2). For each scenario, we compare 16 impact categories, leading to a total of 336 environmental potentials, as shown in Fig. 4.In the following subsections, we first discuss the general results focusing on the performance of the recycling options compared to all conventional treatments. Since each conventional treatment has its own characteristics, we then discuss them separately in detail.
3.1 General discussion of results
Of all 336 calculated environmental potentials, 309 are positive, while only 27 are negative, highlighting the significant potential of PU-rigid-foam recycling. Chemical Recycling to MDI + polyol (option 1) is the best-performing recycling option because it has the highest number of positive environmental potentials and the highest environmental potential in all but one impact category compared to all other analyzed recycling options. Only for the impact category “human toxicity – non-carcinogenic”‡ does recycling to MDI + polyol (option 1) perform slightly worse than recycling to polyol (option 2). This performance difference is attributed to the difference between recycling MDI (option 1) and incinerating it as part of residual wastes (option 2). Energy recovered from the incineration of MDI avoids more environmental burden in “human toxicity – non-carcinogenic” than recovering the MDI.Comparing the recycling to either polyol (option 2) or MDI (option 3), i.e., solely one of the direct PU-rigid-foam precursors, recycling to MDI (option 3) has a higher environmental potential in 13 out of 16 impact categories. The performance difference between those two options can be primarily attributed to two aspects: first, recycling MDI avoids higher burdens from its fossil production than recycling the polyol in almost all impact categories, except the two impact categories of “human toxicity – non-carcinogenic” and “ecotoxicity – freshwater”. Consequently, in those two impact categories, recycling to polyol (option 2) has a higher environmental potential than recycling to MDI (option 3). Second, recycling to polyol (option 2) is associated with an increase in emissions of nitrogen-based compounds. The increased emissions of nitrogen-based compounds result from the residual waste treatment: all mass not contained in the products is incinerated, and as polyol contains no nitrogen, all nitrogen present in the PU rigid foam waste is incinerated when only polyol is recovered (option 2). Those two aspects are the dominating factors for the performance differences in recycling to polyol (option 2) or MDI (option 3) in all but one impact category, “eutrophication – freshwater”. For “eutrophication – freshwater”, recycling to polyol (option 2) has higher avoided burdens from the residual waste energy recovery than recycling to MDI (option 3) leading to a higher environmental potential for recycling to polyol (option 2).
Going back through the value chain of the MDI precursor (i.e., options 3 to 6), a general trend can be observed across the recycling options: the environmental potential decreases when moving further down the value chain, from late precursors, such as MDI or MDA, with high environmental potential to early precursors, such as benzene and aniline, with a lower environmental potential. This trend can be attributed to two aspects: first, the earlier the produced precursors are located in the PU-rigid-foam value chain, the lower the avoided burdens of conventional production, and second, recycling options producing earlier precursors yield more incinerated residual wastes, leading to higher direct emissions.
Among all recycling options, recycling to benzene (option 6), the earliest considered precursor, has the lowest avoided burdens of conventional production and the highest amount of direct emissions, including an increase in emissions of nitrogen-compounds. Consequently, recycling to benzene (option 6) has the lowest environmental potentials of all options, making it the worst-performing recycling option. Notably, recycling to benzene yields 13 negative potentials. Thus, e.g., even an ideal chemical recycling process to benzene would emit more GHG emissions than its fossil production and landfilling or incinerating the PU waste in cement kilns. Nonetheless, recycling to benzene (option 6) still offers a positive environmental potential in half the impact categories compared to all 3 analyzed conventional treatment options, highlighting that even the worst-performing option in this analysis still offers the potential of PU-rigid-foam recycling to reduce environmental impacts.
The recycling to the patent mixture (option 7) offers a positive environmental potential in at least 14 out of the 16 impact categories. As this option is based on experimental results, the high number of positive environmental potentials showcases real-world environmental impact reduction possibilities and thus motivates further research of this option. Notably, recycling to this patent mixture (option 7) outperforms recycling to benzene (option 6) although recycling to this patent mixture results in the highest amount of incinerated residual waste. This finding suggests that even partly recycling higher value precursors (i.e., MDA and aniline) and also recycling chemicals beyond direct precursors (i.e., p-toluidine) might be beneficial, even if this recycling results in more residual waste. Future work should further explore such options, including open-loop options that utilize synergies with other value chains.
Although a general trend can be identified across all options, ranking the recycling options is challenging because the rankings depend on the impact categories (e.g., recycling to polyol (option 2) has a higher potential than recycling to aniline (option 5) in “climate change” but not in “acidification”.) Given that no impact category can be objectively considered more important than another one (or even of equal importance), we refrain from any attempt to unambiguously rank all recycling options across all impact categories.
Overall, the chemical recycling of PU rigid foam has a high number of positive environmental potentials compared to conventional waste treatment, highlighting the possibilities offered by chemical recycling to reduce environmental impacts, even for recycling products that are not direct precursors of PU rigid foam. Moreover, positive environmental potentials for many impact categories also exist for a patent mixture with open-loop recycling products, motivating further research of the patented technology. The general analysis of all options shows that 3 primary factors influence the environmental potential: first, the avoided burdens recovering the precursor; second, the direct emissions from the residual waste incineration; and third, the avoided burdens by the recovered energy from the residual incineration. Nonetheless, not all recycling options offer identical environmental potentials; some options exhibit negative environmental potentials showcasing expected burden shifting that might occur when chemically recycling PU rigid foam. If and to what extent burden shifting occurs depends on the conventional treatment considered as the benchmark. Thus, for assessing recycling options, it is important to consider for which conventional waste treatment a PU-rigid-foam waste stream was originally destined: while a recycling option (e.g., recycling to aniline (option 5)) might exhibit negative environmental potentials when the PU rigid foam waste was destined for municipal solid waste incinerators, the same recycling option may have no negative environmental potentials when the waste was destined for landfilling. Therefore, the following subsections analyze the three conventional waste treatment options in more detail and highlight the recycling options with no negative environmental potentials when the respective conventional waste treatment is chosen as a benchmark.
3.2 Chemical recycling compared to WtE
For recycling when the treatment of PU rigid in municipal solid waste incinerators is considered as the benchmark (top third of Fig. 4), recycling to MDI + polyol (option 1) and polyol (option 2) have solely positive environmental potentials, showing the great potential of recycling PU rigid foam to its direct precursors when sourcing PU destined to incineration with energy recovery. All other analyzed recycling options have at least one negative environmental potential and burden shifting occurs. For recycling to MDI (option 3), burden shifting occurs for the impact category of “Human toxicity – non-carcinogenic”.For recycling to MDA, aniline and the patent mixture (options 4, 5, and 7), burden shifting occurs additionally for the impact category “eutrophication – freshwater”. For those two impact categories, the incineration of PU rigid foam with energy recovery is more beneficial than PU-rigid-foam recycling since recovered energy avoids the production of heat (here assumed to be natural gas-based) and electricity (current European grid, primarily natural gas and coal-based). The extraction and mining of natural gas and coal cause significant environmental impacts in the two aforementioned impact categories. The expected shift in energy provision to renewables will, however, reduce these benefits from the avoided burdens in conventional waste treatment and increase the environmental potential of recycling.
For recycling to benzene (option 6), burden shifting occurs for 7 out of the 16 impact categories. This increase in the number of impact categories with burden shifting results from a significant increase in emissions of nitrogen-based compounds as described in Section 3.1 combined with the lower avoided burdens from recycling the benzene. However, even for this worst case, recycling to benzene (option 6) offers 9 impact categories with positive environmental impacts.
3.3 Chemical recycling compared to landfilling
For recycling when landfilling of PU rigid foam is considered as the benchmark (middle third of Fig. 4), recycling to MDI and its precursors down to aniline (options 1, 3, 4, 5) as well as recycling to the patent mixture (option 7) exhibit solely positive environmental potential in all impact categories, highlighting the great potential of recycling PU rigid foam when sourcing PU destined to landfilling. Landfilling of PU rigid foam has low emissions but no byproducts and thus no credits for avoided burdens, leading to a net environmental impact close to zero. In contrast, recycling PU rigid foam to precursors causes a high credit for avoiding conventional precursor production. As a result, chemical recycling of PU rigid foam performs well compared to landfilling.Only for recycling to polyol (option 2) and to benzene (option 6), burden shifting occurs. For recycling to polyol (option 2), burden shifting occurs in the impact category “eutrophication – terrestrial” only because of the low net environmental impact of landfilling PU rigid foam and high emission of treating nitrogen containing residuals. (cf. Section 3.1). Similarly, for recycling to benzene (option 6), burden shifting occurs for the impact categories “climate change”, “photochemical ozone formation”, and “eutrophication – terrestrial” only because of low credits for recycling benzene and overall high emissions from residual waste treatment.
3.4 Chemical recycling compared to cement kilns
The utilization of PU rigid foam in cement kilns (bottom third of Fig. 4) is considered as a worst-case benchmark, as PU rigid foam waste is assumed to be replaced by lignite in cement kilns and the incineration of lignite has high environmental impacts in all impact categories. Remarkably, PU-rigid-foam recycling still offers positive environmental potentials for almost all impact categories. A negative potential is only identified in the impact category “eutrophication – freshwater” for all recycling options: as mining the lignite causes high environmental impacts in “eutrophication – freshwater”, any potential credits from the avoided production of the chemical recycling products are smaller than those mining impacts. Additionally, for recycling to benzene (option 6), burden shifting occurs for the impact categories “climate change” and “eutrophication – marine” due to low credits for benzene and high emissions from residual waste treatment, as discussed in Section 3.1.To account for an alternative scenario for cement kilns with lower environmental impacts, we also investigate the replacement of PU rigid foam waste with biomass in cement kilns. For this purpose, we use miscanthus instead of lignite in an additional benchmark scenario shown in Section 5 of the ESI.† If miscanthus replaces PU rigid foam waste in cement kilns, all analyzed recycling options can potentially reduce the environmental impact also in the “eutrophication – freshwater” category. However, in this scenario, the environmental potential in the impact category “land use” is negative for all analyzed recycling options due to the large impact of the cultivation of miscanthus on land use, a common trade-off observed for the switch to bio-based processess.3
4 Discussion
The analysis presented in Section 3 highlights the potential to reduce environmental impacts and possible trade-offs that chemical recycling of PU rigid foam waste could offer compared to conventional waste treatment. By assuming ideal chemical recycling, we robustly identify recycling options with the potential to reduce the environmental impacts compared to conventional treatment options, independently of the technology and actual recycling process. For all analyzed options, environmental impacts could be reduced in at least half of the impact categories. However, we also highlight the differences between the analyzed options and identify options with negative environmental potentials. Hence, research efforts can be targeted at promising options with a high number of positive and large environmental potentials to reduce the environmental impacts in all categories, e.g., recycling PU rigid foam to MDA or aniline.Our results are in line with previous studies assessing the environmental performance of chemical recycling of polymers. Meys et al.41 show that chemical recycling of polymers could offer reductions in environmental impacts, especially when monomers are recovered. Similarly, Klotz et al.27 highlight that the chemical recycling options pyrolysis and gasification have the ability to achieve substantial benefits over incineration if their output products can substitute high-impact chemicals. Schwarz et al.56 calculate the environmental impact of treating PU waste in multiple technologies for the category “climate change”. Among their analyzed technologies are incineration with energy recovery and pyrolysis to monomers, with monomers representing an open-loop mixture of aliphatic compounds, propylene, BTX, and diesel substitutes. The difference between their calculated “climate change” impact values for those two technologies (i.e., their environmental potential) is 1.5 kgCO2eq. kg−1 PU treated. This value falls in between our calculated values of recycling to aniline (option 5) and recycling to benzene (option 6) when compared to municipal solid waste incineration with energy recovery.
4.1 Analysis of the minimal required conversion & maximum energy demands
A positive environmental potential does not necessarily guarantee a reduction of environmental impacts when the corresponding chemical recycling options are implemented in real processes. Real processes often cause additional environmental impacts beyond the ideal chemical recycling as they rarely reach the assumed 100% conversion to the targeted product and require additional energy for process steps up- and downstream, such as sorting, shredding, or downstream product purification.For reference, recent experimental work on PU rigid foam recycling have reported widely varying conversions of 59%47 up to 98%.57 The additional energy demand of recycling processes can also vary substantially depending on the recycling technology and the recovered products. For PET, PP, PE, PS, process simulations of chemical recycling determine energy demands of 2–25 MJ kg−1 plastic waste,58 showcasing that the energy demand is often substantially higher than the reaction enthalpy.
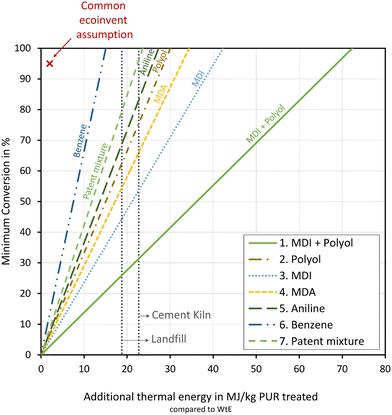
To provide targets for process development, we calculate the minimal conversion of each recycling option that has to be reached to have a positive environmental potential in a real process. We then approximate the additional process steps by an additional thermal energy demand supplied with natural gas and analyze the trade-off between the minimum conversion rate and the additional thermal energy demand (Fig. 5). Real chemical recycling processes that produce the considered products must reach this minimum conversion rate and use less than the corresponding maximal heat demand to achieve lower environmental impacts than conventional waste treatment.
The minimum conversion rate in Fig. 5 is calculated for the comparison of chemical recycling and incineration with energy recovery of PU rigid foam in the impact category “climate change”. For a single impact category, the environmental potentials of the other two conventional treatment options (landfilling and incineration in cement kilns) differ from incineration with energy recovery only by a fixed absolute value, shifting the y-axis in Fig. 5. Hence, we added two vertical lines at 10 MJ kg−1 PU treated and 23 MJ kg−1 PU treated for landfilling and incineration in cement kilns, respectively, indicating the shift of the y-axis for these conventional recycling options. Details regarding the minimum conversion rate calculation can be found in the ESI, Section 6.†
In Fig. 5, a conversion of 0% represents a scenario where all PU rigid foam waste ends up in the residual waste incineration, leading to an environmental potential of zero, as both residual incineration and the conventional WtE receive identical credits from the energy recovery. Consequently, all graphs of the recycling options start at 0% conversion and an additional thermal energy demand of 0 MJ kg−1 PU treated. Recycling to MDI + polyol (option 1) exhibits the lowest increase in required minimum conversion with increasing additional thermal energy, which is in line with the results presented in Section 3, where recycling to MDI + polyol has the highest environmental potential in climate change. A maximum additional thermal energy of around 72 MJ kg−1 PU treated can be supplied for additional process steps if 100% conversion is achieved. In contrast, recycling to benzene (option 6) exhibits the steepest increase in the required minimum conversion and only an additional energy demand of 14 MJ kg−1 PU treated can be supplied before the minimal required conversion rate reaches 100%.
If waste is taken from cement kilns to recycling, the minimum required conversion increases significantly for all options. Therefore, no feasible solution can be identified for recycling to benzene (option 6), because the environmental potential is already negative for the ideal case with 100% conversion and no additional thermal energy demand (cf. Section 3.4). Moreover, compared to cement kilns, recycling to the patent mixture (option 7), requires conversions of at least 97% to have a positive environmental potential.
Environmental impacts of unknown chemical processes are often estimated based on average proxy values for key process metrics.59 For example, the ecoinvent database assumes a 95% conversion, a thermal energy demand of 2 MJ kg−1 and a electricity demand of 1.2 MJ kg−1 for chemical processes estimated from stoichiometric reactions in their version 2 database.60 Those values represent an average of the Gendorf chemical site in Germany61 and are often used and suggested for the estimation of unknown chemical processes.59 According to Fig. 5, all recycling options would still offer a positive environmental potential for climate change, when the corresponding recycling process would be estimated using a 95% conversion and a thermal energy demand of 2 MJ kg−1. Only when compared to PU-rigid-foam utilization in cement kilns would recycling to benzene and to the patent mixture offer no positive environmental potential for climate change.
4.2 Environmental potentials in the scope of future sustainable processes
In this work, the environmental potential is calculated for the current status quo of conventional production. This status quo is expected to change, e.g. with the introduction of novel carbon sources for chemical production or cleaner electricity grid mixes. This change would alter the avoided burdens and, thus, the environmental potential. Further studies should address the quantification of such future changes. However, the general trends identified in the present study are likely to persist, as changes in the status-quo production of earlier precursors will propagate to later precursors, meaning that changes in the avoided burdens will maintain a consistent trend.Moreover, environmental impacts are not the only feasibility criterion for the success of a recycling technology. Market dynamics and economics play a significant role in the feasibility of a recycling technology. A recycling option with a lower environmental potential might produce a more commonly traded product with better handling infrastructure. Therefore, targeting such a product from chemical recycling might prove beneficial over options with higher environmental potential, and thus, future studies should incorporate market and economic considerations. Finally, full LCA studies based on industrial data are needed to quantify the actual environmental impact of the reduction of chemical recycling of PU rigid foams.
5 Conclusion
In this work, we analyze 7 waste recycling options for PU rigid foam and compare their environmental potential to 3 conventional treatments across 16 impact categories. For this purpose, we extend the environmental potential method introduced by Meys et al.41 to incorporate a broad range of recycling reactions and products.All analyzed chemical recycling options of PU rigid foam can reduce most environmental impacts when compared to conventional waste treatment options, showcasing the potential of PU rigid foam recycling for more sustainable plastic value chains. The highest environmental potential across all impact categories is achieved for chemical recycling PU rigid foam to MDI + polyol, the direct precursors of PU rigid foam production in our study. If PU rigid foam can only be recycled to either MDI or polyol, recycling to MDI has higher environmental potentials, due to, among other things, recovering nitrogen present in PU rigid foam. When considering recycling to MDI precursors, the environmental potential is lower the earlier in the production chain a precursor is located, sometimes even turning negative.
Nonetheless, even for chemical recycling to benzene, which is the earliest analyzed precursor associated with the highest amount of negative environmental potentials across the impact categories, environmental impacts could be reduced in at least half of the impact categories. Still, recycling PU rigid foam to benzene does not seem promising as a recycling option for PU as the environmental potential robustly shows that many environmental impacts will increase, including climate change.
Moreover, the environmental potential of recycling options depends on the conventional waste treatment option. Consequently, when implementing a recycling option, environmental burdens shifts depend on the conventional waste treatment the recycling option under consideration would replace.
Our environmental potential analysis can direct the focus of chemical recycling research in both academia and industry at an early stage of development when minimal data is available, to avoid wasting valuable research resources. The suggested stoichiometry-based calculation of the environmental potential can be extended to other types of plastics beyond PU rigid foams. Such an extension would provide a broad picture of the reduction potentials in environmental impacts for various plastic recycling options across the plastic industry, ultimately, supporting the transition to a more sustainable industry.
Author contributions
Martin Pillich: Conceptualization, methodology, investigation, data curation, visualization, validation, writing – original draft, writing – review & editing. Johannes Schilling: Conceptualization, methodology, writing – review & editing, writing – original draft, supervision. Luca Bosetti: Data curation, validation, writing – original draft, writing – review & editing. André Bardow: Conceptualization, methodology, writing – original draft, writing – review & editing, supervision, funding acquisition.Data availability
The data supporting this study are available within the article and as part of the ESI.†Conflicts of interest
The authors and Covestro, patent holder of the patent mixture analyzed in this work, collaborate in a project titled ‘CIRCULAR FOAM’ (Grant Agreement No. 101036854), supported by the European Union Horizon 2020 Research and Innovation Programme.A. B. has ownership interests in firms that render services to industry, some of which may produce polymers. A. B. has served on review committees for research and development at ExxonMobil and TotalEnergies, oil and gas companies that are also active in polymer production.
Acknowledgements
This work was funded by the European Union's Horizon 2020 research and innovation programme as part of the project CIRCULAR FOAM under grant agreement No. 101036854. This work reflects only the authors’ views. It does not represent the view of the European Commission and the Commission is not responsible for any use that may be made of the information it contains. The authors would like to thank Lisa Smeaton for proofreading the manuscript and Benedikt Winter as well as Stefanie Eiden and Yolanda Campos from Covestro for their valuable contributions to discussions.References
- PlasticsEurope, Plastics – the Facts 2022, Plasticseurope technical report, 2022 Search PubMed.
- S. R. Nicholson, N. A. Rorrer, A. C. Carpenter and G. T. Beckham, Manufacturing Energy and Greenhouse Gas Emissions Associated with Plastics Consumption, Joule, 2021, 5, 673–686 CrossRef CAS.
- M. Bachmann, C. Zibunas, J. Hartmann, V. Tulus, S. Suh, G. Guillén-Gosálbez and A. Bardow, Towards Circular Plastics within Planetary Boundaries, Nat. Sustain., 2023, 6, 599–610 CrossRef.
- E. Danopoulos, M. Twiddy, R. West and J. M. Rotchell, A Rapid Review and Meta-Regression Analyses of the Toxicological Impacts of Microplastic Exposure in Human Cells, J. Hazard. Mater., 2022, 427, 127861 CrossRef CAS PubMed.
- S. R. Kahane-Rapport, M. F. Czapanskiy, J. A. Fahlbusch, A. S. Friedlaender, J. Calambokidis, E. L. Hazen, J. A. Goldbogen and M. S. Savoca, Field Measurements Reveal Exposure Risk to Microplastic Ingestion by Filter-Feeding Megafauna, Nat. Commun., 2022, 13, 6327 CrossRef CAS PubMed.
- FAO, Assessment of Agricultural Plastics and Their Sustainability: A Call for Action, Food and Agriculture Organization of the United Nations technical report, 2021 Search PubMed.
- D. Azoulay, P. Villa, Y. Arellano, M. Gordon, K. Miller and K. Thompson, Plastic and Health: The Hidden Costs of a Plastic Planet, Center for international environmental law technical report, 2019 Search PubMed.
- A. Demetrious and E. Crossin, Life Cycle Assessment of Paper and Plastic Packaging Waste in Landfill, Incineration, and Gasification-Pyrolysis, J. Mater. Cycles Waste Manage., 2019, 21, 850–860 CrossRef CAS.
- M. Anshassi, H. Sackles and T. G. Townsend, A Review of LCA Assumptions Impacting Whether Landfilling or Incineration Results in Less Greenhouse Gas Emissions, Resour., Conserv. Recycl., 2021, 174, 105810 CrossRef CAS.
- EU, A European Strategy for Plastics in a Circular Economy, 2018 Search PubMed.
- EU, A New Circular Economy Action Plan For a Cleaner and More Competitive Europe, 2020 Search PubMed.
- H. Alhazmi, F. H. Almansour and Z. Aldhafeeri, Plastic Waste Management: A Review of Existing Life Cycle Assessment Studies, Sustainability, 2021, 13, 5340 CrossRef.
- T. Thiounn and R. C. Smith, Advances and Approaches for Chemical Recycling of Plastic Waste, J. Polym. Sci., 2020, 58, 1347–1364 CrossRef CAS.
- I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Beyond Mechanical Recycling: Giving New Life to Plastic Waste, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS.
- K. M. Knauer, Energy Transition: Climate Action and Circularity, American Chemical Society, 2022, vol. 1412, pp. 567–585 Search PubMed.
- C. Jehanno, J. W. Alty, M. Roosen, S. De Meester, A. P. Dove, E. Y.-X. Chen, F. A. Leibfarth and H. Sardon, Critical Advances and Future Opportunities in Upcycling Commodity Polymers, Nature, 2022, 603, 803–814 CrossRef CAS PubMed.
- P. Garcia-Gutierrez, A. Amadei, D. Klenert, S. Nessi, D. Tonini, D. Tosches, F. Ardente and H. Saveyn, Environmental and Economic Assessment of Plastic Waste Recycling: A Comparison of Mechanical, Physical, Chemical Recycling and Energy Recovery of Plastic Waste, Publications office of the european union technical report, 2023 Search PubMed.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Benson, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown, V. S. Cecon, J. B. Curley, G. W. Curtzwiler, S. Dong, S. Gaddameedi, J. E. Estela-García, I. Hermans, M. S. Kim, J. Ma, L. O. Mark, M. Mavrikakis, O. O. Olafasakin, T. A. Osswald, K. G. Papanikolaou, H. Radhakrishnan, M. A. Sanchez-Castillo, K. L. Sánchez-Rivera, K. N. Tumu, R. C. Van Lehn, K. L. Vorst, M. M. Wright, J. Wu, V. M. Zavala, P. Zhou and G. W. Huber, Expanding Plastics Recycling Technologies: Chemical Aspects, Technology Status and Challenges, Green Chem., 2022, 24, 8899–9002 RSC.
- G. S. Kulkarni, Recycling of Polyurethane Foams, William Andrew Publishing, 2018, pp. 1–16 Search PubMed.
- ISO 15270:2008, Plastics—Guidelines for the Recovery and Recycling of Plastics Waste.
- K. Ragaert, C. Ragot, K. M. Van Geem, S. Kersten, Y. Shiran and S. De Meester, Clarifying European Terminology in Plastics Recycling, Curr. Opin. Green Sustain. Chem., 2023, 44, 100871 CrossRef.
- agilyx , Plastic Recycling Technologies – Chemical Recycling, https://www.agilyx.com/, 2023, accessed: 01.11.2023.
- BASF , Chemisches Recycling von Kunststoffabfällen, https://www.basf.com/ch/de/who-we-are/sustainability/we-drive-sustainable-solutions/circular-economy/mass-balance-approach/chemcycling.html, accessed: 22.01.2024.
- dow , Dow and Orrion to Begin Recycling Polyurethane Foam in 2021, https://www.mrchub.com/news/371357-dow-and-orrion-to-begin-recycling-polyurethane-foam-in-2021, 2021, accessed: 22.01.2024.
- B. Liu, Z. Westman, K. Richardson, D. Lim, A. L. Stottlemyer, T. Farmer, P. Gillis, V. Vlcek, P. Christopher and M. M. Abu-Omar, Opportunities in Closed-Loop Molecular Recycling of End-of-Life Polyurethane, ACS Sustainable Chem. Eng., 2023, 11, 6114–6128 CrossRef.
- L. Polo Fonseca, A. Duval, E. Luna, M. Ximenis, S. De Meester, L. Avérous and H. Sardon, Reducing the Carbon Footprint of Polyurethanes by Chemical and Biological Depolymerization: Fact or Fiction?, Curr. Opin. Green Sustain. Chem., 2023, 41, 100802 CrossRef.
- M. Klotz, M. Haupt and S. Hellweg, Potentials and Limits of Mechanical Plastic Recycling, J. Ind. Ecol., 2023, 27, 1043–1059 CrossRef.
- A. Kemona and M. Piotrowska, Polyurethane Recycling and Disposal: Methods and Prospects, Polymers, 2020, 12, 1752 CrossRef CAS PubMed.
- M. Grdadolnik, B. Zdovc, A. Drinčić, O. C. Onder, P. Utroša, S. G. Ramos, E. D. Ramos, D. Pahovnik and E. Žagar, Chemical Recycling of Flexible Polyurethane Foams by Aminolysis to Recover High-Quality Polyols, ACS Sustainable Chem. Eng., 2023, 11, 10864–10873 CrossRef CAS PubMed.
- PUReSmart , PUReSmart, https://www.puresmart.eu/, accessed: 22.01.2024.
- Covestro , Evocycle® CQ – Our Program for Innovative Recycling, https://solutions.covestro.com/en/highlights/articles/stories/2022/evocycle-cq, accessed: 22.01.2024.
- CircularFoam , Circular Foam, https://circular-foam.eu/, 2024, accessed: 10.08.2024.
- BASF , Go! Create Something New from Old PUR and Close the Material Loop for PUR Rigid Foams, https://www.basf.com/global/en/media/news-releases/2022/10/p-22-350.html, accessed: 22.01.2024.
- C. Liang, U. R. Gracida-Alvarez, E. T. Gallant, P. A. Gillis, Y. A. Marques, G. P. Abramo, T. R. Hawkins and J. B. Dunn, Material Flows of Polyurethane in the United States, Environ. Sci. Technol., 2021, 55, 14215–14224 CrossRef CAS PubMed.
- G. Rossignolo, G. Malucelli and A. Lorenzetti, Recycling of Polyurethanes: Where We Are and Where We Are Going, Green Chem., 2024, 26, 1132–1152 RSC.
- D. Simón, A. M. Borreguero, A. de Lucas and J. F. Rodríguez, Recycling of Polyurethanes from Laboratory to Industry, a Journey towards the Sustainability, Waste Manage., 2018, 76, 147–171 CrossRef.
- E. M. Zakharyan and A. L. Maksimov, Pyrolysis of Polyurethanes. Process Features and Composition of Reaction Products, Russ. J. Appl. Chem., 2022, 95, 191–255 CrossRef CAS.
- A. Sheel and D. Pant, in Recycling of Polyurethane Foams, ed. S. Thomas, A. V. Rane, K. Kanny, V. K. Abitha and M. G.Thomas, William Andrew Publishing, 2018, pp. 67–75 Search PubMed.
- S. Bhandari and P. Gupta, Recycling of Polyurethane Foams, ed. S. Thomas, A. V. Rane, K. Kanny, V. K. Abitha and M. G.Thomas, William Andrew Publishing, 2018, pp. 77–87 Search PubMed.
- R. K. Padhan, Recycling of Polyurethane Foams, ed. S. Thomas, A. V. Rane, K. Kanny, V. K. Abitha and M. G.Thomas, William Andrew Publishing, 2018, pp. 89–96 Search PubMed.
- R. Meys, F. Frick, S. Westhues, A. Sternberg, J. Klankermayer and A. Bardow, Towards a Circular Economy for Plastic Packaging Wastes – the Environmental Potential of Chemical Recycling, Resour., Conserv. Recycl., 2020, 162, 105010 CrossRef.
- G. Brereton, R. M. Emanuel Jr, R. Lomax, K. Pennington, T. Ryan, H. Tebbe, M. Timm, P. Ware, K. Winkler, T. Yuan, Z. Zhu, N. Adam, G. Avar, H. Blankenheim, W. Friederichs, M. Giersig, E. Weigand, M. Halfmann, F.-W. Wittbecker, D.-R. Larimer, U. Maier, S. Meyer-Ahrens, K.-L. Noble and H.-G. Wussow, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Ltd, 2019, pp. 1–76 Search PubMed.
- R. Heiran, A. Ghaderian, A. Reghunadhan, F. Sedaghati, S. Thomas and A. H. Haghighi, Glycolysis: An Efficient Route for Recycling of End of Life Polyurethane Foams, J. Polym. Res., 2021, 28, 22 CrossRef CAS.
- H. Wiesinger, Z. Wang and S. Hellweg, Deep Dive into Plastic Monomers, Additives, and Processing Aids, Environ. Sci. Technol., 2021, 55, 9339–9351 CrossRef CAS.
- S. Eiden, R. Bellinghausen, A. Wolf, C. Hahn, T. Loddenkemper and C. Jendrzok, Pyrolyse von Material mit Polyurethanverbindung zur Wiedergewinnung von Rohstoffen, World Intellectual Property Organization, WO2022253873A, 2022 Search PubMed.
- O. Dogu, M. Pelucchi, R. Van de Vijver, P. H. M. Van Steenberge, D. R. D'hooge, A. Cuoci, M. Mehl, A. Frassoldati, T. Faravelli and K. M. Van Geem, The Chemistry of Chemical Recycling of Solid Plastic Waste via Pyrolysis and Gasification: State-of-the-art, Challenges, and Future Directions, Prog. Energy Combust. Sci., 2021, 84, 100901 CrossRef.
- T. B. Bech, B. S. Donslund, S. K. Kristensen and T. Skrydstrup, Chemical Separation of Polyurethane via Acidolysis – Combining Acidolysis with Hydrolysis for Valorisation of Aromatic Amines, Green Chem., 2024, 26, 8395–8404 RSC.
- M. B. Johansen, B. S. Donslund, S. K. Kristensen, A. T. Lindhardt and T. Skrydstrup, Tert-Amyl Alcohol-Mediated Deconstruction of Polyurethane for Polyol and Aniline Recovery, ACS Sustainable Chem. Eng., 2022, 10, 11191–11202 CrossRef CAS.
- S. K. Kristensen, A. Ahrens, B. S. Donslund and T. Skrydstrup, Perspective on the Development of Monomer Recovery Technologies from Plastics Designed to Last, ACS Org. Inorg. Au, 2024, 4, 373–386 CrossRef CAS.
- P. Zhu, Z. Cao, Y. Chen, X. Zhang, G. Qian, Y. Chu and M. Zhou, Glycolysis Recycling of Rigid Waste Polyurethane Foam from Refrigerators, Environ. Technol., 2014, 35, 2676–2684 CrossRef CAS.
- R. Donadini, C. Boaretti, A. Lorenzetti, M. Roso, D. Penzo, E. Dal Lago and M. Modesti, Chemical Recycling of Polyurethane Waste via a Microwave-Assisted Glycolysis Process, ACS Omega, 2023, 8, 4655–4666 CrossRef CAS PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, The Ecoinvent Database Version 3 (Part I): Overview and Methodology, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- S. Fazio, F. Biganzoli, L. V. De, L. Zampori, S. Sala and E. Diaconu, Supporting Information to the Characterisation Factors of Recommended EF Life Cycle Impact Assessment Methods, Version 2, from ILCD to EF 3.0, 2018 Search PubMed.
- G. Doka, Updates to Life Cycle Inventories of Waste Treatment Services – Part II: Waste Incineration, Doka life cycle assessments technical report, 2013 Search PubMed.
- L. Stellner, A. Kätelhön, O. Vögler, R. Hermanns, S. Sangwon, A. Bardow and R. Meys, Methodology Cm.Chemicals Version 1.01, Carbon minds gmbh technical report, 2022 Search PubMed.
- A. E. Schwarz, T. N. Ligthart, D. Godoi Bizarro, P. De Wild, B. Vreugdenhil and T. van Harmelen, Plastic Recycling in a Circular Economy; Determining Environmental Performance through an LCA Matrix Model Approach, Waste Manage., 2021, 121, 331–342 CrossRef.
- R. Miguel-Fernández, I. Amundarain, A. Asueta, S. García-Fernández, S. Arnaiz, N. L. Miazza, E. Montón, B. Rodríguez-García and E. Bianca-Benchea, Recovery of Green Polyols from Rigid Polyurethane Waste by Catalytic Depolymerization, Polymers, 2022, 14, 2936 CrossRef.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, Technical, Economic, and Environmental Comparison of Closed-Loop Recycling Technologies for Common Plastics, ACS Sustainable Chem. Eng., 2023, 11, 965–978 CrossRef.
- T. Langhorst, B. Winter, D. Roskosch and A. Bardow, Stoichiometry-Based Estimation of Climate Impacts of Emerging Chemical Processes: Method Benchmarking and Recommendations, ACS Sustainable Chem. Eng., 2023, 11, 6600–6609 CrossRef.
- H.-J. Althaus, R. Hischier, A. Primas, M. Osses, N. Jungbluth and M. Chudacoff, Life Cycle Inventories of Chemicals, 2007 Search PubMed.
- Gendorf, Umwelterklaerung 2020, Werk gendorf technical report, 2020 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc02594f |
| ‡ The European Commission's Joint Research Centre (JRC) classifies this impact category as “recommended, but to be applied with caution” as it includes large uncertainties.53 |
| This journal is © The Royal Society of Chemistry 2024 |