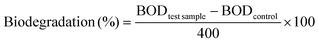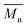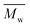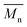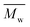Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enzymatic bulk synthesis, characterization, rheology, and biodegradability of biobased 2,5-bis(hydroxymethyl)furan polyesters†
Cornelis
Post
 ab,
Dina
Maniar
ab,
Dina
Maniar
 a,
Jesse A.
Jongstra
c,
Daniele
Parisi
a,
Jesse A.
Jongstra
c,
Daniele
Parisi
 c,
Vincent S. D.
Voet
b,
Rudy
Folkersma
b and
Katja
Loos
c,
Vincent S. D.
Voet
b,
Rudy
Folkersma
b and
Katja
Loos
 *a
*a
aMacromolecular Chemistry & New Polymeric Materials, University of Groningen, Nijenborgh 3, 9747 AG Groningen, the Netherlands. E-mail: k.u.loos@rug.nl
bCircular Plastics, NHL Stenden University of Applied Sciences, Van Schaikweg 94, 7811 KL Emmen, the Netherlands
cDepartment of Chemical Engineering, Engineering and Technology Institute Groningen (ENTEG), University of Groningen, Nijenborgh 4, 9747 AG Groningen, the Netherlands
First published on 29th May 2024
Abstract
The synthesis of biobased polyesters based on 2,5-bis(hydroxymethyl)furan (BHMF) is challenging due to the limited stability of this interesting furanic monomer. In this work, a series of BHMF-based polyesters were produced via a green and efficient bulk polymerization process, by using either an enzyme (iCALB) or a commercially available catalyst (DBTO). The polymerization methods were compared and shown to both be successful, with 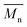 values up to 14
values up to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. The number of methylene units in the aliphatic comonomer was varied from 2 to 8, and the influence of this on the thermal behavior and stability of the polymers was investigated. The degree of crystallinity of compression molded discs was found to be in the range from 13 to 27%, and the contact angles were determined to be in the region from 63 to 73°, confirming that these polyesters are hydrophilic. An oscillatory shear rheology investigation demonstrated significant differences between the melt behavior of the BHMF-based polyesters, ranging from a Newtonian liquid to a shear thinning material with a 3 orders of magnitude higher complex viscosity. A clear inversely proportional correlation between the low-frequency complex viscosity and the number of methylene units in the aliphatic segment was observed. Finally, a biodegradability test revealed that the synthesized BHMF-based polyesters had a biodegradable character over time, wherein different biodegradation rates were observed related to the length of aliphatic segments in the repeating units. The sustainability of both synthesis routes was analyzed based on atom economy (AE), reaction mass efficiency (RME), E-factor and EcoScale. This work emphasizes that renewable BHMF-based polyesters can be produced via a solvent-free and sustainable process, which show biodegradable behavior and that their thermal and rheological properties can be tailored by varying the number of methylene groups in the aliphatic unit.
000 g mol−1. The number of methylene units in the aliphatic comonomer was varied from 2 to 8, and the influence of this on the thermal behavior and stability of the polymers was investigated. The degree of crystallinity of compression molded discs was found to be in the range from 13 to 27%, and the contact angles were determined to be in the region from 63 to 73°, confirming that these polyesters are hydrophilic. An oscillatory shear rheology investigation demonstrated significant differences between the melt behavior of the BHMF-based polyesters, ranging from a Newtonian liquid to a shear thinning material with a 3 orders of magnitude higher complex viscosity. A clear inversely proportional correlation between the low-frequency complex viscosity and the number of methylene units in the aliphatic segment was observed. Finally, a biodegradability test revealed that the synthesized BHMF-based polyesters had a biodegradable character over time, wherein different biodegradation rates were observed related to the length of aliphatic segments in the repeating units. The sustainability of both synthesis routes was analyzed based on atom economy (AE), reaction mass efficiency (RME), E-factor and EcoScale. This work emphasizes that renewable BHMF-based polyesters can be produced via a solvent-free and sustainable process, which show biodegradable behavior and that their thermal and rheological properties can be tailored by varying the number of methylene groups in the aliphatic unit.
Introduction
Polymers and plastics are inseparable from today's society since they are applied in numerous fields: textiles, packaging, construction, fibers, pharmaceuticals, cosmetics, and automotive industries.1,2 The current plastics market is mainly represented by commodity polymers due to their favorable properties and cost-effectiveness, namely, poly(ethylene) (PE), poly(propylene) (PP), poly(vinyl chloride) (PVC), poly(styrene) (PS), and poly(ethylene terephthalate) (PET).3 However, they are usually not biobased or biodegradable and may therefore contribute to a larger extent to greenhouse gas emissions and environmental pollution.4–6Biobased polymers are derived from renewable sources and are therefore more sustainable when greenhouse gas emissions are considered.7 Hence, these green materials are therefore seen as part of the transition to sustainable plastics and are expected to play an important role in the circular plastic economy.2 While biodegradability presents advantages in specific contexts, such as the utilization of poly(butylene succinate) (PBS) within agriculture, biomedical fields, or packaging, its significance often diminishes in many applications.8,9 In most applications, durability and proper recycling of polymers are often preferred.10
However, only a limited amount of the produced polymers are recycled, and they might end up in the environment.4,11 This leads to environmental pollution and the formation of microplastics.12 Therefore, it is essential to find a balance between polymer durability and design for degradation, i.e. biodegradability, which could then be optimized for each application.
Polyesters are a very interesting and sustainable class of polymers due to their good recyclability via either chemical, enzymatic or mechanical routes, which embraces an atom economy.13–15 Polyesters can be fully aliphatic, such as PBS and poly(lactic acid) (PLA), or contain aromatic or furanic units, such as poly(ethylene terephthalate) (PET), poly(ethylene furanoate) (PEF) and poly(butylene adipate terephthalate) (PBAT). Although linear aliphatic polyesters are more prone to biodegradation, their thermal and mechanical properties are often limited.16–18 This issue can be addressed by introducing aromatic units into the backbone to provide chain rigidity and improve thermal and mechanical properties.17,19 For certain (partly) aromatic polyesters, biodegradability could even be retained, for example, in the case of PBAT.20
A promising sustainable rigid furanic molecule is 2,5-bis(hydroxymethyl)furan (BHMF), which is structurally similar to furandicarboxylic acid (FDCA), which is used for the synthesis of PEF.21,22 BHMF is a biobased building block that can be obtained in high yield from renewable resources such as fructose and hydroxymethylfurfural (HMF).23–27 Furthermore, BHMF can act as a monomer, and can be used for the synthesis of different types of aliphatic-aromatic polymers.28 On the other hand, aliphatic comonomers, including succinic acid, glutaric acid and adipic acid, can be obtained from natural resources such as glucose.29–31 This implies that fully biobased BHMF-based polyesters can be produced via (co)polymerization with these biobased aliphatic comonomers.
The synthesis of BHMF-based polymers by either enzymatic or organo-catalyzed solution polymerization has been previously reported. For example, both Jiang et al. (2014) and Pellis et al. (2020) used the immobilized enzyme Candida antarctica lipase B (iCALB) as a biocatalyst in a three- or two -stage process to polymerize BHMF with various aliphatic comonomers.32,33 Although enzymatic solution polymerization was proven to be successful, only limited molecular weights were achieved (2100–3100 g mol−1). Slightly higher molecular weights, up to 7200 g mol−1, were reached by the group of Yoshie, who used three equivalents of both N,N-dimethyl-4-aminopyridine (DMAP) and N,N′-diisopropylcarbodiimide (DIC) to polymerize BHMF with similar aliphatic comonomers.34,35 In addition, copolymerization of BHMF-based polyesters was also reported by incorporating FDCA or lactide units, using iCALB or the combination of DMAP and DIC as catalyst.36,37
Polymerization using iCALB aligns well with the green chemistry guidelines, since this biocatalyst is very efficient, works at relative low temperatures, is able to be separated and avoids the use of toxic chemicals.38,39 However, the reported polymerizations were, carried out in solution, which is non-sustainable and less attractive for future commercialization. Bulk polymerization stands out as the industry's favored method due to its solvent-free nature and the elimination of needed separation and purification steps. In addition, the solvent-free conditions are more sustainable and less sustainable hazardous according to the 12 principles of Green Chemistry. However, implementing this technique for BHMF polymerization presents challenges due to the limited thermal and chemical stability of this furanic building block.28
In this work, to the best of the authors’ knowledge, BHMF was polymerized for the first time in bulk by using either the enzyme iCALB or the commercially available catalyst dibutyltin(IV) oxide (DBTO). The use of DBTO as a catalyst for the synthesis of polyesters has been previously reported, for example, in the synthesis of PEF and sorbitol-based polyesters.40,41 Various aliphatic comonomers were used to alter the length of the aliphatic segment in the repeating unit. Both catalyzed synthesis routes are straight forward and resulted in high yield polymers, i.e. minimizing waste. The effect of an increased molecular weight on the thermal properties will be investigated. In addition, the macromolecular properties, including the rheology and water contact angle, are analyzed for the first time. Finally, a biodegradability test is performed to demonstrate that these furanic polyesters are more environmentally friendly than nonbiodegradable polyesters.
Experimental
Materials
Lipase acrylic resin (Candida antartica lipase B (iCALB), 5000 U g−1, recombinant, expressed in Aspergillus niger), dimethyl succinate (DMSuc, 98%), dimethyl glutarate (DMGlu, 99%), dimethyl adipate (DMAd, >99%), dimethyl pimelate (DMPim, 99%), dimethyl suberate (DMSub, 99%), chloroform (amylene stabilized, HPLC grade, >99.8%), and dibutyltin(IV) oxide (DBTO, 98%) were purchased from Sigma-Aldrich. Dimethyl azelate (DMAze, >98%) and dimethyl sebacate (DMSeb, >98%) were purchased from TCI EUROPE. 2,5-Bis(hydroxymethyl)furan (BHMF, >97%) was purchased from Apollo Scientific. Chloroform (HPLC grade) was supplied by macron fine chemicals and diethyl ether was obtained from Honeywell Research Chemicals. All chemicals were used as received. The activated sludge was kindly supplied by the wastewater treatment facility in Glimmen, The Netherlands.Synthesis of BHMF-based polyesters
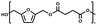
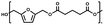
The setup consisted of a 25 ml three-necked round bottom flask equipped with a magnetic stirrer and fitted to a distillation line. The system was connected to a Schlenk line, to switch between vacuum and argon flow. As an example, the enzymatic synthesis of poly(2,5-furandimethylene succinate) is given; 2.00 g BHMF (1.0 eq.), 2.30 g DMSuc (1.01 eq.) and 10% w/w iCALB were added to the flask. The reaction mixture was maintained under a mild argon flow, magnetically stirred in an oil bath and heated to 70 °C for 26 h. Subsequently, the argon flow was closed, and the system was gently switched to vacuum (2 × 10−2 mbar), while the temperature was kept at 70 °C for another 21 h. Then, the temperature was set to 100 °C and thereafter slowly stepwise increased in steps of 10 °C to a final temperature of 130 °C. The total reaction time was 100 h.The synthesis process of the metal-catalyzed synthesis of BHMF-based polyesters was similar to the enzymatic synthesis process. The same comonomers were used in identical molar ratios and masses; however, DBTO was used here as a metal-based catalyst (5% w/w). The difference is the reaction time, where the iCALB experiments were run for 100 h and reached 130 °C, and the DBTO experiments were stopped after 75 h at a final temperature of 120 °C. This was done to avoid crosslinking/degradation of the polymer mixture.
The obtained polyesters were dissolved in chloroform, separated from the immobilized enzyme particles using a thin needle and syringe, and subsequently precipitated in diethyl ether. In the DBTO synthesis route, the polyesters were dissolved in chloroform and directly precipitated in diethyl ether. The polymers were collected via centrifugation (4500 rpm, 10 min, 1 °C) and dried in a fume hood. The experimental procedures for the synthesis of the other BHMF-based polyesters were identical. Only the masses of the aliphatic dimethyl esters and iCALB varied to maintain a constant stoichiometry (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
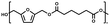
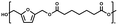
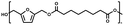
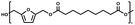
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.01) for BHMF and the comonomer and constant catalyst concentration (10 and 5% w/w for iCALB and DBTO, respectively). The general synthetic route is shown in Fig. 1.
1.01) for BHMF and the comonomer and constant catalyst concentration (10 and 5% w/w for iCALB and DBTO, respectively). The general synthetic route is shown in Fig. 1.
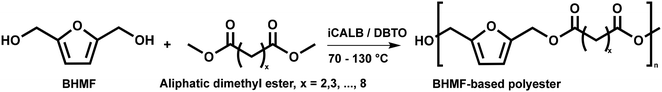 | ||
| Fig. 1 Bulk polymerization of BHMF and an aliphatic dimethyl ester using either CALB or DBTO as catalysts. | ||
Characterization
Proton nuclear magnetic resonance (1H-NMR) measurements were recorded on a 400 MHz Varian VXR spectrometer using DMSO-D6 as the solvent.The molecular weights of the synthesized polyesters were determined via gel permeation chromatography (GPC). The device, a Malvern Viscotek GPCmax, was fitted with triple detection: a Malvern Dual detector and a Schambeck RI2912 refractive index detector. The separation was performed by utilizing two PL gel 5 mm MIXED-C 300 mm columns from Agilent Technologies at 35 °C. Amylene-stabilized HPLC grade chloroform was used as the eluent at a flow rate of 0.5 mL min−1. Molecular weight calculations were based on a universal calibration curve from narrow dispersity polystyrene standards (Agilent and Polymer Laboratories, 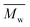 ranging from 625 to 3
ranging from 625 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001
001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and performed using the software Viscotek OmniSec (version 5.0.). Concentrations of 2 μg ml−1 were prepared and filtered over a 0.20 μm PTFE filter prior to measurement.
000 g mol−1) and performed using the software Viscotek OmniSec (version 5.0.). Concentrations of 2 μg ml−1 were prepared and filtered over a 0.20 μm PTFE filter prior to measurement.
The thermal stability of the polyesters was analyzed via thermogravimetric analysis (TGA), which was conducted on a TA-Instruments Discovery TGA 5500. The samples were heated from room temperature to 700 °C at a constant rate of 10 °C min−1 under an inert atmosphere (N2).
Differential scanning calorimetry (DSC) analysis was carried out to determine the glass transition temperatures (Tg) and melting points (Tm). The measurements were performed on a TA-Instruments Q1000 DSC using a heating and cooling rate of 10 °C min−1, under a mild nitrogen flow, for a temperature range of −70 to 190 °C and a 5 mg sample size.
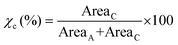
The relative crystallinities of the enzymatically produced BHMF-based polyesters were obtained via wide-angle X-ray diffraction (WAXD) analysis of compression molded discs (25 mm diameter, 1.5 mm thickness). The discs were pressed at a Fontijne LabManual 300 hot press for approximately 10 min at 50 kN and allowed to slowly cool down in the press without pressure applied. The samples were stored at room temperature for at least 4 days prior to measurement. The measurements were repeated after 6 to 8 weeks. The WAXD measurements were performed on a Bruker D8 Advance diffractometer (CuKα radiation, λ = 0.1541 nm, 40 kV, 40 mA) in the angular range (2θ) of 4 to 51 at ambient conditions. The obtained WAXD patterns were analyzed with the software Origin 2018, wherein the crystalline and amorphous peaks were separated via peak deconvolution. The degree of crystallinity (χc) was calculated from the following formula (eqn (1)) below, where Areac and Areaa are the sum of the integrated crystalline and amorphous peaks, respectively.
 | (1) |
The same compression molded discs were used for water contact angle measurements. These experiments were performed on a Dataphysics OCA 15EC device, and the software SCA20 was used to determine the left, right and mean water contact angles. A water droplet of 5.00 μL was dispensed on the surface at a rate of 5.00 μL s−1. The droplet was directly photographed, and each polymer sample was measured in triplicate.
The compressed polymer discs were finally used for the rheological analysis. The experiments were performed on an HR-2 rheometer (TA Instruments, New Castle, United States) equipped with a plate-plate geometry with a diameter of 25 mm and constant gaps between 0.9 and 1.2 mm. The temperature was controlled via a convection oven and set constant at 30 °C degrees above the melting point of each polyester. An inert atmosphere was ensured by using a mild nitrogen flow in the convection oven. A dynamic strain sweep experiment was performed at a constant oscillation frequency (100 rad s−1) while the strain was increased from 0.1 to 20% to determine the linear viscoelastic regime. Subsequently, a dynamic frequency sweep was conducted at a strain of 1%, well within the linear viscoelastic regime, in the frequency range 0.01–100 rad s−1.
The biodegradability of the enzymatically produced BHMF-based polyesters was analyzed using a Lovibond® Water testing BOD-System BD 600 setup. The procedure was performed according to OECD 301F manometric respirometry test guidelines.42,43 The principle of the test is based on the biological oxygen demand (BOD), which is the amount of oxygen that is consumed by the microorganisms from the activated sludge to degrade the test sample. The produced CO2 was absorbed in a KOH solution, which was placed inside a rubber seal cup. The decrease in pressure is converted into a BOD value (mg L−1) and is defined in eqn (2):44
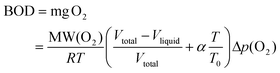 | (2) |
The experiments are executed for a BOD range from approximately 0 to 400 mg L−1 and a total liquid volume of 157 mL. The theoretical oxygen demand (ThOD) is the amount of oxygen that is needed to oxidize a compound completely and is based on the molecular weight of the repeating unit of the polyester. The ThOD is expressed as mg of oxygen which is needed per mg of test sample. The ThOD for the model compound CcHhClclNnNanaOoPpSs is defined as:42
 | (3) |
The amount of test sample was derived from the ThOD value to reach the maximum BOD value (400 mg L−1).
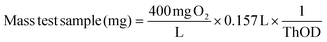 | (4) |
The percentage of biodegradation was calculated based on the BOD value of the test sample and corrected for the BOD value of the control experiment (without test sample), expressed as a percentage of the theoretical maximum BOD.
The activated sludge was stored in a refrigerator (6 °C) and aerated for 3 days prior to use. One liter of test medium consisted of 25 mL of activated sludge, stock solutions A, B, C and D (each 1 mL) and 975 mL of distilled water. Stock solution A (1 L) consisted of 8.50 g KH2PO4, 21.75 g K2HPO4, 33.30 g Na2HPO4·2H2O and 0.50 g NH4Cl dissolved in distilled water. Stock solution B (1 L) was prepared by dissolving 27.50 g CaCl in distilled water. Similarly, stock solution C (1 L) consisted of 22.50 g MgSO4·7H2O in distilled water. Finally, stock solution D (1 L) was prepared by dissolving 0.25 g FeCl3·6H2O and 1 drop of concentrated HCl in distilled water. Each test bottle was equipped with a magnetic stirring bar and 5 drops of nitrification inhibitor. The setup was placed in a dark environment in a temperature-controlled room (21 °C), and the experiments were performed for 28 days. Each polymer was tested in duplicate, while three control experiments were performed. The averaged values of the samples were used for the calculation of the percentage of biodegradability. The two most similar results of the control experiments, showing the highest BOD values during the test, were considered the most reliable and used for the average control values in the calculations. In addition, two sodium acetate test samples were used as a reference substance to validate the biological activity of the activated sludge.
Results and discussion
Bulk polymerization
A series of polyesters were successfully synthesized from BHMF and aliphatic dimethyl esters, in which the length of the methylene units varied from 2 to 8 (Fig. 1). Although the use of these diacid derivatives violates with the principles of green chemistry, it was necessary to use dimethyl esters since BHMF appeared to degrade under acidic conditions. However, the methanol which is produced during the reaction could be recycled and used again for esterification of the diacids.The enzymatic bulk polymerization consisted of a two-stage process: oligomerization under an argon flow and polyesterification under high vacuum conditions. Temperature control was the important key aspect due to BHMF's limited thermal stability and to balance the catalytic activity and degradation of iCALB. The optimized process of enzymatic polymerization was also applied for the metal-catalyzed polycondensation of the same series of BHMF-based polyesters; however, it was 25 h shorter in time and had a maximum temperature of 120 °C. This adaptation was necessary to prevent degradation/crosslinking of the reaction mixture at elevated temperature and extended reaction time. It is expected that this phenomenon is caused by side reactions introduced by the catalyst (DBTO) with BHMF or impurities, or due to premature degradation of BHMF, since the DBTO catalyzed reactions turned brown and DBTO was found to be less active compared to iCALB at relatively low temperature conditions (70 °C) during the first part of the synthesis. Nevertheless, DBTO is cost-effective and was found to be the most active catalyst out of a range of tested commercially available catalysts (Ti, Zn, Sb, Sn and organic-based catalysts) for the polymerization of BHMF with aliphatic dimethyl esters under the given conditions.
The successful synthesis of all polyesters was confirmed by 1H-NMR. The spectra of the iCALB catalyzed BHMF-dimethyl suberate polyester (PFSub) are presented in Fig. 2a and b as an example. The formation of the ester bonds was clearly visualized by the disappearance of the hydroxyl end groups of BHMF (“1”, 5.14–5.23 ppm), the appearance of the methylene protons next to the ester bonds (“2 + 4”, 5.00 ppm) and the decrease in intensity of both the methoxy groups of the dimethyl ester (“8”, 3.55–3.58 ppm) and methylene protons next to the hydroxyl end groups of the furanic unit (“2′”, 4.33–4.37 ppm).
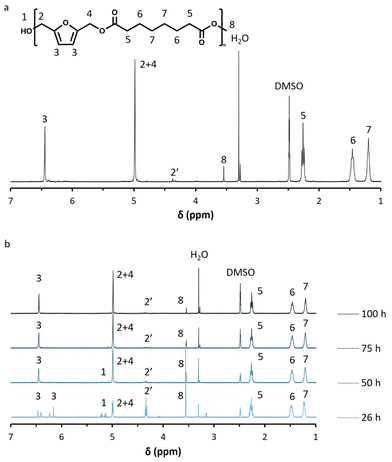 | ||
| Fig. 2 (a) Normalized H-NMR spectrum of poly(2,5-furandimethylene suberate) (PFSub) in DMSO-d6, (b) H-NMR spectra of PFSub during iCALB-catalyzed polycondensations at different times. | ||
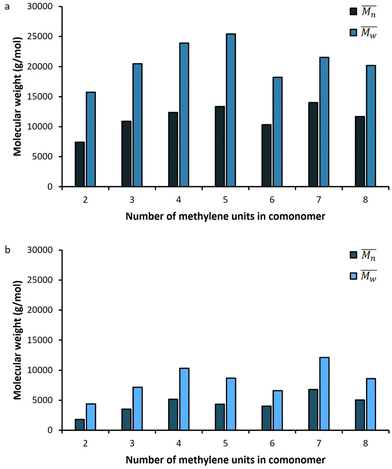
The molar masses of all obtained polyesters were determined by GPC analysis and were relative to polystyrene (PS) standards. The number average molecular weight (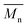 ) and the weight average molecular weight (
) and the weight average molecular weight (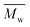 ) of both the iCALB and DBTO series are illustrated in Fig. 3. The average
) of both the iCALB and DBTO series are illustrated in Fig. 3. The average 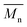 of all iCALB-synthesized polyesters was determined to be 11
of all iCALB-synthesized polyesters was determined to be 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1, while the average
400 g mol−1, while the average 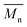 of the DBTO series reached 4400 g mol−1. The obtained molecular weights in both series are significantly higher than the enzymatic solution polymerization reported in the literature (
of the DBTO series reached 4400 g mol−1. The obtained molecular weights in both series are significantly higher than the enzymatic solution polymerization reported in the literature (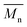 = 2100–3100 g mol−1).32,33 The higher molecular weights are assigned to the benefits of bulk polymerization compared to solution polymerization. In the absence of a solvent, a significantly higher vacuum can be applied, and higher temperatures can be reached without running the risk of evaporating the solvent.
= 2100–3100 g mol−1).32,33 The higher molecular weights are assigned to the benefits of bulk polymerization compared to solution polymerization. In the absence of a solvent, a significantly higher vacuum can be applied, and higher temperatures can be reached without running the risk of evaporating the solvent.
The 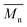 averages of both series also indicate that the enzymatic pathways yield higher molecular weights than the metal-catalyzed process. However, the DBTO experiments were only performed for a shorter period of time compared to the iCALB experiments, 75 h and 100 h, respectively. Based on the 1H-NMR samples taken to monitor the progress of the polymerization, it was observed that iCALB is more active than DBTO at the initial stage. The methylene protons next to the ester bonds (“4”) were clearly higher after 26 h for the iCALB-synthesized polyesters compared to the DBTO-catalyzed polyesters (ESI†). Nevertheless, this phenomenon is expected to be reversed at higher temperatures. Although iCALB is used for aliphatic-furanic solution polycondensations up to 140 °C, it is also known that protein denaturation and deactivation already occur at temperatures of approximately 90 to 100 °C,45 while DBTO polycondensations are usually performed at temperatures of approximately 140 °C.41,46
averages of both series also indicate that the enzymatic pathways yield higher molecular weights than the metal-catalyzed process. However, the DBTO experiments were only performed for a shorter period of time compared to the iCALB experiments, 75 h and 100 h, respectively. Based on the 1H-NMR samples taken to monitor the progress of the polymerization, it was observed that iCALB is more active than DBTO at the initial stage. The methylene protons next to the ester bonds (“4”) were clearly higher after 26 h for the iCALB-synthesized polyesters compared to the DBTO-catalyzed polyesters (ESI†). Nevertheless, this phenomenon is expected to be reversed at higher temperatures. Although iCALB is used for aliphatic-furanic solution polycondensations up to 140 °C, it is also known that protein denaturation and deactivation already occur at temperatures of approximately 90 to 100 °C,45 while DBTO polycondensations are usually performed at temperatures of approximately 140 °C.41,46
In more detail, Fig. 3a and Table 1 reveal that generally a higher number of methylene units in the comonomer resulted in an increased molecular weight and degree of polymerization (DP), and an optimum was found for PFPim, which contains 5 methylene units in the aliphatic segment. This phenomenon has also been observed before in the enzymatic polymerization of aliphatic furanic polyesters.36,45 It was reported that iCALB prefers adipic acid over succinic acid, glutaric acid and pimelic acid.47 This slightly deviates from the results reported here, but it could be that the preference of iCALB is different in the case of the dimethyl esters of these acids.
Similar results are obtained for the DBTO-catalyzed polyesters, where PFSuc and PFGlu clearly have the lowest molecular weight and degree of polymerization. This might indicate that the reactivity of the aliphatic comonomer or the behavior of the polymer (i.e., flexibility, viscosity and thermal properties) could also have an influence. Furthermore, the catalytic reaction mechanism of DBTO is proposed to proceed via Sn coordination of the ester group or an exchange/insertion pathway of the diol.48 Hence, since the mechanism is not fully understood, the effect of the size of the dimethyl ester cannot be contested.
Enzymatic polymerization of a series of furanic-aliphatic polyesters in typically only performed with the comonomers containing “even” number of methylene units, and is studied in detail.32,33,36,39 In this work, it was found that no significant difference is observed in the degree of polymerization between “odd” and “even” aliphatic dimethyl esters during bulk polymerization with BHMF, according to the data in Table 1.
The average polydispersity index (Đ) of the iCALB-catalyzed polyesters is a bit lower compared to the average Đ of the DBTO series, 1.8 and 1.9, respectively. Both values are lower than 2.0 which is the expected value from the Flory–Schulz distribution for ideal step-growth polymerization. In addition, the PDI was higher for PFSuc in both series, which might indicate that some side reactions, such as branching, have occurred or lower molecular weight chains were lost during purification.
Only the iCALB synthesized polyesters are discussed in the next paragraphs. These polyesters have higher molecular weights and the catalyst particles (enzyme beads) are removed; therefore, they should provide more accurate results for subsequent examination.
Thermal analysis and crystallization
The thermal stability of BHMF was one of the main limiting factors during the optimization of the polymerization process. The thermal stability of each polyester was estimated by TGA analysis and quantified by determining the Td5% and Td50% values; the temperature at which 5 and 50% weight loss is reached, respectively. As shown in Fig. 4, the thermal stabilities of the BHMF-based polyesters are significantly higher than that of the BHMF monomer. The thermal degradation of BHMF already initiates at approximately 104 °C (Td5%). However, this temperature is still well above a safe temperature to start bulk polymerization, which was experimentally determined at approximately 70 °C to 90 °C, depending on the reaction rate and catalyst concentration. The thermal stability of the BHMF-based polymers increased significantly up to values above 250 °C (Td5%). This indicates that the conversion of the hydroxyl groups to ester bonds is crucial to improve the thermal stability of the furan units. Another interesting observation is the shift in decomposition temperature; BHMF-based polymers containing longer aliphatic segments possess a higher thermal stability. This could be correlated with a relatively lower concentration of reactive groups, i.e., ester and furan groups.32 Furthermore, all polyesters showed a single thermal degradation step, except for PFSub, PFAze and PFSeb, which had a second minor degradation step.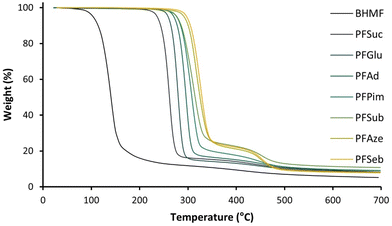 | ||
| Fig. 4 Thermogravimetric analysis of BHMF and enzymatically synthesized BHMF-based polyesters containing different numbers of methylene units in the comonomer segment. | ||
The thermophysical behavior of the series of polyesters was analyzed by DSC, and the Tg and Tm values are plotted in Fig. 5. The highest Tg was found for PFSuc (2 methylene units), which decreased quadratically with an increasing number of methylene units in the comonomer to reach a minimum Tg value for PFAze (7 methylene units) and subsequently increased again for PFSeb (8 methylene units). A similar quadratic behavior was reported in the work of Jiang et al.; however, their Tg values were lower and reached a minimum for PFSub (6 methylene units).32 Although the polyesters were structurally identical, their molecular weights were significantly lower, which is expected to be the cause for their lower Tg values. Similar results were obtained for the DBTO-synthesized polyesters with a minimum Tg observed for PFSeb (Table 2) due to the rendered lower molecular weights compared to the iCALB-synthesized polyesters.
 | ||
| Fig. 5 Glass transition temperature (Tg) and melting point (Tm) of enzymatically produced BHMF-based polyesters containing different numbers of methylene units in the comonomer segment. | ||
The Tg is a second-order like transition and corresponds to the amorphous region of the polyesters. In addition, it is related to both chain mobility and flexibility.49 The decrease in Tg from 16.3 °C to −31.9 °C for 2 to 7 methylene units in the aliphatic unit could be explained by the relative decrease in furan content, which results in fewer restrictions and therefore more chain flexibility. Furthermore, the increase in Tg values from −31.9 °C to −25.9 °C (for 7 to 8 methylene units) is expected to be a consequence of a higher degree of crystallinity of the polymer powders, as observed by Jiang et al.32 A higher degree of crystallinity is a consequence of more favorable interactions between the chains, resulting in less chain mobility. In summary, the quadratic behavior of the Tg value is due to a balance between increasing chain flexibility, influenced by the relative furan content, and decreasing chain mobility.
Similar to the Tg value, the Tm values of the enzymatically produced polyesters were influenced by the number of methylene units in the aliphatic segment. The highest Tm was observed for PFSuc, namely, 97.9 °C, while the lowest Tm was observed for PFGlu (62.9 °C) which has only one CH2 group extra in the repeating unit. Subsequently, the Tm increased gradually with an increasing number of methylene units (Fig. 5). This is in contrast to FDCA-based polyesters, where the melting points decrease as the carbon number of the diol increases.50 As the number of methylene units increases, the relative furan content decreases, and the polymer structure becomes increasingly similar to polyethylene (PE). This could justify that the melting points of linear furanic-aliphatic polyesters, whether based on BHMF or FDCA, approach the melting point of PE (125–135 °C) as the number of methylene groups in the repeating unit increases.51
T m is a first-order transition and is a property of the crystalline region, meaning that all produced BHMF-based polyester products are semicrystalline. The increase in Tm was explained by a higher chain packing capability for the longer aliphatic segments.32 The relatively high melting point of PFSuc is an exception compared to the other BHMF-based polyesters and could be caused by favorable pi-pi interactions between the furan units, since the Tm generally increases by introducing aromatic groups. For example, the melting point of poly(butylene adipate) (PBA) is approximately 58 °C, while poly(butylene-adipate-co-terephthalate) (PBAT) melts at approximately 115 °C to 125 °C.19,52
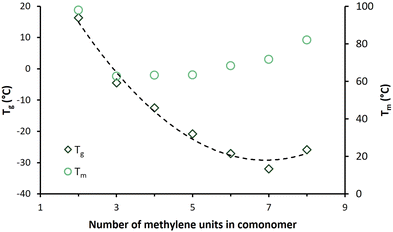
The melting endotherms of the semicrystalline BHMF-based polyester powders are clearly visible in Fig. 6a. PFGlu, PFAd and PFPim demonstrated one broad melting point, while two or more overlapping melting endotherms were observed for the other polyesters. The glass transition was difficult to detect in the first heating curve, but easily derived from the second heating curve, as shown in Fig. 6b.
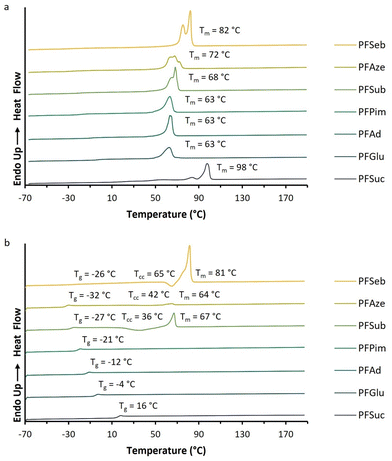 | ||
| Fig. 6 DSC curves of the enzymatically produced BHMF-based polyesters containing different numbers of methylene units in the comonomer segment: (a) first heating curves and (b) second heating curves. | ||
Fig. 6b demonstrates that only PFSub and PFSeb showed a clear melting endotherm in the second heating curve, while for PFAze, only a relatively small melting endotherm appeared. In addition, these three polyesters showed a cold crystallization point (Tcc) in their second heating curve, and only PFSeb showed a crystallization exotherm in the cooling curve. This visualizes the significant differences in crystallization kinetics between the BHMF-based polyesters. The same phenomenon was already observed for the low molecular weight, but similar polyesters of Jiang et al.32
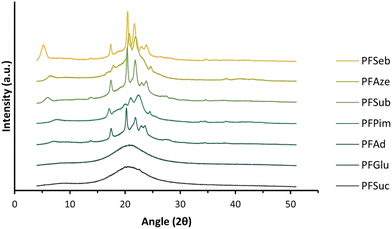
The WAXD patterns, obtained after a short waiting time (at least 4 days) after processing, of the enzymatically produced polyester series are shown in Fig. 7. The WAXD curves of the compression-molded PFSuc and PFGlu discs lack sharp crystalline peaks and are therefore completely amorphous. On the other hand, PFAd, PFPim, PFSub, PFAze and PFSeb all clearly show both crystalline and amorphous areas, meaning that these processed polyesters are semicrystalline. The patterns of PFSuc and PFGlu provided insight into the amorphous curvatures and were used to distinguish between the amorphous and crystalline regions of the other polyesters via peak deconvolution. This is used to calculate the degree of crystallinity for the compression molded polymer discs, as depicted in Fig. 8. It is clearly shown that after a short waiting time (see Experimental section), there is a threshold for the amorphous polyesters and semicrystalline polyesters, which all had a degree of crystallinity in the range of 15.6 to 25.3%.
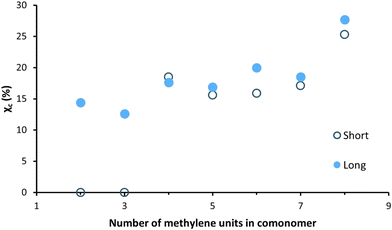 | ||
| Fig. 8 Degree of crystallinity versus the number of methylene units in the comonomer of the enzymatically produced BHMF-based polyester discs after a short and long waiting period after processing. | ||
These WAXD results are not in accordance with the DSC results (Fig. 6a and b), where it was shown that all unprocessed polyester products were semicrystalline. In addition, Jiang et al. made a similar BHMF-based polyester series and determined the degree of crystallinity of the unprocessed polymer powders.32 They found that all BHMF-based polyester powders, with either 2, 3, 4, 6, 8 or 10 methylene units in the comonomer, were semicrystalline, and the degree of crystallinity increased gradually from 34 to 65%. Furthermore, the DSC curves implied that the polyesters crystallized at varied rates. As a result, it is envisaged that all polyester discs would eventually be semicrystalline; however, crystallization is relatively slow.
To support this, the same polymer discs were subjected to WAXD analysis again after a longer waiting period, i.e., 6 to 8 weeks, to verify whether the degree of crystallinity changed over time. The polymers PFSuc and PFGlu also turned into semicrystalline polymers after a longer period of time, while the other polyesters maintained a constant value.
The polymers containing an even number of methylene units in the repeating unit have a slightly higher degree of crystallinity relative to the general increasing trend. This could be attributed to the fact that the melting points of even linear saturated aliphatic dicarboxylic acids are generally higher than those of odd carbon diacids, which is related to the in-plane orientation of the carbonyl groups.53 This might lead to more favorable interactions between the chains and therefore a higher degree of crystallinity.
Either way, these WAXD results provide a proper indication of the crystallization behavior of these BHMF-based polyesters after processing. The crystallization behavior of the processed polymers will be studied in more detail in the future.
Hydrophilicity
Fig. 9 depicts the mean and standard deviations of the measured water contact angles in triplicate of the enzymatically synthesized BHMF-based polyesters. All water contact angles are clearly below 90°, meaning that the polyester discs represent a hydrophilic surface. In addition, the values are more or less in the same range, from approximately 63 to 73°. The water contact angles of several FDCA-based furanic-aliphatic polyesters were obtained from the literature and added to Fig. 9.54 The water contact angle of PEF is found to be very similar to PFSuc. Furthermore, the other FDCA-based polyesters, containing a higher number of methylene groups in the diol, demonstrate a clearly higher water contact angle but are still hydrophilic.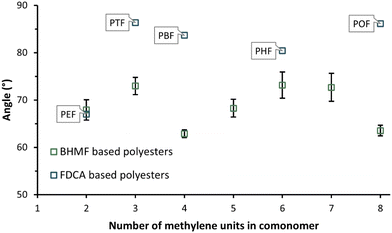 | ||
| Fig. 9 Average water contact angle of the enzymatically produced BHMF-based polyester discs (this work) and FDCA-based polyesters: poly(ethylene 2,5-furandicarboxylate) (PEF), poly(trimethylene 2,5-furandicarboxylate) (PTF), poly(butylene 2,5-furandicarboxylate) (PBF), poly(hexylene 2,5-furandicarboxylate) (PHF), and poly(octylene 2,5-furandicarboxylate) (POF), obtained from.54 | ||
Rheology
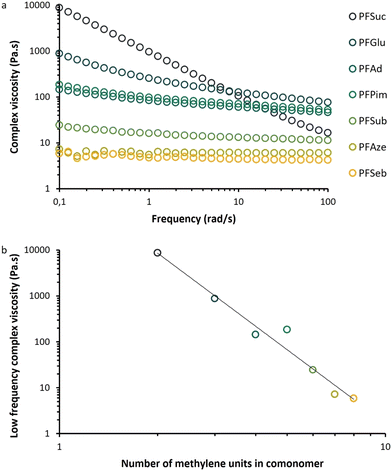
Fig. 10a illustrates the melt behavior of the BHMF-based polyesters at various frequencies. Polymers PFSeb, PFAze and PFSub behaved like low viscous Newtonian fluids as the complex viscosity was basically frequency independent. PFPim, PFAd, PFGlu and PFSuc all exhibited a non-Newtonian effect as the viscosity decreased for higher frequencies, although this shear thinning effect was more apparent for PFSuc and PFGlu. In addition, not only did the behavior change, but also the value of the complex viscosity at low frequency also increased by 3 orders of magnitude from PFSeb to PFSuc.This phenomenon is depicted in Fig. 10b, where a strong inversely proportional correlation was observed between the complex viscosity and the number of methylene units in the comonomer. It is expected that a longer aliphatic part in the repeating unit results in more flexibility and therefore less resistance to flow, i.e., a lower viscosity. This indicates that the structural composition of the BHMF-based polymers has a significant influence on the behavior in the melt phase.
An additional note has to be made about the rheological behavior of PFSuc. The initial measurements, according to the experimental procedure, lead to significantly lower complex viscosity values and a minor shear thinning effect during the dynamic frequency sweep. However, when this polymer was first subjected to a dynamic time sweep (100 rad s−1, 1% strain, 4 h), a different behavior was observed during the subsequently performed dynamic frequency sweep and a major increase in low-frequency complex viscosity, as presented in Fig. 10a. This behavior was observed only for PFSuc and is expected to be caused by the introduction of π–π interactions in the melt phase over time. This is in line with previous findings about the relatively high melting point of PFSuc compared to the other BHMF-based polyesters. It would be highly desirable to explore the field of crystallization and structuring in the melt phase of BHMF polyesters in more detail.
Biodegradation
The biodegradability of polymers is dependent on multiple different parameters, including thermal properties, polymer structure, degree of crystallinity and hydrophilicity.28,55,56 In the previous sections, it was shown that the polymeric properties can be tailored by varying the length of the aliphatic comonomer in BHMF-based polyesters. Furthermore, the hydrophilic character of the polyester surfaces is expected to be beneficial for biodegradation.57The averages of the obtained BOD values of each polyester are plotted versus time in Fig. 11a. First, the BOD values of all polyesters are higher than those of the control experiments, and the curves are similar in shape. The BOD curves versus time were expected to show an increasing behavior, with a diminishing increase over time. However, after 13 and 27 days, a peak in BOD is observed for all samples. This could be explained by a difference in surrounding temperature, which led to a difference in pressure and subsequently to a deviating BOD value. To correct this, and the fact that the activated sludge itself consumes oxygen as well, the percentage of biodegradability over time was determined with respect to the control (Fig. 11b). PFAze, PFSub, PFSeb and PFAd show a percentage of biodegradability between approximately 20 and 40% after 28 days. The polymers PFGlu, PFSub and PFPim show only values between 3 and 12% biodegradability after 28 days. Generally, BHMF-based polyesters containing a higher number of methylene units in the repeating unit show higher biodegradability activity. These polyesters also showed lower Tg values, but strong correlations between the biodegradability and polymeric properties, such as the hydrophilicity, degree of crystallinity and thermal transition points, cannot be drawn from these data. Nevertheless, it is clearly demonstrated that BHMF-based polyesters show a biodegradable character over time, based on OECD 301 F test guidelines. In addition, where other furanic polyesters, such as PEF, are considered nonbiodegradable, BHMF-based polyesters open a new window of applications for these furanic polyesters, which show different rates of biodegradability.18 The biodegradable behavior aligns well with the principles of green chemistry to design safer chemicals and to control hazard and risk at the end of life of a polymer product.
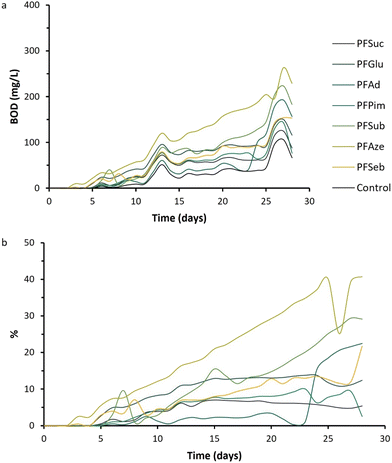 | ||
| Fig. 11 (a) BOD values versus time of the enzymatically produced BHMF-based polyesters and the control experiment. (b) Percentage of biodegradability versus time. | ||
Green characteristic analysis
The sustainability of the bulk polymerization synthesis routes was assessed based on atom economy (AE), reaction mass efficiency (RME), E-factor and EcoScale, which are given in Table 3. The AE is correlated to the remaining atoms in the product, and is expressed as the ratio between the molecular weight of the desired products and the reactants. The AE ranges from 0.77 to 0.82 for the BHMF-based polyesters due to formation of methanol as a condensation product. The RME is similar to AE, but assesses the experimental efficiency since it contains the yield, whereas the AE delivers the reaction's theoretical maximum efficiency.58 Hence, the RME values are lower than the AE numbers, but are similar for the iCALB- and DBTO-synthesized polyesters due to the similarities in yield. The E-factor is more comprehensive and takes waste production into account, and is defined as the mass ratio of the waste to the desired products. The E-factors are very high since solvents are used to dissolve and precipitate the polymers. In the event of a scale-up, the solvents would be recycled or the polymers may be simply collected straight from the reactor, eliminating the need for isolation and purification steps and thereby lowering the E-factor considerably in both situations. The EcoScale, which assesses the yield, cost, safety, setup, temperature and duration of the reaction, workup and purification, is the most useful tool for evaluating the sustainability of these polymerization reactions.59 The EcoScale ranges from 0 to 100 (ideal reaction), by applying penalty points based on given criteria. The calculated EcoScale values of the iCALB synthesized polymers were in the top of the range from 50 to 75, which is classified as “acceptable”. The moderate yields (59–77%) and the safety penalty points for methanol are the main limiting factors. Additionally, the polyesters catalyzed by DBTO are also rated as “acceptable”, however their ranking is lower because DBTO has additional safety penalty points.| Polymer | Yield (%) | AE | RME | E-factor | EcoScale |
|---|---|---|---|---|---|
| (a) | |||||
| PFSuc | 77 | 0.77 | 0.59 | 131 | 71 |
| PFGlu | 59 | 0.78 | 0.45 | 161 | 62 |
| PFAd | 79 | 0.79 | 0.62 | 112 | 73 |
| PFPim | 65 | 0.80 | 0.51 | 130 | 65 |
| PFSub | 66 | 0.81 | 0.53 | 121 | 66 |
| PFAze | 67 | 0.81 | 0.54 | 113 | 66 |
| PFSeb | 74 | 0.82 | 0.60 | 98 | 70 |
| (b) | |||||
| PFSuc | 76 | 0.77 | 0.58 | 133 | 61 |
| PFGlu | 60 | 0.78 | 0.46 | 158 | 53 |
| PFAd | 74 | 0.79 | 0.58 | 120 | 60 |
| PFPim | 76 | 0.80 | 0.61 | 110 | 61 |
| PFSub | 72 | 0.81 | 0.58 | 110 | 59 |
| PFAze | 57 | 0.81 | 0.46 | 132 | 52 |
| PFSeb | 76 | 0.82 | 0.62 | 95 | 61 |
These sustainability metrics demonstrate that, when it comes to catalyzing the synthesis of BHMF-based polymers, iCALB is more environmentally friendly than DBTO. In addition, when the process would be scaled-up and further optimized, it is expected that higher sustainability scores would be attained due to the absence of purification steps and higher yields.
Conclusion
In this study, BHMF-based polyesters were synthesized at relatively high molecular weights via an efficient and sustainable bulk polymerization process for the first time. Both the enzyme iCALB and the commercially available catalyst DBTO are shown to be active at low temperatures, which is needed to prevent premature degradation of BHMF. The solvent-free conditions, use of renewable monomers and efficient catalysts at relative low temperature, high yields, and straightforward synthesis process are in good agreement with the principles of green chemistry.Two series of furanic-aliphatic polyesters were produced wherein the number of methylene units in the comonomer varied. The enzyme-catalyzed reactions reached higher molecular weights than the DBTO-catalyzed polymers. DSC measurements demonstrated that both the Tg and Tm values of the BHMF-based polymers are influenced by the length of the aliphatic comonomer. The Tg values showed quadratic behavior and reached a minimum for PFAze due to a balance between chain rigidity and mobility. The detected melting points of the semicrystalline powders were in the range of 63 to 98 °C. PFSuc and PFGlu were initially completely amorphous, while the other five polymers, containing a higher length of the aliphatic comonomer, had a degree of crystallinity in the range of 16 to 25%. A rheological analysis of the melt behavior demonstrated a significant difference between the polyesters, i.e., a shift from Newtonian fluid behavior to shear thinning behavior with a low-frequency complex viscosity of 3 orders of magnitude higher. Furthermore, it was found that an increase in the number of methylene units in the comonomer results in a decrease in low-frequency complex viscosity. The biodegradability study revealed that these BHMF-based polyesters are biodegradable over time under OECD 301 F conditions. A higher number of methylene units in the aliphatic comonomer generally resulted in a higher percentage of biodegradability, meaning that the biodegradation rate could be tailored by varying the polymer composition. Finally, the sustainability of the synthesis routes is assessed based on the atom economy (AE), reaction mass efficiency (RME), E-factor and EcoScale, from where is concluded that iCALB is a more sustainable catalyst than DBTO in this process.
This study confirms the high potential of these renewable BHMF-based polyesters to play a role in a circular and sustainable plastic market due to their varying properties, green synthesis and biodegradable character.
Author contributions
C. Post: investigation, conceptualization, methodology, formal analysis, data curation, visualization, writing – original draft. D. Maniar: conceptualization, methodology, supervision, writing – review & editing, J. A. Jongstra: investigation, methodology, formal analysis, writing – review & editing. D. Parisi: conceptualization, supervision, formal analysis, visualization, writing – review & editing. V. S. D. Voet, R. Folkersma, K. Loos: conceptualization, supervision, resources, project administration, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Fur4Sustain COST network, Greenwise Campus, Regio Deals, Province of Drenthe, and Municipality of Emmen. We would like to express our gratitude to Patrizio Raffa and Cor Koning, from the Engineering and Technology Institute Groningen (ENTEG), University of Groningen, The Netherlands, to allow us to use their biodegradability test facilities.References
- Y. J. Sohn, H. T. Kim, K. A. Baritugo, S. Y. Jo, H. M. Song and S. Y. Park, et al., Recent Advances in Sustainable Plastic Upcycling and Biopolymers, Biotechnol. J., 2020, 15(6), 1900489 CrossRef CAS PubMed.
- R. A. Sheldon and M. Norton, Green chemistry and the plastic pollution challenge: Towards a circular economy, Green Chem., 2020, 22(19), 6310–6322 RSC.
- A. L. Andrady and M. A. Neal, Applications and societal benefits of plastics, Philos. Trans. R. Soc., B, 2009, 364(1526), 1977–1984 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), 25–29 Search PubMed.
- A. A. Shah, F. Hasan, A. Hameed and S. Ahmed, Biological degradation of plastics: A comprehensive review, Biotechnol. Adv., 2008, 26(3), 246–265 CrossRef CAS PubMed.
- B. C. Almroth and H. Eggert, Marine plastic pollution: Sources, impacts, and policy issues, Rev. Environ. Econ. Policy, 2019, 13(2), 317–326 CrossRef.
- T. Iwata, Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics, Angew Chem., Int. Ed., 2015, 54(11), 3210–3215 CrossRef CAS PubMed.
- M. Gigli, M. Fabbri, N. Lotti, R. Gamberini, B. Rimini and A. Munari, Poly(butylene succinate)-based polyesters for biomedical applications: A review in memory of our beloved colleague and friend Dr. Lara Finelli, Eur. Polym. J., 2016, 75, 431–460, DOI:10.1016/j.eurpolymj.2016.01.016.
- M. Puchalski, G. Szparaga, T. Biela, A. Gutowska, S. Sztajnowski and I. Krucińska, Molecular and supramolecular changes in polybutylene succinate (PBS) and polybutylene succinate adipate (PBSA) copolymer during degradation in various environmental conditions, Polymers, 2018, 10(3), 1–12 CrossRef PubMed.
- E. Feghali, L. Tauk, P. Ortiz, K. Vanbroekhoven and W. Eevers, Catalytic chemical recycling of biodegradable polyesters, Polym. Degrad. Stab., 2020, 179, 109241, DOI:10.1016/j.polymdegradstab.2020.109241.
- Y. Chen, A. K. Awasthi, F. Wei, Q. Tan and J. Li, Single-use plastics: Production, usage, disposal, and adverse impacts, Sci. Total Environ., 2021, 752, 141772, DOI:10.1016/j.scitotenv.2020.141772.
- M. Padervand, E. Lichtfouse, D. Robert and C. Wang, Removal of microplastics from the environment. A review, Environ. Chem. Lett., 2020, 18(3), 807–828, DOI:10.1007/s10311-020-00983-1.
- B. Shojaei, M. Abtahi and M. Najafi, Chemical recycling of PET: A stepping-stone toward sustainability, Polym. Adv. Technol., 2020, 31(12), 2912–2938 CrossRef CAS.
- A. Carniel, V. A. de Waldow and A. M. de Castro, A comprehensive and critical review on key elements to implement enzymatic PET depolymerization for recycling purposes, Biotechnol. Adv., 2021, 52, 107811, DOI:10.1016/j.biotechadv.2021.107811.
- Z. O. G. Schyns and M. P. Shaver, Mechanical Recycling of Packaging Plastics: A Review, Macromol. Rapid Commun., 2021, 42(3), 1–27 CrossRef PubMed.
- V. Tserki, P. Matzinos, E. Pavlidou, D. Vachliotis and C. Panayiotou, Biodegradable aliphatic polyesters. Part I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate), Polym. Degrad. Stab., 2006, 91(2), 367–376 CrossRef CAS.
- C. Pang, J. Zhang, G. Wu, Y. Wang, H. Gao and J. Ma, Renewable polyesters derived from 10-undecenoic acid and vanillic acid with versatile properties, Polym. Chem., 2014, 5(8), 2843–2853 RSC.
- Z. Terzopoulou, V. Tsanaktsis, D. N. Bikiaris, S. Exarhopoulos, D. G. Papageorgiou and G. Z. Papageorgiou, Biobased poly(ethylene furanoate-: Co -ethylene succinate) copolyesters: Solid state structure, melting point depression and biodegradability, RSC Adv., 2016, 6(87), 84003–84015, 10.1039/C6RA15994J.
- J. Jian, Z. Xiangbin and H. Xianbo, An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT, Adv. Ind. Eng. Polym. Res., 2020, 3(1), 19–26, DOI:10.1016/j.aiepr.2020.01.001.
- Q. Liu, Y. Wang, J. Liu, X. Liu, Y. Dong and X. Huang, et al., Degradability and Properties of PBAT-Based Biodegradable Mulch Films in Field and Their Effects on Cotton Planting, Polymers, 2022, 14(15), 1–15 Search PubMed.
- Z. Terzopoulou, E. Karakatsianopoulou, N. Kasmi, M. Majdoub, G. Z. Papageorgiou and D. N. Bikiaris, Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester, J. Anal. Appl. Pyrolysis, 2017, 126, 357–370, DOI:10.1016/j.jaap.2017.05.010.
- E. De Jong, M. A. Dam, L. Sipos and G. J. M. Gruter, Furandicarboxylic acid (FDCA), A versatile building block for a very interesting class of polyesters, ACS Symp. Ser., 2012, 1105, 1–13 CrossRef CAS.
- T. Wang, J. Wei, H. Liu, Y. Feng, X. Tang and X. Zeng, et al., Synthesis of renewable monomer 2, 5-bishydroxymethylfuran from highly concentrated 5-hydroxymethylfurfural in deep eutectic solvents, J. Ind. Eng. Chem., 2020, 81, 93–98 CrossRef CAS.
- G. Trapasso, G. Mazzi, B. Chícharo, M. Annatelli, D. Dalla Torre and F. Aricò, Multigram Synthesis of Pure HMF and BHMF, Org. Process Res. Dev., 2022, 26(10), 2830–2838 CrossRef CAS PubMed.
- F. Aricò, Synthetic approaches to stable bio-based diol, Pure Appl. Chem., 2021, 93(5), 551–560 CrossRef.
- R. Kumar, H. Lee, E. Chen, Y. Du, C. Lin and W. Prasanseang, et al., Facile synthesis of the atomically dispersed hydrotalcite oxide supported copper catalysts for the selective hydrogenation of 5 – hydroxymethylfurfural into 2, 5-bis (hydroxymethyl) furan, Appl. Catal., B, 2023, 329122547 Search PubMed.
- S. Zhang, C. Ma, Q. Li, Q. Li and Y. C. He, Efficient chemoenzymatic valorization of biobased D-fructose into 2,5-bis(hydroxymethyl)furan with deep eutectic solvent Lactic acid:Betaine and Pseudomonas putida S12 whole cells, Bioresour. Technol., 2022, 344, 126299 CrossRef CAS PubMed.
- C. Post, D. Maniar, V. S. D. Voet, R. Folkersma and K. Loos, Biobased 2,5-Bis(hydroxymethyl)furan as a Versatile Building Block for Sustainable Polymeric Materials, ACS Omega, 2023, 8(10), 8991–9003 CrossRef CAS PubMed.
- Z. Dai, F. Guo, S. Zhang, W. Zhang, Q. Yang and W. Dong, et al., Bio-based succinic acid: an overview of strain development, substrate utilization, and downstream purification, Biofuels, Bioprod. Biorefin., 2020, 14(5), 965–985 CrossRef CAS.
- C. M. Rohles, L. Gläser, M. Kohlstedt, G. Gießelmann, S. Pearson and A. Del Campo, et al., A bio-based route to the carbon-5 chemical glutaric acid and to bionylon-6,5 using metabolically engineered: Corynebacterium glutamicum, Green Chem., 2018, 20(20), 4662–4674 RSC.
- E. Skoog, J. H. Shin, V. Saez-Jimenez, V. Mapelli and L. Olsson, Biobased adipic acid – The challenge of developing the production host, Biotechnol. Adv., 2018, 36(8), 2248–2263, DOI:10.1016/j.biotechadv.2018.10.012.
- Y. Jiang, A. J. J. Woortman, G. O. R. Alberda Van Ekenstein, D. M. Petrović and K. Loos, Enzymatic synthesis of biobased polyesters using 2,5-bis(hydroxymethyl) furan as the building block, Biomacromolecules, 2014, 15(7), 2482–2493 CrossRef CAS PubMed.
- A. Pellis, S. Weinberger, M. Gigli, G. M. Guebitz and T. J. Farmer, Enzymatic synthesis of biobased polyesters utilizing aromatic diols as the rigid component, Eur. Polym. J., 2020, 130, 109680 CrossRef CAS.
- C. Zeng, H. Seino, J. Ren, K. Hatanaka and N. Yoshie, Bio-Based Furan Polymers with Self-Healing Ability, Polymer, 2013, 54(20), 5351–5357 CrossRef CAS.
- T. Ikezaki, R. Matsuoka, K. Hatanaka and N. Yoshie, Biobased poly(2,5-furandimethylene succinate-co-butylene succinate) crosslinked by reversible Diels-Alder reaction, J. Polym. Sci., Part A: Polym. Chem., 2014, 52(2), 216–222 CrossRef CAS.
- D. Maniar, Y. Jiang, A. J. J. Woortman, J. van Dijken and K. Loos, Furan-Based Copolyesters from Renewable Resources: Enzymatic Synthesis and Properties, ChemSusChem, 2019, 12(5), 990–999 CrossRef CAS PubMed.
- S. Cai, Z. Qiang, C. Zeng and J. Ren, Multifunctional poly(lactic acid) copolymers with room temperature self-healing and rewritable shape memory properties via Diels-Alder reaction, Mater. Res. Express, 2019, 6(4), 45701, DOI:10.1088/2053-1591/aafba3.
- C. Herrlé, S. Fadlallah, S. Toumieux, A. Wadouachi and F. Allais, Sustainable mechanosynthesis of diamide tetraols monomers and their enzymatic polymerization, Green Chem., 2024, 26, 1462–1470 RSC.
- F. Silvianti, D. Maniar, L. Boetje and K. Loos, Green Pathways for the Enzymatic Synthesis of Furan - Based Polyesters and Polyamides. In: Sustain Green Polym Chem Vol 2 Biocatal Biobased Polym Part 1 – Green Pathways Enzym Synth Furan-Based Polyesters Polyam, 2020, vol. 2 Search PubMed.
- Z. Terzopoulou, E. Karakatsianopoulou, N. Kasmi, M. Majdoub, G. Z. Papageorgiou and D. N. Bikiaris, Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester, J. Anal. Appl. Pyrolysis, 2017, 126, 357–370, DOI:10.1016/j.jaap.2017.05.010.
- L. Gustini, C. Lavilla, W. W. T. J. Janssen and M. De Ilarduya, Green and selective polycondensation methods toward linear sorbitol-based polyesters : enzymatic versus organic and metal-based catalysis, ChemSusChem, 2016, 916, 2250–2260 Search PubMed.
- OECD Guideline for tetsing of chemicals, OECD 301 - Ready Biodegradability. OECD Guidel Test Chem. 1992.
- J. Chrobak, J. Iłowska, R. Grabowski, M. Szmatoła, H. Studnik and K. Korasiak, et al., The use of modified vegetable oil from Crambe abyssinica as a lubricant base for the food industry, Environmental Protection and Natural Resources, 2020, 31(1), 8–13 CrossRef.
- P. Reuschenbach, U. Pagga and U. Strotmann, A critical comparison of respirometric biodegradation tests based on OECD 301 and related test methods, Water Res., 2003, 37(7), 1571–1582 CrossRef CAS PubMed.
- Y. Jiang, A. J. J. Woortman, G. O. R. Alberda Van Ekenstein and K. Loos, A biocatalytic approach towards sustainable furanic-aliphatic polyesters, Polym. Chem., 2015, 6(29), 5198–5211 RSC.
- C. Lavilla, A. Alla, A. M. De Ilarduya, E. Benito, J. A. Galbis and S. Mu, Carbohydrate-Based Polyesters Made from Bicyclic Acetalized Galactaric Acid, Biomacromolecules, 2011, 127, 2642–2652 Search PubMed.
- R. W. McCabe and A. Taylor, An investigation of the acyl-binding site of Candida antarctica lipase B, Enzyme Microb. Technol., 2004, 35(5), 393–398 CrossRef CAS.
- I. Gavrila, P. Raffa and F. Picchioni, Acetalised galactarate polyesters: Interplay between chemical structure and polymerisation kinetics, Polymers, 2018, 10(3), 1–20 CrossRef PubMed.
- K. Balani, V. Verma, A. Agarwal and R. Narayan, Physical, Thermal, and Mechanical Properties of Polymers, Biosurfaces, 2015, 329–344 CAS.
- J. Wang, L. Sun, Z. Shen, J. Zhu, X. Song and X. Liu, Effects of Various 1,3-Propanediols on the Properties of Poly(propylene furandicarboxylate), ACS Sustainable Chem. Eng., 2019, 7(3), 3282–3291 CrossRef CAS.
- H. Shr-Ru and R. S. S. Thein Kyu, Characterization and Properties of Polyethylene Blends I. Linear Low-Density Polyethylene with High-Density Polyethylene, J. Polym. Sci., Part B: Polym. Phys., 1987, 25, 71–87 CrossRef.
- M. S. Nikolic and J. Djonlagic, Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene adipate)s, Polym. Degrad. Stab., 2001, 74(2), 263–270 CrossRef CAS.
- R.W Johnson, C.M Pollock and R.R Cantrell. Dicarboxylic Acids, In: Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, 2010 Search PubMed.
- M. Jiang, Q. Liu, Q. Zhang, C. Ye and G. Zhou, A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources, J. Polym. Sci., Part A: Polym. Chem., 2012, 50(5), 1026–1036 CrossRef CAS.
- Y. Tokiwa, B. P. Calabia, C. U. Ugwu and S. Aiba, Biodegradability of plastics, Int. J. Mol. Sci., 2009, 10(9), 3722–3742 CrossRef CAS PubMed.
- M. Naeimirad, B. Krins and G. J. M. Gruter, A Review on Melt-Spun Biodegradable Fibers, Sustainability, 2023, 15(19), 14474 CrossRef CAS.
- C. Park, E. Y. Kim, Y. T. Yoo and S. S. Im, Effect of hydrophilicity on the biodegradability of polyesteramides, J. Appl. Polym. Sci., 2003, 90(10), 2708–2714 CrossRef CAS.
- S. Calderon-Ardila, D. Morvan, O. Péruch, V. Bellière-Baca, M. Dusselier and B. F. Sels, Methionine and its hydroxy analogues: the paths toward their sustainable chemical synthesis, Green Chem., 2024, 26(8), 4242–4269 RSC.
- K. Van Aken, L. Strekowski and L. Patiny, EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters, Beilstein J. Org. Chem., 2006, 2, 1–7 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc01512f |
| This journal is © The Royal Society of Chemistry 2024 |