 Open Access Article
Open Access ArticleCatalytic production of 1,4-pentanediol from lignocellulosic biomass
Shanhui
Zhu
 *ab,
Zexiang
Lv
ab,
Jiamin
Wang
ab,
Xiangyu
Jia
ab,
Xiaoming
Li
ab,
Mei
Dong
*ab,
Zexiang
Lv
ab,
Jiamin
Wang
ab,
Xiangyu
Jia
ab,
Xiaoming
Li
ab,
Mei
Dong
 a,
Jianguo
Wang
a,
Jianguo
Wang
 *b and
Weibin
Fan
*b and
Weibin
Fan
 *a
*a
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, PR China. E-mail: zhushanhui@sxicc.ac.cn; fanwb@sxicc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, PR China. E-mail: iccjgw@sxicc.ac.cn
First published on 11th March 2024
Abstract
Sustainable production of 1,4-pentanediol (1,4-PDO) from biomass is of great importance because 1,4-PDO is a monomer of degradable polyesters and plastics. This paper reviews the conversion of biomass-derived levulinic acid (LA) and furfural as well as their derivatives alkyl levulinate, γ-valerolactone (GVL) and furfuryl alcohol (FAL) into 1,4-PDO. MoOx or ReOx modified noble metal catalysts and non-noble metal catalysts with core–shell and alloy structures show a high 1,4-PDO yield and stability in LA hydrogenation under severe hydrothermal conditions. The conversion of furfural to 1,4-PDO involves the initial C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond hydrogenation to form FAL, the consecutive acid-catalyzed ring-opening and the final hydrogenation. Therefore, it needs a bifunctional catalyst composed of metallic species and acid sites to improve the 1,4-PDO yield. Efforts are made here to highlight the employed catalysts, structure–performance relationship, reaction pathway and reaction mechanism in detail. The current challenges for large-scale applications of conversion of LA and furfural to 1,4-PDO have been proposed, including process development, catalyst cost, solvent and active site distribution. We believe that this review will provide new opportunities and expand the options for the production of 1,4-PDO via biorefining processes.
O bond hydrogenation to form FAL, the consecutive acid-catalyzed ring-opening and the final hydrogenation. Therefore, it needs a bifunctional catalyst composed of metallic species and acid sites to improve the 1,4-PDO yield. Efforts are made here to highlight the employed catalysts, structure–performance relationship, reaction pathway and reaction mechanism in detail. The current challenges for large-scale applications of conversion of LA and furfural to 1,4-PDO have been proposed, including process development, catalyst cost, solvent and active site distribution. We believe that this review will provide new opportunities and expand the options for the production of 1,4-PDO via biorefining processes.
1. Introduction
Conversion of huge amounts of biomass into fuels and chemicals is a potentially effective strategy for achieving carbon neutrality and is a great challenge.1–6 Non-edible and renewable lignocellulose consists of cellulose, hemicellulose and lignin.7 It accounts for more than 95% of biomass resources that are annually produced, about 170 billion tons.7 Although it is highly significant to valorize sustainable production of chemicals and biofuels from lignocellulose, technological advances are important and necessary. Reductive catalytic fractionation, known as the lignin first strategy, involves lignocellulose solvolysis, extraction and stabilization of reactive lignin fragments and subsequent conversion, which has been demonstrated to be powerful technology with potential to increase the profitability and sustainability of future biorefineries.4,5 Sels et al.5 successfully converted birch wood into high value-added phenol, propylene, oligomers and pulp using this approach. Barta et al.6 sought an effective deep eutectic solvent composed of choline chloride, oxalic acid and ethylene glycol for lignocellulose fractionation, in which the obtained lignin with a high β-O-4 content can be efficiently converted into distinct monophenolic compounds, while the cellulose residues were readily depolymerized to 95.9% glucose yield through enzymatic catalysis. Besides, a variety of catalytic methods and innovative biorefinery approaches have been developed to produce various platform chemicals, such as furfural,8,9 levulinic acid (LA),10,11 5-hydroxymethylfurfural,12,13 glycerol,14 guaiacol15 and so on. These platform chemicals can be further converted into many types of value-added products through hydrogenation, oxidation, esterification and even complex cascade processes.1,4-Pentanediol (1,4-PDO), like other bio-diols, is a building block of degradable polyesters and plastics.16,17 1,4-PDO can integrate with long-chain diacids (>C12) to produce crystalline polyesters that are amenable to develop shape-memory polymers due to their low switching temperature (<0 °C).18 This unique thermosensitive adhesive can hold wide potential applications in the packaging of temperature-sensitive goods, adhesives, coatings, and elastomeric/rubbery materials. Besides, 1,4-PDO is widely used as an industrial solvent. Currently, 1,4-PDO is commercially produced by the condensation of hydroquinone and acetic anhydride over homogeneous NaOH or H2SO4 catalysts. The total European and USA production was 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons and its market price can reach $3000 per ton.19
000 tons and its market price can reach $3000 per ton.19
Currently, optically active 2,4-pentanediol has been employed as a chiral linking bridge to synthesize enantiomerically pure reagents, such as binaphthol and 2-hydroxycyclohexanone acetal.20,21 1,4-PDO has one asymmetric carbon and a similar structure to 2,4-pentanediol. Thus, the greener 1,4-PDO holds the potential to substitute 2,4-pentanediol as a highly efficient and widely applicable stereocontroller in the future. Obviously, it is urgent and necessary to develop the production process of optically pure 1,4-PDO.
In light of the heavy pollution from the conventional petroleum route, two platform chemicals, i.e. cellulose-derived LA and hemicellulose-derived furfural, have been employed to produce 1,4-PDO from renewable biomass. As shown in Scheme 1, LA is sequentially hydrogenated into 1,4-PDO. In alcohol solvent, alkyl levulinate is formed by LA esterification. It can be hydrogenated to γ-valerolactone (GVL) that is further converted into 1,4-PDO through consecutive hydrogenation. Conversion of furfural to 1,4-PDO is achieved through consecutive hydrogenations and a ring-opening reaction with the formed FAL alcoholysis to alkyl levulinate that is converted to 1,4-PDO via GVL. This shows that one route for the production of 1,4-PDO is from LA and its derivatives of alkyl levulinate and GVL as feedstocks, and the other route involves the use of furfural and its derivatives FAL, 2-methylfuran (2-MF) and 2-methyltetrahydrofuran (2-MTHF) as starting materials. The two routes require heterogeneous catalysts with different types of active sites.22–24
 | ||
| Scheme 1 Reaction pathways for the production of 1,4-PDO from cellulose and hemicellulose derivatives. AL: alkyl levulinate. | ||
The employed catalyst systems, structure–performance relationship, reaction pathway and catalytic mechanism are reviewed here in detail, and the challenges for large-scale production of 1,4-PDO are described, including reactors, catalyst cost, solvent and active site distribution. In addition, the future prospects and mechanic investigation for rational and precise catalyst design are envisaged by using Earth-abundant metals and developing more benign yet competitive catalysts.
2. Conversion of LA and its derivatives into 1,4-PDO
2.1. Conversion of LA into 1,4-PDO
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of LA.25 Thus, Ru-based catalysts are mostly used for conversion of biomass-based LA, particularly with water as the solvent.26–29 Many research results have demonstrated that water adsorbed on the Ru site generates a proton, which can participate in the LA hydrogenation.30,31 The nanoporous metal possesses a high surface-to-volume ratio and abundant low-coordinated surface sites, and thus, shows excellent performance. Lv et al.32 developed a highly active and selective nanoporous Ru catalyst, which gave a 1,4-PDO yield of 78.8% at 100 °C in water solvent (entry 1, Table 1). The suitable acid sites and lattice planes of this catalyst promote the C
O bond of LA.25 Thus, Ru-based catalysts are mostly used for conversion of biomass-based LA, particularly with water as the solvent.26–29 Many research results have demonstrated that water adsorbed on the Ru site generates a proton, which can participate in the LA hydrogenation.30,31 The nanoporous metal possesses a high surface-to-volume ratio and abundant low-coordinated surface sites, and thus, shows excellent performance. Lv et al.32 developed a highly active and selective nanoporous Ru catalyst, which gave a 1,4-PDO yield of 78.8% at 100 °C in water solvent (entry 1, Table 1). The suitable acid sites and lattice planes of this catalyst promote the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond activation of LA and lactone group of GVL, consequently resulting in a high 1,4-PDO selectivity. The catalyst support greatly affects Ru dispersion, electron structure, catalyst acidity, and hence, catalytic performance. Ru loaded on Al2O3–Co3O4 gave a 1,4-PDO yield of ca. 35% with a major by-product of 2-butanol at 220 °C.33 The large number of acid sites on the Al2O3–Co3O4 support and the high reaction temperature caused serious decarboxylation of the 4-hydroxylpentanoic acid intermediate to 2-butanol.
O bond activation of LA and lactone group of GVL, consequently resulting in a high 1,4-PDO selectivity. The catalyst support greatly affects Ru dispersion, electron structure, catalyst acidity, and hence, catalytic performance. Ru loaded on Al2O3–Co3O4 gave a 1,4-PDO yield of ca. 35% with a major by-product of 2-butanol at 220 °C.33 The large number of acid sites on the Al2O3–Co3O4 support and the high reaction temperature caused serious decarboxylation of the 4-hydroxylpentanoic acid intermediate to 2-butanol.
| Entry | Catalyst | Temperature (°C) | H2 pressure (MPa) | Solvent | Conv. (%) | 1,4-PDO Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Nanoporous Ru | 100 | 6 | H2O | 100 | 78.8 | 32 |
| 2 | RuRe/C | 140 | 15 | H2O | 100 | 82 | 37 |
| 3 | Rh–MoOx/SiO2 | 80 | 6 | H2O | 100 | 70 | 38 |
| 4 | Ru–MoOx/AC | 70 | 4 | H2O | 99.9 | 96.7 | 39 |
| 5 | Ru–MoOx/TiO2 | 110 | 4 | H2O | 100 | 91 | 40 |
| 6 | Pt–MoOx/HAP | 130 | 5 | H2O | >99 | 93 | 41 |
| 7 | Pdn/NC@SBA-15 | 80 | 0.1 | Ethanol | >99 | >99 | 47 |
| 8 | Au/TiO2 | 200 | 5 | Free | 100 | 100 | 49 |
| 9 | Cu/MgO | 170 | 3 | Ethanol | 100 | 53.6 | 24 |
| 10 | hcp-Co@G | 220 | 5 | H2O | 100 | 89.5% | 52 |
| 11 | Cu–Ni–Zn/HZSM-5 | 130 | 2.5 | H2O | 97.4 | 93.4 | 51 |
| 12 | CuCo@N–CNT | 200 | 5 | 1,4-Dioxane | 100 | 74.1 | 17 |
ReOx- and MoOx-modified noble metal catalysts are often employed for hydrodeoxygenation of biomass,34–36 because ReOx and MoOx can effectively tune the electronic structure and improve the dispersion of metal species. In addition, partially reduced ReOx and MoOx possess considerable numbers of oxygen vacancies that can behave as acid sites to activate the GVL intermediate. Pinel's group reported a series of heterogeneous Re-promoted noble–metal (Ru, Pd or Pt) catalysts for the hydrogenation of bio-based carboxylic acids.37 Among these catalysts, RuRe/C gave the highest 1,4-PDO yield of 82% at 140 °C in water (entry 2, Table 1). It was found that LA was initially hydrogenated to GVL at a high rate, and then underwent ring-opening to transform into 1,4-PDO at a very slow rate. Kinetic studies corroborated that GVL ring-opening was the rate-determining step in the conversion of LA into 1,4-PDO. Another notable catalyst is Rh–MoOx/SiO2 (Fig. 1), which shows nearly 100% conversion with a 1,4-PDO yield of 70% at a low temperature of 80 °C (entry 3) due to the synergistic effect between closely contacted Rh and Mo species.38 MoOx enhances carboxyl acid adsorption through interaction with the lone-pair electrons of the oxygen atoms in the carbonyl group, and hence, drastically increases the carboxyl group hydrogenation rate. Recently, a MoOx-decorated Ru/AC catalyst was prepared, and it exhibited a 1,4-PDO yield as high as 96.7% at 70 °C (entry 4).39 In addition, its catalytic performance can be well maintained within 200 h in a continuous fixed-bed reactor. Rodiansono and co-workers found that the high catalytic performance (entry 5) of MoOx-modified Ru/TiO2 in LA hydrogenation to 1,4-PDO was due to the presence of Brφnsted acid sites produced by surface Mo6+–OH species.40 However, Kaneda's group assumed that the oxygen vacancies in reduced Mo species acted as Lewis acid sites to activate carbonyl moieties of GVL,41 which highlighted the promotional role of MoOx for Pt (entry 6). These studies show that the catalytic roles of ReOx and MoOx still remain under debate, which needs to be clarified by further research.
 | ||
| Fig. 1 LA hydrogenation to 1,4-PDO over Rh/MoOx/SiO2. Reproduced from ref. 38 with permission from the Royal Society of Chemistry, copyright (2014). | ||
The increase of noble metal dispersion and exposure of more active sites by structural optimization are the most used strategies to improve their utilization efficiency and lower the catalyst preparation cost. Atomically dispersed metal atoms that can form strong interfacial interaction with the support achieve its utilization efficiency of 100% and high specific activity.42–44 In comparison, metal nanoclusters provide multiple adsorption sites and adjacent metal bonds,45,46 thus offering a variety of structural possibilities and benefiting complex biomass conversion. Li and co-workers found that single atomic-layered, low-nuclearity Pd clusters on N-doped carbon-coated SBA-15 (Pdn/NC@SBA-15) showed excellent intrinsic activity with a turnover frequency (TOF) of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 h−1 and a 1,4-PDO yield of >99% at 80 °C and 0.1 MPa (entry 7).47 Kinetic studies revealed that Pd nanoclusters exhibited a much lower apparent activation energy (62.2 kJ mol−1) than Pd single atoms (82.5 kJ mol−1) and Pd nanoparticles (NPs) (105.2 kJ mol−1). Theoretic calculations showed that the synergetic interaction between adjacent Pd atoms in layered Pd clusters facilitated the adsorption and activation of LA and GVL as a result of a substantial decline of the energy barrier of the rate-determining step for cleavage of the C1–O1 bond of GVL (0.59 eV) (Fig. 2), significantly lower than that of the C4–O1 bond (1.56 eV). In addition, Pdn/NC@SBA-15 can be well reused for at least ten cycles and was evaluated on a larger scale (100-fold), evidencing its potential use in industrial applications.
480 h−1 and a 1,4-PDO yield of >99% at 80 °C and 0.1 MPa (entry 7).47 Kinetic studies revealed that Pd nanoclusters exhibited a much lower apparent activation energy (62.2 kJ mol−1) than Pd single atoms (82.5 kJ mol−1) and Pd nanoparticles (NPs) (105.2 kJ mol−1). Theoretic calculations showed that the synergetic interaction between adjacent Pd atoms in layered Pd clusters facilitated the adsorption and activation of LA and GVL as a result of a substantial decline of the energy barrier of the rate-determining step for cleavage of the C1–O1 bond of GVL (0.59 eV) (Fig. 2), significantly lower than that of the C4–O1 bond (1.56 eV). In addition, Pdn/NC@SBA-15 can be well reused for at least ten cycles and was evaluated on a larger scale (100-fold), evidencing its potential use in industrial applications.
 | ||
| Fig. 2 (a) C–O cleavage configurations and the corresponding reaction energy on Pd3/NC. (b) The relative energy profiles of intermediates (int) and transition states (TS) in the C–O cleavage of GVL on Pd3/NC. IS: initial state and FS: final state. The blue, green, brown, red and white balls in the configurations represent Pd, N, C, O and H atoms, respectively. Reproduced from ref. 47 Copyright (2023), with permission from Elsevier. | ||
Although a decrease of Au particle size or enhancement of Au–support interactions can significantly increase the oxidation activity, Au-based catalysts are rarely applied for biomass hydrogenation due to chemical inertness.48 Nevertheless, Bucciol et al. recently demonstrated that Au/TiO2 could convert LA into 1,4-PDO through microwave catalysis.49 Unlike thermal catalysts, microwave catalysts can harvest the rapidly produced internal heating energy of microwaves and considerably accelerate chemical transformation, in which there are direct interactions between the electromagnetic field and reactant molecules, intermediates and/or transition states. Under microwave conditions, Au/TiO2 gave 100% LA conversion and promoted the key ring-opening step of the GVL intermediate to 1,4-PDO.49 In particular, 100% of 1,4-PDO yield was achieved at 200 °C under solvent-free conditions (entry 8). The ionization induced by microwaves promotes the formation of abundant H+ species that could spillover on the surface of TiO2. These H+ species can rapidly react with pre-absorbed LA and GVL, thus enhancing the catalytic performance. In this case, Au acts as an active component for hydrogenation, while the TiO2 support provides Lewis acid sites for 4-hydroxylpentanoic acid lactonization and ring-opening of GVL. Addition of water is unfavorable for the formation of 1,4-PDO due to its competitive adsorption on Lewis acid sites with the substrate of LA.
The acidic carboxylic groups in LA molecules cause severe corrosion and sintering of non-noble metals in the reaction process. Thus, the challenge is to construct a highly active and stable base metal catalyst, particularly for metastable metal crystals. We encapsulated metastable hexagonal closed-packed (hcp) Co NPs in a few-layer thick graphene shell through pyrolysis of a metal–organic framework material of the Co3[Co(CN)6] compound under a reductive atmosphere.52 This hcp-Co@G catalyst could resist the corrosion of LA and, hence, showed a high 1,4-PDO yield (89.5%, entry 10) and catalytic stability. After six repeated recycles, its catalytic performance did not obviously decrease as it was well preserved by the graphene shell. It needs to be pointed out that the graphene shell contains large numbers of defective voids,53 which allowed LA and organic molecules to freely penetrate through the graphene layer and access hcp-Co NPs.
LA has two oxygen-containing groups, the carbonyl and carboxylic groups. Compared to the carboxylic group, the carbonyl group of LA preferentially undergoes hydrogenation to produce 4-hydroxylpentanoic acid (Scheme 2). Most of the researchers reported that 4-hydroxylpentanoic acid could not be rapidly converted into 1,4-PDO,47,52 but proceeds to lactonization to GVL. This is because reduction of the carboxylic group needs higher activation energy than lactonization. The generated GVL undergoes C–O bond cleavage (ring-opening) to form 1,4-PDO. Nevertheless, many by-products, e.g. 1-pentannol and 2-pentanol, are simultaneously produced due to GVL over-hydrogenolysis and 2-MTHF dehydration.
 | ||
| Scheme 2 Reaction pathway for LA hydrogenation to 1,4-PDO. HPA: 4-hydroxylpentanoic acid. Reproduced from ref. 52 Copyright (2019), with permission from Elsevier. | ||
Another attractive approach to improve non-noble metal-based catalyst stability is by alloying with other metals. Formation of alloys generates new metal bonds, and hence, retards sintering and leaching during the reaction process.54 Kim et al. prepared Zn-modified CuNi alloy on ZSM-5, which led to a 1,4-PDO yield of 93.4% at 130 °C in LA hydrogenation (entry 11).51 CuNi alloy facilitates H2 spillover, suppresses leaching of CuNi species, and thus decreases CuNi NP sintering, consequently resulting in a remarkable improvement in catalytic activity and recyclability. After five cycles, only a small decrease was detected in LA conversion and 1,4-PDO yield. In the presence of strong acidic HZSM-5, a different reaction pathway was proposed for LA hydrogenation to-1,4-PDO (Fig. 3).51 LA might initially undergo intramolecular cyclization to produce pseudo-levulinic acid, followed by dehydration on Brφnsted acid sites. The formed α-angelica lactone could be rapidly hydrogenated to GVL by active H* species that were produced by dissociating H2 on metal sites. Then, the GVL adsorbed on Lewis acid sites undergoes ring-opening through C–O bond cleavage to produce 3-acetyl-1-propanol, which is subsequently hydrogenated to 1,4-PDO on CuNi alloy. The improvement of catalytic performance is also demonstrated by the formation of CuCo alloy.17 Compared to Co@CN (27.7%) and Cu/CN (2.0%), CuCo@N–CNTs gave a 1,4-PDO yield of 74.1% at 200 °C as a result of the electron transfer from Cu to Co (entry 12). Electron-enriched Co enhances H2 activation, while electron-deficient Cu species generate plentiful acid sites for adsorbing the GVL intermediate and promoting its ring opening. These two factors cooperatively result in excellent catalytic performance. Moreover, 1,4-PDO selectivity only mildly decreased from 85% to 75% within five reaction runs over CuCo@N–CNTs owing to the CuCo NPs confined in N-doped carbon nanotubes.
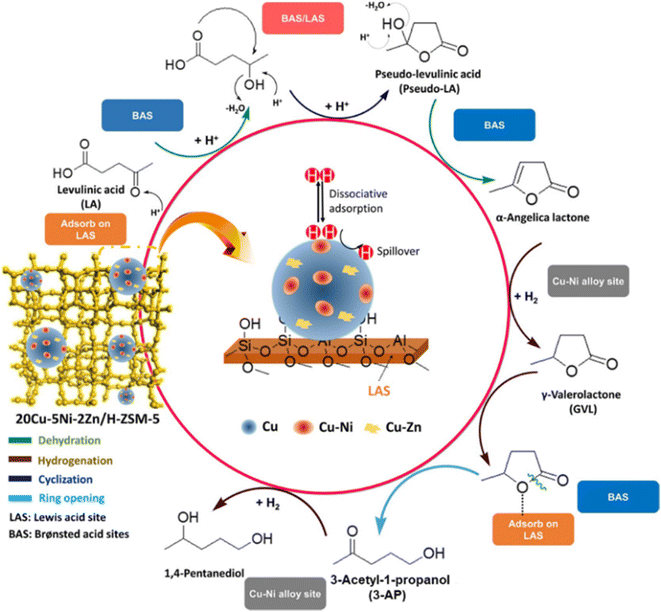 | ||
| Fig. 3 Reaction pathway for LA hydrogenation to 1,4-PDO over the Cu–Ni–Zn/HZSM-5 catalyst. Reproduced from ref. 51 Copyright (2021), with permission from American Chemical Society. | ||
Table 1 summarizes the representative results for the catalytic hydrogenation of LA to 1,4-PDO over various catalysts. Noble metal catalysts give a high 1,4-PDO yield at a temperature ≤140 °C due to their high intrinsic activity. High H2 pressure is generally required to achieve superior performance except that Pdn/NC@SBA-15 possesses very strong H2 activation ability at ambient pressure. In contrast, base metal catalysts are generally operated at high reaction temperature. Among the used solvents, H2O is highly promising, because biomass itself contains much water, which can save the water separation process during biomass pretreatment. It is more desirable to develop a free-solvent system to save the separation process, which needs the design of an extraordinarily active catalyst with strong acid-resistance ability.
2.2. Conversion of alkyl levulinate into 1,4-PDO
Compared to LA, alkyl levulinate does not cause catalyst component leaching and reactor corrosion as its acidic carboxyl group has been esterified. This makes cheap non-noble catalysts a better choice for the hydrogenation of alkyl levulinate to 1,4-PDO. Ethyl levulinate and methyl levulinate as feeds are mostly used, as they can be sustainably obtained in high yields by alcoholysis of cellulose or FAL and esterification of LA with ethanol and methanol.55–59 In EL hydrogenation, the 1,4-PDO yield reached as high as 98% at 160 °C on the ternary skeletal CuAlZn catalyst.60 A recent research study shows that the bimetallic Cu2Co1/Al2O3 catalyst is more active and selective than monometallic Cu/Al2O3,61 as it has a lower activation energy (65.1 kJ mol−1) in the rate-determining step. As a result, it exhibited not only a 1,4-PDO selectivity of 93% but also high catalytic stability, as demonstrated by no obvious decrease of 1,4-PDO yield within 200 h. Introduction of CoOx not only improves Cu dispersion and enhances H2 activation ability due to its strong interfacial electronic interaction with Cu that modifies the Cu0/Cu+ ratio, evidencing the synergistic effect of Cu and electron-deficient CoOx. Another notable catalyst is Cu/ZrO2,62 which yielded 39% of 1,4-PDO at 200 °C in the transfer hydrogenation of methyl levulinate with 2-propanol. The transfer hydrogenation does not require high pressure H2, thus improving the safety of the entire process. The Cu/MgO prepared by the co-precipitation method gave a 1,4-PDO yield of 97.7% in isopropanol solvent.63 MgO not only serves as support, but also provides abundant basic sites, thus enabling the GVL ring-opening to form 1,4-PDO. However, the H* production rate in the transfer hydrogenation process is much lower than in H2 dissociation, which greatly lowers the reaction efficiency and retards its large-scale application. However, Cu/MgO underwent deactivation after four reaction cycles at a high Cu content owing to Cu NP agglomeration.In the reaction process of alkyl levulinate hydrogenation to 1,4-PDO, alkyl 4-hyxoxyvalerate is another preferentially formed key intermediate, as the alkyl levulinate carbonyl group is much more easily hydrogenated than its ester group. Hu and coworkers have investigated the ethyl levulinate hydrogenation pathway on the CoMgAl catalyst by a kinetic study.64 The carbonyl group in ethyl levulinate is firstly hydrogenated to ethyl 4-hydroxyvalerate, which is then lactonized to GVL (Scheme 3). The activation energy of the latter step is higher than that of the former one (78.0 kJ mol−1vs. 63.5 kJ mol−1). A more significant activation energy (84.0 kJ mol−1) is required for the ring-opening of GVL to 1,4-PDO, showing that this elementary reaction is the rate-determining step of EL hydrogenation to 1,4-PDO.
 | ||
| Scheme 3 Reaction pathway of ethyl levulinate hydrogenation to 1,4-PDO along with the apparent activation energies of various reaction steps. Reproduced from ref. 64 Copyright (2022), with permission from Elsevier. | ||
2.3. Conversion of GVL into 1,4-PDO
Besides biomass-derived LA and ethyl levulinate, GVL is another available feedstock to synthesize 1,4-PDO. In this process, Cu-based catalysts are widely used for selective hydrogenation of esters, such as dimethyl oxalate, ethyl lactate, and γ-butyrolactone, to alcohols.65–67 This is because Cu is efficient for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–O bond hydrogenation, but inactive for C–C bond scission. Cao et al. found that Cu/ZrO2 prepared by the oxalate gel co-precipitation method shows excellent catalytic performance for the hydrogenation of GVL into 1,4-PDO.68 The Cu/ZrO2 with 30 wt% of Cu gave a GVL conversion of 97% and a 1,4-PDO selectivity of 99%. This catalyst even outperforms supported Ru-based catalysts under the same reaction conditions. With the inspiration of this catalyst, a variety of effective Cu-based catalysts have been fabricated for hydrogenation of GVL to 1,4-PDO. Li's group prepared a series of Cux/Mg3−xAlO catalysts based on layered double hydroxides.69 An excellent 1,4-PDO yield was achieved on Cu1.5/Mg1.5AlO due to the synergistic effect of well-dispersed Cu NPs and nearby appropriate surface base sites. Interestingly, a good linear relationship has been well established between the TOF value and Lewis basic site concentration (Fig. 4A). The basic sites enhance the interaction of the catalyst surface with GVL through the lone-pair electrons of the carbonyl oxygen and the π* acceptor orbital of the carbonyl carbon. The active sites of Cu0 enable the adsorbed H2 to dissociate into H*.
O/C–O bond hydrogenation, but inactive for C–C bond scission. Cao et al. found that Cu/ZrO2 prepared by the oxalate gel co-precipitation method shows excellent catalytic performance for the hydrogenation of GVL into 1,4-PDO.68 The Cu/ZrO2 with 30 wt% of Cu gave a GVL conversion of 97% and a 1,4-PDO selectivity of 99%. This catalyst even outperforms supported Ru-based catalysts under the same reaction conditions. With the inspiration of this catalyst, a variety of effective Cu-based catalysts have been fabricated for hydrogenation of GVL to 1,4-PDO. Li's group prepared a series of Cux/Mg3−xAlO catalysts based on layered double hydroxides.69 An excellent 1,4-PDO yield was achieved on Cu1.5/Mg1.5AlO due to the synergistic effect of well-dispersed Cu NPs and nearby appropriate surface base sites. Interestingly, a good linear relationship has been well established between the TOF value and Lewis basic site concentration (Fig. 4A). The basic sites enhance the interaction of the catalyst surface with GVL through the lone-pair electrons of the carbonyl oxygen and the π* acceptor orbital of the carbonyl carbon. The active sites of Cu0 enable the adsorbed H2 to dissociate into H*.
 | ||
| Fig. 4 (A) Linear relationship between the TOF value and the Lewis basic site concentration on different Cu/MgAlO catalysts. Reproduced from ref. 69 Copyright (2019), with permission from Elsevier. (B) Dependence of the acid site amount and 1,4-PDO selectivity on the Zn/Cu molar ratio of ZnCu/Al2O3 catalysts. Reproduced from ref. 70 with permission from the Royal Society of Chemistry, copyright (2020). (C) Catalytic results of CuLax/Al2O3 catalysts for conversions of various lactones into diols. Reproduced from ref. 67 Copyright (2022), with permission from Elsevier. (D) Proposed GVL adsorption and 1,4-PDO desorption mechanism on Cu/SiO2A and Cu/SiO2B catalysts. Reproduced from ref. 63 (2021), with permission from Elsevier. | ||
Doping of other elements into a Cu-based catalyst can stabilize and facilitate the dispersion of Cu NPs. Zhao et al. demonstrated the remarkable promotional effect of Zn species on the Cu/Al2O3 catalyst.70 The 1,4-PDO selectivity increased from 46% on Cu/Al2O3 to >98% on ZnCu/Al2O3. The presence of Zn species decreases surface Lewis acid sites and suppresses the formation of by-product 2-MTHF through dehydration of 1,4-PDO (Fig. 4B). In addition, the formation of the ZnCu alloy and metallic Zn prevents metallic Cu0 from being oxidized into Cu2+ species during the reaction process, and thus, improves the activity and stability. ZnCu/Al2O3 can be recycled 10 times without obvious deactivation, while GVL conversion decreased from 98% to 50% over Cu/Al2O3 after 5 cycles. Without ZnO doping, the active Cu species were covered by Al(OH)3 owing to the formed more water originated from dehydration of 1,4-PDO. These researchers further found the promoting effect of LaOx on Cu/Al2O3 catalyst,67 which is attributed to the formation of Cu+–LaOx interfacial sites that enhance the GVL adsorption and activation. The CuLax/Al2O3 catalyst shows extensive substrate tolerance to various lactones (Fig. 4C), no matter whether they have alkyl substituents or not.
Solvent influences H2 solubility, active metal stability, and even catalyst acidity and alkalinity. Zaccheria's team highlighted the role of the solvent in GVL hydrogenolysis to 1,4-PDO over the Cu/SiO2 catalyst.71 A change of the solvent from dioxane to cyclopentyl methyl ether substantially improved the 1,4-PDO selectivity due to the increase of acidic sites by 24%. The authors thought that the surface acid sites facilitated the adsorption of GVL via the carbonyl group (Fig. 4D), not in agreement with other reports.47,69 This shows that the active sites for adsorption of GVL are still in debate. Acidic, basic and metallic sites are usually considered as active sites to adsorb and activate the carbonyl group of GVL, which is highly dependent on the catalyst support. In addition, it had been demonstrated that high hydrophobicity of SiO2 support promotes 1,4-PDO desorption.
3. Conversion of furfural and its derivatives into 1,4-PDO
3.1. Conversion of furfural into 1,4-PDO
Furfural is one of a few industrially produced biomass-based platform chemicals. It is made from acid-catalyzed hemicellulose degradation. Every year, more than 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of furfural are produced in the world.8,72 It is interesting to convert furfural into value-added products. Early attempts by Leuck et al.73 showed that furfural hydrogenation in the mixed ethanol and water solvent gave a 1,4-PDO yield of 40% on RANEY® Ni catalyst. Zhang's group made significant progress in the selective production of 1,4-PDO from furfural. They reported that Ru supported on ordered mesoporous carbon (CMK-3) showed a 1,4-PDO yield of 90% in one-pot hydrogenation of furfural at 80 °C in water under pressurized H2 and CO2 (entry 1, Table 2).23 As is displayed in Scheme 4, furfural is firstly hydrogenated to FAL on metallic Ru sites, and then, undergoes acid-catalyzed Piancatelli rearrangement to generate 3-acetyl-1-propanol. 3-Acetyl-1-propanol is finally hydrogenated to 1,4-PDO. The key for the formation of 1,4-PDO is to construct a weakly acidic environment that promotes the FAL Piancatelli rearrangement. The positively charged Ru species and the dissolved pressurized CO2 in water provide necessary weakly acidic sites. Afterwards, the same group developed a continuous process with a 1,4-PDO yield of 86% by combining Amberlyst-15 and Ru–FeOx/AC (entry 2).74 Such a dual-component catalyst exhibited a long catalytic life of 175 h without obvious deactivation. Addition of FeOx to Ru/AC switched the product from LA to 1,4-PDO, which is probably due to the decrease of Lewis acid sites.
000 tons of furfural are produced in the world.8,72 It is interesting to convert furfural into value-added products. Early attempts by Leuck et al.73 showed that furfural hydrogenation in the mixed ethanol and water solvent gave a 1,4-PDO yield of 40% on RANEY® Ni catalyst. Zhang's group made significant progress in the selective production of 1,4-PDO from furfural. They reported that Ru supported on ordered mesoporous carbon (CMK-3) showed a 1,4-PDO yield of 90% in one-pot hydrogenation of furfural at 80 °C in water under pressurized H2 and CO2 (entry 1, Table 2).23 As is displayed in Scheme 4, furfural is firstly hydrogenated to FAL on metallic Ru sites, and then, undergoes acid-catalyzed Piancatelli rearrangement to generate 3-acetyl-1-propanol. 3-Acetyl-1-propanol is finally hydrogenated to 1,4-PDO. The key for the formation of 1,4-PDO is to construct a weakly acidic environment that promotes the FAL Piancatelli rearrangement. The positively charged Ru species and the dissolved pressurized CO2 in water provide necessary weakly acidic sites. Afterwards, the same group developed a continuous process with a 1,4-PDO yield of 86% by combining Amberlyst-15 and Ru–FeOx/AC (entry 2).74 Such a dual-component catalyst exhibited a long catalytic life of 175 h without obvious deactivation. Addition of FeOx to Ru/AC switched the product from LA to 1,4-PDO, which is probably due to the decrease of Lewis acid sites.
 | ||
| Scheme 4 Reaction pathway of furfural hydrogenation to 1,4-PDO over Ru/CMK3. 4-HCP: 4-hydroxy2-cyclopentenone. Reproduced from ref. 23 with permission from the Royal Society of Chemistry, copyright (2018). | ||
| Entry | Catalyst | Temperature (°C) | H2 pressure (MPa) | Solvent | Conv. (%) | 1,4-PDO yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Ru/CMK-3 | 80 | 1 | H2O | 100 | 90 | 23 |
| 2 | Amberlyst-15+ Ru–FeOx/AC | 80 | 0.2 | H2O | 100 | 86 | 74 |
| 3 | PtI/Al2O3 | 100 | 2 | Water | 99.2 | 80.1 | 77 |
| 4 | Ru/SC–SBA-15 | 140 | 1.5 | Water | 100 | 87 | 78 |
| 5 | Ni–Sn alloy | 160 | 3 | Ethanol–H2O | 100 | 92 | 16 |
| 6 | Cu/SiO2 | 120 | 4 | Ethanol | 99.9 | 86.2 | 22 |
As is known, to achieve excellent catalytic performance, the distribution of metal and acid sites on the catalyst need to be tailored in molecular-level proximity or formed metal–acid interface.75,76 In addition, it has been recognized that the type of acid site plays an important role in selectivity control in furfural to 1,4-PDO. Deng et al. fabricated an iodine-modified Pt-based catalyst with abundant Pt–I pairs.77 The 1,4-PDO yield reached 80.1% on this catalyst at 100 °C and 2.0 MPa H2 (entry 3). The in situ hydrogen spillover-induced H−Pt–I–H+ pairs not only generate hydrogenation sites, but also supply Brφnsted acid sites, thus enabling strong synergistic catalysis for tandem conversion of furfural to 1,4-PDO. Brφnsted acid sites promote FAL ring-opening to produce the key intermediate of 3-acetyl-1-propanol. Sulfonation is an efficient method to create a large number of Brφnsted acid sites. Ru NPs anchored on sulfonated carbon layer-coated SBA-15 (Ru/SC-SBA-15) exhibited a high 1,4-PDO yield of 87% under optimum conditions (entry 4).78 The coordination of sulfonic acid groups to Ru influences Ru species electronic properties, which is beneficial for H2 dissociation and sequential hydrogenation of the acid-hydrolyzed ring-opening intermediate. The kinetic study definitely demonstrates that the reaction preferentially occurs via the furfural hydrogenation to FAL, FAL to 3-acetyl-1-propanol, and finally 3-acetyl-1-propanol to 1,4-PDO in terms of obtained activation energies and rate constants.
The above-mentioned Ru and Pt-based catalysts are able to promote the conversion of furfural to 1,4-PDO under gentle conditions. However, Ru and Pt are noble and rare metals. In comparison, transition metals such as Ni and Cu are cheap metals, which hold great potential for high catalytic performance upon elaborately structural designs and controls. Shimazu et al.16 achieved a 1,4-PDO yield of 92% over the bulk Ni–Sn alloy catalyst at 160 °C and 3 MPa H2 in an ethanol–water system (Table 2). The catalytic reaction mechanism involves in the furfural hydrogenation to FAL (Fig. 5), and protonation, dehydration and sequential hydrogenation to 2-MF. The generated 2-MF undergoes partial hydrogenation to yield 2-methyl-4,5-dihydrofuran, which is further hydrogenated to 2-hydroxy-2-methyltetrahydrofuran (2H2MTHF) on the Ni–Sn alloy surface. Upon ring-chain tautomerism via an equilibration reaction, 3-acetyl-1-propanol is formed and facilely hydrogenated to 1,4-PDO. Various characterization techniques confirm that Ni–SnOx species exhibit Brφnsted acidity that can promote dehydration, and the Ni3Sn2 alloy serves as a hydrogenation active site. These two species synergistically contribute to the hydrogenation of furfural and dehydration of FAL to key 2-MF.79
 | ||
| Fig. 5 Reaction mechanism for the conversion of furfural into 1,4-PDO over the bulk Ni–Sn alloy catalyst. EtOH: ethanol, 3-AP: 3-acetyl-1-propanol. 1,4-PeD: 1,4-pentanediol (1,4-PDO). Reproduced from ref. 16 with permission from the Royal Society of Chemistry, copyright (2019). | ||
To date, several mechanisms have been proposed for one-pot conversion of furfural into 1,4-PDO depending on the employed solvent and catalyst. Very recently, Wang and co-workers reported a new pathway to produce 1,4-PDO with a high yield of 86.2% via ethanol-induced transformation of furfural (entry 6).22 The Cu/SiO2 catalyst derived from copper phyllosilicate enables high Cu dispersion and adequate numbers of acid sites that originated from coordinatively unsaturated Cu2+ and surface hydroxyl groups of copper phyllosilicate. As displayed in Fig. 6, the metallic Cu sites are capable of promoting furfural hydrogenation to FAL. This is followed by acid-catalyzed etherification between FAL and ethanol to produce 2-ethoxymethyl-furan (2-(EoM)F), which is then degraded into ethyl levulinate. Ethyl levulinate undergoes sequential hydrogenation to 1,4-PDO over Cu sites via formation of GVL and 3-acetyl-1-propanol intermediates.
 | ||
| Fig. 6 Reaction pathway for the conversion of furfural into 1,4-PDO over the Cu/SiO2–AE catalyst. 3-AP: 3-acetyl-1-propanol. EL: ethyl levulinate. Reproduced from ref. 22 with permission from the Royal Society of Chemistry, copyright (2023). | ||
3.2. Conversion of furfural derivatives into 1,4-PDO
FAL can be facilely produced from furfural hydrogenation over Cu-based catalysts.80,81 Direct hydrogenolysis of FAL into 1,2-PDO and 1,5-PDO has been reported with satisfactory yields under mild conditions,82,83 but one-step conversion of FAL into 1,4-PDO is a challenge for its complex reaction pathways catalyzed by metal-acid bifunctional catalysts. At high pressure of H2, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of FAL is preferentially hydrogenated to generate 2-MF and 2-MTHF and the C–O bond undergoes hydrogenolysis to produce 1,2-PDO and 1,5-PDO over metal or metal–acid bifunctional catalysts, which greatly retards the formation of 1,4-PDO.82,84 Zhu et al.85 separately loaded HZSM-5 and CuMgAl catalysts in the upper and lower layers of a fixed-bed reactor, respectively. HZSM-5 promoted FAL alcoholysis with ethanol to ethyl levulinate, which can be exclusively produced without the occurrence of hydrogenation by-reaction of FAL (Scheme 5). The formed ethyl levulinate is further hydrogenated to 1,4-PDO over the CuMgAl catalyst in the lower layer. Interestingly, the 1,4-PDO yield reached 94.3%. Moreover, no obvious deactivation was observed within 220 h. FAL alcoholysis was conducted in the confined acidic pores of HZSM-5 and an increased electron cloud density around Cu in the CuMgAl facilitates the activation of the EL carbonyl group.
C bond of FAL is preferentially hydrogenated to generate 2-MF and 2-MTHF and the C–O bond undergoes hydrogenolysis to produce 1,2-PDO and 1,5-PDO over metal or metal–acid bifunctional catalysts, which greatly retards the formation of 1,4-PDO.82,84 Zhu et al.85 separately loaded HZSM-5 and CuMgAl catalysts in the upper and lower layers of a fixed-bed reactor, respectively. HZSM-5 promoted FAL alcoholysis with ethanol to ethyl levulinate, which can be exclusively produced without the occurrence of hydrogenation by-reaction of FAL (Scheme 5). The formed ethyl levulinate is further hydrogenated to 1,4-PDO over the CuMgAl catalyst in the lower layer. Interestingly, the 1,4-PDO yield reached 94.3%. Moreover, no obvious deactivation was observed within 220 h. FAL alcoholysis was conducted in the confined acidic pores of HZSM-5 and an increased electron cloud density around Cu in the CuMgAl facilitates the activation of the EL carbonyl group.
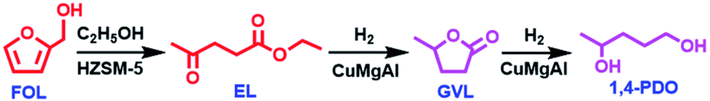 | ||
| Scheme 5 Reaction pathway for the conversion of FAL into 1,4-PDO over HZSM-5 and CuMgAl catalysts. EL: ethyl levulinate. Reproduced from ref. 85 with permission from the Royal Society of Chemistry, copyright (2022). | ||
2-MF can be sustainably obtained from furfural hydrogenation.86,87 Thus, it is a biomass-related industrial product, and the development of processes for the conversion of 2-MF into value-added products such as 1,4-PDO is of interest. In 1947, Schniepp and co-workers made an attempt to produce 1,4-PDO from 2-MF hydrogenation in the presence of water and minor formic acid,88 and about 50%–60% of 1,4-PDO yield was obtained. Very recently, water was demonstrated to participate in the furan ring-opening reaction in the process of 2-MF to 1,4-PDO over the PtNi bimetallic species supported on multi-walled carbon nanotubes (MWCNTs).89 PtNi/MWCNTs gave a 1,4-PDO yield of about 69% with the aid of water. As shown in Fig. 7, 2-MF is preferentially adsorbed on the PtNi surface through the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, which is rapidly hydrogenated to 2-methyl-2,3-dihydrofuran (2-M-2,3-DIF). The H218O isotope labeling experiment and kinetic study results definitely confirm that water provides abundant hydroxyl groups at the C(2) position of 2-M-2,3-DIF for the formation of the key intermediate 2-methyltetrahydrofuran-2-ol (2-ME-2OL). Subsequently, intra-molecular hydrogen transfers from hydroxyl groups of the C(2) site to the O (1) group. Simultaneously, the α C–O bond is cleaved to produce 1-hydroxy-4-pentanone (1-H-4-PeO) via the ring-opening reaction with the H+ attack. This process is the rate-determining step of 2-MF to 1,4-PDO. The formed 1-H-4-PeO is subsequently hydrogenated to the aimed product of 1,4-PDO. Notably, MWCNTs were superior to other supports (ZrO2, Al2O3 and Nb2O5) in the improvement of 1,4-PDO selectivity due to the weak surface acid sites. Additionally, PtNi/MWCNTs showed high stability for at least 8 consecutive cycles, in which the 1,4-PDO yield only gently decreased from 69% to 51% because of slight Pt and Ni leaching.
C bond, which is rapidly hydrogenated to 2-methyl-2,3-dihydrofuran (2-M-2,3-DIF). The H218O isotope labeling experiment and kinetic study results definitely confirm that water provides abundant hydroxyl groups at the C(2) position of 2-M-2,3-DIF for the formation of the key intermediate 2-methyltetrahydrofuran-2-ol (2-ME-2OL). Subsequently, intra-molecular hydrogen transfers from hydroxyl groups of the C(2) site to the O (1) group. Simultaneously, the α C–O bond is cleaved to produce 1-hydroxy-4-pentanone (1-H-4-PeO) via the ring-opening reaction with the H+ attack. This process is the rate-determining step of 2-MF to 1,4-PDO. The formed 1-H-4-PeO is subsequently hydrogenated to the aimed product of 1,4-PDO. Notably, MWCNTs were superior to other supports (ZrO2, Al2O3 and Nb2O5) in the improvement of 1,4-PDO selectivity due to the weak surface acid sites. Additionally, PtNi/MWCNTs showed high stability for at least 8 consecutive cycles, in which the 1,4-PDO yield only gently decreased from 69% to 51% because of slight Pt and Ni leaching.
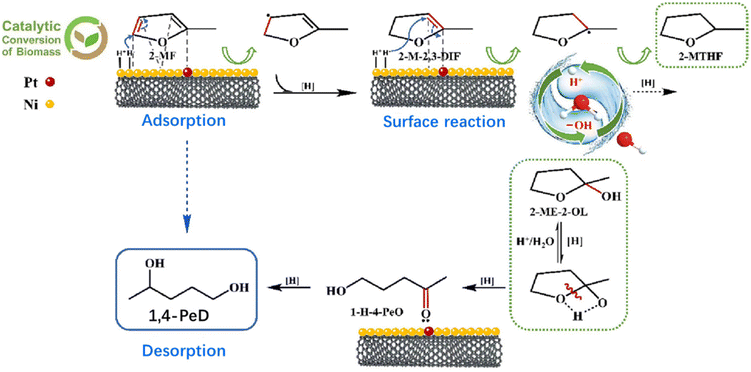 | ||
| Fig. 7 Proposed reaction pathway for water-promoted hydrogenolysis of 2-MF to 1,4-PDO over the PtNi/MWNT catalyst. 1,4-PeD: 1,4-pentanediol. Reproduced from ref. 89 Copyright (2023), with permission from Elsevier. | ||
2-MTHF is currently produced through 2-MF hydrogenation or furfural sequential hydrogenation.90,91 It has been reported that 2-MTHF can be efficiently produced from LA and its derivatives of alkyl levulinate and GVL.92–94 Fu et al. developed a two-step method to convert 2-MTHF into 1,4-PDO with a total yield of 77.9%,95 as displayed in Scheme 6. In the presence of acetic acid (AcOH), 2-MTHF was converted into 1,4-diacetoxypentane with a yield of 82% on the Sc(OTf)3 catalyst. Once the residual AcOH was separated from the reactor, the formed 1,4-diacetoxypentane was facilely transformed into 1,4-PDO via saponification when adding NaOH solution.
 | ||
| Scheme 6 Conversion of 2-MTHF into 1,4-PDO. MeOH: methanol. 1,4-PDA: 1,4-diacetoxypentane. Reproduced from ref. 95 Copyright (2018), with permission from Wiley-VCH. | ||
The industrial production of LA and its derivatives are facing many challenges from the hydrolysis/alcoholysis of cellulose. The purification of LA from water solution needs high energy consumption. Compared to LA, furfural and its derivatives have been commercially produced from hemicellulose, which has formed a very complete industrial chain. However, the abundant furfural residue and the strongly corrosive sulfuric acid process bring about severe environmental pollution. Thus, furfural has much cheaper price than LA, but shows relatively low greenness. Additionally, the acidic LA usually corrodes the catalyst and leads to the leaching of active species. However, the synthesis of 1,4-PDO from furfural requires to undergo complex tandem reactions and forms humins in water, resulting in a relatively low 1,4-PDO yield.
4. Current challenges and future prospects for 1,4-PDO production from biomass
Regardless of these great advances on the production of 1,4-PDO from biomass, many challenges should be faced, which has retarded large-scale commercial applications. (1) Although the catalytic conversion LA or furfural and their derivatives achieve high yields of 1,4-PDO under mild conditions, most of the catalytic reactions and processes are performed in batch reactors with low feed concentrations. These non-continuous processes have low production efficiency, bear strong labor load, and require high H2 pressure. Additionally, the used catalysts unavoidably need to separate with reaction products, which can lose partial catalysts and likely raises operation costs. (2) Most of processes employ relatively rare and expensive noble metal catalysts in order to achieve good performance and improve the hydrothermal stability during high temperature and pressure environment. Particularly, the most used Ru element occupies just 0.00004 parts per million in Earth's crust. (3) Water is a green and low-price solvent that is naturally involved in biomass. Thus, water is an unavoidable and promising solvent for 1,4-PDO production. However, the use of water can lead to the formation of humins, particularly for the conversion of furan compounds, which can seriously affect the carbon balance and greatly enhance the separation cost. The hydrothermal condition and strong acid of LA in aqueous solution result in serious sintering and leaching of the metal catalyst. Moreover, the role of water on the catalyst structure and reaction mechanism has not been thoroughly investigated. (4) The production of 1,4-PDO from either LA or furfural involves several individual metal and acid-catalyzed reactions, and therefore the synergistic effect between metal and acid sites is crucial for enhancing the production rate of 1,4-PDO. The currently used catalysts don't consider the proximity of metal and acid sites, which remarkably affects the diffusion of reaction intermediates and reaction rate owing to spatially separated metal NPs and acid sites. (5) Previous research studies predominantly focused on the catalytic performance (activity, selectivity and stability) and reaction condition optimization, while the reaction mechanism has been rarely investigated in detail. The complex cascade reaction process as well as harsh reaction conditions greatly enhance the difficulties to probe catalyst structure evolution during the reaction process by in situ or operando characterization techniques.Biomass is an abundant renewable carbon source with carbon neutrality. Conversion of biomass-based LA, furfural and their derivatives is an effective way to decline the reliance on fossil resources. To achieve this goal, many challenging and long-standing issues should be resolved by tailoring an advanced catalyst and deep recognition of the reaction mechanism. Future research is emphasized in following directions that could bring about significant progress in 1,4-PDO production. (1) Continuous flow or slurry bed reactors can significantly enhance the production efficiency of 1,4-PDO, which is beneficial for large-scale application. Measurement of accurate and meaningful kinetic parameters enables the design of an optimal reactor. Meanwhile, it is necessary to probe the best reaction route in the thermodynamic state space. Tracking a fluid element on its way through the reactor, manipulating the flux profiles and minimizing the residence time are also essential prerequisites for reactor optimization.96 (2) Further efforts are needed to decline the catalyst cost that is still a main limitation for commercial production of 1,4-PDO. A single atom catalyst offers the maximum atom efficiency and 100% metal dispersion.42,97 Thus, this is an ideal strategy to create single metal atoms anchored on supports, which can remarkably reduce the cost of using expensive noble metals. Additionally, the development of Earth-abundant alloy catalysts can save the costs and construct a robust structure via the formation of relatively strong alloy bonds to stabilize metal atoms against dissolution. Alloying can also tune the electronic structure and decrease the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group adsorption energy of LA and furfural, which has been shown to enhance catalytic activity.53 (3) The use of a high substrate concentration can obviously decline the production costs. It is indispensable to exploit efficient separation methods such as using a two-phase system with immiscible organics or a deep eutectic solvent, which can simplify difficult and energy-intensive isolation procedures. Additionally, fabrication of water-resistant catalysts is an effective method to synthesize 1,4-PDO in water. Many catalysts with core–shell, alloy, or confinement structures are endowed with good hydrothermal stability.53,98 Alternatively, active metal leaching by acidic LA can be circumvented by selectively covering hydrophobic support. It is also necessary to probe the role of water in catalyst leaching and the reaction mechanism by advanced techniques such as operando Raman spectroscopy and D2O-isotope labeling. (4) To achieve catalytic benefits from bifunctional active sites outperforms those from a single optimal active site, because the cooperation between different types of active sites can remarkably enhance the 1,4-PDO yield for cascade conversion of LA/furfural.99 To improve the proximity of metal and acid sites for maximizing their synergy effect, it is essential to tailor the distribution of catalytic sites and control the catalyst structure with the formation of a metal–acid interface. Encapsulation of metal NPs into the pores of zeolites can fabricate the geometrically closest metal NPs with acid sites, which greatly shortens the diffusion distance of LA/furfural molecules to access the metal–acid active sites.100,101 Construction of well-separated metal–MoOx (or ReOx) pair catalysts can form abundant metal–acid interfaces,99 in which pairs of active sites work synergistically to promote 1,4-PDO production from LA or furfural. Additionally, the geometry structure of the metal–MoOx interface, adaptive reconstruction and inter-site communication are crucial for dual catalytic behavior during the reaction process.99 (5) Powerful operando characterization techniques or density functional theory (DFT) calculations tools are applied to elucidate the cascade reaction mechanism of LA or furfural to 1,4-PDO. Operando liquid nuclear magnetic and X-ray absorption spectra that are adaptable to high-temperature and high-pressure liquid media can be used to trap key intermediates (GVL and 3-acetyl-1-propanol) and obtain kinetic data. DFT calculation based on machine learning molecular dynamics simulation has been used as a general and automated way to visualize and understand the surface reaction mechanism referring to each elementary reaction29,102 The metal–acid dual site structure directly involves in the catalytic cycle by adsorbing and activating LA/furfural as well as intermediates. The reaction process including the transition state configuration and the energy change during every step can be traced and quantitatively calculated at the atomic level. (6) The harness of biomass via emerging photo-/electro-catalytic approaches may foster the development of green H2 production. Combining furfural or LA hydrogenation at the anode and the hydrogen evolution reaction at the cathode in a biomass electrolysis or photo-reforming reactor perhaps affords an appealing strategy for producing 1,4-PDO with low environmental costs and carbon footprint.103
O group adsorption energy of LA and furfural, which has been shown to enhance catalytic activity.53 (3) The use of a high substrate concentration can obviously decline the production costs. It is indispensable to exploit efficient separation methods such as using a two-phase system with immiscible organics or a deep eutectic solvent, which can simplify difficult and energy-intensive isolation procedures. Additionally, fabrication of water-resistant catalysts is an effective method to synthesize 1,4-PDO in water. Many catalysts with core–shell, alloy, or confinement structures are endowed with good hydrothermal stability.53,98 Alternatively, active metal leaching by acidic LA can be circumvented by selectively covering hydrophobic support. It is also necessary to probe the role of water in catalyst leaching and the reaction mechanism by advanced techniques such as operando Raman spectroscopy and D2O-isotope labeling. (4) To achieve catalytic benefits from bifunctional active sites outperforms those from a single optimal active site, because the cooperation between different types of active sites can remarkably enhance the 1,4-PDO yield for cascade conversion of LA/furfural.99 To improve the proximity of metal and acid sites for maximizing their synergy effect, it is essential to tailor the distribution of catalytic sites and control the catalyst structure with the formation of a metal–acid interface. Encapsulation of metal NPs into the pores of zeolites can fabricate the geometrically closest metal NPs with acid sites, which greatly shortens the diffusion distance of LA/furfural molecules to access the metal–acid active sites.100,101 Construction of well-separated metal–MoOx (or ReOx) pair catalysts can form abundant metal–acid interfaces,99 in which pairs of active sites work synergistically to promote 1,4-PDO production from LA or furfural. Additionally, the geometry structure of the metal–MoOx interface, adaptive reconstruction and inter-site communication are crucial for dual catalytic behavior during the reaction process.99 (5) Powerful operando characterization techniques or density functional theory (DFT) calculations tools are applied to elucidate the cascade reaction mechanism of LA or furfural to 1,4-PDO. Operando liquid nuclear magnetic and X-ray absorption spectra that are adaptable to high-temperature and high-pressure liquid media can be used to trap key intermediates (GVL and 3-acetyl-1-propanol) and obtain kinetic data. DFT calculation based on machine learning molecular dynamics simulation has been used as a general and automated way to visualize and understand the surface reaction mechanism referring to each elementary reaction29,102 The metal–acid dual site structure directly involves in the catalytic cycle by adsorbing and activating LA/furfural as well as intermediates. The reaction process including the transition state configuration and the energy change during every step can be traced and quantitatively calculated at the atomic level. (6) The harness of biomass via emerging photo-/electro-catalytic approaches may foster the development of green H2 production. Combining furfural or LA hydrogenation at the anode and the hydrogen evolution reaction at the cathode in a biomass electrolysis or photo-reforming reactor perhaps affords an appealing strategy for producing 1,4-PDO with low environmental costs and carbon footprint.103
5. Conclusions
This review summarizes the conversion of biomass-derived LA and furfural as well as their derivatives into valuable 1,4-PDO. The used catalysts, structure–performance correlation, reaction pathway and mechanism have been well discussed in detail. Comparatively, the reaction pathway of LA-to-1,4-PDO has been well identified, in which LA initially hydrogenates to 4-hydroxylpentanoic acid, which proceeds with lactonization to GVL, and it undergoes ring-opening to 1,4-PDO. However, the reaction pathway of furfural to 1,4-PDO is still a debate, which highly depends on the used catalyst and reaction solvent. Conversion of LA suffers from catalyst deactivation owing to the acidic properties of LA, and thus there are emerging ReOx/MoOx modified noble metal catalysts or non-noble metals with special structures such as core–shell or alloy. Conversion of furfural to 1,4-PDO involves a complex and variable cascade reaction, which greatly enhances the difficulty in understanding the reaction mechanism.Although 1,4-PDO synthesis from biomass has achieved notable advances, particularly for obtaining a high yield of 1,4-PDO, a variety of challenges, such as process development, catalysts, solvent, active site distribution and reaction mechanism, should be faced ahead of the industrial production. We provide various strategies for improving the possibility of large-scale application of 1,4-PDO and driving future research toward the development of profitable biorefinery operations for highly-efficient conversion of biomass into 1,4-PDO. In summary, it is of great significance to exploit an economically viable technique for expanding the biomass conversion roadmap of 1,4-PDO for achieving both carbon neutrality and environmental protection, which will offer potential opportunities for multidisciplinary experts involving material scientists, chemists, engineers and economists.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We greatly acknowledge the financial support by the National Natural Science Foundation of China (22278416), the Fundamental Research Program of Shanxi Province for Excellent Young Scholars (20210302122015), and ICC CAS (SCJC-DT-2023-02).References
- Y. Jing, Y. Guo, Q. Xia, X. Liu and Y. Wang, Chem, 2019, 5, 2520–2546 CAS.
- M. He, Y. Sun and B. Han, Angew. Chem., Int. Ed., 2022, 61, e202112835 CrossRef CAS PubMed.
- L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed.
- M. M. Abu-Omar, K. Barta, G. T. Beckham, J. S. Luterbacher, J. Ralph, R. Rinaldi, Y. Román-Leshkov, J. S. M. Samec, B. F. Sels and F. Wang, Energy Environ. Sci., 2021, 14, 262–292 RSC.
- Y. Liao, S.-F. Koelewijn, G. V. D. Bossche, J. V. Aelst, S. V. D. Bosch, T. Renders, K. Navare, T. Nicolaï, K. V. Aelst, M. Maesen, H. Matsushima, J. M. Thevelein, K. V. Acker, B. Lagrain, D. Verboekend and B. F. Sels, Science, 2020, 367, 1385–1390 CrossRef CAS PubMed.
- Y. Liu, N. Deak, Z. Wang, H. Yu, L. Hameleers, E. Jurak, P. J. Deuss and K. Barta, Nat. Commun., 2021, 12, 5424 CrossRef CAS PubMed.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef PubMed.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sadaba and M. L. Granados, Energy Environ. Sci., 2016, 9, 1144–1189 RSC.
- J.-P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150–166 CrossRef CAS PubMed.
- D. Di Menno Di Bucchianico, Y. Wang, J.-C. Buvat, Y. Pan, V. Casson Moreno and S. Leveneur, Green Chem., 2022, 24, 614–646 RSC.
- Z. Yu, X. Lu, J. Xiong, X. Li, H. Bai and N. Ji, ChemSusChem, 2020, 13, 2916–2930 CrossRef CAS PubMed.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- Q. Wang, J. Feng, L. Zheng, B. Wang, R. Bi, Y. He, H. Liu and D. Li, ACS Catal., 2020, 10, 1353–1365 CrossRef CAS.
- B. Katryniok, H. Kimura, E. Skrzynska, J.-S. Girardon, P. Fongarland, M. Capron, R. Ducoulombier, N. Mimura, S. Paul and F. Dumeignil, Green Chem., 2011, 13, 1960–1979 RSC.
- Y.-K. Hong, D.-W. Lee, H.-J. Eom and K.-Y. Lee, Appl. Catal., B, 2014, 150–151, 438–445 CrossRef CAS.
- Rodiansono, M. Dewi Astuti, T. Hara, N. Ichikuni and S. Shimazu, Green Chem., 2019, 21, 2307–2315 RSC.
- Y. Wei, J. Lu, S. Zhang, C. Wu, X. Nong, J. Li, C.-L. Liu and W.-S. Dong, Chem. Commun., 2023, 59, 2477–2480 RSC.
- B. M. Stadler, A. Brandt, A. Kux, H. Beck and J. G. de Vries, ChemSusChem, 2020, 13, 556–563 CrossRef CAS PubMed.
- E. Benisvy-Aharonovicha, A. Zandanya, A. Saadya, Y. Kinel-Tahanb, Y. Yehoshuab and A. Gedanken, Bioresour. Technol. Rep., 2020, 11, 100514 CrossRef PubMed.
- T. Sugimura, H. Iguchi, R. Tsuchida, A. Tai, N. Nishiyama and T. Hakushi, Tetrahedron: Asymmetry, 1998, 9, 1007–1013 CrossRef CAS.
- T. Sugimura, H. Yamada, S. Inoue and A. Tai, Tetrahedron: Asymmetry, 1997, 8, 649–655 CrossRef CAS.
- Y. Zheng, J. Zang, Q. Zhang, X. Wu, S. Qiu, Q. Meng and T. Wang, Green Chem., 2023, 25, 1128–1136 RSC.
- F. Liu, Q. Liu, J. Xu, L. Li, Y.-T. Cui, R. Lang, L. Li, Y. Su, S. Miao, H. Sun, B. Qiao, A. Wang, F. Jérôme and T. Zhang, Green Chem., 2018, 20, 1770–1776 RSC.
- Y. Shao, K. Sun, Q. Li, Q. Liu, S. Zhang, Q. Liu, G. Hu and X. Hu, Green Chem., 2019, 21, 4499–4511 RSC.
- C. Michel and P. Gallezot, ACS Catal., 2015, 5, 4130–4132 CrossRef CAS.
- S. Shao, Y. Yang, K. Sun, S. Yang, A. Li, F. Yang, X. Luo, S. Hao and Y. Ke, ACS Catal., 2021, 11, 12146–12158 CrossRef CAS.
- W. Cao, W. Luo, H. Ge, Y. Su, A. Wang and T. Zhang, Green Chem., 2017, 19, 2201–2211 RSC.
- K. Zhang, Q. Meng, H. Wu, T. Yuan, S. Han, J. Zhai, B. Zheng, C. Xu, W. Wu, M. He and B. Han, Green Chem., 2021, 23, 1621–1627 RSC.
- X. Gao, S. Zhu, M. Dong, J. Wang and W. Fan, J. Catal., 2020, 389, 60–70 CrossRef CAS.
- J. Tan, J. Cui, T. Deng, X. Cui, G. Ding, Y. Zhu and Y. Li, ChemCatChem, 2015, 7, 508–512 CrossRef CAS.
- V. K. Velisoju, G. B. Peddakasu, N. Gutta, V. Boosa, M. Kandula, K. V. R. Chary and V. Akula, J. Phys. Chem. C, 2018, 122, 19670–19677 CrossRef CAS.
- J. Lv, Z. Rong, L. Sun, C. Liu, A.-H. Lu, Y. Wang and J. Qu, Catal. Sci. Technol., 2018, 8, 975–979 RSC.
- R. Wang, L. Chen, X. Zhang, Q. Zhang, Y. Li, C. Wang and L. Ma, RSC Adv., 2018, 8, 40989–40995 RSC.
- M. Tamura, R. Tamura, Y. Takeda, Y. Nakagawa and K. Tomishige, Chem. – Eur. J., 2015, 21, 3097–3107 CrossRef CAS PubMed.
- Z. Wang, G. Li, X. Liu, Y. Huang, A. Wang, W. Chu, X. Wang and N. Li, Catal. Commun., 2014, 43, 38–41 CrossRef CAS.
- Y. Takeda, T. Shoji, H. Watanabe, M. Tamura, Y. Nakagawa, K. Okumura and K. Tomishige, ChemSusChem, 2015, 8, 1170–1178 CrossRef CAS PubMed.
- L. Corbel-Demailly, B.-K. Ly, D.-P. Minh, B. Tapin, C. Especel, F. Epron, A. Cabiac, E. Guillon, M. Besson and C. Pinel, ChemSusChem, 2013, 6, 2388–2395 CrossRef CAS PubMed.
- M. Li, G. Li, N. Li, A. Wang, W. Dong, X. Wang and Y. Cong, Chem. Commun., 2014, 50, 1414–1416 RSC.
- J. Cui, J. Tan, Y. Zhu and F. Cheng, ChemSusChem, 2018, 11, 1316–1320 CrossRef CAS PubMed.
- Rodiansono, A. S. Azzahra, H. P. Dewi, I. B. Adilina and K. C. Sembiring, Catal. Sci. Technol., 2023, 13, 4466–4476 RSC.
- T. Mizugaki, Y. Nagatsu, K. Togo, Z. Maeno, T. Mitsudome, K. Jitsukawa and K. Kaneda, Green Chem., 2015, 17, 5136–5139 RSC.
- P. Liu, Y. Zhao, R. Qin, S. Mo, G. Chen, L. Gu, D. M. Chevrier, P. Zhang, Q. Guo, D. Zang, B. Wu, G. Fu and N. Zheng, Science, 2016, 352, 797–800 CrossRef CAS PubMed.
- L. Lin, W. Zhou, R. Gao, S. Yao, X. Zhang, W. Xu, S. Zheng, Z. Jiang, Q. Yu, Y.-W. Li, C. Shi, X.-D. Wen and D. Ma, Nature, 2017, 544, 80–83 CrossRef CAS PubMed.
- A. J. Therrien, A. J. R. Hensley, M. D. Marcinkowski, R. Zhang, F. R. Lucci, B. Coughlin, A. C. Schilling, J.-S. Mc Ewen and E. C. H. Sykes, Nat. Catal., 2018, 1, 192–198 CrossRef CAS.
- J. Zhang, M. Wang, Z. Gao, X. Qin, Y. Xu, Z. Wang, W. Zhou and D. Ma, J. Am. Chem. Soc., 2022, 144, 5108–5115 CrossRef CAS PubMed.
- X. Zhang, M. Zhang, Y. Deng, M. Xu, L. Artiglia, W. Wen, R. Gao, B. Chen, S. Yao, X. Zhang, M. Peng, J. Yan, A. Li, Z. Jiang, X. Gao, S. Cao, C. Yang, A. J. Kropf, J. Shi, J. Xie, M. Bi, J. A. van Bokhoven, Y.-W. Li, X. Wen, M. Flytzani-Stephanopoulos, C. Shi, W. Zhou and D. Ma, Nature, 2021, 589, 396–401 CrossRef CAS PubMed.
- X. Lu, T. Luo, M. Zhang, J. Hugh Horton, Q. Wu, W. Wu, M. Qiao, Y. Wang and Z. Li, Chem. Eng. J., 2023, 464, 142647 CrossRef CAS.
- W. Zhan, Q. He, X. Liu, Y. Guo, Y. Wang, L. Wang, Y. Guo, A. Y. Borisevich, J. Zhang, G. Lu and S. Dai, J. Am. Chem. Soc., 2016, 138, 16130–16139 CrossRef CAS PubMed.
- F. Bucciol, S. Tabasso, G. Grillo, F. Menegazzo, M. Signoretto, M. Manzoli and G. Cravotto, J. Catal., 2019, 380, 267–277 CrossRef CAS.
- T. J. Korstanje, J. Ivar van der Vlugt, C. J. Elsevier and B. de Bruin, Science, 2015, 350, 298–302 CrossRef CAS PubMed.
- N. Karanwal, M. G. Sibi, M. K. Khan, A. A. Myint, B. Chan Ryu, J. W. Kang and J. Kim, ACS Catal., 2021, 11, 2846–2864 CrossRef CAS.
- X. Gao, S. Zhu, M. Dong and W. Fan, J. Catal., 2021, 399, 201–211 CrossRef CAS.
- S. Zhu, H. Liu, S. Wang, X. Gao, P. Wang, J. Wang and W. Fan, Appl. Catal., B, 2021, 284, 119698 CrossRef CAS.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355 Search PubMed.
- K.-I. Tominaga, A. Mori, Y. Fukushima, S. Shimada and K. Sato, Green Chem., 2011, 13, 810–812 RSC.
- D. Zhao, P. Prinsen, Y. Wang, W. Ouyang, F. Delbecq, C. Len and R. Luque, ACS Sustainable Chem. Eng., 2018, 6, 6901–6909 CrossRef CAS.
- J.-P. Lange, W. D. van de Graaf and R. J. Haan, ChemSusChem, 2009, 2, 437–441 CrossRef CAS PubMed.
- H. Tian, Y. Shao, C. Liang, Q. Xu, L. Zhang, S. Zhang, S. Liu and X. Hu, Renewable Energy, 2020, 162, 1576–1586 CrossRef CAS.
- S. Zhu, C. Chen, Y. Xue, J. Wu, J. Wang and W. Fan, ChemCatChem, 2014, 6, 3080–3083 CrossRef CAS.
- D. Ren, X. Wan, F. Jin, Z. Song, Y. Liu and Z. Huo, Green Chem., 2016, 18, 5999–6003 RSC.
- J. Wu, G. Gao, P. Sun, X. Long and F. Li, ACS Catal., 2017, 7, 7890–7901 CrossRef CAS.
- Y. Yang, X. Xu, W. Zou, H. Yue, G. Tian and S. Feng, Catal. Commun., 2016, 76, 50–53 CrossRef CAS.
- M. Fan, Y. Shao, K. Sun, Q. Li, S. Zhang, Y. Wang, J. Xiang, S. Hu, S. Wang and X. Hu, Mol. Catal., 2021, 510, 111680 CrossRef CAS.
- Y. Shao, S. Ba, K. Sun, G. Gao, M. Fan, J. Wang, H. Fan, L. Zhang and X. Hu, Chem. Eng. J., 2022, 429, 132433 CrossRef CAS.
- Y. Zhu, Y. Zhu, G. Ding, S. Zhu, H. Zheng and Y. Li, Appl. Catal., A, 2013, 468, 296–304 CrossRef CAS.
- L. Huang, Y. Zhu, H. Zheng, M. Du and Y. Li, Appl. Catal., A, 2008, 349, 204–211 CrossRef CAS.
- Q. Xu, C. Wang, Z. Shang, C. Zhang, X. Wang, Q. Liu, L. Dang, Y. Liu and F. Zhao, Appl. Catal., B, 2022, 317, 121689 CrossRef CAS.
- X.-L. Du, Q.-Y. Bi, Y.-M. Liu, Y. Cao, H.-Y. He and K.-N. Fan, Green Chem., 2012, 14, 935–939 RSC.
- J. Wu, G. Gao, Y. Li, P. Sun, J. Wang and F. Li, Appl. Catal., B, 2019, 245, 251–261 CrossRef CAS.
- Q. Liu, Z. Zhao, M. Arai, C. Zhang, K. Liu, R. Shi, P. Wu, Z. Wang, W. Lin, H. Cheng and F. Zhao, Catal. Sci. Technol., 2020, 10, 4412–4423 RSC.
- D. Cavuoto, N. Ravasio, N. Scotti, A. Gervasini, S. Campisi, M. Marelli, G. Cappelletti and F. Zaccheria, Mol. Catal., 2021, 516, 111936 CrossRef CAS.
- S. Zhu, Y. Cen, J. Guo, J. Chai, J. Wang and W. Fan, Green Chem., 2016, 18, 5667–5675 RSC.
- G. J. Leuck, J. Pokorny and F. N. Peters, US Pat., 2097493, Nov. 2, 1937 Search PubMed.
- Q. Liu, B. Qiao, F. Liu, L. Zhang, Y. Su, A. Wang and T. Zhang, Green Chem., 2020, 22, 3532–3538 RSC.
- H. J. Cho, D. Kim, S. Li, D. Su, D. Ma and B. Xu, ACS Catal., 2020, 10, 3340–3348 CrossRef CAS.
- J. He, Z. Wu, Q. Gu, Y. Liu, S. Chu, S. Chen, Y. Zhang, B. Yang, T. Chen, A. Wang, B. M. Weckhuysen, T. Zhang and W. Luo, Angew. Chem., Int. Ed., 2021, 60, 23713–23721 CrossRef CAS PubMed.
- L. Zhang, X. Li, J. Wang, Z. Zeng, W. Yang, S. Deng and Q. Deng, AIChE J., 2023, 69, e17996 CrossRef CAS.
- K. Cui, W. Qian, Z. Shao, X. Zhao, H. Gong, X. Wei, J. Wang, M. Chen, X. Cao and Z. Hou, Catal. Lett., 2021, 151, 2513–2526 CrossRef CAS.
- Rodiansono, A. S. Azzahra, P. R. Ansyah, S. Husain and S. Shimazu, RSC Adv., 2023, 13, 21171–21181 RSC.
- P. Cao, L. Lin, H. Qi, R. Chen, Z. Wu, N. Li, T. Zhang and W. Luo, ACS Catal., 2021, 11, 10246–10256 CrossRef CAS.
- K. Chen, J.-L. Ling and C.-D. Wu, Angew. Chem., Int. Ed., 2020, 59, 1925–1931 CrossRef CAS PubMed.
- B. Zhang, Y. Zhu, G. Ding, H. Zheng and Y. Li, Green Chem., 2012, 14, 3402–3409 RSC.
- R. Ma, X.-P. Wu, T. Tong, Z.-J. Shao, Y. Wang, X. Liu, Q. Xia and X.-Q. Gong, ACS Catal., 2017, 7, 333–337 CrossRef CAS.
- Y. Zhu, W. Zhao, J. Zhang, Z. An, X. Ma, Z. Zhang, Y. Jiang, L. Zheng, X. Shu, H. Song, X. Xiang and J. He, ACS Catal., 2020, 10, 8032–8041 CrossRef CAS.
- H. Wang, Y. Wang, L. Huang, A. Geng, F. Yi, Y. Zhu and Y. Li, Sustainable Energy Fuels, 2022, 6, 2449–2461 RSC.
- Y. Deng, R. Gao, L. Lin, T. Liu, X.-D. Wen, S. Wang and D. Ma, J. Am. Chem. Soc., 2018, 140, 14481–14489 CrossRef CAS PubMed.
- S. Iqbal, X. Liu, O. F. Aldosari, P. J. Miedziak, J. K. Edwards, G. L. Brett, A. Akram, G. M. King, T. E. Davies, D. J. Morgan, D. K. Knight and G. J. Hutchings, Catal. Sci. Technol., 2014, 4, 2280–2286 RSC.
- L. E. Schniepp, H. H. Geller and R. W. V. Korff, J. Am. Chem. Soc., 1947, 69, 672–674 CrossRef CAS.
- M. Wu, T. Wang, W. Li, Q. Zhang, B. Zhang, K. Chen, S. Peng, G. Li, J. Huang, Q. Wang and C. Wang, Chem. Eng. J., 2023, 461, 141944 CrossRef CAS.
- P. Liu, L. Sun, X. Jia, C. Zhang, W. Zhang, Y. Song, H. Wang and C. Li, Mol. Catal., 2020, 490, 110951 CrossRef CAS.
- F. Dong, Y. Zhu, G. Ding, J. Cui, X. Li and Y. Li, ChemSusChem, 2015, 8, 1534–1537 CrossRef CAS PubMed.
- A. Phanopoulos, A. J. P. White, N. J. Long and P. W. Miller, ACS Catal., 2015, 5, 2500–2512 CrossRef CAS.
- Y.-B. Huang, A.-F. Liu, Q. Zhang, K.-M. Li, W. B. Porterfield, L.-C. Li and F. Wang, ACS Sustainable Chem. Eng., 2020, 8, 11477–11490 CrossRef CAS.
- Z. Xie, B. Chen, H. Wu, M. Liu, H. Liu, J. Zhang, G. Yang and B. Han, Green Chem., 2019, 21, 606–613 RSC.
- K. Zhang, X.-L. Li, S.-Y. Chen, H.-J. Xu, J. Deng and Y. Fu, ChemSusChem, 2018, 11, 726–734 CrossRef CAS PubMed.
- A. Peschel, H. Freund and K. Sundmacher, Ind. Eng. Chem. Res., 2010, 49, 10535–10548 CrossRef CAS.
- L.-N. Chen, K.-P. Hou, Y.-S. Liu, Z.-Y. Qi, Q. Zheng, Y.-H. Lu, J.-Y. Chen, J.-L. Chen, C.-W. Pao, S.-B. Wang, Y.-B. Li, S.-H. Xie, F.-D. Liu, D. Prendergast, L. E. Klebanoff, V. Stavila, M. D. Allendorf, J. Guo, L.-S. Zheng, J. Su and G. A. Somorjai, J. Am. Chem. Soc., 2019, 141, 17995–17999 CrossRef CAS PubMed.
- N. Wang, Q. Sun, R. Bai, X. Li, G. Guo and J. Yu, J. Am. Chem. Soc., 2016, 138, 7484–7487 CrossRef CAS PubMed.
- I. Ro, J. Qi, S. Lee, M. Xu, X. Yan, Z. Xie, G. Zakem, A. Morales, J. G. Chen, X. Pan, D. G. Vlachos, S. Caratzoulas and P. Christopher, Nature, 2022, 609, 287–292 CrossRef CAS PubMed.
- Z. Jin, X. Yi, L. Wang, S. Xu, C. Wang, Q. Wu, L. Wang, A. Zheng and F.-S. Xiao, Appl. Catal., B, 2019, 254, 560–568 CrossRef CAS.
- J. Yang, Y. He, J. He, Y. Liu, H. Geng, S. Chen, L. Lin, M. Liu, T. Chen, Q. Jiang, B. M. Weckhuysen, W. Luo and Z. Wu, ACS Catal., 2022, 12, 1847–1856 CrossRef CAS.
- Z. Zeng, F. Wodaczek, K. Liu, F. Stein, J. Hutter, J. Chen and B. Cheng, Nat. Commun., 2023, 14, 6131 CrossRef CAS PubMed.
- H. Luo, J. Barrio, N. Sunny, A. Li, L. Steier, N. Shah, I. E. L. Stephens and M. Titirici, Adv. Energy Mater., 2021, 11, 2101180 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |



