Open Access Article
Open Access ArticleEnvironmental impact of different scenarios for the pyrolysis of contaminated mixed plastic waste†
Guillermo
Garcia-Garcia
 *ab,
María Ángeles
Martín-Lara
*ab,
María Ángeles
Martín-Lara
 *a,
Mónica
Calero
*a,
Mónica
Calero
 a and
Gabriel
Blázquez
a and
Gabriel
Blázquez
 a
a
aDepartment of Chemical Engineering, Faculty of Sciences, University of Granada, 18071 Granada, Spain. E-mail: guillermo.garcia@ugr.es; marianml@ugr.es
bDepartment of Agrifood Chain Economics, Institute of Agricultural and Fisheries Research and Training (IFAPA), Centre ‘Camino de Purchil’, 18080 Granada, Spain
First published on 21st February 2024
Abstract
Every day, large amounts of plastic are disposed of all over the world. Most of it is not recycled and ends up polluting the environment. Therefore, waste collection and management must be improved to reduce the environmental impact caused by plastic waste. Pyrolysis has been explored as an alternative to treat contaminated mixed plastic waste and obtain valuable materials, such as oil and char. These materials can effectively substitute fuel and activated carbon, respectively. However, the pyrolysis process also has a significant environmental impact, mainly due to gas emissions. It is important to quantify this environmental impact and compare it with alternative treatment methods to identify the best management strategy for contaminated mixed plastic waste. This study applies the Life-Cycle Assessment methodology to evaluate the environmental impact and compare it with the conventional practice of landfilling. Three different pyrolysis scenarios are considered: one in which the char is used as fuel and therefore combusted, and two in which the char is activated by carbon dioxide and potassium hydroxide, respectively, to be used as an adsorbent. Our results show that pyrolysis is environmentally superior to landfilling for the treatment of contaminated mixed plastic waste. This is mainly due to the production of oil, which substitutes commercial diesel, the production of which has a high environmental impact. Pyrolysis followed by char combustion has the lowest environmental impact of all pyrolysis scenarios considered.
1. Introduction
The wide range of applications of plastics in many different industrial and commercial sectors has made the production of plastics soar in the last decades. This has led to a massive increase in the amount of plastic waste worldwide. Global plastic waste generation more than doubled between 2000 and 2019, with current levels exceeding 350 Mt, with only 9% of plastic waste being recycled, while 19% is incinerated, 50% is landfilled and the rest evades waste management systems.1 This results in huge environmental impacts that seriously affect ecosystems and human health.2 Currently, 93% of plastics are produced from fossil fuels, with only 6% produced from recycled plastics and a negligible amount from biomass.3 Most fossil plastics do not biodegrade, so they persist in nature for long periods of time. There is therefore an urgent need to optimise the collection and management of plastic waste.4 The United Nations Environment Programme is currently working on an international legally binding global agreement on plastic pollution.5 Several countries, regions and cities have recently introduced regulations and legislation primarily aimed at use and disposal of plastic.6Mechanical recycling is the most common recycling option for plastic waste. Mechanical recycling involves washing, separating plastic waste by colour and polymer type, re-melting, forming pellets and using these pellets to produce new plastic products by melting and moulding.7 However, the new plastic produced has different properties to virgin plastic, so the applications of these new plastics are limited. Chemical recycling, on the other hand, makes it possible to produce products from plastic waste for a variety of uses, such as fuels and chemical feedstocks.8,9 Chemical recycling is particularly advantageous for the treatment of contaminated and/or mixed plastic waste due to the economic and technical limitations of mechanical recycling.
Pyrolysis has been successfully used as an environmentally friendly alternative to deal with plastic waste that cannot be treated by mechanical recycling10 and to support a more circular economy.11 Pyrolysis is a thermochemical conversion process that takes place in the absence of oxygen at temperatures between 400 and 600 °C. The products of the chemical reactions that occurs in the pyrolysis process are gases, liquid oil and solid char.12,13 The gases consist mainly of methane, carbon monoxide and hydrogen; the oil is mostly hydrocarbons; and the char is a carbon-rich solid material. The oil can be used as a direct fuel14–16 and the char as a precursor for the production of activated carbon.17,18 As for the gases, they usually provide the energy needed to run the pyrolysis process.19
Contaminated mixed plastic waste can be pyrolyzed respecting the principles of green chemistry,20 particularly the atom economy (Principle 2) and design for energy efficiency (Principle 6). With regard to the former, pyrolysis allows virtually all the atoms present in the waste material to be reused to produce useful chemicals in the form of gas, oil and char. Regarding the latter, although pyrolysis requires high temperatures, the heat generated by the combustion of the pyrolysis gas can be recovered and used in the pyrolysis process itself. Furthermore, the issue of the E-factor, which is closely related to the principles of green chemistry, can also be addressed by pyrolysis, as this process reduces the amount of final waste produced. The E-factor measures the mass of waste per mass of product.21
Nevertheless, the pyrolysis process still has environmental impacts, mainly due to its gas emissions and energy requirements (heat and/or electricity). Life-Cycle Assessment (LCA) is a widely used methodology to calculate the environmental impact of processes. There are recent studies that have assessed the life-cycle environmental impacts of different scenarios for the pyrolysis of plastic waste.22–24 These articles highlight the potential environmental benefits of pyrolysis of plastic waste compared to other waste treatment methods.
This study examines three different scenarios for the pyrolysis of plastic waste: one in which the char is used as a fuel and therefore combusted, and two in which the char is activated with carbon dioxide and potassium hydroxide, respectively, to be used as an adsorbent. LCA is used to calculate the environmental impacts of these three scenarios and compare them with the most common conventional practice of landfilling. In this way, it can be determined whether pyrolysis of plastic waste is environmentally better than the current landfilling and what the best pyrolysis scenario is.
2. Methodology
This article assesses the environmental impact of the pyrolysis and landfilling of contaminated mixed plastic waste from the non-selectively recovery fraction of municipal solid waste by the LCA methodology, following the standards ISO 14040:2006 and ISO 14044:2006.25,26 This study followed a similar approach, regarding the experimental pyrolysis process and LCA methodology, to that described in our previous article.27 The goal and scope and life-cycle inventory are described in the next two subsections, while the life-cycle impact assessment and interpretation are presented in Section 3.2.1. Goal and scope
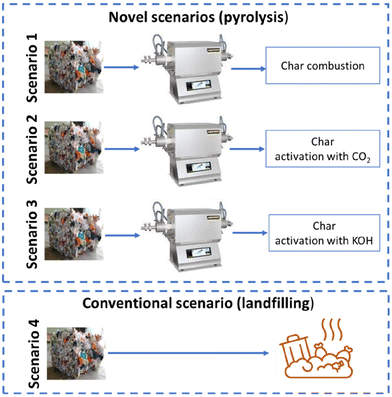
The goal of this LCA study was to calculate the environmental impact of the pyrolysis of contaminated mixed plastic waste from the non-selectively recovery fraction of municipal solid waste. The functional unit was set as the treatment of 1 kg of contaminated mixed plastic waste. The following three scenarios were considered:1. Pyrolysis with combustion of the char.
2. Pyrolysis with activation of the char with carbon dioxide.
3. Pyrolysis with activation of the char with potassium hydroxide.
The results obtained from the analysis of these scenarios were compared with the most common conventional practice, i.e. landfilling, which forms scenario 4.
These four scenarios are depicted in Fig. 1.
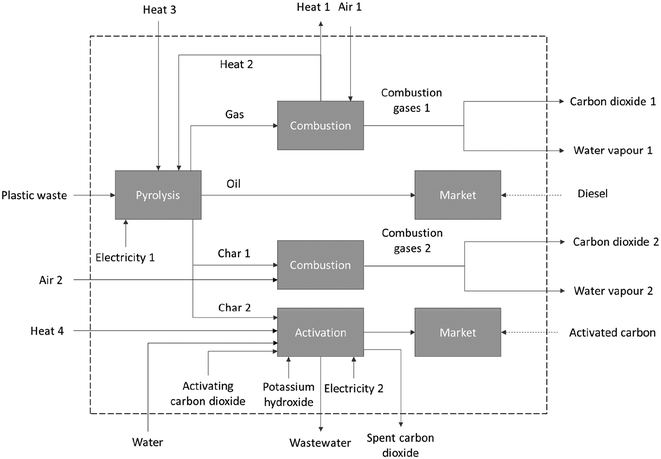
Fig. 2 represents the scope of the study, which includes all processes as well as material and energy flows considered in the foreground system. Table 1 lists the values for each flow within each scenario considered. Arrows indicate material or energy flows, while boxes represent processes. The “market” boxes signify replacing marketed goods product (i.e. diesel or activated carbon) with ones made through the pyrolysis process. Therefore, the pyrolysis oil is considered to be used as a commercial diesel substitute, based on its Higher Heating Value (HHV), whereas the char, after activation, substitutes commercial activated carbon according to its chemical and surface properties.
| Flow name | Scenario 1 | Scenario 2 | Scenario 3 | Unit |
|---|---|---|---|---|
| Plastic waste | 1000 | 1000 | 1000 | g |
| Gas | 367 | 367 | 367 | g |
| Oil | 567 | 567 | 567 | g |
| Char 1 | 66 | 0 | 0 | g |
| Char 2 | 0 | 66 | 66 | g |
| Air 1 | 6375 | 6375 | 6375 | g |
| Combustion gases 1 | 1855 | 1855 | 1855 | g |
| Carbon dioxide 1 | 941 | 941 | 941 | g |
| Water vapour 1 | 914 | 914 | 914 | g |
| Diesel | 558 | 558 | 558 | g |
| Air 2 | 344 | 0 | 0 | g |
| Combustion gases 2 | 121 | 0 | 0 | g |
| Carbon dioxide 2 | 118 | 0 | 0 | g |
| Water vapour 2 | 3 | 0 | 0 | g |
| Water | 0 | 0 | 1320 | g |
| Activating carbon dioxide | 0 | 370 | 0 | L |
| Spent carbon dioxide | 0 | 370 | 0 | L |
| Potassium hydroxide | 0 | 0 | 23 | g |
| Wastewater | 0 | 0 | 1345 | g |
| Activated carbon | 0 | 23 | 23 | g |
| Electricity 1 | 0.30 | 0.30 | 0.30 | kW h |
| Electricity 2 | 0 | 0.36 | 0.36 | kW h |
| Heat 1 | 6.70 | 6.70 | 6.70 | MJ |
| Heat 2 | 9.90 | 9.90 | 9.90 | MJ |
| Heat 3 | 10.70 | 10.70 | 10.70 | MJ |
| Heat 4 | 0 | 0.23 | 0.23 | MJ |
The emissions and resource depletion associated with all the processes required to perform the pyrolysis were included within the system boundaries, as shown in Fig. 2. The emissions and resource depletion associated with the materials and processes that are within the system boundaries, e.g., use of heat and electricity, were also considered in the analysis. The feedstock of the pyrolysis, i.e., plastic waste, was considered to enter the system with no environmental impact associated, according to a zero-burden approach also followed by other LCA studies of waste management, for example by Garcia-Garcia and Rahimifard.28
2.2. Life-cycle inventory
Data used to build the life-cycle inventory were collected from experimental work in our laboratory and the commercial database ecoinvent 3.7. Experimental work included the pyrolysis of 20 g of contaminated mixed plastic waste as well as the char activation. Although we shredded the contaminated mixed plastic waste before introducing them in the pyrolysis oven, this process was excluded from the inventory since no shredding is expected at an industrial scale. The experimental method followed to pyrolyze the char and measure the gas, oil and char generated in the pyrolysis can be found in our previous article.27 The composition of these three mass flows for each scenario is given in the ESI (Tables S1–S3†). HHVgas was determined as 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 MJ kg−1 = 45
319 MJ kg−1 = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 252 MJ Nm−3, based on its composition. HHVoil was determined as 44.89 MJ kg−1, based on its elemental analysis and the formula by Channiwala and Parikh.29
252 MJ Nm−3, based on its composition. HHVoil was determined as 44.89 MJ kg−1, based on its elemental analysis and the formula by Channiwala and Parikh.29
The pyrolysis gas was combusted. Part of the heat released was recovered and fed back to the pyrolysis (Heat 2), like in similar studies.30–32 This combustion did not need external heat. The HHV of the gas was calculated from the gas composition (Table S1†) and then used to calculate the heat that the combustion releases (Heat 1 and Heat 2). Assuming that the combustion was complete, just carbon dioxide and water were produced and released into the environment. The stoichiometry of the chemical reaction was used to calculate the composition of this combustion gas. Following this approach, an input of oxygen was found out to be required for the combustion to take place. The mass ratio of oxygen to air was used to calculate the air input required for combustion.
It was assumed that 40.4% of the heat from the gas combustion was lost (Heat 1), as per work by Zhang et al.33 The remaining heat (Heat 2) was recovered and used to heat the pyrolysis oven. The heat needed in the pyrolysis was assumed to be 20.6 MJ kg−1, as in previous work by Zhang et al.,33 who pyrolyzed polyethylene at 500 °C. Therefore, additional heat was needed (Heat 3), which was calculated as the difference between Heat 2 and 20.6 MJ kg−1. Heat was also needed to activate the char in scenarios 2 and 3 (Heat 4). The average heat for char activation from other studies was used: 3.55 MJ kg−1 char.34–36 Considering the functional unit, the calculated value for Heat 4 was 0.23 MJ kg−1 plastic waste.
The amount of commercial diesel that the oil might replace was calculated based on its composition. According to earlier studies,15,16 the oil produced by the pyrolysis of plastic waste shares many characteristics with diesel and can be used as fuel in diesel engines without the need for any modifications. Arjharn et al.15 did note some variations in the exhaust gas emissions and combustion properties of the two fuels used in a diesel engine. More nitrogen oxides and carbon emissions are produced by pyrolysis oil than by diesel fuel. As a result, using pyrolysis oil has slightly different impacts than using regular diesel fuel. We calculated the emissions using the elemental composition of the pyrolysis oil and theoretical combustion reactions. These explanations support the decision to use oil in direct replacement of commercial diesel, in spite of some further processing being necessary in some particular cases. Based on the method by Channiwala and Parikh,29 the HHV of the oil was estimated for this substitution, yielding a value of HHV = 44.89 MJ kg−1 (Table S2†). The HHV of commercial diesel was determined as 45.6 MJ kg−1.37 Thus, it was calculated that 0.98 kg of commercial diesel can be replaced by 1 kg of oil.
In scenario 1, the char is combusted, and it is assumed that only CO2 and H2O is released as an emission to air (nitrogen content corresponds to only 0.86%, as shown in Table S3†). In scenarios 2 and 3, the char was activated to substitute commercial activated carbon. In scenario 2, the char was activated using carbon dioxide, while in scenario 3 the activation was undertaken with potassium hydroxide and water. The composition and Brunauer–Emmett–Teller (BET) surface area of two commercial activated carbons can be seen in ESI (Table S4†). Based on these compositions and that of the activated char (Table S3†), similar to that of commercial activated carbon, we set a substitution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Kodera et al.38 calculated that 60 kW of power are needed for the pyrolysis of 200 kg h−1 of polypropylene and laminates of polypropylene with polyethylene terephthalate, which is the value we used to model our pyrolysis plant.
Background data was taken from the ecoinvent database. Whenever possible, processes and materials from Spain were used in the model. When this information was not available, processes and materials from Europe were used.
Table 1 lists the life-cycle inventory data, scaled up to the functional unit of 1 kg of contaminated mixed plastic waste. It must be noted that the experimental data were obtained from the pyrolysis of 20 g of contaminated mixed plastic waste, shown in ESI (Table S5†). The products and processes from the ecoinvent database that we used in our study are listed in ESI (Table S6†).
3. Results and discussion
The results of the life-cycle impact assessment are presented in Section 3.1 and the interpretation of these results is presented in Section 3.2.3.1. Life-cycle impact assessment
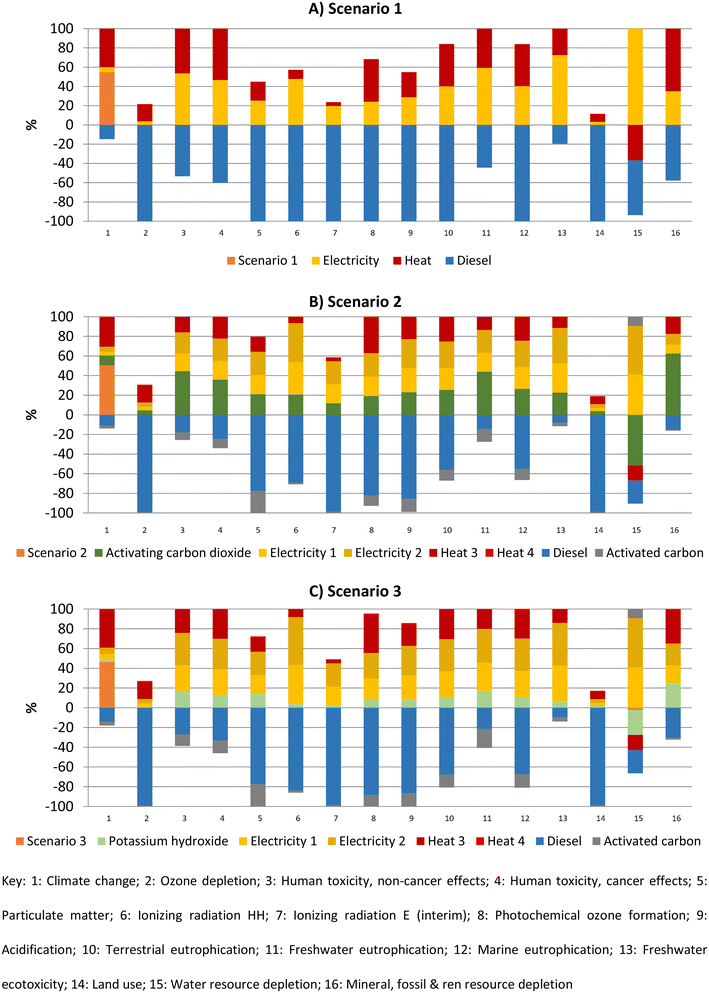
The commercial software SimaPro 9.4 (PRé Sustainability) was used to perform the life-cycle impact assessment. The method used was ILCD 2011 Midpoint + V1.10/EC-JRC Global equal weighting (updated to June 2017), including long-term emissions and infrastructure.Fig. 3 shows the characterised results for scenarios 1–3. Avoiding the production of commercial diesel significantly reduces the environmental impact in most categories for the three scenarios due to the high environmental impact generated during the production of commercial diesel. The avoided production of the activated carbon in scenarios 2 and 3 also reduces the environmental impact, but to a much smaller extent than diesel does. Electricity generation and distribution contribute significantly to the impacts in most categories for all scenarios. Electricity creates high freshwater ecotoxicity and human toxicity (cancer effects) impacts. Heat generation also creates a significant environmental impact, particularly in scenario 1. For scenario 2, an important contributor to the environmental impact is the activating carbon dioxide, which was assumed to be purchased as commercial liquid carbon dioxide. The activating carbon dioxide creates high toxicity impacts (human toxicity (cancer and non-cancer effects) and freshwater ecotoxicity). In scenario 3, the production and distribution of potassium hydroxide generates an environmental impact, but small compared to that created by the electricity and heat. The pyrolysis process emits carbon dioxide, contributing to climate change, where it is the most impacting process in the three scenarios.
The absolute results for the three scenarios, as well as the conventional scenario (landfilling), are shown in Table 2. Within each environmental impact category, the largest value is in either scenario 2 or the conventional scenario, except for water resource depletion. In scenarios 1–3, negative values (which means favourable results) are obtained for several environmental impact categories, due to the avoided production of diesel and activated carbon. The conventional scenario gets positive environmental impact scores (which means unfavourable results) for all impact categories, meaning that this scenario provides no environmental benefit.
| Impact category | Scenario 1 | Scenario 2 | Scenario 3 | Conventional scenario | Unit |
|---|---|---|---|---|---|
| Climate change | 1.65 × 10 | 2.24 × 10 | 1.66 × 10 | 9.52 × 10−2 | kg CO2 eq. |
| Ozone depletion | −3.00 × 10−7 | −2.66 × 10−7 | −2.81 × 10−7 | 2.87 × 10−9 | kg CFC-11 eq. |
| Human toxicity, non-cancer effects | 3.25 × 10−8 | 1.54 × 10−7 | 8.38 × 10−8 | 4.90 × 10−7 | CTUh |
| Human toxicity, cancer effects | 6.36 × 10−9 | 2.59 × 10−8 | 1.56 × 10−8 | 1.76 × 10−9 | CTUh |
| Particulate matter | −1.57 × 10−4 | −7.46 × 10−5 | −1.02 × 10−4 | 1.19 × 10−5 | kg PM2.5 eq. |
| Ionizing radiation HH | −6.01 × 10−2 | 5.95 × 10−2 | 2.34 × 10−2 | 1.31 × 10−3 | kBq U235 eq. |
| Ionizing radiation E (interim) | −7.18 × 10−7 | −3.94 × 10−7 | −4.86 × 10−7 | 7.78 × 10−9 | CTUe |
| Photochemical ozone formation | −5.55 × 10−4 | 1.56 × 10−4 | −8.98 × 10−5 | 1.23 × 10−4 | kg NMVOC eq. |
| Acidification | −1.62 × 10−3 | 4.45 × 10−5 | −5.88 × 10−4 | 9.56 × 10−5 | molc H+ eq. |
| Terrestrial eutrophication | −6.85 × 10−4 | 2.52 × 10−3 | 1.22 × 10−3 | 3.40 × 10−4 | molc N eq. |
| Freshwater eutrophication | 4.73 × 10−5 | 1.89 × 10−4 | 1.04 × 10−4 | 1.87 × 10−6 | kg P eq. |
| Marine eutrophication | −6.15 × 10−5 | 2.33 × 10−4 | 1.08 × 10−4 | 4.08 × 10−4 | kg N eq. |
| Freshwater ecotoxicity | 3.51 × 10 | 9.37 × 10 | 7.53 × 10 | 7.34 × 10−1 | CTUe |
| Land use | −4.56 × 10 | −4.21 × 10 | −4.32 × 10 | 1.63 × 10−1 | kg C deficit |
| Water resource depletion | 4.19 × 10−5 | 1.58 × 10−4 | 5.55 × 10−4 | 2.61 × 10−5 | m3 water eq. |
| Mineral, fossil & ren resource depletion | 3.06 × 10−6 | 2.32 × 10−5 | 9.33 × 10−6 | 2.46 × 10−7 | kg Sb eq. |
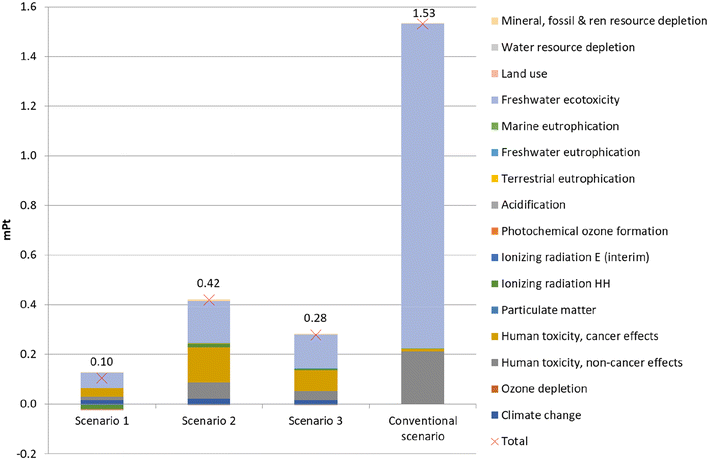
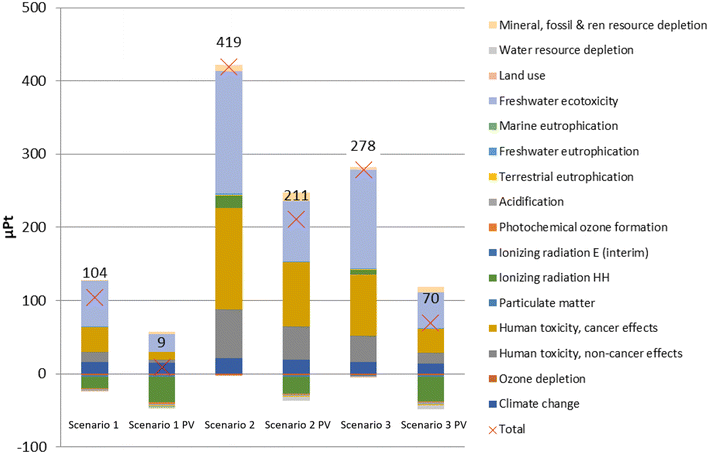
To further compare the impacts of the four scenarios, the life-cycle environmental impact results were normalised and aggregated into a single score (marked with a red cross in Fig. 4). The conventional scenario has by far the highest environmental impact. Therefore, it can be concluded that the pyrolysis of the contaminated mixed plastic waste is environmentally better than landfilling. The high impact of landfilling is mostly due to the high impact of freshwater ecotoxicity. The conventional scenario has a significantly higher environmental impact due to its long-term emissions. Between scenarios 1–3, scenario 2 creates the highest environmental impact due to the high human toxicity (cancer and non-cancer effects) and freshwater ecotoxicity caused by the activating carbon dioxide. Combustion of the char (scenario 1) is environmentally preferable to activating it and using it as a substitute for activated carbon (scenarios 2 and 3).
3.2. Interpretation
The LCA results we obtained prove that the pyrolysis scenarios (scenarios 1–3) have a better environmental performance than landfilling (conventional scenario) thanks to the production of avoided products. The conventional scenario was determined to be the worst environmentally, mostly due to its high impact on freshwater ecotoxicity. However, using the ReCiPe 2016 Endpoint (H) V1.03 method, the conventional scenario performs better than scenarios 1–3, due to a much smaller impact to human health (mostly due to a smaller value for global warming), and to a smaller extent to ecosystems. Scenarios 1–3 obtain negative values (i.e., favourable results) for the area of protection resources, but this does not compensate the larger impacts for ecosystems and particularly for human health. With the ReCiPe 2016 Midpoint (H) V1.03 method, the largest value for each environmental impact category is in either scenario 2 or the conventional scenario, as with the ILCD 2011 Midpoint + V1.10. The ReCiPe 2016 Midpoint (H) V1.03 method also attributes a high environmental impact to the conventional scenario due to the freshwater ecotoxicity (as with ILCD 2011 Midpoint + V1.10), but especially due to marine ecotoxicity (not included in ILCD 2011 Midpoint + V1.10). Normalised results by the ReCiPe 2016 Midpoint (H) V1.03 method seem to indicate that the conventional scenario creates the highest environmental impact, as opposed to the single score results obtained by the ReCiPe 2016 Endpoint (H) V1.03 method. Using the CML-IA baseline V3.05/EU25, the results are very similar to those obtained by the ReCiPe 2016 Midpoint (H) V1.03. In conclusion, scenarios 1–3 perform better than the conventional scenarios when using ILCD 2011 Midpoint + V1.10, ReCiPe 2016 Midpoint (H) V1.03 and CML-IA baseline V3.05 methods, but worse when using the ReCiPe 2016 Endpoint (H) V1.03 method.The generation and distribution of electricity used in the process modelled has a high environmental impact of electricity, so an alternative source of electricity was considered in order to investigate how this energy source affects the environmental impact results for scenarios 1–3. Electricity is often selected for sensitivity analysis due to its substantial impact on the results.10 Photovoltaic (PV) electricity was chosen due to the continuous increase in PV electricity generation in Spain in the last years, with a fivefold increase in installed capacity over 2018–2022.39 The single-score results for scenarios 1–3 with and without PV energy are shown in Fig. 5. Unsurprisingly, the overall environmental impact is reduced in all scenarios, as well as within each environmental impact category, with the exception of land use (due to the large area needed for PV plants) and mineral, fossil and renewable resource depletion (due to the materials needed to manufacture the PV plants). The greatest reduction in overall environmental impact (>90%) occurs in scenario 1, with an overall environmental impact approaching 0 Pt. Yet, the ranking of the scenarios does not change, with scenario 1 performing best and scenario 2 performing worst.
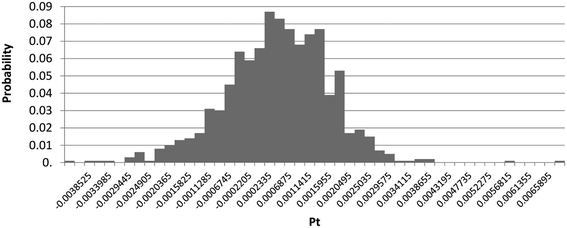
Next, the Monte Carlo method was used to assess the absolute uncertainty of the model. The specific steps followed to perform the Monte Carlo analysis is described in our previous work.27 The distribution results are shown in Fig. 6.
The Monte Carlo analysis showed that 369![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433 materials and processes were used in the model. 64.4% of them were assigned a lognormal distribution, 35.5% were undefined, 0.051% were assigned a triangle distribution and 0.003% were assigned a normal distribution. The single score was within the range of −0.00212 and 0.00256 Pt, at a confidence interval of 95%. The mean value obtained for scenario 2 was 0.42 mPt (which is the single score value previously obtained and indicated in Fig. 4), while the median was 0.471 mPt. A large coefficient of variation was obtained, but this is explained by the mean being close to zero and the values obtained being both negative and positive. In addition, the source of electricity generation and distribution, which contributes the most to the overall environmental impact in this scenario, has a significantly high coefficient of variation in ecoinvent. The environmental impact category that contributes the most to the overall model uncertainty, with the highest coefficient of variation, is water resource depletion. The standard deviation was 0.00118 and the standard error of mean was 0.0000375, which is below 0.01 and therefore acceptable.40 The conclusion from this absolute uncertainty analysis is that the model and LCA results obtained are reliable and consistent.
433 materials and processes were used in the model. 64.4% of them were assigned a lognormal distribution, 35.5% were undefined, 0.051% were assigned a triangle distribution and 0.003% were assigned a normal distribution. The single score was within the range of −0.00212 and 0.00256 Pt, at a confidence interval of 95%. The mean value obtained for scenario 2 was 0.42 mPt (which is the single score value previously obtained and indicated in Fig. 4), while the median was 0.471 mPt. A large coefficient of variation was obtained, but this is explained by the mean being close to zero and the values obtained being both negative and positive. In addition, the source of electricity generation and distribution, which contributes the most to the overall environmental impact in this scenario, has a significantly high coefficient of variation in ecoinvent. The environmental impact category that contributes the most to the overall model uncertainty, with the highest coefficient of variation, is water resource depletion. The standard deviation was 0.00118 and the standard error of mean was 0.0000375, which is below 0.01 and therefore acceptable.40 The conclusion from this absolute uncertainty analysis is that the model and LCA results obtained are reliable and consistent.
The main conclusion of this study, i.e. that pyrolysis outperforms landfilling to reduce the environmental impacts of plastic waste management, agrees with other studies.22,23,41 More specifically, Gear et al.42 concluded that the benefits of plastic waste pyrolysis over other treatment methods relies on the substitution of commercial products by the of pyrolysis products, which also agrees with our study.
The climate change impact for the pyrolysis described in our article is between 1.65 kg CO2 eq. per kg plastic waste (scenario 1) and 2.24 kg CO2 eq. per kg plastic waste (scenario 2). These values are of the same order of magnitude, but higher, than those reported by similar studies,42–44 who reported values of 0.7–1.0 kg CO2 eq. per kg plastic waste. Another study gives similar values to ours: 1.7–2.0 kg CO2 eq. per kg plastic waste.45 As Jeswani et al.44 noted, differences between modelling choices make comparisons difficult, such as system boundaries, data sources, assumptions for system credits, energy mix, and impact assessment methods used, among others.
Furthermore, some studies highlight the weaknesses of previous LCA studies on plastic waste management. These weaknesses include lack of consistency and representativeness,46 no consideration of the feedstock composition, lack of information on the modelling scope, lack of uncertainty and sensitivity analyses,41 and lack of life-cycle inventory databases.23 Therefore, there is a need for more well-reported studies to calculate the environmental impact of the pyrolysis of contaminated mixed plastic waste. Our study aims to contribute in this regard.
On another note, there are some challenges for a wider implementation of the pyrolysis process for the treatment of plastic waste on a large scale, such as unavailability and inconsistent quality of plastic feedstock, inefficient and expensive sorting, and lack of market due to the lack of standardised products and ambiguous regulatory frameworks for plastic waste management.23 Policy support and clearer legislation, together with more effective waste sorting, could support the large-scale implementation of pyrolysis systems to treat contaminated mixed plastic waste.
4. Conclusions
The massive amount of plastic waste being disposed of around the world has made it imperative to find alternative ways to manage it other than the conventional practice of landfilling. This article has analysed three pyrolysis scenarios for the treatment of contaminated mixed plastic waste: one in which the char is combusted, and two in which the char is activated by carbon dioxide and potassium hydroxide, respectively. An environmental impact assessment showed that all pyrolysis scenarios had a lower environmental impact than landfilling. The environmental savings of the pyrolysis scenarios are mainly due to the substitution of commercial diesel with pyrolysis oil. This makes the pyrolysis scenarios to have a negative environmental impact for several impact categories, which means that these results are favourable. Among the pyrolysis scenarios, the one where the char is activated with carbon dioxide has the highest impact, while the one where the char is combusted has the lowest impact. This means that out of all the scenarios considered, pyrolysis of the contaminated mixed plastic waste and combustion of the char is environmentally preferable. In all pyrolysis scenarios, electricity generation and distribution contributes significantly to the overall environmental impact due to its high impact on freshwater ecotoxicity and human toxicity (cancer effects). Therefore, replacing the electricity used in the process from the Spanish electricity grid mix with photovoltaic electricity significantly reduces the overall environmental impact.In conclusion, this study supports the use of alternative management methods, such as pyrolysis, to deal with contaminated mixed plastic waste. Currently, the amount of contaminated mixed plastic waste being pyrolyzed is negligible. Policy support, for example in the form of subsidies, as well as clearer legislation, could help speed up the implementation of such a waste management solution. Scaling up successful laboratory and pilot plant systems is also challenging and needs important economic investments. However, it is expected that these investments will provide economic returns, based on the commercial applications of the char and oil. Nevertheless, further study of scaled-up pyrolysis systems to manage contaminated mixed plastic waste is needed to support this hypothesis with accurate data. Another challenge for large-scale pyrolysis of contaminated mixed plastic waste is the heterogeneity of the feedstock. More effective waste sorting could facilitate pyrolysis and the production of homogenised pyrolysis oil, which could more easily replace commercial diesel.
Author contributions
Guillermo Garcia-Garcia: formal analysis, investigation, methodology, software, visualization, writing – original draft; María Ángeles Martín-Lara: conceptualization, data curation, funding acquisition, project administration, writing – review & editing; Mónica Calero: conceptualization, data curation, funding acquisition, project administration, resources, supervision; Gabriel Blázquez: conceptualization, data curation, funding acquisition, methodology, resources.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work has received funding from the project PID2019-108826RB-I00/SRA (State Research Agency)/10.13039/501100011033, the project B-RNM-78-UGR20 (FEDER/Junta de Andalucía-Consejería de Transformación Económica, Industria, Conocimiento y Universidades) and the project P20_00167 (FEDER/Junta de Andalucía-Ministry of Economy, Transformation, Industry, and Universities). Guillermo Garcia-Garcia is grateful for the grant “Juan de la Cierva Incorporación” funded by MCIN/AEI/10.13039/501100011033 and “ESF Investing in your future”, and the Grant ‘Marie Skłodowska-Curie Actions (MSCA) Postdoctoral Fellowship’ with Grant agreement ID: 101052284. We acknowledge funding for open access provided by Universidad de Granada/CBUA.References
- OECD, Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options, OECD, 2022 Search PubMed.
- N. Evode, S. A. Qamar, M. Bilal, D. Barceló and H. M. N. Iqbal, Case Stud. Chem. Environ. Eng., 2021, 4, 100142 CrossRef CAS.
- OECD, Climate change and plastics pollution. Synergies between two crucial environmental challenges. Policy highlights, 2023.
- McKinsey & Company, Addressing the challenges of plastic waste: Circularity and leakage, https://www.mckinsey.com/industries/chemicals/our-insights/addressing-the-challenges-of-plastic-waste-circularity-and-leakage, (accessed 15 February 2024).
- UNEP, Zero draft text of the international legally binding instrument on plastic pollution, including in the marine environment. UNEP/PP/INC.3/4. Intergovernmental negotiating committee to develop an international legally binding instrument on plastic pollution, including in the marine environment. Third session. Nairobi, 13–19 November 2023. Preparation of an international legally binding instrument on plastic pollution, including in the marine environment., 2023.
- Principles for Responsible Investment, The plastics landscape: regulations, policies and influencers, 2019.
- Chemical Recycling Europe, 10 Questions and Answers to Better Understand Chemical Recycling, https://www.chemicalrecyclingeurope.eu/copy-of-about-chemical-recycling, (accessed 15 February 2024).
- T. Thiounn and R. C. Smith, J. Polym. Sci., 2020, 58, 1347–1364 CrossRef CAS.
- C. Salah, S. Cobo, J. Pérez-Ramírez and G. Guillén-Gosálbez, ACS Sustainable Chem. Eng., 2023, 11, 3238–3247 CrossRef CAS PubMed.
- M. S. Qureshi, A. Oasmaa, H. Pihkola, I. Deviatkin, A. Tenhunen, J. Mannila, H. Minkkinen, M. Pohjakallio and J. Laine-Ylijoki, J. Anal. Appl. Pyrolysis, 2020, 152, 104804 CrossRef CAS.
- X. Zhao, M. Korey, K. Li, K. Copenhaver, H. Tekinalp, S. Celik, K. Kalaitzidou, R. Ruan, A. J. Ragauskas and S. Ozcan, Chem. Eng. J., 2022, 428, 131928 CrossRef CAS.
- K. Kohli, S. R. Chandrasekaran, R. Prajapati, B. Kunwar, S. Al-Salem, B. R. Moser and B. K. Sharma, Energies, 2022, 15, 8821 CrossRef CAS.
- Supriyanto, P. Ylitervo and T. Richards, J. Anal. Appl. Pyrolysis, 2021, 158, 105248 CrossRef CAS.
- S. Huang, H. Wang, W. Ahmad, A. Ahmad, N. I. Vatin, A. M. Mohamed, A. F. Deifalla and I. Mehmood, Int. J. Environ. Res. Public Health, 2022, 19, 4556 CrossRef CAS PubMed.
- W. Arjharn, P. Liplap, S. Maithomklang, K. Thammakul, S. Chuepeng and E. Sukjit, ACS Omega, 2022, 7, 9720–9729 CrossRef CAS PubMed.
- P. Sushma, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 455, 012066 Search PubMed.
- Z. Kadirova, Y. Kameshima, A. Nakajima and K. Okada, J. Hazard. Mater., 2006, 137, 352–358 CrossRef CAS PubMed.
- C. Song, B. Zhang, L. Hao, J. Min, N. Liu, R. Niu, J. Gong and T. Tang, Green Energy Environ., 2022, 7, 411–422 CrossRef CAS.
- D. Czajczyńska, R. Krzyżyńska, H. Jouhara and N. Spencer, Energy, 2017, 134, 1121–1131 CrossRef.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 2000 Search PubMed.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- M. G. Davidson, R. A. Furlong and M. C. McManus, J. Cleaner Prod., 2021, 293, 126163 CrossRef CAS.
- T. Xayachak, N. Haque, R. Parthasarathy, S. King, N. Emami, D. Lau and B. K. Pramanik, J. Environ. Chem. Eng., 2022, 10, 108865 CrossRef CAS.
- A. A. Kartika, L. S. Chun and S. Ismail, Int. J. Adv. Eng. Res. Nat. Sci. Eng. Res., 2023, 7, 371–376 Search PubMed.
- ISO, ISO 14040:2006. Environmental management - Life cycle assessment - Principles and framework, https://www.iso.org/standard/37456.html, (accessed 1 March 2017).
- ISO, ISO 14044:2006. Preview Environmental management - Life cycle assessment - Requirements and guidelines, https://www.iso.org/standard/38498.html, (accessed 1 March 2017).
- G. Garcia-Garcia, M. Á. Martín-Lara, M. Calero, F. Ortega and G. Blázquez, Sci. Total Environ., 2023, 895, 165063 CrossRef CAS PubMed.
- G. Garcia-Garcia and S. Rahimifard, Resour., Conserv. Recycl., 2019, 149, 1–11 CrossRef.
- S. A. Channiwala and P. P. Parikh, Fuel, 2002, 81, 1051–1063 CrossRef CAS.
- A. Chamkalani, S. Zendehboudi, N. Rezaei and K. Hawboldt, Renewable Sustainable Energy Rev., 2020, 134, 110143 CrossRef CAS.
- C. Li, X. Yuan, Z. Sun, M. Suvarna, X. Hu, X. Wang and Y. S. Ok, Bioresour. Technol., 2022, 346, 126582 CrossRef CAS PubMed.
- J. F. Peters, D. Iribarren and J. Dufour, Fuel, 2015, 139, 441–456 CrossRef CAS.
- Y. Zhang, G. Ji, D. Ma, C. Chen, Y. Wang, W. Wang and A. Li, Process Saf. Environ. Prot., 2020, 142, 203–211 CrossRef CAS.
- B. A. Mohamed, N. R. Nicomel, H. Hamid and L. Y. Li, Sci. Total Environ., 2023, 874, 162392 CrossRef CAS PubMed.
- A. Vilén, P. Laurell and R. Vahala, J. Environ. Manage., 2022, 324, 116356 CrossRef PubMed.
- J. Shaheen, Y. H. Fseha and B. Sizirici, Heliyon, 2022, 8, e12388 CrossRef CAS PubMed.
- The Engineering Toolbox, Fuels - Higher and Lower Calorific Values, https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html, (accessed 15 February 2024).
- Y. Kodera, T. Yamamoto and E. Ishikawa, Fuel Communications, 2021, 7, 100016 CrossRef.
- Red Eléctrica, Potencia Instalada Renovable por Tecnología/Combustible (MW) | Sistema Eléctrico: Nacional. Del 2018 al 2022. Solar fotovoltaica, https://www.ree.es/es/datos/generacion/estructura-renovables, (accessed 15 February 2024).
- M. Goedkoop, M. Oele, J. Leijting, T. Ponsioen and E. Meijer, Introduction to LCA with SimaPro, 2016, https://pre-sustainability.com/legacy/download/SimaPro8IntroductionToLCA.pdf Search PubMed.
- A. Antelava, S. Damilos, S. Hafeez, G. Manos, S. M. Al-Salem, B. K. Sharma, K. Kohli and A. Constantinou, Environ. Manage., 2019, 64, 230–244 CrossRef PubMed.
- M. Gear, J. Sadhukhan, R. Thorpe, R. Clift, J. Seville and M. Keast, J. Cleaner Prod., 2018, 180, 735–747 CrossRef.
- H. H. Khoo, Resour., Conserv. Recycl., 2019, 145, 67–77 CrossRef.
- H. Jeswani, C. Krüger, M. Russ, M. Horlacher, F. Antony, S. Hann and A. Azapagic, Sci. Total Environ., 2021, 769, 144483 CrossRef CAS PubMed.
- S. Das, C. Liang and J. B. Dunn, Waste Manage., 2022, 153, 81–88 CrossRef CAS PubMed.
- L. Pires Costa, D. M. Vaz de Miranda and J. C. Pinto, ACS Sustainable Chem. Eng., 2022, 10, 3799–3807 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc04396g |
| This journal is © The Royal Society of Chemistry 2024 |