 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aqueous sodium tosylate: a sustainable medium for alkylations†
Sem
Bleus
 a,
Jeltzlin
Semerel
ab and
Wim
Dehaen
*a
a,
Jeltzlin
Semerel
ab and
Wim
Dehaen
*a
aSustainable Chemistry for Metals and Molecules, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, P.O. Box 2404, B-3001 Leuven, Belgium. E-mail: wim.dehaen@kuleuven.be
bSISSTEM Program, Faculty of Arts and Science, University of Aruba, J. Irausquin Plein 4, Oranjestad, Aruba
First published on 13th February 2024
Abstract
Typical alkylation reactions produce significant amounts of undesired salt and solvent waste. Herein, we report an efficient alkylation protocol utilising aqueous sodium tosylate (NaTos) solutions as a hydrotrope-containing medium. The methodology represents an inexpensive and straightforward approach for preparing alkyl aryl ethers, thioethers and N-alkylated tosyl amides in high yields under mild conditions. The generated reaction waste, aqueous NaTos salt, was repurposed as a reaction medium in subsequent steps. This medium was recycled ten times without any significant change in the yield, providing an improved environmental factor over various literature examples. Under similar conditions, commonly applied solvents furnished the products with lower yields and more waste.
Introduction
The O-alkylation of phenolic substrates gives access to many relevant compounds (e.g., perfumes, pheromones and pharmaceuticals), but its sustainability requires improvement. Significant advances have already been made in the starting materials, as various phenols can be bio-sourced.1 However, the corresponding alkyl phenyl ethers have been predominantly prepared via Williamson's synthesis-type procedures, which often produce harmful and diverse waste by relying on non-benign solvents2 and highly toxic alkylating agents, including alkyl halides3 or dialkyl sulfates.4 Furthermore, stoichiometric salt quantities are typically generated as by-products5 requiring direct disposal due to their limited further use. Dialkyl carbonates are regarded as sustainable alternatives, but their weak alkylating potential necessitates a high energy input6 and often needs dedicated equipment and catalysts.7 These factors often favour simpler, unsustainable alkylations, especially within academia, making the development of efficient, straightforward and sustainable methods desirable.As solvents are the prime source of waste in chemical reactions, considerable attention has been devoted to their substitution with water.8 Nevertheless, the low solubilisation of organic compounds in aqueous media represents an intrinsic problem. Hydrotropes, non-amphiphilic organic solubilising agents (Fig. 1), can provide a solution but have received limited attention. Nevertheless, successful organic syntheses in aqueous hydrotrope solutions9 indicate that the products often precipitate during the reaction, facilitating their solventless isolation. Alternatively, dilution can induce phase separation, as the dissolution process is highly hydrotrope-concentration dependent.10 Unfortunately, few studies have thus far systematically investigated comparisons with other solvents.11 Moreover, hydrotrope recycling has barely been evaluated but would be mandatory considering the high salt loading.11,12
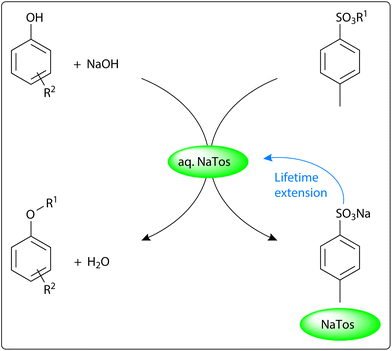
Based on the successful occurrence of SN2 reactions in aqueous hydrotrope solutions,11 we aimed to develop and evaluate a hydrotropic alkylation strategy (Scheme 1) with a solventless work-up. Alkyl tosylates and NaOH were selected as the electrophile and the green base,13 respectively, since the only side products generated would be water and sodium tosylate (NaTos), a common hydrotrope. As a result, NaTos was preferred as the hydrotrope to minimise waste diversification. The reaction waste was repurposed as a reaction medium in further runs, extending its life cycle. The aqueous hydrotrope solution was benchmarked against several alternative solubilising agents and prior literature examples.
Initially, 3-nitrophenol 1aa was reacted with an excess of MeTos and NaOH in 30 wt% aq. NaTos. The promising 94% NMR yield of 3-nitroanisole 3aa prompted us to optimise the reaction conditions regarding the process sustainability (Table S1†). Under the final conditions (20 wt% NaTos, 1.0 equiv. of MeTos and 1.2 equiv. of NaOH stirred at 50 °C for 6 h), significantly less waste and a slightly decreased 87% isolated yield resulted (Scheme 2; entry 14, Table S1†). No excess alkylating agent was required and 3aa conveniently precipitated out of the reaction mixture, facilitating a solventless work-up by simple filtration. A control experiment in pure water (entry 15, Table S1†) afforded a significantly lower 50% NMR yield. Here, 3aa could not be isolated without solvents due to unconverted 1aa being present. Direct alkylation with tosyl chloride and excess methanol according to the reduce derivates principle was unsuccessful, as it selectively resulted in the tosylated phenol.
The optimised conditions were subjected to a phenol scope to assess their generality, as summarised in Scheme 2. Both electron-rich and electron-poor phenols were evaluated, generally furnishing the products in high yields with spontaneous phase separation from the aqueous mixture in each case. The reaction towards 2-nitroanisole 3ab only proceeded with 48% conversion, which may be attributed to the encumbered oxygen reactivity resulting from the intramolecular hydrogen bonding of 2-nitrophenol.14 From the data, two characteristics regarding the hydrotrope-containing medium became apparent. First, the interference by the aqueous medium seems low as no or limited hydrolysis occurred for the amide 3ae and the esters 3af and 3at, respectively. Second, the results indicate a correlation between the solubilisation and yield. Polar substrates with hydrogen-accepting capabilities generally resulted in lower yields (3aa–3af) due to the significant solubilisation of the products in the aqueous hydrotrope solution, as corroborated by 1H-NMR analysis of the aqueous phase. The results for compounds 3aj–3ap indicate that the medium partially dissolves the products with smaller alkyl chains (3aj–3am), whereas phase separation is near-quantitative when several or larger aliphatic groups are present (3an–3ap). More polarisable halide substituents (3ag–3ai) and even larger fused ring systems (3as–3au) were tolerated well, portraying the high solubilisation of the phenolate salts in the aqueous medium.
Several other nucleophiles were evaluated (Scheme 2). Under nitrogen atmosphere, aromatic and aliphatic thiols could be methylated quantitatively within 3 h (3ba–3bb). Tosyl amides also seemed to be well tolerated, given a 90% yield resulted for the methylation of N-methyl-p-toluenesulfonamide 2bc to 3bc. Methyl ester 3bd could be obtained from the corresponding carboxylate 2bd using 1.0 equiv. of NaOH in a moderate 63% yield. The low nucleophilicity of 2bd increased the reaction time and led to significant (tosylate) ester hydrolysis by the base, as indicated by crude NMR. Moreover, washing with NaOH was required to remove the residual acid, rendering carboxylic acids less suitable substrates.
The tosylate esters were varied next, using 2-naphthol as a nucleophile (Scheme 2). Whereas the methylation of 2as furnished the product in a 94% yield within 6 h, the ethylation towards 3cb required a threefold increased reaction time to attain a slightly lower yield. The effect was even more pronounced for 3cs since the limited conversion of propyl tosylate 2cs in 30 wt% NaTos led to an inseparable mixture. Complete conversion was achieved using 40 wt% NaTos, yielding 2cs in 68%, mainly because it tended to liquefy when filtered. The alkylation using 2-hydroxyethyl tosylate 2ds was completed in 6 h with 20 wt% NaTos in good yield, indicating that solubility is the decisive factor. The developed methodology thus mainly suits methylations or the introduction of relatively polar groups. Other cases require additional optimisation.
Given the high hydrotrope content, the medium recyclability was evaluated for the synthesis of 3as. The first cycle was performed with 1.0 equiv. of MeTos and 1.2 equiv. of NaOH in 94% yield. The undiluted medium was readily recovered during filtration and reused without intermediate purification. The reaction was repeated with this filtrate after loading with stoichiometric MeTos, 2-naphthol and base, considering the excess NaOH present to facilitate product isolation would be recoverable. This process could be repeated for nine additional cycles (Fig. 2) in a 93% average yield, using 1.0 equiv. of the base per run and a total hydrotrope-containing water volume of 60 mL. As NaTos is generated in situ, the medium becomes gradually enriched in hydrotrope. Nevertheless, the consistently high yields indicate that the product remains sparingly soluble at a high hydrotrope loading. Saturation occurred after six runs at a theoretical NaTos concentration of 62 wt%, which correlates with the available solubility data.15 The precipitated salt could readily be removed from the product by washing with water, although increasing overall water consumption. Alternatively, lowering the reactant concentrations or diluting the reaction medium could delay the precipitation.
The E factors after one, six and ten cycles were determined to be 5.0, 2.1 and 1.9, showing the pronounced effect of medium recycling on the overall waste generation. In comparison (Table 1), the relatively green solvents16 acetonitrile and DMSO, commonly employed for alkylations,17 produce over four times the waste of a single run in NaTos solution. For ethylene glycol, the value was sixfold higher than that in hydrotrope-containing water. Although pure water and solventless conditions generate less salt waste than the hydrotrope, their low conversions necessitate chromatography or energy-intensive distillation. Moreover, the E factors without purification are 57–220% higher than that for the aqueous hydrotrope solution used six times. After ten cycles, the hydrotrope-containing medium even provided an 18% improvement over 1 wt% aqueous sodium dodecyl sulfate (SDS). Moreover, the complete E factor18 of a single run was 11% lower. In each case, the aqueous NaTos solution provided a significantly higher yield, making it a valuable alkylation medium, especially in routine syntheses.
| Entry | Solvent | Yield (%) | E factor |
|---|---|---|---|
| a Conditions: 2.5 mmol scale, 20 wt% NaTos, 1.2 equiv. of NaOH, 1.0 equiv. of MeTos, 6 h, 50 °C. b After 1 run at the 25 mmol scale. c After 6 runs at the 25 mmol scale. d After 10 runs at the 25 mmol scale. e Precipitated by water, followed by filtration. f Excluding work-up. | |||
| 1 | 20 wt% NaTos | 93b | 5.0 |
| 2 | Recycled NaTos | 93c | 2.1 |
| 3 | Recycled NaTos | 93d | 1.9 |
| 4 | Acetonitrile | 65 | 21.0 |
| 5 | DMSO | 86 | 21.1e |
| 6 | Ethylene glycol | 62 | 30.1 |
| 7 | Water | 56 | 3.3f |
| 8 | Neat | 31 | 6.8f |
| 9 | 1 wt% SDS | 80 | 2.2 |
The developed methodology was compared to literature methylations of 2as. Upon saturation (run 6), the E factor is significantly lower than these reported in various literature protocols (Table S2†). In particular, our method provides one of the few examples with an E factor < 2.0. DMC-based catalytic strategies producing less waste exist (Scheme 3) but do not consider product degradation or solvent usage during the work-up as only GC yields are reported.19,20 These methods are more energy intensive due to the high reaction temperatures and distillation to remove solvents, purify products and recover catalysts. Additionally, these reactions need a protective nitrogen atmosphere19 or flow equipment.20 Our method represents an energetically favourable alternative with a more straightforward reaction set-up, product isolation and recycling.
Similar to DMC, the formed side products in our approach (NaTos and water) are reusable, non-toxic, have a low potential towards bioaccumulation and are readily biodegradable.21 MeTos, despite being more toxic than DMC, has a lower toxicity than other common alkylating agents22 and is less volatile, limiting exposure through inhalation. The main disadvantage is that current green preparations of alkyl tosylates regularly involve reagent excesses and produce stoichiometric halogenated waste.23 Nevertheless, alkylation of the green tosylic acid16 with water as a sole by-product seems viable,24 potentially providing a suitable and greener alternative.
Conclusion
In summary, we successfully developed an aqueous alkylation method relying on sodium tosylate as the solubilising agent, with the only by-products being water and the hydrotrope itself. The optimised conditions tolerated a broad phenol scope and could be extended to other nucleophiles and alkyl groups. The reaction proved scalable and recyclable for at least ten consecutive cycles. In addition, the aqueous hydrotrope solution outperformed several alternative green solubilisation methods with respect to the E factor and is among the better alkylation methods in terms of waste generation and energy consumption in the literature. Our research represents one of the few examples in which hydrotropes have been evaluated in-depth regarding their recyclability and performance compared to other media.Author contributions
Conceptualisation: W. D. and S. B.; formal analysis: S. B. and J. Z. S.; funding acquisition: W. D.; investigation: S. B. and J. Z. S.; methodology: W. D. and S. B.; resources: W. D.; supervision: W. D.; visualisation: S. B.; writing – original draft: S. B.; writing – review and editing W. D., S. B. and J. Z. S.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the Research Foundation – Flanders (FWO; grant 11G8123N) and the European Union (FED/2019/406-549) for financially supporting this work. We acknowledge Bart Van Huffel and Gert Steurs for their technical assistance in NMR spectroscopy and Alejandra Moreno Ramirez for conducting preliminary experiments.References
- J. Yan, Q. Meng, X. Shen, B. Chen, Y. Sun, J. Xiang, H. Liu and B. Han, Sci. Adv., 2020, 6, eabd1951 CrossRef CAS PubMed.
- Y. Zhong, I. Douair, T. Wang, C. Wu, L. Maron and D. Cui, Angew. Chem., Int. Ed., 2019, 59, 4947 CrossRef CAS PubMed; P. S. Hellwig, A. M. Barcellos, R. Cargnelutti, T. Barcellos and G. Perin, J. Org. Chem., 2022, 87, 15050 CrossRef PubMed; N. V. Tzouras, L. P. Zorba, E. Kaplanai, N. Tsoureas, D. J. Nelson, S. P. Nolan and G. C. Vougioukalakis, ACS Catal., 2023, 13, 8845 CrossRef.
- W. H. Gardiner, M. Camilleri, L. A. Martinez-Lozano, S. P. Bew and G. R. Stephenson, Chem. – Eur. J., 2018, 24, 19089 CrossRef CAS PubMed; I. Medina-Mercado, A. Colin-Molina, J. E. Barquera-Lozada, B. Rodríguez-Molina and S. Porcel, ACS Catal., 2021, 11, 8968 CrossRef; A. Senior, K. Ruffell and L. T. Ball, Nat. Chem., 2022, 15, 386 CrossRef PubMed.
- J. Alan, M. Schade, M. Wagener, F. Christian, S. Nordhoff, B. Merla, T. R. Dunkern, G. Bahrenberg and P. Ratcliffe, J. Med. Chem., 2019, 62, 6391 CrossRef CAS PubMed; G. H. M. de Kruijff, T. Goschler, N. Beiser, A. Stenglein, O. M. Türk and S. R. Walgvogel, Green Chem., 2019, 21, 4815 RSC.
- F. Chang, C. Wang, Q. Chen, Y. Zhang and G. Liu, Angew. Chem., Int. Ed., 2021, 61, e202114809 CrossRef CAS PubMed; C. A. Franco, T. I. da Silva, M. G. Dias, B. W. Ferreira, B. L. de Sousa, G. M. Bousada, R. W. Barreto, B. G. Vaz, G. da, S. Lima, M. H. dos Santos, J. A. S. Grossi and E. V. Vieira Varejão, J. Agric. Food Chem., 2022, 70, 2806 CrossRef PubMed.
- J. A. Barrett, Y. Gao, C. M. Bernt, M. Chui, A. T. Tran, M. B. Foston and P. C. Ford, ACS Sustainable Chem. Eng., 2016, 4, 6877 CrossRef CAS; Y. W. Lui, B. Chan and M. Y. Lui, ChemSusChem, 2022, 15, e202102538 CrossRef PubMed.
- T. N. Glasnov, J. D. Holbrey, C. O. Kappe, K. R. Seddon and T. Yan, Green Chem., 2012, 14, 3071 RSC; U. Tilstam, Org. Process Res. Dev., 2012, 16, 1150 CrossRef CAS; A. Dhakshinamoorthy, A. Sharmila and K. Pitchumani, Chem. – Eur. J., 2010, 16, 1128 CrossRef PubMed.
- M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC; B. H. Lipshutz, F. Gallou and S. Handa, ACS Sustainable Chem. Eng., 2016, 4, 5838 CrossRef CAS; T. Kitanosono, K. Masuda, P. Xu and S. Kobayashi, Chem. Rev., 2018, 118, 679 CrossRef PubMed; M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237 RSC.
- T. Sela and A. Vigalok, Adv. Synth. Catal., 2012, 354, 2407 CrossRef CAS; S. N. Jadhav, A. S. Kumbhar, C. V. Rode and R. S. Salunkhe, Green Chem., 2016, 18, 1898 RSC; C.-X. Shi, Y.-T. Guo, Y.-H. Wu, Z.-Y. Li, Y.-Z. Wang, F.-S. Du and Z.-C. Li, Macromolecules, 2019, 52, 4260 CrossRef.
- M. R. Thakker, J. K. Parikh and M. A. Desai, ACS Sustainable Chem. Eng., 2018, 6, 3215 CrossRef CAS; A. Mazaud, R. Lebeuf, M. Lageurre and V. Nardello-Rataj, ACS Sustainable Chem. Eng., 2020, 8, 15268 CrossRef.
- T. Sela, X. Lin and A. Vigalok, J. Org. Chem., 2017, 82, 11609 CrossRef CAS PubMed.
- A. Kumbhar, S. Kamble, M. Barge, G. Rashinkar and R. Salunkhe, Tetrahedron Lett., 2012, 22, 2756 CrossRef.
- R. K. Henderson, A. P. Hill, A. M. Redman and H. F. Sneddon, Green Chem., 2015, 17, 945 RSC.
- S. R. Thawarkar, B. Thombare, B. S. Munde and N. D. Khupse, RSC Adv., 2018, 8, 38384 RSC.
- K.-K. Pei, R.-X. Zhao, G.-L. Zhang, Q. Xia and F.-B. Zhang, J. Chem. Eng. Data, 2018, 63, 1556 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288 RSC.
- For reactions in acetonitrile, see: R. R. Milburn and V. Snieckus, Angew. Chem., Int. Ed., 2004, 43, 888 CrossRef CAS PubMed; M. Y. Lui, A. K. L. Yuen, A. F. Masters and T. Maschmeyer, ChemSusChem, 2016, 9, 2312 CrossRef PubMed; Q. Zheng, H. Xu, H. Wang, W.-G. H. Du, N. Wang, H. Xiong, Y. Gu, L. Noodleman, K. B. Sharpless, G. Yang and P. Wu, J. Am. Chem. Soc., 2021, 143, 3753 CrossRef PubMed . For reactions in dimethylsulfoxide, see: X. Shen, M. Zhou, C. Ni, W. Zhang and J. Hu, Chem. Sci., 2014, 5, 117 RSC; J. B. Washington, M. Assante, C. Yan, D. McKinney, V. Juba, A. G. Leach, S. E. Baillie and M. Reid, Chem. Sci., 2021, 12, 6949 RSC; X. Dong, Q. Y. Li and T. P. Yoon, Org. Lett., 2021, 23, 5703 CrossRef PubMed.
- R. A. Sheldon, Green Chem., 2023, 25, 1704 RSC.
- Z. L. Shen, X. Z. Jiang, W. M. Mo, B. X. Hu and N. Sun, Green Chem., 2005, 7, 97 RSC.
- S. Ouk, S. Thiébaud, E. Borredon and P. Le Gars, Green Chem., 2002, 4, 431 RSC.
- J. Dreyfuss, J. M. Shekosky and J. J. Ross Jr., Toxicol. Appl. Pharmacol., 1971, 20, 548 CrossRef CAS PubMed; W. F. Bergfeld, D. V. Belsito, C. D. Klaassen, R. Hill, D. Liebler, J. G. Marks Jr., R. C. Shank, T. J. Slaga, P. W. Snyder and F. A. Andersen, Int. J. Toxicol., 2011, 30, 270S CrossRef PubMed; K. Stanton, C. Tibazarwa, H. Certa, W. Greggs, D. Hildebold, L. Jovanovich, D. Woltering and R. Sedlak, Integr. Environ. Assess. Manage., 2009, 6, 155 CrossRef PubMed.
- J. McCann, E. Choi, E. Yamasaki and B. N. Ames, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 5135 CrossRef CAS PubMed.
- J. Morita, H. Nakatsuji, T. Misaki and Y. Tanabe, Green Chem., 2005, 7, 711 RSC; F. Kazemi, A. R. Massah and M. Javaherian, Tetrahedron, 2007, 63, 5083 CrossRef CAS; K. Asano and S. Matsubara, Org. Lett., 2009, 11(8), 1757 CrossRef PubMed.
- X. Dong, R. Sang, Q. Wang, X.-Y. Tang and M. Shi, Chem. – Eur. J., 2013, 19, 16910 CrossRef CAS PubMed; B. M. Choudary, N. S. Chowdari and M. L. Kantam, Tetrahedron, 2000, 56, 7291 CrossRef; S. Velusamy, J. S. K. Kumar and T. Punniyamurthy, Tetrahedron Lett., 2004, 45, 203 CrossRef; B. Das, V. S. Reddy and M. R. Reddy, Tetrahedron Lett., 2004, 45, 6717 CrossRef; J. Chandra, R. Chaudhuri, S. R. Manne, S. Mondal and B. Mandal, ChemistrySelect, 2017, 2, 8471 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterisation data. See DOI: https://doi.org/10.1039/d3gc04206e |
| This journal is © The Royal Society of Chemistry 2024 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 h, N2 atmosphere; b
3 h, N2 atmosphere; b

