Open Access Article
Open Access ArticleOrganocatalytic Friedel–Crafts arylation of aldehydes with indoles utilizing N-heterocyclic iod(az)olium salts as halogen-bonding catalysts†
Eirini M.
Galathri
a,
Thomas J.
Kuczmera
 b,
Boris J.
Nachtsheim
*b and
Christoforos G.
Kokotos
b,
Boris J.
Nachtsheim
*b and
Christoforos G.
Kokotos
 *a
*a
aLaboratory of Organic Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis, Athens 15771, Greece. E-mail: ckokotos@chem.uoa.gr
bInstitut für Organische und Analytische Chemie, Universität Bremen, Leobener Straße NW2C, 28359 Bremen, Germany
First published on 15th November 2023
Abstract
The Friedel–Crafts arylation is among the most known organic reactions, usually being promoted by a Lewis acid, that have been employed for the synthesis of bis-indolyl methanes. Herein, we report a mild, inexpensive, green and organocatalytic protocol for the promotion of a Friedel–Crafts-type reaction between indoles and aldehydes, where N-heterocyclic iod(az)olium salts are utilized as halogen-bonding catalysts, leading to the double addition of the indole motif. A variety of aliphatic and aromatic aldehydes were converted into diarylmethanes in good to high yields, while the scope of indoles was also investigated. Water was employed as the solvent, while the reaction time was short. The reaction mechanism was also studied.
Introduction
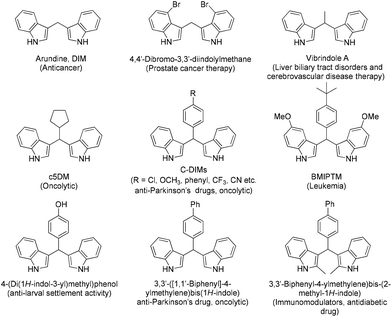
Among the most common reactions, taught in almost all undergraduate courses around the world, the Friedel–Crafts arylation has a prominent place. It was first introduced in the literature in 1877 by Friedel and Crafts,1 presenting a new way, at the time, to attach substituents onto aromatic rings. Since then, the reaction has been thoroughly studied.2 Bis-indolyl methanes (BIMs) and their analogues constitute an important class of compounds that exhibit various medicinal and pharmacological properties and are usually employed as anti-cancer, anti-oxidant, anti-bacterial, anti-inflammatory and anti-proliferative agents,3 while one of the metabolites of indole-3-carbinol, its dimer 3,3′-bis-indolyl-methane (arundine or DIM), plays an important role in the prevention of breast cancer (Fig. 1).4Since BIMs present so many different properties, a variety of synthetic routes have been devised for their synthesis, although the Friedel–Crafts-type reaction between aldehydes and indoles is the most common approach (Scheme 1). Most frequently, an acid, either Lewis or Brønsted, is employed, usually presenting major disadvantages, such as the use of high temperature or toxic reagents (Scheme 1A).5 In order to provide greener approaches towards the synthesis of BIMs, green solvents, neat conditions, and sonochemistry or microwave chemistry have been employed.6 In 2002, the use of a catalytic amount of NBS was proposed (Scheme 1B),7 while attempts to anchor the acidic catalyst on a solid support, in order to recycle the catalyst, were also performed by other researchers.8 In 2020, Bez and co-workers described the first aminocatalytic approach for the synthesis of BIMs, utilizing a prolinamide catalyst at elevated temperatures (Scheme 1C).9 In the same year, Yuan and co-workers described an electrochemically promoted synthesis of BIMs, proposing an autocatalytic process (Scheme 1D).10 In 2019, Badillo and co-workers described a very elegant photochemical protocol, where they employed the organocatalytic properties of Schreiner's thiourea, in order to promote a photo-acidic process using blue LED irradiation (Scheme 1E).11 In 2023, the Kokotos’ group, in collaboration with the research groups of Fagnoni and Protti, proposed a fast, versatile and efficient procedure for the visible-light-driven synthesis of diarylmethanes via Friedel–Crafts-type coupling of aldehydes and (hetero)arenes, utilising arylazo sulfones as photoacid generators (PAGs) (Scheme 1F).12 Among the different applications of arylazo sulfones in synthesis and chemistry of materials, their use as non-ionic photoacid generators (PAGs) is able to generate methanesulfonic acid in oxygen-saturated or air equilibrated solutions.
 | ||
| Scheme 1 Common synthetic pathways for the Friedel–Crafts-type reaction between indoles and aldehydes. | ||
Halogen bonding (XB) is the interaction of electrophilic halogen substituents with Lewis bases (LBs) and has been extensively studied in the past two decades.13 Halogen bonding has been successfully applied in crystal engineering,14 anion recognition,15 organic synthesis15 and organocatalysis.16 Halogen-bond catalysis has attracted increasing attention, due to growing awareness of the problems associated with metal catalysts in recent years.17 Among them, halogen-bond catalysis is largely led by iodine-derived catalysts. Since halogen bonding catalysts have the advantages of being relatively cheap, stable, green and easy-to-handle,18 they have been gradually established in organocatalysis as an area of interest, receiving increased attention from 2008 and onwards, when Bolm and co-workers reported an example of using perfluoroiodoalkanes as halogen-bond catalysts.19 It is known that iodine(I)-based halogen bond catalysts have been used in a variety of organic transformations, ranging from polyfluorinated arenes20 to imidazolium salt21 or triazolium salt derivatives.22 The iodobenzimidazolium group is one of the most potent available iodine(I)-based halogen bond catalysts, and iodine(III)-based halogen bond catalysts were proven to exhibit high catalytic activity in halide abstractions, Diels–Alder reactions, Nazarov cyclizations and Michael addition reactions.23 The research group of Nachtsheim has investigated in detail the application of N-heterocyclic iod(az)olium salts (NHISs) as halogen-bonding catalysts.24 Thus, in 2019, Toy and co-workers proposed the use of a diiodine-based molecule as the potential catalyst for the Friedel–Crafts-type coupling of aldehydes with indoles.25a The authors employed a low catalyst loading of 1 mol%, but prolonged reaction times (up to 72 h), heating at 70 °C and MeCN as the solvent had to be applied. The reaction mechanism was recently studied by DFT calculations.25b Also, in 2020, Herrera and co-workers introduced the use of iodo-alkynes as potential catalysts for the same reaction.25c A high catalyst loading of 20 mol%, along with toluene as the solvent and a reaction time of 48 h, was necessary, while only aromatic aldehydes reacted successfully. In both cases, the products were purified by column chromatography.
We thus decided to merge the experience of Kokotos’ group with that of Nachtsheim's group to investigate whether halogen bonding can be employed in introducing a fast, green and efficient procedure for the organocatalytic synthesis of diarylmethanes via Friedel–Crafts-type coupling of aldehydes with (hetero)arenes, utilizing NHISs as catalysts (Scheme 1G).
Results and discussion
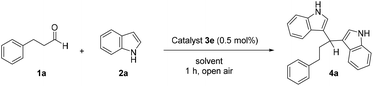
We began our investigations by studying the reaction between 3-phenylpropanal (1a) and 1H-indole (2a) in CH2Cl2 at room temperature to form 3,3′-(3-phenylpropane-1,1-diyl)bis(1H-indole) (4a) (Scheme 2). The reaction in the absence of a catalyst does not lead to product formation. Then, we screened various substituted N-heterocyclic iod(az)olium salts (NHISs) (3a–3e).24g These compounds have decreased electron density at the N-heterocycle which strengthens the XB-bonding capability of the hypervalent iodine atom, through an increased electron-pull, which is initiated by the charged N-heterocycle. Among the compounds tested, catalyst 3e afforded the most satisfactory yield (90%), when employed in a very low catalyst loading (0.5 mol%, Scheme 2). The catalysts with the highest yields were 3c and 3e, having a significant difference in terms of the counter anion (BArF instead of B(C6F5)4), as well as an N- vs. C-bound heterocycle.Next, we screened a number of common solvents (Table 1). Most organic solvents afforded moderate to excellent yields; however, water proved to be the best (Table 1, entry 9). In the case of water as the solvent, the desired product could be isolated by simple extraction from the reaction mixture, with high enough purity. In further investigation regarding the catalyst loading, using 0.1 mol% catalyst loading after 5 h led to the desired product in 79% yield. Thus, we concluded that when reducing the catalyst loading, the reaction time had to be increased. Taking this into account, we carried out reactions with 0.1, 0.01 and 0.005 mol% catalyst loading for 18 h and the product was obtained in 95%, 87% and 60% yields, respectively. In the framework of these experiments, a gram-scale reaction was also carried out with 0.01 mol% catalyst loading, affording the desired product in 79% yield.
| Entry | Solvent | Yielda (%) |
|---|---|---|
| a Yields determined by 1H NMR using an internal standard. Yield of 4a after isolation by column chromatography is given in parenthesis. The reaction was performed with 3-phenylpropanal (1a) (26 mg, 0.20 mmol), indole (2a) (52 mg, 0.44 mmol), and catalyst 3e (0.5 mol%, 1.0 μmol) in solvent (0.5 mL) for 1 h. | ||
| 1 | CH2Cl2 | 93 |
| 2 | CHCl3 | 90 |
| 3 | MeCN | 93 |
| 4 | EtOAc | 89 |
| 5 | DMSO | 35 |
| 6 | Toluene | 96 |
| 7 | Pet. Eth. | 80 |
| 8 | THF | 50 |
| 9 | H 2 O | 97 (93) |
| 10 | Et2O | 55 |
| 11 | MeOH | 53 |
| 12 | Cyrene | — |
| 13 | 2-Me-THF | 76 |
Having in hand the optimum reaction conditions utilizing N-heterocyclic iod(az)olium salt 3e as the organocatalyst and water as the solvent, we turned our attention to exploring the substrate scope (Schemes 3 and 4). Initially, we employed indole (2a) as a representative heterocycle, with a variety of aliphatic and aromatic aldehydes (Scheme 3). We began our investigations using aliphatic derivatives, isolating product 4a in 93% yield. Moving to linear aliphatic aldehydes, bis-indoles 4b and 4c, as well as the anticancer bis-indole 4d (arundine), were isolated in good to high yields. Also, we explored three different α,α-disubstituted aldehydes, and in all cases, the desired products 4e–4g were obtained in very good yields (74–98%, Scheme 3). We then explored the scope of the aliphatic aldehydes having double or triple bonds. Oleyl aldehyde 1h was employed successfully, leading to 4h in 80% yield, whereas, in the case of aldehyde 1i which contains a triple bond, the reaction was more problematic, leading to product 4i in only 20% yield after 1 h or 18 h. Once we realized that the use of aliphatic aldehydes is possible, we explored the scope of aromatic aldehydes. Benzaldehyde provided the double addition product 4j in a good yield. Substitution either at the para-position or the ortho-position of the aromatic ring was well tolerated, leading to products 4k or 4l in good to excellent yields. Both electron-withdrawing and electron-donating groups were well tolerated and products 4m–r were isolated in good to excellent yields. CF3- and NO2-substituted aldehydes 1o and 1r required a prolonged reaction time (18 h). Cyclohexanone as a model ketone reacted as well, but for a prolonged reaction time of 18 h, affording 4s in 96% yield. However, acetophenone did not react under the optimized conditions. Once the substrate scope of the aldehyde counterpart was investigated, we moved to testing the scope of substituted indoles (Scheme 4). By utilizing 3-phenylpropanal (1a) as a common starting material, a variety of N-substituted indoles were tested. Sterically hindered 2-methyl indole was a competent nucleophile, providing access to 4t in 75% yield. Then, the substitution pattern on the nitrogen of the indole was probed. Simple alkyl substituents, such as methyl (4u), butyl (4y) or benzyl (4v), secondary alkyl substituents, such as isobutyl (4w), and aryl substituents, such as phenyl (4x) or allyl (4z), were well tolerated, leading to good to excellent yields. Substituted indoles at the 2-position, such as N-methyl-2-phenyl indole, afforded 4aa in 78% yield. Also, we tested other arenes, such as pyrrole, thiophene, benzothiophene, thiazole or furan, but only pyrrole afforded product 4ab in 60% yield.
Mechanistic studies
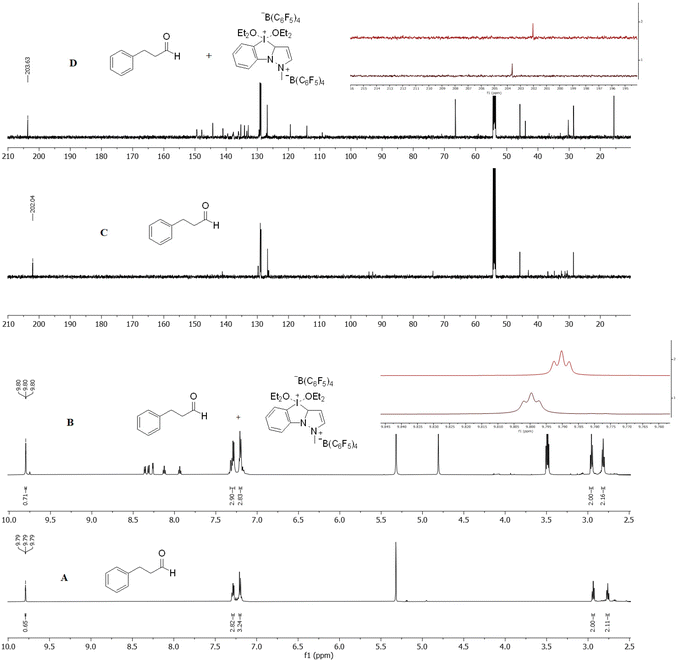
After studying the substrate scope, we turned our attention into studying the reaction mechanism. Having the literature as a strong inspiration24 and in order to have a better understanding, we performed 1H- and 13C NMR mechanistic studies (Fig. 2). Initially, the 1H NMR (600 MHz, DMSO-d6) spectrum of 3-phenyl-propanal (1a) was recorded (Fig. 2A). The triplet peak of the proton of the carbonyl group resonates at 9.79 ppm. The addition of 1.0 equiv. of catalyst 3e to 1a resulted in a slight shift in 1H NMR from 9.79 ppm to 9.80 ppm (Fig. 2B). Moving to 13C NMR (150 MHz, DMSO-d6), the carbon of the carbonyl moiety of 3-phenyl-propanal (1a) resonates at 202.04 ppm (Fig. 2C). The mixture of 1a to 3a presents a significant shift for the carbon on the carbonyl moiety to 203.63 ppm (Fig. 2D). This low-field shift of 1.59 ppm is indicative of the halogen bonding between the oxygen of the carbonyl group of the aldehyde and the iodine of the iodonium catalyst 3e, supporting the formation of an aldehyde–catalyst complex. We also performed similar NMR studies with indole and other NHIS catalysts 3a–d.26Taking all these data into account, the following mechanism is proposed (Scheme 5). Iodonium catalyst 3e can enhance the electrophilicity of aldehyde 1, through halogen bonding, leading to complex A (Scheme 5). This complexation facilitates the nucleophilic addition of indole to afford tetrahedral intermediate B. This, in turn, can collapse, losing a molecule of water via the protonated alcohol C, regenerating the organocatalyst and generating azafulvene intermediate D. This can react with another molecule of 2 in a conjugate addition, to afford the desired product 4.
Conclusions
In conclusion, a simple, green and efficient organocatalytic protocol was developed, activating aldehydes for their reaction with indoles, leading to diarylmethanes. This method relies on a small organic molecule activating efficiently both aliphatic and aromatic aldehydes, leading to diarylmethanes in good to high yields. Based on extensive mechanistic studies, the interaction between the iodonium catalysts and aldehydes by halogen bonding has been verified. Among the key features of this protocol are the extremely low catalyst loading (0.5 mol%), the use of water as the solvent and the quite fast reaction times (1 h).Experimental
General procedure for the organocatalytic reaction between indoles and aldehydes
In a glass vial, catalyst (3e) (1.8 mg, 1.0 μmol) in H2O (0.5 mL), aldehyde (0.20 mmol) and indole (0.44 mmol) were added consecutively. The reaction mixture was stirred for 1 h. After reaction completion, the reaction mixture was diluted with EtOAc (2 mL). The organic layer was separated, dried over Na2SO4 and concentrated in vacuo. The desired product was isolated by column chromatography.Author contributions
Conceptualization: C.G.K. and B.N.; reaction optimization, substrate scope and compound characterization: E.G.; synthesis of catalysts: T.K.; writing – original draft: E.G. and C.G.K.; writing, reviewing and editing: B.N. and C.G.K.; and supervision and project administration: C.G.K.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Hellenic Foundation for Research and Innovation (HFRI) for financial support through a grant, which is financed by the 1st Call for H.F.R.I. Research Projects to Support Faculty Members & Researchers and a procurement of high-cost research equipment grant (grant number 655).References
- (a) C. Friedel and J. M. Crafts, Compt. Rend., 1877, 84, 1392–1395 Search PubMed; (b) C. Friedel and J. M. Crafts, Compt. Rend., 1877, 84, 1450–1454 Search PubMed.
- For selected and recent reviews on the Friedel–Crafts reaction, see: (a) M. Rueping and B. J. Nachtsheim, Beilstein J. Org. Chem., 2010, 6, 6 CrossRef PubMed; (b) R. Sunke, S. B. Nallapati, J. S. Kumar, K. S. Kumar and M. Pal, Org. Biomol. Chem., 2017, 17, 4042–4057 RSC.
- (a) M. Kobayashi, S. Aoki, K. Gato, K. Matsunami, M. Kurosu and I. Kitagawa, Chem. Pharm. Bull., 1994, 42, 2449–2451 CrossRef CAS; (b) G. Sivaprasad, P. T. Perumal, V. R. Prabavathy and N. Mathivanan, Bioorg. Med. Chem. Lett., 2006, 16, 6302–6305 CrossRef CAS; (c) M. Damodiran, D. Muralidharan and P. T. Perumal, Bioorg. Med. Chem. Lett., 2009, 19, 3611–3614 CrossRef CAS PubMed; (d) A. Kamal, M. N. A. Khan, K. S. Reddy, Y. V. V. Srikanth, S. K. Ahmed, K. P. Kumar and U. S. N. Murthy, J. Enzyme Inhib. Med. Chem., 2009, 24, 559–565 CrossRef CAS; (e) K. Abdelbaqi, N. Lack, E. T. Guns, L. Kotha, S. Safe and T. Sanderson, Prostate, 2011, 71, 1401–1412 CrossRef CAS; (f) K. Yoon, S. Lee, S. Cho, K. Kim, S. Khan and S. Safe, Carcinogenesis, 2011, 32, 836–842 CrossRef CAS; (g) G. S. S. Kumar, S. Kumaresan, A. A. M. Prabhu, N. Bhuvanesh and P. G. Seethalakshmi, Spectrochim. Acta, Part A, 2013, 101, 255–263 CrossRef.
- (a) Y. S. Kim and J. A. Milner, J. Nutr. Biochem., 2005, 16, 65–73 CrossRef CAS PubMed; (b) E. G. Rogan, In Vivo, 2006, 20, 221–228 CAS.
- For a review, see: (a) M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250–2293 CrossRef CAS PubMed. For selected examples, see: (b) J. S. Yadav, B. V. S. Reddy, C. V. S. R. Murthy, G. M. Kumar and C. Madan, Synthesis, 2001, 783–787 CrossRef CAS; (c) R. Nagarajan and P. T. Perumal, Tetrahedron, 2002, 58, 1229–1232 CrossRef CAS; (d) G. Gupta, G. Chaudhari, P. Tomar, Y. Gaikwad, R. Azad, G. Pandya, G. Waghulde and K. Patil, Eur. J. Chem., 2012, 3, 475–479 CrossRef CAS; (e) J. Beltrá, M. C. Gimeno and R. P. Herrera, Beilstein J. Org. Chem., 2014, 10, 2206–2214 CrossRef; (f) H. Veisi, B. Maleki, F. H. Eshbala, H. Veisi, R. Masti, S. S. Ashrafi and M. Baghayeri, RSC Adv., 2014, 4, 30683–30688 RSC.
- For selected examples, see: (a) G. Penieres-Carrillo, J. G. García-Estrada, J. L. Gutiérrez-Ramirez and C. Alvarez-Toledano, Green Chem., 2003, 5, 337–339 RSC; (b) J. Li, H. Dai, W. Xu and T. Li, Ultrason. Sonochem., 2006, 13, 24–27 CrossRef CAS; (c) N. Azizi, L. Torkian and M. R. Saidi, J. Mol. Catal. A: Chem., 2007, 275, 109–112 CrossRef CAS; (d) M. Zahran, Y. Abdin and H. Salama, ARKIVOC, 2008, 256–265 Search PubMed; (e) A. K. Chakraborti, S. R. Roy, D. Kumar and P. Chopra, Green Chem., 2008, 10, 1111–1118 RSC; (f) J. Li, M. Sun, G. He and X. Xu, Ultrason. Sonochem., 2011, 18, 412–414 CrossRef CAS; (g) S. Handy and N. M. Westbrook, Tetrahedron Lett., 2014, 55, 4969–4971 CrossRef CAS; (h) U. N. Yadav and G. S. Shankarling, J. Mol. Liq., 2014, 191, 137–141 CrossRef CAS.
- H. Koshima and W. Matsusaka, J. Heterocycl. Chem., 2009, 39, 1089–1091 CrossRef.
- S. B. Kamble, R. K. Swami, S. S. Sakate and C. V. Rode, ChemPlusChem, 2013, 78, 1393–1399 CrossRef CAS.
- G. Basumatary, R. Mohanta, S. D. Baruah, R. C. Deka and G. Bez, Catal. Lett., 2020, 150, 106–111 CrossRef CAS.
- C. Liu, Z. Xiao, S. Wu, Y. Shen, K. Yuan and Y. Ding, ChemSusChem, 2020, 13, 1997–2001 CrossRef CAS PubMed.
- (a) Z. M. Salem, J. Saway and J. J. Badillo, Org. Lett., 2019, 21, 8528–8532 CrossRef CAS PubMed. For a similar use of Schreiner's thiourea for acetalization of aldehydes, see: (b) N. Spiliopoulou, N. F. Nikitas and C. G. Kokotos, Green Chem., 2020, 22, 3539–3545 RSC.
- E. M. Galathri, L. Di Terlizzi, M. Fagnoni, S. Protti and C. G. Kokotos, Org. Biomol. Chem., 2023, 21, 365–369 RSC.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS.
- A. Mukherjee, S. Tothadi and G. R. Desiraju, Acc. Chem. Res., 2014, 47, 2514–2524 CrossRef CAS PubMed.
- M. S. Taylor, Coord. Chem. Rev., 2020, 413, 213270 CrossRef CAS.
- D. Bulfield and S. M. Huber, Chem. – Eur. J., 2016, 22, 14434–14450 CrossRef CAS PubMed.
- (a) A. Bauza, T. J. Mooibroek and A. Frontera, ChemPhysChem, 2015, 16, 2496–2517 CrossRef CAS; (b) M. Breugst and J. J. Koenig, Eur. J. Org. Chem., 2020, 5473–5487 CrossRef CAS; (c) J. Bamberger, F. Ostler and O. G. Mancheno, ChemCatChem, 2019, 11, 5198–5211 CrossRef CAS.
- (a) R. L. Sutar and S. M. Huber, ACS Catal., 2019, 9, 9622–9639 CrossRef CAS; (b) H. Yang and M. W. Wong, Molecules, 2020, 25, 1045 CrossRef CAS.
- C. Bolm, A. Bruckmann and M. Pena, Synlett, 2008, 900–902 CrossRef.
- (a) F. Heinen, D. L. Reinhard, E. Engelage and S. M. Huber, Angew. Chem., Int. Ed., 2021, 60, 5069–5073 CrossRef CAS; (b) F. Kniep, S. H. Jungbauer, Q. Zhang, S. M. Walter, S. Schindler, I. Schnapperelle, E. Herdtweck and S. M. Huber, Angew. Chem., Int. Ed., 2013, 52, 7028–7032 CrossRef CAS PubMed.
- (a) C. W. Kee and M. W. Wong, J. Org. Chem., 2016, 81, 7459–7470 CrossRef CAS; (b) S. H. Jungbauer, S. M. Walter, S. Schindler, L. Rout, F. Kniep and S. M. Huber, Chem. Commun., 2014, 50, 6281–6284 RSC; (c) S. H. Jungbauer and S. M. Huber, J. Am. Chem. Soc., 2015, 137, 12110–12120 CrossRef CAS PubMed; (d) A. Dreger, P. Wonner, E. Engelage, S. M. Walter, R. Stoll and S. M. Huber, Chem. Commun., 2019, 55, 8262–8265 RSC; (e) Y. C. Chan and Y. Y. Yeung, Org. Lett., 2019, 21, 5665–5669 CrossRef CAS PubMed; (f) V. P. N. Nziko and S. Scheiner, J. Org. Chem., 2016, 81, 2589–2597 CrossRef CAS; (g) J. P. Gliese, S. H. Jungbauer and S. M. Huber, Chem. Commun., 2017, 53, 12052–12055 RSC.
- (a) M. Breugst, D. von der Heiden, E. Detmar and R. Kuchta, Synlett, 2017, 29, 1307–1313 CrossRef; (b) R. Haraguchi, S. Hoshino, M. Sakai, S. G. Tanazawa, Y. Morita, T. Komatsu and S. I. Fukuzawa, Chem. Commun., 2018, 54, 10320–10323 RSC; (c) M. Kaasik, A. Metsala, S. Kaabel, K. Kriis, I. Jarving and T. Kanger, J. Org. Chem., 2019, 84, 4294–4303 CrossRef CAS PubMed; (d) R. A. Squitieri, K. P. Fitzpatrick, A. A. Jaworski and K. A. Scheidt, Chem. – Eur. J., 2019, 25, 10069–10073 CrossRef CAS.
- (a) R. L. Sutar, E. Engelage, R. Stoll and S. M. Huber, Angew. Chem., Int. Ed., 2020, 59, 6806–6810 CrossRef CAS PubMed; (b) A. J. To and G. K. Murphy, New J. Chem., 2022, 46, 15313–15320 RSC; (c) Y. Zhang, J. Han and Z.-J. Liu, RSC Adv., 2015, 5, 25485–25488 RSC; (d) F. Heinen, E. Engelage, A. Dreger, R. Weiss and S. M. Huber, Angew. Chem., Int. Ed., 2018, 57, 3830–3833 CrossRef CAS; (e) A. Boelke, T. J. Kuczmera, E. Lork and B. J. Nachtsheim, Chem. – Eur. J., 2021, 27, 13128–13134 CrossRef CAS PubMed; (f) R. Robidas, D. L. Reinhard, C. Y. Legault and S. M. Huber, Chem. Rec., 2021, 21, 1912–1927 CrossRef CAS PubMed.
- (a) A. Boelke, E. Lork and B. J. Nachtsheim, Chem. – Eur. J., 2018, 24, 18653–18657 CrossRef CAS; (b) A. Boelke, Y. A. Vlasenko, M. S. Yusubov, B. J. Nachtsheim and P. S. Postnikov, Beilstein J. Org. Chem., 2019, 15, 2311–2318 CrossRef CAS; (c) A. Boelke, T. J. Kuczmera, L. D. Caspers, E. Lork and B. J. Nachtsheim, Org. Lett., 2020, 22, 7261–7266 CrossRef CAS; (d) A. Boelke and B. J. Nachtsheim, Adv. Synth. Catal., 2020, 362, 184–191 CrossRef CAS; (e) A. H. Abazid and B. J. Nachtsheim, Angew. Chem., Int. Ed., 2020, 59, 1479–1484 CrossRef CAS PubMed; (f) A. H. Abazid, N. Clamor and B. J. Nachtsheim, ACS Catal., 2020, 10, 8042–8048 CrossRef CAS; (g) A. Boelke, T. J. Kuczmera, E. Lork and B. J. Nachtsheim, Chem. – Eur. J., 2021, 27, 13128–13134 CrossRef CAS PubMed.
- (a) X. Liu, S. Ma and P. H. Toy, Org. Lett., 2019, 21, 9212–9216 CrossRef CAS PubMed; (b) C. Zhao, Y. Li, X. Li and Y. Zeng, Phys. Chem. Chem. Phys., 2023, 25, 21100–21108 RSC; (c) J. V. Alegre-Requena, A. Valero-Tena, I. G. Sonsona, S. Uriel and R. P. Herrera, Org. Biomol. Chem., 2020, 18, 1594–1601 RSC.
- For more details, see the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Full optimisation studies, experimental procedures and characterisation data of starting materials and products, mechanistic studies and NMR traces. See DOI: https://doi.org/10.1039/d3gc03687a |
| This journal is © The Royal Society of Chemistry 2024 |