Photoinitiators from bio-sourced naphthoquinone – the application of naphthoquinone-based vitamins K1 and K3 in free radical photopolymerization†
Timur
Borjigin
 ab,
Ji
Feng
ab,
Michael
Schmitt
ab,
Di
Zhu
ab,
Ji
Feng
ab,
Michael
Schmitt
ab,
Di
Zhu
 c,
Fabrice
Morlet-Savary
ab,
Pu
Xiao
c,
Fabrice
Morlet-Savary
ab,
Pu
Xiao
 *d and
Jacques
Lalevée
*d and
Jacques
Lalevée
 *ab
*ab
aUniversité de Haute-Alsace, CNRS, IS2M UMR 7361, F-68100 Mulhouse, France. E-mail: jacques.lalevee@uha.fr
bUniversité de Strasbourg, F-67000 Strasbourg, France
cResearch School of Chemistry, Australian National University, Canberra, ACT 2601, Australia
dState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, P. R. China. E-mail: p.xiao@mail.sic.ac.cn
First published on 28th November 2023
Abstract
Two common bio-sourced naphthoquinone derivatives, vitamin K1 (phylloquinone) and vitamin K3 (menadione), were employed in free radical photopolymerization under LED light exposure at 405 nm. In comparison with juglone, a previously reported natural naphthoquinone derivative, both vitamins exhibited enhanced properties in inducing free radical photopolymerization as sole initiators (monocomponent type II) and in combination with amines, and they can act as typical type II photoinitiators. In a three-component photoinitiating system consisting of an iodonium salt, amine and the vitamin, the polymerization process of TMPTA was significantly accelerated/improved too. These three-component systems resulted in faster reactions than the system that solely contains the iodonium salt or amine as the coinitiator, and they can additionally act as sensitizing dyes. Notably, the more polar vitamin K3 alone can initiate PEGDA polymerization with a high conversion rate, suggesting its potential utility in the development of biopolymer materials. The analysis of steady-state photolysis experiments revealed that K3 rapidly reacted with the electron-rich amine under light but was also more likely to generate by-products. In contrast, the inclusion of the iodonium salt in the three-component system significantly accelerated the photolysis of K3, demonstrating the superior chemical reactivity of the three-component system. Electron spin resonance spin trapping experiments successfully captured the radicals that initiated the photopolymerization reaction. Hence, these results facilitated the further deduction of potential reaction pathways. Finally, K1/iodonium salt and K3/amine, two systems that demonstrated strong performance in free radical polymerization experiments, were successfully applied to direct laser write experiments.
With the gradual advancement of photochemical research, various systems have been developed aiming to convert light energy into chemical energy.1 Researchers have drawn inspiration from nature, either by studying natural products or by artificially simulating photochemical reaction systems in nature.2–4 For instance, researchers in the field of photocatalysis have attempted to construct an artificial Z-scheme that mimics the photosynthesis of green plants, which converts carbon dioxide into clean fuels such as methane.5 Similarly, researchers in the field of photopolymerization have extracted chlorophyll from green leaves and used it as a photoinitiator for free radical polymerization.6,7 These research efforts have been motivated by the principles of green chemistry, which emphasize low energy consumption and environmental protection.
Photopolymerization technology involves the polymerization of polymerizable monomers by light irradiation. The photopolymerization reaction system comprises photoinitiating systems (PISs), polymerizable monomers, and light sources.8,9 As a critical component of the system, the photoinitiator (PI) must absorb light energy, release active species, and trigger the polymerization reaction.10 Based on the different initiation mechanisms, photoinitiators can be divided into type I and type II photoinitiators. When exposed to light, type I photoinitiators immediately produce initiating radicals due to the process of homolytic cleavage. On the other hand, type II photoinitiators yield radicals via a method that involves electron/proton exchange between the photoinitiator (in an excited state) and the compounds that donate hydrogen, commonly referred to as coinitiators.11 However, the synthesis routes of many photoinitiators are complex, and designing photoinitiators that can be directly prepared from natural products remains a significant challenge.12 Consequently, the development of environmentally friendly and multifunctional photoinitiators is a major focus of research in this field.13 For instance, anthraquinone derivatives are widely distributed in nature and are used as dyes in industrial production.14,15 Numerous studies have been reported on the use of anthraquinone dyes, such as hydroxyl-substituted anthraquinone derivatives15,16 and amino-substituted anthraquinone,17–23 as PIs or photosensitizers in photoinitiating systems. Interestingly, a photoinitiating system based on 2,6-diaminoanthraquinone (AQD) could initiate cationic polymerization, and the resulting coatings exhibited antimicrobial properties.14 In addition, naphthoquinone derivatives are prevalent in plants and microorganisms.24,25 Compared to anthraquinones, most naphthoquinone derivatives, such as phylloquinone, juglone, and lawsone, can be extracted without the need for complex synthetic steps.26 The quinone structure in these compounds possesses excellent photobiological properties.27 Moreover, phylloquinone is a vital electron acceptor in plant photosynthesis.28 Therefore, it is expected that natural naphthoquinone derivatives can be effectively utilized in various photochemical fields such as photopolymerization.29 However, there have been limited reports24,30 on the application of natural naphthoquinone derivatives in photopolymerization.
Phylloquinone (vitamin K1) is commonly found in chloroplasts31 and comprises one naphthoquinone ring and one semi-unsaturated phytoconjugate side chain (Scheme 1). In the natural photosynthetic photosystem II (PSII), phylloquinone oxidizes H2O molecules to produce oxygen while simultaneously being reduced to plastid quinol.32 Menadione (vitamin K3) is present in the metabolites of some bacteria and is often synthesized artificially.33 It facilitates a rapid redox cycle in the cell, resulting in the production of reactive oxygen species.34 Both phylloquinone and menadione exhibit excellent chemical activities. Despite this, most current research on these compounds has mainly focused on biomedical applications,35 and their potential in photopolymerization has not been well explored.
This study introduces two bio-sourced naphthoquinone derivatives, phylloquinone (vitamin K1) and menadione (vitamin K3), as free radical photoinitiators (PIs) in photopolymerization. Additionally, the photoinitiation ability of juglone, another bio-sourced naphthoquinone derivative which was previously reported as a photoinitiator,24 was also studied for reference. In the three-component PISs used for free radical polymerization (FRP), the iodonium salt, bis-(4-t-butylphenyl)-iodonium hexafluorophosphate (Iod = Speedcure 938®), and the amine salt, ethyl 4-(dimethylamino)benzoate (Speedcure EDB®), were selected as co-initiators, while trimethylolpropane triacrylate (TMPTA) and poly(ethylene glycol) diacrylate with an average Mn of 600 (PEGDA600) were used as benchmark monomers. Scheme 1 displays the chemical structures of various compounds utilized in this research, including phylloquinone, menadione, juglone, and additional substances. In addition, steady-state photolysis and electron spin resonance (ESR) techniques were conducted to investigate the photochemical properties and the photoinitiation mechanism of PISs. Finally, direct laser writing (DLW: 50 μm spot size, 110 mW and 405 nm) was carried out to study the practical application of two-component PISs in 3D printing.
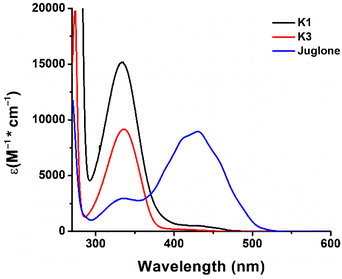
Fig. 1 illustrates the UV-vis absorptions of K1, K3, and juglone in chloroform, and Table S1† provides details on the maximum absorption wavelengths (λmax) and the molar extinction coefficients at the corresponding maximum absorption wavelengths (εmax) and at a wavelength of 405 nm (ε405 nm). The maximum absorption wavelengths (λmax) of both K1 and K3 were in the near-UV range (333–335 nm), Fig. 1. Both vitamins have a broad and similar absorption range and a red flanked shoulder/plateau, indicating light absorption up to 460 nm, which does overlap with the light absorption spectra of blue LEDs. The molar extinction coefficients of vitamin K3 have been experimentally documented in the pertinent literature.36 To verify this, the UV absorption spectrum of K3 in ethanol was obtained and is illustrated in Fig. S1.† The experimentally obtained molar extinction coefficient of K3 at a wavelength of 334 nm was found to be 6200 M−1 cm−1, which is significantly higher than the value of 2700 M−1 cm−1 presented in the literature. Concurrently, the maximum absorption peak of K3 falls around 334 nm in the wavelength range from 300 nm to 400 nm and a small absorption shoulder at a wavelength > 400 nm is observed for both the K3 referenced in the literature and the K3 analyzed in this study. Hence, the accuracy of the light absorption data of K3 presented in this study can be corroborated. Similarly, the accuracy of the light absorption data of K1 was verified by comparing the absorption peak shape of K1 across multiple sources.37,38 In contrast, juglone's light absorption was primarily concentrated at a wavelength of 430 nm. Compared to K3 and juglone, K1 exhibited much better light absorption performance (εmax = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1), which can be attributed to the presence of phytane (2,6,10,14-tetramethylhexadecane) in K1.39 Juglone illustrated better light absorption at a wavelength of 405 nm (ε405 nm = 7700 M−1 cm−1). Given that K1 and K3 demonstrated absorbance at 405 nm, it was appropriate to use an LED at 405 nm as the irradiation source for photopolymerization reactions.
200 M−1 cm−1), which can be attributed to the presence of phytane (2,6,10,14-tetramethylhexadecane) in K1.39 Juglone illustrated better light absorption at a wavelength of 405 nm (ε405 nm = 7700 M−1 cm−1). Given that K1 and K3 demonstrated absorbance at 405 nm, it was appropriate to use an LED at 405 nm as the irradiation source for photopolymerization reactions.
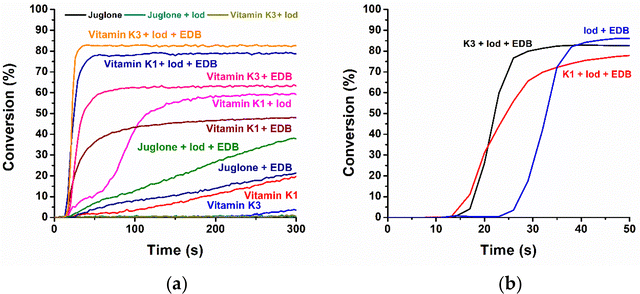
The free radical photopolymerization kinetics of TMPTA upon exposure to an LED at 405 nm was assessed by monitoring the final acrylate function conversion (FC) and the rate of photopolymerization through real-time Fourier-transform infrared spectroscopy (RT-FTIR).40Fig. 2 illustrates the polymerization profiles, and the FC values are presented in Table S2.† The photoinitiation abilities of three natural naphthoquinone derivatives in one-component, two-component, and three-component PISs were studied and compared (see Fig. 2A). Notably, K3 exhibited the best photoinitiation ability in both naphthoquinone/EDB and naphthoquinone/Iod/EDB systems, in terms of FC and the photopolymerization rate. Specifically, after 290 s irradiation under air at room temperature, the FC of TMPTA initiated using the K3/Iod/EDB PIS reached 83%. Similarly, the FC of TMPTA polymerization initiated by the K1/Iod/EDB PIS was 79%. In the two-component systems, the K1/Iod PIS had a relatively good FC (59%) although it exhibited unusual polymerization profiles, Fig. 2. We will discuss the possible reasons in the next section where we discuss the results for the PEGDA samples. For K3/Iod and juglone/Iod PISs, no significant photoinitiation abilities for photopolymerization were observed within the TMPTA samples. For K1/EDB and K3/EDB PISs, the photochemical reactions between K1 or K3 and EDB are more pronounced compared to juglone. Interestingly, K1 can act as a one-component type II PI, but the corresponding FC of TMPTA was only about 20%. When EDB was added as a co-initiator, the photopolymerization conversion was greatly improved (FC(K1) = 20%; FC(K1/EDB) = 48%). Similarly, the PIS with K3 alone did not effectively initiate the polymerization of TMPTA. It was worth noting that the photoinitiation ability of the K3/EDB PIS (FC(K3/EDB) = 63%) was excellent, and the samples polymerized using the K3/EDB PIS appeared almost transparent and colorless (see Table S3†), which greatly facilitated the transmission of light. Moreover, both K1 and K3 demonstrated significant advantages in terms of photoinitiation ability compared to the reported juglone photoinitiators.24
The three-component systems exhibited a significant enhancement in the FC of acrylate functional groups, as well as an increased rate of polymerization compared to the other two-component systems. To demonstrate the key role played by K1 and K3 in the PIS, the Iod/EDB PIS was utilized as a control group in the photopolymerization experiments (see Fig. 2B). After 50 s irradiation, the control group resulted in a FC of 86%, which was only slightly higher than the FC of the K3/Iod/EDB PIS (83%). However, when comparing the photopolymerization rates, it can be observed that the K3/Iod/EDB PIS completed the polymerization of TMPTA in about 15 s of irradiation, whereas the Iod/EDB PIS required approximately 35 s of irradiation. Similarly, the polymerization rate was also significantly enhanced using the K1/Iod/EDB system. The time taken for completing the polymerization of TMPTA was reduced by 15 s after the addition of K1 to the Iod/EDB PIS.
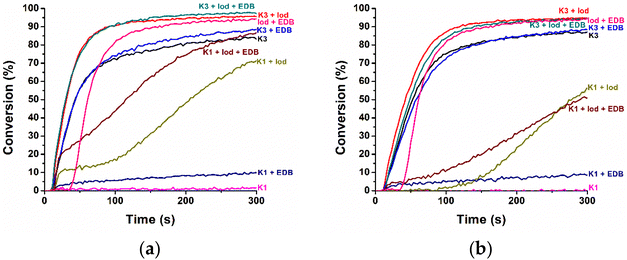
To further investigate the versatility of K1- and K3-based PISs, poly(ethylene glycol) diacrylate with an average Mn of 600 (PEGDA), a difunctional monomer, was also employed as a monomer in the polymerization experiments. Table S2† shows the FCs of the acrylate functional groups of PEGDA using different PISs. As depicted in Fig. 3, except for vitamin K1 containing a large aliphatic chain and K1/EDB systems, all other PISs were more effective at initiating the polymerization of PEGDA monomers compared to initiating the photopolymerization of TMPTA monomers under the irradiation of an LED at 405 nm. A comparison of the photoinitiation abilities of the K1- and K3-based PISs revealed that the K3-based PISs were far superior to the K1-based systems for the polymerization of PEGDA. All K1-based reactive systems show non-sigmoidal polymerization profiles, with a fast phase immediately after light onset, followed by a slower increase/plateau and a rise again, Fig. 3a. A possible reason for these effects could be the inhomogeneity of the solution of the vitamin (sterically induced influence of the bimolecular reaction) and the diffusion and solubility of oxygen. Given the substantial molar mass disparity between K1 (450.7 g mol−1) and K3 (172.2 g mol−1), we aimed to minimize discrepancies when comparing their capability to initiate the polymerization of PEGDA monomers. To ensure accuracy, K1 and K3 were used in equivalent molar quantities under consistent conditions, and the photoinitiation systems were separately prepared and tested in repeated experiments. Fig. 3b clearly demonstrates that, when used in equivalent molar quantities, K3's initiation performance significantly outperforms K1's. Even though K1 has a larger molecular mass—resulting in a higher mass percentage in the K1-based systems (greater than 0.1 wt%)—compared to K3 when the molar count is equivalent, its initiation capability is surprisingly diminished, as shown in Fig. 3a, which indicates the non-perfect solubility of the PI or its partial phase separation in the mixture. The performance of the K3-based PISs demonstrated the excellent FC and conversion rate of the monomer for single-component (FC(K3) = 83%), two-component (FC(K3/Iod) = 96%; FC(K3/EDB) = 87%), and three-component (FC(K3/Iod/EDB) = 97%) systems even though the absorbance is much lower. Similarly, the Iod/EDB system was still regarded as a control group. From the results, it was observed that compared to Iod/EDB, the K3/Iod/EDB system significantly enhanced the initiation performance due to the addition of K3, which was reflected by the higher FC (FC(Iod/EDB) = 94%) and polymerization rate of the monomer than those of the control group. It was noteworthy that the excellent performance of the K3 one-component system made it feasible to dispense with the addition of co-initiators. Considering the fact that K3 can be used as a human vitamin K supplement in medicine and can be applied in clinical applications41 and that poly-PEG is widely used as a dental shaping material,42 the combination of the two can foster new non-toxic and non-hazardous methods for the fabrication of medical composites.
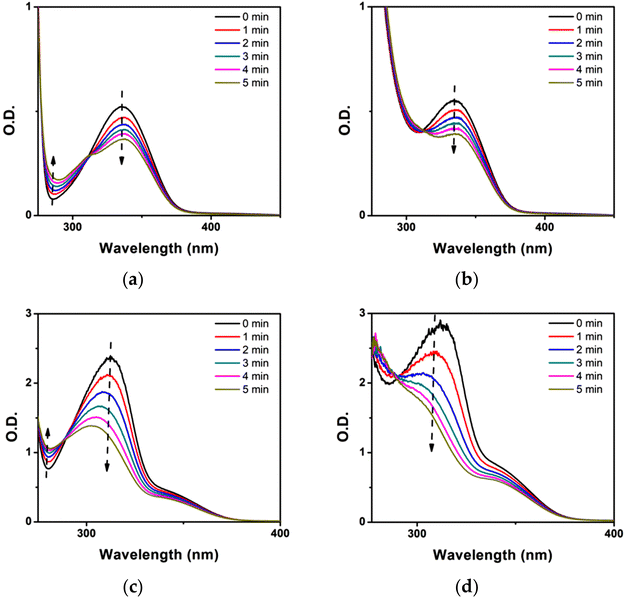
To explore the interaction between K3 and the additives in detail, we conducted steady-state photolysis with K3-based PISs. As a result, the K3 absorption peak at 335 nm diminished upon continuous exposure to an LED at 405 nm for 5 minutes, as shown in Fig. 4A, while the absorption peak at 275 nm experienced a noticeable increase. These changes can be ascribed to the formation of by-products resulting from the photochemical oxidation and reduction of K3.43 For the K3/Iod system (see Fig. 4B), the rate of K3 degradation was comparable to that of K3 alone, but it was evident that the absorption peaks of by-products vanished; thus, it could be inferred that Iod might react with the by-products of K3 photolysis. Conversely, the decrease in the absorption peak of K3 in the photolysis of the K3/EDB system (see Fig. 4C) was more pronounced. Moreover, the rate of decrease of the K3 absorption peak in the photolysis of the three-component system (see Fig. 4D) was considerably higher than that of the other experimental groups, and the absorption peak of K3 virtually disappeared after 5 minutes of irradiation. Compared to the photolysis outcomes of the K3/Iod system, the addition of EDB significantly enhanced the photoreactivity of the system, and the interaction among K3, Iod, and EDB could more efficiently boost the initiation performance of the system.
Further examination of the fluorescence characteristics of K1 and K3 was conducted (Fig. S2†), revealing that the tested samples display no discernible peaks in their fluorescence spectrum, indicating that both K1 and K3 lack significant fluorescence properties, making the calculation of the singlet excited state energy (ES1) not possible from the crossing point of normalized absorption/fluorescence spectra. To elucidate the redox processes and free energy changes of K1 and K3 during the photochemical reactions, we undertook cyclic voltammetry experiments. The resulting data are depicted in Fig. S3.† Some parameters characterizing the electron transfer reactions in the photoinitiating systems are given in Table 1, i.e. the singlet excited state energy (ES1), the Gibbs free energy change (ΔG) (for detailed calculations of the free energy, please refer to the formulas provided in the ESI†), and the triplet state energy (calculated at the DFT level) of K1 and K3. Given the absence of a fluorescence spectrum, the value of ES1 is derived from the edge of the UV-vis absorption spectrum, specifically at the longest absorption wavelength. Due to the lack of clear oxidation peaks in cyclic voltammetry experiments for K1 and K3, some data remain unknown. This later behavior can suggest a high oxidation potential. Therefore, the data presented in Table 1 suggest that K1 and K3 have a higher propensity to chemically interact with EDB compared to Iod, aligning with observations from the photolysis experiments. Additionally, K1 and K3 are more likely to react in the singlet excited state than in the triplet excited state (ΔG is always more favorable with S1).
| E s1 | E T1 | E ox | E red | ΔGs1Iod | ΔGT1Iod | ΔGs1EDB | ΔGT1EDB | |
|---|---|---|---|---|---|---|---|---|
| a No oxidation peak (Eox) clearly observed (see in the ESI†); ΔG for the photooxidation by Iod cannot be evaluated. | ||||||||
| K1 | 2.63 eV | 2.08 eV | −0.98 V | −0.65 eV | −0.1 eV | |||
| K3 | 2.74 eV | 2.24 eV | −0.86 V | −0.88 eV | −0.38 eV | |||
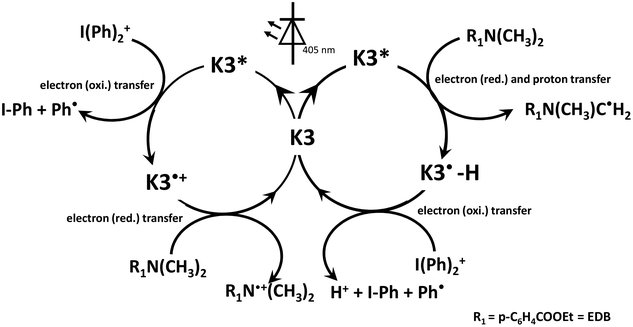
To gain deeper insights into the photoinitiation mechanism, ESR-ST experiments were conducted to investigate the free radicals generated from the relevant PISs. Fig. 5A presents the ESR-ST spectrum obtained from the K3/Iod system under light irradiation, which indicated the generation of radical adducts with hyperfine coupling constants, aN = 14.2 G and aH = 2.2 G. These values aligned with aryl (Ar˙)/PBN radical adducts (47.8%).44 Analogously, Fig. 5B shows that aminoalkyl radicals were generated in the experiments of the K3/EDB system (aN = 14.3 G and aH = 2.2 G, 74.7%). Moreover, oxygen-centered radicals (aN = 13.5 G, aH = 1.8 G, 52.2% and aN = 14.0 G, aH = 4.6 G, 25.3%) were discerned in both the K3/Iod and K3/EDB systems. The successful detection of the aforementioned free radicals unequivocally confirmed that K3 can be employed as a photoinitiator for free radical polymerization. Simultaneously, we can infer potential reactions within the K3 PISs (Scheme 2).
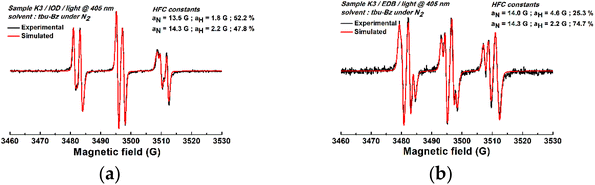 | ||
| Fig. 5 ESR-ST spectra of the radical adducts obtained in the presence of the (a) K3/Iod PIS and (b) K3/EDB PIS exposed to LED light at 405 nm. | ||
As the polymerization of PEGDA can be initiated solely by K3, ESR-ST experiments were conducted with K3/PEGDA under LED irradiation at 405 nm as a comparative investigation. The acquired spectra are illustrated in Fig. S4.† The spectral comparison before and after irradiation demonstrated that PBN effectively captured the free radicals responsible for the photopolymerization of PEGDA, as evidenced by the changes observed in the spectra.45,46 There was a high probability that the free radical comes from the chemical reaction of K3 with PEG as a hydrogen donor. The PEG chain can act as a hydrogen donor due to its ether structure.47 Supporting this conclusion, a previous report by Nie et al. detailed the interaction between PEG and an initiator. Their study demonstrated that the initiator, termed “BFC”, formed a complex with PEG owing to electrostatic interactions. Additionally, a hydrogen transfer interaction was observed between the two entities.48
Based on the above conclusion, by comparing the PEGDA monomer conversion data of two different systems, K3/Iod (FC = 93%) and K3/Iod/EDB(FC = 95%), it is evident that there is only a very small difference in the final conversions. This can indirectly prove that in the absence of amine (EDB) in the system, PEG in some cases replaces the role of amine as a hydrogen donor in the photopolymerization reaction, thus driving the continuation of the reaction, as described in the previous section. Meanwhile, it is important to note that the mere generation of free radicals in the ESR solvent does not guarantee the occurrence of free radical polymerization reactions. The quantity (too low) of free radicals generated may be insufficient to efficaciously initiate polymerization. The K3/Iod system generated radicals upon irradiation, capable of initiating the polymerization of the PEGDA monomer, though not TMPTA. When PEG is present, the internal interaction of the two-component system, K3/Iod, more closely resembles that of the three-component system, K3/Iod/amine. In contrast, in TMPTA, the K3/Iod system is purely a two-component system (with no other hydrogen donor). From the UV spectra, it is clear that the by-products produced after the irradiation of K3 react with Iod, leading us to speculate that the chemical reaction priority of the complex interaction between K3, Iod and amine is higher than that of the combination of K3 photolysis products with Iod, which affects the quantity of free radicals produced. Therefore, the K3/Iod/EDB system can successfully initiate the photopolymerization of TMPTA, while the K3/Iod system cannot initiate the photopolymerization of TMPTA because it cannot produce a high enough free radical concentration in TMPTA. For K1, the long aliphatic chain may influence the homogeneity of the distribution within the resins and have steric effects. The higher concentration measurements, Fig. 3, also support the assumption of non-perfect solubility or phase separation of the PI in the resin blends. Compared with PEGDA, TMPTA is less polar and more suitable for the dispersion of K1. This observation was particularly noteworthy when comparing the performance of all K1-based PISs in TMPTA photopolymerization and PEGDA photopolymerization, respectively, Table S2.† Here, the FC and polymerization rate of TMPTA (a less polar resin) tended to be lower when utilizing the same initiation systems.
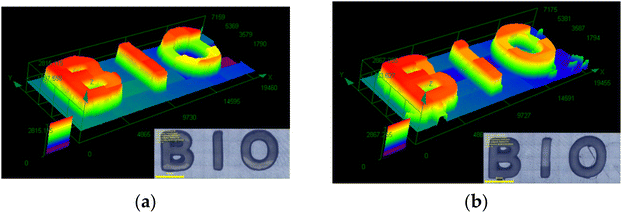
The K1/Iod and K3/EDB systems, which exhibited good capabilities in the FRP of TMPTA, were utilized for 3D printing experiments. A numerical control laser diode at 405 nm was used to fabricate the 3D pattern “BIO” via direct laser writing. The digital images of the resulting 3D patterns were examined using digital optical microscopy. As demonstrated in Fig. 6, the 3D patterns showed effective photopolymerization and high spatial resolution (within the size of the laser beam, approximately 50 μm).
In summary, this study introduced bio-sourced naphthoquinone derivatives phylloquinone (vitamin K1) and menadione (vitamin K3) as photoinitiators in free radical photopolymerization. The application of these two vitamins, commonly found in daily life, as photoinitiators is reported for the first time. Compared with the previously reported juglone, K1 and K3 demonstrated superior performance as type II photoinitiators. Notably, the more polar vitamin K3 alone can photoinitiate PEGDA polymerization with a high conversion. The formulations composed of two low-toxic compounds can be better used in biopolymer materials. When combined with Iod/EDB, the polymerization rate and double bond conversion of TMPTA can be significantly improved with K1 or K3. From the free energy calculations related to the photopolymerization reaction, it becomes evident that K1 and K3 have a good propensity to interact with EDB. This observation aligns with the findings from both photolysis and practical polymerization experiments. ESR experiments captured free radicals capable of initiating photopolymerization reactions across various systems. Specifically, for the K3/PEGDA system, coupled with insights from earlier reports, it was further evidenced that hydrogen transfer occurs between the PEG chain and K3 because of their electronic interactions. Consequently, even as a one-component system, K3 remains an exemplary type II photoinitiator. The photoinitiation mechanism was discussed in detail, and the efficiencies of K1/Iod and K3/EDB systems in 3D printing were demonstrated. The experiments indicated that K1 and K3 exhibited high photoinitiation abilities, even considering the low optical absorbance under the irradiation of an LED at 405 nm.
The use of natural products as photoinitiators presents an interesting and growing area of research, particularly in the fields of green chemistry and sustainable materials science. Traditionally, all current commercial photoinitiators are synthetic and an increasing number of them are now considered as harmful to the environment or human health. Natural products are often biodegradable and can sometimes be less toxic. This makes them more environmentally friendly and safer for use in various applications. The use of natural products or products synthesized from natural products with “low” toxicity as photoinitiators offers several potential advantages and application prospects. To be considered environmentally friendly, not only the reactants, but also the synthesis (possible optimizations such as reducing solvent consumption, catalysts, and recycling) and of course the end-of-life environmental impact must be factored in. For example, due to their low-toxicity, vitamins K1 and K3 as photoinitiators can be particularly advantageous in biomedical applications such as tissue engineering, dental materials, and drug delivery systems, where biocompatibility is crucial. They can be used in eco-friendly coatings, paints, and printing inks, especially for applications that require low-VOC (volatile organic compounds) or low-toxicity formulations. In additive manufacturing and 3D printing, vitamins K1 and K3 as photoinitiators can contribute to more sustainable production processes. Unlike other bio-based compounds such as chlorophyll, these vitamins are readily available and, at less than 77 Euro per g, are much cheaper than the green dye priced at >400k Euro per g. The development of non-toxic and eco-friendly natural naphthoquinones as photoinitiators can contribute to the progress of green chemistry and offer a reference for the development of synthetic naphthoquinone photoinitiators.
Despite these prospects, there are additional challenges (e.g. carbon balance and the environmental impact of synthesis and/or purification) that need to be addressed for the widespread application of vitamins K1 and K3 as photoinitiators. They can be sensitive to environmental conditions, and their stability and shelf life may be lower compared to their synthetic counterparts. Overall, the application prospects of vitamins K1 and K3 as photoinitiators are promising, particularly in the context of sustainable and green chemistry. Continued research and development in this area are essential to overcome current challenges and fully realize the potential of these environmentally friendly alternatives.
Experimental part
The ultraviolet-visible (UV-vis) absorption characteristics of various PISs were scrutinized using a JASCO V730 spectrophotometer. The acrylate group conversion was continuously monitored through real-time Fourier-transform infrared spectroscopy (RT-FTIR, JASCO FTIR 4100) at approximately 6120 cm−1 for the acrylate functionalities in trimethylolpropane triacrylate (TMPTA) and poly(ethylene glycol) diacrylate (PEGDA).29 The irradiation process commenced at t = 10 s. The fluorescence experiments were performed using a JASCO FP-6200 spectrometer. The Gaussian 16 suite of programs was used to perform computational chemistry calculations at the UB3LYP/6-31G* level. Electron spin resonance spin trapping (ESR-ST) experiments were conducted using an X-band spectrometer (Bruker EMX-Plus). Nitrogen-saturated solutions of the studied initiator and spin trap N-tert-butyl-1-phenylmethanimine oxide (PBN) in tert-butylbenzene were irradiated with an LED light source at 405 nm and ambient temperature in the cavity of the ESR device. 3D laser writing experiments (simulated 3D printing experiments) were performed using a homemade DLW printer. The resulting three-dimensional patterns were examined using a digital optical microscope (OLYMPUS DSX-HRSU).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research project is supported by China Scholarship Council (CSC) (No. 202007030005; Timur Borjigin as Distinguished International Students Scholarship).References
- T. Borjigin, M. Schmitt, F. Morlet-Savary, P. Xiao and J. Lalevée, Low-Cost and Recyclable Photocatalysts: Metal Oxide/Polymer Composites Applied in the Catalytic Breakdown of Dyes, Photochemistry, 2022, 2, 733–751 CAS.
- N. Corrigan, J. Yeow, P. Judzewitsch, J. Xu and C. Boyer, Seeing the Light: Advancing Materials Chemistry through Photopolymerization, Angew. Chem., Int. Ed., 2019, 58, 5170–5189 CrossRef CAS.
- B. Kumru, Light-Enabled Access to Oxidative Polymerization: A Short Perspective on Bioinspired Oxidative Photopolymerization, Sol. RRL, 2022, 6, 2200728 CrossRef CAS.
- M. Liang, T. Borjigin, Y. Zhang, B. Liu, H. Liu and H. Guo, Controlled assemble of hollow heterostructured g-C3N4@CeO2 with rich oxygen vacancies for enhanced photocatalytic CO2 reduction, Appl. Catal., B, 2019, 243, 566–575 CrossRef CAS.
- M. Liang, T. Borjigin, Y. Zhang, H. Liu, B. Liu and H. Guo, Z-Scheme, Au@Void@g-C3N4/SnS Yolk–Shell Heterostructures for Superior Photocatalytic CO2 Reduction under Visible Light, ACS Appl. Mater. Interfaces, 2018, 10, 34123–34131 CrossRef CAS.
- S. Shanmugam, J. Xu and C. Boyer, Utilizing the electron transfer mechanism of chlorophyll a under light for controlled radical polymerization, Chem. Sci., 2015, 6, 1341–1349 RSC.
- C. Wu, S. Shanmugam, J. Xu, J. Zhu and C. Boyer, Chlorophyll a crude extract: efficient photo-degradable photocatalyst for PET-RAFT polymerization, Chem. Commun., 2017, 53, 12560–12563 RSC.
- T. Borjigin, G. Noirbent, D. Gigmes, P. Xiao, F. Dumur and J. Lalevée, The new LED-Sensitive photoinitiators of Polymerization: Copper complexes in free radical and cationic photoinitiating systems and application in 3D printing, Eur. Polym. J., 2022, 162, 110885 CrossRef CAS.
- S. Liu, T. Borjigin, M. Schmitt, F. Morlet-Savary, P. Xiao and J. Lalevée, High-Performance Photoinitiating Systems for LED-Induced Photopolymerization, Polymers, 2023, 15, 342 CrossRef CAS PubMed.
- N. S. Allen, Photoinitiators for UV and visible curing of coatings: Mechanisms and properties, J. Photochem. Photobiol., A, 1996, 100, 101–107 CrossRef CAS.
- M.-A. Tehfe, F. Dumur, B. Graff, F. Morlet-Savary, D. Gigmes, J.-P. Fouassier and J. Lalevée, Design of new Type I and Type II photoinitiators possessing highly coupled pyrene–ketone moieties, Polym. Chem., 2013, 4, 2313–2324 RSC.
- G. Noirbent and F. Dumur, Photoinitiators of polymerization with reduced environmental impact: Nature as an unlimited and renewable source of dyes, Eur. Polym. J., 2021, 142, 110109 CrossRef CAS.
- F. Dumur, Recent advances on benzylidene ketones as photoinitiators of polymerization, Eur. Polym. J., 2022, 178, 111500 CrossRef CAS.
- S. Tomane, P. Sautrot-Ba, P.-E. Mazeran, J. Lalevée, B. Graff, F. Morlet-Savary, S. Abbad-Andaloussi, V. Langlois and D.-L. Versace, Photoinitiating Systems Based on Anthraquinone Derivatives: Synthesis of Antifouling and Biocide Coatings, ChemPlusChem, 2017, 82, 1298–1307 CrossRef CAS.
- J. Zhang, J. Lalevée, J. Zhao, B. Graff, M. H. Stenzel and P. Xiao, Dihydroxyanthraquinone derivatives: natural dyes as blue-light-sensitive versatile photoinitiators of photopolymerization, Polym. Chem., 2016, 7, 7316–7324 RSC.
- J. Zhang, N. Hill, J. Lalevée, J.-P. Fouassier, J. Zhao, B. Graff, T. W. Schmidt, S. H. Kable, M. H. Stenzel and M. L. Coote, et al., Multihydroxy-Anthraquinone Derivatives as Free Radical and Cationic Photoinitiators of Various Photopolymerizations under Green LED, Macromol. Rapid Commun., 2018, 39, 1800172 CrossRef.
- J. Zhang, K. Launay, N. S. Hill, D. Zhu, N. Cox, J. Langley, J. Lalevée, M. H. Stenzel, M. L. Coote and P. Xiao, Disubstituted Aminoanthraquinone-Based Photoinitiators for Free Radical Polymerization and Fast 3D Printing under Visible Light, Macromolecules, 2018, 51, 10104–10112 CrossRef CAS.
- J. Zhang, J. Lalevée, N. S. Hill, K. Launay, F. Morlet-Savary, B. Graff, M. H. Stenzel, M. L. Coote and P. Xiao, Disubstituted Aminoanthraquinone-Based Multicolor Photoinitiators: Photoinitiation Mechanism and Ability of Cationic Polymerization under Blue, Green, Yellow, and Red LEDs, Macromolecules, 2018, 51(20), 8165–8173 CrossRef CAS.
- G. Wang, N. S. Hill, D. Zhu, P. Xiao, M. L. Coote and M. H. Stenzel, Efficient Photoinitiating System Based on Diaminoanthraquinone for 3D Printing of Polymer/Carbon Nanotube Nanocomposites under Visible Light, ACS Appl. Polym. Mater., 2019, 1, 1129–1135 CrossRef CAS.
- P. Xiao, F. Dumur, B. Graff, J. P. Fouassier, D. Gigmes and J. Lalevée, Cationic and Thiol–Ene Photopolymerization upon Red Lights Using Anthraquinone Derivatives as Photoinitiators, Macromolecules, 2013, 46, 6744–6750 CrossRef CAS.
- J. Zhang, J. Lalevée, N. S. Hill, X. Peng, D. Zhu, J. Kiehl, F. Morlet-Savary, M. H. Stenzel, M. L. Coote and P. Xiao, Photoinitiation Mechanism and Ability of Monoamino-Substituted Anthraquinone Derivatives as Cationic Photoinitiators of Polymerization under LEDs, Macromol. Rapid Commun., 2019, 40, 1900234 CrossRef.
- J. Zhang, J. Lalevée, F. Morlet-Savary, B. Graff and P. Xiao, Photopolymerization under various monochromatic UV/visible LEDs and IR lamp: Diamino-anthraquinone derivatives as versatile multicolor photoinitiators, Eur. Polym. J., 2019, 112, 591–600 CrossRef CAS.
- J. Zhang, J. Lalevée, N. S. Hill, J. Kiehl, D. Zhu, N. Cox, J. Langley, M. H. Stenzel, M. L. Coote and P. Xiao, Substituent Effects on Photoinitiation Ability of Monoaminoanthraquinone-Based Photoinitiating Systems for Free Radical Photopolymerization under LEDs, Macromol. Rapid Commun., 2020, 41, 2000166 CrossRef CAS.
- X. Peng, D. Zhu and P. Xiao, Naphthoquinone derivatives: Naturally derived molecules as blue-light-sensitive photoinitiators of photopolymerization, Eur. Polym. J., 2020, 127, 109569 CrossRef CAS.
- E. S. Ahmadi, A. Tajbakhsh, M. Iranshahy, J. Asili, N. Kretschmer, A. Shakeri and A. Sahebkar, Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity, Mini-Rev. Med. Chem., 2020, 20, 2019–2035 CrossRef CAS.
- R. H. Thomson, Distribution of naturally occurring quinones, Pharm. Weekbl., 1991, 13, 70–73 CrossRef CAS.
- H. M. Awad, M. G. Boersma, S. Boeren, P. J. van Bladeren, J. Vervoort and I. M. C. M. Rietjens, Structure−Activity Study on the Quinone/Quinone Methide Chemistry of Flavonoids, Chem. Res. Toxicol., 2001, 14, 398–408 Search PubMed.
- F. MacMillan, J. Hanley, L. van der Weerd, M. Knüpling, S. Un and A. W. Rutherford, Orientation of the Phylloquinone Electron Acceptor Anion Radical in Photosystem I, Biochemistry, 1997, 36(31), 9297–9303 CrossRef CAS.
- T. Borjigin, M. Schmitt, N. Giacoletto, A. Rico, H. Bidotti, M. Nechab, Y. Zhang, B. Graff, F. Morlet-Savary and P. Xiao, et al., The Blue-LED-Sensitive Naphthoquinone-Imidazolyl Derivatives as Type II Photoinitiators of Free Radical Photopolymerization, Adv. Mater. Interfaces, 2023, 10, 2202352 CrossRef CAS.
- C. Elian, V. Brezová, P. Sautrot-Ba, M. Breza and D.-L. Versace, Lawsone Derivatives as Efficient Photopolymerizable Initiators for Free-Radical, Cationic Photopolymerizations, and Thiol—Ene Reactions, Polymers, 2021, 13(12), 2015 CrossRef CAS.
- L. P. Kegel and F. L. Crane, Vitamin K1 in Chloroplasts, Nature, 1962, 194, 1282–1282 CrossRef CAS.
- W.-D. Schubert, O. Klukas, W. Saenger, H. T. Witt, P. Fromme and N. Krauß, A common ancestor for oxygenic and anoxygenic photosynthetic systems: A comparison based on the structural model of photosystem, in Journal of Molecular Biology, ed. R. Huber, 1998, vol. 280, pp. 297–314 Search PubMed.
- D. Altwiley, T. Brignoli, A. Edwards, M. Recker, J. C. Lee and R. C. Massey, A functional menadione biosynthesis pathway is required for capsule production by Staphylococcus aureus, Microbiology, 2021, 167(11), 001108 CrossRef CAS PubMed.
- T. W. Gant, D. N. Ramakrishna Rao, R. P. Mason and G. M. Cohen, Redox cycling and sulphydryl arylation; Their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes, Chem.-Biol. Interact., 1988, 65, 157–173 CrossRef CAS.
- X. Fu, S. L. Booth and D. E. Smith, Vitamin K Contents of Rodent Diets: A Review, J. Am. Assoc. Lab. Anim. Sci., 2007, 46, 8–12 CAS.
- J. Szejtli, É. Bolla-Pusztai and M. Kajtár, The β-cyclodextrin inclusion complex of menadione (vitamin K3), Pharmazie, 1982, 37, 725–728 CAS.
- D. T. Ewing, J. M. Vandenbelt and O. Kamm, The ultraviolet absorption of vitamins K1, K2, and some related compounds, J. Biol. Chem., 1939, 131, 345–356 CrossRef CAS.
- U. Hubicka, A. Padiasek, B. Żuromska-Witek and M. Szlósarczyk, Determination of Vitamins K1, K2 MK-4, MK-7, MK-9 and D3 in Pharmaceutical Products and Dietary Supplements by TLC-Densitometry, Processes, 2020, 8(7), 870 CrossRef CAS.
- J. B. Johnston, P. M. Kells, L. M. Podust and P. R. Ortiz de Montellano, Biochemical and structural characterization of CYP124: A methyl-branched lipid ω-hydroxylase from Mycobacterium tuberculosis, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20687–20692 CrossRef CAS PubMed.
- C. Dietlin, S. Schweizer, P. Xiao, J. Zhang, F. Morlet-Savary, B. Graff, J.-P. Fouassier and J. Lalevée, Photopolymerization upon LEDs: new photoinitiating systems and strategies, Polym. Chem., 2015, 6, 3895–3912 RSC.
- H. Bai, H. Arai, K. Ikuta, S. Ishikawa, Y. Ohtani, K. Iwashita, N. Okada, H. Shirakawa, M. Komai and F. Terada, et al., Effects of dietary vitamin K3 supplementation on vitamin K1 and K2 (menaquinone) dynamics in dairy cows, Anim. Sci. J., 2022, 93, e13680 CrossRef CAS PubMed.
- T. D. Jones, A. Kefi, S. Sun, M. Cho and S. B. Alapati, An Optimized Injectable Hydrogel Scaffold Supports Human Dental Pulp Stem Cell Viability and Spreading, Adv. Med., 2016, 2016, 7363579 CAS.
- S. Izawa, Photochemical reduction and oxidation of menadione (vitamin K3) in chloroplasts, Plant Cell Physiol., 1960, 1, 269–279 CAS.
- M.-A. Tehfe, J. Lalevée, F. Morlet-Savary, B. Graff, N. Blanchard and J.-P. Fouassier, Tunable Organophotocatalysts for Polymerization Reactions Under Visible Lights, Macromolecules, 2012, 45, 1746–1752 CrossRef CAS.
- P. Pal, A. Saha, A. K. Mukherjee and D. C. Mukherjee, Study of charge transfer complexes of menadione (vitamin K3) with a series of anilines, Spectrochim. Acta, Part A, 2004, 60, 167–171 CrossRef.
- S. J. Duthie and M. H. Grant, The role of reductive and oxidative metabolism in the toxicity of mitoxantrone, adriamycin and menadione in human liver derived Hep G2 hepatoma cells, Br. J. Cancer, 1989, 60, 566–571 CrossRef CAS PubMed.
- M. A. Tasdelen, N. Moszner and Y. Yagci, The use of poly(ethylene oxide) as hydrogen donor in type II photoinitiated free radical polymerization, Polym. Bull., 2009, 63, 173–183 CrossRef CAS.
- J. Li, X. Zhang, J. Nie and X. Zhu, Visible light and water-soluble photoinitiating system based on the charge transfer complex for free radical photopolymerization, J. Photochem. Photobiol., A, 2020, 402, 112803 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc03551d |
| This journal is © The Royal Society of Chemistry 2024 |