Open Access Article
Open Access ArticleDeciphering the potential of Cymbopogon citratus (DC.) Stapf as an anti-obesity agent: phytochemical profiling, in vivo evaluations and molecular docking studies†
Omnia
Aly‡
a,
Reham Hassan
Mekky‡
 b,
Florbela
Pereira‡
b,
Florbela
Pereira‡
 c,
Yasser M.
Diab
d,
Mohamed A.
Tammam
c,
Yasser M.
Diab
d,
Mohamed A.
Tammam
 *d and
Amr
El-Demerdash
*d and
Amr
El-Demerdash
 *ef
*ef
aDepartment of Medical Biochemistry, National Research Centre, Cairo 12622, Egypt. E-mail: mat01@fayoum.edu.eg
bDepartment of Pharmacognosy, Faculty of Pharmacy, Egyptian Russian University, Badr City, Cairo-Suez Road, 11829, Cairo, Egypt
cLAQV REQUIMTE, Department of Chemistry, NOVA School of Science and Technology, Universidade Nova de Lisboa, 2829516 Caparica, Portugal
dDepartment of Biochemistry, Faculty of Agriculture, Fayoum University, Fayoum 63514, Egypt
eSchool of Chemistry, Pharmacy and Pharmacology, University of East Anglia, Norwich Research Park, Norwich NR4 7TJ, UK. E-mail: a_eldemerdash83@mans.edu.eg; A.Eldemerdash@uea.ac.uk; Mohamed Tammam
fFaculty of Sciences, Mansoura University, Mansoura 35516, Egypt
First published on 25th November 2024
Abstract
Based on its anti-inflammatory and antioxidant properties, Cymbopogon citratus (DC) Stapf is commonly used in traditional and modern medicine to cure different diseases. The present study investigates the potential of C. citratus organic extract as an anti-obesity drug in a HCHFD (high-carbohydrate, high-fat diet) model for obese rats. Its negative hypolipidemic effect has been confirmed through biochemical and histological methods. Fifty male albino rats were randomly divided into five groups (10 rats each) Group I (Control group), Group II (HCHFD group), Group III (C. citratus group), Group IV (HCHFD + C. citratus group) and Group V (HCHFD + Orlistat group). Serum glucose levels and lipid profiles were quantified using a spectrophotometer. Insulin, apelin, and adiponectin parameters were measured using ELISA (enzyme-linked immunosorbent assay) kits, while real-time PCR following extraction and purification was used for apelin, apelin receptor genes (APJ), and adiponectin gene expression evaluation. Besides, C. citratus methanolic extract was subjected to untargeted metabolic profiling via RP-HPLC-QTOF-MS and MS/MS, disclosing the presence of 52 secondary metabolites where they mainly belonged to phenolic compounds viz., flavones and hydroxycinnamic acids, among other metabolites with predominance of derivatives of luteolin and O-coumaroyl-O-feruloylglycerol. Our findings were further strengthened by computational-based virtual screening protocols that included molecular docking (MDock) and Structure–Activity Relationships (SARs). The MDock studies revealed that the three main flavone-containing metabolites, each with a luteolin C6-glycosylation core featuring two sugar units (16, 25, and 31), outperformed the positive control (8EH, a triazole derivative) known to bind to the APJ protein. These metabolites exhibited exceptional binding affinities, with estimated free binding energy (ΔGB) values of −9 kcal mol−1 or lower, likely due to potential hydrogen bond interactions with the Arg168 residue of the APJ protein. Additionally, the pharmacokinetic, physicochemical, and toxicity profiles of the 11 major metabolites from C. citratus leaf extract were assessed, revealing a profile like that of the positive control in the three selected flavone metabolites. Based on the acquired data, it can be concluded that C. citratus shows strong potential as a hypolipidemic agent and could play a significant role in managing obesity and mitigating its associated complications.
1. Introduction
On a global scale, obesity is a major and urgent public health problem, resulting in a considerable burden of disability as well as mortality. The condition of obesity is not just characterized by excessive weight. Still, it's an inflammatory systemic disease as well that is associated with diabetes, insulin resistance, cancer, heart disease, chronic renal disease, and other metabolic disorders. Adipose tissue (AT), which consists of stromal vascular cells and adipocytes, is pivotal in the development of obesity and metabolic disorders. Prolonged periods of inactivity and insufficient levels of physical exercise significantly contribute to the development of obesity and associated disorders. Within the axillary thyroid, certain adipokines, released by this endocrine gland, have a role in the development of several diseases and alter lipid and glucose metabolism significantly.1Undoubtedly, being the largest endocrine gland, adipose tissue releases several bio-effective peptides widely referred to as adipokines. Recombinant APLN (Apelin) is a novel adipokine produced from preproapelin, consisting of 77 amino acids. By deriving a 55-amino-acid fragment from preproapelin, smaller bioactive isoforms like APLN-36, APLN-17, APLN-13, and the pyroglutamyl version of APLN-13 (Pyr-APLN-13) are generated. Smaller isoforms (Pyr) APLN-13 and -17 exhibit higher activity and are more commonly found in the bloodstream. Beyond adipose tissue, apelin and its receptor APJ are widely distributed throughout the body and synthesized in varying quantities in nearly all tissues, notably in the brain, blood vessels, heart, lung, spleen, gut, reproductive tract, and breast. Additionally, APLN and APJ play a role in other fundamental biological metabolomics pathways, including cell division, angiogenesis, cardiovascular activity, fluid balance, control of energy metabolism, and food consumption.2
Furthermore, over the past 25 years, since the mid-1990s, adiponectin, a 28 kDa protein adipocytokine mostly synthesized and released into the bloodstream by lean adipocytes, has been extensively researched. The principal role of adiponectin is to control the metabolism of carbohydrates and lipids. Nevertheless, the complete scope of its biological activity has yet to be clarified, encompassing a broad range of impacts on various cell and tissue categories. The protective functions of adiponectin against various disease states associated with obesity, including immunomodulatory, insulin-sensitizing, antidiabetic, anti-obesogenic, anti-inflammatory, anti-atherogenic, anti-fibrotic, cardio, and neuroprotective properties, have led to its initial classification as a guardian angel adipocytokine.3
Indeed, as a substitute for traditional therapies for obesity and related issues, natural products, such as pure compounds or extracts derived from medicinal plants, are readily available in the market. These phytochemicals can elicit their anti-obesity effects by various mechanisms, including the inhibition of digestive enzyme activities (pancreatic lipase and amylase), regulation of appetite, and reduction of white adipose tissue (WAT) formation or enhancement of WAT browning. Furthermore, it has been shown that the phytoconstituents present in various plants exhibit a variety of supplementary modes of action against obesity. Usually, these natural compounds restrict the development of adipose tissue by preventing the differentiation of adipocytes and adipogenesis and reducing levels of triacylglycerol by enhancing the breakdown of fats or reducing metabolic pathways involved in fat production.4
In particular, lemongrass, scientifically known as Cymbopogon. citratus (DC.) Stapf, is a plant extensively employed for phytoremediation because of its extraordinary resistance to certain heavy metals. The cultivated plant is largely of commercial significance to the cosmetics and perfumes sectors due to its essential oils, which consist mostly of citral. Citral is composed of two geometric isomers, geranial and neral, and has a distinctive lemon fragrance. Furthermore, C. citratus possesses minerals, vitamins, and bioactive substances (such as alkaloids, terpenoids, flavonoids, phenols, saponins, and tannins) that are accountable for its pharmacological characteristics (antioxidant, antifungal, anticancer, antihypertensive, antidiabetic, and anxiolytic action).5
Traditionally, the leaves of C. citratus were utilized as tea or decoction in Asia, South America, and Africa as they possessed anti-inflammatory, antiseptic, anti-dyspeptic, and anti-fever effects. They also have antispasmodic, analgesic, antipyretic, tranquillizer, anti-hermetic, diuretic, antidiabetic, and antihyperlipidemic activities. In certain regions of Asia and African countries, it has been employed to deter snakes and reptiles.6,7 There traditional uses provoked several researchers to disclose the phytochemical composition and biological activities of C. citratus, in this sense, Madi et al., explored the phytochemical composition of the leaves via UPLC-Orbitrap HRMS revealing the occurrence of 21 compounds including flavonoids. The leaves also exhibited a neuroprotective effect of leaves against AlCl3−induced neurotoxicity in rats.8 Moreover, Costa et al., proved a strong topical anti-inflammatory ability by the carrageenan-induced rat paw edema model of the HPLC standardized organic extract of lemon grass leaves characterized by the presence of hydroxycinnamic acids and flavones.9 Also, the leaves have a high content of essential oil with a majority of citral, among other terpenoids, where the composition of the essential oil varied according to the geographical origin, season of harvesting, extraction methods, and genetic disparities, among others.6 Besides, a plethora of studies investigated the biological potentials of the leaves, viz., antimicrobial, anti-inflammatory, antimalarial, insecticidal, antihypertensive, and anti-obesity, etc.6,7
With emphasis on the antiobesity activity of C. citratus, several studies focused on the antiobesity activity of lemongrass.10 In this context, Da Ressurreição et al.11 investigated the effect of C. citratus leaves extract, phenolic fraction, and flavonoids on the micellar solubility of cholesterol where a significant micellar destruction was noticed indicating that the intake of lemon grass could eventually disrupt various processes associated with intraluminal lipid processing, including enzymatic hydrolysis, micelle formation, and the absorption of lipid digestion products in the colon. Moreover, Adeneye and Agbaje12 investigated the effect of administration of a single, daily oral dosage of 125–500 mg kg−1 of fresh leaf aqueous C. citratus extract in normal, male Wistar rats for 42 days where it caused weight loss in rats, reduced fasting plasma glucose and lipid parameters (total cholesterol), LDL-c (low-density lipoprotein-cholesterol), and VLD-c (Verl low-density lipoprotein cholesterol), and increased plasma HDL-c (high-density lipoprotein-cholesterol) levels (p < 0.05) dose-dependently, without affecting plasma triglycerides. Furthermore, Kumar et al.13C. citratus oil's antihyperlipidaemic efficacy against dexamethasone-induced hyperlipidaemia in adult male Wistar albino rats. Treatment with C. citratus oil (100 and 200 mg kg−1, po.) significantly inhibits dexamethasone hyperlipidaemia by maintaining normal blood cholesterol, triglycerides, and atherogenic index levels.
Based on the above-mentioned data and in the context of our ongoing research program on pharmacologically active plant and marine-derived natural products,1,14–20 we were motivated to examine the C. citratus organic extract activity as an anti-obesity agent on obese rats using adiponectin, apelin, and its receptor APJ as biomarkers for obesity as well as investigate its active constitutes using reversed-phase high-performance liquid chromatography, additionally supporting our data by a virtual screening protocol including SARs (Structure–Activity Relationships) and MDock (molecular docking). Furthermore, the pharmacokinetic profiles and physicochemical properties, as well as the toxicity profiles of the 11 major metabolites from C. citratus leaf extract (one amino acid (1), one hydroxycinnamic acid (39), eight flavones (14i, 14ii, 16, 24, 25, 31, 37, 40), and one fatty acid (48)) in our screening library were evaluated using the Deep-PK online webtool.
2. Materials and methods
2.1. Plant material
C. citratus leaves were collected in the flowering stage from the Fayoum governate, Egypt. A voucher specimen of the collected sample has been settled at the plant collection of Biochemistry Department, Faculty of Agriculture, Fayoum University, Fayoum Governorate, Egypt (FAY/TP0100). The airdried leaves were extensively extracted with MeOH at room temperature, to afford after evaporation of the solvents in vacuo a crude extract (16.8 g).2.2. In vivo deciphering of the anti-obesity properties of C. citratus extract
The levels of serum apelin (Phoenix Pharmaceuticals, Burlingame, Calif) and adiponectin were measured using ELISA kits adhering to the methodology presented by Mellouk et al.30 The target genes Adelin, Apj, and Adiponectin, as well as the internal reference gene β-actin, were subjected to relative quantitative analysis utilizing the real-time PCR system Light-Cycler 480 from Roche, Germany. Dedicated primer sets (Bioneer, South Korea) developed explicitly for this work are listed in Table 1. The used technique was previously mentioned in detail by Tammam et al.1
| Target | Sequence |
|---|---|
| β-Actin | F: 5′-AGGGAAATCGTGCGTGACAT-3′ |
| R: 5′-GAACCGCTCATTGCCGATAG-3′ | |
| Apelin | F: 5′-TGGAAGGGAGTACAGGGATG-3′ |
| R: 5′-TCCTTATGCCCACT-3′ | |
| APJ | F: 5′-GGACTCCGAATTCCCTTCTC-3′ |
| R: 5′-CTTGTGCAAGGTCAACCTCA-3′ | |
| Adiponectin | F: 5′- CTA CTG TTG CAA GCT CTC C-3′ |
| R: 5′- CTT CAC ATC TTT CAT GTA CAC C-3′ |
All data was analyzed using mean ± SEM. A normal state test was conducted using the SPSS program, version 26, to verify the presence of a normal distribution in the data. Trials with more than two groups and one dependent variable were evaluated for statistical significance using one-way analysis of variance (ANOVA) and post hoc Bonferroni hypothesis testing. Computed Pearson's correlation coefficient was successfully obtained. The criterion for experimental significance (P value) was set at a level lower than 0.05.31
2.3. Metabolic profiling C. citratus (DC.) Stapf leaves extract by LC-MS and tandem MS/MS
2.4. Preparation of the protein structures and molecular docking (MDock)
The 3D X-ray crystal structure of the apelin receptor (APJ receptor) in complex with the small molecule 8EH ((1R,2S)-N-[4-(2,6-dimethoxyphenyl)-5-(6-methylpyridin-2-yl)-1,2,4-triazol-3-yl]-1-(5-methylpyrimidin-2-yl)-1-oxidanyl-propane-2-sulfonamide) was obtained from the Protein Data Bank (PDB ID: 7SUS). PDBQT files were used for docking to the human APJ receptor with AutoDock Vina (version 1.2.3).37 Prior to docking, water molecules, ions, and ligands were removed from 7SUS using AutoDockTools (https://mgltools.scripps.edu/, accessed on 22 May 2024). The 3D structures of the eleven major metabolites from C. citratus leaf extract, as well as the positive control (8EH), were optimized using the RDKit function MMFFOptimizeMolecule with arguments mmffVariant = ‘MMFF94’ and maxIters = 5000 in Python.38The search space coordinates for the APJ receptor (7SUS) were set to encompass the entire macromolecule for docking, with the search space centered at X: −40.361, Y: 5.622, Z: 50.205, and dimensions of X: 20.000, Y: 20.000, Z: 20.000. Ligand tethering to the APJ receptor was achieved by adjusting the genetic algorithm (GA) parameters, utilizing 10 runs of the GA criteria. The docking poses were visualized with the PyMOL Molecular Graphics System (Version 2.0 Schrödinger, LLC), UCSF ChimeraX (version 1.7.1),39 and the Protein–Ligand Interaction Profiler (PLIP) web tool.402.5. Physicochemical properties, pharmacokinetic and toxicity profiles and in silico prediction
The physicochemical properties, pharmacokinetic profiles, and toxicity profiles of the 11 major metabolites from C. citratus leaf extract (one amino acid (1), one hydroxycinnamic acid (39), eight flavones (14i, 14ii, 16, 24, 25, 31, 37, 40), and one fatty acid (48)) in our screening library were calculated using the Deep-PK online web tool (https://biosig.lab.uq.edu.au/deeppk/, accessed on 22 May 2024).41 The Deep-PK tool encompasses nine general properties: boiling point (°C), hydration free energy (which indicates the drug's aqueous solubility), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 (the logarithm of the n-octanol/water distribution coefficient, representing the lipophilicity of a molecule at pH 7.4), log
D7.4 (the logarithm of the n-octanol/water distribution coefficient, representing the lipophilicity of a molecule at pH 7.4), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (the logarithm of the n-octanol/water distribution coefficient), log
P (the logarithm of the n-octanol/water distribution coefficient), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S (the logarithm of aqueous solubility at a temperature of 20–25 °C), log
S (the logarithm of aqueous solubility at a temperature of 20–25 °C), log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VP (the logarithm of the vapor pressure, representing the volatility of a molecule at 25 °C), melting point (°C), pKa acid, and pKa basic (which control its pharmacokinetic properties).
VP (the logarithm of the vapor pressure, representing the volatility of a molecule at 25 °C), melting point (°C), pKa acid, and pKa basic (which control its pharmacokinetic properties).
Seven absorption properties (Caco-2 permeability, Human oral bioavailability, Human intestinal absorption (HIA), Madin–Darby Canine Kidney cells (MDCK) permeability, skin permeability, P-glycoprotein substrate, P-glycoprotein I inhibitor), four distribution properties (BBB (blood–brain barrier) permeability, fraction unbound (human), Plasma protein binding (PPB), Steady State Volume of Distribution (SSVD)), seven metabolism properties (CYP2D6 substrate, CYP3A4 substrate, CYP1A2 inhibitor, CYP2C19 inhibitor, CYP2C9 inhibitor, CYP2D6 inhibitor, CYP3A4 inhibitor), and three excretion properties (total clearance, Half-life, renal OCT2 substrate) were available through the Deep-PK tool.
The 33 available toxicity properties (AMES mutagenesis, avian toxicity, honey bee toxicity, bioconcentration factor, biodegradation, carcinogenicity, crustacean toxicity, liver injury I, liver injury II, eye corrosion and irritation, maximum tolerated dose (human), hERG inhibitor, Daphnia magna toxicity, micronucleus formation, NR-Aryl hydrocarbon Receptor (AhR), NR-Androgen Receptor (AR), NR-Androgen Receptor (AR) Ligand-Binding Domain (LBD) activation, NR-aromatase inhibition, NR-Estrogen Receptor (ER), NR-Estrogen Receptor (ER) Ligand-Binding Domain (LBD), NR-Glucocorticoid Receptor (GR), NR-Peroxisome Proliferator-Activated Receptor Gamma (PPAR-gamma), NR-Thyroid Receptor (TR), oral rat acute toxicity (LD50), oral rat chronic toxicity (LOAEL), Fathead Minnow toxicity, respiratory disease, skin sensitization, SR-Antioxidant Responsive Element (ARE), SR-ATAD5 (ATPase Family AAA Domain Containing 5) gene, SR-Heat Shock Sequence (HSE) elements, SR-Mitochondrial Membrane Potential (MMP), and SR-p53 pathway) in Deep-PK were calculated to predict the potential toxicity profiles of these compounds.
3. Results and discussion
3.1. In vivo deciphering of the anti-obesity properties of C. citratus extract
One of the leading causes of cardiovascular diseases is obesity. Systemic metabolic dysfunction and cardiovascular and inflammation problems can result from obesity, which can affect the production of adipokines generated from adipose tissue.42 Current research indicates that the cardiovascular systems of overweight rats exhibit dysregulated expression or secretion patterns of Apelin and its receptor, Apj. Apelin system expression was examined in relation to C. citratus in this study using overweight rats that were fed a high-carbohydrate, high-fat diet.42In Table 2, the comparison between the HCHFD group and the control group revealed a substantial increase (P < 0.05) in fasting blood glucose and HOMA-IR, as well as a substantial decrease (P < 0.05) in insulin level. All other treatment groups revealed a significant reduction (P < 0.05) in fasting blood glucose and HOMA-IR, together with an increase (P < 0.05) in insulin saturation, when compared to the HCHFD group. In addition, there was no discernible difference between the control group and the C. citratus group; nevertheless, there was a striking amount of variation between the groups who received either orlistat alone or in combination with HCHFD. Our findings demonstrate that the group administered HCHFD plus C. citratus demonstrated remarkable improvements in the levels of glucose, insulin, and HOMA-IR.
| Control | HCHFD | C. citratus | HCHFD + C. citratus | HCHFD + Orlistat | |
|---|---|---|---|---|---|
| SE Mean is the statistical distribution used to represent values. For each group, n is the total number of rats, n = 10. A p-value of less than 0.05 was taken to indicate statistical significance. C. citratus group, and HCHFD + C. citratus group at P < 0.05, respectively. a, b, c and d significant when compared to control group, HCHFD group, C. citratus group and HCHFD + C. citratus at P < 0.05, respectively. | |||||
| Glucose (mg dl−1) | 89.44 ± 1.16t | 171.47 ± 2.88acd | 91.01 ± 1.2bd | 107.86 ± 2.85abc | 127.55 ± 3.92abcd |
| Insulin (μIU ml−1) | 12.91 ± 0.68 | 9.72 ± 0.84acd | 12.85 ± 0.55b | 11.64 ± 0.42b | 10.37 ± 0.25abc |
| HOMA-IR | 2.84 ± 0.14 | 4.08 ± 0.32acd | 2.94 ± 0.15b | 3.29 ± 0.18 ab | 3.47 ± 0.12ab |
Indeed, the findings of our study are consistent with previous research,43 indicating that obese rats experience an accumulation of inflammatory cytokines and free fatty acids in their bloodstream. Deviation from the equilibrium between insulin synthesis and insulin responsiveness might arise when these parameters restrict the uptake and use of glucose in peripheral tissues. This, in turn, leads to high blood glucose levels. As a result of impaired insulin sensitivity, hyperinsulinemia causes the body to create an excess of insulin. On the other hand, insulin production can decline as pancreatic beta cells are fatigued. Inhibition of insulin signaling pathways by inflammatory cytokines generated by adipose tissue can impede the absorption and utilization of glucose-induced by insulin in target tissues. Overweight rats may develop insulin resistance due to a combination of factors, i.e., include dysregulated signaling pathways involved in glucose metabolism, changed adipokine production, modified adipokine secretion from adipose tissue, elevated liberation of free fatty acids from adipose tissue, and chronic low-grade inflammation.1
According to previous studies44 polyphenols improved glucose absorption by cells, which led us to believe that polyphenols were responsible for the observed decreases in glucose and HOMA-IR levels. The ability to regulate blood sugar levels is conferred upon C. citratus by its enhanced glucose absorption, which may improve insulin sensitivity. This discovery lends credence to the research of Adeneye and Agbaje45 and Ewenighi et al., which demonstrated that C. citratus restored glucose levels to normal in four weeks of treatment in rats,46 which demonstrated that C. citratus restored glucose levels to normal in four weeks of treatment in rats.
In comparison to the control group, the HCHFD group exhibited a notable rise (P < 0.05) in triglycerides, LDL-cholesterol, and cholesterol, as well as a notable fall (P < 0.05) in HDL-cholesterol, according to the findings in Table 3.
| Control | HCHFD | C. citratus | HCHFD + C. citratus | HCHFD + Orlistat | |
|---|---|---|---|---|---|
| SE Mean is the statistical distribution used to represent values. For each group, n is the total number of rats, n = 10. A p-value of less than 0.05 was taken to indicate statistical significance. C. citratus group, and HCHFD + C. citratus group at P < 0.05, respectively. a, b, c and d significant when compared to control group, HCHFD group, C. citratus group and HCHFD + C. citratus at P < 0.05, respectively. | |||||
| Cholesterol (mg dl−1) | 94.72 ± 1.53 | 169.3 ± 2.19acd | 94.43 ± 1.14bd | 117.76 ± 1.68abc | 143.33 ± 2.53abcd |
| Triglyceride (mg dl−1) | 76.53 ± 0.38 | 153.07 ± 5.02acd | 79.39 ± 0.95bd | 99.18 ± 0.49abc | 116.28 ± 2.25abcd |
| HDL-cholesterol (mg dl−1) | 61.31 ± 0.37 | 28.27 ± 0.33acd | 63.45 ± 0.24bd | 51.23 ± 0.89abc | 45.70 ± 0.64abcd |
| LDL-cholesterol (mg dl−1) | 22.30 ± 1.56 | 96.42 ± 3.44acd | 21.10 ± 1.75bd | 46.70 ± 2.19abc | 74.37 ± 2.76abcd |
The results observed in Table 3, showed that as compared to the HCHFD group, all treatment groups had significantly lower levels of cholesterol, triglycerides, and LDL-cholesterol, and significantly higher levels of HDL-cholesterol (P < 0.05). Furthermore, there was considerable fluctuation, but no significant difference, between the control groups and the C. citratus group. Moreover, when comparing the groups given orlistat to those given HCHFD + C. citratus, lipid profiles improved in the groups given orlistat following obesity induction. In contrast, lipid profiles improved significantly in the group given C. citratus extract following obesity induction.
It can be concluded that C. citratus extract exhibits hypoglycemic properties.47 In Wistar rats, a daily dose ranging from 125 to 500 mg per kilogram is found to decrease total cholesterol, HDL, and fasting plasma glucose (FPG). Moreover, C. citratus can be used to treat type 2 diabetes because the dose employed thus far did not exhibit any harm.45 Furthermore, a 4-week course of therapy with C. citratus extracts on diabetic rats’ results in decreased blood glucose, TG, cholesterol, and LDL levels. The same procedure caused a decrease in body weight. The essential oils (EO) demonstrated a hypocholesterolemic impact that was mediated via post-transcriptional down-regulation by the regulatory enzyme HMG-CoA reductase.48 This way, the EO inhibits the hepatic 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which plays a vital role in cholesterol formation.49
Additionally, medical practitioners have employed C. citratus to treat neurological diseases associated with etiology. Due to the presence of antioxidant components, it aids in lowering oxidative stress, which is crucial in the development of several neurological disorders. Extracts of C. citratus contain the phenolic chemicals quercetin, gallic acid, quercetin, and rutin. These later offer defense against oxidative stress brought on by several pro-oxidants that cause lipid peroxidation. As a result, C. citratus may be useful in preventing several neurological conditions linked to oxidative stress.50
Furthermore, the results in Tables 4 and 5, showed a significant decrease (P < 0.05) in serum apelin, adiponectin, and gene expression of apelin, Apj, and adiponectin when comparing the HCHFD group to the control group. On the other hand, when compared to the HCHFD group, all treatment groups exhibited a substantial rise (P < 0.05) in serum apelin, adiponectin, and gene expression of apelin, Apj, and adiponectin. In addition, there was no discernible difference between the control and C. citratus groups; nevertheless, there was a striking disparity between the orlistat-treated group and the HCHFD + C. citratus group. Both the orlistat and C. citratus groups demonstrated improvements in the apelin system and adiponectin levels following obesity induction, although the C. citratus extract group exhibited significantly more improvement.
| Control | HCHFD | C. citratus | HCHFD + C. citratus | HCHFD + Orlistat | |
|---|---|---|---|---|---|
| SE Mean is the statistical distribution used to represent values. For each group, n is the total number of rats, n = 10. A p-value of less than 0.05 was taken to indicate statistical significance. C. citratus group, and HCHFD + C. citratus group at P < 0.05, respectively. a, b, c and d significant when compared to control group, HCHFD group, C. citratus group and HCHFD + C. citratus at P < 0.05, respectively. | |||||
| Apelin (ng ml−1) | 174.03 ± 2.16 | 134.21 ± 2.71acd | 172.88 ± 1.95bd | 158.95 ± 2.13abc | 141.67 ± 1.80abcd |
| Adiponectin (ng ml−1) | 15.90 ± 0.46 | 6.49 ± 0.28acd | 16.18 ± 0.34bd | 11.94 ± 0.38abc | 8.55 ± 0.22abcd |
| Control | HCHFD | C. citratus | HCHFD + C. citratus | HCHFD + Orlistat | |
|---|---|---|---|---|---|
| SE Mean is the statistical distribution used to represent values. For each group, n is the total number of rats, n = 10. A p-value of less than 0.05 was taken to indicate statistical significance. C. citratus group, and HCHFD + C. citratus group at P < 0.05, respectively. a, b, c and d significant when compared to control group, HCHFD group, C. citratus group and HCHFD + C. citratus at P < 0.05, respectively. | |||||
| Apelin | 1.00 ± 0.00 | 0.43 ± 0.02 acd | 1.31 ± 0.08abd | 0.64 ± 0.01abc | 0.57 ± 0.02abcd |
| Apj | 1.00 ± 0.00 | 0.39 ± 0.04acd | 1.25 ± 0.06abd | 0.71 ± 0.01abc | 0.64 ± 0.04abcd |
| Adiponectin | 1.00 ± 0.00 | 0.33 ± 0.01acd | 1.52 ± 0.07abd | 0.57 ± 0.01abc | 0.46 ± 0.01abcd |
Additionally, the obtained results in Table 5, indicate that apelin's gene expression tends to be lower in circulation levels, which may be related to decreased apelin production in adipose tissue, a significant source of apelin production. It is thought that these alterations are linked to the emergence of insulin resistance and problems resulting from obesity. Insulin sensitivity control has been linked to apelin. In peripheral tissues, including skeletal muscle and adipose tissue, insulin signaling, and glucose uptake can be improved. This implies that apelin plays a part in controlling how glucose is metabolized and how insulin resistance develops.51 Furthermore, the expression of the APJ receptor is frequently changed in obesity, albeit the direction of the shift varies according to the tissue or cell type. For instance, APJ receptor expression may be downregulated in adipose tissue, which could explain why apelin signaling is less effective in this tissue. It has been demonstrated that apelin affects immunological response and inflammation. It can control the synthesis and release of several inflammatory chemokines and cytokines. Apelin may act in a pro- or anti-inflammatory manner.52
Our results contradicted the findings of other research that indicated a significant increase in obesity was associated with serum apelin levels and its genes. Obese humans and animals have high concentrations of plasma apelin.53,54 Boucher et al., demonstrated in 2005 that apelin was secreted and produced by adipocytes, as well as that apelin and insulin had a close association both in vivo and in vitro. In the adipose tissue (AT) of obese animal models, apelin expression rose in tandem with hyperinsulinemia.54 Additionally, they found that apelin level and body mass index were positively correlated. Since obesity-related elevations in inflammatory cytokines can hasten apelin production and release.51
Moreover, Adiponectin is a protein that is particular to adipocytes and increases the sensitivity of the liver and muscle to the effects of insulin.55,56 Numerous studies indicate that adiponectin has anti-atherosclerotic, anti-insulin resistance, and anti-inflammatory characteristics.57 Adiponectin appears to have anti-inflammatory and protective metabolic qualities that prevent atherosclerosis, and it may be a marker for coronary artery disease.58 A significant decrease in adiponectin during obesity was observed due to the ability of adiponectin to increase the oxidation of free fatty acids, insulin effectiveness, decrease gluconeogenetic enzymes, enhance phosphorylation of acetyl Co-A carboxylase, enhance the production of certain cytokines, and enhance the metabolism of glucose and lactate. In conclusion, adiponectin, has significant anti-diabetic benefits.59 Similarly, adiponectin was observed to be lower in obese rats compared to controls; however, following treatment with C. citratus, there was an increase in serum adiponectin levels.60
In accordance with our findings, Chakraborti proposed that increased TNF-α and IL-6 production, a hypoxic microenvironment created in larger adipocytes, and increased production of insulin-like growth factor binding protein-3 which is obesity-induced and inhibits adiponectin transcription could be the cause of the reduction in adiponectin in obesity.61
In particular, C. citratus has been investigated for possible impacts on adipocyte metabolism and function; in obese rats, it may help restore normal function of the adipose tissue by encouraging apelin and adiponectin synthesis, release, and gene expression. C. citratus may affect insulin sensitivity, according to certain research.62C. citratus may indirectly affect the levels and functionality of these variables by increasing insulin sensitivity in obese rats.63 Additionally, C. citratus includes a variety of antioxidant chemicals, including flavonoids and phenolic compounds. APJ receptor, apelin, adiponectin, and adiponectin gene expression can all be negatively impacted by oxidative stress in terms of production and signaling. In obese rats, the antioxidant qualities of C. citratus may help lower oxidative stress and maintain normal levels and functioning of these variables.64
Furthermore, an enzyme known as AMP-activated Protein Kinase (AMPK) is essential for energy metabolism and the control of several metabolic functions, including apelin synthesis and release, APJ receptor function, adiponectin, and adiponectin gene expression, according to some research, C. citratus active biocomponents can activate AMPK, which could aid obese rats’ levels and functionality of these variables.63
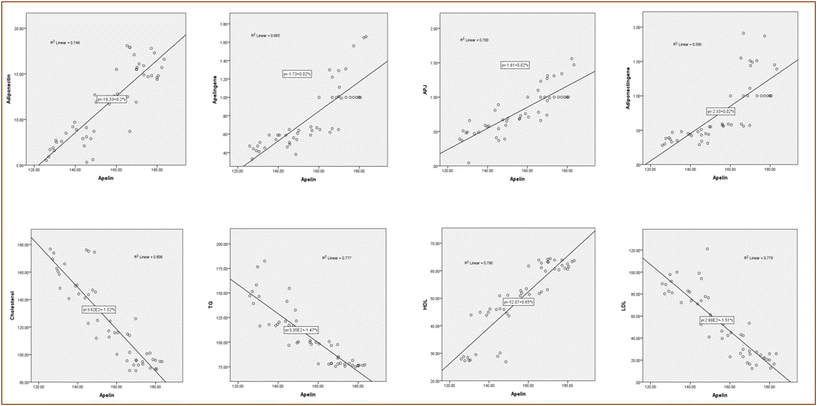
Pearson's correlation was calculated for the concerned parameters along the studied groups as shown in Fig. 1 and 2 as well as Table S1.† Where the calculated correlation showed a statistically positive correlation in apelin when correlated with adiponectin, apelin gene, APJ, adiponectin gene, and HDL, r was 0.865, 0.826, 0.836, 0.774, and 0.889, respectively. In contrast, it showed a negative correlation with cholesterol, TG, LDL, and glucose; r was −0.898, −0.881, −0.883, and 0.829, respectively. Moreover, a statistically significant positive correlation was observed when correlating adiponectin with the apelin gene, APJ, adiponectin gene, and HDL, r was 0.846, 0.849, 0.852, and 0.921, respectively. However, it showed a negative correlation with cholesterol, TG, LDL, and glucose, r was −0.940, −0.892, −0.931, and −0.882 respectively.
Herein, apelin has an inverse relationship with glucose, HOMA-IR, cholesterol, TG, and LDL but a direct relationship with insulin, HDL, Apj, and adiponectin. Additionally, adiponectin has an inverse relationship with glucose, HOMA-IR, cholesterol, TG, and LDL but a direct relationship with insulin, HDL, Apj, and apelin. Accordingly, it can be concluded that apelin and adiponectin may have a strong correlation with lipid profile levels, insulin resistance, and cardiovascular health via different pathways.
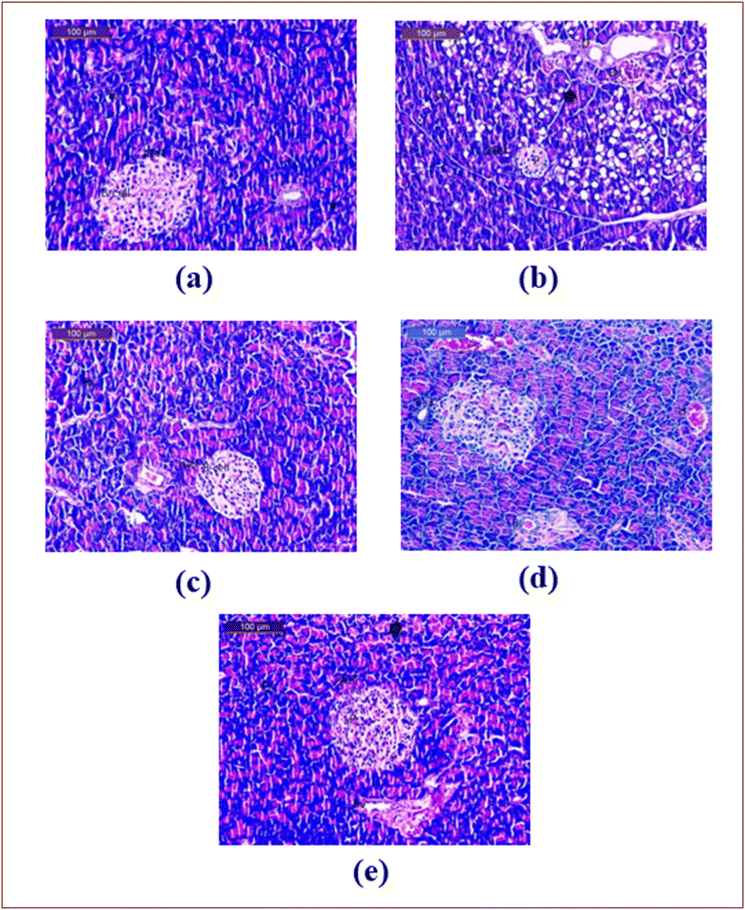
The obtained results of the histological examination supported our biochemical results (Fig. 3 and 4), which demonstrated that the sections of liver from the control group displayed normal hepatic architecture features, such as rounded vesicular nuclei with blood sinusoids and hepatocytes arranged in cords radiating from the central veins (Fig. 3a).
On the other hand, while histological analysis of the liver tissues from the HCHFD group revealed fatty cells, necrosis, focal infiltration of mononuclear cells with pyknotic nuclei, degenerative alterations surrounding the major vein, and modest activation of Kupffer cells (Fig. 3b), C. citratus displayed almost normal structure in group C, along with a slight initiation of Kupffer cells (Fig. 3c). The hepatic tissues belonging to HCHFD + C. citratus group, showed virtually normal structure with minor activation of Kupffer cells, mononuclear cell infiltration, slightly dilated blood sinusoids with few pyknotic nuclei, and slight degenerative alterations around the central vein (Fig. 3d). A section of the hepatic tissue from the HCHFD + Orlistat group revealed minor activation of Kupffer cells, a few adipose cells with pyknotic nuclei within slightly dilated blood sinusoids, and nearly normal structure coupled with degenerative alterations around the major vein (Fig. 3e).
In terms of the pathological analysis of the pancreatic sections that were taken, the sections from the control group demonstrated normal histoarchitecture and islets of Langerhans (Islets) with pale, ovoid, rounded β-cells implanted in the exocrine region of the pancreas (Fig. 3a). In contrast, the sections from the HCHFD group revealed disorganization in the endocrine and exocrine glands, shrunken islets of Langerhans (Islets), visible degeneration and necrosis of the islet-making cells, vacuolation, exocrine acini's degeneration, fatty changes, and ductal slight dilatation surrounded by mildly infiltrating inflammatory cells (Fig. 4b). Furthermore, the pancreatic slices from the C. citratus group showed nearly normal islet organization, with exocrine acini and β cells positioned in the center (Fig. 4c). Furthermore, interstitial bleeding and congestion were seen in some blood arteries, and the pancreatic sections from the HCHFD + C. citratus group showed normal islets of Langerhans, despite the detection of some cells with small pyknotic nuclei in the islet's canter, some of which were still degenerating, and nearly normal exocrine acini (Fig. 4d). The objective is to restore the typical structure of the pancreas, which consists of pancreatic islets of a typical size with few pyknotic nuclei and almost normal exocrine acini, was another indication of a somewhat improved pancreatic tissue structure in the HCHFD + Orlistat group's examination results (Fig. 4e). However, some of the clogged blood arteries were still degenerating.
3.2. Metabolic profiling C. citratus (DC.) Stapf leaves extract by LC-MS and tandem MS/MS
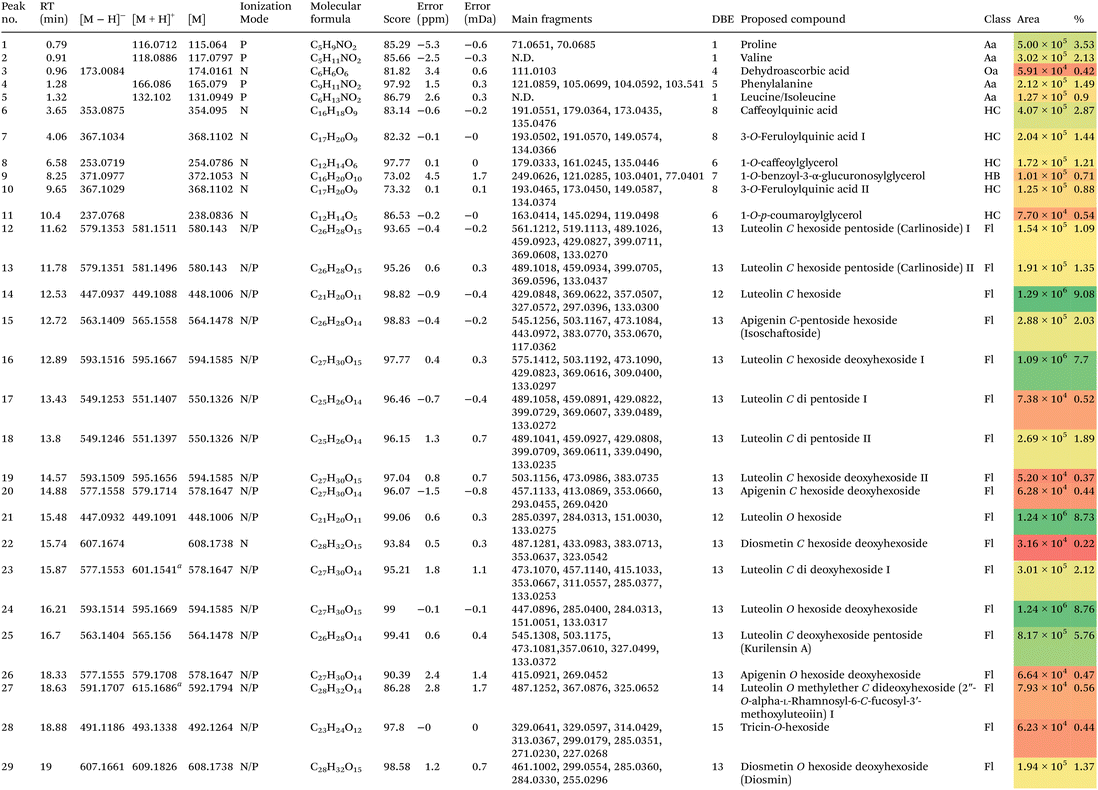
C. citratus (DC.) Stapf leaves extract profiling utilizing RP-HPLC-MS and tandem MS/MS in the negative and positive ionization mode portrayed the presence of 52 metabolites. Within this particular framework, Fig. 5a, illustrates the base peak chromatogram of the extract. The characterization approach of the metabolites was based on observations of retention times (RT), derived molecular formulas, double bond equivalence (DBE), molecular ion peaks (m/z), neutral losses, and peak areas (Fig. 5b), as described in earlier publications,65,66 consulting relevant literature and databases.8,9,67–69 In total, 52 metabolites were detected (Fig. 5 and 6, Table 6), the annotated metabolites were grouped into flavones (27, Fig. S1–S3), hydroxycinnamic acids (10, Fig. S4), hydroxybenzoic acid (1, Fig. S5), fatty acids (8, Fig. S6), amino acids (4, Fig. S7), an organic acid (1, Fig. S8), and a diterpenoid (1, Fig. S9†).
a Ion with sodium adduct, N; negative, P; positive, Aa; amino acids, Dt; diterpenoid, HC; hydroxycinnamic acids, Hb, hydroxybenzoic acids, Fa, fatty acids, Fl; flavones, Oa, organic acids, N.D., undetected, DBE, double bond equivalence, compounds in bold indicate new proposed structures. Peak area: lowest value  highest value. highest value. |
|---|
|

|
As for flavones, they were derivatives of luteolin, luteolin O-methyl ether (Fig. S5), apigenin (Fig. S6), and tricin (Fig. S7†). They represented the major class of annotated metabolites with 27 derivatives qualitatively and 61.51% of the relative abundance (Table 6). In this regard, two constitutional isomers of apigenin hexoside deoxyhexoside were observed with m/z = 577.16−/579.17+ and molecular formula C27H30O14 and yet both showed different fragmentation patterns where the first isomer exhibited the C-glycosylation pattern with neutral loss of n CHOH groups of (n × 30 Da),66 whereas the second one exerted the neutral loss of a hexosyl and a deoxyhexosyl moieties and hence they were described as apigenin C-hexoside deoxyhexoside and apigenin O hexoside deoxyhexoside that were mentioned in the family Poaceae as vitexin 2′′-O-rhamnoside and apigenin-7-O-β-D-rutinoside, respectively.68 Indeed, advanced methods such as RP-HPLC-ESI-QTOF-MS and -MS/MS enabled the distinction of constitutional isomers with closely related chemical formulae. Similarly, peak 15 showed a C-glycosylation pattern and the characteristic fragment ion 117.03 [1,3B]−, and hence it was characterized as apigenin C-pentoside hexoside which was identified as isoschaftoside in C. citratus68 (Table 6, and Fig. S10a†). Regarding luteolin derivatives, 15 glycosides were noticed, with a relative abundance of about 53% of the total characterized metabolites. They exhibited O-glycosylation, characterized by the neutral loss of the sugar moiety, resulting in the formation of the aglycone at m/z = 285.04,33 and C-glycosylation where the loss of n CHOH (30 Da) groups occurred,34 alongside the characteristic fragment ions of [1,3A]−, [1,3B]−, and [0,2B]− which were noticed at m/z = 151, 133 and 135, respectively.70
In this sense, two constitutional isomers of C21H20O11 were characterized as luteolin C hexoside and luteolin O hexoside (Fig. S10b†) according to their fragmentation pattern (Table 6) where they described in C. citratus as orientin/isoorientin and luteolin 7-O-glucoside.68 In the same manner, peaks 16, 19, and 24 were characterized as luteolin C hexoside deoxyhexoside isomers I-II (Fig. S10c†) and luteolin O-hexoside deoxyhexoside, respectively. It bears noting that they were mentioned in C. citratus as isoorientin 2′′-O-rhamnoside and luteolin 7-neohesperidoside.68 Luteolin C dipentoside isomers I–II were characterized (Table 6). They were described in the family Poaceae as Kurilensin B (Luteolin 6 C (2-O-(β-D-xylopyranosyl)-α-L-arabinofuranosyl)).68 Likewise, peak 25 was annotated as luteolin C-deoxyhexoside pentoside that was observed in C. citratus as Kurilensin A,68 (Table 6). Additionally, two isomers of luteolin C hexoside pentoside I–II and luteolin C di-deoxyhexoside I–II were detected in C. citratus extract that were described as luteolin 6-C-β-D-glucopyranoside-8-C-α-L-arabinopyranoside and in C. citratus and as luteolin 8-C-rhamnoside-7-O-rhamnoside, respectively.68 Peak 31, with a molecular formula C27H28O14 and m/z = 575.14−/577.16+ expressed C-glycosylation fragmentation pattern and hence was tentatively characterized as cassiaoccidentalin B (luteolin C-6-deoxy-2-O-(6-deoxy-α-L-mannopyranosyl)-β-L-ribo-hexopyranos-3-ulosyl).68
Concerning luteolin O-methyl ether (diosmetin) derivatives, two isomers of luteolin O methylether (C28H32O14) were conjugated with two deoxyhexosides in a C-glycosylation pattern and hence were characterized as luteolin O-methylether C-dideoxyhexoside isomers I–II which were described in Zea mays as (2′′-O-α-L-rhamnosyl-6-C-fucosyl-3′-methoxyluteolin).68 Besides, peak 34 with m/z 589.16−/591.17+ and molecular formula C28H30O14 was annotated as 3′-O-methylmaysin.68 In this concern, two constitutional isomers with molecular formula C28H32O15 exhibited different fragmentation patterns for C-glycosylation and O-glycosylation and were characterized as Luteolin O methyl ether C hexoside deoxyhexoside which was identified as 2′′-O-α-L-rhamnosyl-4′-O-methylorientin (Fig. S11a) and diosmetin O-hexoside deoxyhexoside, which was described as diosmin (Fig. S11b†).67
With regards to tricin derivatives, their presence was described for the first time in C. citratus,67,68 in this regard, tricin O-hexoside, tricin O-hexoside deoxyhexoside were observed exerting the neutral loss of the conjugated sugar with the appearance of the ion of tricin m/z = 329.06 followed by the sequential loss of two methyl moieties (15 Da × 2).67,68 Furthermore, peaks 42 and 43 showed the neutral loss of a phenylhexoside moiety (238 Da) followed by the typical fragmentation of tricin. Consequently, they were characterized as tricin O-phenylhexoside I–II, considered new proposed structures. As a matter of fact, the occurrence of phenylhexoside was described in the family Poaceae as phenyl β-D-glucopyranoside.68 Moreover, two isomers of tricin 4′-O-(β-guaiacyl-glyceryl) ether I–II were noticed with neutral loss of guaiacyl-glyceryl moiety (196.08 Da) and tricin fragmentation pattern and the appearance of guaiacyl glyceryl m/z = 195.07 and guaiacyl glyceryl –CHOH m/z = 165.06 (Fig. S11c†). It bears noting that they were described in Zizania latifolia, in the family Poaceae.67
The occurrence of hydroxycinnamic acids was mainly as coumaric acid, caffeic acids and the O-methylated derivatives of the latter as ferulic acid derivatives. The aforementioned derivatives are either conjugated with glycerol, quinic acid, or sugars. The conjugation is mono hydroxycinnamic acid or di-hydroxycinnamic acid (Table 6). Peak 11, in this context, displayed the neutral loss of a glyceryl moiety (74 Da) followed by the characteristic fragmentation pattern of coumaric acid. The fragmentation pattern began with the molecular ion of coumaric acid at m/z = 163.04, followed by its dehydrated and decarboxylated ions at m/z = 145.3 and 119.05, consecutively,34,66 and hence it was characterized as 1-O-p-coumaroyl glycerol which was described in the family Poaceae.67 In the same manner, peak 38 with m/z = 383.11−/407.11+ (ion sodium adduct) exhibited a similar fragmentation pattern to the compound above with an additional neutral loss of a coumaroyl moiety. Consequently, it was characterized as di-O-coumaroyl glycerol. Also, peak 8 showed a neutral loss of a glyceryl moiety followed by caffeic acid ion m/z = 179.03 alongside its dehydrated and decarboxylated ions at m/z = 161.02 and 135.04, respectively.33 It was characterized as 1-O-caffeoylglycerol.67 Moreover, peaks 36 and 39 portrayed a glycerol moiety neutral loss followed by the fragmentation pattern of coumaric acid and caffeic acid for the former one and ferulic acid for the latter one, and hence they were tentatively identified as O-coumaroyl-O-caffeoyl glycerol (Fig. S12a and Table 6) and O-coumaroyl-O-feruloyl glycerol (Fig. S12b† and Table 6).69 Accordingly, peak 41 with m/z = 443.13−/467.13+ (ion with sodium adduct) and molecular formula C23H24O9 was characterized as di-O-feruloyl glycerol.69
In line with quinic acid conjugates with hydroxycinnamic acid, caffeoyl quinic acid and feruloyl quinic acid isomers I–II were noticed in C. citratus extract, figuring out the ions of m/z 191.05 and m/z = 173.04 accounting for quinic acid and quinic acid-H2O accompanied with either caffeic acid or ferulic acid fragmentation (Table 6).33,34,67,69
Furthermore, peak 33 with m/z = 639.20- and molecular formula C32H38O17 exhibited the neutral loss of feruloyl moiety as well as two hexosyl moieties with the presence of a ferulic acid fragmentation pattern (Fig. S3c†). Therefore, it was annotated as di-O-feruloyl sucrose.67
Regarding hydroxybenzoic acid, it is noteworthy that peak 10 with m/z = 377.10− showed the neutral loss of benzoic acid with the appearance of benzoic acid ion m/z = 121.03 as well as its dehydrated (m/z = 103.04) and decarboxylated (m/z = 77.03) fragments and was identified as 1-O-benzoyl-3-α-glucuronosyl glycerol (Fig. S13a†).67
The presence of palmitic acid (C16:0) and stearic acid (C18:0) was seen in conjunction with the unsaturated isomers of the latter, namely oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3).65,71 Moreover, hydroxylinoleic acid (Fig. S13b†) and hydroxylinolenic acid isomers I–II were also detected (Table 6). Four amino acids were present in C. citratus extract, namely proline, valine, phenylalanine, and leucine/isoleucine, in agreement with previous studies.35,36 Besides, gibberellin A9 and dehydroascorbic acid (Fig. S13c†) were detected (Table 6).
3.3. Molecular docking (MDock), binding energies studies and structure–activity relationships (SARs) analysis
Molecular docking was utilized to examine the binding interactions of eleven major metabolites derived from C. citratus leaf extract with the human APJ protein (PDB ID: 7SUS), aiming at treating obesity. The dataset of metabolites from C. citratus includes: one amino acid (1), one hydroxycinnamic acid (39), eight flavones (14i, 14ii, 16, 24, 25, 31, 37, 40), and one fatty acid (48), as depicted in Fig. 6 and Table 7, presents the outcomes of molecular docking conducted using AutoDock Vina software on the APJ protein.| Class | # | Name | ΔGBa | Interaction | ||
|---|---|---|---|---|---|---|
| Hydrophobic residues | H-bond residues | π-Stacking residues | ||||
| a In kcal mol−1. b (1R,2S)-N-[4-(2,6-Dimethoxyphenyl)-5-(6-methylpyridin-2-yl)-1,2,4-triazol-3-yl]-1-(5-methylpyrimidin-2-yl)-1-oxidanyl-propane-2-sulfonamide, APJ ligand. | ||||||
| Amino acid | 1 | Proline | −4.80 | — | — | — |
| Hydroxycinnamic acid | 39 | O-Coumaroyl-O-feruloylglycerol | −8.44 | Trp85, Ile109, Phe110, Val267, Lys268, Tyr271, Phe291 | Met183 | Tyr271, Phe291 |
| Flavones | 14i | Luteolin C 6-hexoside 1 | −8.01 | Trp85, Ile109, Thr295 | Arg168, Try264, Ser298 | — |
| 14ii | Luteolin C 8-hexoside 2 | −7.86 | Tyr93, Tyr271 | Tyr88, Arg168, Tyr185, Lys268 | — | |
| 16 | Luteolin C 6-hexoside deoxyhexoside I | −9.47 | Trp85, Ile109, Tyr264, Tyr271, Phe291, Thr295 | Arg168, Lys268, Ser298 | — | |
| 24 | Luteolin O 7-hexoside deoxyhexoside | −8.82 | Ile109, Tyr182 | Ser105, Arg168, Tyr185, Tyr264, Lys268 | — | |
| 25 | Luteolin C 6-deoxyhexoside pentoside (Kurilensin A) | −9.01 | Ile109, Phe110, Tyr264, Phe291, Pro292, Thr295, Tyr299 | Tyr93, Arg168, Tyr264, Ser298 | — | |
| 31 | Cassiaoccidentalin B | −10.02 | Trp85, Ile109, Tyr264, Thr295 | Arg168, Lys268, Ser298 | — | |
| 37 | Tricin-4′-O-(erythro-beta-guaiacylglyceryl) ether I | −8.16 | Trp85, Tyr93, Ile109, Tyr299 | Tyr93, Arg168, Ser298 | — | |
| 40 | Tricin-4′-O-(erythro-beta-guaiacylglyceryl) ether II | −8.24 | Phe110, Tyr264, Tyr271, Phe291, Tyr299 | Arg168, Tyr264, Ser298 | — | |
| Fatty acids | 48 | Linolenic acid | −6.32 | Phe78, Trp85, Tyr88, Trp95, Ile109, Thr295, Tyr299 | — | — |
| Positive Controlb | — | 8EH (triazole derivative) | −9.00 | Ile109, Tyr271, Phe291, Thr295, Tyr299 | Arg168, Tyr264, Lys268 | Trp85, Phe291 |
As shown in Table 7, the metabolites with the lowest calculated ΔGB values, indicating the most promising candidates, are flavones with a luteolin C6-glycosylation core featuring two sugar units. Specifically, luteolin C 6-deoxyhexoside pentoside (25), luteolin C 6-hexoside deoxyhexoside I (16), and cassiaoccidentalin B (31) have estimated ΔGB values less than or equal to −9 kcal mol−1, with precise values of −9.01, −9.47, and −10.02 kcal mol−1, respectively. It is also worth noting that the positive control (8EH), a known ligand of the APJ protein, has a calculated ΔGB value of −9 kcal mol−1. As shown in Fig. 7, the best-docked pose for the positive control (8EH), was demonstrated on APJ protein.
In Fig. 8, the best-docked poses for the three most probable lead-like anti-obesity APJ inhibitors, 16, 25 and 31, were shown. These excellent binding affinities could be attributed to potential hydrogen bond interactions with the residue Arg168 of the APJ protein, both in the positive control (Fig. 7) and in the three flavone derivatives proposed as anti-obesity agents (Fig. 8).
For example, in the three flavones with a luteolin C-6-glycosylation core featuring two sugar units (16, 25, and 31), there appear to be hydrogen bond interactions between the oxygen atom of the hydroxyl group at position 5 of the benzene ring (ring A) for 16, the hydroxyl group at position 7 of the benzene ring (ring A) for 25, or the oxygen atom of the carbonyl group of the heterocyclic pyran ring (ring C) for 31 of the 4H-chromen-4-one core, and the two amine groups of the guanidino moiety of the residue Arg168. The length of the hydrogen bonds varies specifically, for 16: 2.38 and 2.46 Å; for 25: 2.61 Å; and for 31: 2.27 and 3.19 Å.
3.4. Pharmacokinetics, toxicity and druglikeness (ADME/Tox), in silico prediction
To examine the physicochemical properties, pharmacokinetic profiles, and toxicity profiles of the 11 principal metabolites derived from C. citratus leaf extract (comprising one amino acid (1), one hydroxycinnamic acid (39), eight flavones (14i, 14ii, 16, 24, 25, 31, 37, 40), and one fatty acid (48)) in our screening library, we utilized a freely available online tool called Deep-PK (https://biosig.lab.uq.edu.au/deeppk/, accessed on May 22, 2024). The tool was employed to analyze their profiles, and the results are detailed in Table S2 in the ESI.†Table 8 presents the physicochemical and ADMET properties of our most promising metabolites, specifically the three flavones with a luteolin C6-glycosylation core featuring two sugar units (16, 25, and 31).| Flavones | Positive control | ||||
|---|---|---|---|---|---|
| ADME/Tox | 16 | 25 | 31 | 8EH | |
| a Non-Bioavailable. b Bioavailable. c Non-absorbed. d Absorbed. e Non-penetrable. f Steady state volume of distribution. g Non-inhibitor. h Non-substrate. i Inhibitor. j Substrate. | |||||
| General Properties | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 D7.4 |
−0.730 | −0.180 | 0.420 | 0.890 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
0.360 | 0.830 | 1.410 | 1.990 | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S S |
−4.330 | −4.500 | −5.170 | −3.380 | |
| Absorption | Caco-2 permeability | −6.430 | −6.480 | −6.710 | −5.390 |
| Human Oral Bioavailability 20% | NBa | NBa | NBa | Bb | |
| Human Intestinal Absorption | NAc | NAc | NAc | Ad | |
| Skin Permeability | 7.170 | 3.880 | 3.670 | −2.360 | |
| Distribution | Blood–Brain Barrier | NPe | NPe | NPe | NPe |
| Plasma Protein Binding | 68.160 | 73.840 | 82.060 | 86.650 | |
| SSVDf | 0.940 | 0.940 | 1.010 | 0.560 | |
| Metabolism | CYP 1A2, CYP 2C19, CYP 2D6, OATP1B1 Inhibitor | NIg | NIg | NIg | NIg |
| CYP 1A2, CYP 2C19, CYP 2D6 Substrate | NSh | NSh | NSh | NSh | |
| CYP 2C9, CYP 3A4 Inhibitor | NIg | NIg | NIg | Ii | |
| CYP 2C9, CYP 3A4 Substrate | NSh | NSh | NSh | Sj | |
| Excretion | Clearance | 11.380 | 13.170 | 14.470 | 1.390 |
| Organic Cation Transporter 2 | NIg | NIg | NIg | NIg | |
| Toxicity | AMES Mutagenesis | Toxic | Toxic | Toxic | Safe |
| Avian | Safe | Safe | Safe | Safe | |
| Bee | Safe | Toxic | Toxic | Toxic | |
| Biodegradation | Safe | Safe | Safe | Safe | |
| Carcinogenesis | Safe | Safe | Safe | Safe | |
| Crustacean | Safe | Safe | Safe | Toxic | |
| Liver Injury I | Safe | Safe | Safe | Safe | |
| Eye Corrosion | Safe | Safe | Safe | Safe | |
| Eye irritation | Safe | Safe | Safe | Safe | |
| Maximum Tolerated Dose | 0.770 | 0.780 | 0.820 | 0.960 | |
| Liver Injury II | Toxic | Toxic | Toxic | Toxic | |
| hERG Blocker | Safe | Safe | Safe | Safe | |
| Skin Sensitization | Safe | Toxic | Toxic | Safe | |
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4, the logarithm of the n-octanol/water distribution coefficient, represents the lipophilicity of a molecule at pH 7.4, impacting both aqueous solubility and membrane permeability. For un-ionizable compounds, log
D7.4, the logarithm of the n-octanol/water distribution coefficient, represents the lipophilicity of a molecule at pH 7.4, impacting both aqueous solubility and membrane permeability. For un-ionizable compounds, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and log
P and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 values will be similar. The optimal range for log
D7.4 values will be similar. The optimal range for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and log
P and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 in orally administered drugs is between 1 and 3.41 All three flavone derivatives and the positive control are predicted to have lower lipophilicity at pH 7.4 (<1). However, the flavone derivative (31) and the positive control (8EH) are predicted to possess adequate pH-independent lipophilicity characteristics. Additionally, all three derivatives and the positive control are predicted to have adequate water solubility characteristics.
D7.4 in orally administered drugs is between 1 and 3.41 All three flavone derivatives and the positive control are predicted to have lower lipophilicity at pH 7.4 (<1). However, the flavone derivative (31) and the positive control (8EH) are predicted to possess adequate pH-independent lipophilicity characteristics. Additionally, all three derivatives and the positive control are predicted to have adequate water solubility characteristics.
The Caco-2 cell monolayer is widely used as an in vitro model of the human intestinal mucosa to predict the absorption of orally administered drugs.41 Low Caco-2 permeability was predicted for all three flavone derivatives (16, 25, and 31) as well as the positive control (8EH). However, only the positive control was estimated to be human oral bioavailable and human intestinally absorbed, whereas the three flavones were predicted to be neither bioavailable nor absorbed. In terms of skin permeability, the behavior is reversed: the three flavone derivatives are predicted to have high skin permeability, while the positive control is predicted to have low skin permeability.
All flavone derivatives (16, 25, and 31) and the positive control (8EH) are estimated to be poorly distributed to the brain. However, an appropriate value for plasma protein binding, which is a therapeutic index related to the amount of free drug in the body, was predicted for all the compounds. Only the positive control (8EH) was predicted to have a low value for the steady-state volume of distribution (SSVD), which is related to drug concentration.
All flavone derivatives (16, 25, and 31) are estimated to be non-inhibitors of cytochrome P450 isoforms (CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4), an important class of detoxification enzymes primarily found in the liver.
In terms of toxicity predictions, all flavone derivatives (16, 25, and 31) are predicted to be toxic in the AMES mutagenicity test, indicating potential mutagenicity and, therefore, possible carcinogenicity. However, all flavone derivatives (16, 25, and 31) are also predicted to be non-carcinogenic. Only flavone (16) and the positive control (8EH) were predicted not to cause skin sensitization.
4. Conclusions
Obesity represents a critical and immediate global public health issue, imposing a significant burden in terms of both disability and mortality. The current study found that HCHFD rats had reduced apelin, Apj and adiponectin expression, and that treatment with C. citratus greatly enhanced these changes, leading to improvements in insulin resistance, body weight, inflammatory markers, lipid profile, serum apelin, adiponectin and insulin levels. C. citratus treatment of overweight rats led to weight loss, improved insulin resistance and inflammatory markers, and upregulation of the Apelin axis. Besides, C. citratus was subjected to untargeted metabolic profiling via RP-HPLC-QTOF-MS and MS/MS disclosing the presence of 52 metabolites where they mainly belonged to phenolic compounds viz., flavones and hydroxycinnamic acids, among other metabolites. Additionally, a molecular docking was utilized to examine the binding interactions of eleven major metabolites derived from C. citratus leaf extract with the human APJ protein (PDB ID: 7SUS), aiming at treating obesity, where the best-docked poses for the three most probable lead-like anti-obesity APJ inhibitors, 16, 25 and 31, were shown. These excellent binding affinities could be attributed to potential hydrogen bond interactions with the residue Arg168 of the APJ protein, both in the positive control and in the three flavone derivatives proposed as anti-obesity agents. Moreover, the pharmacokinetic, physicochemical, and toxicity profiles of the 11 major metabolites from C. citratus leaf extract were evaluated, uncovering a profile similar to that of the positive control in the three selected flavone metabolites. Based on these findings, C. citratus may represent a new regulator of the Apelin axis, which could alleviate some of the problems associated with obesity in a rat model.Ethics approval
All procedures were conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986 and related guidelines, EU Directive 2010/63/EU for animal experiments, and the National Research Council's Guide for the Care and Use of Laboratory Animals. The animal study protocol was approved by the Ethics Committee of National Research Center, Dokki, Cairo, Egypt, approval number (015420824).Abbreviations
| 2-ΔΔCt method | The delta-delta Ct method |
| Aa | Amino acids |
| ADME/Tox | Pharmacokinetics, toxicity and druglikeness |
| AhR | NR-Aryl hydrocarbon receptor |
| AlCl3 | Aluminum trichloride |
| AMPK | AMP-activated Protein Kinase |
| ANOVA | One-way analysis of variance |
| APJ | Apelin receptor genes |
| APLN | Apelin |
| AR | NR-androgen receptor |
| ARE | SR-antioxidant responsive element |
| AT | Adipose tissue |
| BBB | Blood–brain barrier |
| BPCs | Base peak chromatograms |
| Bv | Congestion blood vessels |
| C. | Cymbopogon |
| cDNA | Complementary DNA |
| CV | Central vein |
| D | Ductal slight dilatation |
| DBE | Double bond equivalence |
| Dt | Diterpenoid |
| ELISA | The enzyme-linked immunosorbent assay |
| EO | Essential oils |
| ER | NR-estrogen receptor |
| Ex | Degeneration exocrine acini |
| Fa | Fatty acids |
| FI | Flavones |
| FPG | Fasting plasma glucose |
| GA | Genetic algorithm |
| GR | NR-glucocorticoid receptor |
| H | Interstitial haemorrhage |
| HB | Hydroxybenzoic acids |
| HC | Hydroxycinnamic acids |
| HCHFD | High-carbohydrate, high-fat diet |
| HDL | High density lipoprotein |
| HIA | Human intestinal absorption |
| HMG-CoA | Hepatic 3-hydroxy-3-methylglutaryl-coenzyme A |
| HOMA-IR formula | Homeostatic model assessment for insulin resistance |
| HPLC | High performance liquid chromatography |
| HSE | SR-heat shock sequence |
| IL-6 | Interleukin 6 |
| Islet | Islets of Langerhans |
| K | Kupffer cells |
| kcal/mol | Kilocalorie per mole |
| LBD | Ligand-binding domain |
| LC-MS | Liquid chromatography–mass spectrometry |
| LD50 | Oral rat acute toxicity |
| LDL | Low density lipoprotein |
| LOAEL | Oral rat acute toxicity |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 D7.4 | The logarithm of the n-octanol/water distribution coefficient, representing the lipophilicity of a molecule at pH 7.4 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P | The logarithm of the n-octanol/water distribution coefficient |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S S | The logarithm of aqueous solubility at a temperature of 20–25 °C |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VP VP | The logarithm of the vapor pressure, representing the volatility of a molecule at 25 °C |
| MDCK | Madin-darby canine kidney cells |
| MDOCK | Molecular docking |
| MIQE | Minimum information for publication of quantitative real-time PCR experiments |
| MMP | SR-mitochondrial membrane potential |
| N | Nucleus |
| N.D. | Not detected |
| Oa | Organic acids |
| P | Pyknotic nuclei |
| PCR | Polymerase chain reaction |
| PDBQT | An extended protein data base (PDB) format for coordinate files, incorporating atomic partial charges and atom types |
| PLIP | Protein–ligand Interaction Profiler |
| PPAR-gamma | NR-peroxisome proliferator-activated receptor gamma |
| PPB | Plasma protein binding |
| qPCRTM | Real-time polymerase chain reaction |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| RP-HPLC-QTOF-MS | Reversed-phase high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry |
| S | Blood sinusoids |
| SAR | Structure–activity relationship |
| SPSS | Statistical software suit |
| SR-ATAD5 | ATPase Family AAA Domain Containing 5 |
| SSVD | Steady state volume of distribution |
| SYBR Green I® | Asymmetrical cyanine dye |
| T-chol | Total cholesterol |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor |
| TR | NR-Thyroid Receptor |
| UPLC-Orbitrap HRMS | Ultra-high-performance liquid chromatography high-resolution mass spectrometry |
| V | Vacuolation |
| ΔGB | Free binding energy |
Author contributions
Conceptualization: M.A.T and A-E.D.; methodology: O.A., R.H.M., F.P., M.A.T., and A-E.D.; software: O.A., R.H.M., and F.P.; formal analysis: O.A., R.H.M., and F.P., investigation: O.A., R.H.M., F.P., M.A.T., and A-E.D.; resources: Y.M.D.; data curation: O.A., R.H.M., F.P., M.A.T., and A-E.D.; writing original draft preparation: O.A., R.H.M., F.P., M.A.T., and A-E.D.; writing review and editing: O.A., R.H.M., F.P., M.A.T., and A-E.D.; visualization: M.A.T and A-E.D. All authors have read and agreed to the published version of the manuscript.Data availability
The data presented in this study are available in the present article and the ESI.†Conflicts of interest
The authors declare that they have no known competing commercial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Florbela Pereira gratefully acknowledges FCT – Fundação para a Ciência e a Tecnologia, I. P., for an Assistant Research Position (CEECIND/01649/2021) and the project UIDB/50006/2020 of the Associated Laboratory for Green Chemistry (LAQV) of the Network of Chemistry and Technology (REQUIMTE). Mohamed A. Tammam is humbly dedicating this work to the soul of his sister Dr Mai A. Tammam who passed away on 19 of March 2022, she was always a kind supporter in all aspects of his life. Amr El-Demerdash is thankful to his home universities, University of East Anglia (UK) and Mansoura University (Egypt) for the unlimited support, inside and outside.References
- M. A. Tammam, O. Aly, F. Pereira, A. Mahdy and A. El-Demerdash, Unveiling the potential of marine-derived diterpenes from the order Alcyonacea as promising anti-obesity agents, Curr. Res. Biotechnol., 2024, 7, 100175 CrossRef CAS.
- B. Shokrollahi, H. Y. Zheng, L. Y. Li, L. P. Tang, X. Y. Ma, X. R. Lu, A. Q. Duan, Y. Zhang, X. H. Tan, C. X. Huang, Y. Y. Xu and J. H. Shang, Apelin and apelin receptor in follicular granulosa cells of buffalo ovaries: Expression and regulation of steroidogenesis, Front. Endocrinol., 2022, 13, 844360 CrossRef PubMed.
- O. Wu, X. Lu, J. Leng, X. Zhang, W. Liu, F. Yang, H. Zhang, J. Li, S. Khederzadeh, X. Liu and C. Yuan, Reevaluating Adiponectin's impact on obesity hypertension: a Chinese case-control study, BMC Cardiovasc. Disord., 2024, 24, 1–15 CrossRef PubMed.
- U. F. Shaik Mohamed Sayed, S. Moshawih, H. P. Goh, N. Kifli, G. Gupta, S. K. Singh, D. K. Chellappan, K. Dua, A. Hermansyah, H. L. Ser, L. C. Ming and B. H. Goh, Natural products as novel anti-obesity agents: insights into mechanisms of action and potential for therapeutic management, Front. Pharmacol., 2023, 14, 1182937 CrossRef PubMed.
- R. M. Sete da Cruz, H. Ferreira, J. M. Jaski, M. C. E. Vieira, M. M. Pinc, S. G. H. de Souza and O. Alberton, Growth and phytochemistry of Cymbopogon citratus Stapf inoculated with plant growth-promoting bacteria under different lead levels, Plants, 2024, 13, 944 CrossRef CAS PubMed.
- O. S. Oladeji, F. E. Adelowo, D. T. Ayodele and K. A. Odelade, Phytochemistry and Pharmacology of Cymbopogon citratus: A review, Sci. Afr., 2019, 6, e00137 Search PubMed.
- G. Shah, R. Shri, V. Panchal, N. Sharma, B. Singh and A. S. Mann, Scientific basis for the therapeutic use of Cymbopogon citratus, Stapf (Lemon grass), J. Adv. Pharm. Technol. Res., 2011, 2, 3–8 CrossRef CAS PubMed.
- Y. F. Madi, M. A. Choucry, S. A. El-Marasy, M. R. Meselhy and E. S. A. El-Kashoury, UPLC-Orbitrap HRMS metabolic profiling of Cymbopogon citratus cultivated in Egypt; neuroprotective effect against AlCl3-induced neurotoxicity in rats, J. Ethnopharmacol., 2020, 259, 112930 CrossRef CAS PubMed.
- G. Costa, J. P. Ferreira, C. Vitorino, M. E. Pina, J. J. Sousa, I. V. Figueiredo and M. T. Batista, Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents, J. Ethnopharmacol., 2016, 178, 222–228 CrossRef CAS PubMed.
- M. W. Mashitah, N. Widodo, N. Permatasari and A. Rudijanto, Anti-obesity activity of Cymbopogon citratus (lemongrass): A systematic review, J. Pharm. Pharmacogn. Res., 2024, 12, 1090–1110 CrossRef.
- S. Da Ressurreição, S. Pedreiro, M. T. Batista and A. Figueirinha, Effect of phenolic compounds from Cymbopogon citratus (DC) Stapf. Leaves on micellar solubility of cholesterol, Molecules, 2022, 27, 7338 CrossRef PubMed.
- A. A. Adeneye and E. O. Agbaje, Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats, J. Ethnopharmacol., 2007, 112, 440–444 CrossRef PubMed.
- V. S. Kumar, M. N. Inamdar and G. L. Viswanatha, Protective effect of lemongrass oil against dexamethasone induced hyperlipidemia in rats: Possible role of decreased lecithin cholesterol acetyl transferase activity, Asian Pac. J. Trop. Med., 2011, 4, 658–660 CrossRef CAS PubMed.
- F. Pereira, L. Bedda, M. A. Tammam, A. K. Alabdullah, R. Arafa and A. El-Demerdash, Investigating the antiviral therapeutic potentialities of marine polycyclic lamellarin pyrrole alkaloids as promising inhibitors for SARS-CoV-2 and Zika main proteases (Mpro), J. Biomol. Struct. Dyn., 2023, 42(8), 3983–4001 CrossRef PubMed.
- M. A. Tammam, F. Pereira, O. Aly, M. Sebak, Y. M. Diab, A. Mahdy and A. El-Demerdash, Investigating the hepatoprotective potentiality of marine-derived steroids as promising inhibitors of liver fibrosis, RSC Adv., 2023, 13, 27477–27490 RSC.
- Y. M. Diab, M. A. Tammam, A. M. Emam, M. A. Mohamed, M. E. Mahmoud, W. M. Semida, O. Aly and A. El-Demerdash, Punica granatum L var nana: A hepatoprotective and curative agent against CCl4 induced hepatotoxicity in rats, Egypt. J. Chem., 2022, 65, 723–733 Search PubMed.
- A. el Demerdash, A. M. Dawidar, E. M. Keshk and M. Abdel-Mogib, Gingerdione from the rhizomes of Curcuma longa, Chem. Nat. Compd., 2012, 48, 646–648 CrossRef CAS.
- A. El-Demerdash, C. Borde, G. Genta-Jouve, A. Escargueil and S. Prado, Cytotoxic constituents from the wheat plant pathogen Parastagonospora nodorum SN15, Nat. Prod. Res., 2019, 36, 1273–1281 CrossRef PubMed.
- A. El-Demerdash, G. Genta-Jouve, M. Bärenstrauch, C. Kunz, E. Baudouin and S. Prado, Highly oxygenated isoprenylated cyclohexanoids from the fungus Parastagonospora nodorum SN15, Phytochemistry, 2019, 166, 112056 CrossRef CAS PubMed.
- C. Moriou, D. Lacroix, S. Petek, A. El-Demerdash, R. Trepos, T. M. Leu, C. Florean, M. Diederich, C. Hellio, C. Debitus and A. Al-Mourabit, Bioactive bromotyrosine derivatives from the Pacific marine sponge Suberea clavata (Pulitzer-Finali, 1982), Mar. Drugs, 2021, 19, 143 CrossRef CAS PubMed.
- P. G. Reeves, Components of the AIN-93 diets as improvements in the AIN-76A diet, J. Nutr., 1997, 127(5 Suppl), 838S–841S CrossRef CAS PubMed.
- S. A. Zaitone and S. Essawy, Addition of a low dose of rimonabant to orlistat therapy decreases weight gain and reduces adiposity in dietary obese rats, Clin. Exp. Pharmacol. Physiol., 2012, 39, 551–559 CrossRef CAS PubMed.
- H. Passing and W. Bablok, A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I, J. Clin. Chem. Clin. Biochem., 1983, 21, 709–720 CAS.
- R. Yalow and W. Bauman, Diabetes Mellitus: Theory and Practice, Insulin in health and disease, in Diabetes Mellitus: Theory and Practice, ed. R. Yalow and W. A. Bauman, Excerpta Medica, New York, 1983 Search PubMed.
- D. R. Matthews, J. P. Hosker, A. S. Rudenski, B. A. Naylor, D. F. Treacher and R. C. Turner, Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia, 1985, 28, 412–419 CrossRef CAS PubMed.
- T. G. Cole, I. Kuisk, W. Patsch and G. Schonfeld, Effects of high cholesterol diets on rat plasma lipoproteins and lipoprotein-cell interactions, J. Lipid Res., 1984, 25, 593–603 CrossRef CAS.
- W. T. Friedewald, R. I. Levy and D. S. Fredrickson, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge, Clin. Chem., 1972, 18, 499–502 CrossRef CAS.
- K.-O. Kim, Y.-A. Kim and H. Lee, Isoflavone-rich bean sprout cookie improves lipid metabolism in hyperlipidemic rat, FASEB J., 2007, 21, A1087–A1087 Search PubMed.
- M. F. Lopes-Virella, P. Stone, S. Ellis and J. A. Colwell, Cholesterol determination in high-density lipoproteins separated by three different methods, Clin. Chem., 1977, 23, 882–884 CrossRef CAS.
- N. Mellouk, C. Rame, J. L. Touzé, E. Briant, L. Ma, D. Guillaume, D. Lomet, A. Caraty, T. Ntallaris, P. Humblot and J. Dupont, Involvement of plasma adipokines in metabolic and reproductive parameters in Holstein dairy cows fed with diets with differing energy levels, J. Dairy Sci., 2017, 100, 8518–8533 CrossRef CAS PubMed.
- R. Levesque, SPSS programming and data management: A guide for SPSS and SAS users, in Ibm Spss, 2005, pp. 1–520 Search PubMed.
- G. Paget and R. Thomson, Standard operating procedures in pathology: including developmental toxicology and quality assurance. 1979.
- R. H. Mekky, E. Abdel-Sattar, A. Segura-Carretero and M. D. M. Contreras, Metabolic profiling of the oil of sesame of the Egyptian cultivar ‘Giza 32′ employing LC-MS and tandem MS-Based untargeted method, Foods, 2021, 10, 298 CrossRef CAS PubMed.
- R. H. Mekky, E. Abdel-Sattar, A. Segura-Carretero and M. Del Mar Contreras, Phenolic compounds from sesame cake and antioxidant activity: A new insight for agri-food residues’ significance for sustainable development, Foods, 2019, 8, 432 CrossRef CAS PubMed.
- R. H. Mekky, M. D. M. Contreras, M. R. El-Gindi, A. R. Abdel-Monem, E. Abdel-Sattar and A. Segura-Carretero, Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: a comparative study, RSC Adv., 2015, 5, 17751–17767 RSC.
- R. H. Mekky, M. M. Thabet, C. Rodríguez-Pérez, D. M. Y. Elnaggar, E. A. Mahrous, A. Segura-Carretero and E. Abdel-Sattar, Comparative metabolite profiling and antioxidant potentials of seeds and sprouts of three Egyptian cultivars of Vicia faba L, Food Res. Int., 2020, 136, 109537 CrossRef CAS PubMed.
- J. Eberhardt, D. Santos-Martins, A. F. Tillack and S. Forli, AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings, J. Chem. Inf. Model., 2021, 61, 3891–3898 CrossRef CAS PubMed.
- RDKit: Open-Source Cheminformatics Software | BibSonomy, https://www.bibsonomy.org/bibtex/28d01fceeccd6bf2486e47d7c4207b108/salotz, (accessed 14 September 2024).
- E. C. Meng, T. D. Goddard, E. F. Pettersen, G. S. Couch, Z. J. Pearson, J. H. Morris and T. E. Ferrin, UCSF ChimeraX: Tools for structure building and analysis, Protein Sci., 2023, 32, e4792 CrossRef CAS PubMed.
- M. F. Adasme, K. L. Linnemann, S. N. Bolz, F. Kaiser, S. Salentin, V. J. Haupt and M. Schroeder, PLIP 2021: expanding the scope of the protein-ligand interaction profiler to DNA and RNA, Nucleic Acids Res., 2021, 49, W530–W534 CrossRef CAS PubMed.
- Y. Myung, A. G. C. de Sá and D. B. Ascher, Deep-PK: deep learning for small molecule pharmacokinetic and toxicity prediction, Nucleic Acids Res., 2024, 2024, 1–7 CrossRef PubMed.
- N. R. Kohan, S. Nazifi, M. R. Tabandeh and M. A. Lari, Effect of L-carnitine supplementation on apelin and apelin receptor (Apj) expression in cardiac muscle of obese diabetic rats, Cell J., 2018, 20, 427–434 Search PubMed.
- M. P. Czech, Insulin action and resistance in obesity and type 2 diabetes, Nat. Med., 2017, 23, 804–814 CrossRef CAS PubMed.
- R. Naz, F. Saqib, S. Awadallah, M. Wahid, M. F. Latif, I. Iqbal and M. S. Mubarak, Food polyphenols and type ii diabetes mellitus: Pharmacology and mechanisms, Molecules, 2023, 28, 3996 CrossRef CAS PubMed.
- A. A. Adeneye and E. O. Agbaje, Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats, J. Ethnopharmacol., 2007, 112, 440–444 CrossRef PubMed.
- C. Ewenighi, U. Dimkpa, J. Onyeanusi, L. Onoh, G. Onoh and U. Ezeugwu, Estimation of glucose level and body weight in alloxan induced diabetic rat treated with aqueous extract of Garcinia kola Seed, Ulutas Med. J., 2015, 1, 26 CrossRef.
- M. Machraoui, Z. Kthiri, M. Ben Jabeur and W. Hamada, Ethnobotanical and phytopharmacological notes on Cymbopogon citratus (DC.) Stapf, J. New Sci., 2018, 55, 3642–3652 Search PubMed.
- B. Middleton and H. Kok-Pheng, Evidence for binding of certain acidic drugs to α1-acid glycoprotein, Biochem. Pharmacol., 1982, 31, 2897–2901 CrossRef CAS PubMed.
- C. E. Elson, G. L. Underbakke, P. Hanson, E. Shrago, R. H. Wainberg and A. A. Qureshi, Impact of lemongrass oil, an essential oil, on serum cholesterol, Lipids, 1989, 24, 677–679 CrossRef CAS PubMed.
- R. P. Pereira, R. Fachinetto, A. De Souza Prestes, R. L. Puntel, G. N. Santos Da Silva, B. M. Heinzmann, T. K. Boschetti, M. L. Athayde, M. E. Bürger, A. F. Morel, V. M. Morsch and J. B. T. Rocha, Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus, Neurochem. Res., 2009, 34, 973–983 CrossRef CAS PubMed.
- N. Ranjbar Kohan, M. R. Tabandeh, S. Nazifi and Z. Soleimani, L-carnitine improves metabolic disorders and regulates apelin and apelin receptor genes expression in adipose tissue in diabetic rats, Physiol. Rep., 2020, 8, e14641 CAS.
- K. Mehri, G. Hamidian, Z. Zavvari Oskuye, S. Nayebirad and F. Farajdokht, The role of apelinergic system in metabolism and reproductive system in normal and pathological conditions: an overview, Front. Endocrinol., 2023, 14, 1193150 CrossRef PubMed.
- P. C. Arica, S. Aydin, U. Zengin, A. Kocael, A. Orhan, K. Zengin, R. Gelisgen, M. Taskin and H. Uzun, The effects on obesity related peptides of laparoscopic gastric band applications in morbidly obese patients, J. Invest. Surg., 2018, 31, 89–95 CrossRef PubMed.
- J. Boucher, B. Masri, D. Daviaud, S. Gesta, C. Guigné, A. Mazzucotelli, I. Castan-Laurell, I. Tack, B. Knibiehler, C. Carpéné, Y. Audigier, J. S. Saulnier-Blache and P. Valet, Apelin, a newly identified adipokine up-regulated by insulin and obesity, Endocrinology, 2005, 146, 1764–1771 CrossRef CAS PubMed.
- Z. Kochan and J. Karbowska, Dehydroepiandrosterone up-regulates resistin gene expression in white adipose tissue, Mol. Cell. Endocrinol., 2004, 218, 57–64 CrossRef CAS PubMed.
- T. R. Kumar, J. Agno, J. A. Janovick, P. M. Conn and M. M. Matzuk, Regulation of FSHbeta and GnRH receptor gene expression in activin receptor II knockout male mice, Mol. Cell. Endocrinol., 2003, 212, 19–27 CrossRef CAS PubMed.
- M. Matsuda, I. Shimomura, M. Sata, Y. Arita, M. Nishida, N. Maeda, M. Kumada, Y. Okamoto, H. Nagaretani, H. Nishizawa, K. Kishida, R. Komuro, N. Ouchi, S. Kihara, R. Nagai, T. Funahashi and Y. Matsuzawa, Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis, J. Biol. Chem., 2002, 277, 37487–37491 CrossRef CAS PubMed.
- N. Ouchi and K. Walsh, Adiponectin as an anti-inflammatory factor, Clin. Chim. Acta, 2007, 380, 24–30 CrossRef CAS PubMed.
- Y. Yamamoto, H. Hirose, I. Saito, M. Tomita, M. Taniyama, K. Matsubara, Y. Okazaki, T. Ishii, K. Nishikai and T. Saruta, Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population, Clin. Sci., 2002, 103, 137–142 CrossRef CAS PubMed.
- S. Lee and H.-B. Kwak, Role of adiponectin in metabolic and cardiovascular disease, J. Exerc. Rehabil., 2014, 10, 54 CrossRef PubMed.
- C. K. Chakraborti, Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity, World J. Diabetes, 2015, 6, 1296 CrossRef PubMed.
- J. A. Falode, T. B. Olofinlade, G. S. Fayeun, A. O. Adeoye, F. A. Bamisaye, O. R. Ajuwon and T. O. Obafemi, Free and bound phenols from Cymbopogon citratus mitigated hepatocellular injury in streptozotocin-induced type 1 diabetic male rats via decrease in oxidative stress, inflammation, and other risk markers, Pharmacol. Res. – Mod. Chin. Med., 2023, 7, 100234 CrossRef.
- B. Y. Sheikh, The role of prophetic medicine in the management of diabetes mellitus: A review of literature, J. Taibah Univ. Med. Sci., 2016, 11, 339–352 Search PubMed.
- S. Hussain, W. Javed, A. Tajammal, M. Khalid, N. Rasool, M. Riaz, M. Shahid, I. Ahmad, R. Muhammad and S. A. A. Shah, Synergistic antibacterial screening of Cymbopogon citratus and Azadirachta indica: Phytochemical profiling and antioxidant and hemolytic activities, ACS Omega, 2023, 8, 16600–16611 CrossRef CAS PubMed.
- A. Tej, R. H. Mekky, M. del M. Contreras, A. Feriani, M. Tir, B. L′taief, M. O. Alshaharni, B. Faidi, K. Mnafgui, Z. Abbes, E. Saadaoui, M. A. Borgi and N. Tlili, Eucalyptus torquata seeds: Investigation of phytochemicals profile via LC-MS and its potential cardiopreventive capacity in rats, Food Biosci., 2024, 59, 103666 CrossRef CAS.
- R. Hassan Mekky, E. Abdel-Sattar, A. Segura-Carretero and M. del M. Contreras, A comparative study on the metabolites profiling of linseed cakes from Egyptian cultivars and antioxidant activity applying mass spectrometry-based analysis and chemometrics, Food Chem., 2022, 395, 133524 CrossRef CAS PubMed.
- Reaxys, https://www.reaxys.com/#/search/quick, (accessed 19 April 2024).
- KNApSAcK Core System, https://www.knapsackfamily.com/knapsack_core/top.php, (accessed 19 April 2024).
- P. Okińczyc, J. Widelski, M. Ciochoń, E. Paluch, A. Bozhadze, M. Jokhadze, G. Mtvarelishvili, I. Korona-Głowniak, B. Krzyżanowska and P. M. Kuś, Phytochemical profile, plant precursors and some properties of Georgian propolis, Molecules, 2022, 27, 7714 CrossRef PubMed.
- F. Cuyckens and M. Claeys, Mass spectrometry in the structural analysis of flavonoids, J. Mass Spectrom., 2004, 39, 1–15 CrossRef CAS PubMed.
- N. M. Fayek, R. H. Mekky, C. N. Dias, M. Kropf, A. G. Heiss, L. A. Wessjohann and M. A. Farag, UPLC-MS metabolome-based seed classification of 16 Vicia Species: A prospect for phyto-equivalency and chemotaxonomy of different accessions, J. Agric. Food Chem., 2021, 69, 5252–5266 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo04602a |
| ‡ These authors are equally contributed. |
| This journal is © The Royal Society of Chemistry 2024 |