 Open Access Article
Open Access ArticleHuman milk metals and metalloids shape infant microbiota†
Eduard
Flores Ventura
 *a,
Manuel
Bernabeu
*a,
Manuel
Bernabeu
 a,
Belén
Callejón-Leblic
a,
Belén
Callejón-Leblic
 b,
Raúl
Cabrera-Rubio
b,
Raúl
Cabrera-Rubio
 a,
Laxmi
Yeruva
a,
Laxmi
Yeruva
 c,
Javier
Estañ-Capell
c,
Javier
Estañ-Capell
 d,
Cecilia
Martínez-Costa
d,
Cecilia
Martínez-Costa
 d,
Tamara
García-Barrera
d,
Tamara
García-Barrera
 b and
María Carmen
Collado
b and
María Carmen
Collado
 *a
*a
aInstitute of Agrochemistry and Food Technology–National Research Council (IATA-CSIC), Valencia, Spain. E-mail: mcolam@iata.csic.es; Tel: +34 96900022
bResearch Centre of Natural Resources, Health and the Environment (RENSMA), Department of Chemistry, Faculty of Experimental Sciences, University of Huelva, Campus El Carmen, Fuerzas Armadas Ave., 21007, Huelva, Spain
cMicrobiome and Metabolism Research Unit, USDA-ARS, SEA, Arkansas Children's Nutrition Center, Little Rock, AR, USA
dDepartment of Pediatrics, University of Valencia, INCLIVA Biomedical Research Institute, Avenida Blasco Ibáñez 15-17, 46010 Valencia, Spain
First published on 11th November 2024
Abstract
Background: The profile of metal(loid)s in human milk is essential for infant growth and development, yet its impact on the development of the infant microbiota remains unclear. Elements, such as manganese, zinc, iron or copper, play crucial roles in influencing infant health. Aim: To investigate the metal(loid) content within human milk and its influence on the infant's gut microbiota within the first 2 months after birth. Methods: Human milk samples and infant stool samples from 77 mother–infant dyads in the MAMI cohort were collected at two time points: the early transitional stage and the mature stage. Metallomic profiling of human milk was conducted using inductively coupled plasma-mass spectrometry (ICP-MS). The infant gut microbiota was profiled through 16S rRNA amplicon sequencing and maternal–infant clinical data were available. Spearman's rank correlation coefficientsprovided insights into metal(loid)–microbiota relationships. Results: Independent cross-sectional analyses of mother–infant pairs at two time points, significant variations in metal concentrations and differences in microbial abundances and diversities were observed. Notably, Bifidobacterium genus abundance was higher during the mature lactation stage. During early lactation, we found a significant positive correlation between infant gut Corynebacterium and human milk nickel concentrations, and negative correlations between Veillonella spp. and antimony, and Enterobacter spp. and copper. Additionally, Simpson's diversity was negatively correlated with iron. In the mature lactation stage, we identified eleven significant correlations between metals and microbiota. Notably, Klebsiella genus showed multiple negative correlations with iron, antimony, and vanadium. Conclusion: Our study highlights the significance of metal(loid)–microbiota interactions in early infant development, indicating that infant gut Klebsiella genus may be particularly vulnerable to fluctuations in metal(loid) levels present in human milk, when compared to other genera. Future research should explore these interactions at a strain level and the implications on infant health and development. This trial was registered as NCT03552939.
Introduction
Human milk (HM) plays a crucial role in infant health, growth, and development, recommended for exclusive breastfeeding during the first 6 months post-birth.1,2 While HM bioactive components interact with gut microbiota,3 HM's mineral composition, which constitutes up to 0.5%,4 also raises questions about the role of metals in influencing the infant's microbiota.5–10 This is due to the predictive role of the infant gut microbiota in long-term health,11–13 and the impact of environmental pollutants.14 Maternal exposome during pregnancy can modulate HM metal composition,15–18 influenced by solute carrier transporter expression and hormone levels during lactation,19,20 making the metal composition dependent on various factors.Metal(loids) influence microbial growth and metabolism, resembling prebiotic and biocide effects, and play a significant role in metallobiology.21–24 Bacteria and the innate immune system compete for control over ions like iron, zinc, and manganese, impacting bacterial abundance.23 Iron and zinc are essential nutrients for specific microbes, shaping the diversity and richness of microbial genera.25,26 HM can dynamically modulate the microbiota through glycoproteins like lactoferrin, which sequesters iron ions, altering bacterial availability.27,28 By adjusting metal concentrations, HM might adapt to infant needs, influencing bacterial survival and microbiota modulation. Furthermore, metal ions present in extracellular environments are readily available for incorporation into major bacterial pathways. These ions become part of metalloproteins that play crucial roles in various metabolic processes. For instance, metal ions such as iron, zinc, nickel, manganese, and copper are integral to transcriptional regulators like the ferric uptake regulator family, as well as enzymes such as succinate dehydrogenase, which is involved in the tricarboxylic acid cycle.29
Metal(loid)–microbiota interactions, enhancing enzyme catalysis and supporting reaction stability, have potential to modify the gut environment.23 This may influence infant health by altering bacterial metabolism,30 and metabolite production, affecting metal bioavailability. Such variations could mediate infant health and developmental outcomes. Metal(loid)s may also influence bacterial virulence development via metal-related processes.31 We hypothesize that differences in early and mature HM metal(loid) concentrations contribute to the infant gut microbial ecosystem. Thus, we investigated the metal(loid) content in HM and its impact on infant gut microbiota within the first 2 months after birth.
Methods
Study design and volunteers
The present study is a double cross-sectional study involving 77 mother–infant dyads from a longitudinal prospective MAMI cohort study.32 Briefly,33 participants were followed during the period of the first two months post-birth (Fig. S1†). Maternal–neonatal clinical parameters were collected including maternal antibiotic exposure, BMI and weight gain during pregnancy, mode of delivery and feeding type. All participants received oral and written information about the study and written consent was obtained. The study was approved by the Hospital Ethics Committees (Hospital Universitario y Politécnico La Fe and Hospital Clínico Universitario de Valencia), and is registered on the ClinicalTrial.gov platform, with the registration number NCT03552939.Human milk samples
A total number of 98 HM samples from 77 healthy women were collected at two different lactational stages: n = 64 samples at an early transitional stage (between 7 and 15 days postpartum), and n = 34 samples at a mature stage (between 30 and 60 days postpartum). Of these, 19 women provided samples at both early and mature stages (Fig. S1†).HM collection followed a standardized protocol, adhering to the recommended procedures as described previously.34 In brief, breast skin was cleaned with water and soap and the first drops were discarded. Subsequently, milk was collected by using a sterile pumper in sterile bottles to normalize the collection among the participants. Morning collection was recommended. Finally, HM samples were stored immediately at −20 °C in a freezer and sent to the hospital to be stored at −80 °C until further analysis.
Human milk metallomic profile
An aliquot of 500 μL of whole HM was used to determine the metallomic profiles by inductively coupled plasma-mass spectrometry (ICP-MS) as described in Arias-Borrego et al.35 Briefly, mineralization was carried out by using MiniXpress polytetrafluoroethylene (PTFE) vessels. Then, 4 mL of nitric acid and 1 mL of hydrogen peroxide (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were added to 500 μL of whole HM samples. After 10 min of pre-mineralization, the PTFE vessels were closed and introduced into a model MARS microwave oven (CEM, Matthews, NC, USA). The power was set at 400 W and a temperature program was applied from room temperature to 160 °C in 15 min and held at this temperature for 20 min.
1, v/v) were added to 500 μL of whole HM samples. After 10 min of pre-mineralization, the PTFE vessels were closed and introduced into a model MARS microwave oven (CEM, Matthews, NC, USA). The power was set at 400 W and a temperature program was applied from room temperature to 160 °C in 15 min and held at this temperature for 20 min.
Isotopes monitored in the ICP-MS analysis were 27Al, 51V, 53Cr, 55Mn, 57Fe, 59Co, 60Ni, 63Cu, 65Cu, 64Zn, 66Zn, 75As, 95Mo, 103Rh, 112Cd, 114Cd, 121Sb, 205Tl, and 208Pb with a dwell time of 0.3 s per isotope. A tuning aqueous solution of Li, Co, Y, and Tl at 1 μg L−1 was used to tune the ICP-MS system. A multi-element calibration standard–2A solution at 10 mg L−1 of Al, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Cd, Tl, and Pb was obtained from Agilent Technologies. Moreover, stock solutions at 1000 mg L−1 of Mo and Sb (Merck Millipore) were used for the preparation of the calibration curves of these elements. Rh at 100 ng L−1 was used as internal standard. Most of the analysed elements required 4.5 mL min−1 flow rate of helium. For As, a mixture of hydrogen (2 mL min−1) and oxygen (40%) was used in the MS/MS mode. The experiments were performed in triplicate. The quality control validation parameters investigated were the limits of detection (LODs), limits of quantification (LOQs), precision, and accuracy.35 Moreover, as only 19 HM samples and 12 infant fecal samples had complete data available for both early and mature lactation stages, visualizations were created to illustrate the trends of metal(loid)s from HM samples, as well as the 10 most abundant bacteria, and alpha diversity indices in the infant's gut (Fig. S2–S4†). To verify the accuracy of the ICP-MS method, a milk powder certified reference material (NIST-1849) was also analysed for the determination of elements such as 66Zn, 64Zn, 65Cu, 63Cu, 57Fe, 55Mn and 53Cr.
Infant gut microbiota profiling by 16S rRNA amplicon sequencing
Infant clinical data and 16S amplicon sequences were available from previous studies.36 In this study, a subsample corresponding to the 77 healthy mother–infant pairs was selected for the integrative analysis. In brief, total DNA was isolated from the fecal samples as described elsewhere,37 using the Master-Pure DNA Extraction Kit (Epicentre, Madison, WI, USA) by following the manufacturer's instructions with some modifications including physical and enzymatic treatments. Gut microbiota composition and diversity were determined by the V3–V4 variable region of the 16S rRNA gene sequencing, following Illumina protocols. A Nextera XT Index Kit (Illumina, CA, USA) was used for the multiplexing step and a Bioanalyzer DNA 1000 chip (Agilent Technologies, CA, USA) was used for checking the polymerase chain reaction (PCR) product quality. Libraries were sequenced using a 2 × 300 bp paired-end run (MiSeq Reagent Kit v3) on a MiSeq-Illumina platform (FISABIO Sequencing Service, Valencia, Spain), according to the manufacturer's instructions (Illumina). The original raw sequences have been re-processed using the DADA2 bioinformatics tool,38 and taxonomically classified using the Silva Small Subunit rRNA Database v132.39 Relative abundances at genus levels and alpha diversity indices, including richness (Observed, Chao1 and ACE) and diversity (Shannon, Simpson, InvSimpson and Fisher), were calculated using the Phyloseq package (version 4.3.1).40Metadata pre-processing and integration of metallomics and 16S rRNA gene sequencing
A Shapiro–Wilk test for normality on HM metal and metalloid concentrations was conducted, and subsequently, an exploration via violin plots revealed the presence of outliers that explained the variability of the data (Fig. 1). Then, metal and metalloid values for concentrations were scaled using the centered log ratio. Finally, an unpaired t-test was conducted to explore differences between the lactation stages for metal and metalloid concentrations in HM. A Spearman's rank correlation coefficient matrix was created to examine the correlation between the HM metal and metalloid concentrations and the infant gut microbiota, filtering bacteria using 0.001% relative abundances. The significance of each correlation coefficient was tested and p-values were adjusted for multiple comparisons using the Benjamini–Hochberg method for false discovery rate (FDR). Moreover, as only 19 HM samples and 12 infant fecal samples had complete data available for both early and mature lactation stages, visualizations were created to illustrate the trends of metal(loid)s from HM samples, as well as the 10 most abundant bacteria, and alpha diversity indices in the infant's gut (Fig. S2–S4†). These figures were created using GraphPad Prism 8 software.41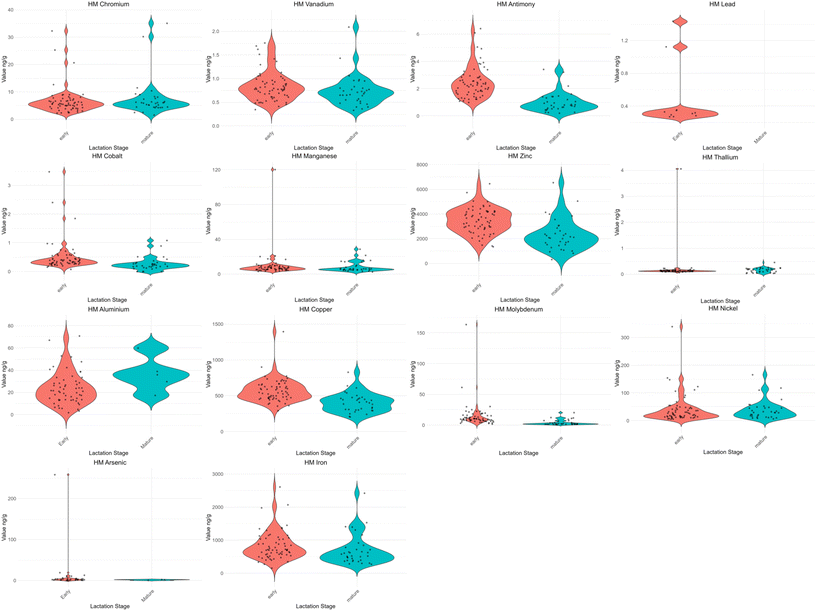 | ||
| Fig. 1 Violin plots of metals and metalloids in human milk samples. Concentrations of metals and metalloids were measured in 98 human milk samples. Among these, 71 samples were analysed for lead (Pb), aluminium (Al), arsenic (As), and cadmium (Cd). Values below the limits of detection were not included in the plots (see Table 2). | ||
Results
Cohort descriptives
Our cohort descriptive analysis included full clinical records from 77 mother–infant dyads. Characteristics of the cohort are presented in Table 1, while data on HM and infant stool samples are detailed in Table 2 and Fig. S5,† respectively. A Shapiro–Wilk test for normality and violin plot visualization of HM metal(loid) concentrations (Fig. 1)—including Sb, Mo, Co, Pb, Cu, Zn, V, Fe, Cr, As, Tl, Mn, Ni, and Al—indicated non-normality in the distributions, with Cd excluded due to its detection in only one sample. Additionally, a longitudinal subset of 19 HM samples and 12 infant fecal samples was isolated for visualization of their differences and trends (Fig. S2–S4†).| Maternal characteristics | Early cross-sectional analysis | Mature cross-sectional analysis | Longitudinal analysis |
|---|---|---|---|
| Mothers in the early lactation stage (N = 64) | Mothers in the mature lactation stage (N = 34) | Longitudinal paired samples (N = 19) | |
| Gestational age (weeks)a | 39 (39–40) | 40 (39–41) | 40 (39–41) |
| Pregestational BMI (kg m−2)a,b | 22.6 (20.5–25.1) | 22.9 (20.9–24.2) | 23.2 (21.7–24.9) |
| Weight gain over pregnancy (kg)a,b | 12 (9.8–14.6) | 13 (11–15) | 13 (10.5–14.5) |
| Antibiotics during pregnancy (no. of cases) | |||
| Yes | 27 | 15 | 5 |
| No | 37 | 19 | 14 |
| Antibiotics during labourb (no. of cases) | |||
| Yes | 16 | 10 | 9 |
| No | 48 | 23 | 10 |
| Not available | 1 | ||
| Delivery mode (n) | |||
| C-section | 25 | 15 | 9 |
| Vaginal | 39 | 19 | 10 |
| Infant characteristicsc | Infants in the early lactation stage (N = 56) | Infants in the mature lactation stage (N = 32) | Longitudinal paired samples (N = 12) |
|---|---|---|---|
| Cohort characteristics for the 77 mother–infant dyads are shown in Table S1.†a Values are expressed as median (quartile 1–quartile 3).b Variables with missing data (n = 1 mother).c n = 10 infants who missed the microbiota analysis. | |||
| Gender (n) | |||
| Females | 31 | 20 | 6 |
| Males | 25 | 12 | 6 |
| Breastfeeding durationa (months) | 5 (0.5–6) | 5.5 (4–6) | 5 (4.9–6) |
| Weight at birtha (kg) | 3.22 (3.01–3.56) | 3.31 (3–3.43) | 3.3 (3.19–3.41) |
| Weight at 7 daysa (kg) | 3.24 (2.95–3.6) | 3.29 (3.96–3.57) | 3.24 (2.99–3.53) |
| Weight at 15 daysa (kg) | 3.49 (3.18–3.75) | 3.52 (3.16–3.81) | 3.54 (3.21–3.68) |
| Weight at 1 montha (kg) | 4.08 (3.74–4.54) | 4.02 (3.72–4.61) | 4.04 (3.79–4.51) |
| BMI z-score at birth | −0.13 (−0.62–0.6) | −0.21 (−0.55–0.5) | −0.1 (−0.29–0.3) |
| Metal(loid)s | HM early stagea (n = 64 samples) | HM mature stagea (n = 34 samples) | p-valuesb | LODc [ng ml−1] | ||
|---|---|---|---|---|---|---|
| Detection | Concentration [ng ml−1] | Detection | Concentration [ng ml−1] | |||
| a The median concentration (interquartile range) of metal(loid) concentrations in ng mL−1 or ng mg−1 was calculated for 98 human milk samples. b Unpaired t-test at a confidence level of 95%. Analysis was not conducted for samples corresponding to the elements lead (Pb), aluminium (Al), arsenic (As), and cadmium (Cd). NA = not available, the values under the limits of detection precluded statistical comparison between lactation stages. c The limit of detection (LOD) for the quality control parameter was determined by calculating three times the standard deviation of the procedural blank obtained from microwave digestion.35 d In total, 71 samples were analysed for Pb, Al, As, and Cd. | ||||||
| Ald | 59/64 | 21.62 (13.5–31.77) | 5/7 | 53.04 (39.36–67.18) | NA | 1.41 |
| V | 64/64 | 0.78 (0.61–0.95) | 34/34 | 0.69 (0.54–0.79) | 0.62 | 0.12 |
| Cr | 64/64 | 5.54 (4.13–6.98) | 34/34 | 5.95 (4.45–7.52) | 0.27 | 0.44 |
| Mn | 64/64 | 6.84 (5.20–8.79) | 34/34 | 5.84 (4.91–8.75) | 0.59 | 0.13 |
| Fe | 64/64 | 700.55 (561.23–1031.73) | 34/34 | 548.8 (391.6–765.6) | 0.49 | 0.22 |
| Co | 63/64 | 0.37 (0.28–0.48) | 28/34 | 0.21 (0.15–0.28) | 0.03 | 0.10 |
| Ni | 64/64 | 24.55 (13.68–44.51) | 34/34 | 29.13 (15.89–47.23) | 0.46 | 0.14 |
| Cu | 64/64 | 543.32 (468.78–651.75) | 34/34 | 404.34 (302.52–464.58) | <0.001 | 0.16 |
| Zn | 64/64 | 3417.7 (2835.2–4172.65) | 34/34 | 2099.4 (1520.7–2808.58) | 0.001 | 1.47 |
| Asd | 32/64 | 2.17 (0.92–4.03) | 3/7 | 7.12 (4.42–12.52) | NA | 0.01 |
| Mo | 64/64 | 9.92 (7.43–15.61) | 34/34 | 2.18 (1.39–4.01) | <0.001 | 0.034 |
| Cdd | 1/64 | 0.3 | 0/7 | <LOD | NA | 0.10 |
| Sb | 64/64 | 2.24 (1.68–2.82) | 34/34 | 0.79 (0.54–1.29) | <0.001 | 0.026 |
| Tl | 55/64 | 0.12 (0.11–0.14) | 24/34 | 0.18 (0.09–0.22) | 0.73 | 0.10 |
| Pbd | 11/64 | 0.32 (0.285–0.35) | 0/7 | <LOD | NA | 0.01 |
This subset of pairs indicates high variability in shifts, showing both minor variations and notable trends in changes in metal and metalloid concentrations, as well as the microbiota profile, from early to mature lactation stages.
HM metallomic profiles differed between lactational stages
In our independent cross-sectional analysis of mother–infant pairs from two categorically separate time points exploring changes in HM metallomic profiles, we observed significantly lower concentrations of Co, Cu, Zn, Mo, and Sb in mature HM samples compared to early HM samples (p-values <0.01) (Table 2).Infant gut microbiota profile in early and mature lactational stages
Among the most abundant genera, Bifidobacterium exhibited the highest relative abundance in infant feces during the mature lactation stage, along with Bacteroides and Streptococcus genus. Conversely, Enterococcus and Klebsiella genus showed lower relative abundances in the infant microbiota during the mature lactation stage (Fig. S5†). However, no statistical differences were observed. Additionally, statistical differences were observed in alpha diversity indices, including Observed and Fisher indices being higher in samples from infants in the mature lactation stage (p-values <0.05) (Table S1†).Early lactation stage and metal–microbiota interactions
In our cross-sectional analysis, significant correlations were identified between HM metal concentrations, infant gut microbiota genera, and alpha diversity indices in the infant fecal samples (Fig. 2A). During the early lactation stage, only one significant positive correlation was found between infant gut Corynebacterium members and HM nickel concentrations (r ≈ 0.15, p-values <0.05). Additionally, negative correlations were observed between Veillonella genus and antimony, and Enterobacter genus and copper (r ≈ −0.2, p-values <0.05). Simpson diversity was also negatively correlated with iron (r ≈ −0.2, p-values <0.05) (Fig. 2B). Furthermore, Table S3† shows the correlations between the detected samples for Pb, Al, and As. Spearman correlations were identified only for Pb, which was moderately and negatively correlated with Shannon (r ≈ −0.63, p-values <0.05), Simpson (r ≈ −0.77, p-values <0.05), and InvSimpson (r ≈ −0.73, p-values <0.05) diversity indices, but not with bacterial abundances.Mature lactation stage and metal–microbiota interactions
The correlations between HM metal concentrations and infant fecal microbiota observed during the mature lactation stage were not replicated when compared to those observed during the early stage. Notably, significant positive correlations were found between Escherichia coli/Shigella and magnesium, as well as Bacteroides genus and zinc (r ≈ 0.2, p-values <0.05). Conversely, negative correlations were observed between Streptococcus genus and cobalt concentrations, and Enterococcus genus with manganese and vanadium. Particularly noteworthy was the interaction of Klebsiella genus with various metals, exhibiting significant negative correlations with iron, antimony, and vanadium. Furthermore, significant negative relationships were identified between Bacteroides and Lacticaseibacillus genera and antimony, with similar correlations observed for Acinetobacter with manganese (r ≈ 0.2, p-values <0.05 for all) (Fig. 2A). Additionally, infant microbiota richness and diversity indices displayed significant negative correlations. These included manganese and nickel with Chao1. Specifically, only manganese exhibited a negative correlation with the Chao1 index (r ≈ −0.3, p-values <0.05) (Fig. 2B).Discussion
Our investigation unveiled significant variations in HM metal concentration and disparities in infant gut microbiota observed between early and mature milk lactation stages, shedding light on potential relationships between individual metals and bacterial abundances, richness, and diversity within the first 2 months postpartum. We identified five significant correlations during early lactation and eleven during mature lactation. Analysis of individual metal–bacteria correlations suggests that Klebsiella genus is the most susceptible genus to metal concentrations. Moreover, variations in HM metals could pose health risks in infants.42 We detected Pb in 15.5% of our samples from the early lactation stage, while all samples from the mature stage were below the limit of detection (LOD). Research has shown that women exposed to metals such as Pb throughout their lives, potentially due to factors like residing in industrial/mining areas or smoking habits, often exceed dietary levels established by the European Food Safety Authorities (EFSA) by threefold and may accumulate these metals in tissues and bones.15 Subsequently, the accumulated metals may be transferred to the mammary gland via the maternal bloodstream.16 Although we identified Pb in all our samples, Freire et al. (2022)43 utilizing ICP-MS identified Pb in only 50.6% of their samples. Heavy metals have not been shown as being involved in any biological process in humans.44 When comparing our values with those reported in the external literature (Table S4†), we observed that the concentrations are highly heterogeneous, varying significantly between populations and across years. This variability warrants further exploration where toxicological guidance values are not yet defined; however, HM samples in this cohort are under toxicological levels as per the established tolerable intakes (Table S4†).In light of exploring potential mechanisms to explain the observed novel metal(loid)–microbiota interactions, we turn to the relevant literature. The most interactive genus was Klebsiella, experiencing negative correlations with iron, antimony, and vanadium. According to the external literature, during pathogenic activity, Ni and Co have been identified as substrates for yersiniabactin to chelate Fe ions,45 and K. pneumoniae can also bind to Ni and Mn. In the context of health and disease, a developed human innate immune system utilizes calprotectin to sequester these metals, limiting the survival of pathogens.46 However, with regard to undeveloped innate immune systems, it remains unclear whether 2-month-old infants can effectively control bacterial pathogenicity in this way. It is important to note that K. pneumoniae strains have higher prevalence of multidrug resistance and metal gene resistance in the context of high concentrations of Fe (approximately 893 μg mL−1).47 Our samples contained outliers with concentrations of iron approximately 1000-fold lower than those in similar contexts. Therefore, these outliers necessitate a more in-depth exploration to understand the features of these strains.
Our exploration also uncovered a novel potential negative relationship between Streptococcus genus with cobalt during the mature stage, showing a decline in Streptococcus abundance observed from the early to mature lactation stages. Although possibly due to a random effect, this trend was consistently reflected in the longitudinal paired sample 7, as shown in Fig. S2 and S3.† Conversely, the literature has shown that S. agalactiae and S. pneumoniae consistently display strong survival mechanisms to handle metal-induced stress.48–50 Studies indicate their effective regulation of stress related to metals, such as Zn in S. agalactiae51 and Mn in S. pneumoniae.52 These mechanisms likely support virulence by enhancing bacterial survival during infections.
Our samples revealed that Bifidobacterium genus had exhibited the highest change in relative abundance, being higher in feces of infants during the mature lactation stage. Interestingly, the literature reports that Zn and Fe can enhance the activity of the metal-dependent phosphoesterase enzyme of B. adolescentis.53 Furthermore, B. longum and B. lactis can effectively remove Cd, Cu, Zn, As, and Pb from the media,54–56 and the growth of B. bifidum var. pennsylvanicus can be facilitated by Fe, Mn, Zn, and Cu.57 Conversely, our samples demonstrated higher concentrations of Bifidobacterium in the mature stage, despite significantly lower levels of copper (Cu) and zinc (Zn) in the mature stage samples.
Additionally, our correlation analysis showed new potential metal–Bacteroides interactions with zinc and antimony. The genus Bacteroides, in particular, has demonstrated specific growth requirements, highlighting distinctions among various organic and metal–organic compounds.58 Investigations have elucidated metal-binding sites in the structure of metallo-lactamases, potentially utilized by Fe, Ni, Cu, Mn, Co, and Zn, thereby enhancing enzymatic activity and contributing to antibiotic resistance, particularly in the case of B. fragilis.59–61 Moreover, B. ovatus has been shown to exhibit a metal-dependent glycosyltransferase featuring metal-binding sites for Mg and Mn, augmenting its ability to metabolize human milk oligosaccharides.62,63 Furthermore, other species such as B. thetaiotaomicron and B. distasonis possess Fe- and Mn-containing superoxide dismutase, fortifying their survival capabilities.64 On the other hand, although research has shown potential interactions with the genus Stenotrophomonas, correlations with metals were not identified. The literature has extensively studied S. maltophilia, revealing that metallo-enzymes are essential for survival.91,92 This includes metallo-beta-lactamase, where Zn is required, and other divalent metals such as Cd and Cu can replace Zn as ligands, indicating an increased potential for the bacteria to survive beta-lactam antibiotics.65–67 The same species has also demonstrated siderophore-mediated metal chelation, responding to Cd-induced toxicity with an exopolysaccharide reaction.65 Nevertheless, S. maltophilia has exhibited mechanisms to overcome metal toxicity derived from Cu(II) and Co(II), such as detoxifying Cd into CdS and reducing oxyanions to nontoxic elemental ions.66,67 The prolonged exposure to high levels of Cd can enhance the production of lipopolysaccharide, resulting in a shift in bacterial abundance and the gut metabolite environment, including a reduction in butyrate production.68
Additionally, previous studies have demonstrated metal toxicity in Escherichia coli when Cd and As ions non-specifically insert into different enzymes, such as molybdoenzymes. This inhibition is also observed for Mo, V, and Cu.69 Consistent with these observations, Cd and As (>1 ng mL−1) have been shown to inhibit the growth of E. coli. However, at lower concentrations of Cd and As, bacterial growth remains unaffected, while antibiotic resistance is increased.70 Further research has explored that Co, Ni, and Fe are related to high pathogenicity islands that secrete yersiniabactin in uropathogenic E. coli71 and extraintestinal E. coli.70 Mn, Co and Cu have been implicated in catalytic enzymes,71–73 and Mn, Fe, Co, Cu, Zn, Cd, and Pb have been identified to increase E. coli's chances of survival in response to antibiotics.74,75 Also, Fe and Zn are associated with enterotoxigenic E. coli pathogenesis,76,77 and Pb may influence mitochondrial apoptosis.78 While research has shown that E. coli can interact with environmental metals and increase virulence factors, our study observed a decrease in the Escherichia/Shigella genus from the early to mature lactation stage, with a significant but weak correlation with manganese.
While we identified potential metal–Corynebacterium relationships, particularly with nickel during early lactation stages, the existing literature suggests more probable interactions between metals and Corynebacterium. Previous research has predominantly focused on the interaction of C. diphtheriae with divalent metal ions, including Mn(II), Co(II), Ni(II), Fe(II), and Zn(II). This interaction serves to shield the bacteria from oxidative damage, while modulating the activity of diphtheria toxin repressor (DtxR).79–85 Furthermore, C. glutamicum has been shown to interact with Ni, Mn, and Co to modulate metal efflux80 and to regulate ZIP family ion transporters.83
Finally, alpha diversity indices were negatively correlated in both lactation stages with HM metal(loids). However, Shen et al. (2022)86 did not find significant associations between childhood and perinatal blood metals and alpha diversity, and although we found significant correlations between alpha diversity and individual metals, research in children did not demonstrate an influence of metal concentrations on gut microbial diversity.86 Nevertheless, in line with our findings, a study among adults showed that long-term exposure to multiple metals, including As, Cd, Cu, Pb, and Zn in metal-polluted areas, was associated with increased alpha diversity.87 Our findings might have been time-dependent rather than concentration-dependent.
Limitations and future directions
During our study, despite it being a longitudinal birth cohort, due to the high variability in breastfeeding duration and practices, we compared samples in a cross-sectional manner at two time points, namely HM from early and mature lactation stages, with only a limited number of women (N = 19) providing samples at both time points. This longitudinal sample size was small considering the different variables that may influence outcomes (secretor status, mode of delivery, infant sex, and also, maternal nutritional status, among others). This study adopts two independent cross-sectional approaches, complemented by longitudinal visualization of paired data available at two time points, to investigate metal–microbiota interactions during the first two months post-birth. The study utilizes ICP-MS for metallomic profiling and 16S rRNA sequencing for microbiota analysis. It identifies novel potential relationships via correlational approaches and holds clinical relevance by offering insights into how metal variations in HM may influence infant gut microbiota establishment. To achieve a better understanding of metal–microbiota interactions, metagenomic sequencing, short-chain fatty production, carbohydrate utilisation and other capabilities should be put to the test with different metal–ion and metal–organic compound concentrations. Most interesting is the mechanism by which some strains respond to high environmental concentrations of metals can lead to the expression of efflux systems to remove the cations from cells and these mechanisms are also attached to antibiotic resistance genes. Thus, higher exposure to metals might be increasing the expression of antibiotic resistance genes,88,89 contributing to a global health issue. Future research needs to consider whole genomes and the complexity of the microbiota. Although a larger sample size would improve generalizability and statistical power, where correlations lost significance after FDR adjustments, external validity is still needed to extrapolate and build predictable models. More detailed maternal information, such as diet and environmental exposure, would improve contextual understanding of observed HM metallomic variations. An additional limitation of this study is the inability to consider potential covariates due to the available data. These covariates include the absorption and utilization of elements by the infants, as well as the specific forms of the elements, such as isotopic composition, electronic or oxidation state, and molecular structure. Moreover, 80% of the women in this cohort were secretors (functional or non-functional variants of the FUT2 gene),90 but the sample size was insufficient to compare secretors and non-secretors. Further research could explore the influence of metalloids on strain functionality in the mother–infant context. Extended follow-up periods may provide insights into this aspect, including potential impacts on infant health and development. Finally, strain specificity is also a factor to consider in future research, where some studies have reported the capability of different strains from the same species interacting differently with metals.Conclusions
Our investigation into the metal(loid) content within HM and its influence on the infant's gut microbiota within the first two months after birth has revealed dynamic changes in metallomic profiles. Our findings suggest the plausibility of Klebsiella genus as the most susceptible genus to changes due to metal(loid) fluctuations in human milk, shedding light on potential microbial responses to environmental exposure. Furthermore, our study contributes new insights into potential relationships between metals and the gut microbiota. Addressing existing knowledge gaps, further research is warranted to delve into the mechanistic aspects of these potential interactions and their long-term implications on gut microbiota and infant well-being. Future studies should extend their focus to organometallic derivatives to provide a comprehensive understanding of metal–microbiota interactions and their impact on infant health.Author contributions
EFV: writing – original draft, conceptualization, methodology, data curation, formal analysis, investigation, validation, visualization, and data interpretation. MB, BCL, RCR, LY and JEC: investigation, methodology, and writing – review and editing. CMC, TGB and MCC: supervision, funding acquisition, resources, conceptualization, interpretation, project administration, and writing – review and editing. All authors contributed to the final version of the manuscript.Data availability
All metadata utilized will be made available upon request to the corresponding authors.Raw sequences are available through the NCBI Sequence Read Archive Database under project accession number BioProject ID PRJNA614975.
All R code necessary to replicate the data analysis is available in the following GitHub repository: https://github.com/EFV1995/BM_metallomic_16S_correlations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the families involved in the MAMI study, as well as all the members of the MAMI cohort study. This research was supported by the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme (ERC starting grant no. 639226) and the Spanish Ministry of Science and Innovation (MAMI-Plus ref. PID2022-139475OB-I00); PID2021-123073NB-C21 from the Spanish Ministry of Science and Innovation (MICIN); the Generación del Conocimiento (MCI/AEI/FEDER “Una manera de hacer Europa”), and UHU-1256905 and UHU-202009 from the FEDER Andalusian Operative Program 2014-2020 (Ministry of Economy, Knowledge, Business and Universities, Regional Government of Andalusia, Spain). E.F.V. is grateful for the predoctoral grant awarded by the Spanish Ministry of Science and Innovation and thanks the European Social Fund Plus (ESF+) for the training of doctors within the framework of the State Plan for Scientific, Technical and Innovation Research 2021–2023 (ref. CEX2021-001189-S-20-1). M.B. is grateful for the post-PhD grant “Juan de la Cierva” (ref. JDC2022-050269-I) supported by the Spanish government MCIU/AEI and the European Union “NextGenerationEU”/PRTR. R.C.R. acknowledges the support from the Generalitat-Valenciana for the grant of the Plan GenT project (CDEIGENT 2020). IATA-CSIC authors also acknowledge the Spanish government MCIN/AEI to the Center of Excellence Accreditation Severo Ochoa (CEX2021-001189-S/MCIN/AEI/10.13039/501100011033).References
- S. M. Reyes, M. M. Brockway, J. M. McDermid, D. Chan, M. Granger, R. Refvik, K. K. Sidhu, S. Musse, C. Monnin, L. Lotoski, D. T. Geddes, F. Jehan, P. Kolsteren, L. H. Allen, D. Hampel, K. G. Eriksen, N. Rodriguez and M. B. Azad, Human Milk Micronutrients and Child Growth and Body Composition in the First 2 years: A Systematic Review, Adv. Nutr., 2024, 15, 100082 CrossRef CAS PubMed.
- WHO, Breastfeeding, https://www.who.int/health-topics/breastfeeding, (accessed 8 June 2023).
- D. Matharu, A. J. Ponsero, M. Lengyel, A. Meszaros-Matwiejuk, K.-L. Kolho, W. M. de Vos, D. Molnar-Gabor and A. Salonen, Human milk oligosaccharide composition is affected by season and parity and associates with infant gut microbiota in a birth mode dependent manner in a Finnish birth cohort, EBioMedicine, 2024, 104 DOI:10.1016/j.ebiom.2024.105182.
- S. Truchet and E. Honvo-Houéto, Physiology of milk secretion, Best Pract. Res., Clin. Endocrinol. Metab., 2017, 31, 367–384 CrossRef CAS PubMed.
- M. Barone, F. D'Amico, P. Brigidi and S. Turroni, Gut microbiome–micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins?, BioFactors, 2022, 48, 307–314 CrossRef CAS PubMed.
- V. Bielik and M. Kolisek, Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome, Int. J. Mol. Sci., 2021, 22, 6803 CrossRef CAS PubMed.
- N. Hadadi, V. Berweiler, H. Wang and M. Trajkovski, Intestinal microbiota as a route for micronutrient bioavailability, Curr. Opin. Endocr. Metab. Res., 2021, 20, 100285 CrossRef CAS PubMed.
- M. Riederer, N. Schweighofer, S. Trajanoski, C. Stelzer, M. Zehentner, B. Fuchs-Neuhold, K. Kashofer, J. A. Mayr, M. Hörmann-Wallner, S. Holasek and M. Van Der Kleyn, Free threonine in human breast milk is related to infant intestinal microbiota composition, Amino Acids, 2022, 54, 365–383 CrossRef CAS PubMed.
- U. Trautvetter, A. Camarinha-Silva, G. Jahreis, S. Lorkowski and M. Glei, High phosphorus intake and gut-related parameters – results of a randomized placebo-controlled human intervention study, Nutr. J., 2018, 17, 23 CrossRef PubMed.
- P. N. Alexandrov, J. M. Hill, Y. Zhao, T. Bond, C. M. Taylor, M. E. Percy, W. Li and W. J. Lukiw, Aluminum-induced generation of lipopolysaccharide (LPS) from the human gastrointestinal (GI)-tract microbiome-resident Bacteroides fragilis, J. Inorg. Biochem., 2020, 203, 110886 CrossRef CAS PubMed.
- M. Kalliomäki, M. C. Collado, S. Salminen and E. Isolauri, Early differences in fecal microbiota composition in children may predict overweight, Am. J. Clin. Nutr., 2008, 87, 534–538 CrossRef PubMed.
- S. Zuffa, P. Schimmel, A. Gonzalez-Santana, C. Belzer, J. Knol, S. Bölte, T. Falck-Ytter, H. Forssberg, J. Swann and R. Diaz Heijtz, Early-life differences in the gut microbiota composition and functionality of infants at elevated likelihood of developing autism spectrum disorder, Transl. Psychiatry, 2023, 13, 257 CrossRef CAS PubMed.
- A. Loughman, A.-L. Ponsonby, M. O'Hely, C. Symeonides, F. Collier, M. L. K. Tang, J. Carlin, S. Ranganathan, K. Allen, A. Pezic, R. Saffery, F. Jacka, L. C. Harrison, P. D. Sly and P. Vuillermin, Gut microbiota composition during infancy and subsequent behavioural outcomes, EBioMedicine, 2020, 52, 102640 CrossRef PubMed.
- S. Mitra, A. J. Chakraborty, A. M. Tareq, T. B. Emran, F. Nainu, A. Khusro, A. M. Idris, M. U. Khandaker, H. Osman, F. A. Alhumaydhi and J. Simal-Gandara, Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity, J. King Saud Univ., Sci., 2022, 34, 101865 CrossRef.
- M. Motas, S. Jiménez, J. Oliva, M. Á. Cámara and M. D. Pérez-Cárceles, Heavy Metals and Trace Elements in Human Breast Milk from Industrial/Mining and Agricultural Zones of Southeastern Spain, Int. J. Environ. Res. Public Health, 2021, 18, 9289 CrossRef PubMed.
- S. Khayat, H. Fanaei and A. Ghanbarzehi, Minerals in Pregnancy and Lactation: A Review Article, J. Clin. Diagn. Res., 2017, 11(9), QE01–QE05 CAS.
- F. M. Rebelo and E. D. Caldas, Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants, Environ. Res., 2016, 151, 671–688 CrossRef CAS PubMed.
- B. S. Karthikeyan, J. Ravichandran, S. R. Aparna and A. Samal, ExHuMId: A curated resource and analysis of Exposome of Human Milk across India, Chemosphere, 2021, 271, 129583 CrossRef CAS PubMed.
- A. M. García-Lino, I. Álvarez-Fernández, E. Blanco-Paniagua, G. Merino and A. I. Álvarez, Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk, Nutrients, 2019, 11(10), 2372 CrossRef PubMed.
- C. S. Kovacs, Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery, Physiol. Rev., 2016, 96, 449–547 CrossRef CAS PubMed.
- H. Dong, L. Huang, L. Zhao, Q. Zeng, X. Liu, Y. Sheng, L. Shi, G. Wu, H. Jiang, F. Li, L. Zhang, D. Guo, G. Li, W. Hou and H. Chen, A critical review of mineral–microbe interaction and co-evolution: mechanisms and applications, Natl. Sci. Rev., 2022, 9(10) DOI:10.1093/nsr/nwac128.
- S. L. Stafford, N. J. Bokil, M. E. S. Achard, R. Kapetanovic, M. A. Schembri, A. G. McEwan and M. J. Sweet, Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper, Biosci. Rep., 2013, 33, e00049 CrossRef PubMed.
- C. C. Murdoch and E. P. Skaar, Nutritional immunity: the battle for nutrient metals at the host–pathogen interface, Nat. Rev. Microbiol., 2022, 20, 657–670 CrossRef CAS PubMed.
- T. E. Kehl-Fie and E. P. Skaar, Nutritional immunity beyond iron: a role for manganese and zinc, Curr. Opin. Chem. Biol., 2010, 14, 218–224 CrossRef CAS PubMed.
- L. Ma, A. Terwilliger and A. W. Maresso, Iron and zinc exploitation during bacterial pathogenesis, Metallomics, 2015, 7, 1541–1554 CrossRef CAS PubMed.
- M. Knez, M. Ranic, J. C. R. Stangoulis and M. Glibetic, in Comprehensive Gut Microbiota, Elsevier, 2022, pp. 230–242 Search PubMed.
- J. C. Yu, H. Khodadadi, A. Malik, B. Davidson, É. D. S. L. Salles, J. Bhatia, V. L. Hale and B. Baban, Innate Immunity of Neonates and Infants, Front. Immunol., 2018, 9, 1759 CrossRef PubMed.
- D. Legrand and J. Mazurier, A critical review of the roles of host lactoferrin in immunity, BioMetals, 2010, 23, 365–376 CrossRef CAS PubMed.
- S. A. Loutet, A. C. K. Chan, M. J. Kobylarz, M. M. Verstraete, S. Pfaffen, B. Ye, A. L. Arrieta and M. E. P. Murphy, in Trace Metals and Infectious Diseases, ed. J. O. Nriagu and E. P. Skaar, MIT Press, Cambridge (MA), 2015 Search PubMed.
- W. Li, B.-M. Lv, Y. Quan, Q. Zhu and H.-Y. Zhang, Associations between Serum Mineral Nutrients, Gut Microbiota, and Risk of Neurological, Psychiatric, and Metabolic Diseases: A Comprehensive Mendelian Randomization Study, Nutrients, 2024, 16, 244 CrossRef CAS PubMed.
- N. German, F. Lüthje, X. Hao, R. Rønn and C. Rensing, in Progress in Molecular Biology and Translational Science, Elsevier, 2016, vol. 142, pp. 27–49 Search PubMed.
- The Power of Maternal Microbes on Infant Health | MAMI Project | Fact Sheet | H2020, https://cordis.europa.eu/project/id/639226, (accessed 30 January 2024).
- I. García-Mantrana, C. Alcántara, M. Selma-Royo, A. Boix-Amorós, M. Dzidic, J. Gimeno-Alcañiz, I. Úbeda-Sansano, I. Sorribes-Monrabal, R. Escuriet, F. Gil-Raga, A. Parra-Llorca, C. Martínez-Costa, M. C. Collado and MAMI team, MAMI: a birth cohort focused on maternal-infant microbiota during early life, BMC Pediatr., 2019, 19, 140 CrossRef PubMed.
- E. Cortes-Macías, M. Selma-Royo, I. García-Mantrana, M. Calatayud, S. González, C. Martínez-Costa and M. C. Collado, Maternal Diet Shapes the Breast Milk Microbiota Composition and Diversity: Impact of Mode of Delivery and Antibiotic Exposure, J. Nutr., 2021, 151, 330–340 CrossRef PubMed.
- A. Arias-Borrego, F. J. Soto Cruz, M. Selma-Royo, C. Bäuerl, E. García Verdevio, F. J. Pérez-Cano, C. Lerin, I. Velasco López, C. Martínez-Costa, M. C. Collado and T. García-Barrera, Metallomic and Untargeted Metabolomic Signatures of Human Milk from SARS–CoV–2 Positive Mothers, Mol. Nutr. Food Res., 2022, 66, 2200071 CrossRef CAS PubMed.
- M. Selma-Royo, M. Calatayud Arroyo, I. García-Mantrana, A. Parra-Llorca, R. Escuriet, C. Martínez-Costa and M. C. Collado, Perinatal environment shapes microbiota colonization and infant growth: impact on host response and intestinal function, Microbiome, 2020, 8, 167 CrossRef CAS PubMed.
- M. Selma-Royo, I. García-Mantrana, M. Calatayud, A. Parra-Llorca, C. Martínez-Costa and M. C. Collado, Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota, Eur. J. Nutr., 2021, 60, 1429–1442 CrossRef CAS PubMed.
- B. J. Callahan, P. J. McMurdie, M. J. Rosen, A. W. Han, A. J. A. Johnson and S. P. Holmes, DADA2: High resolution sample inference from Illumina amplicon data, Nat. Methods, 2016, 13, 581–583 CrossRef CAS PubMed.
- C. Quast, E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, J. Peplies and F. O. Glöckner, The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 2013, 41, D590–D596 CrossRef CAS PubMed.
- P. J. McMurdie and S. Holmes, phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data, PLoS One, 2013, 8, e61217 CrossRef CAS PubMed.
- Prism - GraphPad, https://www.graphpad.com/features, (accessed 13 February 2024).
- Y.-C. Lin, W.-H. Chang, T.-C. Li, O. Iwata and H.-L. Chen, Health Risk of Infants Exposed to Lead and Mercury Through Breastfeeding, Exposure Health, 2023, 15, 255–267 CrossRef CAS.
- C. Freire, L. M. Iribarne-Durán, F. Gil, P. Olmedo, L. Serrano-Lopez, M. Peña-Caballero, J.-A. Hurtado, N. E. Alvarado-González, M. F. Fernández, F. M. Peinado, F. Artacho-Cordón and N. Olea, Concentrations and determinants of lead, mercury, cadmium, and arsenic in pooled donor breast milk in Spain, Int. J. Hyg. Environ. Health, 2022, 240, 113914 CrossRef CAS PubMed.
- Z. Fu and S. Xi, The effects of heavy metals on human metabolism, Toxicol. Mech. Methods, 2020, 30, 167–176 CrossRef CAS PubMed.
- A. E. Robinson, J. E. Lowe, E.-I. Koh and J. P. Henderson, Uropathogenic enterobacteria use the yersiniabactin metallophore system to acquire nickel, J. Biol. Chem., 2018, 293, 14953–14961 CrossRef CAS PubMed.
- T. G. Nakashige, E. M. Zygiel, C. L. Drennan and E. M. Nolan, Nickel Sequestration by the Host-Defense Protein Human Calprotectin, J. Am. Chem. Soc., 2017, 139, 8828–8836 CrossRef CAS PubMed.
- J. P. R. Furlan, E. A. Savazzi and E. G. Stehling, Genomic insights into multidrug-resistant and hypervirulent Klebsiella pneumoniae co-harboring metal resistance genes in aquatic environments, Ecotoxicol. Environ. Saf., 2020, 201, 110782 CrossRef CAS PubMed.
- M. J. Sullivan, K. G. K. Goh and G. C. Ulett, Regulatory cross-talk supports resistance to Zn intoxication in Streptococcus, PLoS Pathog., 2022, 18, e1010607 CrossRef CAS PubMed.
- M. L. Korir, R. S. Doster, J. Lu, M. A. Guevara, S. K. Spicer, R. E. Moore, J. D. Francis, L. M. Rogers, K. P. Haley, A. Blackman, K. N. Noble, A. J. Eastman, J. A. Williams, S. M. Damo, K. L. Boyd, S. D. Townsend, C. H. Serezani, D. M. Aronoff, S. D. Manning and J. A. Gaddy, Streptococcus agalactiae cadD alleviates metal stress and promotes intracellular survival in macrophages and ascending infection during pregnancy, Nat. Commun., 2022, 13, 5392 CrossRef CAS PubMed.
- L. R. Burcham, R. A. Hill, R. C. Caulkins, J. P. Emerson, B. Nanduri, J. W. Rosch, N. C. Fitzkee and J. A. Thornton, Streptococcus pneumoniae metal homeostasis alters cellular metabolism†, Metallomics, 2020, 12, 1416–1427 CrossRef CAS PubMed.
- M. J. Sullivan, K. G. K. Goh and G. C. Ulett, Cellular Management of Zinc in Group B Streptococcus Supports Bacterial Resistance against Metal Intoxication and Promotes Disseminated Infection, mSphere, 2021, 6(3) DOI:10.1128/msphere.00105-21.
- J. W. Johnston, D. E. Briles, L. E. Myers and S. K. Hollingshead, Mn2+-Dependent Regulation of Multiple Genes in Streptococcus pneumoniae through PsaR and the Resultant Impact on Virulence, Infect. Immun., 2006, 74, 1171–1180 CrossRef CAS PubMed.
- G. W. Han, J. Ko, C. L. Farr, M. C. Deller, Q. Xu, H.-J. Chiu, M. D. Miller, J. Sefcikova, S. Somarowthu, P. J. Beuning, M.-A. Elsliger, A. M. Deacon, A. Godzik, S. A. Lesley, I. A. Wilson and M. J. Ondrechen, Crystal structure of a metal-dependent phosphoesterase (YP_910028.1) from Bifidobacterium adolescentis: Computational prediction and experimental validation of phosphoesterase activity, Proteins, 2011, 79, 2146–2160 CrossRef CAS PubMed.
- T. Halttunen, S. Salminen and R. Tahvonen, Rapid removal of lead and cadmium from water by specific lactic acid bacteria, Int. J. Food Microbiol., 2007, 114, 30–35 CrossRef CAS PubMed.
- A field study on the composition, structure, and function of endophytic bacterial community of Robinia pseudoacacia at a composite heavy metals tailing - PubMed, https://pubmed.ncbi.nlm.nih.gov/35940266/, (accessed 17 January 2024).
- R. M. Elsanhoty, I. A. Al-Turki and M. F. Ramadan, Application of lactic acid bacteria in removing heavy metals and aflatoxin B1 from contaminated water, Water Sci. Technol., 2016, 74, 625–638 CrossRef CAS PubMed.
- A. Bezkorovainy and N. Topouzian, The effect of metal chelators and other metabolic inhibitors on the growth of Bifidobacterium bifidus var. Pennsylvanicus, Clin. Biochem., 1981, 14, 135–141 CrossRef CAS PubMed.
- D. R. Caldwell and C. Arcand, Inorganic and Metal-Organic Growth Requirements of the Genus Bacteroides, J. Bacteriol., 1974, 120, 322–333 CrossRef CAS PubMed.
- T. Khushi, D. J. Payne, A. Fosberry and C. Reading, Production of metal dependent β-lactamases by clinical strains of Bacteroides fragilis isolated before 1987, J. Antimicrob. Chemother., 1996, 37, 345–350 CrossRef CAS PubMed.
- M. Dal Peraro, A. J. Vila and P. Carloni, Protonation State of Asp120 in the Binuclear Active Site of the Metallo-β-Lactamase from Bacteroides fragilis, Inorg. Chem., 2003, 42, 4245–4247 CrossRef CAS PubMed.
- E. G. Orellano, J. E. Girardini, J. A. Cricco, E. A. Ceccarelli and A. J. Vila, Spectroscopic Characterization of a Binuclear Metal Site in Bacillus cereus β-Lactamase II, Biochemistry, 1998, 37, 10173–10180 CrossRef CAS PubMed.
- T. T. K. Pham, B. Stinson, N. Thiyagarajan, M. Lizotte-Waniewski, K. Brew and K. R. Acharya, Structures of Complexes of a Metal-independent Glycosyltransferase GT6 from Bacteroides ovatus with UDP-N-Acetylgalactosamine (UDP-GalNAc) and Its Hydrolysis Products *, J. Biol. Chem., 2014, 289, 8041–8050 CrossRef CAS PubMed.
- P. Tumbale and K. Brew, Characterization of a Metal-independent CAZy Family 6 Glycosyltransferase from Bacteroides ovatus*, J. Biol. Chem., 2009, 284, 25126–25134 CrossRef CAS PubMed.
- Y. Chen and E. M. Gregory, In vivo metal substitution in Bacteroides fragilis superoxide dismutase, Free Radical Res. Commun., 1991, 12–13(Pt 1), 313–318 CrossRef CAS PubMed.
- Y. Kim, N. Maltseva, M. Wilamowski, C. Tesar, M. Endres and A. Joachimiak, Structural and biochemical analysis of the metallo-β-lactamase L1 from emerging pathogen Stenotrophomonas maltophilia revealed the subtle but distinct di-metal scaffold for catalytic activity, Protein Sci., 2020, 29, 723–743 CrossRef CAS PubMed.
- J. D. Garrity, A. L. Carenbauer, L. R. Herron and M. W. Crowder, Metal Binding Asp-120 in Metallo-β-lactamase L1 from Stenotrophomonas maltophilia Plays a Crucial Role in Catalysis*, J. Biol. Chem., 2004, 279, 920–927 CrossRef CAS PubMed.
- D. Xu, H. Guo and Q. Cui, Antibiotic Deactivation by a Dizinc β-Lactamase: Mechanistic Insights from QM/MM and DFT Studies, J. Am. Chem. Soc., 2007, 129, 10814–10822 CrossRef CAS PubMed.
- S. Ramakrishnan, T. Muruganraj, R. Majumdar and S. Sugumar, Study of Cadmium Metal Resistance in Stenotrophomonas maltophilia, Indian J. Microbiol., 2023, 63, 91–99 CrossRef CAS PubMed.
- D. Pages, J. Rose, S. Conrod, S. Cuine, P. Carrier, T. Heulin and W. Achouak, Heavy Metal Tolerance in Stenotrophomonas maltophilia, PLoS One, 2008, 3, e1539 CrossRef PubMed.
- K. Yang and Y. Zhang, Reversal of heavy metal-induced antibiotic resistance by dandelion root extracts and taraxasterol, J. Med. Microbiol., 2020, 69, 1049–1061 CrossRef CAS PubMed.
- A. E. Robinson, J. R. Heffernan and J. P. Henderson, The iron hand of uropathogenic Escherichia coli: the role of transition metal control in virulence, Future Microbiol., 2018, 13, 745–756 CrossRef CAS PubMed.
- B. S. Kelly, W. E. Antholine and O. W. Griffith, Escherichia coli γ-Glutamylcysteine Synthetase: TWO ACTIVE SITE METAL IONS AFFECT SUBSTRATE AND INHIBITOR BINDING*, J. Biol. Chem., 2002, 277, 50–58 CrossRef CAS.
- M. A. Smith, P. Pirrat, A. R. Pearson, C. R. P. Kurtis, C. H. Trinh, T. G. Gaule, P. F. Knowles, S. E. V. Phillips and M. J. McPherson, Exploring the Roles of the Metal Ions in Escherichia coli Copper Amine Oxidase, Biochemistry, 2010, 49, 1268–1280 CrossRef CAS PubMed.
- Y. Huang, Y. Li, J. Chen, J. Lin, L. Liu, J. Ye and Y. Su, Promoting effect of Fe3+ on gentamicin resistance in Escherichia coli, Biochem. Biophys. Res. Commun., 2022, 625, 134–139 CrossRef CAS PubMed.
- G. Na, Z. Lu, H. Gao, L. Zhang, Q. Li, R. Li, F. Yang, C. Huo and Z. Yao, The effect of environmental factors and migration dynamics on the prevalence of antibiotic-resistant Escherichia coli in estuary environments, Sci. Rep., 2018, 8, 1663 CrossRef.
- M. C. Kiefer, N. I. Motyka, J. D. Clements and J. P. Bitoun, Enterotoxigenic Escherichia coli Heat-Stable Toxin Increases the Rate of Zinc Release from Metallothionein and Is a Zinc- and Iron-Binding Peptide, mSphere, 2020, 5(2) DOI:10.1128/msphere.00146-20.
- E. Velasco, S. Wang, M. Sanet, J. Fernández-Vázquez, D. Jové, E. Glaría, A. F. Valledor, T. V. O'Halloran and C. Balsalobre, A new role for Zinc limitation in bacterial pathogenicity: modulation of α-hemolysin from uropathogenic Escherichia coli, Sci. Rep., 2018, 8, 6535 CrossRef PubMed.
- S. K. Palanirajan and S. N. Gummadi, Heavy-Metals-Mediated Phospholipids Scrambling by Human Phospholipid Scramblase 3: A Probable Role in Mitochondrial Apoptosis, Chem. Res. Toxicol., 2020, 33, 553–564 Search PubMed.
- E. D. Peng, L. R. Lyman and M. P. Schmitt, Analysis of the Manganese and MntR Regulon in Corynebacterium diphtheriae, J. Bacteriol., 2021, 203(20) DOI:10.1128/jb.00274-21.
- W.-W. Choi, H. Jeong, Y. Kim and H.-S. Lee, Gene nceA encodes a Ni/Co-sensing transcription factor to regulate metal efflux in Corynebacterium glutamicum, Metallomics, 2022, 14(12) DOI:10.1093/mtomcs/mfac094.
- J. A. D'Aquino, J. Tetenbaum-Novatt, A. White, F. Berkovitch and D. Ringe, Mechanism of metal ion activation of the diphtheria toxin repressor DtxR, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 18408–18413 CrossRef PubMed.
- X. Tao, H. Y. Zeng and J. R. Murphy, Transition metal ion activation of DNA binding by the diphtheria tox repressor requires the formation of stable homodimers., Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 6803–6807 CrossRef CAS PubMed.
- K. F. Smith, L. A. Bibb, M. P. Schmitt and D. M. Oram, Regulation and Activity of a Zinc Uptake Regulator, Zur, in Corynebacterium diphtheriae, J. Bacteriol., 2009, 191, 1595–1603 CrossRef CAS PubMed.
- Z. Wang, M. P. Schmitt and R. K. Holmes, Characterization of mutations that inactivate the diphtheria toxin repressor gene (dtxR), Infect. Immun., 1994, 62, 1600–1608 CrossRef CAS PubMed.
- M. P. Schmitt, Analysis of a DtxR-Like Metalloregulatory Protein, MntR, from Corynebacterium diphtheriae That Controls Expression of an ABC Metal Transporter by an Mn2+-Dependent Mechanism, J. Bacteriol., 2002, 184, 6882–6892 CrossRef CAS PubMed.
- Y. Shen, H. E. Laue, M. J. Shrubsole, H. Wu, T. R. Bloomquist, A. Larouche, K. Zhao, F. Gao, A. Boivin, D. Prada, D. J. Hunting, V. Gillet, L. Takser and A. A. Baccarelli, Associations of Childhood and Perinatal Blood Metals with Children's Gut Microbiomes in a Canadian Gestation Cohort, Environ. Health Perspect., 2022, 130, 017007 CrossRef CAS PubMed.
- M. Shao and Y. Zhu, Long-term metal exposure changes gut microbiota of residents surrounding a mining and smelting area, Sci. Rep., 2020, 10, 4453 CrossRef CAS.
- T. H. T. Nguyen, H. D. Nguyen, M. H. Le, T. T. H. Nguyen, T. D. Nguyen, D. L. Nguyen, Q. H. Nguyen, T. K. O. Nguyen, S. Michalet, M.-G. Dijoux-Franca and H. N. Pham, Efflux Pump Inhibitors in Controlling Antibiotic Resistance: Outlook under a Heavy Metal Contamination Context, Molecules, 2023, 28, 2912 CrossRef CAS PubMed.
- F. Aslam, A. Yasmin and T. Thomas, Essential Gene Clusters Identified in Stenotrophomonas MB339 for Multiple Metal/Antibiotic Resistance and Xenobiotic Degradation, Curr. Microbiol., 2018, 75, 1484–1492 CrossRef CAS PubMed.
- M. Selma-Royo, S. González, M. Gueimonde, M. Chang, A. Fürst, C. Martínez-Costa, L. Bode and M. C. Collado, Maternal Diet Is Associated with Human Milk Oligosaccharide Profile, Mol. Nutr. Food Res., 2022, 66, e2200058 CrossRef PubMed.
- U. Guzik, K. Hupert-Kocurek, K. Sałek and D. Wojcieszyńska, Influence of metal ions on bioremediation activity of protocatechuate 3,4-dioxygenase from Stenotrophomonas maltophilia KB2, World J. Microbiol. Biotechnol., 2013, 29, 267–273 CrossRef CAS PubMed.
- W. Xiong, C. Yin, Y. Wang, S. Lin, Z. Deng and R. Liang, Characterization of an efficient estrogen-degrading bacterium Stenotrophomonas maltophilia SJTH1 in saline-, alkaline-, heavy metal-contained environments or solid soil and identification of four 17β-estradiol-oxidizing dehydrogenases, J. Hazard. Mater., 2020, 385, 121616 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo01929f |
| This journal is © The Royal Society of Chemistry 2024 |

