Open Access Article
Open Access ArticleDevelopment of a food frequency questionnaire for the estimation of dietary (poly)phenol intake†
Yong
Li
,
Yifan
Xu
 ,
Melanie
Le Sayec
,
Nur Najiah Zaidani
Kamarunzaman
,
Haonan
Wu
,
Jiaying
Hu
,
Shan
Li
,
Rachel
Gibson
and
Ana
Rodriguez-Mateos
,
Melanie
Le Sayec
,
Nur Najiah Zaidani
Kamarunzaman
,
Haonan
Wu
,
Jiaying
Hu
,
Shan
Li
,
Rachel
Gibson
and
Ana
Rodriguez-Mateos
 *
*
Department of Nutritional Sciences, School of Life Course and Population Sciences, Faculty of Life Sciences and Medicine, King's College London, London, UK. E-mail: ana.rodriguez-mateos@kcl.ac.uk
First published on 18th September 2024
Abstract
Background: (Poly)phenol intake has been associated with reduced risk of non-communicable diseases in epidemiological studies. However, there are currently no dietary assessment tools specifically developed to estimate (poly)phenol intake in the UK population. Objectives: This study aimed to develop a novel food frequency questionnaire (FFQ) to capture the dietary (poly)phenol intake in the UK and assess its relative validity with 7 day diet diaries (7DDs) and plasma and urine (poly)phenol metabolites. Methods: The KCL (poly)phenol FFQ (KP-FFQ) was developed based on the existing EPIC (European Prospective Investigation into Diet and Cancer)-Norfolk FFQ, which has been validated for energy and nutrient intake estimation in the UK population. Participants aged 18–29 years (n = 255) completed both the KP-FFQ and the EPIC-Norfolk FFQ. In a subgroup (n = 60), 7DD, spot urine, and fasting plasma samples were collected. An in-house (poly)phenol database was used to estimate (poly)phenol intake from FFQs and 7DDs. Plasma and urinary (poly)phenol metabolite levels were analysed using a validated ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry method. The agreements between (poly)phenol intake estimated using the KP-FFQ, EPIC-Norfolk FFQ and 7DDs, as well as plasma and urinary biomarkers, were evaluated by intraclass correlation coefficients (ICC), weighted kappa, quartile cross-classification, and Spearman's correlations, and the associations were investigated using linear regression models adjusting for energy intake and multiple testing (false discovery rate (FDR) < 0.05). Results: The mean (standard deviation, SD) of total (poly)phenol intake estimated from KP-FFQs was 1366.5 (1151.7) mg d−1. Fair agreements were observed between ten (poly)phenol groups estimated from KP-FFQs and 7DDs (kappa: 0.41–0.73), including total (poly)phenol intake (kappa = 0.45), while the agreements for the rest of the 17 classes and subclasses were poor (kappa: 0.07–0.39). Strong positive associations with KP-FFQ were found in ten (poly)phenols estimated from 7DDs, including dihydroflavonols, theaflavins, thearubigins, flavones, isoflavonoids, ellagitannins, hydroxyphenylacetic acids, total stilbenes, resveratrol, and tyrosols with stdBeta ranged from 0.61 (95% confidence interval CI: 0.42 to 0.81) to 0.95 (95% CI: 0.86 to 1.03) (all FDR adjusted p < 0.05). KP-FFQs estimated (poly)phenol intake exhibited positive associations with 76 urinary metabolites (stdBeta: 0.28 (95% CI: 0.07–0.49) to 0.81 (0.62–1.00)) and 19 plasma metabolites (stdBeta: 0.40 (0.17–0.62)–0.83 (0.64–1.02)) (all FDR p < 0.05). The agreement between KP-FFQs and the EPIC-Norfolk FFQs was moderate (ICC 0.51–0.69) for all (poly)phenol subclasses after adjusting for energy intake. Compared with the EPIC-Norfolk FFQs estimated (poly)phenol intake, stronger and more agreements and associations were found in KP-FFQs estimated (poly)phenol with 7DDs and biomarkers. Conclusion: (Poly)phenol intake estimated from KP-FFQ exhibited fair agreements and moderate to strong associations with 7DDs and biomarkers, indicating the novel questionnaire may be a promising tool to assess dietary (poly)phenol intake.
1. Introduction
(Poly)phenols are broadly distributed in the plant kingdom.1 Flavonoids, phenolic acids, lignans, and stilbenes are among the major groups of (poly)phenols, with more than 8000 phenolic structures identified so far.2 As abundant secondary metabolites of plants, (poly)phenols are ubiquitous in the human diet, especially in fruits, vegetables, wholegrains, legumes, nuts, seeds, cocoa and soy products, coffee, and tea. The evidence from randomized clinical trials and observational studies on their health benefits is continuously accumulating, for instance, in cardiovascular health3,4 and age-related cognitive protection.5 However, the lack of accurate assessment data may hinder the establishment of valid relationships between (poly)phenol and health at the population level.4,6,7It is challenging to measure the dietary intake of (poly)phenols due to their complex nature, which includes a wide range of structures from single aromatic-ring monomer molecules to intricate condensed polymer tannins found in food.8,9 Different (poly)phenols can accumulate in certain plants, resulting in distinct profiles of (poly)phenols in foods. Some (poly)phenols, for instance, quercetin, are widely found in many types of plant foods, including fruits, vegetables, cereals, tea, and wine, whereas some (poly)phenols are specifically abundant in certain foods, for instance, flavanones in citrus fruit and isoflavones in soya.10 The complexity and variability of (poly)phenol abundance in the human diet require a comprehensive and targeted food list included in assessment tools.11
Currently, dietary (poly)phenol intake information from large cohort studies is mainly collected through food frequency questionnaires (FFQs), due to the low burden on both participants and researchers.12–15 Comparison studies between FFQs and 7 day diet diaries (7DDs) indicated that the agreements were poor for the groups that contribute small percentages to total (poly)phenol intake, such as anthocyanins, chalcones, flavones, and hydroxyphenylacetic acids, which might be due to the difficulty of capturing such food sources with these tools.6 Since these tools were designed to estimate habitual nutrient intake, they do not necessarily capture well all the (poly)phenol food sources, so tools with a more comprehensive and detailed list of food items or food groups to accurately evaluate all the (poly)phenol subclasses are needed. In addition, only a few of them have been validated for (poly)phenol intake,16 which restricts the understanding of dietary assessment tools’ performance in evaluating (poly)phenol intake.17,18 Therefore, a dietary assessment tool that has been specifically developed and validated for estimating habitual (poly)phenol intake would be valuable to advancing research on the exposure and health impact of (poly)phenol consumption in the population.
No objective ‘gold standard’ reference biomarker tool has been established for evaluating (poly)phenol exposure and only a few biomarkers have been partially validated for individual (poly)phenols, including flavan-3-ols19,20 and isoflavones.21 However, total urinary (poly)phenols, and in particular 24 h urine, have been proposed to reflect total (poly)phenol intake,22 and urine measurements have been used as the reference tool to strengthen the relative validation of 7DDs.23 Thus, quantitative targeted metabolomics including a comprehensive panel of (poly)phenol metabolites hold the potential to serve as a surrogate marker for (poly)phenol intake.24–29
The primary objectives of this work were to (1) develop a FFQ to capture habitual (poly)phenol intake in the free-living UK population (KCL (poly)phenol FFQ or KP-FFQ), (2) conduct a relative validity study with 7DDs, and (3) test agreements between (poly)phenol intake estimated from the KP-FFQ with objective (poly)phenol metabolites from 24 h urine and plasma samples (Fig. 1). The secondary objectives of this study were to compare the novel KP-FFQ with an established FFQ (the EPIC (European Prospective Investigation into Diet and Cancer)-Norfolk FFQ) in the estimation of (poly)phenol and nutrient intake (Fig. 1).
 | ||
| Fig. 1 Primary and secondary research objectives regarding the development and relative validation of the KCL (poly)phenol FFQ. | ||
2. Methods
2.1 Tool development
The KP-FFQ is adapted from the EPIC-Norfolk FFQ. This FFQ is a well-established tool in the UK, having been validated against nutrient intakes in the EPIC Norfolk study.30 The EPIC-Norfolk FFQ was designed to estimate habitual nutrient intake in the UK EPIC-Norfolk study.31 It consists of 130 food items typically consumed in the UK with nine frequency options from “Never or less than once a month” to “more than six times per day”. Through extensive experience using the EPIC-Norfolk FFQ in our laboratory as part of clinical trials32–34 and cross-sectional studies,6,35,36 we have identified certain challenges in accurately estimating (poly)phenol intake during its application. This recognition has led us to develop a specialized (poly)phenol-focused version, adapted from the original EPIC-Norfolk FFQ.The KP-FFQ aims to distinguish between food items with different (poly)phenol content and composition, by either (1) disaggregation of distinct food items listed in the EPIC-Norfolk FFQ as one entry (e.g., single food entry for strawberries, raspberries, and kiwi fruit); (2) differentiation of food groups with different colours (e.g., red, white, and yellow onion); (3) differentiation of parts of the same food source (e.g., peel and pulp); or (4) addition of additional food sources not listed in the EPIC-Norfolk FFQ, e.g., blueberries, based on their (poly)phenol content. In all, food items that met the criteria of ‘providing more than 1 mg of total (poly)phenols per serving’37 or considered to be rich in (poly)phenols were included in the KP-FFQ through expert agreement (ARM, RG, and YX). Food items identified as the main contributors to (poly)phenol intake among children and adults in the UK were also included.38 Food groups (e.g., meat and fish) and food items (e.g., Horlicks, honey) not rich in (poly)phenols from the original EPIC-Norfolk FFQ were also included in the KP-FFQ to retain the structure for estimating overall nutrient intake.
The KP-FFQ required participants to report their average intake of the food items over the last year, ranging from a frequency of ‘never or less than once per month’ to ‘6 + per day’ which were numerically coded as ‘1’ to ‘9’. Missing data were recorded as ‘−9’ throughout the data entry process. Data on the frequency of intake were manually entered into Microsoft Excel sheet as numeric codes. The average daily intake was derived by multiplying the frequency of intake with default portion sizes. The procedure used for the development of the KP-FFQ is shown in Fig. 2.
 | ||
| Fig. 2 Summary of processes followed in KCL (poly)phenol FFQ development. EPIC: European Prospective Investigation into Diet and Cancer, FFQ: food frequency questionnaire. | ||
The final KP-FFQ included 442 food items. The food items under each food group in the EPIC-Norfolk FFQ and KP-FFQ are compared in ESI Table 1.†
2.2 (Poly)phenol database/estimation of (poly)phenol intake
The (poly)phenol content of all food items was established based on publicly available databases, including Phenol-Explorer,39 the United States Department of Agriculture (USDA) database, and an in-house (poly)phenol database35 that includes common foods and beverages in the UK diet calculated from recipes. Each potential (poly)phenol-rich food item was verified against these databases prior to being included in the KP-FFQ.The total (poly)phenol content of foods/beverages was calculated using the default average portion sizes, which reflected the average portion sizes of the UK population. The portion sizes were selected based on available UK data (EPIC-Norfolk portion sizes, https://www.epic-norfolk.org.uk), Nutritics (https://www.nutritics.com/en/), and product information in three major supermarkets in the UK: Sainsbury's™ (https://food-to-order.sainsburys.co.uk/category/allfood), Tesco™ (https://www.tesco.com/) and Morrisons™ (https://groceries.morrisons.com/navigation). (Poly)phenol intake of each food item (mg d−1) was calculated by multiplying the total (poly)phenol content (mg per 100 g) with the default portion size (g) and the intake frequency factor as below:
| Ptotal = ∑Pi = ∑(Ci × Qi ÷ 100) |
| Qi = Di × F |
Of which: Ptotal: total (poly)phenol intake (mg d−1); Pi: the (poly)phenol intake of food item (i) in FFQ, mg d−1; Ci: (poly)phenol content of the food item (i), mg per 100 g, Qi: intake quantity of food item (i) (g d−1); Di: default portion size of food item (i); F: frequency factor (Never or less than once/month: 0; 1–3 per month: 0.07; once a week: 0.14; 2–4 per week: 0.43, 5–6 per week: 0.79, once a day: 1; 2–3 per day: 2.5; 4–5 per day:4.5; 6 + per day: 6)
2.3 Pilot study
The KP-FFQ was piloted in 20 participants that were recruited from the student population at King's College London. Participants provided written informed consent and were provided with an envelope consisting of the EPIC-Norfolk FFQ, KP-FFQ and a blank 7DD with an allocated randomised code to ensure that no identifiable information was collected by the General Data Protection Regulation 2016 (Research Ethics Minimal Risk Registration number MRA-20/21-24013). Participants were given one week to complete the questionnaires. They were directed to complete the KP-FFQ on the first day and the EPIC-Norfolk FFQ on the seventh day during the 7 day period of recording the 7DD. They were also required to record the time taken to complete each FFQ. The envelopes were returned by the participants to an allocated box in the department office anonymously.2.4 Evaluation study
Participants enrolled in the KCL (poly)phenol FFQ pilot study, a randomized controlled trial and an observational study conducted from 2021 to 2024 at the Metabolic Research Unit of the Department of Nutritional Sciences, King's College London, UK completed the KP-FFQ along with other baseline dietary measurements. The three studies applied the same dietary assessment protocols and tools, including EPIC-Norfolk FFQ, KP-FFQ, and 7DD, to obtain the dietary intake of each participant. Healthy participants aged 18–29 years old (n = 262) were included to evaluate the KP-FFQ. The studies were approved by the Research Ethics Committee of King's College London (Ethics numbers: KCL (poly)phenol FFQ pilot study: MRA-20/21-24013; FOODMOOD study: HR/DP-21/22-28880; CRANMOOD study HR/DP-21/22-26721; validation of a new FFQ to estimate (poly)phenol intake in CRANMOOD study: LRS/DP-21/22-32445) and conducted following the Declaration of Helsinki. The participants were excluded if no available dietary assessment data from any of the EPIC-Norfolk/KP-FFQs or 7DDs were collected (n = 27). Finally, 235 participants with both FFQs and 7DDs were included in the analysis.A detailed flow chart of this process is exhibited in Fig. 3.
 | ||
| Fig. 3 Flowchart of the study. 7DDs: 7 day diet diaries, FFQs: Food Frequency Questionnaires, EPIC: European Prospective Investigation into Diet and Cancer. | ||
2.5 Study procedures
At the baseline visit, the weight, height, and body composition data of each participant were collected using standard anthropometric methods. Their demographic characteristic, smoking status, alcohol consumption, and physical activity level were collected using case report forms and the International Physical Activity Questionnaire Long Form (IPAQ-LF) to classify participants into high, moderate, or low levels of activity.40 Participants were asked to complete three dietary assessment tools, including KP-FFQ, EPIC-Norfolk FFQ, and 7DDs (usually dispensed 1 week prior to the baseline visit). The KP-FFQ was collected to capture their habitual (poly)phenol intake. The EPIC-Norfolk FFQ (version 6, CAMB/PQ/6/1205)30 was also collected at the baseline, to test the agreements with our newly developed FFQ for nutrients and (poly)phenol intake data. 7DDs were given to participants to record their daily consumption for seven consecutive days at baseline,30 one week before the first visit. There are six-time slots in each day included in each diary, including before breakfast, breakfast, mid-morning, lunch, tea, evening meal, and later evening. Participants were required to report their daily type and amount of food and beverage consumption in detail. The instructions with standard photos of portion sizes or grams were described at the beginning pages of the paper-based diaries booklet.6 Furthermore, spot urine and fasting (at least 12 h) plasma samples were also collected at baseline.2.6 Estimation of (poly)phenol intake
The EPIC-Norfolk FFQs were coded with the Microsoft Access software (Access 2019, Microsoft, USA) and transformed into daily food and nutrient intake levels by the FFQ EPIC Tool for Analysis (FETA) software.41 Nutrient composition was obtained from McCance and Widdowson's “The Composition of Foods (5th edition)” and ESI.†42 The 7DDs were coded into standard food codes and portions by trained coders using the Nutritics software (Nutritics Research Edition v 5.76, Nutritics, Dublin, Ireland). A standard protocol was followed by all coders to minimize the coding error and improve the quality and consistency of the data. The KP-FFQs were coded following a standard protocol using Excel (Microsoft, USA) software and transformed into daily food and nutrient intake levels by Excel (Microsoft, USA) and R (version 4.1.2)43 (melt() function with the “reshape2” package, version 1.44).An in-house database was established based on the online open-access Phenol-Explorer database,39 the USDA database, and several published papers44–66 to estimate the (poly)phenol content of each food item. Information regarding this database has been previously described.35 (poly)phenol intake (mg d−1) was calculated using the estimated food intake (g d−1) multiplied by the corresponding (poly)phenol intake from the in-house database (mg per 100 g) and divided by 100. The classes and subclasses of (poly)phenols were extracted by adding all individual compounds within the group.
2.7 Sample collection, processing and (poly)phenol metabolite analyses
Baseline spot urine (n = 41) and plasma samples (n = 36) were collected as part of the CRANMOOD cohort. The spot urine and plasma samples were collected, stored, processed, and analysed following a validated method described in detail previously67,68 with ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry (UHPLC-Q-q-Q MS) (TSQ Vantage, Thermo Fisher Scientific, Waltham, MA, USA). Metabolites were identified and quantified by authentic standards,21 and a total of 110 urinary and 89 plasma metabolites were selected in the statistical analysis. The TraceFinder software (TraceFinder 5.0, Thermo Fisher Scientific, Waltham, MA, USA) was employed for data analysis and calculation. The urinary creatinine levels were analysed by Affinity Biomarker Labs (London, UK) using the Jaffe method, and the concentrations of the metabolites (nM) were adjusted by the creatinine levels (mg L−1) into mmol g−1 creatinine.2.8 Statistical analysis
The statistical analysis in this research was performed using R (version 4.1.2).43 (Poly)phenol intake was reported as mean (standard deviation, SD) and median (interquartile range, IQR). The significant levels were adjusted for multiple comparisons using the false discovery rate (FDR) method, and p < 0.05 was used as the significant level.The relative validation of KP-FFQs used 7DDs, and biomarkers from spot urine and plasma samples were employed to strengthen the validity. The association and agreement between the KP-FFQs against 7DDs/biomarkers were investigated. The association was tested by linear regression with lm.beta R package (version 1.7.2) and adjusted for energy intake. To investigate the agreements in ranking participants into quartiles, weighted Kappas were calculated with the “psych” package in R. A weighted kappa value over 0.75 was considered an excellent agreement, 0.40–0.75 was considered a fair to good agreement, and lower than 0.40 was considered as a poor agreement.69 The 95% confidence intervals (CI) were calculated for kappa. The percentages of participants grouped into the same, adjacent or opposite quartiles were also calculated to show agreements between the two methods. For comparison, the association and agreement between the EPIC-Norfolk FFQs against 7DDs/biomarkers were also explored with the above methods. Weighted kappa and cross-classification were used to assess the relative validity of KP-FFQs against 7DDs on nutrient intake.
Agreements between (poly)phenol and nutrient intake estimated from the KP-FFQs and EPIC-Norfolk FFQs were presented as two-way mixed effects intraclass correlation coefficients (ICC). The consistency (ICC-C) and agreement (ICC-A) models in ICC were calculated with the “irr” package in R. The consistency model ignores the systematic difference between the two FFQs, while the agreement model compares the absolute values of the estimated intake. The ICC values lower than 0.5 were considered poor agreement, and between 0.50–0.75 were considered moderate agreement, 0.75–0.90 good agreement, and above 0.90 excellent agreement.70 In addition, the weighted Kappas were also employed, and Spearman's analysis was also adopted to test the correlation between these two tools. (Poly)phenol intake was further adjusted for energy intake by the residual method and calculated for the above values. The 95% CI was extracted for ICC, kappa, and Spearman's rho values.
3. Results
3.1 Characteristics of the study population
No demographic information was collected as part of the pilot study (n = 20). As for the demographic characteristics of the CRANMOOD and FOODMOOD studies, 235 participants were included with 36 males and 199 females (Table 1). The average age of subjects was 22.6 (SD 2.7) years, and nearly half of the population were postgraduate students (55.3). The majority of subjects were from the Asian ethnic group (66.0%), with a BMI lower than 25 kg m−2 (86.0%) and high or moderate physical activity levels (50.2% and 44.3%, respectively). Most of them reported non-smoking (88.5%) and alcohol consumption lower than five units per week (46.4%) or non-alcohol consumption (44.3%). There were 225 EPIC-Norfolk FFQs and 201 KP-FFQs collected. The collection of 7DDs was not incorporated into the design of the FOODMOOD study, therefore only 65 participants from the CRANMOOD cohort provided 7DDs.| Characteristics | Mean (SD)/n (%) | Missingness (%) |
|---|---|---|
| 7DDs: 7 day diet diaries, EPIC: European Prospective Investigation into Diet and Cancer, FFQs: food frequency questionnaires; SD: standard deviation. | ||
| Age (years) | 22.6 (2.7) | 0 |
| Ethnicity | 2.0 | |
| White | 53 (22.6) | |
| Black | 13 (5.5) | |
| Asian | 155 (66.0) | |
| Mixed | 9 (3.8) | |
| Sex | 0 | |
| Male | 36 (15.3) | |
| Female | 199 (84.7) | |
| Physical activity level | 0.9 | |
| Low | 11 (4.7) | |
| Moderate | 104 (44.3) | |
| High | 118 (50.2) | |
| Smoking status | 0.4 | |
| Never | 208 (88.5) | |
| Current & ex-smoker | 26 (11.1) | |
| Alcohol consumption | 0.4 | |
| Not drinking | 104 (44.3) | |
| ≤5 unit per week | 109 (46.4) | |
| >5 unit per week | 21 (8.9) | |
| BMI | 0.4 | |
| <25 kg m−2 | 202 (86.0) | |
| ≥25 kg m−2 | 32 (13.6) | |
| Education level | 0.9 | |
| Bachelor | 87 (37.0) | |
| Master | 130 (55.3) | |
| PhD | 16 (6.8) | |
| Dietary assessment tools | ||
| EPIC-Norfolk FFQs | 225 (95.7) | 4.3 |
| KCL (poly)phenol FFQs | 201 (85.5) | 14.5 |
| 7DDs | 65 (27.7) | 72.3 |
3.2 The completion time of the KCL (poly)phenol FFQ from the pilot study
The pilot study (n = 20) found that the average time to complete EPIC-Norfolk FFQs and KP-FFQs was 13.9 ± 6.8 and 36.9 ± 21.4 min, respectively. No adjustments to the questionnaire were made as a result of the pilot study.3.3 (Poly)phenol intake estimated from the KCL (poly)phenol FFQ
The total (poly)phenol intake from KP-FFQ stratified by baseline characteristics is listed in Table 2. The mean (SD) and median (IQR) of total (poly)phenol intake estimated from the KP-FFQ was 1366.5 (1151.7) and 1073.2 (1022.1) mg d−1, respectively in Table 3. Regarding the contribution of different (poly)phenol subclasses (Table 3), flavonoids were the main type of dietary (poly)phenols (47.1%), followed by phenolic acids (45.5%). As for food sources (Table 4), non-alcoholic beverages were the main food sources of total (poly)phenols (42.0%), followed by fruits (9.7%) (apple 5.8%, blueberries 2.4%, strawberries 1.5%), and Chocolate (5.3%) (drinking chocolate powder 3.8%, dark chocolate 1.5%). As for the individual foods, coffee contributed the most to the total (poly)phenol intake (29.4%), followed by tea (12.6%). Multiple subtypes of tea and coffee in the KP-FFQ contributed to the total (poly)phenol intake, including black tea (10.1%) (English breakfast tea (4.4%), Assam (3.2%), Earl grey (2.5%)), green tea (2.5%) in tea category and decaffeinated (10.5%), infusion (9.4%), espresso (6.2%), filtered (3.3%) in coffee category. The average coffee intake was 1.1 ± 0.4 cup per d (203.6 ± 78.6 g d−1) from KP-FFQ, whereas the average tea intake was 0.8 ± 0.2 cup per d (147.0 ± 28.6) g d−1 (standardized as 190 g per cup for both coffee and tea according to default portion size in the EPIC-Norfolk FFQ). The KP-FFQ lists 442 items, and 312 of them were not listed in the EPIC-Norfolk FFQ. The food/beverages unique to the KP-FFQ contributed 72.7% of the total (poly)phenol intake estimated from the KP-FFQs (74.6% of flavonoids, 67.7% phenolic acids, 87.5% stilbenes, 72.2% lignans, and 93.3% of the other (poly)phenols intake).| Characteristics | N | Mean (SD) | Median (IQR) | |
|---|---|---|---|---|
| FFQs: food frequency questionnaires, BMI: body mass index, IPAQ: International Physical Activity Questionnaire, IQR: interquartile range, SD: standard deviation. N: number of participants in each group. | ||||
| Sex | Men | 31 | 1340.3 (1555.2) | 944.8 (922.1) |
| Women | 170 | 1398.9 (1103.6) | 1085.8 (1073.2) | |
| Age group | 18–22 | 97 | 1532.8 (1429.0) | 1121.3 (1117.6) |
| 23–29 | 104 | 1256.5 (878.2) | 1065.3 (978.4) | |
| Ethnicity | White | 43 | 1594.2 (1384.7) | 1139.0 (1092.3) |
| Black | 11 | 1062.8 (672.0) | 842.6 (788.7) | |
| Asian | 138 | 1339.3 (1140.8) | 1057.4 (1063.9) | |
| Mixed | 9 | 1587.6 (1127.0) | 1274.4 (659.9) | |
| BMI | <25 kg m−2 | 174 | 1333.8 (1043.8) | 1071.3 (1054.7) |
| ≥25 kg m−2 | 27 | 1750.8 (1810.1) | 1137.2 (1041.6) | |
| IPAQ | Low | 10 | 938.2 (723.5) | 745.2 (575.5) |
| Moderate | 91 | 1146.3 (828.5) | 997.3 (873.9) | |
| High | 100 | 1656.6 (1413.8) | 1266.1 (1129.1) | |
| Smoking | Never | 180 | 1359.3 (1208.3) | 1048.0 (1025.1) |
| Current & Ex-smoker | 21 | 1651.5 (918.5) | 1347.7 (1335.1) | |
| Alcohol consumption | Not drinking | 96 | 1328.4 (1211.9) | 981.3 (1104.2) |
| ≤5 unit per week | 87 | 1401.8 (1193.5) | 1096.2 (898.1) | |
| >5 unit per week | 18 | 1659.6 (928.7) | 1457.2 (1478.8) | |
| Education level | Bachelor | 79 | 1531.6 (1562.4) | 1042.2 (1031.5) |
| Master | 106 | 1248.3 (767.2) | 1088.2 (1050.8) | |
| PhD | 16 | 1627.4 (1179.7) | 1213.5 (899.3) | |
| (Poly)phenols (mg d−1) | Mean (SD) | Median (IQR) | % |
|---|---|---|---|
| FFQs: food frequency questionnaires, IQR: inter-quartile range, SD: standard deviation. %: percentage of contribution to the total (poly)phenol intake. | |||
| Total (poly)phenols | 1366.5 (1151.7) | 1073.2 (1022.1) | 100.0 |
| Total flavonoids | 643.0 (517.6) | 496.3 (464.6) | 47.1 |
| Anthocyanins | 53.4 (71.5) | 37.5 (41.3) | 3.9 |
| Chalcones | 0.0 (0.0) | 0.0 (0.0) | 0.0 |
| Dihydroflavonols | 0.2 (0.4) | 0.0 (0.3) | 0.0 |
| Dihydrochalcones | 2.9 (3.7) | 1.9 (3.3) | 0.2 |
| Total flavan-3-ols | 445.0 (400.0) | 315.6 (408.2) | 32.6 |
| Flavan-3-ol monomers | 91.4 (104.6) | 56.5 (87.7) | 6.7 |
| Theaflavins | 9.8 (20.2) | 1.7 (10.1) | 0.7 |
| Thearubigins | 64.3 (132.3) | 11.2 (66.9) | 4.7 |
| Proanthocyanidins | 279.6 (228.3) | 220.3 (231.3) | 20.5 |
| Flavanones | 60.1 (77.0) | 39.1 (57.5) | 4.4 |
| Flavones | 10.0 (11.3) | 6.8 (7.3) | 0.7 |
| Flavonols | 59.9 (49.2) | 50.2 (38.0) | 4.4 |
| Isoflavonoids | 11.6 (22.6) | 4.7 (11.8) | 0.8 |
| Total phenolic acids | 622.4 (631.2) | 418.5 (632.3) | 45.5 |
| Hydroxybenzoic acids | 58.8 (69.0) | 40.4 (43.2) | 4.3 |
| Ellagitannins | 2.4 (9.8) | 0.0 (1.3) | 0.2 |
| Hydroxycinnamic acids | 563.5 (602.3) | 378.0 (575.9) | 41.2 |
| Hydroxyphenylacetic acids | 0.0 (0.1) | 0.0 (0.0) | 0.0 |
| Total stilbenes | 0.3 (0.4) | 0.2 (0.4) | 0.0 |
| Resveratrol | 0.2 (0.2) | 0.2 (0.2) | 0.0 |
| Total lignans | 4.8 (7.6) | 2.4 (3.1) | 0.4 |
| Other (poly)phenols | 96.0 (221.4) | 40.8 (60.8) | 7.0 |
| Tyrosols | 6.4 (6.1) | 5.2 (7.4) | 0.5 |
| Alkylmethoxyphenols | 2.6 (3.2) | 1.8 (2.8) | 0.2 |
| Alkylphenols | 16.0 (24.5) | 8.8 (14.8) | 1.2 |
| (Poly)phenols (mg d−1) | (Poly)phenol FFQs estimated (poly)phenol food sources (% to total) |
|---|---|
| Total (poly)phenols | Coffee (decaffeinated 10.5%, infusion 9.4%, espresso 6.2%, filtered 3.3%) (29.4%), black tea (English breakfast tea 4.4%, Assam 3.2%, Earl grey 2.5%) (10.1%), apple (red & green) (5.8%), chocolate (drinking chocolate powder 3.8%, dark chocolate 1.5%) (5.3%), cloves (3.0%), green tea (2.5%), blueberries (2.4%), white rice (2.0%), strawberries (1.5%), orange juice (1.5%) |
| Total flavonoids | Black tea (English breakfast tea 8.4%, Assam 6.1%, Earl grey 4.7%) (19.2%), apple (red & green) (11.0%), chocolate (drinking chocolate powder 7.9%, dark chocolate 3.0%) (10.9%), green tea (4.5%), citrus (orange 2.2%, mandarins 1.5%) (3.7%), blueberries (3.4%), hazelnut milk (2.9%), orange juice (2.8%), strawberries (2.6%), tomatoes (2.1%), spinach (2.0%), grapes (1.6%) |
| Anthocyanins | Blueberries (13.2%), cherries (12.4%), Cabbage (purple & red) (20.5%), aubergine (8.5%), grapes (7.2%), strawberries (7.2%), blackberries (3.9%), blackcurrants (3.0%), black beans (3.0%) |
| Chalcones | Broad beans (70.2%), Ale, beer (23.0%), regular, beer (6.8%) |
| Dihydroflavonols | Wine (red 84.6%, white 7.6%, rose 4.2%), moussaka (3.5%) |
| Dihydrochalcones | Apple (red, flesh and skin 41.0%, flesh only 16.9%, green flesh and skin 14.5%, flesh only 9.5%), apple juice (concentrate 9.6%, pure juice 7.9%), apple chutney (0.5%), pomegranate juice (0.1%) |
| Total flavan-3-ols | Black tea (English breakfast tea 11.9%, Assam 8.7%, Earl grey 6.7%) (27.3%), apple (red & green) (14.8%), drinking chocolate powder (11.4%), green tea (6.3%), chocolate (4.3%), Hazelnut milk (4.2%), blueberries (3.1%), strawberries (2.8%), almond (2.1%), hazelnuts (2.1%) |
| Flavan-3-ol monomers | Black tea (English breakfast tea 18.5%, Assam 13.6%, Earl grey 10.4%) (42.5%), green tea (27.7%), apple (red & green) (6.2%), chocolate (drinking chocolate powder 4.4%, dark chocolate 1.2%) (5.6%), broad beans (2.5%), mint tea (1.6%), grape (1.1%) |
| Theaflavins | Black tea (English breakfast tea 43.5%, Assam 32.0%, Earl grey 24.6%) (100%) |
| Thearubigins | Black tea (English breakfast tea 43.2%, Assam 31.8%, Earl grey 24.4%) (99.4%), green tea (0.6%) |
| Proanthocyanidins | Chocolate (drinking chocolate powder 16.8%, dark chocolate 6.5%) (23.3%), apple (red & green) (21.6%), hazelnut milk (6.7%), black tea (English breakfast tea 3.4%, Assam 1.0%, Earl grey 0.8%) (5.2%), blueberries (5.0%), strawberries (4.3%), grapes (green & black) (4.2%), hazelnuts (3.3%), almond (3.2%) |
| Flavanones | Citrus (orange 23.9%, mandarins 15.2%, lemons 11.1%, limes 3.3%, grapefruit 2.7%) (56.2%), citrus juice (orange juice 28.7%, grapefruits juice 1.6%, blood orange juice 1.5%, lemon juice 0.7%) (32.5%), mints (8.1%), tomatoes (cherry 1.1%, raw 0.4%, ketchup 0.1%) (1.6%) |
| Flavones | Parsley (11.0%), mint (8.7%), tortilla (wholemeal flour & wheat flour) (9.8%), orange juice (9.0%), bagel (plain & Wholemeal) (9.5%), citrus (mandarins 3.3%, lemon 2.4%, blood orange 0.8%) (6.5%), pizza (cheese and tomatoes) (4.6%), croissant (butter, chocolate, almond) (4.3%), spinach (1.8%), celery (1.6%) |
| Flavonols | Tomatoes (raw 22.0%, ketchup 6.6%, soup 4.6%, cherry 1.3%) (34.5%), spinach (21.7%), onion (red 3.2%, yellow 2.1%, white 0.7%) (6.0%), black tea (English breakfast tea 1.6%, Assam 1.2%, Earl grey 0.9%) (3.7%), broccoli (3.1%), vegetable soup (2.0%), lettuce (red & green) (2.1%), green tea (1.9%), blueberries (1.6%) |
| Isoflavonoids | Soy milk (32.2%), tofu (16.1%), edamame bean (15.8%), beansprouts (8.9%), soy based (Greek style & low-fat yoghurt) (11.2%), tempeh (6.6%), soya mince (4.2%) |
| Total phenolic acids | Coffee (infusion 20.5%, decaffeinated, espresso based 16.3%, espresso 13.5%, filtered 7.2%, decaffeinated, instant or ground 6.5%, decaffeinated, filtered 3.5%) (67.5%), white & brown rice (5.1%), black tea (English breakfast tea 1.0%, Assam 0.8%, Earl grey 0.6%) (2.4%), chestnut (2.1%), blueberries (1.8%) |
| Hydroxybenzoic acids | Chestnuts (22.6%), black tea (English breakfast tea 9.4%, Assam 6.9%, Earl grey 5.3%) (21.6%), green tea (6.5%), strawberries (6.7%), garlic (6.4%), white rice (3.8%), pomegranate juice (concentrate 3.5% and pure juice 1.4%) (4.9%), clove (2.1%) |
| Ellagitannins | Pomegranate juice (72.1%), low fat yoghurt, raspberry (18.6%), full fat yoghurt, raspberry (5.5%), yoghurt drinks, raspberry (3.9%) |
| Hydroxycinnamic acids | Coffee (infusion 22.6%, decaffeinated, espresso based 18.0%, espresso 14.9%, filtered 8.0%, decaffeinated, instant or ground 7.2%, decaffeinated, filtered 3.9%) (74.6%), white & brown rice (5.3%), apple (red & green) (2.0%), blueberries (1.9%) |
| Hydroxyphenylacetic acids | Regular beer (49.3%), wine (red 20.4%, white 10.5%) (30.9%), olive oil (extra virgin 9.6%, virgin 4.2%, refined 3.0%) (16.8%) |
| Total stilbenes | Wine (red 35.5%, white 5.2%, rose 2.4%) (43.1%), citrus (mandarins 16.8%, lemon 12.4%) (29.2%), strawberries (9.1%), grape (black 4.5%, green 4.1%) (8.6%), low fat (mixed berries 1.0%, peach 0.5%, mango 0.4%) (1.9%), moussaka (1.5%) |
| Resveratrol | Citrus (mandarins 24.7%, lemon 18.1%) (42.8%), wine (red 12.0%, rose 3.2%, white 3.1%) (18.3%), strawberries (13.5%), grape (black 6.4%, green 6.1%) (12.5%), low fat (mixed berries 1.5%, peach 0.7%, mango 0.6%) (2.8%), redcurrant (1.6%) |
| Total lignans | Flaxseed (33.8%), sesame seeds (13.3%), bread, seeded (10.1%), potatoes (boiled 9.0%, roast 4.1%, crisps 1.7%) (14.8%), broccoli (5.0%) |
| Other (poly)phenols | Cloves (41.4%), turmeric (20.6%), olive oil (extra virgin 3.1%, virgin 1.4%, refined 1.0%) (5.5%), break cereal (5.0%), star anise (4.8%), bread (wholemeal 1.9%, rye 1.6%, pitta 0.8%) (4.3%), coffee (decaffeinated 1.4%, infusion 1.1%, espresso 0.7%, filtered 0.4%) (3.6%), spaghetti (wholemeal & white) (3.2%), curry powder (2.5%) |
| Tyrosols | Olive oil (extra virgin 47.2%, virgin 20.5%, refined 14.8%) (82.5%), pesto (green & red) (6.8%), red wine (2.4%), pizza, pesto (2.2%) |
| Alkylmethoxyphenols | Coffee (decaffeinated, espresso based 27.0%, infusion 17.2%, espresso 11.3%, filtered 6.1%, decaffeinated, instant or ground 10.7%, decaffeinated, filtered 5.9%) (78.2%), rapeseed oil (18.3%) |
| Alkylphenols | Breakfast cereal (30.0%), bread (wholemeal 11.6%, rye 9.3%, pitta 4.9%) (25.8%), spaghetti (wholemeal & white) (19.2%), tortilla (wholemeal & wheat flour) (8.9%), bagel (wholemeal & plain) (5.7%), pizza (pesto 0.8%, cheese and tomato 0.6%, vegetarian 0.2%) (1.6%) |
3.4 Relative validity with 7DDs estimated (poly)phenol and nutrients intake
The agreements between KP-FFQs and 7DDs in ranking participants in quartiles of (poly)phenol levels are shown in Table 5. Fair agreement was found in ten (poly)phenol groups, including total (poly)phenol intake (kappa: 0.45, 95% CI: 0.25–0.66), chalcones (kappa: 0.41, 95% CI: 0.18–0.65), isoflavonoids (kappa: 0.48, 95% CI: 0.29–0.67), total phenolic acids (kappa: 0.73, 95% CI: 0.64–0.83), hydroxycinnamic acids (kappa: 0.73, 95% CI: 0.64–0.83), hydroxyphenylacetic acids (kappa: 0.52, 95% CI: 0.31–0.73), resveratrol (kappa: 0.47, 95% CI: 0.26–0.67), alkymethoxyphenols (kappa: 0.45, 95% CI: 0.24–0.66), and tyrosols (kappa: 0.49, 95% CI: 0.29–0.70). The agreements of the rest (poly)phenols were poor.
| (Poly)phenols | Kappa | (95% CI) | Same quartile (%) | Adjacent quartile (%) | Correctly classifieda (%) | Opposite quartile (%) |
|---|---|---|---|---|---|---|
| a Correctly classified (%): correctly classified the (poly)phenols into the same or adjacent quartiles (%). FFQs: food frequency questionnaires, 7DDs: 7 day diet diaries, kappa: weighted kappa coefficient (linear weights). CI: confidence interval. | ||||||
| Total (poly)phenols | 0.45 | (0.25, 0.66) | 33.33 | 51.67 | 85.00 | 5.00 |
| Total flavonoids | 0.08 | (−0.19, 0.35) | 36.67 | 30.00 | 66.67 | 13.33 |
| Anthocyanins | 0.16 | (−0.08, 0.40) | 21.67 | 48.33 | 70.00 | 8.33 |
| Chalcones | 0.41 | (0.18, 0.65) | 46.67 | 33.33 | 80.00 | 6.67 |
| Dihydroflavonols | 0.33 | (0.06, 0.60) | 48.33 | 30.00 | 78.33 | 10.00 |
| Dihydrochalcones | 0.31 | (0.10, 0.52) | 28.33 | 43.33 | 71.66 | 3.33 |
| Total flavan-3-ols | 0.09 | (−0.15, 0.34) | 25.00 | 38.33 | 63.33 | 8.33 |
| Flavan-3-ol monomers | 0.24 | (0.01, 0.47) | 25.00 | 45.00 | 70.00 | 5.00 |
| Theaflavins | 0.27 | (0.00, 0.54) | 41.67 | 33.33 | 75.00 | 10.00 |
| Thearubigins | 0.24 | (−0.03, 0.51) | 41.67 | 36.67 | 78.34 | 13.33 |
| Proanthocyanidins | 0.09 | (−0.16, 0.34) | 23.33 | 43.33 | 66.66 | 10.00 |
| Flavanones | 0.19 | (−0.06, 0.43) | 30.00 | 36.67 | 66.67 | 6.67 |
| Flavones | 0.11 | (−0.15, 0.36) | 30.00 | 38.33 | 68.33 | 11.67 |
| Flavonols | 0.31 | (0.07, 0.54) | 30.00 | 46.67 | 76.67 | 6.67 |
| Isoflavonoids | 0.48 | (0.29, 0.67) | 43.33 | 35.00 | 78.33 | 1.67 |
| Total phenolic acids | 0.73 | (0.64, 0.83) | 43.33 | 53.33 | 96.66 | 0.00 |
| Hydroxybenzoic acids | 0.16 | (−0.08, 0.40) | 28.33 | 36.67 | 65.00 | 6.67 |
| Ellagitannins | 0.41 | (0.17, 0.66) | 43.33 | 43.33 | 86.66 | 10.00 |
| Hydroxycinnamic acids | 0.73 | (0.64, 0.83) | 43.33 | 53.33 | 96.66 | 0.00 |
| Hydroxyphenylacetic acids | 0.52 | (0.31, 0.73) | 51.67 | 30.00 | 81.67 | 3.33 |
| Total stilbenes | 0.39 | (0.17, 0.60) | 38.33 | 36.67 | 75.00 | 3.33 |
| Resveratrol | 0.47 | (0.26, 0.67) | 50.00 | 25.00 | 75.00 | 1.67 |
| Total lignans | 0.37 | (0.19, 0.56) | 26.67 | 48.33 | 75.00 | 1.67 |
| Other (poly)phenols | 0.37 | (0.16, 0.59) | 35.00 | 40.00 | 75.00 | 3.33 |
| Tyrosols | 0.49 | (0.29, 0.70) | 38.33 | 48.33 | 86.66 | 5.00 |
| Alkylmethoxyphenols | 0.45 | (0.24, 0.66) | 38.33 | 45.00 | 83.33 | 5.00 |
| Alkylphenols | 0.37 | (0.16, 0.59) | 33.33 | 45.00 | 78.33 | 5.00 |
| Nutrients | Kappa | (95% CI) | Same quartile (%) | Adjacent quartile (%) | Correctly classifieda (%) | Opposite quartile (%) |
|---|---|---|---|---|---|---|
| a Correctly classified (%): correctly classified the (poly)phenols into the same or adjacent quartiles (%). FFQs: food frequency questionnaires, 7DDs: 7 day diet diaries, kappa: weighted kappa coefficient (linear weights). CI: confidence interval. | ||||||
| Energy (kcal) | −0.01 | (−0.27, 0.24) | 20.00 | 41.67 | 61.67 | 11.67 |
| Fibre (g d−1) | 0.40 | (0.19, 0.61) | 36.67 | 40.00 | 76.67 | 3.33 |
| Calcium (mg d−1) | 0.13 | (−0.08, 0.35) | 20.00 | 40.00 | 60.00 | 3.33 |
| Iron (mg d−1) | 0.27 | (0.03, 0.5) | 30.00 | 43.33 | 73.33 | 6.67 |
| Potassium (mg d−1) | 0.19 | (−0.04, 0.42) | 23.33 | 48.33 | 71.66 | 8.33 |
| Retinol (μg d−1) | 0.16 | (−0.06, 0.38) | 20.00 | 45.00 | 65.00 | 5.00 |
| Carotene (μg d−1) | 0.28 | (0.03, 0.53) | 41.67 | 31.67 | 73.34 | 8.33 |
| Vitamin C (mg d−1) | 0.23 | (0.01, 0.44) | 21.67 | 48.33 | 70.00 | 5.00 |
| Fat (g d−1) | 0.08 | (−0.15, 0.31) | 23.33 | 36.67 | 60.00 | 6.67 |
| Cholesterol (mg d−1) | −0.12 | (−0.37, 0.13) | 20.00 | 38.33 | 58.33 | 15.00 |
| MUFA (g d−1) | 0.11 | (−0.15, 0.36) | 26.67 | 40.00 | 66.67 | 10.00 |
| PUFA (g d−1) | 0.09 | (−0.14, 0.33) | 18.33 | 50.00 | 68.33 | 10.00 |
| SFA (g d−1) | 0.12 | (−0.10, 0.34) | 20.00 | 41.67 | 61.67 | 5.00 |
| Protein (g d−1) | −0.01 | (−0.28, 0.25) | 33.33 | 26.67 | 60.00 | 13.33 |
| Total carbohydrate (g d−1) | 0.07 | (−0.16, 0.29) | 16.67 | 41.67 | 58.34 | 5.00 |
| Sugars (g d−1) | 0.16 | (−0.06, 0.38) | 21.67 | 40.00 | 61.67 | 3.33 |
| Fructose (g d−1) | −0.01 | (−0.27, 0.24) | 26.67 | 30.00 | 56.67 | 10.00 |
| Galactose (g d−1) | 0.24 | (0.00, 0.48) | 26.67 | 48.33 | 75.00 | 8.33 |
| Glucose (g d−1) | 0.05 | (−0.20, 0.30) | 23.33 | 40.00 | 63.33 | 10.00 |
| Starch (g d−1) | 0.29 | (0.05, 0.54) | 30.00 | 48.33 | 78.33 | 8.33 |
| Sucrose (g d−1) | 0.16 | (−0.06, 0.38) | 25.00 | 38.33 | 63.33 | 5.00 |
| Lactose (g d−1) | 0.29 | (0.06, 0.53) | 31.67 | 43.33 | 75.00 | 6.67 |
| Maltose (g d−1) | 0.32 | (0.09, 0.55) | 35.00 | 38.33 | 73.33 | 5.00 |
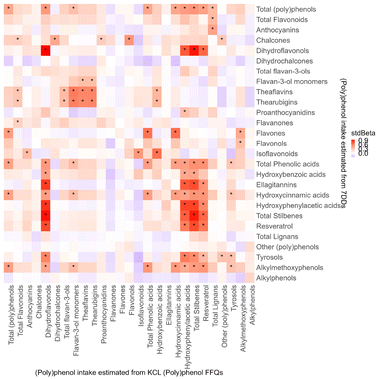
3.5 Validity against objective (poly)phenol metabolites from urine and plasma
Fig. 5 shows the association between (poly)phenol intakes estimated from KP-FFQs and objective (poly)phenol metabolites from urine (Fig. 5a) and plasma (Fig. 5b). (Poly)phenol intake from KP-FFQs showed positive associations with 76 subgroup and individual urinary metabolites with standard beta ranging from 0.28 (95% CI: 0.07–0.49) between urinary 3-methoxybenzoic acid-4-sulfate and total flavonoids intake to 0.81 (95% CI: 0.62–1.00) between urinary (−)-epicatechin-3′-sulfate and ellagitannins intake (all FDR adjusted p values < 0.05) (Fig. 5a). As for plasma metabolites, KP-FFQs estimated intake showed positive associations with 19 subgroup and individual metabolites with standard beta ranging from 0.40 (95% CI: 0.17–0.62, between 3-hydroxybenzoic acid and flavones intake) to 0.83 (95% CI: 0.64–1.02, between (−)-epicatechin and total flavan-3-ols from plasma and ellagitannins intake estimated from FFQs) (all FDR adjusted p values < 0.05) (Fig. 5b).The agreements between dietary assessment and metabolites in ranking participants in quartiles are shown in Table 7. Poor agreements were seen for all groups of (poly)phenols, including total (poly)phenols, total flavonoids, total lignans, total stilbenes, and total other (poly)phenols between biomarkers in urine and plasma samples and KP-FFQ (kappa < 0.40). Regarding the agreement between total (poly)phenol metabolite levels and the total estimated (poly)phenol intake, metabolites from plasma (kappa = 0.29) showed a slightly better agreement than urinary metabolites (kappa = 0.16) with KP-FFQ, although all these agreements were poor.
| Metabolite levels | Groups | Kappa | (95% CI) | Same quartile (%) | Adjacent quartile (%) | Correctly classified a (%) | Opposite quartile (%) |
|---|---|---|---|---|---|---|---|
| a Correctly classified (%): correctly classified the (poly)phenols into the same or adjacent quartiles (%). FFQs: food frequency questionnaires, kappa: weighted kappa coefficient (linear weights). CI: confidence interval. | |||||||
| Urine sample | Total (poly)phenols | 0.16 | (−0.14, 0.46) | 26.83 | 46.34 | 73.17 | 12.20 |
| Total flavonoids | 0.06 | (−0.25, 0.37) | 29.27 | 39.02 | 68.29 | 14.63 | |
| Total dihydrochalcones | 0.12 | (−0.20, 0.44) | 31.71 | 36.59 | 68.29 | 12.20 | |
| Total flavan-3-ols | −0.21 | (−0.51, 0.10) | 19.51 | 41.46 | 60.98 | 21.95 | |
| Total flavanones | −0.02 | (−0.33, 0.30) | 31.71 | 29.27 | 60.98 | 14.63 | |
| Total flavonols | 0.04 | (−0.26, 0.34) | 19.51 | 46.34 | 65.85 | 12.20 | |
| Total isoflavonoids | −0.05 | (−0.38, 0.27) | 34.15 | 26.83 | 60.98 | 17.07 | |
| Total phenolic acids | 0.23 | (−0.04, 0.51) | 29.27 | 41.46 | 70.73 | 7.32 | |
| Total hydroxybenzoic acids | −0.03 | (−0.34, 0.27) | 26.83 | 34.15 | 60.98 | 14.63 | |
| Total cinnamic acids | 0.06 | (−0.26, 0.38) | 26.83 | 46.34 | 73.17 | 17.07 | |
| Total phenylacetic acids | −0.11 | (−0.42, 0.20) | 31.71 | 29.27 | 60.98 | 19.51 | |
| Total stilbenes | 0.08 | (−0.24, 0.40) | 34.15 | 34.15 | 68.29 | 14.63 | |
| Total lignans | 0.04 | (−0.24, 0.32) | 24.39 | 31.71 | 56.10 | 7.32 | |
| Total other (poly)phenol | −0.17 | (−0.46, 0.12) | 19.51 | 36.59 | 56.10 | 17.07 | |
| Total tyrosols | −0.25 | (−0.54, 0.05) | 19.51 | 34.15 | 53.66 | 19.51 | |
| Plasma sample | Total (poly)phenols | 0.29 | (0.01, 0.57) | 27.78 | 41.67 | 69.44 | 2.78 |
| Total flavonoids | −0.13 | (−0.44, 0.18) | 19.44 | 36.11 | 55.56 | 13.89 | |
| Total dihydrochalcones | −0.09 | (−0.37, 0.19) | 5.56 | 58.33 | 63.89 | 13.89 | |
| Total flavan-3-ols | 0.22 | (−0.10, 0.54) | 30.56 | 41.67 | 72.22 | 8.33 | |
| Total flavanones | −0.16 | (−0.50, 0.19) | 25.00 | 36.11 | 61.11 | 19.44 | |
| Total flavonols | 0.07 | (−0.26, 0.39) | 30.56 | 33.33 | 63.89 | 11.11 | |
| Total isoflavonoids | −0.13 | (−0.46, 0.19) | 19.44 | 36.11 | 55.56 | 13.89 | |
| Total phenolic acids | 0.22 | (−0.10, 0.54) | 38.89 | 30.56 | 69.44 | 8.33 | |
| Total hydroxybenzoic acids | −0.18 | (−0.51, 0.15) | 30.56 | 22.22 | 52.78 | 16.67 | |
| Total cinnamic acids | 0.04 | (−0.29, 0.38) | 22.22 | 47.22 | 69.44 | 13.89 | |
| Total phenylacetic acids | −0.09 | (−0.4, 0.22) | 22.22 | 36.11 | 58.33 | 13.89 | |
| Total stilbenes | 0.24 | (−0.07, 0.56) | 27.78 | 47.22 | 75.00 | 8.33 | |
| Total lignans | −0.07 | (−0.38, 0.25) | 11.11 | 52.78 | 63.89 | 13.89 | |
| Total other (poly)phenol | −0.02 | (−0.36, 0.32) | 13.89 | 52.78 | 66.67 | 13.89 | |
| Total tyrosols | −0.16 | (−0.47, 0.16) | 27.78 | 27.78 | 55.56 | 16.67 | |
3.6 Comparison between KCL (poly)phenol FFQs and EPIC-Norfolk FFQs
There was moderate reliability between EPIC-Norfolk and KP-FFQs estimated total (poly)phenol in absolute values (ICC-A: 0.54, 95% CI: 0.33–0.68). As for classes and subclass intakes, strong agreement was found between flavonols (ICC-A:0.77, 95%CI: 0.63, 0.84), and moderate agreements were found between total flavonoids, dihydrochalcones, flavan-3-ols, flavan-3-ol monomers, theaflavins, thearubigins, isoflavonoids, total phenolic acids, hydroxycinnamic acids, and hydroxyphenylacetic acids with ICC-A ranging from 0.55 (isoflavonoids 95% CI: 0.41–0.66) to 0.69 (hydroxyphenylacetic acids 0.56–0.78) (Table 8). In the ability to rank participants according to (poly)phenol intake levels, the reliabilities between flavonols exhibited high reliability (ICC-C: 0.79, 95% CI: 0.73–0.84). Moderate reliability was exhibited in total (poly)phenols, total flavonoids, dihydrochalcones, flavan-3-ols, flavan-3-ol monomers, theaflavins, thearubigins, proanthocyanidins, isoflavonoids, total phenolic acids, hydroxycinnamic acids and hydroxyphenylacetic acids with ICC-C ranging from 0.53 (proanthocyanidins, 95% CI: 0.39–0.64) to 0.71 (hydroxyphenylacetic acids, 0.62–0.78). When sorting participants into quartiles by intake, a poor agreement between the FFQs was exhibited, including anthocyanins, chalcones, flavanones, flavones, ellagitannins, other (poly)phenol, tyrosols, and alkylphenols with kappa ranging from 0.04 (ellagitannins, −0.09, 0.17) to 0.39 (anthocyanins and chalcones, 0.27–0.51), whereas the agreements between the total (poly)phenol (kappa: 0.54, 0.44–0.64) and all other classes and subclasses were fair (kappa: from 0.42 (resveratrol, 0.30–0.54) to 0.65 (hydroxycinnamic acids, 0.57–0.73)) (Table 8). After adjusting for energy intake, all the agreements were increased to moderate for ICC-A and ICC-C among each group of (poly)phenols (ESI Table 4†).
| (Poly)phenols (mg d−1) | ICC-A | (95% CI) | ICC-C | (95% CI) | Kappa | (95% CI) | Same quartile (%) | Adjacent quartile (%) | Correctly classifieda (%) | Opposite quartile (%) | Spearman's Rho |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Correctly classified (%): correctly classified the (poly)phenols into the same or adjacent quartiles (%). ICC-C: intraclass correlation coefficient-consistency model: when the systematic difference between EPIC-Norfolk FFQ and KCL (poly)phenol FFQ estimated (poly)phenol intakes were not relevant. ICC-A: intraclass correlation coefficient-agreement model: when the systematic difference between EPIC-Norfolk FFQ and KCL (poly)phenol FFQ estimated (poly)phenol intakes were relevant. EPIC: European Prospective Investigation into Diet and Cancer, FFQ: food frequency questionnaire, kappa: weighted kappa coefficient (linear weights). CI: confidence interval. a p < 0.001. b p > 0.05. | |||||||||||
| Total (poly)phenols | 0.54 | (0.33, 0.68) | 0.58 | (0.45, 0.68) | 0.54 | (0.44, 0.64) | 43.13 | 42.65 | 85.78 | 2.84 | 0.60 a |
| Total flavonoids | 0.58 | (0.29, 0.73) | 0.64 | (0.52, 0.72) | 0.53 | (0.43, 0.64) | 44.55 | 39.81 | 84.36 | 2.84 | 0.56 a |
| Anthocyanins | 0.09 | (−0.14, 0.29) | 0.13 | (−0.14, 0.33) | 0.39 | (0.27, 0.51) | 37.44 | 41.23 | 78.67 | 5.21 | 0.44 a |
| Chalcones | 0.39 | (0.20, 0.54) | 0.39 | (0.20, 0.53) | 0.39 | (0.27, 0.50) | 27.96 | 55.45 | 83.41 | 6.16 | 0.38 a |
| Dihydroflavonols | 0.10 | (−0.15, 0.30) | 0.11 | (−0.16, 0.32) | 0.56 | (0.45, 0.67) | 53.55 | 30.81 | 84.36 | 3.32 | 0.56 a |
| Dihydrochalcones | 0.63 | (0.51, 0.72) | 0.64 | (0.53, 0.73) | 0.52 | (0.41, 0.63) | 48.82 | 35.07 | 83.89 | 3.79 | 0.57 a |
| Total flavan-3-ols | 0.62 | (0.44, 0.73) | 0.65 | (0.54, 0.73) | 0.55 | (0.45, 0.66) | 48.34 | 37.44 | 85.78 | 3.32 | 0.56 a |
| Flavan-3-ol onomers | 0.68 | (0.58, 0.76) | 0.69 | (0.60, 0.77) | 0.56 | (0.46, 0.66) | 43.60 | 44.08 | 87.68 | 3.32 | 0.63 a |
| Theaflavins | 0.61 | (0.49, 0.71) | 0.62 | (0.50, 0.71) | 0.49 | (0.38, 0.60) | 41.71 | 41.71 | 83.41 | 3.79 | 0.52 a |
| Thearubigins | 0.61 | (0.49, 0.71) | 0.62 | (0.50, 0.71) | 0.56 | (0.46, 0.67) | 47.39 | 39.34 | 86.73 | 3.32 | 0.57 a |
| Proanthocyanidins | 0.42 | (−0.09, 0.66) | 0.53 | (0.39, 0.64) | 0.47 | (0.36, 0.58) | 44.55 | 35.55 | 80.09 | 3.32 | 0.54 a |
| Flavanones | 0.23 | (−0.01, 0.41) | 0.25 | (0.02, 0.43) | 0.38 | (0.26, 0.50) | 40.28 | 35.55 | 75.83 | 4.27 | 0.41 a |
| Flavones | 0.14 | (−0.11, 0.34) | 0.17 | (−0.08, 0.37) | 0.35 | (0.24, 0.47) | 34.12 | 41.23 | 75.36 | 4.27 | 0.35 a |
| Flavonols | 0.77 | (0.63, 0.84) | 0.79 | (0.73, 0.84) | 0.59 | (0.49, 0.68) | 46.45 | 39.34 | 85.78 | 1.42 | 0.68 a |
| Isoflavonoids | 0.55 | (0.41, 0.66) | 0.55 | (0.41, 0.66) | 0.55 | (0.45, 0.65) | 48.34 | 33.65 | 81.99 | 1.42 | 0.57 a |
| Total phenolic acids | 0.59 | (0.46, 0.68) | 0.59 | (0.46, 0.69) | 0.64 | (0.55, 0.73) | 50.71 | 38.86 | 89.57 | 1.90 | 0.67 a |
| Hydroxybenzoic acids | 0.42 | (0.22, 0.57) | 0.44 | (0.27, 0.57) | 0.49 | (0.38, 0.60) | 43.60 | 38.86 | 82.46 | 3.79 | 0.49 a |
| Ellagitannins | 0.38 | (0.19, 0.53) | 0.38 | (0.19, 0.53) | 0.04 | (−0.09, 0.17) | 22.75 | 41.23 | 63.98 | 10.90 | 0.03 b |
| Hydroxycinnamic acids | 0.60 | (0.47, 0.69) | 0.60 | (0.48, 0.69) | 0.65 | (0.57, 0.73) | 49.76 | 40.28 | 90.05 | 1.42 | 0.68 a |
| Hydroxyphenylacetic acids | 0.69 | (0.56, 0.78) | 0.71 | (0.62, 0.78) | 0.54 | (0.43, 0.64) | 46.45 | 38.39 | 84.83 | 3.32 | 0.57 a |
| Total stilbenes | 0.16 | (−0.12, 0.36) | 0.20 | (−0.05, 0.39) | 0.48 | (0.37, 0.59) | 41.23 | 42.18 | 83.41 | 4.27 | 0.49 a |
| Resveratrol | 0.25 | (−0.09, 0.47) | 0.32 | (0.10, 0.48) | 0.42 | (0.30, 0.54) | 41.23 | 37.91 | 79.15 | 4.74 | 0.44 a |
| Total lignans | 0.08 | (−0.17, 0.28) | 0.09 | (−0.20, 0.30) | 0.44 | (0.33, 0.55) | 34.60 | 48.34 | 82.94 | 4.74 | 0.49 a |
| Other (poly)phenols | 0.03 | (−0.23, 0.24) | 0.03 | (−0.27, 0.26) | 0.27 | (0.15, 0.39) | 31.75 | 39.34 | 71.09 | 5.21 | 0.29 a |
| Tyrosols | 0.04 | (−0.13, 0.21) | 0.09 | (−0.20, 0.30) | 0.30 | (0.17, 0.42) | 32.70 | 40.76 | 73.46 | 5.69 | 0.31 a |
| Alkylmethoxyphenols | 0.45 | (0.25, 0.59) | 0.47 | (0.31, 0.60) | 0.54 | (0.44, 0.65) | 45.02 | 41.71 | 86.73 | 3.79 | 0.57 a |
| Alkylphenols | 0.19 | (−0.06, 0.38) | 0.19 | (−0.06, 0.38) | 0.37 | (0.25, 0.50) | 40.76 | 37.91 | 78.67 | 6.64 | 0.35 a |
Regarding the agreements in differentiating participants into quartiles in both specimens, there were 10 out of 15 groups of (poly)phenol intake estimated from EPIC-Norfolk FFQ, which exhibited a lower correctly classified percentage (ranking subjects into the same or adjacent quartile) than the KP-FFQ estimated (poly)phenol intake groups (ESI Table 6†).
| Nutrients | ICC-A | (95% CI) | ICC-C | (95% CI) | Kappa | (95% CI) | Same quartile (%) | Adjacent quartile (%) | Correctly classifieda (%) | Opposite quartile (%) | Spearman's Rho |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Correctly classified (%): correctly classified the (poly)phenols into the same or adjacent quartiles (%). ICC-C: intraclass correlation coefficient-consistency model: when the systematic difference between EPIC and KCL (poly)phenol FFQs estimated (poly)phenol intakes was not relevant. ICC-A: intraclass correlation coefficient-agreement model: when the systematic difference between EPIC-Norfolk and KCL (poly)phenol FFQs estimated (poly)phenol intakes were relevant. EPIC: European Prospective Investigation into Diet and Cancer, FFQs: food frequency questionnaires, kappa: weighted kappa coefficient (linear weights). CI: confidence interval. a p < 0.001. | |||||||||||
| Energy (kcal) | 0.59 | (0.23, 0.76) | 0.66 | (0.27, 0.74) | 0.55 | (0.45, 0.65) | 44.81 | 40.09 | 84.90 | 2.36 | 0.61 a |
| Fibre (g d−1) | 0.60 | (0.21, 0.77) | 0.68 | (0.41, 0.75) | 0.69 | (0.62, 0.77) | 53.77 | 37.74 | 91.51 | 0.94 | 0.72 a |
| Calcium (mg d−1) | 0.54 | (0.40, 0.65) | 0.54 | (0.16, 0.65) | 0.38 | (0.26, 0.50) | 39.15 | 38.21 | 77.36 | 5.19 | 0.43 a |
| Iron (mg d−1) | 0.52 | (0.11, 0.72) | 0.61 | (0.29, 0.70) | 0.62 | (0.53, 0.71) | 45.75 | 42.92 | 88.67 | 1.42 | 0.65 a |
| Potassium (mg d−1) | 0.67 | (0.50, 0.78) | 0.71 | (0.36, 0.78) | 0.60 | (0.50, 0.70) | 51.42 | 35.38 | 86.80 | 2.36 | 0.64 a |
| Retinol (μg d−1) | 0.27 | (0.04, 0.44) | 0.27 | (−0.09, 0.44) | 0.39 | (0.27, 0.51) | 40.57 | 37.26 | 77.83 | 5.19 | 0.44 a |
| Carotene (μg d−1) | 0.67 | (0.48, 0.77) | 0.70 | (0.45, 0.77) | 0.69 | (0.62, 0.77) | 49.53 | 43.40 | 92.93 | 0.94 | 0.76 a |
| Vitamin C (mg d−1) | 0.47 | (0.07, 0.67) | 0.55 | (0.29, 0.66) | 0.56 | (0.46, 0.66) | 46.23 | 39.15 | 85.38 | 2.36 | 0.62 a |
| Fat (g d−1) | 0.55 | (0.09, 0.74) | 0.64 | (0.21, 0.73) | 0.54 | (0.44, 0.64) | 43.87 | 39.62 | 83.49 | 1.89 | 0.56 a |
| Cholesterol (mg d−1) | 0.36 | (−0.25, 0.65) | 0.52 | (0.18, 0.63) | 0.48 | (0.37, 0.59) | 41.98 | 39.15 | 81.13 | 3.30 | 0.53 a |
| MUFA (g d−1) | 0.58 | (0.31, 0.72) | 0.63 | (0.22, 0.72) | 0.52 | (0.42, 0.62) | 41.51 | 40.09 | 81.60 | 1.42 | 0.54 a |
| PUFA (g d−1) | 0.55 | (0.26, 0.70) | 0.60 | (0.29, 0.70) | 0.49 | (0.38, 0.60) | 42.92 | 41.51 | 84.43 | 4.72 | 0.49 a |
| SFA (g d−1) | 0.66 | (0.53, 0.75) | 0.68 | (0.23, 0.76) | 0.52 | (0.42, 0.63) | 41.98 | 42.45 | 84.43 | 2.83 | 0.60 a |
| Protein (g d−1) | 0.67 | (0.54, 0.76) | 0.69 | (0.34, 0.77) | 0.58 | (0.49, 0.68) | 47.17 | 38.21 | 85.38 | 1.42 | 0.65 a |
| Carbohydrate (g d−1) | 0.64 | (0.45, 0.76) | 0.68 | (0.37, 0.76) | 0.58 | (0.49, 0.68) | 48.58 | 36.79 | 85.37 | 1.89 | 0.62 a |
| Sugars (g d−1) | 0.69 | (0.52, 0.79) | 0.72 | (0.40, 0.79) | 0.59 | (0.50, 0.68) | 45.28 | 41.04 | 86.32 | 1.42 | 0.64 a |
| Fructose (g d−1) | 0.63 | (0.33, 0.77) | 0.69 | (0.41, 0.76) | 0.62 | (0.53, 0.70) | 45.28 | 42.45 | 87.73 | 0.94 | 0.67 a |
| Galactose (g d−1) | 0.21 | (−0.02, 0.4) | 0.22 | (−0.05, 0.41) | 0.38 | (0.26, 0.51) | 42.45 | 34.91 | 77.36 | 5.66 | 0.41 a |
| Glucose (g d−1) | 0.72 | (0.53, 0.82) | 0.76 | (0.51, 0.82) | 0.62 | (0.53, 0.71) | 46.70 | 42.45 | 89.15 | 1.89 | 0.69 a |
| Starch (g d−1) | 0.59 | (0.41, 0.71) | 0.62 | (0.36, 0.71) | 0.51 | (0.40, 0.62) | 49.06 | 32.55 | 81.61 | 3.30 | 0.56 a |
| Sucrose (g d−1) | 0.74 | (0.67, 0.81) | 0.74 | (0.44, 0.80) | 0.63 | (0.54, 0.72) | 48.58 | 40.09 | 88.67 | 1.42 | 0.68 a |
| Lactose (g d−1) | 0.28 | (−0.18, 0.54) | 0.39 | (0.13, 0.54) | 0.36 | (0.24, 0.48) | 35.38 | 42.92 | 78.30 | 6.13 | 0.37 a |
| Maltose (g d−1) | 0.15 | (−0.09, 0.35) | 0.18 | (−0.09, 0.37) | 0.32 | (0.20, 0.45) | 34.43 | 42.92 | 77.35 | 7.08 | 0.34 a |
4. Discussion
This is the first study that has developed and validated a FFQ to capture (poly)phenol intake in a UK population. A comprehensive and (poly)phenol-focused food list was established based on the EPIC-Norfolk FFQ and was expanded to 442 items. Non-(poly)phenol items were included to retain the structure of the EPIC-Norfolk FFQ, thus allowing for the estimation of overall nutrient intake as well. The KP-FFQ was evaluated against 7DDs as the dietary referent assessment, and objective (poly)phenol biomarkers from urine and plasma samples. Moderate agreements were shown in 10 groups of (poly)phenols estimated from KP-FFQs and 7DDs, and strong associations were found in 10 (poly)phenols estimated from 7DDs. A moderate agreement was found in fibre between KP-FFQs and 7DDs. As for biomarkers, KP-FFQs estimated (poly)phenol intake exhibited positive associations with 78 urinary metabolites and 19 plasma metabolites. Total (poly)phenol, total phenolic acids, and cinnamic acids from the urine sample and flavan-3-ols and stilbenes from the plasma sample exhibited more than 70% correct classification in the same or adjacent quartiles. Although moderate agreements were shown in all (poly)phenol intake levels from KP-FFQs and the EPIC-Norfolk FFQs after adjusting for energy intake, stronger and more agreements and associations were found in KP-FFQs estimated (poly)phenol with 7DDs and biomarkers than the (poly)phenol intake from EPIC-Norfolk FFQs.In this study, the total (poly)phenol intake levels estimated from KP-FFQs were higher than those derived from EPIC-Norfolk FFQs (1366.5 mg d−1vs. 962.1 mg d−1). The results from KP-FFQs were similar to the intakes reported by the EPIC-calibration study using 24 h recalls (around 1600 and 1750 mg d−1 for women and men, respectively),15 despite the differences between the databases in the EPIC and our study,15 for instance, the (poly)phenols were in the form of glycosides in the EPIC study rather than aglycone equivalents in the current study. Data from the UK NDNS (National Diet and Nutrition Survey) (2008 to 2014) shows that total (poly)phenol intake was around 600 mg d−1 from 4 day food diaries in a similar age group (19–34 years old) as in our study,38 which was lower than the intake from the KP-FFQ but similar to the intake from EPIC-Norfolk FFQ in our study. However, the result reported from the NDNS might be lower than the actual intake since it only used Phenol-Explorer as the data source and did not include lignans and other (poly)phenols in the estimation of total (poly)phenol intake.15 In the EPIC-Norfolk FFQ, phenolic acids were identified as the primary contributors (53.6%) to total (poly)phenol intake rather than flavonoid intake (44.6%) in accordance with our prior research estimated by EPIC-Norfolk FFQ.6 However, flavonoids represent the highest contributor compared with phenolic acids in the total (poly)phenol intake estimated from KP-FFQ as reported in the NDNS research,38 which is likely due to the different coffee and tea contribution between the two FFQs in our cohort. Here, in the KP-FFQ, coffee and tea contributed 31.0% (1.1 ± 0.4 cup per d (203.6 ± 78.6 g d−1)) and 12.7% (0.8 ± 0.2 cup per d (147.0 ± 28.6) g d−1) of the total (poly)phenol intake, respectively, whereas in the EPIC-Norfolk FFQ, coffee and tea contributed 41.0% (0.8 ± 1.0 cup per d (145.3 ± 187.1 g d−1)), and 23.6% (0.7 ± 1.1 cup per d (129.8 ± 200.0 g d−1)) to the total (poly)phenol intake (standardized as 190 g per cup according to the default portion size in the EPIC-Norfolk FFQ). This varying ratio of tea and coffee consumption could partially elucidate the differential contributions of phenolic acids and flavonoids to the overall intake of (poly)phenols. Notably, the population we evaluated comprises young individuals (19–29 years old) with high education level (college students) and high percentage of Asian than the general UK population, and a higher proportion of coffee consumers (76.2 and 79.6% from EPIC-Norfolk and KP-FFQs, respectively) than that in the UK adults (62.0%).71 Moreover, due to the large proportion of Asian people in the targeted population, white rice, as the major food source, represents a good source of dietary (poly)phenols, contributing 2% to the total (poly)phenol intake estimated from KP-FFQ.
The estimated class and subclass of (poly)phenol intake levels were different between the two FFQs, with 23 out of 26 (poly)phenol groups being higher in the KP-FFQs, which aligns with our expectations. Compared with EPIC-Norfolk FFQs, KP-FFQs captured more food sources of (poly)phenols, for instance, blueberry, grape, aubergine, olive, herbs and spices, seeds, alternative milk such as almond, oat, soy milk, and sauces such as soy sauce. Anthocyanins, a subclass of (poly)phenols, play a role in the skin colouring of fruits such as apples.72 The (poly)phenol content of fruits differs depending on whether the skin is included, such as apple and pear (apple: peeled 26.52, non-peeled 55.94 mg per 100 g; pear: peeled 0.58, non-peeled 1.65 mg per 100 g fresh weight of total (poly)phenol (aglycone equivalent) from in-house database6,36), while this was not distinguished in the EPIC-Norfolk FFQ. Besides, the fruits or vegetables with diverse colours can have diverse (poly)phenol content, which are also not distinguished in the EPIC-Norfolk FFQ, for instance, grapes (green: 10.30, black: 7.49 mg per 100 g fresh weight of total (poly)phenol (aglycone equivalent)6,36), and onions (red: 2.60, white: 0.34, yellow: 1.73 mg per 100 g fresh weight of total (poly)phenol (aglycone equivalent)6,36). Moreover, several food items with distinct (poly)phenol levels or profiles were grouped in one question in the EPIC-Norfolk FFQs, for instance, tea (black, green, and herbal tea), wine (white, rose, or red wine), “strawberries, raspberries, kiwi fruit”, “peanuts or other nuts”, and “dried lentils, beans, peas”. Participants may interpret the questions differently, while in analysis, those foods were transformed into a combination of default items.6 The above issues could all lead to potential underestimation of (poly)phenols in the EPIC-Norfolk FFQs compared with the KP-FFQs. In addition, due to the expanded food list in the KP-FFQs, it has a longer completion time than the EPIC-Norfolk FFQs (36.9 ± 21.4 min vs. 13.9 ± 6.8 min). While the amount of time needed is acceptable, it may lead to over or underestimation of (poly)phenol consumption. As a result, the agreement between the two FFQs was moderate. Agreements were extremely poor for the groups contributing a small percentage of the total intake, including anthocyanins, dihydroflavonols, flavones, total other (poly)phenols, and tyrosols, which required a more detailed measurement tool6 such as the KP-FFQ to be fully captured.
The food records are not limited to a predefined food list, which enables more specificity as (poly)phenol content is linked to individual food items rather than less food groups in the FFQs and captures day-to-day variabilities and less common foods.16 Regarding the relative validity against 7DDs, moderate agreements were exhibited in limited (poly)phenols, including dihydroflavonols, dihydrochalcones, total phenolic acids, hydroxycinnamic acids, and alkylmethoxyphenols, and only alkylphenols from 7DDs was significantly associated with the intake from EPIC-Norfolk FFQs. 7DDs do not have predefined food lists and allow matching individual food items with (poly)phenol content,16 which allow more accurate estimation of the (poly)phenol intake. However, 7DD only captures intake for a short period (1 week), which are prone to inter-day/seasonal variations in the diet. These difference in tools could all lead to the discrepancies between the two FFQs and 7DDs in our results. Previous validation studies between EPIC-Norfolk FFQs and 3 day food records found an ICC of 0.489 for total (poly)phenol intake73 and a cross-classification test of 30.2%73 to 36.6%74 of the same quartiles for total (poly)phenols and 18.0% to 31.0% for flavonoids subclasses,75 which is in accordance with our results (33.3% for total (poly)phenol and 21.7% to 48.3% for flavonoids subclasses). In comparison, a 0.73 kappa for total phenolic acids and hydroxycinnamic acids was estimated from KP-FFQs and 7DDs with a cross-classification test of 96.7% in the same and adjacent quartile with no opposite quartile. This high agreement may be due to the similar food source and contribution in the two measurements, with coffee, rice, tea, chestnuts, and blueberries contributing 67.5%, 5.1%, 2.4%, 2.1%, and 1.8% in KP-FFQs and 65.7%, 4.2%, 2.0%, 1.3% and 2.2% in 7DDs for total phenolic acids, and coffee, rice, apple, and blueberries contributing 74.6%, 5.3%, 2.0%, and 1.9% in KP-FFQs and 72.3%, 4.7%, 1.8%, and 2.3% in 7DDs for hydroxycinnamic acids, respectively. In addition, compared to the agreements between EPIC-Norfolk FFQs and 7DDs, more (poly)phenols with moderate agreements between KP-FFQs and 7DDs were exhibited, including total (poly)phenol and subclass of (poly)phenols from four out of five classes, for instance, chalcones, isoflavonoids, ellagitannins, hydroxyphenylacetic acids, resveratrol, tyrosols, and alhylmethoxyphenols. The 442 food items in KP-FFQs cover the most important (poly)phenol dietary sources in the UK diet through the NDNS study 2008–2014.38 It integrates closely with the free-living eating habits and agrees more with the detailed food source of (poly)phenols captured by 7DDs, which contributed to better agreements than the EPIC-Norfolk FFQs. Coherently, strong associations were exhibited in ten (poly)phenols from four classes estimated from 7DDs with (poly)phenols from KP-FFQs, including dihydroflavonols, theaflavins, thearubigins, flavones, isoflavonoids, ellagitannins, hydroxyphenylacetic acids, total stilbenes, resveratrol, and tyrosols. To note, no gold standard method has been established to measure (poly)phenol intake. The food records also require repeat measurements to capture a period of dietary estimation, for instance, conducted in different seasons to represent yearly diet intake.76,77 Compared with the time coverage of the past year in the FFQs, the lack of yearly representative of food records may contribute to the poor agreements of several (poly)phenols estimated from KP-FFQs.
A large panel of plasma and urine (poly)phenol metabolites were used to enhance the validity testing of FFQs against 7DDs. Regarding the association with urinary and plasma metabolites, stronger significant relationships were observed in KP-FFQs estimated intake compared to EPIC-Norfolk FFQs, which aligns with the better association result of KP-FFQs estimated intake with 7DDs. Compared with urinary metabolites, fewer positive associations were found with plasma metabolites, which may be attributed to the collection time of plasma with more than 8 hours of fasting, resulting in the removal of many metabolites from circulation. Despite the better associations between urinary metabolites with (poly)phenol intake than plasma, spot urine in our research only provided a snapshot of excreted (poly)phenols compared with 24 hour urine, which is able to capture more comprehensive information due to the longer collection time. The agreements were poor between metabolites in urine and plasma and estimated dietary (poly)phenol intake from both EPIC-Norfolk FFQ and KP-FFQ. These poor agreements may be attributed to the extensive metabolism of dietary (poly)phenols after ingestion, including phase II metabolisms into glucuronides, sulfates, and ring fissions into smaller molecules by the gut microbiota.78 Some phenolic compounds with small molecular weight, such as phenolic acids, benzaldehydes, and benzenes, would be present in food and be generated by the gut microbiota from various types of (poly)phenol molecules. Therefore, the endogenous pathways of phenolic metabolites and inter-individual variability in (poly)phenol gut microbial metabolism may lead to poor agreements between the (poly)phenols of the same class/subclass from diet and in biosamples. Different half-lives of the various (poly)phenols and the sample collection time concerning the dietary assessment further influenced the agreements. For example, FFQs reflect habitual intakes, whereas the spot urine and fasting plasma rather reflect recent (poly)phenol intake in the past 24 hours. In addition, the restrictions in the reporting accuracy of the dietary assessment methods, the limited number of specimen samples, and diverse sources of (poly)phenol exposure, such as food additives, also contributed to the discrepancies. Further validation in larger cohorts with higher specimen sample sizes is required.
As for the nutrient intake, including energy, fibre, and macronutrients, the agreement between EPIC-Norfolk and KP-FFQs was moderate, with the ICC, kappa, and Spearman's correlation higher than 0.5. More than 80% of participants were found correctly classified into the same or adjacent quartiles in our study, from fat (83.5%) to fibre (91.5%), which is similar to the previous findings between FFQ and 3 day dietary record with a fair agreement of more than 70% correct classification from fat (70.3%) to energy (84.2%).79 In addition, less than 2.5% of the participants were grossly misclassified (in the opposite quartile), with only 0.89% of misclassification of fibre intake. Plant-based foods are sources of dietary (poly)phenols, which are also important sources of fibre in the human diet.80 The (poly)phenol-focused food list of the questionnaire contributed to the fair agreement in fibre intake. When comparing the nutrients estimated from KP-FFQs against 7DDs, the agreements were poor in general, and only fibre in KP-FFQs showed moderate agreement with 7DDs (kappa = 0.40). However, more than 50% of the participants were correctly classified into the same or adjacent quartiles. Less than 10% of the participants were grossly misclassified, except for energy (11.67%), protein (13.33%) and animal-related nutrient cholesterol (15.0%). The validation study of macronutrient intakes from the EPIC FFQ compared against 24 hour dietary recalls reported a higher percentage of correctly classified nutrients, including energy, protein, fat, and carbohydrate (73.1%, 74.8%, 75.3%, and 71.7%) compared to our study (61.7%, 60.0%, 60.0%, and 58.3%).81 However, the percentage of correctly classified fibre intake was lower in their research compared to ours (66.4% vs. 76.7%).81 Dietary validation studies recommended that more than half of the correct classification, less than 10% grossly misclassification, and weighted kappa values above 0.4 are desirable for nutrients of interest to minimise the false-negative associations between diet and health outcome.82 In the present study, overall agreements for energy and nutrients between two FFQs were considered fair and reasonable, but the agreement between the KP-FFQs and the 7DDs was less satisfactory. The discrepancy in the agreements may be due to the small sample size of the validation study against 7DDs (n = 60), though a sample size of at least 50 has been suggested for validity testing.11 A larger sample would be warranted in future studies for the validation test against dietary records. The finding also implied that KP-FFQs might overestimate the dietary intake due to the expanded food list, which may also explain the fair agreement with EPIC-Norfolk FFQs, which are also prone to overreport.83,84
The strengths and limitations should be noted when interpreting the results. To our knowledge, this is the first study to develop an FFQ to capture the dietary (poly)phenol among a young adult UK population. The (poly)phenol metabolites estimated from 24 hour urine and plasma were conducted as the reference method to strengthen the relative validity assessment power of 7DDs since no objective ‘gold standard’ reference measurement tool has been developed for dietary assessment. In addition, the collection of KP-FFQ was prior to the reference tool of 7DDs following the guidance of Cade et al. on the order of validation, administrating the test instrument before the reference instrument to avoid drawing participants’ attention to their diets.11 The KP-FFQ extended the (poly)phenol-rich food list and kept the (poly)phenol-free food items included in the EPIC-Norfolk FFQ, such as meat products. Since dietary (poly)phenol consumption cannot be captured fully with a finite food list, the extending of the questionnaire length is unavoidable.11 It disaggregated the combined questions of plant-rich foods and included more plant food groups, which may potentially overestimate the intake of plant food and nutrients than the established EPIC-Norfolk FFQ. Since the 442 food items may result in a higher burden on participants and lead to misreport, we tested the average time to complete the EPIC-Norfolk and KP-FFQs in the pilot study with 13.9 and 36.9 minutes, respectively. Considering the much longer completion time than EPIC-Norfolk FFQs, the proper order of food groups is significant. The food groups of particular interest, (poly)phenol-rich food, should be placed at the beginning of the FFQs,11 and the (poly)phenol-free food groups towards the end since the accuracy of responses may decline due to boredom or fatigue.11 Other limitations are mainly related to the general characteristics of FFQs to estimate dietary components. FFQs are prone to self-reporting errors in determining frequencies over the long-term and pre-quantified food portion size.17 Clear instructions on completion and photos of portion sizes85–87 may help estimate the portion size.11 In addition, the prolonged reference period of FFQs has been proved to overestimate healthy food intake, such as fruits and vegetables, prominent sources of (poly)phenols,17,88 which may also contribute to errors in the present study. Another limitation relates to the reference (poly)phenol database. Although our in-house database included the well-established Phenol-Explorer database,32 the USDA database and several published papers,36–58 it is important to acknowledge that the limited information on the influence of harvest conditions, food processing, storage, and cooking methods on the (poly)phenol content of foods restricted the proper interpretation of dietary (poly)phenol intake data.89 Moreover, the lack of representative population in our study should also be noted for ethnicity, and especially the narrow age range, which primarily consists of university students with high education levels. A larger and more representative population for the further reliability test of the newly developed FFQ may be warranted.
5. Conclusion
In conclusion, a novel KP-FFQ developed to estimate dietary (poly)phenol intake in the UK population demonstrated satisfactory validation results, exhibiting moderate to strong agreements and associations with 7DDs and biofluid metabolites. KP-FFQ performs better than established EPIC-Norfolk FFQ for (poly)phenol intake when tested against biomarkers and 7DDs. However, limitations were identified in KP-FFQ for estimating nutrient intake, as it potentially overestimates plant-based food intake due to disaggregation and inclusion of more plant food groups compared with the established FFQ. Future studies should aim to validate this tool against repeated food diaries or weighed food records collected across several seasons to assess the instrument's ability to capture seasonal dietary (poly)phenol intake more accurately and potentially improve the validity for measuring nutrients. A representative UK population group is warranted to test the further usability.Author contributions
Conceptualisation, Y. L., A. R. M., and R. G.; data curation, Y. L., Y. X., M. L. S., N. N. Z. K., H. W., J. H. and S. L.; formal analysis and writing – original draft, Y. L.; methodology, Y. L., Y. X., A. R. M. and R. G.; writing – review & editing; supervision, A. R. M., and R. G.; project administration and funding acquisition, A. R. M.Data availability
Due to privacy and ethical concerns, the raw data supporting the findings of this study may be subject to restrictions. Detailed information on the dietary assessment tools, (poly)phenol intake estimates, and biomarker analysis methods can be provided to interested researchers in accordance with institutional and ethical guidelines.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We extend our highest gratitude to the participants of all the studies. YL and YX are funded by the King's-China Scholarship Council (K-CSC) joint scholarship.References
- J. Dai and R. J. Mumper, Plant phenolics: extraction, analysis and their antioxidant and anticancer properties, Molecules, 2010, 15(10), 7313–7352 CrossRef CAS PubMed.
- M. M. Piergiorgio Pietta and L. Bramati, Plant Polyphenols: Structure, Occurrence and Bioactivity, in Studies in Natural Products Chemistry, ed. A.-u. Rahman, 2003, vol. 28 Search PubMed.
- A. Micek, et al., Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis, Mol. Nutr. Food Res., 2021, 65(6), e2001019 CrossRef PubMed.
- C. Del Bo', et al., Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern?, Nutrients, 2019, 11(6), 1355–1409 CrossRef PubMed.
- D. J. Lamport and C. M. Williams, Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses, Brain Plast., 2021, 6(2), 139–153 Search PubMed.
- Y. Xu, et al., Comparison between dietary assessment methods and biomarkers in estimating dietary (poly)phenol intake, Food Funct., 2023, 14(3), 1369–1386 RSC.
- R. L. Bailey, Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies, Curr. Opin. Biotechnol, 2021, 70, 91–96 CrossRef CAS PubMed.
- A. Bertelli, et al., Polyphenols: From Theory to Practice, Foods, 2021, 10(11), 2595–2609 CrossRef CAS PubMed.
- V. Cheynier, Polyphenols in foods are more complex than often thought, Am. J. Clin. Nutr., 2005, 81(1 Suppl), 223S–229S CrossRef CAS PubMed.
- C. Manach, et al., Polyphenols: food sources and bioavailability, Am. J. Clin. Nutr., 2004, 79(5), 727–747 CrossRef CAS PubMed.
- J. Cade, et al., Development, validation and utilisation of food-frequency questionnaires - a review, Public Health Nutr., 2002, 5(4), 567–587 CrossRef PubMed.
- R. Zamora-Ros, et al., Dietary flavonoid intake and colorectal cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort, Int. J. Cancer, 2017, 140(8), 1836–1844 CrossRef CAS PubMed.
- R. Zamora-Ros, et al., Dietary intake of total polyphenol and polyphenol classes and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, Eur. J. Epidemiol., 2018, 33(11), 1063–1075 CrossRef CAS PubMed.
- R. Zamora-Ros, et al., The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study, Diabetes Care, 2013, 36(12), 3961–3970 CrossRef CAS PubMed.
- R. Zamora-Ros, et al., Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study, Eur. J. Nutr., 2016, 55(4), 1359–1375 CrossRef CAS PubMed.
- Y. Xu, et al., Dietary Assessment Methods to Estimate (Poly)phenol Intake in Epidemiological Studies: A Systematic Review, Adv. Nutr., 2021, 12(5), 1781–1801 CrossRef CAS PubMed.
- K. Kent and K. E. Charlton, Development, validation and reproducibility of a food frequency questionnaire to measure flavonoid intake in older Australian adults, Nutr. Diet., 2018, 75(1), 106–116 CrossRef PubMed.
- Y. Yue, et al., Dietary flavonoids and flavonoid-rich foods: validity and reproducibility of FFQ-derived intake estimates, Public Health Nutr., 2020, 23(18), 3295–3303 CrossRef PubMed.
- J. I. Ottaviani, et al., Evaluation of (-)-epicatechin metabolites as recovery biomarker of dietary flavan-3-ol intake, Sci. Rep., 2019, 9(1), 13108 CrossRef PubMed.
- J. I. Ottaviani, et al., Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies, Sci. Rep., 2018, 8(1), 9859 CrossRef PubMed.
- M. R. Ritchie, et al., Investigation of the reliability of 24 h urine excretion as a biomarker of isoflavone exposure over time and over a wide range of isoflavone intakes, Eur. J. Clin. Nutr., 2004, 58(9), 1286–1289 CrossRef CAS PubMed.
- A. Medina-Remon, et al., Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake, Anal. Chim. Acta, 2009, 634(1), 54–60 CrossRef CAS PubMed.
- G. Rogerson, et al., Development and assessment of relative validity and reliability of the Workplace Beverage Intake Questionnaire in UK office workers, J. Hum. Nutr. Diet., 2023, 36(5), 2036–2049 CrossRef PubMed.
- R. Zamora-Ros, et al., Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study, Sci. Rep., 2016, 6, 26905 CrossRef CAS PubMed.
- L. I. Mennen, et al., Urinary excretion of 13 dietary flavonoids and phenolic acids in free-living healthy subjects - variability and possible use as biomarkers of polyphenol intake, Eur. J. Clin. Nutr., 2008, 62(4), 519–525 CrossRef CAS PubMed.
- J. Radtke, J. Linseisen and G. Wolfram, Fasting plasma concentrations of selected flavonoids as markers of their ordinary dietary intake, Eur. J. Nutr., 2002, 41(5), 203–209 CrossRef CAS PubMed.
- J. Cao, et al., The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake, Br. J. Nutr., 2010, 103(2), 249–255 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, et al., Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 60(10), 2130–2140 CrossRef CAS PubMed.
- G. Istas, et al., Effects of aronia berry (poly)phenols on vascular function and gut microbiota: a double-blind randomized controlled trial in adult men, Am. J. Clin. Nutr., 2019, 110(2), 316–329 CrossRef PubMed.
- S. A. Bingham, et al., Nutritional methods in the European Prospective Investigation of Cancer in Norfolk, Public Health Nutr., 2001, 4(3), 847–858 CrossRef CAS PubMed.
- N. M. McKeown, et al., Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort, Am. J. Clin. Nutr., 2001, 74(2), 188–196 CrossRef CAS PubMed.
- A. Cheok, et al., Betalain-rich dragon fruit (pitaya) consumption improves vascular function in men and women: a double-blind, randomized controlled crossover trial, Am. J. Clin. Nutr., 2022, 115(5), 1418–1431 CrossRef PubMed.
- E. Wood, et al., Wild blueberry (poly)phenols can improve vascular function and cognitive performance in healthy older individuals: a double-blind randomized controlled trial, Am. J. Clin. Nutr., 2023, 117(6), 1306–1319 CrossRef CAS PubMed.
- M. Le Sayec, et al., The effects of Aronia berry (poly)phenol supplementation on arterial function and the gut microbiome in middle aged men and women: Results from a randomized controlled trial, Clin. Nutr., 2022, 41(11), 2549–2561 CrossRef CAS PubMed.
- Y. Li, et al., (Poly)phenol intake, plant-rich dietary patterns and cardiometabolic health: a cross-sectional study, Food Funct., 2023, 14(9), 4078–4091 RSC.
- Y. Xu, et al., Development of a novel (poly)phenol-rich diet score and its association with urinary (poly)phenol metabolites, Food Funct., 2023, 14(21), 9635–9649 RSC.
- J. Perez-Jimenez, et al., Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database, Eur. J. Clin. Nutr., 2010, 64(Suppl 3), S112–S120 CrossRef CAS PubMed.
- N. Ziauddeen, et al., Dietary intake of (poly)phenols in children and adults: cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014), Eur. J. Nutr., 2019, 58(8), 3183–3198 CrossRef CAS PubMed.
- Phenol-Explorer database. Available from: https://phenol-explorer.eu/.
- C. L. Craig, et al., International physical activity questionnaire: 12-country reliability and validity, Med. Sci. Sports Exercise, 2003, 35(8), 1381–1395 CrossRef PubMed.
- A. A. Mulligan, et al., A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability, BMJ Open, 2014, 4(3), e004503 CrossRef PubMed.
- R. A. McCance and E. M. Widdowson, McCance and Widdowson's The composition of foods, Royal Society of Chemistry, Cambridge, 6th edn, 2002 Search PubMed.
- R. C. Team, R: A Language and Environment for Statistical Computing. 2022 Search PubMed.
- S. Bhagwat and D. B. Haytowitz, in USDA Database for the Flavonoid Content of Selected Foods, Release 3.2, ed. N. D. Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA, 2015 Search PubMed.
- S. Bhagwat and D. B. Haytowitz, in USDA Database for the Isoflavone Content of Selected Foods, ed. N. D. Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA, 2015 Search PubMed.
- S. Bhagwat and D. B. Haytowitz, in USDA Database for the Proanthocyanidin Content of Selected Foods, ed. N. D. Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA, 2015 Search PubMed.
- J. I. Alonso-Esteban, et al., Chemical composition and biological activities of whole and dehulled hemp (Cannabis sativa L.) seeds, Food Chem., 2022, 374, 131754 CrossRef CAS PubMed.
- L. Alvarez-Jubete, et al., Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking, Food Chem., 2010, 119(2), 770–778 CrossRef CAS.
- R. L. Bertin, et al., Nutrient composition and, identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC-ESI-MS/MS, Food Res. Int., 2014, 55, 404–411 CrossRef CAS.
- H. Y. Cai, et al., Phenolic profile and antioxidant activity of Chinese rice wine fermented with different rice materials and starters, LWT-Food Sci. Technol., 2019, 111, 226–234 CrossRef CAS.
- A. V. Carvalho, et al., Chemical composition and antioxidant capacity of acai (Euterpe oleracea) genotypes and commercial pulps, J. Sci. Food Agric., 2017, 97(5), 1467–1474 CrossRef CAS PubMed.
- N. Cicero, et al., Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market, Food Res. Int., 2018, 109, 517–525 CrossRef CAS PubMed.
- Q. H. Gao, et al., Textural Characteristic, Antioxidant Activity, Sugar, Organic Acid, and Phenolic Profiles of 10 Promising Jujube (Ziziphus jujuba Mill.) Selections, J. Food Sci., 2012, 77(11), C1218–C1225 CrossRef CAS PubMed.
- M. Gundogdu, Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. fruits, Adv. Environ. Biol., 2013, 7(2), 344–348 CAS.
- M. A. Hassan, et al., Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review, Phytomedicine, 2021, 93, 153771 CrossRef PubMed.
- A. N. Karunasiri, et al., Antioxidant and Nutritional Properties of Domestic and Commercial Coconut Milk Preparations, Int. J. Food Sci., 2020, 2020, 3489605 Search PubMed.
- M. Kaspar, et al., Comparison of Phenolic Profile of Balsamic Vinegars Determined Using Liquid and Gas Chromatography Coupled with Mass Spectrometry, Molecules, 2022, 27(4), 1356–1370 CrossRef CAS PubMed.
- M. Y. Kim, et al., Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea, J. Agric. Food Chem., 2008, 56(16), 7265–7270 CrossRef CAS PubMed.
- Q. Lv, et al., Identification of Proanthocyanidins from Litchi (Litchi chinensis Sonn.) Pulp by LC-ESI-Q-TOF-MS and Their Antioxidant Activity, PLoS One, 2015, 10(3), e0120480 CrossRef PubMed.
- N. Miceli, et al., Phenolic composition and biological activities of Juniperus drupacea Labill. berries from Turkey, Food Chem. Toxicol., 2011, 49(10), 2600–2608 CrossRef CAS PubMed.
- A. Mocan, et al., Polyphenols from Lycium barbarum (Goji) Fruit European Cultivars at Different Maturation Steps: Extraction, HPLC-DAD Analyses, and Biological Evaluation, Antioxidants, 2019, 8(11), 562–575 CrossRef CAS PubMed.
- W. C. B. Muala, Z. S. C. Desobgo and N. E. Jong, Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract, Heliyon, 2021, 7(4), e06744 CrossRef CAS PubMed.
- P. S. Prasanthi, et al., Compositional variability of nutrients and phytochemicals in corn after processing, J. Food Sci. Technol., 2017, 54(5), 1080–1090 CrossRef CAS PubMed.
- M. J. Rahman, A. C. de Camargo and F. Shahidi, Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities, J. Funct. Foods, 2017, 35, 622–634 CrossRef CAS.
- A. Rueda, et al., Combination of Analytical and Chemometric Methods as a Useful Tool for the Characterization of Extra Virgin Argan Oil and Other Edible Virgin Oils, Role of Polyphenols and Tocopherols, J. AOAC Int., 2016, 99(2), 489–494 CrossRef CAS PubMed.
- L. Xu, D. Bin and B. J. Xu, A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China, Food Chem., 2015, 174, 202–213 CrossRef CAS PubMed.
- Y. Li, et al., Interplay between the (Poly)phenol Metabolome, Gut Microbiome, and Cardiovascular Health in Women: A Cross-Sectional Study from the TwinsUK Cohort, Nutrients, 2023, 15(8), 1900–1916 CrossRef CAS PubMed.
- M. Dominguez-Fernandez, et al., Quantitative Assessment of Dietary (Poly)phenol Intake: A High-Throughput Targeted Metabolomics Method for Blood and Urine Samples, J. Agric. Food Chem., 2021, 69(1), 537–554 CrossRef CAS PubMed.
- J. L. Fleiss, B. Levin and M. C. Paik, Statistical methods for rates and proportions, Wiley, Newy York, 2003 Search PubMed.
- T. K. Koo and M. Y. Li, A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research, J. Chiropr. Med., 2016, 15(2), 155–163 CrossRef PubMed.
- R. Poole, et al., Misclassification of coffee consumption data and the development of a standardised coffee unit measure, BMJ Nutr. Prev. Health., 2019, 2(1), 11–19 CrossRef PubMed.
- M. P. G. Barnett, et al., A Polyphenol Enriched Variety of Apple Alters Circulating Immune Cell Gene Expression and Faecal Microbiota Composition in Healthy Adults: A Randomized Controlled Trial, Nutrients, 2021, 13(4), 1092–1109 CrossRef CAS PubMed.
- I. Vian, et al., Development and validation of a food frequency questionnaire for consumption of polyphenol-rich foods in pregnant women, Matern. Child Nutr., 2015, 11(4), 511–524 CrossRef PubMed.
- S. Shahar, et al., Development and Validation of Food Frequency Questionnaire (FFQ) for Estimation of the Dietary Polyphenol Intake Among Elderly Individuals in Klang Valley, Jurnal Sains Kesihatan Malaysia, 2014, 12(2), 33–40 CrossRef.
- S. Somerset and K. Papier, A food frequency questionnaire validated for estimating dietary flavonoid intake in an Australian population, Nutr. Cancer, 2014, 66(7), 1200–1210 CrossRef CAS PubMed.
- Y. Zhang, et al., Reproducibility and relative validity of a food frequency questionnaire to assess intake of dietary flavonol and flavone in Chinese university campus population, Nutr. Res., 2010, 30(8), 520–526 CrossRef CAS PubMed.
- K. Kent, et al., Estimation of Flavonoid Intake in Older Australians: Secondary Data Analysis of the Blue Mountains Eye Study, J. Nutr. Gerontol. Geriatr., 2015, 34(4), 388–398 CrossRef PubMed.
- D. Del Rio, et al., Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases, Antioxid. Redox Signal., 2013, 18(14), 1818–1892 CrossRef CAS PubMed.
- F. Fatihah, et al., Development and validation of a food frequency questionnaire for dietary intake assessment among multi-ethnic primary school-aged children, Singap. Med. J., 2015, 56(12), 687–694 CrossRef PubMed.
- T. M. Barber, et al., The Health Benefits of Dietary Fibre, Nutrients, 2020, 12(10), 3209–3225 CrossRef CAS PubMed.
- A. Kroke, et al., Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24 h dietary recall methods, Am. J. Clin. Nutr., 1999, 70(4), 439–447 CrossRef CAS PubMed.
- L. F. Masson, et al., Statistical approaches for assessing the relative validity of a food-frequency questionnaire: use of correlation coefficients and the kappa statistic, Public Health Nutr., 2003, 6(3), 313–321 CrossRef CAS PubMed.
- M. Ferraroni, et al., Reproducibility and validity of coffee and tea consumption in Italy, Eur. J. Clin. Nutr., 2004, 58(4), 674–680 CrossRef CAS PubMed.
- R. Takechi, et al., Assessing self-reported green tea and coffee consumption by food frequency questionnaire and food record and their association with polyphenol biomarkers in Japanese women, Asia Pac. J. Clin. Nutr., 2018, 27(2), 460–465 CAS.
- M. R. French, L. U. Thompson and G. A. Hawker, Validation of a phytoestrogen food frequency questionnaire with urinary concentrations of isoflavones and lignan metabolites in premenopausal women, J. Am. Coll. Nutr., 2007, 26(1), 76–82 CrossRef CAS PubMed.
- S. Ranka, et al., Development of a food frequency questionnaire for the assessment of quercetin and naringenin intake, Eur. J. Clin. Nutr., 2008, 62(9), 1131–1138 CrossRef CAS PubMed.
- G. Grosso, et al., Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study, Eur. J. Nutr., 2018, 57(4), 1535–1544 CrossRef CAS PubMed.
- M. Jessri, W. Y. Lou and M. R. L'Abbe, Evaluation of different methods to handle misreporting in obesity research: evidence from the Canadian national nutrition survey, Br. J. Nutr., 2016, 115(1), 147–159 CrossRef CAS PubMed.
- G. G. C. Kuhnle, Nutrition epidemiology of flavan-3-ols: The known unknowns, Mol. Aspects Med., 2018, 61, 2–11 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo03546a |
| This journal is © The Royal Society of Chemistry 2024 |