Open Access Article
Open Access ArticleThe colonic polyphenol catabolite dihydroferulic acid (DHFA) regulates macrophages activated by oxidized LDL, 7-ketocholesterol, and LPS switching from pro- to anti-inflammatory mediators†
Oscar J.
Lara-Guzmán
 ,
Ángela
Arango-González
,
Diego A.
Rivera
,
Katalina
Muñoz-Durango
and
Jelver A.
Sierra
,
Ángela
Arango-González
,
Diego A.
Rivera
,
Katalina
Muñoz-Durango
and
Jelver A.
Sierra
 *
*
Vidarium - Nutrition, Health and Wellness Research Center, Nutresa Business Group, Calle 8 Sur No. 50-67, Medellin, Colombia. E-mail: ojlara@serviciosnutresa.com; bioscar85@gmail.com; jasierra@serviciosnutresa.com
First published on 16th September 2024
Abstract
Macrophage activation plays a central role in the development of atherosclerotic plaques. Interaction with oxidized low-density lipoprotein (oxLDL) leads to macrophage differentiation into foam cells and oxylipin production, contributing to plaque formation. 7-Ketocholesterol (7KC) is an oxidative byproduct of cholesterol found in oxLDL particles and is considered a factor contributing to plaque progression. During atherosclerotic lesion regression or stabilization, macrophages undergo a transformation from a pro-inflammatory phenotype to a reparative anti-inflammatory state. Interleukin-10 (IL-10) and PGE1 appear to be crucial in resolving both acute and chronic inflammatory processes. After coffee consumption, the gut microbiota processes non-absorbed chlorogenic acids producing various lower size phenolic acids. These colonic catabolites, including dihydroferulic acid (DHFA), may exert various local and systemic effects. We focused on DHFA's impact on inflammation and oxidative stress in THP-1 macrophages exposed to oxLDL, 7KC, and lipopolysaccharides (LPS). Our findings reveal that DHFA inhibits the release of several pro-inflammatory mediators induced by LPS in macrophages, such as CCL-2, CCL-3, CCL-5, TNF-α, IL-6, and IL-17. Furthermore, DHFA reduces IL-18 and IL-1β secretion in an inflammasome-like model. DHFA demonstrated additional benefits: it decreased oxLDL uptake and CD36 expression induced by oxLDL, regulated reactive oxygen species (ROS) and 8-isoprostane secretion (indicating oxidative stress modulation), and selectively increased IL-10 and PGE1 levels in the presence of inflammatory stimuli (LPS and 7KC). Finally, our study highlights the pivotal role of PGE1 in foam cell inhibition and inflammation regulation within activated macrophages. This study highlights DHFA's potential as an antioxidant and anti-inflammatory agent, particularly due to its ability to induce PGE1 and IL-10.
Introduction
Atherosclerosis is characterized by the accumulation of immune cells like macrophages and oxidized LDL (oxLDL) in arterial walls, leading to plaque formation.1 During this process, macrophages contribute to plaque destabilization and rupture by secreting inflammatory cytokines and matrix metalloproteinases (MMPs).2 Additionally, macrophage surface overexpression and activation of scavenger receptors such as CD36 and pathogen recognition receptors such as Toll-like receptors (i.e., TLR2, -4, and -6) are key activating inflammatory and oxidative stress pathways that promote plaque development.3,4 Ligands of these receptors such as lipopolysaccharides (LPS) activate NF-κB and lead to pro-inflammatory gene expression, and in combination with CD36 ligands, promote sterile inflammation and coordinate the activation of the NLRP3 inflammasome.5In contrast, the expression of immunosuppressive mediators (e.g., IL-10) promotes atheroma resolution by stimulating angiogenesis and phagocytosis and reducing inflammation. Several studies indicate that IL-10 has a strong anti-inflammatory effect on macrophages and its role in atherogenesis may be related to the deactivation of inflammatory cells.6 In transgenic mice, IL-10 blocks atherosclerotic lesion formation.7,8 In peripheral blood mononuclear cells (PBMCs) from patients with acute coronary syndrome, IL-10 inhibits the release of inflammatory cytokines and MMPs, promoting plaque stabilization,9 and in oxLDL-stimulated THP-1 cells and monocyte-derived macrophages from acute coronary syndrome patients, IL-10 contributed to plaque stabilization in part by counteracting apoptosis.10 Prostaglandins are also crucial in immune system regulation, and PGs modulate immune cell function and response orientation.11 Among prostaglandins, PGE1 is a powerful vasodilator and platelet aggregation inhibitor, with therapeutic effects in advanced human atherosclerosis12 and peripheral vascular disease. Dihomo-gamma-linolenic acid, which is mainly metabolized to PGE1, displays an anti-atherosclerotic effect in apoE-deficient mice.13 PGE1 has been shown to reduce cholesterol and decrease the LDL entry into the rabbit arterial wall;14,15 it also suppresses TNFα production in LPS-stimulated PBMCs, while leaving IL-6 unchanged and increasing IL-10,16 and prevents liver damage caused by Escherichia coli infection in mice by raising IL-10 levels, decreasing circulating IL-12, and inducing a dominant Th2-like response.17 These findings suggest that PGE1 plays a role in anti-inflammatory regulation, potentially through the induction of IL-10. However, the precise mechanisms by which PGE1 influences macrophage shift and modulates inflammatory responses via IL-10 require further elucidation, particularly in defining its impact on the macrophage phenotype and the broader inflammatory landscape.
Recent studies show that oxysterols, natural oxidation products released from cholesterol by oxidative stress, can promote a pro-inflammatory macrophage phenotype in atherosclerotic plaques.18 Among oxysterols, 7-ketocholesterol (7KC) is the most abundant and a key player in this switch.19 It has been shown in in vitro models that 7KC induces inflammatory pathways, including activation of cell proliferation through the epidermal growth factor receptor, endoplasmic reticulum stress, stimulation of Akt, TLR4, as well as NF-κB-mediated cytokine production, reactive oxygen species (ROS) induction, and inflammasome activation.19,20 One of the most well-studied inflammasome complexes sensing metabolic imbalances is NOD-like receptor-family pyrin domain containing 3 (NLRP3).21 Its activation requires two signals; the first signal involves the interaction between TLRs and their ligands, leading to the activation of NF-κB and the synthesis of NLRP3, pro-IL-1β and pro-IL-18. Then, a second signal triggers caspase-1 autoactivation, the cleavage of pro-cytokines, and the release of mature IL-1β and IL-18.21 LPS have been confirmed to provide both activation signals in human monocytes and macrophages, leading to IL-1β and IL-18 secretion.22,23 Studies show that various dietary phenolic compounds, including flavonoids (e.g., quercetin, epigallocatechin, morin, baicalin, apigenin, anthocyanins), stilbenes (e.g., resveratrol), and phenolic acids like ferulic acid can target the NLRP3 inflammasome24–27 through various molecular mechanisms, such as decreasing caspase-1 activity, inhibiting TLR4-IκB-NFκB and TLR-MyD88-NFκB pathways, and hindering NLRP3 assembly. Consequently, these compounds lead to a reduction in production and release of IL-1β and IL-18.
Despite its therapeutic potential, physiological effects of dietary bioactive components are often limited by their bioavailability and biotransformation in the organism. In particular, chlorogenic acids (CGAs) from coffee are poorly absorbed and the circulating fraction hydrolyzed into caffeic and ferulic acids, reaching the maximum levels in plasma close to 0.1 μM.28 The low bioavailability of CGAs (∼30%) means that unabsorbed compounds reach the large intestine, where the colon microbiota produces several small phenolic acids, in which dihydrocaffeic (DHCA) and dihydroferulic (DHFA) can reach physiologically relevant concentrations in the intestine and are found in plasma at levels of up to 0.7 and 1 μM, respectively, 5–10 hours after the intake of 400 ml of coffee containing approximately 400 mg CGAs.29 These compounds exhibit diverse activities. Specifically, DHFA, a dietary compound with potential anti-inflammatory properties, has been shown to have diverse biological activities, including antioxidant and anti-inflammatory effects, protection of neuronal cells from ischemic injury, inhibition of in vitro platelet activation, and induction of antioxidant genes.30–33 In a previous study, we observed that pre-treatment with DHFA, DHCA, and several CGAs mitigated the in vitro production of isoprostanes (IsoPs) and prostaglandins induced by oxLDL in foam cells.34 These findings demonstrate the dual antioxidant and anti-inflammatory activities of these compounds.
Building on this knowledge, we aimed to study the activity of DHFA as a molecule with anti-inflammatory/antioxidant activities in an in vitro experimental model of activated human macrophages. Here, differentiated THP-1 macrophages were treated with pathological concentrations of oxLDL, 7KC, or LPS, which can stimulate inflammatory or oxidative stress responses, and were challenged with DHFA at concentrations from 1 μM. Since DHCA is also one of the most common catabolites from CGAs produced in the colon, we compared the biological activity of DHFA with DHCA and also evaluated the mixture of DHFA/DHCA. We focused in particular on the ROS production and release of 8-isoprostane (8-IsoP), inflammasome activation by the release of IL-1β and IL-18, the modulation of pro-inflammatory cytokines (TNF-α, IL-6, IL-8, IL-12, IL-17, and INFγ), chemokines (CCL-2, -3 and -5), vascular cell adhesion molecule (VCAM-1), and metalloproteinases (MMP-1, -2, -3, -7, -9, and -13), the impact on regulatory cytokines (IL-4, IL-10, and IL-13), inhibition of the atherogenic receptor CD36 and the foam cell formation, and prostaglandin E1 (PGE1) induction, a mediator of immune regulation.
Materials and methods
Chemicals and materials
The E coli O111:B4 LPS was provided by Sigma-Aldrich (St Louis, Missouri, USA). Chloromethyl 2′,7′-dichlorofluorescein diacetate (CM-H2DCFDA) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) were obtained from Invitrogen Life Technologies (Carlsbad, California, USA). The 7-ketocholesterol (5-cholesten-3β-ol-7-one) was purchased from Steraloids Inc. (Newport, Rhode Island, USA). Prostaglandin E1 (PGE1) was from Cayman Chemicals (Ann Arbor, Michigan, USA). The dihydroferulic acid (DHFA; 96%; CAS number 1135-23-5) was purchased from Sigma-Aldrich (St Louis, Missouri, USA) and dihydrocaffeic acid (DHCA; 95% CAS number 1078-61-1) was from Extrasynthese S.A. (Lyon, Auvernia-Ródano-Alpes, FR); Benzoic (>98%; CAS number 65-85-0), 3- phenyl propionic (>98%; CAS number 501-52-0), 3-hydroxybenzoic (>98%; CAS number 99-06-9), 4- hydroxybenzoic (>98%; CAS number 99-96-7), 4-hydroxyphenyl acetic (>98%; CAS number 156-38-7), 3-hydroxyphenyl acetic (>98%; CAS number 621-37-4), and 3-(4-hydroxyphenyl) propionic (>98%; CAS number 501-97-3) acids were purchased from TCI America, Ltd (Tokyo, Kanto, JP). The 3,4-dihydroxybenzoic (>98%; CAS number 99-50-3), and 3-(3-hydroxyphenyl) propionic acids (ND%; CAS number 621-54-5) were obtained from Pfaltz Bauer, Inc. (Waterbury, Connecticut, USA). Phenolic acids stock solutions were prepared at 35 mM in DMSO and stored at −80 °C until further use. The work solutions were prepared at 1 mM in PBS, and sterilized by filtration with 0.22 μm acrodisc syringe filters.LDL isolation, oxidation and DiI labelling
LDL was purified from human plasma by using a discontinuous density gradient using a Beckman XL-100 ultracentrifuge (Brea, California, USA) according to methods previously described.35 Briefly, the LDL fraction was collected and desalted by ultrafiltration with ultra-centrifugal filters (0.5 ml, Ultracel 3K; Amicon; Tullagreen, Cork, IRL) against five-fold PBS at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C. Desalted LDL was diluted in PBS, filtered (0.22 μm, sterile) and stored at 4 °C until use. The protein fraction of LDL was quantified using the bicinchoninic acid assay (Pierce, Rockford, Illinois, USA). The oxLDL used in all experiments was obtained by oxidation of LDL with 5 μM CuSO4 at 37 °C for 6 h.35 For uptake experiments, LDL (1 mg ml−1) was labeled with 0.1 μg ml−1 DiI for 12 h at 37 °C under a nitrogen atmosphere in the dark,34 and then the DiI–LDL complex (0.5 mg ml−1) was oxidized as described above. The unbound DiI and copper ions were removed from the oxLDL–DiI particles by ultrafiltration using Ultracel 3K ultra-centrifugal filters.
000g for 10 min at 4 °C. Desalted LDL was diluted in PBS, filtered (0.22 μm, sterile) and stored at 4 °C until use. The protein fraction of LDL was quantified using the bicinchoninic acid assay (Pierce, Rockford, Illinois, USA). The oxLDL used in all experiments was obtained by oxidation of LDL with 5 μM CuSO4 at 37 °C for 6 h.35 For uptake experiments, LDL (1 mg ml−1) was labeled with 0.1 μg ml−1 DiI for 12 h at 37 °C under a nitrogen atmosphere in the dark,34 and then the DiI–LDL complex (0.5 mg ml−1) was oxidized as described above. The unbound DiI and copper ions were removed from the oxLDL–DiI particles by ultrafiltration using Ultracel 3K ultra-centrifugal filters.
Macrophage culture and treatments
The inductive effect of oxLDL, 7KC, and LPS, as well as the regulatory response to PGE1 and DHFA were assessed on THP-1 monocyte-derived macrophages (ATCC TIB-202) cultured and differentiated as described by Lara-Guzmán.34,35 Cells were seeded and treated according to their experimental setup: 5.0 × 104 cells per well in 96-well plates for cell viability; 1 × 105 cells per well in 96-well black plates for ROS, and 2 × 105 cells per well in 24-well plates for flow cytometry, ELISA and Luminex immunoassays. Before activation with oxLDL, macrophages were exposed to 1 or 10 μM of each phenolic acid, based on the maximum levels reported in plasma,28,29 and an equivalent volume of solvent (<0.05% DMSO) was used as the negative control. The exposure times of cells to DHFA and PGE1 varied according to the assay as follows: 1 h for ROS, 4–12 h for CD36 expression, for oxLDL uptake, for 8-IsoP and cytokine secretion, and 24–48 h for cell viability; the time of exposure to inductors for macrophage activation was: 36 h for of cell viability and oxLDL receptor assays; 6 h for oxLDL uptake; 1 h for ROS production; and 24 h for ELISA and Luminex immunoassays. For the inflammasome-like model, macrophages were primed with 20 μM 7KC for 4 hours, and then, activated with 10 ng ml−1 LPS for additional 20 hours.DiI-oxLDL (DiI) uptake, surface CD36 expression (FITC), and ROS production (DCF) in macrophages were assessed by flow cytometry using LSRFortessa™ apparatus (Beckton Dickinson; San Jose, California, USA), acquiring at least 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events. Cytometric analysis was performed with FlowJo software. The data were calculated by subtracting cell's autofluorescence and/or isotype control fluorescence from the fluorescence of the treated samples. The results are expressed as the mean fluorescence intensity (MFI).
000 events. Cytometric analysis was performed with FlowJo software. The data were calculated by subtracting cell's autofluorescence and/or isotype control fluorescence from the fluorescence of the treated samples. The results are expressed as the mean fluorescence intensity (MFI).
DiI-oxLDL uptake
After treatments, the cells in microplates were washed three times with PBS and harvested with 0.25% trypsin and 0.02% EDTA. Then, the macrophages were centrifuged at 300g for 5 min, washed once with RPMI-1640 and 10% FBS at 37 °C and twice with PBS at 300g for 5 min at 4 °C. Finally, the cells were suspended in cold PBS and analyzed using a flow cytometer.CD36 expression
After treatments, the macrophage cultures were washed with PBS and harvested from the wells with cold PBS supplemented with EDTA (0.2%) with the aid of cell scrapers. Nonspecific binding of antibodies was blocked by incubating cells in 10% FBS-PBS. Cells were incubated with 1 μL per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells of purified antihuman monoclonal antibodies (R&D Systems; Minneapolis, Minnesota, USA), CD36/SR-B3-FITC (clone 255606; Cat# FAB19551F, RRID: AB 1026194) or the isotype control, rat IgG2B-FITC (clone 141945; Cat# IC013F, RRID: AB_357258). Finally, the cells were washed and suspended in cold PBS and analyzed using a flow cytometer.
000 cells of purified antihuman monoclonal antibodies (R&D Systems; Minneapolis, Minnesota, USA), CD36/SR-B3-FITC (clone 255606; Cat# FAB19551F, RRID: AB 1026194) or the isotype control, rat IgG2B-FITC (clone 141945; Cat# IC013F, RRID: AB_357258). Finally, the cells were washed and suspended in cold PBS and analyzed using a flow cytometer.
Macrophage ROS production
Cells were incubated with a 2 μM fluorescent ROS-sensitive probe CM-H2DCFDA for 30 minutes. Then, the cells were washed twice, suspended in PBS, and analyzed by either flow cytometry or spectrofluorometry at 488 nm excitation and 525 nm emission wavelengths.Cell viability
Lactate dehydrogenase (LDH) was quantified in the supernatants collected after the treatments (LDH assay kit; Promega, Madison, USA) at 340 nm using a synergy HT multimode microplate reader (BioTek Instruments Inc.; Winooski, Vermont, USA). The samples and controls were evaluated in triplicate, and the results are expressed as the percent of control.Oxidative stress markers in cell culture supernatants
The 8-isoprostane and PGE1 levels were measured using commercially available ELISA kits from Cayman and Novus biologicals, respectively (Cayman, Ann Arbor, Michigan, USA; Novus biologicals, Denver, Colorado, USA), following the manufacturer's instructions.Inflammation markers in cell culture supernatants
The cytokines IL-1β, IL-18, TNF-α, IL-6, IL-8, MMP-9 and IL-10 were measured using Human DuoSet ELISA kits (R&D Systems, Minneapolis, MN), following the manufacturer's instructions. The levels of CCL2/JE/MCP-1, CCL3/MIP-1α, CCL5/RANTES, IL-1β/IL-1F2, IL-4, IL-12p70, IL-13, IL-17/IL-17A, MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-13, myeloperoxidase/MPO and VCAM-1/CD106 were measured using a customized human magnetic premixed multiplex (17-plex) assay kit (R&D Systems, Minneapolis, MN, USA) for Magpix™ (Luminex Corp, Austin, TX, USA). Briefly, 50 μL of mixed magnetic microspheres were added to the sample and standards at the indicated dilutions. The plate was incubated for 2 h at room temperature, kept away from light. After incubation, samples were washed with wash buffer by applying a magnet to the bottom of the microplate. Then, a biotin-cocktail detection antibody (50 μL) was added to all assay wells and incubated at room temperature for one hour. Finally, a streptavidin–PE (50 μL) conjugate was added and incubated for 30 min. After this period, the microparticles in each well were washed and suspended in wash buffer (100 μL). The plate was incubated for two minutes on a shaker (800 ± 50 rpm) before data acquisition using the Magpix system. Data were analyzed with the Luminex Xponent program v4.3. The results were expressed as pg ml−1 culture supernatants. The limits of detection for CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, IL-1β, IL-4, IL-12p70, IL-13, IL-17, MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-13, myeloperoxidase/MPO, VCAM-1/CD106, and INFγ were 9.9, 16.2, 1.8, 0.8, 9.3, 20.2, 36.6, 1.8, 2.7, 108, 5.3, 23.2, 13.6, 19, 26.2, 238, and 0.22 pg ml−1, respectively.Statistical analysis
Data are reported as the means ± SD. The normality of the continuous variables in each group was evaluated by the Shapiro–Wilk test. To evaluate the effect of treatments, an unpaired t-test or one-way analysis of variance (ANOVA) was performed using GraphPad Prism version 10 for Windows (GraphPad Software, Inc., San Diego, California, USA). The raw data for statistical analyses are available on Github (https://github.com/vidarium/Lipidox). P values ≤ 0.05 were considered statistically significant. When differences were observed in ANOVA, post hoc Dunnett's multiple comparison tests were performed.Results
THP-1 macrophages are activated with 7KC, oxLDL, and LPS stimuli, and release of pro-inflammatory cytokines, proteases and ROS
Macrophages are important players in the progression of atherosclerosis and promote inflammation through the release of pro-inflammatory cytokines and proteases in response to danger signals. First, we examined whether LPS, 7KC and oxLDL affected the macrophage viability and then the effects of 7KC, oxLDL, and LPS on macrophages’ inflammatory profile were compared. After being treated with LPS for 24 hours, about 20% of the cells were dead. In contrast, neither 7KC nor oxLDL or their combination induced macrophage death after 36 h of treatment (ESI Fig. 1†). In contrast to cells under the basal conditions, LPS strongly activated macrophages, causing them to release primarily pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-4, IL-6, IL-8, IL-17, IL-18, and INF-γ), MPO, MMP-13, VCAM-1, and the chemokines CCL-2, CCL-3, and CCL-5 but not PGE1 and IL-10 (Fig. 1A). The 7KC treatment significantly induced the secretion of PGE1, and the two prominent pro-inflammatory cytokines generated by inflammasome (IL-1β and IL-18), while reduced the release of MPO, MMP-13, IL-4 and IL-10 (p < 0.01). Remarkably, the treatment with oxLDL only induced macrophages to produce PGE1.Two signals are needed for inflammasome assembly. In the inflammasome-like model, as depicted in Fig. 1B, THP-1 macrophages primed with 7KC (a TLR ligand) released significant amounts of IL-1β (p < 0.05) and IL-18 (p < 0.01), after the second signal with LPS.20,22,23 In this model, when comparing the response of macrophages treated solely with 7KC or LPS, there is a synergistic increase in the release of IL-18 when cells were treated with a combination of LPS and 7KC.
Finally, because ROS are important mediators for the activation of pro-inflammatory signaling pathways, the ROS responses of macrophages treated with 7KC, oxLDL, and LPS were also evaluated. oxLDL treatment induced a potent ROS response in spite of its limited ability to induce macrophages to release pro-inflammatory cytokines and chemokines (Fig. 1C). When macrophages were exposed to 20 μM 7KC, they also produced ROS (p < 0.01), although the activation was less than that of oxLDL or LPS at physiologically relevant concentrations.
7KC enhances macrophage atherogenicity
Oxidation of the cholesterol in LDL generates several oxidation products including 7KC, which is the most abundant oxysterol present in oxLDL. To investigate the role of 7KC in ROS production, we compared the ability of oxLDL and that of free 7KC to trigger ROS production by macrophages using flow cytometry. Although a significant ROS production was found with the exposure to 7KC and oxLDL (p < 0.001) (Fig. 1C), the 24 h exposure to free 7KC alone was associated with the macrophage secretion of 8-isoprostane (8-IsoP) in culture supernatants (Fig. 1D), and not with the combination of 7KC and oxLDL. This might be due to the strong ROS production by oxLDL itself, masking any additional effect from 7KC.OxLDL uptake by macrophages via scavenger receptors such as CD36 and their subsequent transformation into foam cells, which accumulate in the artery wall, are essential factors underlying atherogenesis. Oil red O staining confirmed the presence of lipid droplets following oxLDL uptake by macrophages (ESI Fig. 2;†Fig. 1E), adding to phenotypic changes observed in flow cytometry dot plots, which included an increase in surface CD36 expression and the appearance of an additional, more autofluorescence cell subset (14–20%) with a higher internal complexity (more granular due to lipid droplet accumulation) (CD36+/FL2+) characteristic of foam cells (Fig. 1F). Following a 36 hour pre-treatment with 7KC, macrophages exhibited enhanced uptake of DiI-oxLDL (Fig. 1E; p < 0.01), along with increased CD36 expression on their surface (CD36+) and in the CD36+/FL2+ cell population (Fig. 1F). However, compared with macrophages exposed to oxLDL alone, neither the CD36 surface expression nor the percentage of CD36+/FL2+ cells were significantly increased by the combination of 7KC and oxLDL (p > 0.05).
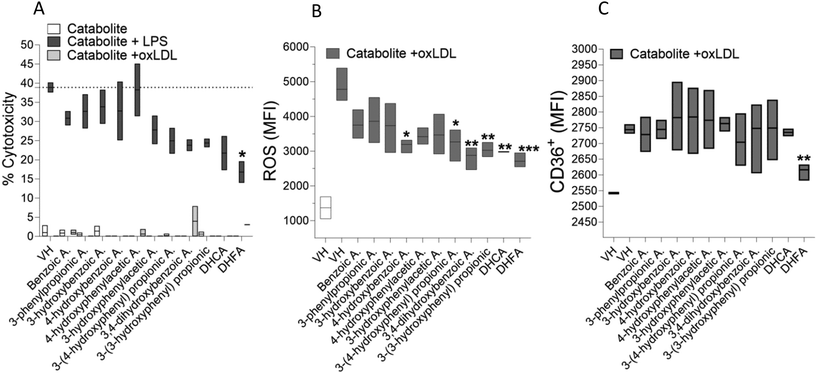
Colonic coffee catabolite DHFA decreases the cell atherogenicity on macrophages activated by oxLDL
Natural compounds, including phenolic acids, have the ability to shift macrophages from pro- to anti-inflammatory phenotypes, potentially aiding in the resolution of pre-existing inflammatory processes. Thus, ROS and CD36 assays were used to characterize the antiatherogenic potential of major colonic catabolites from chlorogenic acid at 1 μM in oxLDL-induced macrophages, as shown in Fig. 2. After 48 hours of exposure, neither the oxLDL and chlorogenic acid catabolites nor their combination caused any toxicity in macrophages; in contrast, lipopolysaccharide (LPS)-induced significant in vitro macrophage cytotoxicity (40%; Fig. 2A), a phenomenon widely reported to be mitigated by the exposure to DHFA. Notably, the reduction of intracellular ROS levels was most effectively achieved by using DHFA and DHCA among all catabolites (Fig. 2B, p < 0.001), with DHFA being the only one decreasing the CD36 expression (Fig. 2C, p < 0.01).Regulatory effect of DHFA on inflammatory markers in macrophages activated with LPS, oxLDL and 7-KC
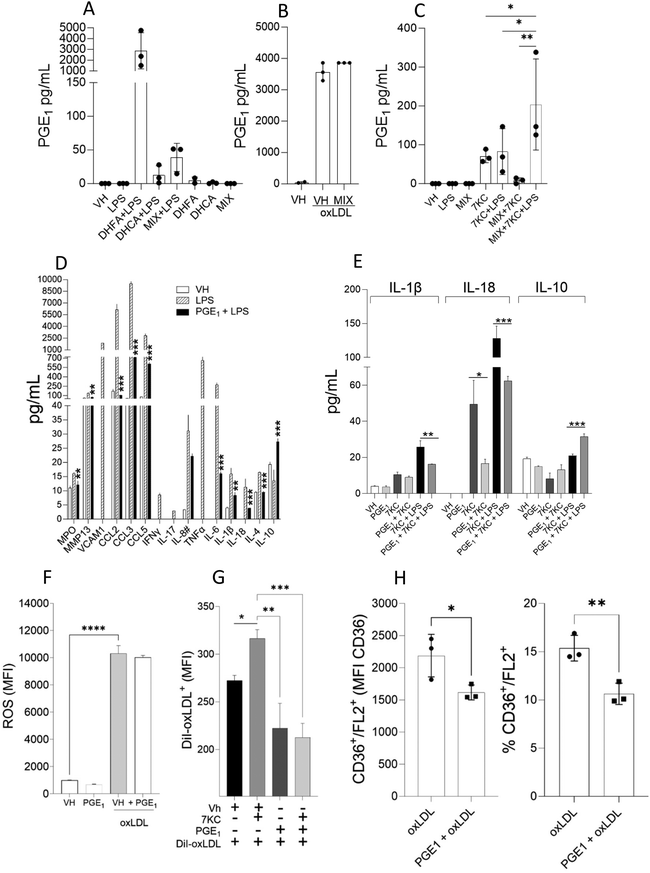
It is already established that DHFA functions upstream by preventing LPS from activating MAPK p38, ERK, and AKT kinase, which in turn reduces and inhibits NF-κB activation affecting the expression of genes including those encoding cytokines and chemokines, and inflammasome regulators in vitro. However, it is unknown if DHFA can modulate the release of anti-inflammatory mediators in activated macrophages. To investigate this, macrophages were pretreated with DHFA prior to LPS, 7KC or oxLDL stimulation. DHFA treatment inhibited the LPS-induced macrophage release of chemokines (i.e., CCL-2, CCL-3, and CCL-5; p < 0.001), and cytokines IL-17 (p < 0.05), IL-18 (p < 0.05), TNF-α (p < 0.001), and IL-6 (p < 0.05) (Fig. 3A and B). Notably, DHFA treatment increased the macrophage release of the regulatory cytokine IL-10 (p < 0.001), but had no effect on IL-4 secretion (Fig. 3C), suggesting that DHFA can inhibit the release of pro-inflammatory mediators and also stimulate regulatory pathways. On the other hand, DHCA pretreatment or the combination of DHCA/DHFA (by mixing 1 μM each) only significantly reduced the release of TNF-α (p < 0.001) and CCL-2 (p < 0.05) by macrophages, but the combination maintained the effect on IL-10 (p < 0.05). These data suggested that DHFA, present in the mixture with DHCA, is the compound contributing to the increased secretion of the anti-inflammatory cytokine IL-10.Similarly, in the two signal inflammasome-like experiment, in which macrophages were pretreated with the DHCA/DHFA mixture, and then primed with 7KC for 4 h and followed by LPS for additional 20 h, the mixture inhibited the secretion of IL-1β and IL-18 (Fig. 3D); this effect was more pronounced on the secretion of IL-18 than on that of IL-1β. These results indicated that DHFA alone, or in a mixture with DHCA could inhibit inflammasome activation by interfering with signal 1 or 2. Finally, the DHCA/DHFA mixture also considerably reduced ROS generation, 8-IsoP secretion and appearance of foam CD36+/FL2+ cells in response to oxLDL (p < 0.05) (Fig. 3E–G).
Effects of PGE1 on inflammatory markers in activated macrophages
PGE1 has been shown to induce immune regulation in a number of cell models and in vivo studies, at least in part by triggering the production of IL-10, a powerful anti-inflammatory mediator. Our results demonstrated that 7KC and oxLDL, as opposed to LPS treatment, caused PGE1 secretion in macrophages (Fig. 1A). Moreover, DHFA demonstrated immunoregulatory properties by inhibiting pro-inflammatory mediators (Fig. 3A and B) and inducing IL-10 (Fig. 3C). Since DHFA can induce IL-10, we hypothesized that it might benefit macrophages in inflammatory or oxidative stress scenarios, even those induced by LPS. To test this hypothesis, we assessed DHFA's ability to induce PGE1 in macrophages treated with oxLDL, 7KC, or LPS, either by itself or in conjunction with DHCA.First, we observed an increased PGE1 production in macrophages treated with both DHFA and the DHFA/DHCA mixture following LPS stimulation (Fig. 4A). Remarkably, PGE1 production was 75 times higher after DHFA than after the DHFA/DHCA mixture treatment, reaching levels comparable to the oxLDL's effect (Fig. 4B). PGE1 secretion induced by oxLDL was not inhibited by the DHFA/DHCA mixture (Fig. 4B). On the other hand, PGE1 levels induced by 7KC alone were significantly reduced by the DHFA/DHCA mixture and PGE1 increased by the presence of DHFA in the inflammasome-like model (7KC + LPS) (Fig. 4C). We then investigated whether PGE1 could regulate macrophage activation by LPS, 7KC, and oxLDL. Interestingly, PGE1 significantly increased macrophages’ IL-10 secretion (p < 0.001), while highly suppressing the secretion of IL-1β and IL-18 induced by LPS (Fig. 4D). PGE1 exhibited a similar suppressive effect on IL-1β and IL-18 production compared to both 7KC and 7KC + LPS stimulation (Fig. 4D). Notably, PGE1 only induced IL-10 secretion when macrophages were exposed to LPS + 7KC, suggesting activation of this regulatory pathway specifically under these co-stimulatory conditions (Fig. 4D). These data support the regulatory roles of PGE1 and DHFA during pro-inflammatory events.
Finally, although PGE1 treatment did not regulate ROS production in oxLDL-activated macrophages (Fig. 4F), it significantly decreased both the uptake of DiI-oxLDL (p < 0.001) and the CD36 expression in the CD36+/FL2+ cell subset (p < 0.05) (Fig. 4G and H). Furthermore, PGE1 significantly reduced the over-uptake of DiI-oxLDL induced by 7KC. Altogether, these results suggested that DHFA treatment in macrophages may be beneficial for preventing inflammation and oxidative stress in this atherosclerosis cell model by acting as an antioxidant and also by promoting anti-inflammatory regulation in macrophages by inducing PGE1 and IL-10 secretion.
Discussion
Macrophages are critical components of the immune system. However, their function becomes a double-edged sword in chronic inflammatory diseases like atherosclerosis. Shifting the macrophage phenotype towards an anti-inflammatory one within plaques could stabilize atherosclerotic lesions and prevent complications, and inhibiting oxidative and pro-inflammatory signals that attract macrophages to the plaque could slow disease progression; these are two therapeutic approaches that could be used to target macrophage physiology in atherosclerosis.36 The current study aimed to investigate the ability of colonic catabolites of chlorogenic acid, specially DHFA to modulate the overexpression of pro-inflammatory mediators, cytokines related to the inflammasome, to regulate the production of ROS and 8-IsoP, to affect the uptake of oxLDL and the expression of CD36, a receptor involved in oxLDL uptake, and to promote the secretion of regulatory mediators like IL-1 and PGE1. THP-1 macrophages activated with LPS, 7KC, and oxLDL at pathological concentrations served as a model system to assess DHFA's bioactivity.34,37,38The atherosclerotic plaque exhibits a high metabolic rate, with increased production of ROS and oxidized lipids such as oxylipins and oxysterols, including 7KC.39 7KC, a molecule generated from cholesterol by oxidative stress, has been linked to various inflammatory diseases, particularly those affecting the cardiovascular and nervous systems.40 It accumulates in areas with high lipoprotein deposits, making it relevant to atherosclerosis. Studies have shown that 7KC primarily triggers inflammation through the TLR4 receptor, activating specific signaling pathways that lead to cytokine production such as AKT-PKC-NF-KB, p38 MAPK, and ER.20 Therefore, interfering with 7KC's inflammatory mechanism holds promise for developing small-molecule therapies. Such therapies could potentially reduce inflammation and delay the onset of chronic diseases like atherosclerosis.
Recent research suggests that 7KC can activate the NLRP3 inflammasome pathway in various cell models, resulting in distinct levels of IL-1β and IL-18 production.41–43 One significant finding of our study was that 7KC primarily increased the release of IL-18, a cytokine associated with the inflammasome complex. Interestingly, IL-1β, another inflammasome-related cytokine, had a weaker response, despite both being enhanced by LPS. Although we did not directly measure the complete inflammasome complex consisting of caspase-1, ASC, and NLRs,41,42 it is well established that the inflammasome activation requires two independent signals: the first activates NF-κB, via TLR signaling, inducing the transcription of pro-IL-1β and pro-IL-18, and the second, often a damage or cellular stress associated signal, leads to the processing and secretion of mature IL 1β and IL-18.41,44 7KC signals through TLR-420 and may act in priming and activation signals required for inflammasome assembly. Further analysis revealed that 7KC also increased the expression of CD36 receptor and ROS production, potentially contributing to the inflammatory response, but most other tested inflammatory mediators, including cytokines like TNF-α and IL-6, chemokines, metalloproteases and adhesion molecules, did not seem to be significantly affected by 7KC. The selective over-production of IL-18 suggests that NLRP3 inflammasome is the primary responder to 7KC-induced inflammation.
LPS, in addition to being a major inductor of the inflammatory process on cell models, may also evoke changes in lipid homeostasis, elicit the inflammasome, and induce oxidative stress in macrophages.45 To establish the present model of LPS-induced inflammation, we evaluated the effects on IL-1β and IL-18 and considered the production of inflammatory mediators cytokines (TNF-α, IL-4, IL-6, IL-8, IL-10, IL-12, IL13, IL-17, and INFγ), chemokines (CCL-2, -3 and -5), vascular cell adhesion molecule (VCAM-1), and MMPs (MMP-1, -2, -3, -7, -9, and -13), together with oxidative stress markers (ROS, and 8-IsoP), and PGE1. Most of the mediators were induced by LPS, except for MMPs, IL-12 and IL-13, and the regulatory molecules such as IL-10 and PGE1. We observed that the co-treatment with 7KC (signal 1) and LPS (signal 2) enhanced the secretion of IL-18 to a greater extent than that of IL-1β.
Results from earlier research on the inflammatory responses induced by oxLDL have been inconsistent and frequently contradictory.46 In part because, the ability of minimally modified LDL (mmLDL) to bind to TLRs and activate TNF-α, IL-6, and IL-1β secretion via NF-kB signaling is superior to that of fully oxidized oxLDL, which causes primarily oxidative stress in macrophages, raising oxysterol accumulation and oxylipin secretion (e.g., prostaglandins and isoprostanes).35,47,48 Oxylipins and oxysterols may increase the expression of several inflammatory mediators in the atherosclerotic plaque and the vascular system by inducing oxidative stress.49,50 In agreement with the current study, exposing macrophages to oxLDL or 7KC increased the production of ROS, CD36, and oxylipins such as 8-IsoP and PGE1, and the co-treatment (oxLDL + 7KC) showed an additive response as well as an increase in oxLDL uptake. It is difficult to compare our findings to previous research because many studies examined inflammation in macrophages through cytokine expression; however, in our experiments, oxLDL did not induce cytokines, chemokines, MMPs, VCAM-1, or MPO.
Macrophages can be influenced by dietary components absorbed from the gut, including bioactive metabolites like DHFA. Studying these metabolites’ effects on macrophages provides valuable insights into their potential health benefits.51–53 Our previous research demonstrated that pre-treating macrophages with 1 μM DHFA before oxLDL exposure protected them from oxidative stress, prevented oxylipin production and decreased the levels of oxLDL receptors (CD36, SR-A, and LOX-1).34 It is worthwhile to highlight the effects of DHFA, which was more active than DCHA, consistent with previous findings in inflammatory models.32 To our knowledge, this is the first study on the effects of this colonic metabolite in cultured human macrophage cells activated by multiple inductors.
Reduced ROS generation and decreasing 8-IsoP in cells treated with DHFA indicate that this compound acts as a primary antioxidant, mitigating the increased levels of ROS and oxidative damage caused by oxLDL-induced stress. These results are consistent with earlier studies conducted in hepatocytes32 and enterocytes.30 On the other hand, the high levels of 8-IsoP induced by oxLDL indicate a state that could result in irreversible oxidative damage to polyunsaturated fatty acids within the cell membranes. Pre-treatment with DFHA partially prevented this dangerous situation, which is consistent with the response observed for hydroxycinnamic acids from coffee.34
In the current study, DFHA was found to be effective in decreasing the majority of the inflammatory mediators tested while increasing IL-10 and PGE1, confirming its anti-inflammatory potential on human macrophages. These findings are especially significant because DFHA is widely detected in human colon, plasma, and urine after consuming various phenolic compounds, including monomeric or oligomeric flavan-3-ols from cocoa,54 anthocyanins from berries,55 and citrus flavanones.56 Previous studies have demonstrated that DHFA attenuated LPS-induced inflammatory response (nitric oxide and NF-κB) in enterocyte-like cells30 and reduced the secretion of IL 6, IL-8, MCP-1, and MIP1α by TNFα-stimulated hepatic cells32 and had a stronger inhibitory effect on P-selectin expression than their phenolic precursors (5-caffeoylquinic acid and 3,5-dicaffeoylquinic acid), suggesting an increased efficacy to modulate platelet activation with the catabolism of the phenolic compounds.33
Our findings suggest that DHFA-induced PGE1 secretion may be a regulatory mechanism in LPS-activated macrophages. PGE1 and DHFA inhibited the majority of pro-inflammatory mediators (cytokines, chemokines, VCAM-1, MPO, and MMP-13). Treatment with DHFA reduced IL-1β and IL-18 secretion in an inflammasome-like assay, similar to other polyphenols such as rutin, quercetin, naringenin, and silymarin that inhibited the NLRP3 inflammasome, suggesting a role in the regulation of the inflammasome multiprotein complex.24,57 Furthermore, in THP-1 macrophages, ferulic acid, one of the parental molecules of DHFA, increased autophagy, inhibited the activation of the NLRP3 inflammasome induced only by LPS, and reduced the expression and release of inflammatory mediators.25 Our results suggest that DHFA + DHCA effectively inhibits IL-1β secretion only in the context of dual stimulation with LPS and 7KC, possibly by modulating inflammasome signaling. This differential response may reflect the distinct roles of LPS and 7KC in inflammasome activation, where LPS provides the secondary signal required for full inflammasome assembly.24,57 Future studies will be essential to elucidate whether DHFA + DHCA specifically disrupts inflammasome complex formation and activation.
Similar to DHFA pre-treatment, PGE1 pre-treatment allowed for the production of IL-10, a key anti-inflammatory cytokine that deactivates activated macrophages.58 As previously reported, PGE is a potent immunomodulator, and the effects on cytokine production by human peripheral blood mononuclear cells activated with LPS are independent of the prostaglandin subtype (i.e. PGE1, PGE2, or PGE3), but IL-10 production is guaranteed.16 PGE differentially deactivated murine macrophages, and IL-10 was found to play a role. These findings suggest that the anti-inflammatory effect of PGE on mononuclear phagocytes is partially mediated by an autocrine feedback mechanism involving IL-10. PGE differentially deactivated murine macrophages, and IL-10 was found to play a role. These findings suggest that the anti-inflammatory effect of PGE on mononuclear phagocytes is partially mediated by an autocrine feedback mechanism involving IL-10.59
In vivo, PGE1 administration protected mice from liver injury following E. coli infection by suppressing circulating IL-12 levels and increasing IL-10 production,17 whereas patients who received PGE1 during cardiac surgery reduced pro-inflammatory cytokine production and increased IL-10 in myocardial reperfusion injury compared to the control group.60 The anti-inflammatory effects of IL-10 have been described in atherogenesis,6 stabilizing the atherosclerotic plaque progression in mouse models, by inhibiting the release of pro-inflammatory cytokines and MMPs in PBMCs and macrophages from patients with acute coronary syndromes.9,10 DHFA from diet is bioavailable after consuming phenolic-rich foods, such as green/roasted coffee,61 whole-grain wheat62 or Cynara scolymus,63 and has been detected in the plasma at concentrations ranging from 0.1 to 1 μM. In our study, we employed DHFA concentrations of up to 1 μM, which, although in the upper limit of the concentrations found in plasma, are necessary to observe a biological effect in our in vitro model. Nevertheless, these concentrations provide valuable insights into the mechanism of action of DHFA, justifying further studies in more complex systems.64 These levels are comparable to those used in in vitro studies, demonstrating the bioactivity of DHFA, including its ability to inhibit platelet activation33 and counteract TNF-α-induced inflammation and oxidative stress in pre-adipocytes.64 DHFA is a metabolite derived from the metabolism of phenolic compounds present in certain foods, particularly those rich in ferulic acid and its derivatives. Foods such as whole grains, coffee, and certain vegetables (e.g., tomatoes, apples) are significant sources of these compounds.65 Regular consumption of these foods has been associated with an increase in plasma DHFA levels, suggesting that dietary intake could modulate the inflammatory responses through DHFA, as demonstrated in our study.62,66,67
Our results have some limitations. First, we did not directly determine which signaling pathways DFHA may have an effect (such as MAPK p38, ERK, and Akt kinase or NLRP3 proteins). On the other hand, it is known that LPS also increases the production of PGE2, affecting the release of TNF-α, IL-6, and IL-10 from macrophages via EP2 and EP4 receptors.68,69 We did not evaluate PGE2 in this study; however, our model with LPS-activated macrophages produced PGE1, but only after pretreatment with DHFA and not with LPS alone. We hypothesize that DHFA might make dihomo-gamma-linolenic acid (DGLA) more readily metabolized by COX enzymes following LPS stimulation.70 Our observation that DHFA induced PGE1 production with LPS co-stimulation suggests a potential role for PGE1 in the anti-inflammatory response mediated by IL-10. It is well-established that IL10 receptor (IL10R) and PGE receptor EP4 signaling synergize to elicit anti-inflammatory response.71 These findings warrant further investigation using animal models or clinical trials to determine whether DHFA's ability to promote both PGE1 and IL-10 production translates to clinically relevant improvements in chronic inflammatory states.
Conclusion
In conclusion, our findings demonstrate that DHFA possesses anti-inflammatory properties in macrophages under different inflammatory and oxidative stress conditions. DHFA treatment not only consistently increased the production of the anti-inflammatory cytokine IL-10, but also mitigated the inflammation induced by LPS and the oxidative stress atherogenic phenotype induced by 7KC and oxLDL. Notably, DHFA's effect appears to be mediated, at least in part, by the induction of PGE1, as it seems to specifically promote the regulatory pathway involving IL-10 secretion. Interestingly, DHFA also reduced the uptake of oxidized LDL and CD36 expression, suggesting its potential role in diminishing atherogenic processes. Overall, these results suggest that DHFA may be a promising candidate for complementing future therapeutic strategies targeting macrophages to combat inflammatory diseases and atherosclerosis.Author contributions
Muñoz-Durango, K., Sierra, J., and Lara-Guzmán, O. J.: conceptualization, investigation, writing – original draft, and writing – review and editing; Lara-Guzmán, O. J.: methodology, formal analysis and data curation. Arango-González, A. and Rivera, D. A: methodology. Muñoz-Durango, K. and Sierra, J.: project supervision and administration.Data availability
The raw data corresponding to the figures presented in this article are available at a Vidarium repository at https://github.com/Vidarium/Lipidox.Conflicts of interest
KM-D, JAS, DAR, and OJL-G are researchers at the Vidarium, Nutrition, Health and Wellness Research Center – Nutresa Business Group. AA-G states that she has no conflicts of interest.Acknowledgements
This study was funded by Vidarium, Nutrition, Health and Wellness Research Center – Nutresa Business Group and supported by the Colombian Ministry of Science, Technology, and Innovation (Minciencias) through tax benefits – administrative act no. 1336–2022. The authors thank the Nutresa Business Group and the Colombian Ministry of Science, Technology, and Innovation.References
- A. Pirillo, G. D. Norata and A. L. Catapano, LOX-1, OxLDL, and Atherosclerosis, Mediators Inflammation, 2013, 2013, 12 CrossRef PubMed.
- A. V. Poznyak, N. G. Nikiforov, A. M. Markin, D. A. Kashirskikh, V. A. Myasoedova, E. V. Gerasimova and A. N. Orekhov, Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis, Front. Pharmacol., 2020, 11, 613780 CrossRef CAS PubMed.
- C. R. Stewart, L. M. Stuart, K. Wilkinson, J. M. van Gils, J. Deng, A. Halle, K. J. Rayner, L. Boyer, R. Zhong, W. A. Frazier, A. Lacy-Hulbert, J. El Khoury, D. T. Golenbock and K. J. Moore, CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer, Nat. Immunol., 2010, 11, 155–161 CrossRef CAS PubMed.
- T. A. Seimon, M. J. Nadolski, X. Liao, J. Magallon, M. Nguyen, N. T. Feric, M. L. Koschinsky, R. Harkewicz, J. L. Witztum, S. Tsimikas, D. Golenbock, K. J. Moore and I. Tabas, Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress, Cell Metab., 2010, 12, 467–482 CrossRef CAS PubMed.
- F. J. Sheedy, A. Grebe, K. J. Rayner, P. Kalantari, B. Ramkhelawon, S. B. Carpenter, C. E. Becker, H. N. Ediriweera, A. E. Mullick, D. T. Golenbock, L. M. Stuart, E. Latz, K. A. Fitzgerald and K. J. Moore, CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation, Nat. Immunol., 2013, 14, 812–820 CrossRef CAS PubMed.
- A. Tedgui and Z. Mallat, Anti-inflammatory mechanisms in the vascular wall, Circ. Res., 2001, 88, 877–887 CrossRef CAS PubMed.
- L. J. Pinderski Oslund, C. C. Hedrick, T. Olvera, A. Hagenbaugh, M. Territo, J. A. Berliner and A. I. Fyfe, Interleukin-10 blocks atherosclerotic events in vitro and in vivo, Arterioscler. Thromb. Vasc. Biol., 1999, 19, 2847–2853 CrossRef CAS PubMed.
- G. Caligiuri, M. Rudling, V. Ollivier, M.-P. Jacob, J.-B. Michel, G. K. Hansson and A. Nicoletti, Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice, Mol. Med., 2003, 9, 10–17 CAS.
- T. Waehre, B. Halvorsen, J. K. Damås, A. Yndestad, F. Brosstad, L. Gullestad, J. Kjekshus, S. S. Frøland and P. Aukrust, Inflammatory imbalance between IL-10 and TNFalpha in unstable angina potential plaque stabilizing effects of IL-10, Eur. J. Clin. Invest., 2002, 32, 803–810 CrossRef CAS PubMed.
- B. Halvorsen, T. Waehre, H. Scholz, O. P. Clausen, J. H. von der Thüsen, F. Müller, H. Heimli, S. Tonstad, C. Hall, S. S. Frøland, E. A. Biessen, J. K. Damås and P. Aukrust, Interleukin-10 enhances the oxidized LDL-induced foam cell formation of macrophages by antiapoptotic mechanisms, J. Lipid Res., 2005, 46, 211–219 CrossRef CAS PubMed.
- M. Luo, N. He, Q. Xu, Z. Wen, Z. Wang, J. Zhao and Y. Liu, Roles of prostaglandins in immunosuppression, Clin. Immunol., 2024, 265, 110298 CrossRef CAS PubMed.
- H. Sinzinger, I. Virgolini and J. O'Grady, Clinical trials of PGE1, PGI2 and mimetics in patients with peripheral vascular disease, Prog. Clin. Biol. Res., 1989, 301, 85–96 CAS.
- S. Takai, D. Jin, H. Kawashima, M. Kimura, A. Shiraishi-Tateishi, T. Tanaka, S. Kakutani, K. Tanaka, Y. Kiso and M. Miyazaki, Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in ApoE-deficient mice, J. Atheroscler. Thromb., 2009, 16, 480–489 CrossRef CAS PubMed.
- A. L. Willis, D. L. Smith and C. Vigo, Suppression of principal atherosclerotic mechanisms by prostacyclins and other eicosanoids, Prog. Lipid Res., 1986, 25, 645–666 CrossRef CAS PubMed.
- H. Sinzinger, I. Virgolini, G. Lupattelli, E. Molinari, A. Gerakakis and P. Angelberger, Prostaglandin E1 decreases the low-density-lipoprotein entry into rabbit arterial wall, Br. J. Pharmacol., 1991, 103, 1626–1628 CrossRef CAS PubMed.
- M. M. B. W. Dooper, L. Wassink, L. M'Rabet and Y. M. F. Graus, The modulatory effects of prostaglandin-E on cytokine production by human peripheral blood mononuclear cells are independent of the prostaglandin subtype, Immunology, 2002, 107, 152–159 CrossRef CAS PubMed.
- Y. Mokuno, M. Takano, T. Matsuguchi, H. Nishimura, J. Washizu, Y. Naiki, Y. Nimura and Y. Yoshikai, Prostaglandin E(1) protects against liver injury induced by Escherichia coli infection via a dominant Th2-like response of liver T cells in mice, Hepatology, 1999, 30, 1464–1472 CrossRef CAS PubMed.
- A. Zmysłowski and A. Szterk, Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols, Lipids Health Dis., 2017, 16, 188 CrossRef PubMed.
- S. Ravi, P. Duraisamy, M. Krishnan, L. C. Martin, B. Manikandan, T. Raman, J. Sundaram, M. Arumugam and M. Ramar, An insight on 7- ketocholesterol mediated inflammation in atherosclerosis and potential therapeutics, Steroids, 2021, 172, 108854 CrossRef CAS PubMed.
- J.-D. Huang, J. Amaral, J. W. Lee and I. R. Rodriguez, 7-Ketocholesterol-induced inflammation signals mostly through the TLR4 receptor both in vitro and in vivo, PLoS One, 2014, 9, e100985 CrossRef PubMed.
- Y. Chen, X. Ye, G. Escames, W. Lei, X. Zhang, M. Li, T. Jing, Y. Yao, Z. Qiu, Z. Wang, D. Acuña-Castroviejo and Y. Yang, The NLRP3 inflammasome: contributions to inflammation-related diseases, Cell. Mol. Biol. Lett., 2023, 28, 51 CrossRef CAS PubMed.
- Y. Yang, H. Wang, M. Kouadir, H. Song and F. Shi, Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors, Cell Death Dis., 2019, 10, 128 CrossRef PubMed.
- Q. Xie, W.-W. Shen, J. Zhong, C. Huang, L. Zhang and J. Li, Lipopolysaccharide/adenosine triphosphate induces IL-1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells, Int. J. Mol. Med., 2014, 34, 341–349 CrossRef CAS PubMed.
- T. P. Domiciano, D. Wakita, H. D. Jones, T. R. Crother, W. A. J. Verri, M. Arditi and K. Shimada, Quercetin Inhibits Inflammasome Activation by Interfering with ASC Oligomerization and Prevents Interleukin-1 Mediated Mouse Vasculitis, Sci. Rep., 2017, 7, 41539 CrossRef CAS PubMed.
- Y. Liu, L. Shi, W. Qiu and Y. Shi, Ferulic acid exhibits anti-inflammatory effects by inducing autophagy and blocking NLRP3 inflammasome activation, Mol. Cell. Toxicol., 2022, 18, 509–519 CrossRef CAS PubMed.
- Y.-S. Yi, Regulatory Roles of Flavonoids on Inflammasome Activation during Inflammatory Responses, Mol. Nutr. Food Res., 2018, 62, e1800147 CrossRef PubMed.
- C. Pellegrini, M. Fornai, L. Antonioli, C. Blandizzi and V. Calderone, Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases, Int. J. Mol. Sci., 2019, 20, 2876 CrossRef CAS PubMed.
- M. Renouf, P. A. Guy, C. Marmet, A. L. Fraering, K. Longet, J. Moulin, M. Enslen, D. Barron, F. Dionisi, C. Cavin, G. Williamson and H. Steiling, Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: small intestine and colon are key sites for coffee metabolism, Mol. Nutr. Food Res., 2010, 54, 760–766 CrossRef CAS PubMed.
- M. Renouf, C. Marmet, F. Giuffrida, M. Lepage, D. Barron, M. Beaumont, G. Williamson and F. Dionisi, Dose–response plasma appearance of coffee chlorogenic and phenolic acids in adults, Mol. Nutr. Food Res., 2014, 58, 301–309 CrossRef CAS PubMed.
- G. Serreli, M. R. Naitza, S. Zodio, V. P. Leoni, M. Spada, M. P. Melis, A. Boronat and M. Deiana, Ferulic Acid Metabolites Attenuate LPS-Induced Inflammatory Response in Enterocyte-like Cells, Nutrients, 2021, 13, 3152 CrossRef CAS PubMed.
- S. H. Le, D. Yeo and J. Hong, Effect of dihydroferulic acid obtained from fermented rice bran extract on neuroprotection and behavioral recovery in an ischemic rat model, Food Sci. Technol., 2020, 40, 475–481 CrossRef.
- A. Sánchez-Medina, M. Redondo-Puente, R. Dupak, L. Bravo-Clemente, L. Goya and B. Sarriá, Colonic Coffee Phenols Metabolites, Dihydrocaffeic, Dihydroferulic, and Hydroxyhippuric Acids Protect Hepatic Cells from TNF-α-Induced Inflammation and Oxidative Stress, Int. J. Mol. Sci., 2023, 24, 1440 CrossRef PubMed.
- G. Baeza, E.-M. Bachmair, S. Wood, R. Mateos, L. Bravo and B. de Roos, The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors, Food Funct., 2017, 8, 1333–1342 RSC.
- O. J. Lara-Guzmán, S. Medina, R. Álvarez, C. Oger, T. Durand, J.-M. Galano, N. Zuluaga, Á. Gil-Izquierdo and K. Muñoz-Durango, Oxylipin regulation by phenolic compounds from coffee beverage: Positive outcomes from a randomized controlled trial in healthy adults and macrophage derived foam cells, Free Radicals Biol. Med., 2020, 160, 604–617 CrossRef PubMed.
- O. J. Lara-Guzmán, Á. Gil-Izquierdo, S. Medina, E. Osorio, R. Álvarez-Quintero, N. Zuluaga, C. Oger, J.-M. Galano, T. Durand and K. Muñoz-Durango, Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages, Redox Biol., 2018, 15, 1–11 CrossRef PubMed.
- S. Eshghjoo, D. M. Kim, A. Jayaraman, Y. Sun and R. C. Alaniz, Macrophage Polarization in Atherosclerosis, Genes, 2022, 13, 756 CrossRef CAS PubMed.
- M. Groeneweg, E. Kanters, M. N. Vergouwe, H. Duerink, G. Kraal, M. H. Hofker and M. P. J. de Winther, Lipopolysaccharide-induced gene expression in murine macrophages is enhanced by prior exposure to oxLDL, J. Lipid Res., 2006, 47, 2259–2267 CrossRef CAS PubMed.
- I. M. Larrayoz, J.-D. Huang, J. W. Lee, I. Pascual and I. R. Rodríguez, 7-ketocholesterol-induced inflammation: involvement of multiple kinase signaling pathways via NFκB but independently of reactive oxygen species formation, Invest. Ophthalmol. Visual Sci., 2010, 51, 4942–4955 CrossRef PubMed.
- I. H. K. Dias, I. Milic, C. Heiss, O. S. Ademowo, M. C. Polidori, A. Devitt and H. R. Griffiths, Inflammation, Lipid (Per)oxidation, and Redox Regulation, Antioxid. Redox Signal., 2020, 33, 166–190 CrossRef CAS PubMed.
- A. Anderson, A. Campo, E. Fulton, A. Corwin, W. G. 3rd Jerome and M. S. O'Connor, 7-Ketocholesterol in disease and aging, Redox Biol., 2020, 29, 101380 CrossRef CAS PubMed.
- G. Shi, S. Chen, W. S. Wandu, O. Ogbeifun, L. F. Nugent, A. Maminishkis, S. J. H. Hinshaw, I. R. Rodriguez and I. Gery, Inflammasomes Induced by 7-Ketocholesterol and Other Stimuli in RPE and in Bone Marrow-Derived Cells Differ Markedly in Their Production of IL-1β and IL-18, Invest. Ophthalmol. Visual Sci., 2015, 56, 1658–1664 CrossRef CAS PubMed.
- X. Li, Y. Zhang, M. Xia, E. Gulbins, K. M. Boini and P.-L. Li, Activation of Nlrp3 inflammasomes enhances macrophage lipid-deposition and migration: implication of a novel role of inflammasome in atherogenesis, PLoS One, 2014, 9, e87552 CrossRef PubMed.
- X. Yuan, O. M. Bhat, A. Samidurai, A. Das, Y. Zhang and P.-L. Li, Reversal of Endothelial Extracellular Vesicle-Induced Smooth Muscle Phenotype Transition by Hypercholesterolemia Stimulation: Role of NLRP3 Inflammasome Activation, Front. Cell Dev. Biol., 2020, 8, 597423 CrossRef PubMed.
- L. González, K. Rivera, M. E. Andia and G. Martínez Rodriguez, The IL-1 Family and Its Role in Atherosclerosis, Int. J. Mol. Sci., 2022, 24, 17 CrossRef PubMed.
- M. J. Page, D. B. Kell and E. Pretorius, The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation, Chronic Stress, 2022, 6, 24705470221076390 CrossRef PubMed.
- J. P. Rhoads and A. S. Major, How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses, Crit. Rev. Immunol., 2018, 38, 333–342 CrossRef PubMed.
- L. Rezende, N. F. Do Couto, W. Fernandes-Braga, Y. Epshtein, J. I. Alvarez-Leite, I. Levitan and L. de O. Andrade, OxLDL induces membrane structure rearrangement leading to biomechanics alteration and migration deficiency in macrophage, Biochim. Biophys. Acta, Biomembr., 2022, 1864, 183951 CrossRef CAS PubMed.
- O. J. Lara-Guzmán, R. Álvarez and K. Muñoz-Durango, Changes in the plasma lipidome of healthy subjects after coffee consumption reveal potential cardiovascular benefits: A randomized controlled trial, Free Radicals Biol. Med., 2021, 176, 345–355 CrossRef PubMed.
- M. A. Nayeem, Role of oxylipins in cardiovascular diseases, Acta Pharmacol. Sin., 2018, 39, 1142–1154 CrossRef CAS PubMed.
- C. Luquain-Costaz and I. Delton, Oxysterols in Vascular Cells and Role in Atherosclerosis, Adv. Exp. Med. Biol., 2024, 1440, 213–229 CrossRef CAS PubMed.
- Y. Kawai, T. Nishikawa, Y. Shiba, S. Saito, K. Murota, N. Shibata, M. Kobayashi, M. Kanayama, K. Uchida and J. Terao, Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids, J. Biol. Chem., 2008, 283, 9424–9434 CrossRef CAS PubMed.
- D. Del Rio, A. Stalmach, L. Calani and A. Crozier, Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols, Nutrients, 2010, 2, 820–833 CrossRef CAS PubMed.
- M. N. Clifford, J. J. J. van der Hooft and A. Crozier, Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols, Am. J. Clin. Nutr., 2013, 98, 1619S–1630S CrossRef CAS PubMed.
- P. Vitaglione, R. Barone Lumaga, R. Ferracane, S. Sellitto, J. R. Morelló, J. Reguant Miranda, E. Shimoni and V. Fogliano, Human bioavailability of flavanols and phenolic acids from cocoa-nut creams enriched with free or microencapsulated cocoa polyphenols, Br. J. Nutr., 2013, 109, 1832–1843 CrossRef CAS PubMed.
- G. Williamson and M. N. Clifford, Colonic metabolites of berry polyphenols: the missing link to biological activity?, Br. J. Nutr., 2010, 104(Suppl), S48–S66 CrossRef CAS PubMed.
- J. K. Aschoff, K. M. Riedl, J. L. Cooperstone, J. Högel, A. Bosy-Westphal, S. J. Schwartz, R. Carle and R. M. Schweiggert, Urinary excretion of Citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study, Mol. Nutr. Food Res., 2016, 60, 2602–2610 CrossRef CAS PubMed.
- Q.-H. Hu, X. Zhang, Y. Pan, Y.-C. Li and L.-D. Kong, Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats, Biochem. Pharmacol., 2012, 84, 113–125 CrossRef CAS PubMed.
- R. de Waal Malefyt, J. Abrams, B. Bennett, C. G. Figdor and J. E. de Vries, Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes, J. Exp. Med., 1991, 174, 1209–1220 CrossRef CAS PubMed.
- S. S. Iyer and G. Cheng, Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease, Crit. Rev. Immunol., 2012, 32, 23–63 CrossRef CAS PubMed.
- T. Kawamura, N. Nara, M. Kadosaki, K. Inada and S. Endo, Prostaglandin E1 reduces myocardial reperfusion injury by inhibiting proinflammatory cytokines production during cardiac surgery, Crit. Care Med., 2000, 28, 2201–2208 CrossRef CAS PubMed.
- A. Stalmach, G. Williamson and A. Crozier, Impact of dose on the bioavailability of coffee chlorogenic acids in humans, Food Funct., 2014, 5, 1727–1737 RSC.
- P. Vitaglione, I. Mennella, R. Ferracane, A. A. Rivellese, R. Giacco, D. Ercolini, S. M. Gibbons, A. La Storia, J. A. Gilbert, S. Jonnalagadda, F. Thielecke, M. A. Gallo, L. Scalfi and V. Fogliano, Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber, Am. J. Clin. Nutr., 2015, 101, 251–261 CrossRef CAS PubMed.
- E. Azzini, R. Bugianesi, F. Romano, D. Di Venere, S. Miccadei, A. Durazzo, M. S. Foddai, G. Catasta, V. Linsalata and G. Maiani, Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: a pilot study, Br. J. Nutr., 2007, 97, 963–969 CrossRef CAS PubMed.
- L. Goya, A. Sánchez-Medina, M. Redondo-Puente, R. Dupak, L. Bravo and B. Sarriá, Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells, Molecules, 2023, 29, 88 CrossRef PubMed.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jiménez, Polyphenols: food sources and bioavailability, Am. J. Clin. Nutr., 2004, 79, 727–747 CrossRef CAS PubMed.
- L. F. Călinoiu and D. C. Vodnar, Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability, Nutrients, 2018, 10, 1615 CrossRef PubMed.
- N. Kumar and V. Pruthi, Potential applications of ferulic acid from natural sources, Biotechnol. Rep., 2014, 4, 86–93 CrossRef PubMed.
- S. Uematsu, M. Matsumoto, K. Takeda and S. Akira, Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway, J. Immunol., 2002, 168, 5811–5816 CrossRef CAS PubMed.
- L. Treffkorn, R. Scheibe, T. Maruyama and P. Dieter, PGE2 exerts its effect on the LPS-induced release of TNF-alpha, ET-1, IL-1alpha, IL-6 and IL-10 via the EP2 and EP4 receptor in rat liver macrophages, Prostaglandins Other Lipid Mediators, 2004, 74, 113–123 CrossRef CAS PubMed.
- S. Kakutani, H. Kawashima, T. Tanaka, A. Shiraishi-Tateishi and Y. Kiso, Uptake of dihomo-gamma-linolenic acid by murine macrophages increases series-1 prostaglandin release following lipopolysaccharide treatment, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2010, 83, 23–29 CrossRef CAS PubMed.
- A. Samiea, J. S. J. Yoon, S. T. Cheung, T. C. Chamberlain and A. L.-F. Mui, Interleukin-10 contributes to PGE2 signalling through upregulation of EP4 via SHIP1 and STAT3, PLoS One, 2020, 15, e0230427 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo02114b |
| This journal is © The Royal Society of Chemistry 2024 |