Probiotic interventions with highly acid-tolerant Levilactobacillus brevis strains improve lipid metabolism and gut microbial balance in obese mice†
Liping
Zhou
a,
Luchan
Gong
a,
Zhihao
Liu
a,
Jinfeng
Xiang
a,
Cong
Ren
 *ab and
Yan
Xu
*ab and
Yan
Xu
 ab
ab
aLab of Brewing Microbiology and Applied Enzymology, Key Laboratory of Industrial Biotechnology of Ministry of Education, Jiangnan University, Wuxi, China. E-mail: congren@jiangnan.edu.cn
bChina Key Laboratory of Microbiomics and Eco-brewing Technology for Light Industry, Wuxi 214122, Jiangsu, China
First published on 2nd December 2024
Abstract
Many studies have shown that specific lactic acid bacteria (LAB) strains can delay obesity, offering a viable alternative to medications and surgeries. However, the mining and development of highly effective LAB strains for obesity control is still limited. In this study, the naturally highly acid-tolerant and gamma-aminobutyric acid-producing Levilactobacillus brevis D17 and its glnR deletion strain were used to investigate their anti-obesity effects. In an 8-week mouse experiment, L. brevis D17 and its glnR-deletion strain D17ΔglnR significantly reduced weight gain by 28.4% and 29.1%, respectively, improving abnormal serum indicators and glucose metabolism caused by a high-fat diet. Furthermore, L. brevis D17 and its glnR-deletion strain D17ΔglnR successfully colonized in the gut. Both D17 and D17ΔglnR interventions significantly restored the relative abundance of Muribaculaceae, Ileibacterium valens, Lactobacillus, Faecalibaculum, Bifidobacterium globosum, Akkermansia muciniphila, and Romboutsia ilealis, whereas they significantly reduced potentially harmful bacteria like Leptogranulimonas, Flintibacter, and Alistipes. Additionally, L. brevis intervention effectively decreased the levels of primary bile acids and increased secondary bile acids in the gut, thus balancing bile acid metabolism. The transcriptional analysis suggested that D17 and D17ΔglnR interventions may activate the AMPK signaling pathway in the liver to inhibit lipogenesis, activate the cAMP pathway to promote lipolysis, and inhibit pro-inflammatory macrophage infiltration to block inflammatory responses. These results indicate that L. brevis D17 and its glnR-deletion mutant strain D17ΔglnR show great potential in combating obesity. Moreover, these results also provide insights into the underlying mechanism behind their anti-obesity properties.
1. Introduction
Obesity has emerged as a global epidemic that necessitates immediate attention. Multiple factors contribute to obesity, including insulin resistance, leptin resistance, gut microbiota imbalances, bile acid metabolism disturbances, and pro-inflammatory macrophage infiltration.1–5 Recent studies have proved the pivotal role of gut microbiota in the development of obesity, with its influence including the following three aspects: firstly, in terms of energy storage, the gut microbiome in obese individuals significantly enhances the host's capacity for energy acquisition and storage.6 For instance, obesity-related gut microbiota amplifies central appetite and reward signals via the gut–brain axis, thereby increasing the host's food consumption.7 Subsequently, regarding the gut microbiota structure, obese individuals exhibit disordered microbiota, characterized by diminished intestinal microbial diversity,8 and an increase in harmful bacteria that promote obesity, such as Bilophila wadsworthia,9Enterobacter cloacae10 and Prevotella copri.11 Lastly, from a metabolic perspective, an imbalanced microbiota impairs bile acid metabolism, negatively impacting lipid metabolism.12 To overcome these challenges, lactic acid bacteria (LAB) intervention, which is devoid of drug-related side effects or surgical risks, presents a safer and more proactive alternative to conventional obesity treatments.Increasing studies suggest that lactic acid bacteria (LAB) have effects on obesity, insulin resistance, and other diseases, with Lactobacillus being the most investigated genus. Many Lactobacillus species are generally recognized as safe strains (GRAS) by the U.S. Food and Drug Administration (FDA)13 and have been granted the qualified presumption of safety (QPS) status by the European Food Safety Authority (EFSA).14 Notably, several Lactobacillus species have been shown to be effective in combating obesity, including L. plantarum, L. fermentum, L. casei, L. rhamnosus and L. reuteri. For instance, a clinical study has shown that L. plantarum K50 can significantly reduce blood lipid levels in obese individuals.15 Similarly, clinical evidence suggests that L. rhamnosus GG substantially slows the progression of obesity-related liver diseases.16 Mechanistically, Lactobacillus exerts its anti-obesity effects through multiple pathways, including restoring gut microbiota, inhibiting pathogens, altering enzymatic activities associated with bile acid metabolism, synthesizing short-chain fatty acids, modulating cytokine production, and engaging with the gut–brain axis to regulate endocrine and neural functions.17
A fundamental requirement for Lactobacillus to exert its anti-obesity effects is its survival and arrival in the intestine in substantial numbers.18 Hence, assessing the acid resistance of Lactobacillus is of critical importance. Lactobacillus mitigates acid stress primarily through six machineries: alkali generation, glutamate decarboxylase (GAD) system, F1-F0-ATPase proton pump, membrane barrier, intracellular macromolecule protection and repair, and pre-adaptation with cross-protection.19–21 Among them, the GAD system emerges as a key acid resistance machinery in multiple Lactobacillus species, including L. brevis, L. plantarum, L. sakei, L. buchneri, and L. reuteri.22,23 Acid stress triggers the efficient expression of the GAD system, including the gadCB operon (gadC encoding the glutamate/GABA antiporter and gadB encoding glutamate decarboxylase) and its transcriptional regulator gadR.24 This results in extracellular transport of glutamate into the cell, followed by decarboxylation of glutamate, a process that consumes H+ ions and generates GABA.25 In other words, the capacity of GABA production is directly correlated with acid resistance capacity. Our previous study identified a novel acid resistance mechanism mediated by glnR-mediated negative regulation on the transcription of gadR and gadCB operons in Lactobacillus under glutamate-rich conditions.26 To create an environment rich in glutamate within living organisms, it is necessary to supplement their diet with this amino acid. However, such supplementation poses potential risks to health and safety if taken in excess.27L. brevis D17, a naturally highly acid-tolerant strain isolated from fermented grains of Chinese liquor making, exhibited significantly higher transcription levels of the gadR and gadCB genes compared to the model strain L. brevis ATCC 367.25 These traits align with the standards for a probiotic, indicating strong potential for probiotic applications. Additionally, following the discovery of the glnR-gadR regulation module on acid tolerance capability of L. brevis, genetically modifying L. brevis D17 to obtain a glnR-deletion strain for further investigation of its physiological functions, particularly in relation to its potential health benefits when consumed as part of a balanced diet without added glutamate, becomes an interesting pursuit.
In this study, we initially constructed a glnR in-frame-deletion strain of L. brevis D17. Subsequently, we explored the anti-obesity functions of L. brevis D17 and its glnR-deletion strain D17ΔglnR through an 8-week high-fat diet intervention in mice. Finally, we investigated the potential anti-obesity mechanisms of L. brevis D17 and D17ΔglnR through gut microbiome analysis, fecal non-targeted metabolomics, and transcriptional analysis for genes related to liver lipid metabolism and inflammation.
2. Materials and methods
2.1 Bacterial strains, media, and growth conditions
Levilactobacillus brevis D17 was obtained from the authors’ lab. Escherichia coli TOP10 was procured from Sangon Biotech. The modified de Man–Rogosa–Sharpe (mMRS) medium contained 10 g L−1 tryptone, 10 g L−1 beef extract, 5 g L−1 yeast extract, 20 g L−1 glucose, 5 g L−1 sodium acetate anhydrous, 2 g L−1 ammonium citrate, 1 g L−1 Tween 80, 30 g L−1 monosodium glutamate, 2 g L−1 KH2PO4, 0.5 g L−1 MgSO4·7H2O, 0.2 g L−1 MnSO4·H2O, and 0.1 g L−1 NaCl. The Luria–Bertani (LB) medium contained 10 g L−1 tryptone, 5 g L−1 yeast extract, and 10 g L−1 NaCl. L. brevis was cultured in mMRS medium and E. coli was cultured in LB medium at 37 °C and 200 rpm.2.2 Construction and validation of the glnR deletion strain
D17ΔglnR, an in-frame glnR deletion strain of L. brevis D17, was constructed by using the following method based on our previous study.26 Initially, two DNA fragments, A and B, adjacent to the glnR gene, were amplified from L. brevis D17 genomic DNA using primer pairs glnR-up-BamHI-F/glnR-up-R and glnR-down-F/glnR-down-HindIII-R (as listed in Table 1), respectively. Each fragment included an additional 21 bp sequence adjacent to the glnR gene. These fragments were then fused using one-step fusion PCR. Subsequently, the A–B fusion fragment and pGID023 plasmid were digested with BamHI and HindIII and ligated at 16 °C for 16 hours. This ligation mixture was transformed into E. coli TOP10 cells to construct the deletion plasmid pGID023-glnR (9.9 kb). The constructed plasmid pGID023-glnR was subsequently introduced into L. brevis cells through electroporation. Following electroporation, Campbell-type integration of the pGID023-glnR plasmid with the L. brevis genome occurred at either the A or B region, resulting in the incorporation of pGID023-glnR into the L. brevis genome. Subsequent intrachromosomal recombination with the other fragment (B or A) resulted in the complete deletion of the glnR open reading frame (ORF). PCR analysis was conducted on two L. brevis strains using primer pairs glnR-up-BamHI-F and glnR-down-HindIII-R. Agarose gel electrophoresis was subsequently carried out to confirm the successful construction of D17ΔglnR.| Primer | Sequence (5′–3′) |
|---|---|
| glnR-up-BamHI-F | AGCGCGGATCCACTGGCCATTAAATTAGCCCAAGCCTTTA |
| glnR-down-HindIII-R | CCCAAGCTTCCAAGCAACATAGACCGGTGCTTCGT |
| glnR-up-R | CGGCCGCTGTTGCTGCCGTAATTCTTTTTCTTTCACCACTAC |
| glnR-down-F | GAAAAAGAATTACGGCAGCAACAGCGGCCGCTTTAAGATATTGATGAG |
2.3 Preparation of bacteria suspension and simulated gastrointestinal fluid tolerance test
The activated L. brevis culture was inoculated into 100 mL of liquid mMRS medium at a 2.5% inoculation rate. This culture was incubated for 10 hours until it reached the mid-logarithmic growth phase (OD600 = 2.0). The cultures were then washed three times with sterile saline, and the cells were resuspended in sterile saline to achieve a cell suspension with a concentration of 109 CFU per mL for further experiments.Simulated gastric juice was prepared by dissolving 3 mg of pepsin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) in 1 mL of sterile saline, adjusting the pH to 2.5 with 0.1 mol L−1 HCl, and filtering the solution through a 0.22 μm sterile membrane. Simulated intestinal fluid was prepared by dissolving 1 mg of trypsin (1
000) in 1 mL of sterile saline, adjusting the pH to 2.5 with 0.1 mol L−1 HCl, and filtering the solution through a 0.22 μm sterile membrane. Simulated intestinal fluid was prepared by dissolving 1 mg of trypsin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250) in 1 mL of sterile saline, adjusting the pH to 8.0 with 0.1 mol L−1 NaOH, adding 0.3% bovine bile salt, and filtering the solution through a 0.22 μm sterile membrane.
250) in 1 mL of sterile saline, adjusting the pH to 8.0 with 0.1 mol L−1 NaOH, adding 0.3% bovine bile salt, and filtering the solution through a 0.22 μm sterile membrane.
The suspension with 109 CFU per mL bacterial cells was divided into several sterile Eppendorf tubes, each containing 1 mL. These aliquots were incubated at 37 °C. For the 3-hour simulated gastric juice tolerance test, cultures were centrifuged and resuspended in simulated gastric juice at 0, 1, 2, 2.5, and 3 h. At the 3-hour mark, bacterial cells were collected through centrifugation. For the 5-hour simulated intestinal fluid tolerance test, cultures were centrifuged and resuspended in simulated intestinal fluid at 0, 1, 2, 3, 4, and 5 h. At the 5-hour mark, bacterial cells were collected through centrifugation. The collected cells from both tests were resuspended in sterile saline and spread on mMRS agar. The plates were incubated at 37 °C for 48 hours. The survival rate was calculated by dividing the colony counts post-stress treatment by the colony counts prior to the stress treatment.
2.4 Animal experiment
Thirty-two 8-week-old C57BL/6J male mice (specific-pathogen free grade, Beijing Vital River Laboratory Animal Technology Co., Ltd) were housed in groups of four mice per cage, with free access to food and water under a strict 12-hour light/dark cycle. Following a one-week acclimatization period, the mice were evenly divided into four groups (n = 8 per group) based on body weight (ensure uniformity in starting conditions) as follows: (1) NFD group: mice were fed a normal diet (XTCON50J, Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd), supplemented with 0.2 mL of sterile saline; (2) HFD group: mice were fed a high-fat diet (XTHF60, Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd, 60% kcal fat), supplemented with 0.2 mL of sterile saline; (3) D17 group: mice were fed a high-fat diet, supplemented with a 0.2 mL saline suspension containing 109 CFU per mL of live L. brevis D17; and (4) D17ΔglnR group: mice were fed a high-fat diet, supplemented with a 200 μL saline suspension containing 109 CFU per mL live L. brevis D17ΔglnR. Mice were orally gavaged daily at 9 am for 8 weeks. The energy distribution and nutritional composition of the NFD and HFD are detailed in Tables S1 and S2.† Food intake, growth, and activity levels of mice were recorded daily during gavage. Each mouse's body weight and average food intake were recorded weekly. Fecal samples were collected at three key points: before initial gavage, mid-trial, and at trial conclusion, and were immediately stored at −80 °C. On the last day of the gavage period, all mice were fasted overnight and sacrificed by decapitation. The liver, kidneys, spleen, epididymal white adipose tissue (eWAT), and inguinal subcutaneous white adipose tissue (iWAT) from each mouse were precisely dissected, weighed, and collected for further analysis. The organ index was calculated as the ratio of organ weight to the final body weight. All of the study protocols were approved by the Ethics Committee of Jiangnan University, China (approval number: JN. no. 20220930c0321220).2.5 Insulin tolerance test (ITT) and oral glucose tolerance test (OGTT)
The ITT was performed at the beginning of week 8, followed by the OGTT three days later. For the ITT, mice were fasted for 6 hours (8 AM–2 PM) followed by intraperitoneal injection of insulin (0.75 IU kg−1, human insulin). Blood samples were collected at 0, 15, 30, 60, and 120 minutes by tail clipping, with blood glucose levels measured using a glucometer (Accu-Chek Performa, Roche Diagnostics, Indianapolis, IN, USA). For the OGTT, mice were fasted overnight (6 PM–6 AM), followed by a gavage of glucose (1.2 g kg−1, 12% glucose solution). Blood samples were collected at 0, 15, 30, 60, and 120 minutes by tail clipping, and blood glucose levels were measured using a glucometer. The area under the glucose curve (AUC) was calculated by plotting blood glucose concentration (mmol L−1) versus time (min).2.6 Biochemical analysis of serum samples
Blood samples were collected from the orbit into 1.5 mL centrifuge tubes and placed at room temperature for one hour. Serum was separated by centrifuging the blood samples at 3000 rpm and 4 °C for 15 minutes, and then stored at −80 °C for biochemical analyses. Leptin and insulin levels were quantified by using an ELISA kit (R&D Systems, Minneapolis, MN, USA). Total cholesterol (TC), triglyceride (TG), fasting blood glucose, serum superoxide dismutase (SOD), malondialdehyde (MDA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), high-density lipoprotein cholesterol (HDL) and low-density lipoprotein cholesterol (LDL) levels were measured using commercial assay kits (Jiancheng Institute of Biological Engineering, Nanjing, China).2.7 Histopathological assessment of liver and adipose tissues
Fresh liver and adipose tissue samples (eWAT and iWAT) were fixed in 4% paraformaldehyde overnight and subsequently sectioned into 5 μm slices. The sections were dried and dewaxed in an oven at 90 °C, followed by 6 minutes of hematoxylin and eosin (H&E) staining. These sections were examined using a fluorescence microscope (Leica, Solms, Germany). ImageJ program was used to quantify lipid droplet sizes within the liver tissue, as well as fat vesicle sizes in the epididymal fat pad and inguinal white adipose tissue.2.8 Fecal DNA extraction and amplicon sequencing
Total DNA was extracted from each mouse's fecal sample using a QIAamp fast DNA stool mini kit (Qiagen GmbH, Hilden, Germany). Gut microbial composition was analyzed by amplifying the V3–V4 region of the 16S rRNA gene using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACNVGGGTWTCTAAT-3′). The PCR amplification reaction was achieved using a thermocycler PCR machine (GeneAmp 9700, ABI, Foster City, CA). Purified PCR products were pooled equimolarly and paired-end sequenced (2 × 250) on an Illumina MiSeq platform (Illumina, San Diego, CA). Raw fastq files were demultiplexed and quality-filtered using QIIME2 (version 2021.11). The de-duplicated paired-end sequences were denoised, merged, and clustered into amplicon sequence variants (ASVs) using the dada2 program in QIIME2. Taxonomic classification of ASVs was achieved using a QIIME2-pretrained Naive Bayes classifier trained on the Silva 138 99% OTU database. The raw sequences of 16S rRNA gene amplicon sequencing have been deposited in the NCBI Sequence Read Archive (SRA) with project number PRJNA1132689.2.9 Metagenomic analysis
Fecal DNA from each group of eight mice was mixed and sequenced using the combined DNA samples. DNA was fragmented using a Covaris S220 Focused-ultrasonicator (Woburn, MA, USA), followed by the preparation of sequencing libraries with a target fragment length of approximately 450 bp. All samples were sequenced on an Illumina HiSeq X instrument in paired-end 150 bp (PE150) mode. The raw sequence reads underwent quality trimming using Trimmomatic (https://www.usadellab.org/cms/uploads/supplementary/Trimmomatic) to eliminate adapter contaminants and low-quality reads.28 Clean reads, free from host-genome contamination and low-quality data, were used for further assembly by Megahit version 1.1.3.79 Metagenomic binning was conducted on contigs from each sample using the software metaBAT2, metadecoder, and semibin.29–31 CheckM v.1.0.3 was used to assess the completeness and contamination of all bins.32 Bins with completeness greater than 90% and contamination less than 10% were categorized as “filtered bins”. To enhance the assembly quality of metagenome-assembled genomes (MAGs), metaSPAdes was employed to re-assemble MAGs using data extracted from clean reads through the BWA MEM method.33 Subsequently, CoverM was used to calculate the abundance of each MAG with default parameters. The raw sequences of metagenomic sequencing have been deposited in the NCBI SRA with project number PRJNA1072169.2.10 Metabolomic analysis of feces
100 mg of thawed fecal samples were placed in 1.5 mL centrifuge tubes, and 500 μL of 80% methanol solution was added. The samples were vortexed and shaken, and then placed in an ice bath for 5 minutes. After that, they were centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes at 4 °C. A portion of the supernatant was diluted with mass spectrometry-grade water to achieve 53% methanol, and then centrifuged again at 15
000 rpm for 20 minutes at 4 °C. A portion of the supernatant was diluted with mass spectrometry-grade water to achieve 53% methanol, and then centrifuged again at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes at 4 °C. The resulting supernatant was collected as the sample extract for UHPLC-MS/MS analysis. Twenty microliters of each prepared sample were extracted and mixed as the quality control sample.
000 rpm for 20 minutes at 4 °C. The resulting supernatant was collected as the sample extract for UHPLC-MS/MS analysis. Twenty microliters of each prepared sample were extracted and mixed as the quality control sample.
UHPLC-MS/MS analysis was performed using a Vanquish UHPLC system (Thermo Fisher Scientific, Germany) coupled with an Orbitrap Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific, Germany). The flow rate was set at 0.2 mL min−1, and the chromatographic column used was a Hypersil Gold (100 × 2.1 mm, 1.9 μm). In the positive polarity mode, the eluents were eluent A (0.1% formic acid) and eluent B (pure methanol). In the negative polarity mode, the eluents were eluent A (5 mM ammonium acetate, pH 9.0) and eluent B (pure methanol). The solvent gradient was as follows: 2% B for 1.5 minutes; 2–100% B over 12 minutes; 100% B for 14 minutes; 100–2% B over 0.1 minutes; and 2% B for 17 minutes. The Q Exactive HF-X mass spectrometer operated in both positive and negative polarity modes, with a spray voltage of 3.2 kV, a capillary temperature of 320 °C, a sheath gas flow rate of 40 arb, and an auxiliary gas flow rate of 10 arb.
2.11 RNA extraction and reverse transcription-quantitative PCR for liver tissues
Frozen liver tissues were rapidly ground in liquid nitrogen, followed by RNA extraction using the TRIzol method. The extracted RNA was reverse-transcribed into cDNA using a PrimeScript RT reagent kit with a gDNA Eraser (Takara, Dalian, China), as follows: (1) the genomic DNA was removed from total RNA extracts with a reaction system consisting of 1 μg of RNA, 2 μL of 5× gDNA Eraser buffer, 1 μL of gDNA Eraser, and RNase-free double-distilled water (ddH2O) to a final volume of 10 μL, followed by incubation at 42 °C for 2 minutes; (2) the DNA-free RNA was reverse transcribed into cDNA by using the reaction mixture from step 1 (10 μL), 1 μL of PrimeScript RT enzyme mix, 1 μL of RT primer mix, 4 μL of 5× PrimeScript buffer, and 4 μL of RNase-free ddH2O, to a total volume of 20 μL, followed by incubation at 37 °C for 15 minutes. Quantitative PCR (qPCR) was performed on a StepOnePlus real-time machine (Applied Biosystems, Foster City, CA, USA) using a fast SYBR green master mix kit (Life Technologies, Gaithersburg, MD, USA). The real-time qPCR reaction system consists of 10 μL of SYBR green master mix, 0.4 μL of each primer, 1 μL of cDNA template, and 8.2 μL of RNase-free ddH2O, totaling 20 μL. The qPCR protocol was as follows: pre-denaturation at 95 °C for 3 minutes, denaturation at 95 °C for 10 s, annealing at 57 °C for 10 s, and extension at 72 °C for 30 s, a total of 40 cycles. The primers used in this study were derived from PrimerBank as shown in Table 2. The relative gene expression levels were normalized to the expression level of the β-actin gene.| Gene name | Forward primer (5′–3′) | Reverse primer (5′–3′) | Product length (bp) |
|---|---|---|---|
| β-Actin | GTGACGTTGACATCCGTAAAGA | GCCGGACTCATCGTACTCC | 123 |
| Srebf1 | GATGTGCGAACTGGACACAG | CATAGGGGGCGTCAAACAG | 104 |
| Fasn | GGAGGTGGTGATAGCCGGTAT | TGGGTAATCCATAGAGCCCAG | 140 |
| Cyp7a1 | GCTGTGGTAGTGAGCTGTTG | GTTGTCCAAAGGAGGTTCACC | 78 |
| Cd36 | ATGGGCTGTGATCGGAACTG | TTTGCCACGTCATCTGGGTTT | 233 |
| Scd1 | TTCTTGCGATACACTCTGGTGC | CGGGATTGAATGTTCTTGTCGT | 98 |
| Adrb3 | GGCCCTCTCTAGTTCCCAG | TAGCCATCAAACCTGTTGAGC | 229 |
| Lipe | CCAGCCTGAGGGCTTACTG | CTCCATTGACTGTGACATCTCG | 106 |
| Il-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG | 131 |
| Tnfα | GACGTGGAACTGGCAGAAGAG | TTGGTGGTTTGTGAGTGTGAG | 228 |
| Mcp-1 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT | 121 |
| Prkaa1 | GTCAAAGCCGACCCAATGATA | CGTACACGCAAATAATAGGGGTT | 100 |
| Pparγ | GGAAGACCACTCGCATTCCTT | GTAATCAGCAACCATTGGGTCA | 121 |
| Cd68 | TGTCTGATCTTGCTAGGACCG | GAGAGTAACGGCCTTTTTGTGA | 75 |
| Cd206 | CTCTGTTCAGCTATTGGACGC | CGGAATTTCTGGGATTCAGCTTC | 132 |
| F4/80 | TGACTCACCTTGTGGTCCTAA | CTTCCCAGAATCCAGTCTTTCC | 111 |
| Ldlr | TGACTCAGACGAACAAGGCTG | ATCTAGGCAATCTCGGTCTCC | 118 |
2.12 Statistical analysis
The significance analyses were conducted at different time points in both the simulated gastric juice tolerance test and the simulated intestinal fluid tolerance test using multiple t-tests. For the other significance analyses, one-way ANOVA with Tukey's post hoc test for significance analysis was used.3. Results
3.1 L. brevis D17 and its glnR-deletion strain D17ΔglnR demonstrated high GABA production efficiency and robust tolerance to gastrointestinal fluid stresses
The in-frame deletion of glnR in L. brevis D17 was confirmed by using agarose gel electrophoresis (Fig. 1a). Then we observed that the deletion of the glnR gene had no impact on the growth of L. brevis D17 (Fig. 1b). During the 48-h fermentation, the final titers of GABA production for L. brevis D17 and its glnR-deletion strain D17ΔglnR were comparable, with yields of 19.2 g L−1 and 20.1 g L−1, respectively. However, the GABA synthesis in D17ΔglnR was more efficient than that in D17. When considering the fermentation within 24 h, the titer of GABA in D17ΔglnR was 18.5 g L−1, 41.2% higher than that in D17 (13.1 g L−1) (Fig. 1b). When considering the fermentation within 12 h, the titer of GABA in D17ΔglnR was 12.4 g L−1, 244.4% higher than that in D17 (3.6 g L−1) (Fig. 1b).It is worth noting that the acid tolerance and conversion efficiency of D17 from glutamate into GABA was much higher than those of other L. brevis strains, such as the model strain ATCC 367.26 However, its tolerance in complex gastrointestinal environments remains unclear. Thus, the tolerance of L. brevis D17 and D17ΔglnR to the gastrointestinal environment was evaluated in simulated gastric juice (pH 2.5) and simulated intestinal juice (pH 8.0). The survival rate of these two strains both in the simulated gastric juice and simulated intestinal juice showed a time-dependent decline, most notably during the first hour post stress exposure (0–1 h) (Fig. 1c and d), suggesting that initial exposure to the stressful environment is extremely detrimental to live cells. Notably, after enduring treatment up to 3 h in simulated gastric juice, the survival rate of D17 and D17ΔglnR remained 18% and 48%, respectively. Following a 5-h exposure to simulated intestinal fluid, the survival rate of D17 and D17ΔglnR remained 10% and 13%, respectively. In summary, L. brevis D17 and D17ΔglnR both exhibited strong tolerance to gastrointestinal environments, with the deletion of glnR further enhancing this tolerance and increasing GABA production.
3.2 L. brevis D17 and its glnR-deletion strain D17ΔglnR significantly delayed obesity development in high-fat diet-induced mice
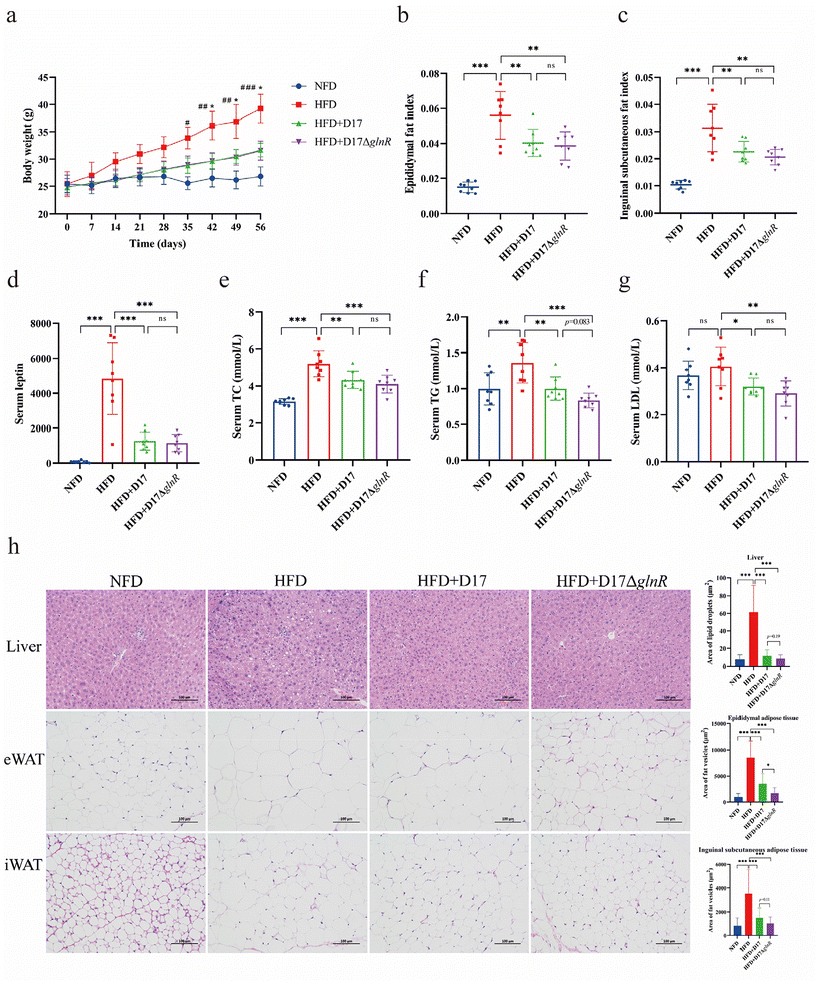
Following a 28-day high-fat diet (HFD), HFD mice exhibited a weight gain by 23.7%, which was 20% higher than that of the non-high-fat diet-fed (NFD) mice, indicating the successful establishment of an obesity model (Fig. 2a). The weight gains persisted throughout the intervention, acuminating to a 46.5% increase for the HFD mice compared with the NFD mice at 56 d. Compared with the control (HFD) mice, oral administration of L. brevis D17 and D17ΔglnR significantly alleviated the weight gain, reducing the weight gain by 28.4% and 29.1%, respectively. Interestingly, although a remarkable difference in body weight between the HFD group and the L. brevis intervention groups was observed, the food consumption within these three groups was found to be similar (Fig. S1a†). We then evaluated the major fat indexes. When administered with a high-fat diet, the epididymal fat index and inguinal subcutaneous index in the HFD + D17 group decreased by 28.6% and 27.8% when compared with the HFD group, respectively (Fig. 2b and c). Similar change patterns were found in the HFD + D17ΔglnR group.Then serum indices were examined. A high-fat diet induced leptin resistance, observed as the elevated levels of serum leptin in HFD mice (Fig. 2d). Meanwhile, significant increases were noted in triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), alanine aminotransferase (ALT), and malondialdehyde (MDA) levels in the HFD group. With the administration of L. brevis D17 and D17ΔglnR, leptin levels were decreased by 73.9% and 76.2%, respectively (Fig. 2d). Likewise, TC levels were decreased by 17.3% and 21.2%, TG levels were decreased by 27.2% and 38.9%, and LDL levels were decreased by 21.9% and 29.3%, respectively (Fig. 2e–g). Conversely, high density lipoprotein (HDL) levels were increased by 12.9% and 13.3% when administering L. brevis D17 and D17ΔglnR, respectively (Fig. S1b†). Supplementing with L. brevis D17 and its glnR-deletion derivative, D17ΔglnR, showed additional benefits. Specifically, serum ALT levels decreased by 27.1% and 33.1%, respectively, while AST levels decreased by 11.8% and 12.4%, respectively. This improvement in liver function was accompanied by a significant increase in superoxide dismutase (SOD) activity of 9.4% with D17 supplementation, indicating enhanced antioxidant defenses. Moreover, malondialdehyde (MDA), a marker of oxidative stress, decreased by 17.4% and 20.9%, respectively, demonstrating the beneficial effects of L. brevis supplementation on liver function and overall oxidative stress levels under high-fat diet intervention34,35 (Fig. S1c–f†).
Moreover, the histological staining revealed substantial changes in the liver and adipose tissues of high-fat diet-induced obese mice. Feeding a high-fat diet caused enlarged adipocytes in the liver, epididymal white adipose tissue (eWAT) and inguinal subcutaneous white adipose tissue (iWAT) (Fig. 2h, HFD panel). Interestingly, supplementation with L. brevis D17 and D17ΔglnR significantly slowed the increase in fat vesicle size. In the epididymal adipose tissue, the increase in the fat vesicle size was delayed by 58.8% and 80.1%, respectively, while in the inguinal subcutaneous adipose tissue, the delay was 57.5% and 70.6%. Additionally, the growth of liver lipid droplets was delayed by 81.1% and 86.2%, respectively (Fig. 2h, “HFD + D17 and HFD + D17ΔglnR quantitative analysis” panels). Therefore, supplementation of either L. brevis D17 or D17ΔglnR significantly reduced the risk of non-alcoholic fatty liver disease (NAFLD).
3.3 L. brevis D17 and its glnR-deletion strain D17ΔglnR significantly attenuated metabolic dysfunction in HFD-fed mice
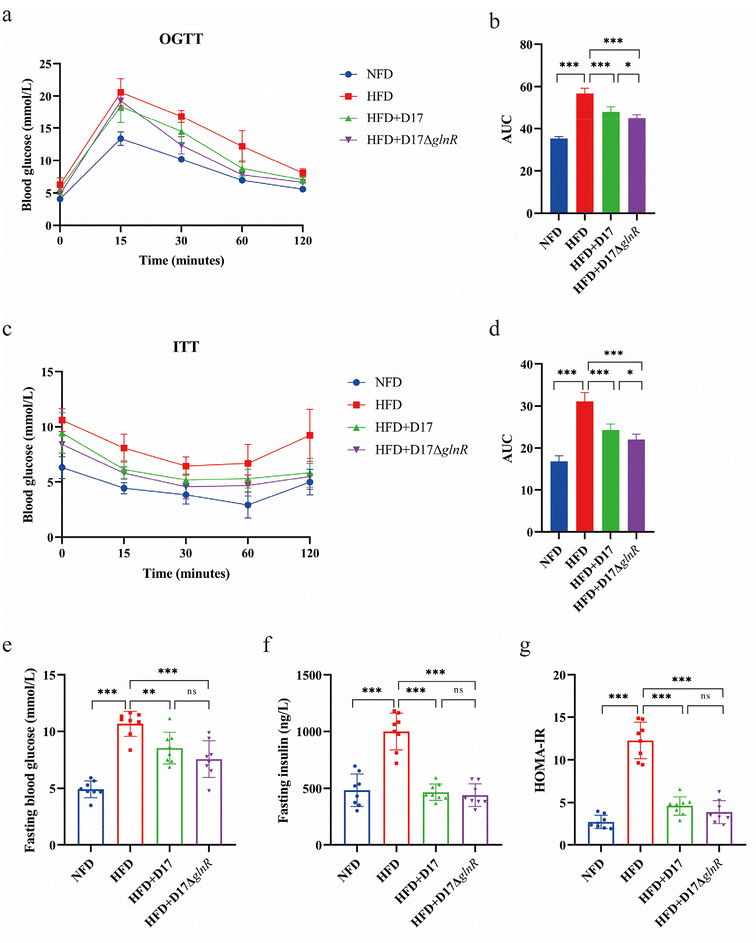
To assess the impact of a high-fat diet on blood glucose metabolism, the oral glucose tolerance test (OGTT) and the insulin tolerance test (ITT) were conducted. In the two-hour OGTT, the blood glucose levels of mice exhibited a rise followed by a decline, attributable to the inherent glucose metabolic response of the mice. Nonetheless, the blood glucose levels of obese mice in the HFD group remained at 8.1 mmol L−1 after two hours of glucose gavage, indicating a development of glucose intolerance (Fig. 3a). The administration of L. brevis D17 and D17ΔglnR moderated blood glucose levels to 7.0 mmol L−1 and 6.6 mmol L−1, respectively, and led to significant reductions in the corresponding area under the curve (AUC) (Fig. 3a and b). In the two-hour ITT, despite exogenous insulin injection, the obese mice in the HFD group maintained higher blood glucose levels (9.2 mmol L−1), indicating a development of insulin resistance. Notably, glucose intolerance and insulin resistance are closely associated with the development of obesity.36,37 However, supplementation of L. brevis D17 and D17ΔglnR normalized blood glucose levels to 5.8 mmol L−1 and 5.5 mmol L−1, respectively, and was accompanied by a significant reduction in the AUC (Fig. 3c and d).Furthermore, compared to the HFD group, supplementation with L. brevis D17 and D17ΔglnR decreased the fasting blood glucose levels of 10.7 mmol L−1 in HFD mice to 8.5 mmol L−1 in HFD + D17 mice and 7.6 mmol L−1 in HFD + D17ΔglnR mice (Fig. 3e), respectively, and restored fasting insulin to normal levels (Fig. 3f). The HOMA-IR (homeostatic model assessment for insulin resistance) index was decreased by 2.66-fold and 3.16-fold in the HFD + D17 group and HFD + D17ΔglnR group (Fig. 3g), respectively. These results indicate that administering L. brevis D17 or its glnR-deletion strain D17ΔglnR significantly reduced the development of metabolic syndrome induced by a high-fat diet.
3.4 L. brevis D17 and its glnR-deletion strain D17ΔglnR positively modulated HFD-induced intestinal dysbiosis
The impact of dietary interventions on the gut microbiota of mice and the colonization ability of supplemented probiotics were analyzed using 16S rRNA gene amplicon sequencing and metagenomic sequencing. The β-diversity using principal coordinates analysis (PCoA) indicated that the high-fat diet significantly altered the gut microbial pattern, markedly differing from that in NFD mice (Fig. 4a). The clusters of gut microbial communities of the probiotic treatment groups (HFD + D17 and HFD + D17ΔglnR) nearly overlapped and closely resembled those of the NFD group. Furthermore, the significant increase in the Chao1 and Shannon indices for gut microbial communities following L. brevis D17 and D17ΔglnR intervention indicated the effectiveness of L. brevis in enhancing gut microbiota richness and species diversity (Fig. S2†).Then the correlation between obesity-related serum markers and gut microbial community was determined. Twenty-one dominant genera with relative abundances exceeding 1% at least within one mouse were demonstrated. As indicated in Fig. 4b, the heat map and correlation map showed that unclassified Muribaculaceae, Faecalibaculum, Akkermansia and Bifidobacterium were negatively correlated with the flowing obesity-related serum markers: leptin, TC, TG, fasting glucose, fasting insulin and HOMA-IR. Conversely, Flintibacter, Thomasclavelia, Alistipes and Acetatifactor were positively correlated with these serum markers. Moreover, the serum marker LDL showed negative correlations with Lactobacillus, Faecalibaculum, Romboutsia, and Lachnospiraceae NK4A136, while it showed positive correlations with Leptogranulimonas and Alistipes. We defined the genera exhibiting negative correlations with obesity development markers as beneficial microbes. Conversely, the genera promoting obesity development were classified as harmful ones. Then the relative abundances of these beneficial and harmful bacterial genera among the four mouse groups were examined. As indicated in Fig. 4c, administration of L. brevis D17 and D17ΔglnR significantly restored the relative abundances of beneficial genera, including unclassified Muribaculaceae (an average relative abundance of 0.5% in the HFD group, 6.3% in the D17 group, and 7.1% in the D17ΔglnR group), Lactobacillus (1.4% in HFD, 7.9% in D17, and 10.0% in D17ΔglnR), Faecalibaculum (0.5% in HFD, 2.0% in D17, and 2.4% in D17ΔglnR), Akkermansia (0.2% in HFD, 2.6% in D17, and 2.3% in D17ΔglnR), Romboutsia (1.6% in HFD, 4.8% in D17, and 5.6% in D17ΔglnR), and Bifidobacterium (0.4% in HFD, 0.8% in D17, and 1.5% in D17ΔglnR). Simultaneously, following the probiotic intervention, the relative abundances of harmful genera were significantly decreased, including Leptogranulimonas (4.6% in HFD, 1.0% in D17, and 0.6% in D17ΔglnR), Flintibacter (10.1% in HFD, 2.0% in D17, and 2.5% in D17ΔglnR), Alistipes (7.9% in HFD, 1.3% in D17, and 0.9% in D17ΔglnR), and Acetatifactor (2.1% in HFD, 0.01% in D17, and 0.003% in D17ΔglnR) (Fig. 4d).
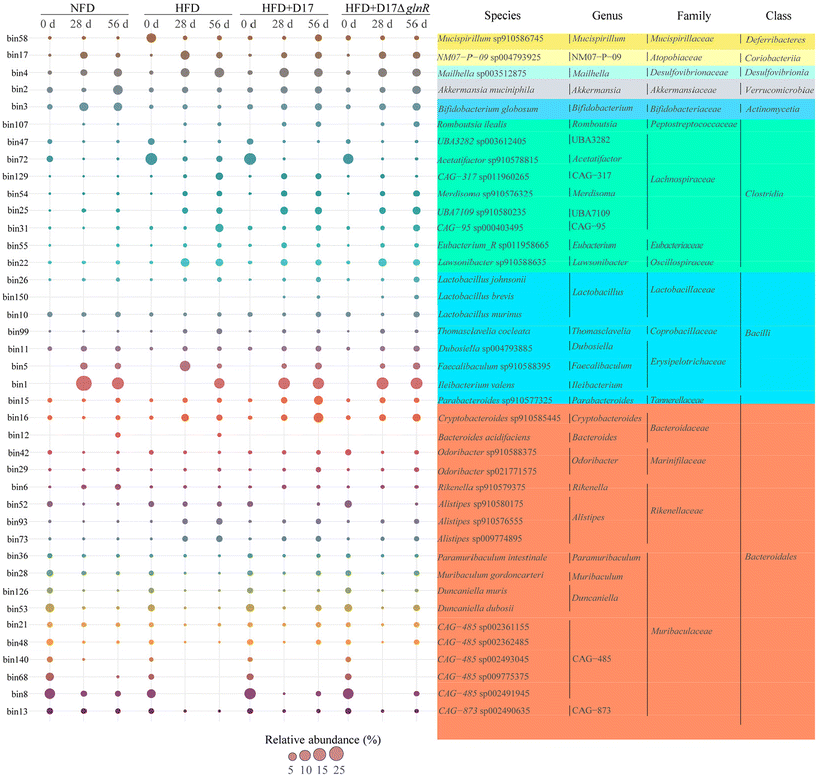
Considering that amplicon sequencing cannot accurately distinguish the gut microbe at the species level, metagenomic sequencing was used to seek the impact of probiotic intervention on the gut microbial species. We achieved 150 high-quality metagenome-assembled genomes (MAGs) under Bacteroidia, Bacilli and Clostridia classes with over 90% completeness and less than 10% contamination. Among these MAGs, 40 MAGs exhibited a relative abundance greater than 1% at least within one mouse group, and were used to create a temporal bubble chart (Fig. 5). Compared with the HFD group, supplementation with L. brevis increased the abundances of Muribaculum gordoncarteri (bin28), Duncaniella dubosii (bin53), Akkermansia muciniphila (bin2), Lactobacillus murinus (bin10), Bifidobacterium globosum (bin3), Romboutsia ilealis (bin107), Dubosiella sp004793885 (bin11), and Faecalibaculum sp910588395 (bin5). Conversely, the abundances of Mailhella sp003512875 (bin4), Alistipes sp009774895 (bin73), Alistipes sp910576555 (bin93), Alistipes sp910580175 (bin52), Thomasclavelia cocleata (bin99), and Lawsonibacter sp910588635 (bin22) were decreased following probiotic supplementation (Fig. 5). In particular, within the Muribaculaceae family, 34 species were successfully annotated. Among these 34 species, the abundances of 29 species (bin8 to bin144, see Table S3†) were decreased following the HFD intervention. Intervention with L. brevis D17 restored the abundances of 20 Muribaculaceae species, including Muribaculum gordoncarteri (bin28), Paramuribaculum intestinale (bin36), Paramuribaculum sp001689565 (bin37), Paramuribaculum sp910579675 (bin38), Duncaniella dubosii (bin53), Duncaniella muris (bin126), Duncaniella sp910578515 (bin66), Duncaniella sp001689575 (bin91), and other 12 unclassified Muribaculaceae species (bin8, bin9, bin13, bin21, bin48, bin59, bin60, bin61, bin71, bin85, bin106 and bin135). Similarly, the intervention with D17ΔglnR restored the abundances of 18 out of these 20 Muribaculaceae species except for Paramuribaculum sp910579675 (bin38) and bin61. Furthermore, neither the guts of NFD mice nor those of HFD-alone mice contained L. brevis. However, following the supplementation of L. brevis, D17 and D17ΔglnR became detectable from the middle to the end of the intervention period. Compared with other L. brevis genomes derived from the NCBI database, the reconstructed L. brevis genomes from mouse feces showed the highest average nucleotide identity values (Table S4†). The reconstructed genomes and average nucleotide identity analysis indicated that the L. brevis in feces was indeed the externally supplemented strains. Collectively, these results show that supplementing with L. brevis D17 and its glnR-deletion strain D17ΔglnR effectively alleviates gut microbiota dysbiosis induced by the high-fat diet.
3.5 Supplementation with L. brevis D17 and its glnR-deletion strain D17ΔglnR markedly altered HFD-induced changes in fecal metabolite profiles
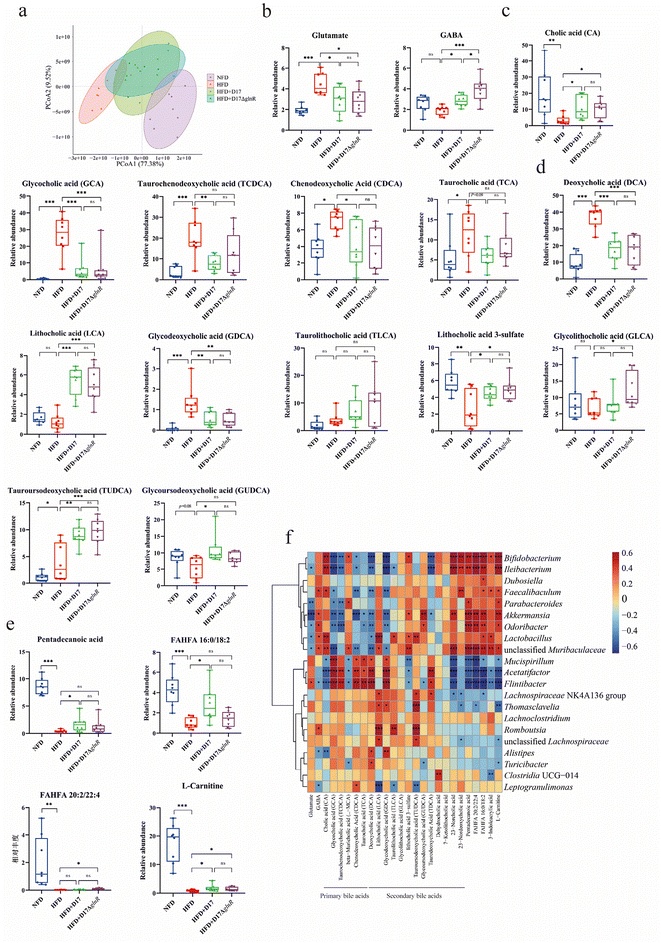
To evaluate the effects of different dietary interventions on the fecal metabolite profiles of mice, non-targeted fecal metabolomics were employed. The PCoA analysis showed that fecal metabolite levels in the probiotic groups were similar to those in the NFD group but significantly differed from the HFD group, indicating that the probiotic supplementation in a high-fat diet significantly altered the fecal metabolome (Fig. 6a). Several key differential metabolites were identified. Firstly, supplementation with L. brevis significantly reduced glutamate levels in the intestinal lumen, reducing it to levels comparable to those in the NFD group. Meanwhile, compared to the HFD group, supplementation with L. brevis significantly elevated GABA levels. The alterations of glutamate and GABA in the intestinal lumen suggested that the supplemented L. brevis might utilize its glutamate decarboxylase system to enhance glutamate metabolism in the gut (Fig. 6b). Secondly, compared to the HFD group, supplementation with L. brevis significantly decreased the levels of primary bile acids in the intestinal lumen, including cholic acid (CA), glycocholic acid (GCA), taurochenodeoxycholic acid (TCDCA), chenodeoxycholic acid (CDCA) and taurocholic acid (TCA) (Fig. 6c). Additionally, it also reduced the levels of two secondary bile acids, i.e. deoxycholic acid (DCA) and glycodeoxycholic acid (GDCA) (Fig. 6d). More importantly, supplementation with L. brevis significantly increased the levels of other secondary bile acids, including lithocholic acid (LCA), taurolithocholic acid (TLCA), glycolithocholic acid (GLCA), tauroursodeoxycholic acid (TUDCA) and glycoursodeoxycholic acid (GUDCA) (Fig. 6d). These findings suggest that L. brevis supplementation effectively restored the bile acid metabolism disrupted by a high-fat diet. Thirdly, when compared to the HFD group, supplementation with L. brevis significantly increased beneficial lipid metabolism metabolites, including pentadecanoic acid, FAHFA 20:2/22:4, FAHFA 16:0/18:2, and L-carnitine (Fig. 6e).Furthermore, a correlation analysis between fecal metabolites and gut microbes was performed. As shown in Fig. 6f, GABA exhibited a strong positive association with genera such as Lactobacillus, Akkermansia and Faecalibaculum, but a significantly negative correlation with Flintibacter, Alistipes and Leptogranulimonas. GCA, TCDCA, CDCA, TCA, and DCA showed positive correlations with Flintibacter, Acetatifactor and Mucispirillum, but showed negative correlations with unclassified Muribaculaceae, Ileibacterium and Akkermansia. LCA was positively correlated with Lactobacillus, Romboutsia, and Lachnospiraceae NK4A136, but was negatively correlated with Leptogranulimonas. It is worth noting that three metabolites (pentadecanoic acid, FAHFA and L-carnitine), which are responsible for fatty homeostasis,38–40 showed positive correlations with unclassified Muribaculaceae, Ileibacterium, Akkermansia and Bifidobacterium. However, these metabolites exhibited negative correlations with Flintibacter, Acetatifactor and Mucispirillum.
3.6 Treatment with L. brevis D17 and its glnR-deletion strain D17ΔglnR improved the expression of genes responsible for lipid metabolism and inflammation in the liver
To further investigate the mechanisms by which L. brevis significantly attenuated obesity, RT-qPCR was performed. Compared to the HFD group, the supplementation of these two strains significantly elevated the expression level of Prkaa1 (encoding protein kinase AMP-activated catalytic subunit α) (Fig. 7a). Prkaa1 is essential for maintaining hepatic lipid homeostasis.41,42 As illustrated in Fig. 7b, the administration of L. brevis resulted in a significant downregulation of genes involved in lipogenesis (Srebf1),43 fatty acid synthesis (Fasn, Scd1),44 fatty acid transport (Cd36),45 and adipocyte differentiation and maturation (Pparγ).46 In contrast, the administration of L. brevis led to an upregulation of lipolysis-related genes, including Adrb3 and Lipe44 (Fig. 7c). Moreover, the administration of L. brevis significantly enhanced the expression of two genes involved in cholesterol metabolism, namely Cyp7a1 and Ldlr,45 thereby promoting cholesterol metabolic processes (Fig. 7d). In addition to modulating lipid and cholesterol metabolisms, the administration of L. brevis was found to significantly downregulate the expression of pro-inflammatory macrophage markers, including Cd68 and F4/80, while upregulating the expression of the anti-inflammatory marker Cd206![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 (Fig. 7e). Furthermore, the administration of L. brevis decreased the expression of the pro-inflammatory cytokine genes including Tnfα, Mcp-1, and Il-6 (Fig. 7f). Taken together, these findings suggest that supplementation with L. brevis D17 and its glnR-deletion strain D17ΔglnR suppress obesity by modulating the expression of genes involved in lipid metabolism and mitigating inflammatory responses.
47 (Fig. 7e). Furthermore, the administration of L. brevis decreased the expression of the pro-inflammatory cytokine genes including Tnfα, Mcp-1, and Il-6 (Fig. 7f). Taken together, these findings suggest that supplementation with L. brevis D17 and its glnR-deletion strain D17ΔglnR suppress obesity by modulating the expression of genes involved in lipid metabolism and mitigating inflammatory responses.
4. Discussion
Obesity is a prevalent chronic disease, and identifying environmentally friendly treatment alternatives is a significant focus of current research. Previous studies have demonstrated that several LAB species, such as L. rhamnosus and L. plantarum, possess beneficial effects on prevention for obesity and metabolic disorders.15,48 However, research on the anti-obesity effects of Levilactobacillus brevis, a species usually with hyper-GABA-production capability within specific strains, was still limited. A recent study shows that L. brevis strain 031 has significant anti-obesity efficacy, slowing weight gain by 11.1%.48 In addition, L. brevis OK56 has been highlighted for its pronounced anti-obesity effects, reducing weight gain by 28%.49 In this study, we found that two L. brevis strains, namely D17 and its glnR-deletion strain D17ΔglnR, demonstrated remarkable anti-obesity effects, delaying weight gain by 28.4% and 29.1%, respectively. Notably, the anti-obesity effect of L. brevis in this study is outstanding not only within the field of L. brevis research but also when compared to other well-known LAB species with comparable benefits. For example, L. rhamnosus 069 was found to reduce weight gain by 18.7%,48 while Lactobacillus plantarum HMRS-6 showed a reduction of approximately 26%.50 Furthermore, Lactobacillus plantarum HT121 and Lactobacillus paracasei 24 resulted in decreases of around 36% and 20.8%, respectively.51,52 Despite these promising findings, the underlying mechanisms of anti-obesity effects of these L. brevis strains remain unclear. In this study, we employ multi-omics technologies to explore the potential mechanisms of L. brevis on controlling obesity.In this study, although exogenous glutamate was not added into diet, the glnR-deficient strain D17ΔglnR still demonstrated more prominent probiotic effects on the serum, tissue, and gut microbiota under low-glutamate conditions in vivo, compared to D17. Specifically, the supplementation of D17ΔglnR resulted in a notable decrease in triglyceride (TG) levels, with a 50% greater reduction compared to the D17 group (p = 0.083). This trend was also observed in low-density lipoprotein (LDL) levels, which showed a 32% greater reduction (p = 0.22). Although these differences were not statistically significant at the conventional criterion of p < 0.05, they still represented notable reductions in lipid levels. Moreover, D17ΔglnR supplementation led to a significant 51.8% reduction in the epididymal adipocyte area (p = 0.018) compared to D17, along with decreases in the hepatic lipid droplet size and subcutaneous fat area by 27.1% (p = 0.19) and 30.8% (p = 0.11), respectively. Furthermore, the D17ΔglnR supplementation improved insulin resistance by 19% more than the D17 group, with a significantly smaller area under the curve (AUC) for insulin resistance. In addition, although there was no significant difference between the two groups for most intestinal microbial indicators, D17ΔglnR showed better improvements such as elevating the relative abundance of Bifidobacterium and decreasing the relative abundance of Leptogranulimonas. This enhanced performance of strain D17ΔglnR could be attributed to three primary reasons: (1) D17ΔglnR has higher tolerance to gastric acid and bile than D17, allowing more viable cells to reach the intestine and exert its function; (2) D17ΔglnR showed a stronger capability to produce GABA (1.2 times higher than the D17 group) within the intestine, which may benefit in terms of anti-obesity and the improvement of blood glucose metabolism. This finding is consistent with previous studies which have also shown that GABA is generally considered to have anti-obesity therapeutic potential and can also reduce high blood glucose induced by high-fat diets;53,54 (3) the supplementation of D17ΔglnR shows a better improvement effect on the gut microbiota disturbed by a high-fat diet than the D17 group.
Although D17ΔglnR significantly improves adipocyte size indicators compared to L. brevis D17, there is no notable difference in body weight effects between the two groups. Similarly, R. Liu et al. reported that intragastric administration of live Bacteroides thetaiotaomicron significantly reduced fat weight and adipocyte volume without impacting the overall body weight,44 suggesting that a smaller adipocyte size may not be a primary factor in weight loss. From an energy perspective, obesity is largely caused by energy intake exceeding energy expenditure, with resting metabolic rate (RMR) playing a major role in total energy expenditure. Studies indicate that RMR typically accounts for 60–75% of total energy expenditure, and a low RMR is associated with obesity. Lean tissue, such as muscle and bone, is the primary contributor to RMR, while fat tissue has a minimal impact (3.9%) on resting energy expenditure. Therefore, reductions in fat mass contribute little to overall weight loss, with lean tissue being the primary driver.55 In our study, supplementation with L. brevis might increase lean tissue mass when decreasing adipocyte size, contributing to weight loss. However, the likely limited increase in lean tissue mass explains why the D17 and D17ΔglnR groups showed no significant differences in body weight. This hypothesis requires further investigation in future studies. However, it is worth noting that the formation of obesity and lipolysis is a very complex process. The lack of a significant effect on body weight with L. brevis D17 and D17ΔglnR could also be due to factors like gavage dosage, mouse sex, and age. We cannot rule out a possibility that, at a lower non-saturating gavage dose, D17ΔglnR might show more pronounced effects than D17. It would be worthwhile to explore whether D17ΔglnR shows a better performance in older mice. Whether D17ΔglnR could exhibit sex- or age-specific effects is unknown. This study indicates that D17ΔglnR can colonize a glutamate-limited gut environment even without external glutamate supplementation and maintain its GABA-producing characteristics, suggesting that glnR-deletion strain has a higher probiotic potency than L. brevis D17. This strain's adaptability shows promising application potential, especially in east Asia where the daily diet is rich in glutamate. Given that stomach pH increases post-meal, supplementation with D17ΔglnR strain might be more effective post meals.
In this study, the acid resistance and bile salt tolerance of L. brevis are not as robust as some other Lactobacillus species. For instance, L. plantarum KC28 shows over a 90% survival rate after enduring 3 hours of simulated gastric fluid stress and 7 hours of simulated intestinal fluid stress.56 However, this study revealed that L. brevis D17 exhibits a superior capability to decrease the levels of blood lipids and blood glucose, and finally reduce fat accumulation compared to previous anti-obesity studies of L. brevis.49,57,58 This could be attributed to L. brevis entering a viable but non-culturable (VBNC) state after stress exposure, suggesting that traditional methods for evaluating survival rates might underestimate their true resilience.59 Hence, VBNC L. brevis cells may still exert anti-obesity effects in the gut. Additionally, several studies have highlighted the benefits of pasteurized Lactobacillus strains.58,60 For instance, heat-killed L. brevis KB290 has been reported to significantly delay fat accumulation.58
The successful colonization of L. brevis leads to more enduring and stable health benefits for the host. Our study confirmed that L. brevis detected in fecal samples was indeed the supplemented strain, as revealed through metagenomic sequencing analysis. Additionally, our fecal metabolome analysis revealed that L. brevis supplementation may have a significant impact on the gut by facilitating the conversion of glutamate into GABA. These findings demonstrate that L. brevis cells can reach and persist in the gut to exert their effects, indicating successful colonization. Notably, our study provides a rare example of intestinal colonization of Lactobacillus in the scientific literature. Typically, high-throughput sequencing and quantitative PCR (qPCR) are used to confirm colonization. For instance, a previous study on the effect of L. plantarum LLY-606 on hyperuricemia relied solely on amplicon sequencing to confirm intestinal colonization.61 To accurately determine whether externally added probiotics can successfully colonize the intestines, a comprehensive approach combining metagenomics and amplicon sequencing is necessary.
This study found that the high-fat diet significantly reduced the abundance of beneficial intestinal microbes such as Muribaculaceae, Ileibacterium valens, Lactobacillus, Faecalibaculum, Bifidobacterium, and Akkermansia muciniphila, while increasing potentially harmful microbes like Alistipes and Acetatifactor. This change aligns with previous studies.62,63 Specifically, the Muribaculaceae family has been previously linked to improving mucosal barriers and inhibiting pro-inflammatory cytokines, making it a key player in intestinal health.64 Our metagenomic analysis identified specific Muribaculaceae species, including Muribaculum gordoncarteri and Duncaniella dubosii. The shifts in Muribaculaceae were found to be due to changes in various species, highlighting the gut microbiota's sensitivity to environmental changes. In contrast, Lachnospiraceae has been generally regarded as beneficial gut bacteria that contribute to delaying obesity.65,66 However, our analysis revealed that several Lachnospiraceae species significantly increased after a high-fat diet, suggesting a potential role in promoting obesity (see Table S3†). These findings highlight the importance of adopting more detailed taxonomic classification, such as species-level identification, to improve the precision and accuracy of gut microbiota research. Our analysis revealed that Romboutsia ilealis is associated with lower LDL, and higher LCA and TLCA levels, suggesting it may help prevent obesity. However, a 19-week study involving a high-fat diet (HFD) found that Romboutsia ilealis may act as an opportunistic pathogen promoting obesity.67 The discrepancy may be due to the varying responses of different gut microbes to short-term and long-term HFD interventions. Notably, our research identified two new genera, Leptogranulimonas and Flintibacter, which are more prevalent in the HFD group and may contribute to obesity. Supplemented L. brevis helps restore balance of gut microbiome through two possible mechanisms. Firstly, L. brevis increases gut acidity, inhibiting the growth of harmful bacteria. Secondly, it competes with harmful microbes for nutrients and attachment sites, reducing their growth and colonization.
The gut microbiome plays a vital role in converting primary bile acids to secondary bile acids.68 Our study showed that supplementing with L. brevis effectively restores the conversion of primary bile acids into secondary bile acids in the intestinal lumen, mediated by enzymes such as bile acid-CoA: amino acid N-acyltransferase (BAAT) and bile acid-CoA synthetase (BACS).12 This is crucial because secondary bile acids are more potent activators of key receptors FXR and TGR5 than primary bile acids.69 FXR plays a crucial role in regulating bile acid levels,70 and its diminished activation by primary bile acids in obese mice disrupts liver bile acid homeostasis and contributes to the development of non-alcoholic fatty liver disease (NAFLD).71 The activation of TGR5 is an important mechanism for maintaining blood glucose stability and preventing the development of chronic inflammatory responses.68 The activation of these receptors plays a crucial role in maintaining liver bile acid homeostasis, blood glucose stability, and suppressing inflammatory responses. Previous studies have demonstrated that several gut microbes, such as Lactobacillus and Bifidobacterium, are capable of producing bile salt hydrolases (BSHs) that deconjugate liver-derived conjugated bile acids into free bile acids, ultimately leading to the formation of secondary bile acids.72 Our research also found a substantial decline of BSH-producing microbes in mice fed with a high-fat diet. Notably, this reduction was reversed following supplementation with Levilactobacillus brevis. Our study found that supplementing with L. brevis increased the abundance of certain gut microbes, such as Lachnospiraceae and Peptostreptococcaceae families, which are known to have bile acid-inducible genes such as Bai-encoded 7α-dehydrogenase that facilitate the dehydrogenation of free bile acids.73 The concentration of secondary bile acid DCA significantly increased in the intestines of obese mice, indicating that high-fat diets favor the metabolic pathway for DCA synthesis. Previous research has shown that enriched DCA in obese mice not only induces DNA damage but also drives macrophage differentiation into the M1 phenotype via toll-like receptor 2 transactivation by the M2 muscarinic receptor, leading to increased pro-inflammatory cytokine production.12,74
The interaction between Levilactobacillus brevis and its host is multifaceted, extending beyond bile acid metabolism to include influences on signaling pathways related to lipid metabolism and inflammation. Supplementing with L. brevis led to significant increases in the expression of Prkaa1 gene, suggesting potential activation of AMPK signaling pathway. Concomitantly, L. brevis supplementation suppressed the expression of genes encoding key proteins in lipid metabolism, such as Srebf1, Fasn, Pparγ, Cd36, and Scd1. These genes are positively regulated by SREBP1C, which is, in turn, negatively regulated by AMPK.75,76 These gene regulatory effects observed with L. brevis align with the characteristics of the AMPK signaling pathway. Thus, L. brevis supplementation may activate this pathway in liver cells, resulting to reduced lipogenesis. It is important to note that, in addition to de novo fat synthesis mediated by Srebf1, Fasn, and Pparγ, the absorption and storage of dietary fat also plays a key role in fat formation. Research has shown that most fatty acids stored in adipocytes are derived from triglycerides absorbed into the bloodstream through the small intestine.40 Additionally, L. brevis may activate the cAMP signaling pathway, promoting lipolysis. Previous research has shown that activation of ADRB3 increases intracellular cAMP levels, which in turn boosts LIPE expression. As a lipase, LIPE hydrolyzes excess stored fat into free fatty acids, which are then further β-oxidized in the mitochondria to generate energy.77 Furthermore, AMPK plays a crucial role in counter-regulating inflammatory signaling pathways in macrophages, promoting a shift toward an anti-inflammatory state from a pro-inflammatory state.78 This suggests that AMPK activation by L. brevis may suppress inflammation triggered by pro-inflammatory macrophages and reduce cytokine production, including Tnfα, Mcp-1, and Il-6. This study proposes that L. brevis supplementation can delay obesity progression by interacting with the gut microbiota and the host, and the potential mechanisms underlying how L. brevis delays obesity are depicted in Fig. 8.
In conclusion, gavaged L. brevis successfully colonized the intestines and had a significant impact on the gut microbiome. This led to delayed weight gain and improved blood glucose metabolism through interactions with intestinal microbes and the host. Specifically, L. brevis supplementation restored levels of beneficial bacteria, including Muribaculaceae, Ileibacterium valens, Lactobacillus, Faecalibaculum, Bifidobacterium globosum, Akkermansia muciniphila, and Romboutsia ilealis, while reducing levels of potential harmful bacteria like Leptogranulimonas, Flintibacter, and Alistipes. The interaction between L. brevis and the host was characterized by two primary ways: firstly, L. brevis supplementation significantly decreased the concentration of primary bile acids and increased secondary bile acids in the intestinal lumen, thereby balancing bile acid metabolism; secondly, L. brevis supplementation may activate the AMPK signaling pathway in liver cells to reduce lipogenesis, enhance the cAMP signaling pathway to facilitate lipolysis, and suppress inflammation by reducing pro-inflammatory macrophage infiltration. However, the detailed mechanisms by which L. brevis establishes a relationship with the host in the intestines remain unclear and warrant further investigation.
Data availability
All data presented in this study are available on request from the corresponding authors. Data other than sequencing data will not be uploaded to a publicly accessible database.Conflicts of interest
All authors declare no conflict of interest in this study.Acknowledgements
The work presented in this study was solely funded by the National First-Class Discipline Program of Light Industry Technology and Engineering (QGJC20230301), hence the acknowledgments within the text are comprehensive and accurate.References
- R. Acin-Perez, S. Iborra, Y. Marti-Mateos, E. C. L. Cook, R. Conde-Garrosa, A. Petcherski, M. D. Munoz, R. M. de Mena, K. C. Krishnan, C. Jimenez, J. P. Bolanos, M. Laakso, A. J. Lusis, O. S. Shirihai, D. Sancho and J. A. Enriquez, Fgr kinase is required for proinflammatory macrophage activation during diet-induced obesity, Nat. Metab., 2020, 2, 974 CrossRef CAS PubMed.
- J. M. Friedman, Leptin and the endocrine control of energy balance, Nat. Metab., 2019, 1, 754–764 CrossRef CAS PubMed.
- Y. Zheng, S. H. Ley and F. B. Hu, Global aetiology and epidemiology of type 2 diabetes mellitus and its complications, Nat. Rev. Endocrinol., 2018, 14, 88–98 CrossRef.
- J. Wang, H. Tang, C. Zhang, Y. Zhao, M. Derrien, E. Rocher, J. E. T. van-Hylckama Vlieg, K. Strissel, L. Zhao, M. Obin and J. Shen, Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice, ISME J., 2014, 9, 1–15 CrossRef.
- Y. Wang, W. F. Yao, B. Li, S. Y. Qian, B. B. Wei, S. Q. Gong, J. Wang, M. Y. Liu and M. J. Wei, Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats, Exp. Mol. Med., 2020, 52, 1959–1975 CrossRef CAS.
- P. J. Turnbaugh, R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis and J. I. Gordon, An obesity-associated gut microbiome with increased capacity for energy harvest, Nature, 2006, 444, 1027–1031 CrossRef.
- C. Torres-Fuentes, H. Schellekens, T. G. Dinan and J. F. Cryan, The microbiota-gut-brain axis in obesity, Lancet Gastroenterol. Hepatol., 2017, 2, 747–756 CrossRef PubMed.
- P. J. Turnbaugh, M. Hamady, T. Yatsunenko, B. L. Cantarel, A. Duncan, R. E. Ley, M. L. Sogin, W. J. Jones, B. A. Roe, J. P. Affourtit, M. Egholm, B. Henrissat, A. C. Heath, R. Knight and J. I. Gordon, A core gut microbiome in obese and lean twins, Nature, 2009, 457, 480–487 CrossRef CAS.
- J. M. Natividad, B. Lamas, H. P. Pham, M. L. Michel, D. Rainteau, C. Bridonneau, G. da Costa, J. V. Vlieg, B. Sovran, C. Chamignon, J. Planchais, M. L. Richard, P. Langella, P. Veiga and H. Sokol, Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice, Nat. Commun., 2018, 9, 15 CrossRef.
- A. Keskitalo, E. Munukka, R. Toivonen, M. Hollmén, H. Kainulainen, P. Huovinen, S. Jalkanen and S. Pekkala, Enterobacter cloacae administration induces hepatic damage and subcutaneous fat accumulation in high-fat diet fed mice, PLoS One, 2018, 13, 15 CrossRef.
- J. T. Gong, Q. J. Zhang, R. Z. Hu, X. Z. Yang, C. K. Fang, L. P. Yao, J. Lv, L. Wang, M. K. Shi, W. T. Zhang, S. Q. Ma, H. K. Xiang, H. F. Zhang, D. X. Hou, Y. L. Yin, J. H. He, L. J. Peng and S. S. Wu, Effects of Prevotella copri on insulin, gut microbiota and bile acids, Gut Microbes, 2024, 16, 12 Search PubMed.
- J. Cai, L. L. Sun and F. J. Gonzalez, Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis, Cell Host Microbe, 2022, 30, 289–300 CrossRef CAS.
- C. Kruger, Y. Z. Hu, Q. Pan, H. Marcotte, A. Hultberg, D. Delwar, P. J. van Dalen, P. H. Pouwels, R. J. Leer, C. G. Kelly, C. van Dollenweerd, J. K. Ma and L. Hammarstrom, In situ delivery of passive immunity by lactobacilli producing single-chain antibodies, Nat. Biotechnol., 2002, 20, 702–706 CrossRef PubMed.
- European Food Safety Authority (EFSA), Introduction of a qualified presumption of safety (QPS) approach for assessment of selected microorganisms referred to EFSA - opinion of the scientific committee, EFSA J., 2007, 5, 587 CrossRef.
- M. Sohn, G. Y. Na, J. Chu, H. Joung, B. K. Kim and S. Lim, Efficacy and safety of Lactobacillus plantarum, K50 on lipids in Koreans with obesity: A randomized, double-blind controlled clinical trial, Front. Endocrinol., 2022, 12, 10 Search PubMed.
- P. Vajro, C. Mandato, M. R. Licenziati, A. Franzese, D. F. Vitale, S. Lenta, M. Caropreso, G. Vallone and R. Meli, Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease, J. Pediatr. Gastroenterol. Nutr., 2011, 52, 740–743 CrossRef PubMed.
- J. Plaza-Diaz, F. J. Ruiz-Ojeda, M. Gil-Campos and A. Gil, Mechanisms of action of probiotics, Adv. Nutr., 2019, 10, S49–S66 CrossRef.
- D. Li, C. Gao, F. Zhang, R. Yang, C. Lan, Y. Ma and J. Wang, Seven facts and five initiatives for gut microbiome research, Protein Cell, 2020, 11, 391–400 CrossRef PubMed.
- Y. P. Liu, H. Z. Tang, Z. L. Lin and P. Xu, Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation, Biotechnol. Adv., 2015, 33, 1484–1492 CrossRef CAS.
- C. Wang, Y. H. Cui and X. J. Qu, Mechanisms and improvement of acid resistance in lactic acid bacteria, Arch. Microbiol., 2018, 200, 195–201 CrossRef CAS PubMed.
- N. Z. Guan and L. Liu, Microbial response to acid stress: Mechanisms and applications, Appl. Microbiol. Biotechnol., 2020, 104, 51–65 CrossRef CAS.
- C. J. Lyu, W. R. Zhao, C. L. Peng, S. Hu, H. Fang, Y. J. Hua, S. J. Yao, J. Huang and L. H. Mei, Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production, Microb. Cell Fact., 2018, 17, 14 CrossRef.
- M. S. Su, S. Schlicht and M. G. Gänzle, Contribution of glutamate decarboxylase in Lactobacillus reuteri to acid resistance and persistence in sourdough fermentation, Microb. Cell Fact., 2011, 10, 12 CrossRef.
- C. Feehily and K. A. G. Karatzas, Role of glutamate metabolism in bacterial responses towards acid and other stresses, J. Appl. Microbiol., 2013, 114, 11–24 CrossRef CAS PubMed.
- L. C. Gong, C. Ren and Y. Xu, Deciphering the crucial roles of transcriptional regulator GadR on gamma-aminobutyric acid production and acid resistance in Lactobacillus brevis, Microb. Cell Fact., 2019, 18, 108 CrossRef PubMed.
- L. C. Gong, C. Ren and Y. Xu, GlnR negatively regulates glutamate-dependent acid resistance in Lactobacillus brevis, Appl. Environ. Microbiol., 2020, 86, 02615–02619 CrossRef.
- H. Ahangari, B. Bahramian, A. Khezerlou, M. Tavassoli, N. Kiani-Salmi, V. Tarhriz and A. Ehsani, Association between monosodium glutamate consumption with changes in gut microbiota and related metabolic dysbiosis-A systematic review, Food Sci. Nutr., 2024, 12, 5285–5295 CrossRef PubMed.
- A. M. Bolger, M. Lohse and B. Usadel, Trimmomatic: A flexible trimmer for Illumina sequence data, Bioinformatics, 2014, 30, 2114–2120 CrossRef CAS PubMed.
- D. W. D. Kang, J. Froula, R. Egan and Z. Wang, MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities, PeerJ, 2015, 3, 15 CrossRef.
- C. C. Liu, S. S. Dong, J. B. Chen, C. Wang, P. Ning, Y. Guo and T. L. Yang, MetaDecoder: A novel method for clustering metagenomic contigs, Microbiome, 2022, 10, 16 CrossRef.
- S. J. Pan, C. K. Zhu, X. M. Zhao and L. P. Coelho, A deep siamese neural network improves metagenome-assembled genomes in microbiome datasets across different environments, Nat. Commun., 2022, 13, 12 CrossRef.
- D. H. Parks, M. Imelfort, C. T. Skennerton, P. Hugenholtz and G. W. Tyson, CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes, Genome Res., 2015, 25, 1043–1055 CrossRef CAS.
- S. Nurk, D. Meleshko, A. Korobeynikov and P. A. Pevzner, metaSPAdes: A new versatile metagenomic assembler, Genome Res., 2017, 27, 824–834 CrossRef CAS PubMed.
- H. M. Parker, N. A. Johnson, C. A. Burdon, J. S. Cohn, H. T. O'Connor and J. George, Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis, J. Hepatol., 2012, 56, 944–951 CrossRef CAS PubMed.
- A. Rauf, M. Imran, I. E. Orhan and S. Bawazeer, Health perspectives of a bioactive compound curcumin: A review, Trends Food Sci. Technol., 2018, 74, 33–45 CrossRef CAS.
- F. M. Wensveen, V. Jelencic, S. Valentic, M. Sestan, T. T. Wensveen, S. Theurich, A. Glasner, D. Mendrila, D. Stimac, F. T. Wunderlich, J. C. Brüning, O. Mandelboim and B. Polic, NK cells link obesity-induced adipose stress to inflammation and insulin resistance, Nat. Immunol., 2015, 16, 376–385 CrossRef CAS PubMed.
- M. C. Soler-Vázquez, M. D. Romero, M. Todorcevic, K. Delgado, C. Calatayud, A. Benitez-Amaro, M. T. L. Lhoëst, P. Mera, S. Zagmutt, M. Bastías-Pérez, K. Ibeas, N. Casals, J. C. Escolà-Gil, V. Llorente-Cortés, A. Consiglio, D. Serra and L. Herrero, Implantation of CPT1AM-expressing adipocytes reduces obesity and glucose intolerance in mice, Metab. Eng., 2023, 77, 256–272 CrossRef.
- K. P. Sunil, P. Hemant, L. C. Ward, J. Waanders and L. Brown, Modulation of tissue fatty acids by L-carnitine attenuates metabolic syndrome in diet-induced obese rats, Food Funct., 2015, 6, 2496–2506 RSC.
- W. C. Wei, C. C. Wong, Z. J. Jia, W. X. Liu, C. A. Liu, F. F. Ji, Y. S. Pan, F. X. Wang, G. P. Wang, L. Y. Zhao, E. S. H. Chu, X. Zhang, J. J. Y. Sung and J. Yu, Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid, Nat. Microbiol., 2023, 8, 1534 CrossRef CAS.
- P. Morigny, J. Boucher, P. Arner and D. Langin, Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics, Nat. Rev. Endocrinol., 2021, 17, 276–295 CrossRef CAS.
- R. Q. Zhao, Y. Ji, X. Chen, Q. H. Hu and L. Y. Zhao, Polysaccharide from Flammulina velutipes attenuates markers of metabolic syndrome by modulating the gut microbiota and lipid metabolism in high fat diet-fed mice, Food Funct., 2021, 12, 6964–6980 RSC.
- S. M. Jeon, Regulation and function of AMPK in physiology and diseases, Exp. Mol. Med., 2016, 48, 13 Search PubMed.
- C. Cheng, X. H. Liu, J. He, J. Gao, J. T. Zhou, J. N. Fan, X. Jin, J. B. Zhang, L. Chang, Z. J. Xiong, J. Yu, S. B. Li and X. M. Li, Apolipoprotein A4 restricts diet-induced hepatic steatosis via SREBF1-mediated lipogenesis and enhances IRS-PI3K-Akt signaling, Mol. Nutr. Food Res., 2022, 66, 14 Search PubMed.
- R. Liu, J. Hong, X. Xu, Q. Feng, D. Zhang, Y. Gu, J. Shi, S. Zhao, W. Liu, X. Wang, H. Xia, Z. Liu, B. Cui, P. Liang, L. Xi, J. Jin, X. Ying, X. Wang, X. Zhao, W. Li, H. Jia, Z. Lan, F. Li, R. Wang, Y. Sun, M. Yang, Y. Shen, Z. Jie, J. Li, X. Chen, H. Zhong, H. Xie, Y. Zhang, W. Gu, X. Deng, B. Shen, X. Xu, H. Yang, G. Xu, Y. Bi, S. Lai, J. Wang, L. Qi, L. Madsen, J. Wang, G. Ning, K. Kristiansen and W. Wang, Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention, Nat. Med., 2017, 23, 859–868 CrossRef CAS.
- Q. Zhang, X. Y. Fan, Y. J. Cao, T. T. Zheng, W. J. Cheng, L. J. Chen, X. C. Lv, L. Ni, P. F. Rao and P. Liang, The beneficial effects of Lactobacillus brevis FZU0713-fermented Laminaria japonica on lipid metabolism and intestinal microbiota in hyperlipidemic rats fed with a high-fat diet, Food Funct., 2021, 12, 7145–7160 RSC.
- Y. H. Qiu, M. L. Gan, X. Y. Wang, T. C. Liao, Q. Y. Chen, Y. H. Lei, L. Chen, J. Y. Wang, Y. Zhao, L. L. Niu, Y. Wang, S. H. Zhang, L. Zhu and L. Y. Shen, The global perspective on peroxisome proliferator-activated receptor γ (PPARγ) in ectopic fat deposition: A review, Int. J. Biol. Macromol., 2023, 253, 14 Search PubMed.
- Y. Y. Wang, H. Jiang, J. Pan, X. R. Huang, Y. C. Wang, H. F. Huang, K. F. To, D. J. Nikolic-Paterson, H. Y. Lan and J. H. Chen, Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury, J. Am. Soc. Nephrol., 2017, 28, 2053–2067 CrossRef CAS PubMed.
- P.-Y. Ho, Y.-C. Chou, Y.-C. Koh, W.-S. Lin, W.-J. Chen, A.-L. Tseng, C.-L. Gung, Y.-S. Wei and M.-H. Pan, Lactobacillus rhamnosus, 069 and Lactobacillus brevis 031: Unraveling strain-specific pathways for modulating lipid metabolism and attenuating high-fat-diet-induced obesity in mice, ACS Omega, 2024, 9, 28520–28533 CrossRef CAS PubMed.
- K.-A. Kim, J.-J. Jeong and D.-H. Kim, Lactobacillus brevis OK56 ameliorates high-fat diet-induced obesity in mice by inhibiting NF-κB activation and gut microbial LPS production, J. Funct. Foods, 2015, 13, 183–191 CrossRef CAS.
- J. W. Zhu, X. Y. Liu, N. Y. Liu, R. C. Zhao and S. S. Wang, Lactobacillus plantarum alleviates high-fat diet-induced obesity by altering the structure of mice intestinal microbial communities and serum metabolic profiles, Front. Microbiol., 2024, 15, 1425764 CrossRef PubMed.
- X. P. Li, Y. M. Huang, L. Q. Song, Y. C. Xiao, S. Lu, J. G. Xu, J. G. Li and Z. H. Ren, Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice, J. Funct. Foods, 2020, 73, 11 Search PubMed.
- Z. J. Liu, X. Zhou, W. Wang, L. Y. Gu, C. B. Hu, H. Sun, C. Xu, J. C. Hou and Z. M. Jiang, Lactobacillus paracasei, 24 attenuates lipid accumulation in high-fat diet-induced obese mice by regulating the gut microbiota, J. Agric. Food Chem., 2022, 70, 4631–4643 CrossRef CAS.
- H. Y. Lee, G. H. Lee, T. H. Hoang, Y. M. Kim, G. H. Jang, C. H. Seok, Y. G. S. Gwak, J. Lim, J. Kim and H. J. Chae, GABA and fermented Curcuma longa L. extract enriched with GABA ameliorate obesity through Nox4-IRE1α Sulfonation-RIDD-SIRT1 decay axis in high-fat diet-induced obese mice, Nutrients, 2022, 14, 18 Search PubMed.
- Z. X. Xie, S. F. Xia, Y. Qiao, Y. H. Shi and G. W. Le, Effect of GABA on oxidative stress in the skeletal muscles and plasma free amino acids in mice fed high-fat diet, J. Anim. Physiol. Anim. Nutr., 2015, 99, 492–500 CrossRef CAS.
- G. Argyrakopoulou, N. Fountouli, M. Dalamaga and A. Kokkinos, Revisiting resting metabolic rate: What is the relation to weight fluctuations?, Curr. Obes. Rep., 2023, 12, 502–513 CrossRef.
- H. Eunchong, K. Seulki, P. Haryung, P. Soyoung, J. Yosep, T. Svetoslav Dimitrov, L. Sang-Dong and W. H. Holzapfel, Modulation of the gut microbiome and obesity biomarkers by Lactobacillus plantarum KC28 in a diet-induced obesity murine model, Probiotics Antimicrob. Proteins, 2021, 13, 677–697 CrossRef.
- E. Patterson, P. M. Ryan, N. Wiley, I. Carafa, E. Sherwin, G. Moloney, E. Franciosi, R. Mandal, D. S. Wishart, K. Tuohy, R. P. Ross, J. F. Cryan, T. G. Dinan and C. Stanton, Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome, Sci. Rep., 2019, 9, 16323 CrossRef CAS PubMed.
- J. Watanabe, N. Hashimoto, T. Yin, B. Sandagdorj, C. Arakawa, T. Inoue and S. Suzuki, Heat-killed Lactobacillus brevis KB290 attenuates visceral fat accumulation induced by high-fat diet in mice, J. Appl. Microbiol., 2021, 131, 1998–2009 CrossRef CAS PubMed.
- M. Derrien and J. Vlieg, Fate, activity, and impact of ingested bacteria within the human gut microbiota, Trends Microbiol., 2015, 23, 354–366 CrossRef CAS PubMed.
- K. O. Jang, J. S. Choi, K. H. Choi, S. Kim, H. Kim and D. K. Chung, Anti-obesity potential of heat-killed Lactiplantibacillus plantarum, K8 in 3T3-L1 cells and high-fat diet mice, Heliyon, 2023, 9, 11 Search PubMed.
- R. J. Shi, J. Ye, H. Fan, C. X. Xiao, D. N. Wang, B. Xia, Z. T. Zhao, B. T. Zhao, X. S. Dai and X. B. Liu, Lactobacillus plantarum LLY-606 supplementation ameliorates hyperuricemia via modulating intestinal homeostasis and relieving inflammation, Food Funct., 2023, 14, 5663–5677 RSC.
- Y. Li, M. L. Chen, Y. X. Ma, Y. Yang, Y. Cheng, H. J. Ma, D. Y. Ren and P. Chen, Regulation of viable/inactivated/lysed probiotic Lactobacillus plantarum, H6 on intestinal microbiota and metabolites in hypercholesterolemic mice, npj Sci. Food, 2022, 6, 50 CrossRef PubMed.
- L. J. den Hartigh, Z. Gao, L. Goodspeed, S. Wang, A. K. Das, C. F. Burant, A. Chait and M. J. Blaser, Obese mice losing weight due to trans-10, cis-12 conjugated linoleic acid supplementation or food restriction harbor distinct gut microbiota, J. Nutr., 2018, 148, 562–572 CrossRef.
- X. Shao, C. Z. Sun, X. Tang, X. S. Zhang, D. Han, S. Liang, R. Qu, X. D. Hui, Y. W. Shan, L. H. Hu, H. Fang, H. D. Zhang, X. Y. Wu and C. B. Chen, Anti-inflammatory and intestinal microbiota modulation properties of Jinxiang garlic (Allium sativum L.) polysaccharides toward dextran sodium sulfate-induced colitis, J. Agric. Food Chem., 2020, 68, 12295–12309 CrossRef CAS PubMed.
- X. Ran, G. Q. Hu, F. D. He, K. F. Li, F. Li, D. W. Xu, J. X. Liu and S. P. Fu, Phytic acid improves hepatic steatosis, inflammation, and oxidative stress in high-fat diet (HFD)-fed mice by modulating the gut-liver axis, J. Agric. Food Chem., 2022, 70, 11401–11411 CrossRef CAS PubMed.
- A. D. Truax, L. Chen, J. W. Tam, N. Cheng, H. Guo, A. A. Koblansky, W. C. Chou, J. E. Wilson, W. J. Brickey, A. Petrucelli, R. R. Liu, D. E. Cooper, M. J. Koenigsknecht, V. B. Young, M. G. Netea, R. Stienstra, R. B. Sartor, S. A. Montgomery, R. A. Coleman and J. P. Y. Ting, The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating gut microbiota homeostasis, Cell Host Microbe, 2018, 24, 364–378 CrossRef CAS PubMed.
- C. H. Zhu, Y. X. Li, Y. C. Xu, N. N. Wang, Q. J. Yan and Z. Q. Jiang, Tamarind xyloglucan oligosaccharides attenuate metabolic disorders via the gut-liver axis in mice with high-fat-diet-induced obesity, Foods, 2023, 12, 17 Search PubMed.
- S. L. Collins, J. C. Stine, J. E. Bisanz, C. D. Okafor and A. D. Patterson, Bile acids and the gut microbiota: Metabolic interactions and impacts on disease, Nat. Rev. Microbiol., 2023, 21, 236–247 CrossRef CAS.
- R. A. G. Pushpass, S. Alzoufairi, K. G. Jackson and J. A. Lovegrove, Circulating bile acids as a link between the gut microbiota and cardiovascular health: impact of prebiotics, probiotics and polyphenol-rich foods, Nutr. Res. Rev., 2022, 35, 161–180 CrossRef CAS.
- Q. M. Shi, X. Yuan, Y. F. Zeng, J. Z. Wang, Y. Q. Zhang, C. Xue and L. J. Li, Crosstalk between gut microbiota and bile acids in cholestatic liver disease, Nutrients, 2023, 15, 2411 CrossRef CAS PubMed.
- J. Z. Chen, M. Thomsen and L. Vitetta, Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics, J. Cell. Biochem., 2019, 120, 2713–2720 CrossRef CAS.
- Z. Song, Y. Cai, X. Lao, X. Wang, X. Lin, Y. Cui, P. K. Kalavagunta, J. Liao, L. Jin, J. Shang and J. Li, Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome, Microbiome, 2019, 7, 9 CrossRef PubMed.
- M. Vital, T. Rud, S. Rath, D. H. Pieper and D. Schlüter, Diversity of bacteria exhibiting bile acid-inducible 7α-dehydroxylation genes in the human gut, Comput. Struct. Biotechnol. J., 2019, 17, 1016–1019 CrossRef CAS.
- S. Yoshimoto, T. M. Loo, K. Atarashi, H. Kanda, S. Sato, S. Oyadomari, Y. Iwakura, K. Oshima, H. Morita, M. Hattori, K. Honda, Y. Ishikawa, E. Hara and N. Ohtani, Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome, Nature, 2013, 499, 97 CrossRef CAS PubMed.
- L. Li, Z. Y. Gu and J. J. Zhang, CTRP9 overexpression attenuates palmitic acid-induced inflammation, apoptosis and impaired migration in HTR8/SVneo cells through AMPK/SREBP1c signaling, Exp. Ther. Med., 2022, 24, 10 Search PubMed.
- D. Y. Kim, H. D. Yuan, I. K. Chung and S. H. Chung, Compound K, intestinal metabolite of Ginsenoside, attenuates hepatic lipid accumulation via AMPK activation in Human hepatoma cells, J. Agric. Food Chem., 2009, 57, 1532–1537 CrossRef CAS.
- S. Lee, A. M. Benvie, H. G. Park, R. Spektor, B. Harlan, J. T. Brenna, D. C. Berry and P. D. Soloway, Remodeling of gene regulatory networks underlying thermogenic stimuli-induced adipose beiging, Commun. Biol., 2022, 5, 16 CrossRef.
- D. Sag, D. Carling, R. D. Stout and J. Suttles, Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype, J. Immunol., 2008, 181, 8633–8641 CrossRef CAS PubMed.
- D. Li, C-M. Liu, R. Luo, K. Sadakane and T.-W. Lam, MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph, Bioinformatics, 2015, 31, 1674–1676 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4fo03417a |
| This journal is © The Royal Society of Chemistry 2025 |